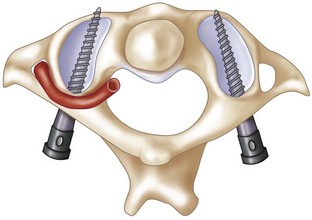
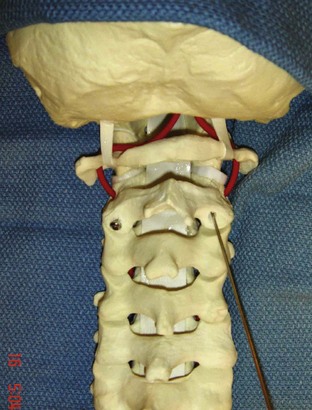
Procedure 11 Posterior C1-C2 Fusion
Harms and Magerl Techniques
Technique A: Posterior C1-2 Polyaxial Screw and Rod Fixation (Harms Technique) (Harms and Melcher, 2001)
Indications
Indications Pearls
• Similar risk of vertebral artery injury compared to transarticular screws (Yoshida et al, 2006).
• Does not require the use of sublaminar wires, thus decreasing the risk of neural injury.
• Screws can assist in the C1-2 reduction.
• Integrity of the posterior arch of C1 is not required.
• Can be incorporated as part of fusions to the occiput and/or the subaxial spine.
Examination/Imaging
 Neurologic and musculoskeletal examination.
Neurologic and musculoskeletal examination.
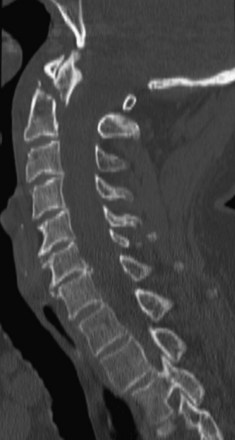
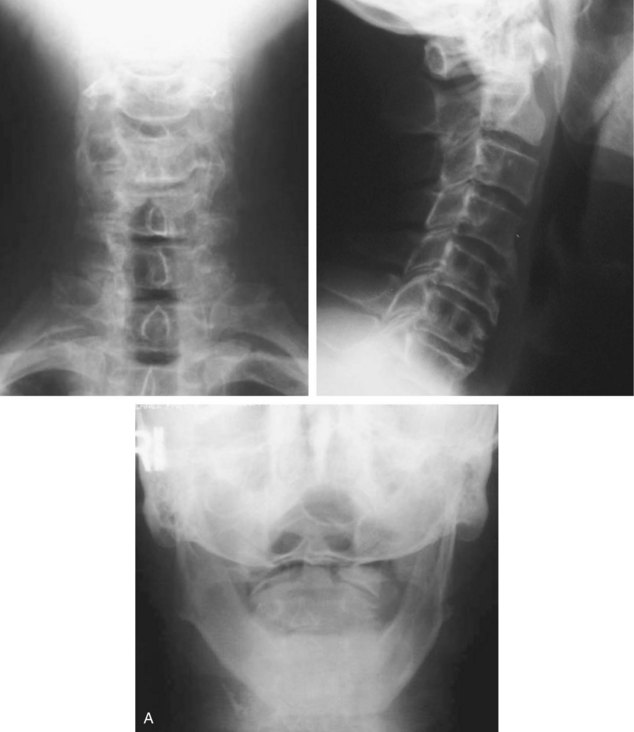
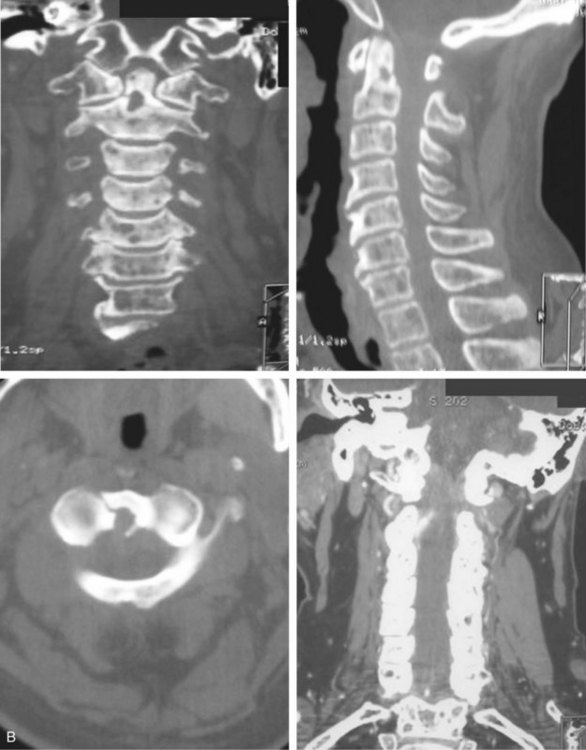
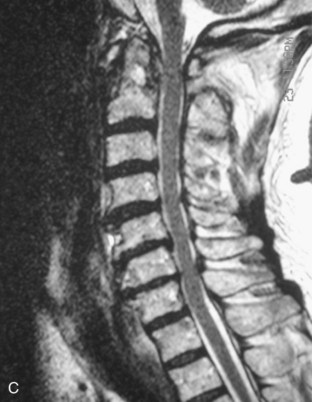
 Preoperative imaging should include plain radiographs (Figure 11-3, A), computed tomography (CT) (Figure 11-3, B), CT angiography, and magnetic resonance imaging (MRI) (Figure 11-3, C) of the cervical spine.
Preoperative imaging should include plain radiographs (Figure 11-3, A), computed tomography (CT) (Figure 11-3, B), CT angiography, and magnetic resonance imaging (MRI) (Figure 11-3, C) of the cervical spine.
 Noninvasive magnetic resonance angiography (MRA) can be utilized to evaluate vertebral artery injury, patency, and/or dominance (in lieu of CT angiography that requires administration of dye contrast).
Noninvasive magnetic resonance angiography (MRA) can be utilized to evaluate vertebral artery injury, patency, and/or dominance (in lieu of CT angiography that requires administration of dye contrast).
Surgical Anatomy
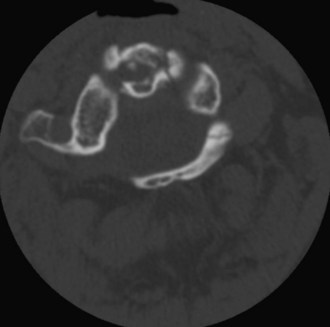
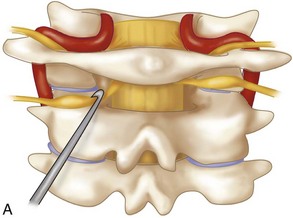
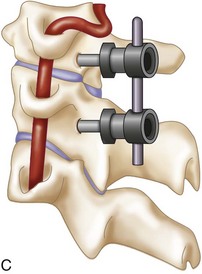
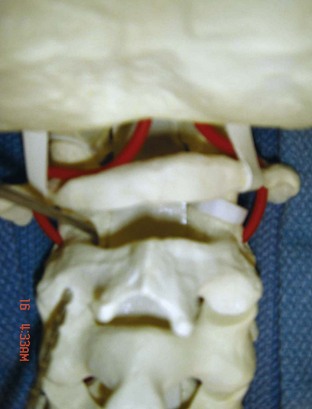
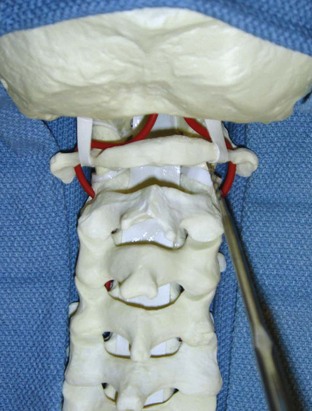
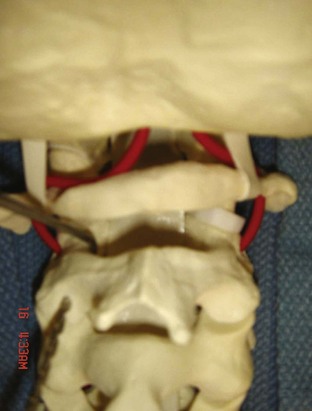
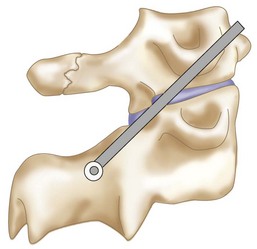
 The posterior arch of C1 and the C1-2 facet joint are key anatomic landmarks for the placement of C1 lateral mass screws. The dorsal root ganglion of C2 lies just posterior to the starting point of the C1 screw and must be gently retracted caudally for adequate exposure (Figure 11-4, A). The starting point for the C1 screw is at the midpoint of the inferior portion of the C1 lateral mass at its junction with the posterior arch. The more superior and medial trajectory of the screws, when compared with transarticular screws, decreases the risk of vertebral artery injury (Figure 11-4, B and C)
The posterior arch of C1 and the C1-2 facet joint are key anatomic landmarks for the placement of C1 lateral mass screws. The dorsal root ganglion of C2 lies just posterior to the starting point of the C1 screw and must be gently retracted caudally for adequate exposure (Figure 11-4, A). The starting point for the C1 screw is at the midpoint of the inferior portion of the C1 lateral mass at its junction with the posterior arch. The more superior and medial trajectory of the screws, when compared with transarticular screws, decreases the risk of vertebral artery injury (Figure 11-4, B and C)
 The ponticulus posticus or congenital arcuate foramen is a common bony anomaly of the atlas (Young et al, 2005) (Figure 11-4, D). It is a bony arch on the cephalad aspect of the C1 lamina that contains the vertebral artery. If present, it can easily be confused with the lamina of C1 and must be identified during the posterior dissection and placement of C1 lateral mass screws to prevent vertebral artery injury.
The ponticulus posticus or congenital arcuate foramen is a common bony anomaly of the atlas (Young et al, 2005) (Figure 11-4, D). It is a bony arch on the cephalad aspect of the C1 lamina that contains the vertebral artery. If present, it can easily be confused with the lamina of C1 and must be identified during the posterior dissection and placement of C1 lateral mass screws to prevent vertebral artery injury.
Positioning
 After an awake fiberoptic nasotracheal intubation is performed, a nasogastric tube is inserted for intraoperative gastric drainage.
After an awake fiberoptic nasotracheal intubation is performed, a nasogastric tube is inserted for intraoperative gastric drainage.
 If the patient is immobilized in a halo vest preoperatively, either the halo can be left in place and attached directly to the Mayfield headholder using an adapter or it can be removed. If the halo ring is removed, the patient is placed in Mayfield tongs and a hard cervical collar before being turned into the prone position. In coordination with anesthesia, the surgeon stands at the head of the hospital bed and stabilizes the patient’s neck. The patient is cautiously turned in the prone position on the operating table with the torso on bolsters or a four-poster frame. The Mayfield tongs or the halo ring is fixed to the operating table using a Mayfield headholder with the neck in a neutral position (Figure 11-5, A and B).
If the patient is immobilized in a halo vest preoperatively, either the halo can be left in place and attached directly to the Mayfield headholder using an adapter or it can be removed. If the halo ring is removed, the patient is placed in Mayfield tongs and a hard cervical collar before being turned into the prone position. In coordination with anesthesia, the surgeon stands at the head of the hospital bed and stabilizes the patient’s neck. The patient is cautiously turned in the prone position on the operating table with the torso on bolsters or a four-poster frame. The Mayfield tongs or the halo ring is fixed to the operating table using a Mayfield headholder with the neck in a neutral position (Figure 11-5, A and B).
 All bony prominences are well padded, and the patient’s arms are secured by their side using a folded sheet that is tucked beneath them.
All bony prominences are well padded, and the patient’s arms are secured by their side using a folded sheet that is tucked beneath them.
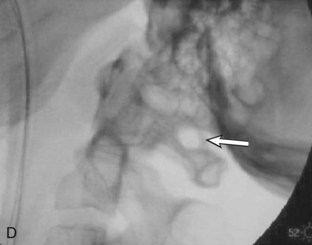
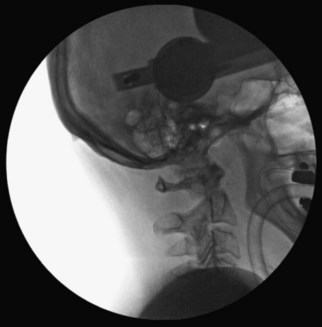
 Using fluoroscopic C-arm, proper alignment of the atlantoaxial bony structures is confirmed with the radiograph centered at C1-2. The lateral fluoroscopic image must not be oblique at C1-2; otherwise, malpositioning of the drill can result in erroneous screw placement (Figure 11-6).
Using fluoroscopic C-arm, proper alignment of the atlantoaxial bony structures is confirmed with the radiograph centered at C1-2. The lateral fluoroscopic image must not be oblique at C1-2; otherwise, malpositioning of the drill can result in erroneous screw placement (Figure 11-6).
 If necessary, adjustments can be made while the patient is in the Mayfield headholder, to obtain reduction. Reduction should be confirmed on fluoroscopic radiograph. If possible, extreme positions of the neck should be avoided.
If necessary, adjustments can be made while the patient is in the Mayfield headholder, to obtain reduction. Reduction should be confirmed on fluoroscopic radiograph. If possible, extreme positions of the neck should be avoided.
 Somatosensory evoked potential (SSEP) and transcranial motor evoked potential (MEP) monitoring are neurophysiologic spinal cord monitoring methods that can be utilized intraoperatively. Baseline readings can be obtained before and after placing the patient in the prone position.
Somatosensory evoked potential (SSEP) and transcranial motor evoked potential (MEP) monitoring are neurophysiologic spinal cord monitoring methods that can be utilized intraoperatively. Baseline readings can be obtained before and after placing the patient in the prone position.
Positioning Pearls
• An open-mouth view is obtained by placing an appropriate-size roll of sterile gauze in the patient’s mouth to facilitate a clear open-mouth view.
• In very osteopenic bone, inverse (negative) radiologic images can be utilized for better bony visualization.
• On the lateral C-arm image, the posterior occiput should be flexed off the posterior arch of C1 to facilitate screw placement at C1.
Portals/Exposures
 An electric razor is used to remove all hair from the patient’s occipital, suboccipital, and neck regions. If a definitive fusion is being performed, the posterior iliac crest is also shaved for bone graft harvesting.
An electric razor is used to remove all hair from the patient’s occipital, suboccipital, and neck regions. If a definitive fusion is being performed, the posterior iliac crest is also shaved for bone graft harvesting.
 The skin surfaces of the neck and posterior iliac crest are prepared and draped in a sterile fashion.
The skin surfaces of the neck and posterior iliac crest are prepared and draped in a sterile fashion.
 Using the inion of the occiput cranially, and the protuberance of the vertebral prominens caudally, the midline is identified and marked from the occiput to C3-4 with a sterile marker.
Using the inion of the occiput cranially, and the protuberance of the vertebral prominens caudally, the midline is identified and marked from the occiput to C3-4 with a sterile marker.
 The subcutaneous skin of the planned skin incision can be infiltrated with 0.5% lidocaine containing epinephrine diluted 1:100,000.
The subcutaneous skin of the planned skin incision can be infiltrated with 0.5% lidocaine containing epinephrine diluted 1:100,000.
 A 10-blade scalpel is used to sharply incise the skin in the midline from the occiput to C3-4.
A 10-blade scalpel is used to sharply incise the skin in the midline from the occiput to C3-4.
 Bovie electrocautery is used for the subcutaneous dissection down to and through the underlying ligamentum nuchae. Midline dissection of the nuchal ligament provides a relatively avascular dissection and decreases the risk of injury to the greater and third occipital nerves. Self-retaining retractors are inserted for adequate visualization.
Bovie electrocautery is used for the subcutaneous dissection down to and through the underlying ligamentum nuchae. Midline dissection of the nuchal ligament provides a relatively avascular dissection and decreases the risk of injury to the greater and third occipital nerves. Self-retaining retractors are inserted for adequate visualization.
 At the cephalad end of the incision, a 1.5-cm fascial cuff of trapezius, along the nuchal ridge, can be elevated to facilitate lateral exposure of C1-2, but this is not usually necessary. Subperiosteal dissection of the paraspinous muscular insertions from the suboccipital bone is completed.
At the cephalad end of the incision, a 1.5-cm fascial cuff of trapezius, along the nuchal ridge, can be elevated to facilitate lateral exposure of C1-2, but this is not usually necessary. Subperiosteal dissection of the paraspinous muscular insertions from the suboccipital bone is completed.
 The midline tubercle of the arch of C1 and the larger spinous process of C2 are used as palpable landmarks during dissection. Starting at the midline, the periosteum of C1 and the tip of the spinous processes of C2 and C3 are incised sharply.
The midline tubercle of the arch of C1 and the larger spinous process of C2 are used as palpable landmarks during dissection. Starting at the midline, the periosteum of C1 and the tip of the spinous processes of C2 and C3 are incised sharply.
 Careful subperiosteal dissection is continued from C3 to C1, starting in the midline and proceeding laterally. Periosteal elevators can facilitate the subperiosteal dissection of the paraspinous muscles as they are swept laterally. The lateral masses and pedicles of C3 and C2 are exposed with care not to disturb the C2-3 facet capsules.
Careful subperiosteal dissection is continued from C3 to C1, starting in the midline and proceeding laterally. Periosteal elevators can facilitate the subperiosteal dissection of the paraspinous muscles as they are swept laterally. The lateral masses and pedicles of C3 and C2 are exposed with care not to disturb the C2-3 facet capsules.
 The C1-2 joint can be exposed with dissection over the superior surface of the C2 pars. Significant venous bleeding can be encountered with dissection around the venous plexus of the C2 nerve. This can effectively be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, cotton pledgets, and various commercial gelatin thrombin preparations.
The C1-2 joint can be exposed with dissection over the superior surface of the C2 pars. Significant venous bleeding can be encountered with dissection around the venous plexus of the C2 nerve. This can effectively be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, cotton pledgets, and various commercial gelatin thrombin preparations.
 To decrease the risk of injuring the vertebral artery on the cephalic surface of the C1 lamina, identify the lamina and follow the caudal edge of the posterior arch during exposure of C1. If present, the ponticulus posticus or congenital arcuate foramen must be identified during the posterior dissection, because it can easily be confused with the lamina of C1 (Young et al, 2005).
To decrease the risk of injuring the vertebral artery on the cephalic surface of the C1 lamina, identify the lamina and follow the caudal edge of the posterior arch during exposure of C1. If present, the ponticulus posticus or congenital arcuate foramen must be identified during the posterior dissection, because it can easily be confused with the lamina of C1 (Young et al, 2005).
 The dissection is complete with exposure of the suboccipital rim of the foramen magnum.
The dissection is complete with exposure of the suboccipital rim of the foramen magnum.
Portals/Exposures Pearls
• The C2 spinous process is an easily identifiable landmark. The C2 spinous process sits more posterior relative to the arch of C1 and can be used to orient your dissection.
• The cephalad orientation of the C2 pars necessitates exposure down to C3. This facilitates the placement of the C2 pars screw.
• The lateral dissection should not be carried past the lateral border of the C1-2 articulation to avoid iatrogenic injury of the vertebral artery.
Procedure
Step 1
 The dorsal root ganglion of C2 must be carefully retracted caudally to expose the starting point for the C1 lateral mass screw. The starting point for the C1 screw is at the midpoint of the inferior portion of the C1 lateral mass at its junction with the posterior arch.
The dorsal root ganglion of C2 must be carefully retracted caudally to expose the starting point for the C1 lateral mass screw. The starting point for the C1 screw is at the midpoint of the inferior portion of the C1 lateral mass at its junction with the posterior arch.
 C-arm imaging can be used to verify the midpoint and trajectory of the C1 lateral mass screw.
C-arm imaging can be used to verify the midpoint and trajectory of the C1 lateral mass screw.
 A 2-mm high-speed burr is used to mark the starting point for the drill and prevent the drill from walking off the convex surface of the posterior inferior lateral mass of C1.
A 2-mm high-speed burr is used to mark the starting point for the drill and prevent the drill from walking off the convex surface of the posterior inferior lateral mass of C1.
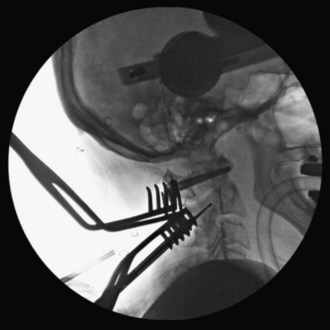
 With the tip of the drill pointing anterior through the lateral mass of C1, a 2-mm drill bit is used to drill a bicortical pilot hole in a straight to slightly convergent trajectory in the anteroposterior plane, and parallel to the posterior arch of C1 in the sagittal plane (Seal et al, 2009). Drill position is confirmed on AP and lateral C-arm fluoroscopic images (Figures 11-7 and 11-8).
With the tip of the drill pointing anterior through the lateral mass of C1, a 2-mm drill bit is used to drill a bicortical pilot hole in a straight to slightly convergent trajectory in the anteroposterior plane, and parallel to the posterior arch of C1 in the sagittal plane (Seal et al, 2009). Drill position is confirmed on AP and lateral C-arm fluoroscopic images (Figures 11-7 and 11-8).
 A depth gauge can be used to confirm the measurement obtained from the preoperative CT scan of the appropriate length screw and can be checked on lateral fluoroscopic radiograph.
A depth gauge can be used to confirm the measurement obtained from the preoperative CT scan of the appropriate length screw and can be checked on lateral fluoroscopic radiograph.
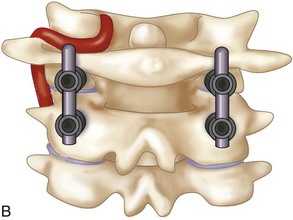
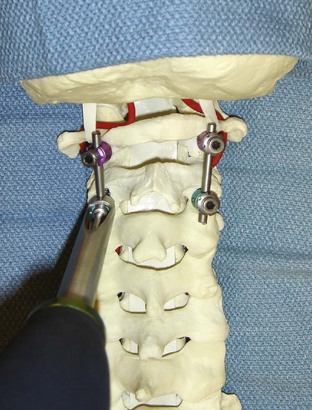
 The drill hole is tapped and the 3.5-mm polyaxial screw is placed into the C1 lateral mass. An 8-mm unthreaded portion of the C1 polyaxial screw sits proud above the bony surface of the lateral mass, allowing the polyaxial portion of the screw to sit above the posterior arch of C1 so that the rod can be linked to the C2 screw head. The proud segment of the screw is unthreaded and theoretically minimizes the risk of irritation of the greater occipital nerve.
The drill hole is tapped and the 3.5-mm polyaxial screw is placed into the C1 lateral mass. An 8-mm unthreaded portion of the C1 polyaxial screw sits proud above the bony surface of the lateral mass, allowing the polyaxial portion of the screw to sit above the posterior arch of C1 so that the rod can be linked to the C2 screw head. The proud segment of the screw is unthreaded and theoretically minimizes the risk of irritation of the greater occipital nerve.
Step 1 Pearls
• Critical landmarks for the accurate placement of C1 lateral mass screws
• The ponticulus posticus or congenital arcuate foramen can be confused with the C1 lamina and must be identified to prevent vertebral artery injury during the posterior dissection and placement of C1 lateral mass screws (Young et al, 2005).
• A superior and slightly medial trajectory (0 to 10 degrees) of the C1 lateral mass screw decreases the risk of vertebral artery injury (Figure 11-11).
Step 2
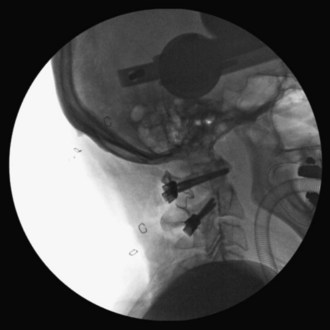
 At C2, a no. 4 Penfield is used to define the medial border of the C2 pars. The starting point for the C2 pars interarticularis screw is in the superior and medial quadrant of the C2 pars. The entry point for placement of the C2 pars screw is marked with the 2-mm high-speed burr (Figure 11-12).
At C2, a no. 4 Penfield is used to define the medial border of the C2 pars. The starting point for the C2 pars interarticularis screw is in the superior and medial quadrant of the C2 pars. The entry point for placement of the C2 pars screw is marked with the 2-mm high-speed burr (Figure 11-12).
 The starting hole and drill bit trajectory can be confirmed under C-arm guidance with open-mouth and lateral views.
The starting hole and drill bit trajectory can be confirmed under C-arm guidance with open-mouth and lateral views.
 A pilot hole is made using a 2-mm drill bit in a 20- to 30-degree convergent and cephalad trajectory, using the superior and medial aspect of the C2 pars as a guide. The integrity of the walls of the pilot drill hole is confirmed with a blunt pedicle probe.
A pilot hole is made using a 2-mm drill bit in a 20- to 30-degree convergent and cephalad trajectory, using the superior and medial aspect of the C2 pars as a guide. The integrity of the walls of the pilot drill hole is confirmed with a blunt pedicle probe.
 A depth gauge can be used to confirm the measurement obtained from the preoperative CT scan of the appropriate length screw and can be checked on lateral fluoroscopic radiograph.
A depth gauge can be used to confirm the measurement obtained from the preoperative CT scan of the appropriate length screw and can be checked on lateral fluoroscopic radiograph.
 The drill hole is tapped, and the 3.5-mm polyaxial screw is placed into the C2 pars (Wait et al, 2009) (Figure 11-13).
The drill hole is tapped, and the 3.5-mm polyaxial screw is placed into the C2 pars (Wait et al, 2009) (Figure 11-13).
Step 2 Pearls
• Intraoperative landmarks, the preoperative thin-cut (1-mm) axial CT scan, and lateral and open-mouth fluoroscopic imaging can all aid in the accurate placement of the C1 lateral mass and C2 pars interarticularis screws.
• Alternative procedures for patients with unilateral vertebral artery anomalies at C2
Step 3
 If reduction of C1 is necessary, the patient’s head can be repositioned before fixation of the rods to the screws. When performed, the reduction is visualized under fluoroscopy.
If reduction of C1 is necessary, the patient’s head can be repositioned before fixation of the rods to the screws. When performed, the reduction is visualized under fluoroscopy.
Step 3 Pearls
• C1-2 reduction can be accomplished by direct manipulation of the C1 and C2 screws. The authors’ recommendation is to obtain a reduction preoperatively, if possible while the patient is awake, to assess neurologic status. Alternatively, reduction can be performed after the patient is positioned prone on the operating table before preparation and draping.
Step 4
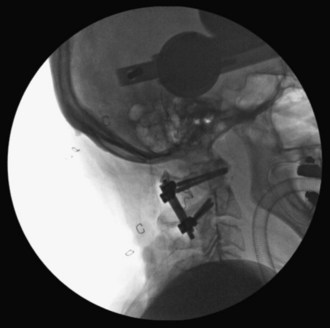
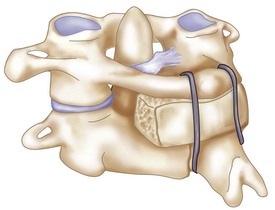
 The interconnecting rods are measured and secured with locking nuts (Figure 11-14).
The interconnecting rods are measured and secured with locking nuts (Figure 11-14).
 Distraction or compression of the construct can be accomplished at this time.
Distraction or compression of the construct can be accomplished at this time.
 The locking nuts are tightened with a torque wrench (Figure 11-15).
The locking nuts are tightened with a torque wrench (Figure 11-15).
Step 4 Pearls
• With this technique, one can avoid damage to the C1-2 facet joints, and the rods and screws can be used as temporary fracture fixation without definitive fusion (i.e., type II and III odontoid fractures) (Harms and Melcher, 2001). Eventual removal of the hardware can allow the patient to regain atlantoaxial motion after fracture healing has occurred.
• The integrity of the posterior arch of C1 is not necessary for stable fixation.
• Patients with rheumatoid arthritis often have instability adjacent to the atlantoaxial region requiring a more extensive fusion. This technique can be incorporated as part of fusions to the occiput and/or the subaxial spine.
Step 5
 For definitive fusion, posterior iliac crest bone graft is harvested. The posterior superior iliac crest is palpated, and an 8-cm line centered over the crest is marked with a sterile marking pen. A 10-blade scalpel is used to incise the skin. Self-retaining retractors are inserted.
For definitive fusion, posterior iliac crest bone graft is harvested. The posterior superior iliac crest is palpated, and an 8-cm line centered over the crest is marked with a sterile marking pen. A 10-blade scalpel is used to incise the skin. Self-retaining retractors are inserted.
 Bovie electrocautery is used to dissect the subcutaneous tissue down to the junction of the lumbodorsal and gluteus maximus fascia.
Bovie electrocautery is used to dissect the subcutaneous tissue down to the junction of the lumbodorsal and gluteus maximus fascia.
 The posterior superior iliac crest is palpated, and the fascia is initially reflected using Bovie electrocautery. A subperiosteal dissection is performed using a Cobb elevator at the superior and lateral margins of the crest. Osteotomes are used to make a cortical window in the crest that is reflected medially. The window is made within 8 cm of the posterior superior iliac crest to avoid injury to the superior cluneal nerves.
The posterior superior iliac crest is palpated, and the fascia is initially reflected using Bovie electrocautery. A subperiosteal dissection is performed using a Cobb elevator at the superior and lateral margins of the crest. Osteotomes are used to make a cortical window in the crest that is reflected medially. The window is made within 8 cm of the posterior superior iliac crest to avoid injury to the superior cluneal nerves.
 Small gouges are used to harvest cancellous bone graft through the cortical window.
Small gouges are used to harvest cancellous bone graft through the cortical window.
 The graft is placed in a sterile cup mixed with the patient’s blood and covered.
The graft is placed in a sterile cup mixed with the patient’s blood and covered.
 Hemostasis is obtained with bone wax. The wound is irrigated and the graft site is packed with Marcaine-soaked Gelfoam, and the cortical window is closed.
Hemostasis is obtained with bone wax. The wound is irrigated and the graft site is packed with Marcaine-soaked Gelfoam, and the cortical window is closed.
 The retractors are removed and the incision is closed in layers.
The retractors are removed and the incision is closed in layers.
Step 6
 The cervical operative field is irrigated with antibiotic solution, and the irrigation fluid is suctioned from the field.
The cervical operative field is irrigated with antibiotic solution, and the irrigation fluid is suctioned from the field.
 If definitive fusion is being performed, the posterior surfaces of C1 and C2 are decorticated with the high-speed burr. Alternatively, decortication of the posterior aspects of C1 and C2 can be performed before insertion of the screws and rods.
If definitive fusion is being performed, the posterior surfaces of C1 and C2 are decorticated with the high-speed burr. Alternatively, decortication of the posterior aspects of C1 and C2 can be performed before insertion of the screws and rods.
 The cancellous bone graft is placed over the decorticated surfaces of C1 and C2.
The cancellous bone graft is placed over the decorticated surfaces of C1 and C2.
 The C1-2 joint surfaces can also be decorticated and packed with bone graft for an intraarticular fusion.
The C1-2 joint surfaces can also be decorticated and packed with bone graft for an intraarticular fusion.
 Alternatively, a piece of iliac crest bone graft can be fashioned and secured between the C1 ring and C2 spinous process.
Alternatively, a piece of iliac crest bone graft can be fashioned and secured between the C1 ring and C2 spinous process.
Step 7
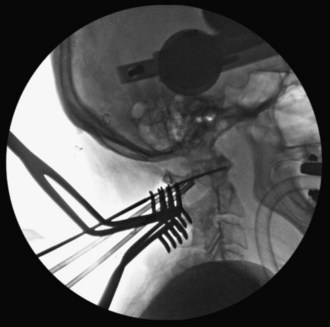
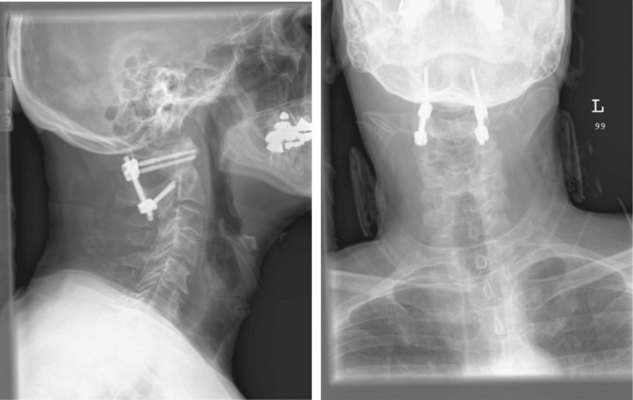
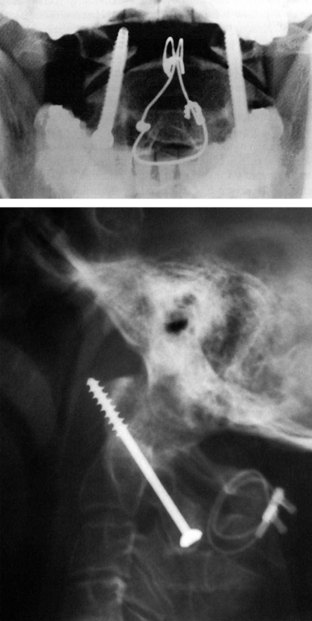
 Final AP and lateral cervical radiographs are obtained to assess placement of the hardware and alignment of the atlantoaxial region.
Final AP and lateral cervical radiographs are obtained to assess placement of the hardware and alignment of the atlantoaxial region.
 The wound is closed securely in a layered fashion obliterating any dead space.
The wound is closed securely in a layered fashion obliterating any dead space.
 A subfascial drain is placed if thought to be needed by the surgeon.
A subfascial drain is placed if thought to be needed by the surgeon.
 Steri-Strips are placed perpendicular to the incision and covered by sterile 4 × 4 gauze and a clear Tegaderm dressing.
Steri-Strips are placed perpendicular to the incision and covered by sterile 4 × 4 gauze and a clear Tegaderm dressing.
 A rigid cervical collar (i.e., Philadelphia or Miami J) is secured in place.
A rigid cervical collar (i.e., Philadelphia or Miami J) is secured in place.
 The surgeon stands at the head of the operating table and is responsible for stabilizing the neck, when the patient is repositioned onto the hospital bed in the supine position. The patient is removed from the Mayfield headholder (Figure 11-16).
The surgeon stands at the head of the operating table and is responsible for stabilizing the neck, when the patient is repositioned onto the hospital bed in the supine position. The patient is removed from the Mayfield headholder (Figure 11-16).
Postoperative Care and Expected Outcomes
 The patient should be taken to the recovery room or the surgical intensive care unit (SICU) for postoperative recovery.
The patient should be taken to the recovery room or the surgical intensive care unit (SICU) for postoperative recovery.
 Supine and upright lateral cervical radiographs should be obtained in the cervical collar to assess stability on postoperative day 1. If atlantoaxial stability has been obtained, the patient can be mobilized (see Figure 11-16).
Supine and upright lateral cervical radiographs should be obtained in the cervical collar to assess stability on postoperative day 1. If atlantoaxial stability has been obtained, the patient can be mobilized (see Figure 11-16).
 On postoperative day 1, or when medically stable, the patient can be transferred to a standard surgical floor.
On postoperative day 1, or when medically stable, the patient can be transferred to a standard surgical floor.
 A postoperative CT scan can be obtained if there is any question concerning screw placement.
A postoperative CT scan can be obtained if there is any question concerning screw placement.
 The patient can be discharged from the hospital when medically stable.
The patient can be discharged from the hospital when medically stable.
 Rigid cervical collar immobilization is used postoperatively.
Rigid cervical collar immobilization is used postoperatively.
 Routine outpatient static lateral and supervised dynamic lateral flexion and extension radiographs can be obtained at approximately 4 weeks to ascertain stability. If stability is obtained, the cervical collar can be weaned from use. Radiographs are obtained at 4- to 6-week intervals to assess stability and fusion.
Routine outpatient static lateral and supervised dynamic lateral flexion and extension radiographs can be obtained at approximately 4 weeks to ascertain stability. If stability is obtained, the cervical collar can be weaned from use. Radiographs are obtained at 4- to 6-week intervals to assess stability and fusion.
 Additionally, a CT scan can be obtained 3 to 6 months postoperatively to assess fusion and fracture healing.
Additionally, a CT scan can be obtained 3 to 6 months postoperatively to assess fusion and fracture healing.
Postoperative Pitfalls
• Screw malposition can result in
Technique B: C1-2 Transarticular Facet Screws (Magerl Technique)
Indications
Indications Pitfalls
• Potential vertebral artery injury
• Maximum biomechanical stability achieved when combined with posterior wiring for three-point fixation (Henriques et al, 2000)
Examination and Imaging
 Complete neurologic and musculoskeletal examination
Complete neurologic and musculoskeletal examination
 Preoperative imaging should include plain radiographs (see Figure 11-3, A), CT (see Figure 11-3, B), CT angiography, and MRI (see Figure 11-3, C) of the cervical spine.
Preoperative imaging should include plain radiographs (see Figure 11-3, A), CT (see Figure 11-3, B), CT angiography, and MRI (see Figure 11-3, C) of the cervical spine.
 Noninvasive magnetic resonance angiography (MRA) can be utilized to evaluate vertebral artery injury, patency, and/or dominance.
Noninvasive magnetic resonance angiography (MRA) can be utilized to evaluate vertebral artery injury, patency, and/or dominance.
Surgical Anatomy
 The cephalad orientation of the C1-2 transarticular screw and the final position of the neck necessary for adequate atlantoaxial alignment may require percutaneous placement of the transarticular facet screws (Figure 11-17).
The cephalad orientation of the C1-2 transarticular screw and the final position of the neck necessary for adequate atlantoaxial alignment may require percutaneous placement of the transarticular facet screws (Figure 11-17).
 The ponticulus posticus or congenital arcuate foramen is a common bony anomaly of the atlas (Young et al, 2005). It is a bony arch on the cephalad aspect of the C1 lamina that contains the vertebral artery. If present, it can easily be confused with the lamina of C1 and must be identified during the posterior dissection to prevent vertebral artery injury.
The ponticulus posticus or congenital arcuate foramen is a common bony anomaly of the atlas (Young et al, 2005). It is a bony arch on the cephalad aspect of the C1 lamina that contains the vertebral artery. If present, it can easily be confused with the lamina of C1 and must be identified during the posterior dissection to prevent vertebral artery injury.
 The gray ramus communicans of the C2 nerve is a reliable landmark for locating the entry point for a screw on the C2 pars (Cavalcanti et al, 2010).
The gray ramus communicans of the C2 nerve is a reliable landmark for locating the entry point for a screw on the C2 pars (Cavalcanti et al, 2010).
Positioning
 After an awake fiberoptic nasotracheal intubation is performed, a nasogastric tube is inserted for intraoperative gastric drainage.
After an awake fiberoptic nasotracheal intubation is performed, a nasogastric tube is inserted for intraoperative gastric drainage.
 If the patient is immobilized in a halo vest, a rigid Philadelphia collar is placed on the patient before halo-vest removal. In coordination with anesthesia, the surgeon stands at the head of the hospital bed and stabilizes the patient’s neck. The patient is cautiously repositioned in the prone position on the operating table with the torso on bolsters or a four-poster frame. The halo ring can be attached to the Mayfield adapter. If the halo ring is removed, the patient is placed in Mayfield tongs before prone positioning. After repositioning the patient prone, the Mayfield tongs are fixed to the operating table using a Mayfield headholder with the neck in a neutral position.
If the patient is immobilized in a halo vest, a rigid Philadelphia collar is placed on the patient before halo-vest removal. In coordination with anesthesia, the surgeon stands at the head of the hospital bed and stabilizes the patient’s neck. The patient is cautiously repositioned in the prone position on the operating table with the torso on bolsters or a four-poster frame. The halo ring can be attached to the Mayfield adapter. If the halo ring is removed, the patient is placed in Mayfield tongs before prone positioning. After repositioning the patient prone, the Mayfield tongs are fixed to the operating table using a Mayfield headholder with the neck in a neutral position.
 All bony prominences are well padded, and the patient’s arms are secured by their side using a folded sheet that is tucked beneath them.
All bony prominences are well padded, and the patient’s arms are secured by their side using a folded sheet that is tucked beneath them.
 Using fluoroscopic C-arm, proper alignment of the atlantoaxial bony structures is confirmed with the radiograph centered at C1-2. The lateral fluoroscopic image must not be oblique at C1-2; otherwise incorrect screw trajectory can result.
Using fluoroscopic C-arm, proper alignment of the atlantoaxial bony structures is confirmed with the radiograph centered at C1-2. The lateral fluoroscopic image must not be oblique at C1-2; otherwise incorrect screw trajectory can result.
 If necessary, adjustments can be made while the patient is in the Mayfield headholder to obtain reduction and should be confirmed on fluoroscopic radiograph. If possible, extreme positions of the neck should be avoided.
If necessary, adjustments can be made while the patient is in the Mayfield headholder to obtain reduction and should be confirmed on fluoroscopic radiograph. If possible, extreme positions of the neck should be avoided.
 Somatosensory evoked potential and transcranial motor evoked potential monitoring are neurophysiologic spinal cord monitoring methods that can be utilized intraoperatively. Baseline readings can be obtained before and after placing the patient in the prone position.
Somatosensory evoked potential and transcranial motor evoked potential monitoring are neurophysiologic spinal cord monitoring methods that can be utilized intraoperatively. Baseline readings can be obtained before and after placing the patient in the prone position.
Portals/Exposures
 An electric razor is used to remove all hair from the patient’s occipital, suboccipital, and neck regions. If transarticular screw fixation with bone graft and sublaminar wiring is being performed, the posterior iliac crest is also shaved for bone graft harvesting.
An electric razor is used to remove all hair from the patient’s occipital, suboccipital, and neck regions. If transarticular screw fixation with bone graft and sublaminar wiring is being performed, the posterior iliac crest is also shaved for bone graft harvesting.
 The skin surfaces of the neck and posterior iliac crest are prepared and draped in sterile fashion.
The skin surfaces of the neck and posterior iliac crest are prepared and draped in sterile fashion.
 Using the inion of the occiput cranially, and the protuberance of the vertebral prominens caudally, the midline is identified and marked from the occiput to C3-4 with a sterile marker.
Using the inion of the occiput cranially, and the protuberance of the vertebral prominens caudally, the midline is identified and marked from the occiput to C3-4 with a sterile marker.
 The subcutaneous skin of the planned skin incision can be infiltrated with 0.5% lidocaine containing epinephrine diluted 1:100,000.
The subcutaneous skin of the planned skin incision can be infiltrated with 0.5% lidocaine containing epinephrine diluted 1:100,000.
 A 10-blade scalpel is used to sharply incise the skin in the midline from the occiput to C3-4.
A 10-blade scalpel is used to sharply incise the skin in the midline from the occiput to C3-4.
 Bovie electrocautery is used for the subcutaneous dissection down to and through the underlying ligamentum nuchae. Midline dissection of the nuchal ligament provides a relatively avascular dissection and decreases the risk of injury to the greater and third occipital nerves. Self-retaining retractors are inserted for adequate visualization.
Bovie electrocautery is used for the subcutaneous dissection down to and through the underlying ligamentum nuchae. Midline dissection of the nuchal ligament provides a relatively avascular dissection and decreases the risk of injury to the greater and third occipital nerves. Self-retaining retractors are inserted for adequate visualization.
 At the cephalad end of the incision, a 1.5-cm fascial cuff of trapezius, along the nuchal ridge, can be elevated to facilitate lateral exposure of C1-2, but this is not usually necessary. Subperiosteal dissection of the paraspinous muscular insertions from the suboccipital bone is completed.
At the cephalad end of the incision, a 1.5-cm fascial cuff of trapezius, along the nuchal ridge, can be elevated to facilitate lateral exposure of C1-2, but this is not usually necessary. Subperiosteal dissection of the paraspinous muscular insertions from the suboccipital bone is completed.
 The midline tubercle of the arch of C1 and the larger spinous process of C2 are used as palpable landmarks during dissection. Starting at the midline, the periosteum of C1 and the tip of the spinous processes of C2 and C3 are incised sharply.
The midline tubercle of the arch of C1 and the larger spinous process of C2 are used as palpable landmarks during dissection. Starting at the midline, the periosteum of C1 and the tip of the spinous processes of C2 and C3 are incised sharply.
 Careful subperiosteal dissection is continued from C3 to C1, starting in the midline and proceeding laterally. Periosteal elevators can facilitate the subperiosteal dissection of the paraspinous muscles as they are swept laterally. C2 and C3 are exposed laterally with care not to disturb the C2-3 facet capsules.
Careful subperiosteal dissection is continued from C3 to C1, starting in the midline and proceeding laterally. Periosteal elevators can facilitate the subperiosteal dissection of the paraspinous muscles as they are swept laterally. C2 and C3 are exposed laterally with care not to disturb the C2-3 facet capsules.
 The C1-2 joint can be exposed with dissection over the superior surface of the C2 pars. The capsule of the C1-2 facet is reflected from caudal to cephalad, using caution not to injure the C2 nerve and surrounding vessels. Significant venous bleeding can be encountered with dissection around the venous plexus of the C2 nerve. This can effectively be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, and cotton pledgets.
The C1-2 joint can be exposed with dissection over the superior surface of the C2 pars. The capsule of the C1-2 facet is reflected from caudal to cephalad, using caution not to injure the C2 nerve and surrounding vessels. Significant venous bleeding can be encountered with dissection around the venous plexus of the C2 nerve. This can effectively be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, and cotton pledgets.
 To decrease the risk of injuring the vertebral artery on the cephalic surface of the C1 lamina, identify the lamina and follow the caudal edge of the posterior arch during exposure of C1.
To decrease the risk of injuring the vertebral artery on the cephalic surface of the C1 lamina, identify the lamina and follow the caudal edge of the posterior arch during exposure of C1.
 The dissection is complete with exposure of the suboccipital rim of the foramen magnum.
The dissection is complete with exposure of the suboccipital rim of the foramen magnum.
Portals/Exposures Pearls
• The C2 spinous process is an easily identifiable landmark. The C2 spinous process sits more posterior relative to the arch of C1 and can be used to orient your dissection.
• If present, the ponticulus posticus or congenital arcuate foramen can easily be confused with the lamina of C1. It must be identified during the posterior dissection to prevent vertebral artery injury.
• The lateral dissection should not be carried past the lateral border of the C1-2 articulation, to avoid iatrogenic injury of the vertebral artery.
Procedure
Step 1
 If posterior bone graft and sublaminar wiring are going to be utilized with transarticular screw fixation, bone graft harvesting and passage of the C1 sublaminar wire should be completed before the insertion of the transarticular screws.
If posterior bone graft and sublaminar wiring are going to be utilized with transarticular screw fixation, bone graft harvesting and passage of the C1 sublaminar wire should be completed before the insertion of the transarticular screws.
 After the dissection, the C1-2 posterior arch interspace and graft size is approximated. A moist sponge is placed in the wound to prevent tissue desiccation while harvesting the bone graft.
After the dissection, the C1-2 posterior arch interspace and graft size is approximated. A moist sponge is placed in the wound to prevent tissue desiccation while harvesting the bone graft.
 The posterior superior iliac crest is palpated, and an 8-cm line centered over the posterior superior iliac crest is marked with a sterile marking pen. A 10-blade scalpel is used to incise the skin. Self-retaining retractors are inserted.
The posterior superior iliac crest is palpated, and an 8-cm line centered over the posterior superior iliac crest is marked with a sterile marking pen. A 10-blade scalpel is used to incise the skin. Self-retaining retractors are inserted.
 Bovie electrocautery is used to dissect the subcutaneous tissue down to the junction of the lumbodorsal and gluteus maximus fascia.
Bovie electrocautery is used to dissect the subcutaneous tissue down to the junction of the lumbodorsal and gluteus maximus fascia.
 The posterior superior iliac crest is palpated, and the fascia is initially reflected using Bovie electrocautery. A subperiosteal dissection is performed using a Cobb elevator at the superior and lateral margins of the crest. Osteotomes and an oscillating saw are used to obtain a tricortical strut graft within 8 cm of the posterior superior iliac crest to avoid injury to the superior cluneal nerves.
The posterior superior iliac crest is palpated, and the fascia is initially reflected using Bovie electrocautery. A subperiosteal dissection is performed using a Cobb elevator at the superior and lateral margins of the crest. Osteotomes and an oscillating saw are used to obtain a tricortical strut graft within 8 cm of the posterior superior iliac crest to avoid injury to the superior cluneal nerves.
 A 1.5 × 4 cm tricortical strut graft is obtained. Small gouges/curettes are used to then harvest additional cancellous bone graft.
A 1.5 × 4 cm tricortical strut graft is obtained. Small gouges/curettes are used to then harvest additional cancellous bone graft.
 Hemostasis is obtained with bone wax. The wound is irrigated, and the graft site is packed with thrombin-soaked Gelfoam. The retractors are removed, the wound is irrigated, and the incision is closed in layers.
Hemostasis is obtained with bone wax. The wound is irrigated, and the graft site is packed with thrombin-soaked Gelfoam. The retractors are removed, the wound is irrigated, and the incision is closed in layers.
 Any soft tissue on the graft is removed with a small Cobb or curette. The graft is placed in a sterile cup mixed with the patient’s blood and covered.
Any soft tissue on the graft is removed with a small Cobb or curette. The graft is placed in a sterile cup mixed with the patient’s blood and covered.
Step 1 Pearls
• Stabilization of the C1-2 segment with transarticular screws can make the passage of the sublaminar wire more difficult and dangerous. Therefore it is the authors’ preference to pass the sublaminar wire before screw fixation.
• The C1 sublaminar wire can aid in reduction of the atlantoaxial segment, thus facilitating placement of the transarticular screws.
Step 2
 To prepare for the passage of the sublaminar wire, the ligamentum flavum on the underlying surfaces of the posterior arches of C1 and C2 is elevated off the superior and inferior surfaces of the lamina using a microcurette.
To prepare for the passage of the sublaminar wire, the ligamentum flavum on the underlying surfaces of the posterior arches of C1 and C2 is elevated off the superior and inferior surfaces of the lamina using a microcurette.
 A Woodson dissector can be used to carefully free any adherent portions of dura.
A Woodson dissector can be used to carefully free any adherent portions of dura.
 A 2-mm Kerrison rongeur is used to remove the ligamentum. Venous plexus bleeding deep to the ligamentum can be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, and pledget packing.
A 2-mm Kerrison rongeur is used to remove the ligamentum. Venous plexus bleeding deep to the ligamentum can be controlled with bipolar electrocautery, thrombin-soaked Gelfoam, and pledget packing.
Step 3
 A no. 4 Penfield elevator is used to define the medial border of the C2 pars. Care must be taken not to violate the dura with the Penfield. The Penfield can be used to protect the C2 nerve root and venous plexus with gentle rostral retraction. The removal of any ligamentum flavum adjacent to the C2 lamina will improve visualization of the C2 pars and atlantoaxial joint and help with orientation for transarticular screw placement.
A no. 4 Penfield elevator is used to define the medial border of the C2 pars. Care must be taken not to violate the dura with the Penfield. The Penfield can be used to protect the C2 nerve root and venous plexus with gentle rostral retraction. The removal of any ligamentum flavum adjacent to the C2 lamina will improve visualization of the C2 pars and atlantoaxial joint and help with orientation for transarticular screw placement.
Step 4
 Using the Gallie technique, a C1 sublaminar wire is passed before the placement of the transarticular screws. A smooth arch is bent into the end of 16- or 18-gauge double-looped wire and conformed to approximate both the length and thickness of the C1 lamina.
Using the Gallie technique, a C1 sublaminar wire is passed before the placement of the transarticular screws. A smooth arch is bent into the end of 16- or 18-gauge double-looped wire and conformed to approximate both the length and thickness of the C1 lamina.
 The wire is carefully passed beneath the C1 lamina. This maneuver can be facilitated by the use of 00-silk suture or a wire-passer. Using both hands, the wire is pulled beneath the C1 lamina. One hand pulls and advances the sublaminar wire, while the other hand provides constant gentle tension on the other end of the wire. This keeps the wire flush against the underside of the lamina, decreasing the risk of impinging the wire upon the cord.
The wire is carefully passed beneath the C1 lamina. This maneuver can be facilitated by the use of 00-silk suture or a wire-passer. Using both hands, the wire is pulled beneath the C1 lamina. One hand pulls and advances the sublaminar wire, while the other hand provides constant gentle tension on the other end of the wire. This keeps the wire flush against the underside of the lamina, decreasing the risk of impinging the wire upon the cord.
Step 5
 Atlantoaxial alignment is necessary for accurate placement of C1-2 transarticular screws. This is confirmed using lateral C-arm fluoroscopy (see Figure 11-17).
Atlantoaxial alignment is necessary for accurate placement of C1-2 transarticular screws. This is confirmed using lateral C-arm fluoroscopy (see Figure 11-17).
 If necessary, anterior atlantoaxial subluxation can be manually realigned intraoperatively with gentle traction on the C1 sublaminar wire and pressure on the axis.
If necessary, anterior atlantoaxial subluxation can be manually realigned intraoperatively with gentle traction on the C1 sublaminar wire and pressure on the axis.
 Posterior atlantoaxial subluxation can be realigned with flexion repositioning of the head in the Mayfield tongs. Alternatively, posterior atlantoaxial subluxation can be reduced with the placement of the bone block graft between C1 and C2. After placement, the graft can be manually manipulated against the posterior arch of C1 to obtain reduction. Reduction can be maintained after the C1 sublaminar wire is secured around the spinous process of C2 and the bone block graft.
Posterior atlantoaxial subluxation can be realigned with flexion repositioning of the head in the Mayfield tongs. Alternatively, posterior atlantoaxial subluxation can be reduced with the placement of the bone block graft between C1 and C2. After placement, the graft can be manually manipulated against the posterior arch of C1 to obtain reduction. Reduction can be maintained after the C1 sublaminar wire is secured around the spinous process of C2 and the bone block graft.
 The cephalad angle of the C1-2 transarticular screw can make placement difficult. To facilitate screw placement the axis can be gently displaced rostrally toward the occiput. This presents the C2 pars at a better angle for accurate placement of the transarticular screws.
The cephalad angle of the C1-2 transarticular screw can make placement difficult. To facilitate screw placement the axis can be gently displaced rostrally toward the occiput. This presents the C2 pars at a better angle for accurate placement of the transarticular screws.
 The position of the neck can also influence the trajectory necessary to obtain optimal placement of the transarticular screws. Percutaneous placement of the C1-2 facet screws may be necessary if intraoperative atlantoaxial alignment precludes the placement of the screws through the wound created by the posterior cervical dissection.
The position of the neck can also influence the trajectory necessary to obtain optimal placement of the transarticular screws. Percutaneous placement of the C1-2 facet screws may be necessary if intraoperative atlantoaxial alignment precludes the placement of the screws through the wound created by the posterior cervical dissection.
Step 6
 If the trajectory requires a percutaneous approach, a stab incision is made with a 15-blade scalpel through the skin into the paraspinal region of T2-3.
If the trajectory requires a percutaneous approach, a stab incision is made with a 15-blade scalpel through the skin into the paraspinal region of T2-3.
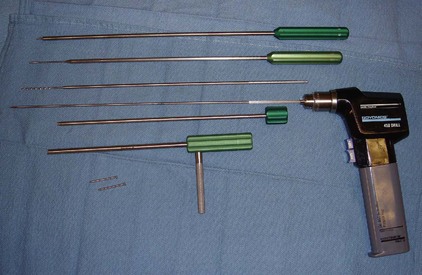
 A pointer tunneler is placed into the soft tissue protection sheath and inserted through the stab incision made at T2-3. The soft tissue protection sheath has a large handle that can be used to manipulate and guide your trajectory through the soft tissues.
A pointer tunneler is placed into the soft tissue protection sheath and inserted through the stab incision made at T2-3. The soft tissue protection sheath has a large handle that can be used to manipulate and guide your trajectory through the soft tissues.
 In the approximate trajectory necessary for transarticular screw placement, a tunnel can carefully be made through the soft tissues of the neck with the instruments exiting at the inferior aspect of the occipitocervical wound.
In the approximate trajectory necessary for transarticular screw placement, a tunnel can carefully be made through the soft tissues of the neck with the instruments exiting at the inferior aspect of the occipitocervical wound.
 The tip of the tissue sheath is placed against the inferior articular process of C2, and the pointed tunneler is removed from the tissue sheath.
The tip of the tissue sheath is placed against the inferior articular process of C2, and the pointed tunneler is removed from the tissue sheath.
Step 7
 The K-wire drill guide is inserted into the soft tissue sheath. A 1.2-mm diameter K-wire is then inserted into the drill guide and secured to a reversible pneumatic drill.
The K-wire drill guide is inserted into the soft tissue sheath. A 1.2-mm diameter K-wire is then inserted into the drill guide and secured to a reversible pneumatic drill.
 If the percutaneous technique is not required, the K-wire drill guide can be inserted directly into the soft tissue sheath and placed in the C2 fossa.
If the percutaneous technique is not required, the K-wire drill guide can be inserted directly into the soft tissue sheath and placed in the C2 fossa.
 The starting point for screw entry is in the C2 fossa, 2 to 3 mm lateral to the junction of the lamina and lateral mass (Figures 11-18 and 11-19).
The starting point for screw entry is in the C2 fossa, 2 to 3 mm lateral to the junction of the lamina and lateral mass (Figures 11-18 and 11-19).
 C-arm fluoroscopy is used to identify all bony atlantoaxial structures and guide the trajectory of the 1.2-mm K-wire. The K-wire is aimed toward the superior border of the lateral mass of C1, just lateral to the C2 pars interarticularis, and parallel to the medial border of the C2 pars interarticularis.
C-arm fluoroscopy is used to identify all bony atlantoaxial structures and guide the trajectory of the 1.2-mm K-wire. The K-wire is aimed toward the superior border of the lateral mass of C1, just lateral to the C2 pars interarticularis, and parallel to the medial border of the C2 pars interarticularis.
 The K-wire is advanced through the pars interarticularis of C2, along the central axis of the C2 pedicle in a 0- to 10-degree convergent trajectory in the AP plane.
The K-wire is advanced through the pars interarticularis of C2, along the central axis of the C2 pedicle in a 0- to 10-degree convergent trajectory in the AP plane.
 A no. 4 Penfield can be used to protect the C2 nerve root and venous plexus with gentle rostral retraction. This allows visualization of the K-wire as it passes across the posterior edge of the atlantoaxial facet joint into the lateral mass of C1. The tip of the K-wire should engage the anterosuperior margin of the C1 lateral mass.
A no. 4 Penfield can be used to protect the C2 nerve root and venous plexus with gentle rostral retraction. This allows visualization of the K-wire as it passes across the posterior edge of the atlantoaxial facet joint into the lateral mass of C1. The tip of the K-wire should engage the anterosuperior margin of the C1 lateral mass.
Step 7 Pearls
• To decrease the risk of vertebral artery injury, the K-wire must not be directed laterally, and must follow the medial wall of the C2 pars.
• Intraoperative bony landmarks, the preoperative thin-cut (1-mm) axial CT scan, and AP and lateral fluoroscopic imaging can all aid in the accurate placement of the K-wires and C1-2 transarticular screws (Weidner et al, 2000).
• Screw fixation with a cannulated 3.5-mm screw system is the authors’ preferred method for transarticular screw placement.
Step 8
 A cannulated drill bit is placed and drilled over the K-wire under C-arm fluoroscopy. Care must be taken as the drill is advanced over the K-wire. Because there is a risk of bending the tip of the K-wire as it crosses the C1-2 facet joint and penetrates the C1 cortical surface, impingement of the K-wire in the cannulated drill can occur. This can result in subsequent advancement of the K-wire into the posterior oropharyngeal fossa.
A cannulated drill bit is placed and drilled over the K-wire under C-arm fluoroscopy. Care must be taken as the drill is advanced over the K-wire. Because there is a risk of bending the tip of the K-wire as it crosses the C1-2 facet joint and penetrates the C1 cortical surface, impingement of the K-wire in the cannulated drill can occur. This can result in subsequent advancement of the K-wire into the posterior oropharyngeal fossa.
 The drill guide is removed from the tissue sheath and a second K-wire of identical length is placed in the tissue sheath until it rests on the posterior surface of C2 adjacent to the other K-wire. The difference in length of the K-wires determines the length of the screw, and it is measured with a ruler.
The drill guide is removed from the tissue sheath and a second K-wire of identical length is placed in the tissue sheath until it rests on the posterior surface of C2 adjacent to the other K-wire. The difference in length of the K-wires determines the length of the screw, and it is measured with a ruler.
 The near cortex is tapped with a cannulated tap through the tissue sheath.
The near cortex is tapped with a cannulated tap through the tissue sheath.
 A cannulated fully threaded 3.5-mm cortical screw of appropriate length (usually 40 to 45 mm) is inserted with a cannulated screwdriver over the K-wire under fluoroscopic visualization. The anterior cortex of the C1 lateral mass should be purchased with the screw. Unicortical screws may be considered to avoid neurovascular injury in cases with satisfactory bone quality (Cyr et al, 2008) (Figure 11-20).
A cannulated fully threaded 3.5-mm cortical screw of appropriate length (usually 40 to 45 mm) is inserted with a cannulated screwdriver over the K-wire under fluoroscopic visualization. The anterior cortex of the C1 lateral mass should be purchased with the screw. Unicortical screws may be considered to avoid neurovascular injury in cases with satisfactory bone quality (Cyr et al, 2008) (Figure 11-20).
 The K-wire is removed and screw placement can be confirmed with C-arm fluoroscopic imaging.
The K-wire is removed and screw placement can be confirmed with C-arm fluoroscopic imaging.
 Steps 5 to 7 are repeated for the contralateral C1-2 transarticular screw.
Steps 5 to 7 are repeated for the contralateral C1-2 transarticular screw.
Step 9
 The future areas of contact of the posterior arches of C1 and C2 with the strut graft are decorticated with the high-speed burr. The inferior cortical margin of the posterior arch of C1 and superior cortical margin of the posterior arch and spinous process of C2 are decorticated with the burr.
The future areas of contact of the posterior arches of C1 and C2 with the strut graft are decorticated with the high-speed burr. The inferior cortical margin of the posterior arch of C1 and superior cortical margin of the posterior arch and spinous process of C2 are decorticated with the burr.
 A notch is made on the inferior margin of the spinolaminar junctions of C2 with an angled Kerrison rongeur for future seating of the sublaminar wire.
A notch is made on the inferior margin of the spinolaminar junctions of C2 with an angled Kerrison rongeur for future seating of the sublaminar wire.
Step 10
 Using a Leksell rongeur, the tricortical strut graft is converted into a bicortical graft by removing the rounded cortical edge.
Using a Leksell rongeur, the tricortical strut graft is converted into a bicortical graft by removing the rounded cortical edge.
 The graft is placed between the posterior arches of C1 and C2 for final sizing and to approximate the midline of the graft. The Leksell rongeur is used to make any final modifications for optimal sizing of the graft.
The graft is placed between the posterior arches of C1 and C2 for final sizing and to approximate the midline of the graft. The Leksell rongeur is used to make any final modifications for optimal sizing of the graft.
 A Kerrison rongeur is used to notch the midline on the inferior aspect of the graft to accommodate the spinous process of C2 for a secure fit.
A Kerrison rongeur is used to notch the midline on the inferior aspect of the graft to accommodate the spinous process of C2 for a secure fit.
Step 11
 The graft is placed back in between the atlas and axis with the notch of the graft sitting on the spinous process of the axis for a secure fit.
The graft is placed back in between the atlas and axis with the notch of the graft sitting on the spinous process of the axis for a secure fit.
 The C1 sublaminar loop of wire is passed below and secured in the notch made on the undersurface of the C2 spinous process (Figure 11-22).
The C1 sublaminar loop of wire is passed below and secured in the notch made on the undersurface of the C2 spinous process (Figure 11-22).
 The free ends of wire are wrapped around the bone graft and secured. A heavy needle driver is used to twist the free ends of wire together and tighten them snugly around the graft.
The free ends of wire are wrapped around the bone graft and secured. A heavy needle driver is used to twist the free ends of wire together and tighten them snugly around the graft.
 Any excess wire is cut with wire cutters and removed. The cut ends of wire are carefully laid flush against the strut graft.
Any excess wire is cut with wire cutters and removed. The cut ends of wire are carefully laid flush against the strut graft.
Step 12
 The posterior cortical surfaces of the C1 posterior arch, C2 lamina, and strut graft are decorticated with the high-speed burr.
The posterior cortical surfaces of the C1 posterior arch, C2 lamina, and strut graft are decorticated with the high-speed burr.
 The bone dust and shavings from the burr are left in the operative field, and the cancellous bone graft is placed over the posterior decorticated surfaces of C1, C2, and strut graft.
The bone dust and shavings from the burr are left in the operative field, and the cancellous bone graft is placed over the posterior decorticated surfaces of C1, C2, and strut graft.
Step 13
 The wound is closed securely in a layered fashion, obliterating any dead space.
The wound is closed securely in a layered fashion, obliterating any dead space.
 Steri-Strips are placed perpendicular to the incision and covered by sterile 4 × 4 gauze and a clear Tegaderm dressing.
Steri-Strips are placed perpendicular to the incision and covered by sterile 4 × 4 gauze and a clear Tegaderm dressing.
 Final AP and lateral cervical radiographs are obtained to assess placement of the hardware and alignment of the atlantoaxial region (Figure 11-23).
Final AP and lateral cervical radiographs are obtained to assess placement of the hardware and alignment of the atlantoaxial region (Figure 11-23).
 A rigid cervical collar (i.e., Philadelphia or Miami-J) is secured in place.
A rigid cervical collar (i.e., Philadelphia or Miami-J) is secured in place.
 The patient is removed from the Mayfield headholder. The surgeon stands at the head of the operating table and is responsible for stabilizing the neck when the patient is repositioned onto the hospital bed in the supine position.
The patient is removed from the Mayfield headholder. The surgeon stands at the head of the operating table and is responsible for stabilizing the neck when the patient is repositioned onto the hospital bed in the supine position.
Postoperative Care and Expected Outcomes
 The patient should be taken to the recovery room or the surgical intensive care unit (SICU) for postoperative recovery.
The patient should be taken to the recovery room or the surgical intensive care unit (SICU) for postoperative recovery.
 Supine and upright lateral cervical radiographs should be obtained in the cervical collar to assess stability on postoperative day one. If atlantoaxial stability has been obtained, the patient can be mobilized.
Supine and upright lateral cervical radiographs should be obtained in the cervical collar to assess stability on postoperative day one. If atlantoaxial stability has been obtained, the patient can be mobilized.
 On postoperative day 1, or when medically stable, the patient can be transferred to a standard surgical floor.
On postoperative day 1, or when medically stable, the patient can be transferred to a standard surgical floor.
 A postoperative CT scan can be obtained if there is any question concerning screw placement.
A postoperative CT scan can be obtained if there is any question concerning screw placement.
 The patient can be discharged from the hospital when medically stable.
The patient can be discharged from the hospital when medically stable.
 Rigid cervical collar immobilization is used for approximately 8 to 12 weeks postoperatively.
Rigid cervical collar immobilization is used for approximately 8 to 12 weeks postoperatively.
 Routine outpatient static lateral radiographs can be obtained 4 weeks postoperatively to ascertain stability. Radiographs can be obtained at 4-week intervals to assess stability and fusion. Supervised dynamic lateral flexion and extension radiographs can be obtained at the end of the 8- to 12-week period to further assess atlantoaxial stability. If stability is obtained, the cervical collar can be weaned from use.
Routine outpatient static lateral radiographs can be obtained 4 weeks postoperatively to ascertain stability. Radiographs can be obtained at 4-week intervals to assess stability and fusion. Supervised dynamic lateral flexion and extension radiographs can be obtained at the end of the 8- to 12-week period to further assess atlantoaxial stability. If stability is obtained, the cervical collar can be weaned from use.
 Additionally, a CT scan can be obtained 3 to 6 months postoperatively to assess fusion and fracture healing.
Additionally, a CT scan can be obtained 3 to 6 months postoperatively to assess fusion and fracture healing.
 Fusion can be achieved in nearly 100% of cases, although 16.7% of patients may experience complications (Finn and Apfelbaum, 2010).
Fusion can be achieved in nearly 100% of cases, although 16.7% of patients may experience complications (Finn and Apfelbaum, 2010).
Postoperative Pitfalls
• Screw malposition can result in:
Cavalcanti D, Agrawal A, Garcia-Gonzalez U, et al. Anterolateral C1-C2 transarticular fixation for atlantoaxial arthrodesis: landmarks, working area, and angles of approach. Operative Neurosurg. 2010;67:38-42.
Currier B, Maus T, Eck J, et al. Relationship of the internal carotid artery to the anterior aspect of the C1 vertebra. Spine. 2008;33:635-639.
Cyr S, Currier B, Eck J, et al. Fixation strength of unicortical versus bicoritcal C1-C2 transarticular screws. Spine J. 2008;8:661-665.
Finn M, Apfelbaum R. Atlantoaxial transarticular screw fixation: update on techniques and outcomes in 269 patients. Neurosurgery. 2010;66A:184-192.
Harms J, Melcher R. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26:2467-2471.
Henriques T, Cunningham B, Olerud C, et al. Biomechanical comparison of five different atlantoaxial posterior fixation techniques. Spine. 2000;25:2877-2883.
Jeanneret B, Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, techniques, and results of transarticular screw fixation. J Spinal Disord. 1992;5:464-475.
Jun BY. Anatomic study for ideal and safe posterior C1-C2 transarticular screw fixation. Spine. 1998;23:1703-1707.
Madawi A, Solanki G, Casey AT, et al. Variation of the groove in the axis vertebra for the vertebral artery: implications for instrumentation. J Bone Joint Surg Br. 1997;79:820-823.
Magerl F, Seemann P-S. Stable posterior fusion of the atlas and axis by transarticular screw fixation. In: Kehr P, Weidner A, editors. Cervical Spine I. New York: Springer Wien; 1986:322-327.
Seal C, Zarro C, Gelb D, et al. C1 lateral mass anatomy: proper placement of lateral mass screws. J Spinal Disord Tech. 2009;22:516-523.
Tan M, Wang H, Wang Y, et al. Morphometric evaluation of screw fixation in atlas via posterior arch and lateral mass. Spine. 2003;28:888-895.
Wait S, Ponce F, Colle K, et al. Importance of the C1 anterior tubercle depth and lateral mass geometry when placing C1 lateral mass screws. Neurosurgery. 2009;65:952-957.
Weidner A, Wahler M, Chiu T, Ullrich C. Modification of C1-C2 transarticular screw fixation by image-guided surgery. Spine. 2000;25:2668-2674.
Yoshida M, Feo M, Fujibayashi S, Nakamura T. Comparison of the anatomical risk for vertebral artery injury associated with the C2-pars interarticularis screw and atlantoaxial transarticular screw. Spine. 2006;31:E513-E517.
Young JP, Young PH, Ackermann MJ, et al. The ponticulus posticus: implications for screw insertion into the first cervical lateral mass. J Bone Joint Surg Am. 2005;87:2495-2498.