CHAPTER 11
Nutritional disorders and their management
CHAPTER OUTLINE
Protein–energy malnutrition in children
Chronic energy deficiency in Western adults
DIET IN THE AETIOLOGY OF DISEASE
THERAPEUTIC DIETS, DIETARY SUPPLEMENTS AND NUTRACEUTICALS
PROVISION OF NUTRITION SUPPORT
INTRODUCTION
The term ‘nutritional disorders’ covers a wide range of conditions. Some of these disorders are primarily nutritional and some are not, albeit that nutrition is still an important factor. This makes their classification somewhat arbitrary, but the following broad categories can be recognized:
• specific deficiency caused by inadequate supply of individual nutrients and occasionally, oversupply of an individual nutrient leading to toxicity, which has been discussed in Chapter 10
• generalized undernutrition, which is an important cause of morbidity and mortality across the world
• generalized overnutrition, in the form of obesity, which has become an important public health problem
• eating disorders are not primarily nutritional disorders, but have important nutritional effects and significant metabolic consequences
• specific diseases in which dietary factors may be important in their aetiology. In some cases there may be scope for dietary modification to alter the course of the disease (e.g. hyperlipidaemia). In others, this is not so (e.g. where carcinogenesis has already been stimulated by a dietary component)
• conditions in which diet has no role in aetiology, but for which specific dietary intervention is an essential part of management (e.g. phenylketonuria)
• primarily non-nutritional diseases in which general nutritional support may improve outcome, especially if patients are, or are likely to become, malnourished during the course of their illness.
MALNUTRITION
‘Malnutrition’ is often taken to mean inadequate nutrition, but this definition also includes dietary excess, so the term ‘undernutrition’ is to be preferred. Undernutrition is a major public health problem in the developing world. Diets of affected populations tend to be deficient in both macronutrients and micronutrients. A high prevalence of bacterial and parasitic disease tends to contribute to undernutrition; in addition, an effect of undernutrition is to increase susceptibility to infection. In children, severe undernutrition is predominantly a state of protein energy malnutrition (PEM). In adults, in whom protein requirements are relatively lower (since growth has ceased), the term chronic energy deficiency (CED) is used. In the UK, severe undernutrition is most commonly seen in adults, usually in association with intercurrent illness and is discussed later in the chapter under nutrition support.
Protein–energy malnutrition in children
Worldwide, PEM remains the most common disorder of infancy and childhood: as many as 200 million children may be significantly affected at any one time and it accounts for over half the deaths in the under-five age group in developing countries. One way of assessing and categorizing undernutrition is by using a weight for height z score, which compares a child’s height to that of a healthy reference population of children of the same height or length. The z score is expressed in terms of standard deviations (SD) from the mean of the reference population. When height or length cannot be measured easily, upper arm circumference, which changes little in healthy children between the ages of six months and five years, can be used as a measure of lean body mass to identify undernutrition. Using these methods, acute undernutrition can be divided into moderate and severe. Moderate undernutrition is defined as a weight for height or length z score between − 2 and − 3SD. Severe undernutrition is defined by a z score of <−3SD, an arm circumference < 110 mm or the presence of nutritional oedema. Moderate or severe undernutrition without nutritional oedema is termed marasmus. Undernutrition with oedema is termed kwashiorkor.
Marasmus tends to occur in younger children who have the greatest energy requirements and susceptibility to infection. Affected children appear emaciated with loss of subcutaneous fat, muscle wasting and triangular facies due to loss of buccal fat pads. In addition to oedema, children with kwashiorkor have enlarged and fatty livers. They may also have dermatoses and depigmentation of hair, the latter being attributed to reduced availability of tyrosine, a precursor of melanin.
The classic explanation for the two different syndromes suggests that marasmus occurs on a diet poor in protein and energy and kwashiorkor on a diet poor in protein but with relatively more carbohydrate. In marasmus, insulin secretion would, therefore, be expected to be low, prompting breakdown of muscle protein and mobilization of amino acids for hepatic synthesis of protein, particularly albumin. In kwashiorkor, relatively higher carbohydrate intake would lead to maintenance of insulin secretion with relative sparing of muscle protein, therefore limiting amino acids available for synthesis of liver proteins. Reduced synthesis of albumin and apolipoproteins would lead to hypoalbuminaemia and oedema, and accumulation of lipids in the liver, respectively. However, recent studies have found marasmus and kwashiorkor to exist in populations where children are eating similar diets. It has been suggested that the clinical syndromes may in fact reflect differences in the metabolic response to starvation, for example varying rates of fatty acid oxidation, rather than the content of the diet.
The treatment of PEM is the provision of appropriate nutrition and treatment of associated infection. Treatment of moderate undernutrition involves the addition to the diet of suitable nutrient rich supplements containing significant energy and the recommended daily allowance of micronutrients; examples include cereal and legume blended flours and lipid rich fortified spreads.
Severe PEM is associated with atrophy of the small bowel mucosa, reducing its capacity for digestion and absorption, hence liquid, milk-based products, supplemented with potassium and phosphate, are favoured for treatment, initially administered as small boluses. Intravenous fluids should be avoided, except in children with profuse diarrhoea. Children with kwashiorkor have increased total body water with sodium retention. Those with marasmus have chronic hypovolaemia with secondary hyperaldosteronism, although tissue breakdown and urinary potassium loss tend to result in hypokalaemia. However, children with marasmus are particularly likely to have a degree of cardiac atrophy and reduced stroke volume, predisposing to cardiac failure if fluid overloaded. In addition, the reduction in subcutaneous fat renders these children susceptible to hypothermia and hypoglycaemia during the recovery process.
Chronic energy deficiency in Western adults
Studies of both medical and surgical inpatients in developed countries indicate that a significant proportion of patients have CED, although variation in the criteria used to define this means that the exact prevalence is uncertain. Caution is required in applying the marasmus/kwashiorkor distinction to such patients, since the presence of hypoalbuminaemia or oedema is more likely to be due to the underlying illness than malnutrition. Nonetheless, severe CED in such patients has important effects. Wasting of respiratory muscles increases the risk of chest infection and may delay weaning from a ventilator. Myocardial function may be impaired and skeletal muscle wasting delays mobilization, with a consequent increased risk of thromboembolism and bed sores. Chronic energy deficiency also results in impaired resistance to infection, and gut permeability may be increased, allowing entry of bacteria and toxins through the gut wall. Apathy and depression impair active efforts at recovery and may also impair appetite, worsening the situation further.
The recognition of undernutrition in adults and its treatment are considered later in this chapter in the section on nutritional support.
OBESITY
Obesity is an excess of body fat. For epidemiological studies and population surveys, as well as clinical assessment of individual patients, body mass index (BMI) is frequently used to define overweight (BMI ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2). Whilst it is a useful index, BMI is not without problems, but alternative methods are expensive and not readily available. In children, there are marked changes in BMI with age. It is recommended that obesity in children is defined with reference to BMI centiles, and charts are available for this purpose, but there is no universally accepted BMI classification system. In the UK, it is suggested that the 91st and 95th centiles should be used in children as indicators of overweight and obesity, respectively. Estimates of the prevalence of obesity are likely to be subject to variation because of differences in the definition, cut-offs and reference standards used.
It is estimated that at least one billion people worldwide are overweight or obese and at least 300 million are obese. In the UK, from 1993 to 2010, the proportion of men and women who were overweight remained stable at 40% for men and 30% for women. However, there was a gradual increase in the proportion classified as obese from 13.2% of men (16.4% of women) in 1993 to 26.2% of men (26.1% of women) in 2010. Obesity is associated with significant health risks and the economic cost has been estimated as 3–8% of total healthcare expenditure. There is a relationship between mortality risk and increased BMI which, although strongest in those below 50 years of age, persists into the ninth decade of life. In addition, various complications are associated with obesity (see Table 11.1).
TABLE 11.1
Complications associated with obesity
| System | Complication |
| Cardiovascular | Coronary heart disease, cardiac failure, hypertension |
| Respiratory | Sleep apnoea, dyspnoea |
| Gastrointestinal | Fatty liver, reflux oesophagitis, gallstones |
| Musculoskeletal | Arthritis, back pain |
| Endocrine and metabolic | Type 2 diabetes mellitus, insulin resistance, polycystic ovary syndrome, amenorrhoea, dyslipidaemia |
| Skin | Acanthosis nigricans, intertrigo |
Aetiology of obesity
The aetiology of obesity is complex and influences on body fat content include genetic, perinatal, behavioural and environmental factors. Epidemiological data indicate that the consumption of a diet that is high in fat and the frequent consumption of ‘fast food’ increase the risk of obesity. This is well demonstrated by the recent rise in obesity in developing countries, whose populations have replaced their indigenous diet with a more energy-dense Westernized alternative. There is also a close relationship between low levels of physical activity and weight gain and the duration of television viewing is a predictor of obesity risk in both adults and children.
Family studies have established that hereditary influences are important in determining body weight, although, to-date, genetic studies have concentrated on severe, early-onset forms of obesity, which are rare. Mutations resulting in leptin (see below) and leptin-receptor deficiencies have been described. Affected children are of normal birth weight, but exhibit extreme hyperphagia and gain weight rapidly. Children with congenital absence of pro-opiomelanocortin (POMC) gene products also become obese, despite glucocorticoid deficiency, an endocrine state normally associated with weight loss. These patients characteristically have pale skin and red hair, resulting from an absence of the action of POMC-derived peptides on melanocytes. A number of heterozygous point mutations in POMC have been found, which increase the risk of obesity, although are not invariably associated with it. Heterozygous mutations in the melanocortin receptor MC4R have been associated with dominantly inherited obesity and have been found in 1–2.5% of those with a BMI of > 30 kg/m2.
There are a number of conditions which have obesity as a feature of their clinical phenotype. The majority of these are associated with short stature; this contrasts with simple obesity where children tend to be tall. Examples of pleiotropic obesity syndromes are shown in Table 11.2.
TABLE 11.2
Pleiotropic obesity syndromes
| Syndrome | Genetic basis | Clinical features |
| Alstrom | Autosomal recessiveALMS gene located at 2p13 | Obesity and type 2 diabetes mellitus Blindness Dilated cardiomyopathy Sensorineural hearing loss Other: hepatic, pulmonary, renal and urological dysfunction |
| Bardet–Biedl | Autosomal recessive 14 genes code for BB proteins involved in ciliary action |
Obesity Hypogonadism Intellectual impairment Polydactyly Retinitis pigmentosa |
| Carpenter | Autosomal recessiveRAB23 gene located at 6p11 | Obesity Cardiac defects Craniosynostosis Polysyndactyly |
| Prader–Willi | 15q 11–13 Imprinted sequence |
Obesity and hyperphagia Hypogonadotrophic hypogonadism Hypotonia Intellectual impairment Short stature Small hands and feet |
Secondary causes of obesity
Investigation of patients to exclude secondary causes of obesity is usually unrewarding, but remains important. Clinical features suggesting endocrine pathology should be pursued, but in-depth dynamic function testing to exclude rare disorders is not usually necessary. Hypothyroidism should be excluded by measuring serum thyroid stimulating hormone and free thyroxine concentrations. The investigation of possible Cushing syndrome may be more difficult, as false positive results have been described in pseudo-Cushing syndrome due to obesity. Growth hormone deficiency may also lead to weight gain, although morbid obesity is not usually present. Associations are also recognized between obesity and polycystic ovary syndrome, hypogonadism and insulinoma.
Some drugs are associated with weight gain. The effect is assumed to be due either to central effects on appetite (e.g. certain anticonvulsants and antipsychotics) or to peripheral metabolic effects (e.g. oral hypoglycaemic agents, protease inhibitors).
Appetite
Energy homoeostasis is the result of a complex, integrated process involving neural, humoral and psychological factors that are integrated by the brain to ensure that nutrient supply remains at appropriate levels for different environmental conditions. In the past, there has not tended to be an evolutionary advantage in weight loss, hence the mechanisms underlying energy homoeostasis are tailored to ensure a powerful drive to eat, especially after weight loss.
Central appetite control
The hypothalamus
The body’s appetite control centres are located in the hypothalamus. The ventromedial hypothalamus is thought to act as the satiety centre and the lateral hypothalamus as the feeding centre. Histochemical and molecular imaging techniques have shown that the hypothalamic nuclei involved in feeding regulation form interconnected circuits, utilizing neuropeptides as well as the classic amine neurotransmitters
The hypothalamus receives both neural and humoral input. At the base of the third ventricle, the arcuate nucleus (Arc; also known as the infundibular nucleus in humans) is able to receive signals from the circulation. The blood–brain barrier (BBB) surrounding the Arc is not complete and this allows hormones such as leptin, secreted by adipocytes, and insulin, from the pancreas, to gain access to the afferent signalling pathway that regulates appetite.
From the Arc, monosynaptic projections are made to many other brain regions, with the projections to the paraventricular nucleus (PVN) being of importance in the regulation of food intake. Integration of peripheral and central signals relating to energy homoeostasis takes place in the Arc. Two neuron populations play a role. The orexigenic (appetite-stimulating) peptides neuropeptide Y (NPY) and Agouti-related peptide (AgRP) are co-localized, while in another population of neurons, the anorectic (appetite-inhibiting) peptide cocaine- and amfetamine-regulated transcript (CART) and pro-opiomelanocortin (POMC, the precursor of α-melanocyte stimulating hormone (αMSH)) are also co-localized. The orexigenic NPY/AgRP neurons inhibit the anorexigenic POMC neurons through γ-aminobutyric acid (GABA)-ergic interneuronal connections. Fasting increases NPY and AgRP, while CART and POMC expression is reduced.
The brain stem
The vagus nerve and sympathetic fibres transmit satiety signals from the gut and liver to the nucleus of the tractus solitarius (NTS) in the brain stem. In areas such as the area postrema, which is in the brain stem adjacent to the NTS at the base of the fourth ventricle, the BBB is incomplete, also allowing circulating hormones access to the brain. The brain stem and hypothalamus are linked by projections from the NTS to the PVN and lateral hypothalamus, and from the raphe nuclei to the arcuate nucleus. Neurons such as those expressing glucagon-like peptide-1 (GLP-1) receive afferents from the vagal and glossopharyngeal nerves, integrating and relaying sensory information to hypothalamic and brain stem centres. Whilst the brain stem plays a role in regulating the size of individual meals, it is thought that the hypothalamus is necessary for long-term energy balance and appetite regulation.
Hypothalamic messengers
Neuropeptide Y
Neuropeptide Y is a 36-amino acid peptide member of the family of peptides comprising NPY, pancreatic polypeptide (PP) and peptide YY (PYY). Neuropeptide Y is one of the most potent stimulators of feeding. At least five distinct G-protein coupled receptors (Y1, Y2, Y4, Y5 and Y6) mediate the actions of NPY, PYY and PP. In rodents, repeated administration of NPY leads to hyperphagia and obesity associated with decreased thermogenesis in brown adipose tissue, hyperinsulinaemia, hypercorticosteronaemia, reduced plasma testosterone concentrations, and insulin resistance in skeletal muscle.
Agouti-related protein
Agouti-related protein increases food intake by acting as an antagonist at central melanocortin-3 and melanocortin-4 receptors (see below). In contrast to the fairly short-lived effects of NPY, central administration of a single dose of AgRP to rodents leads to an increase in food intake for up to one week. Repeated administration leads to hyperphagia and obesity.
Melanocortins
Melanocortins are peptides that are cleaved from the POMC precursor molecule by tissue-specific post-translational cleavage (e.g. in the anterior pituitary, POMC gives rise to adrenocorticotrophic hormone, ACTH). They bind to a family of melanocortin receptors (MC1-R to MC5-R). In the arcuate nucleus, αMSH is released from POMC-expressing neurons that project to the PVN, where it acts through MC3-R and MC4-R to inhibit food intake. The endogenous antagonist AgRP (see above) is released from the terminals of arcuate NPY/AgRP neurons at the PVN: AgRP stimulates food intake by blocking the anorectic effect of αMSH. The MC4 receptor, in particular, seems to be critical to regulation of body weight; thus far, the most common cause of monogenic obesity in humans is mutation in MC4-R.
Cocaine- and amfetamine-regulated transcript
Cocaine- and amfetamine-regulated transcript is co- expressed with POMC in arcuate neurons, and these neurons are directly stimulated by leptin (see later). Central administration of CART to rats inhibits feeding and completely blocks the feeding response stimulated by NPY. Food-deprived animals show decreased expression of CART mRNA in the arcuate nucleus. CART may thus be another endogenous inhibitor of food intake.
5-Hydroxytryptamine
5-Hydroxytryptamine (5-HT) is a monoamine neurotransmitter that is synthesized in both the central nervous system and in the chromaffin cells of the gastrointestinal tract. There are numerous 5-HT receptor subtypes: in the brain these receptors occur mainly in the limbic system and the hypothalamus. 5-Hydroxytryptamine modifies mood and behaviour, and a number of different types of drug that increase the effects of 5-HT are used as antidepressants. Drugs that either mimic 5-HT at its receptors or inhibit its reuptake at synapses generally also reduce feeding. The mechanism for this is not clear.
Peripheral signals of appetite
Peripheral signals influencing food intake can be broadly divided into those that cause satiety and those that are secreted in proportion to the amount of fat in the body. The gut and the brain seem to have a close evolutionary relationship. Peptides discovered in the hypothalamus have also been identified in the gut, and gut peptides have been found to be produced by the brain.
Gastric emptying and stretching
Slow gastric emptying increases stomach distension, which activates stretch receptors. The vagus nerve carries afferent signals related to stomach distension to the NTS, facilitating satiety by projections to the appetite-regulating nuclei of the hypothalamus, for example the PVN. Cholecystokinin (CCK) is a potent inhibitor of gastric emptying by vagal afferent-mediated central mechanisms, and this may explain its anorectic actions. Peptide YY and GLP-1 (see below) may also cause anorexia by reducing gastric emptying in this way.
The main neural connection from the gastrointestinal tract to the brain is through the vagal afferent fibres, via the nodose ganglion. Sympathetic afferent fibres in the spinal nerves also carry satiety signals to the brain stem, as previously described.
Hormones
The endocrinological capacity of the gut is diverse, as diffuse populations of endocrine cells are scattered throughout the mucosa. Primary gastrointestinal functions such as motility, secretion and absorption are regulated by gut hormones, which simultaneously provide feedback to the CNS on the availability of nutrients, thereby regulating food intake.
Insulin
Insulin was the first hormonal candidate, related to adipose tissue, to be postulated as the circulating factor that regulates the hypothalamic control of food intake. Insulin was also thought to be important in achieving the usual long-term stability of body weight and fat mass. The rise in circulating insulin in response to a glucose load is proportional to fat mass. Insulin reaches the CNS via receptor-mediated transport across the BBB and through areas of relative permeability. Central nervous system administration of insulin to rodents causes a reduction in food intake, while brain-specific insulin receptor knockout mice and insulin receptor substrate-2 knockout mice develop obesity. However, when insulin is commenced in patients with type 2 diabetes, weight gain rather than weight loss is observed, possibly as a result of a loss of the anorexigenic effect of hyperglycaemia and the lipogenic actions of insulin.
Cholecystokinin
Cholecystokinin was the first gut hormone described to relay the signal of nutrient intake to the brain, thus leading to the inhibition of further food intake. Cholecystokinin is produced by endocrine cells (I cells) present within the jejunum and duodenum and it is also found in enteric nerves in the ileum and colon. Plasma concentrations of CCK increase in response to the intraluminal presence of the digestion products of protein and fats. Gastric emptying is potently inhibited by CCK through a vagal afferent-mediated central mechanism. In addition to causing satiety via vagally mediated pathways, CCK can cross the BBB and bind directly to specific receptors in the area postrema.
Peptide YY
Peptide YY is secreted from the endocrine L cells of the small and large bowel. It is a 36-amino acid peptide related to NPY. The highest PYY tissue concentrations are in the distal gastrointestinal tract. Postprandial PYY concentrations are proportional to meal energy content. Two isoforms exist, and both PYY1–36 and PYY3–36 may have local effects on gut motility, inhibit secretion of gastric acid and pancreatic enzymes, and inhibit gallbladder emptying. Obese individuals have lower circulating concentrations of PYY.
It is proposed that PYY3–36, released into the circulation after a meal, inhibits appetite by acting directly on the arcuate nucleus via the Y2R, a presynaptic inhibitory autoreceptor, leading to an inhibition of the NPY neurons and a possible reciprocal stimulation of the POMC neurons. There is a tonic GABA-mediated inhibition of POMC neurons by NPY neurons, and thus decreased GABA-mediated tone as produced by leptin may lead to disinhibition of POMC neurons. Thus, peripheral PYY3–36 reduces the expression of NPY mRNA and increases that of POMC. Exogenous PYY3–36 reduces food intake in rodents, rhesus monkeys and in normal weight and obese humans.
Pancreatic polypeptide (PP)
Pancreatic polypeptide (PP) is thought to have arisen by gene duplication of the PYY gene, as PP and PYY are closely related structurally. The PP cells of the pancreatic islets produce PP in response to ingestion of food. This release is in proportion to the energy ingested, and postprandial concentrations remain elevated for up to 6 h. Obese subjects have low PP concentrations, while high concentrations of PP have been demonstrated in patients with anorexia nervosa. Peripheral administration of PP to rodents and humans has been shown to reduce food intake.
Leptin
Leptin is a 167 kDa peptide, the product of the ob gene, and is produced predominantly by adipose tissue. Circulating leptin concentrations are directly proportional to adiposity and correlate better with total fat mass than with body weight. Central or peripheral administration of leptin in rodents causes a profound decrease in food intake and weight loss. The ob/ob mouse, completely deficient in leptin, is hyperphagic, hyperinsulinaemic and very obese, and the leptin receptor-defective db/db mouse has a similar phenotype. Chronic administration of leptin to the ob/ob mouse results in sustained reduction in body weight and reduced food intake, but has no effect on the db/db mouse.
Leptin deficiency in humans is rare, although it has been suggested that some obese humans have lower leptin concentrations than would be expected. Most obese people have normal leptin genes and have elevated leptin concentrations, reflecting their high adiposity.
Ghrelin
Ghrelin is a 28-amino acid peptide identified in 1999 as the endogenous agonist at the growth hormone secretagogue receptor, and is present in the circulation of healthy individuals. Ghrelin is synthesized in the endocrine cells of the stomach and (in much smaller amounts) in the hypothalamus. Ghrelin has been shown to increase NPY and AgRP in the hypothalamic arcuate nucleus, thus being postulated to increase appetite and act as an initiator of feeding. Plasma concentrations of ghrelin peak before a meal and fall rapidly after nutrient ingestion, supporting the hypothesis that it has a role as a stimulus to eating by signalling pre-meal hunger. A state of negative energy balance increases fasting plasma ghrelin concentrations, while concentrations are reduced by positive energy balance. This suggests that ghrelin is also important in long-term regulation of body weight in normal individuals. Exogenous administration of ghrelin increases appetite and ad libitum caloric intake, as well as growth hormone secretion.
Insulin resistance has been postulated to play a role in determining low fasting ghrelin concentrations. Attenuated postprandial reduction of ghrelin concentrations has been shown in the obese, while in normal weight individuals, postprandial suppression of ghrelin secretion is proportional to the calories consumed.
Glucagon-like peptide-1
Processing of proglucagon in the pancreas results in the formation of glucagon, glicentin-related pancreatic polypeptide (GRPP) and major proglucagon fragments. In intestinal L-cells, proglucagon processing results in glicentin, oxyntomodulin (OXM), GLP-1, spacer peptide-2 (SP-2) and glucagon-like peptide-2 (GLP-2). Glucagon-like peptide-1 is co-secreted with PYY in response to the presence of nutrients in the gut.
Glucagon-like peptide-1 is an incretin, stimulating the release of insulin, while also inhibiting the release of glucagon; GLP-1 receptor agonists are used in the treatment of type 2 diabetes. Glucagon-like peptide-1 reduces gastric emptying and inhibits gastric acid secretion, while also regulating food intake. Central administration of GLP-1 in rodents strongly inhibits feeding, and GLP-1 also seems to produce a small, dose-dependent reduction in food intake in humans.
Management of obesity
The objectives of the management of obesity are to reduce body weight and then to maintain a lower body weight in the longer term. A criterion for success of a reduction of 5–15% in body weight has been proposed. Various approaches can be used to achieve this, alone, or in combination.
Non-surgical options
Dietary approaches offer the obese patient education in the principles of healthy eating and a regimen intended to achieve a deficit in energy balance. Very low calorie diets (400–500 kcal/24 h) have been shown to produce greater initial weight loss than low calorie diets (1000–1500 kcal/24 h), but results after one year are not significantly different.
It is difficult to achieve weight loss merely through physical exercise. For example, a man of average build who completes a marathon in 4.5 h will only burn about 2000 kcal, approximately the same amount of energy that he would be expected to consume during an entire less active day. He would have to run a marathon every ten days, without an increase in energy intake, to lose a kilogram a month. However, physical activity can contribute towards an energy deficit and is important in increasing cardiorespiratory fitness.
For some obese patients, learned patterns of eating behaviour may be amenable to behaviour modification. Establishment of new patterns of eating and physical activity makes other strategies for weight loss more likely to be successful.
Various drugs have been used for the treatment of obesity. Many have been discontinued due to unacceptable side-effects, including recently, sibutramine, a centrally acting agent affecting reuptake of serotonin, norepinephrine and dopamine; and rimonabant, an inhibitor of cannabinoid receptor-1. Orlistat is a potent inhibitor of pancreatic lipase and, therefore, increases faecal fat loss when taken orally with food. Adverse effects include abdominal distension, flatulence, liquid stools and occasional faecal incontinence. Some of the weight loss in those taking the drug may be a consequence of a conscious decrease in fat intake to avoid these side-effects. Less than 1% of an oral dose is absorbed, so it has minimal interaction with most drugs. However, absorption of fat soluble vitamins may be affected and supplementation may be required. Methylcellulose is available for use as a bulking agent, with the idea that it produces a feeling of satiety, but there is little evidence to support its use. Thyroid hormones have no role in the treatment of obesity, except in patients with biochemically proven hypothyroidism.
Bariatric surgery
The limited long-term success of behavioural and drug therapy for obesity has led to increased use of surgical treatments, with considerable success. Bariatric surgery is considered an option for selected patients with a BMI ≥ 40 kg/m2, or those with a BMI > 35 kg/m2 with significant comorbidities (e.g. diabetes mellitus), when less invasive methods of weight loss have been unsuccessful and the patient is at high risk of obesity-associated complications.
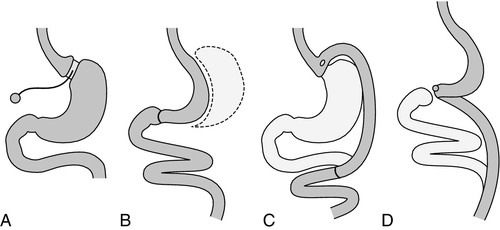
Possible contraindications to surgery include significant mental health problems, an intercurrent medical condition likely to influence surgical risk, a history of poor compliance with medical treatment and pregnancy. Patients should be managed by a multidisciplinary team, including the surgeon, a metabolic physician, dietitian and psychiatrist. Bariatric procedures tend to be described as either restrictive or malabsorptive according to the nature of the surgery involved (see Figure 11.1). However, these descriptions do not define the mechanism of weight loss.
FIGURE 11.1 Bariatric surgery procedures. (A) Adjustable gastric band (AGB). (B) Vertical sleeve gastrectomy (VSG). (C) Roux-en-Y gastric bypass (RYGB). (D) Biliopancreatic diversion with a duodenal switch (BPD–DS).
Restrictive procedures
Laparoscopic gastric banding involves insertion of an inflatable silicon gastric band horizontally around the proximal part of the stomach. The gastric band is connected to a subcutaneous injection port which, when inflated with saline, leads to constriction and the formation of a gastric pouch.
In a laparoscopic sleeve gastrectomy, over three-quarters of the stomach is removed. The gastric remnant is used to fashion a sleeve of stomach, rather than a pouch, which extends from the oesophagus to the duodenum.
Combined restrictive and malabsorptive procedures
Roux-en-Y gastric bypass requires the formation of a small proximal gastric pouch, which empties into a segment of jejunum brought up to the pouch as a Roux-en-Y loop. It results in a degree of malabsorption as a result of bypass of the upper small intestine.
Biliopancreatic diversion with or without duodenal switch involves removal of part of the stomach, together with an alteration in the path of the small intestine.
Most patients report a reduction in appetite after bariatric surgery, an effect more marked after biliopancreatic procedures. Weight loss after bariatric surgery is often reported as the percentage of excess weight loss (%EWL), where excess weight is the total amount above ideal weight. In some series, the %EWL has been reported to be up to 46% after gastric banding, 60% after Roux-en-Y bypass and 64% after biliopancreatic diversion. In addition to weight loss, there is resolution of type 2 diabetes mellitus in many patients and improvement in dyslipidaemia, hypertension and obstructive sleep apnoea. The percentage of patients with resolution of diabetes varies according to the procedure performed, being highest after biliopancreatic diversion, but it is not directly related to %EWL. This resolution can be very rapid. This suggests that changes in glucose homeostasis are operation-specific and may be determined by intestinal factors. The stomach is the major producer of the orexigenic hormone ghrelin and the functional area of the stomach is reduced in the majority of these procedures. However, postoperative changes in ghrelin are variable and its role in weight loss is unclear. The concentration of the anorectic hormone PYY increases early after malabsorptive procedures, probably due to the re-routing of gut contents, and is thought to be an important contributor to the subsequent weight loss. Incretins, for example GLP-1 and oxyntomodulin, are thought to play a synergistic role in postoperative weight loss.
The mortality rate after bariatric surgery is < 1% for younger patients with a BMI < 50 kg/m2 and average complication rates are < 10%. Early complications are related to the surgery itself, for example postoperative bleeding and anastomotic leak. Longer-term problems include erosion from, or displacement of, a gastric band, anastomotic stricture and dumping syndrome owing to the rapid passage of food into the intestine, as well as various metabolic problems, for example metabolic bone disease and kidney stones.
Following bariatric surgery, long-term follow-up is essential. After surgery, patients should be advised to follow a well balanced, calorie-controlled diet. They are usually advised to avoid carbonated drinks, high sugar foods and foods with textures that are difficult to chew such as tough meat and bread. It is also recommended that solid food be chosen for meals, with drinks taken in between. Vitamin and mineral supplementation is recommended after bariatric surgery. After combined restrictive and malabsorptive procedures, but not laparoscopic banding, biochemical assessment of vitamin and trace elements should form part of ongoing review. One suggested scheme for such monitoring is shown in Table 11.3. It is now recognized that a significant proportion of obese patients are deficient in vitamins and trace elements preoperatively. The aetiology includes nutritional deficiency from a diet that, whilst excessive in energy, is likely to have been of poor quality.
TABLE 11.3
Suggested monitoring scheme after bariatric surgery
| Roux-en-Y gastric bypass | Biliopancreatic bypass |
| First year: 3–6-monthly Thereafter: annually |
First year: 3-monthly Thereafter: 3–6-monthly depending on results and clinical status |
| Full blood count Renal profile Liver profile Plasma glucose Lipid profile Iron, ferritin, vitamin B12 Vitamin D Also consider: PTH, thiamin, folate |
Full blood count Renal profile Liver profile Plasma glucose Lipid profile Iron, ferritin, vitamin B12 Fat soluble vitamins (6–12-monthly) A, E, D and prothrombin time Trace elements (annually) Zinc, selenium Metabolic bone evaluation (6–12-monthly) PTH, 24 h urinary calcium Metabolic stone evaluation (12-monthly) 24 h urine calcium, citrate, urate and oxalate |
EATING DISORDERS
Eating disorders, which include anorexia nervosa, bulimia nervosa and atypical eating disorders, are a range of syndromes involving physical, psychological and social features. About 1 in 250 females and 1 in 2000 males suffer from anorexia nervosa and about five times as many from bulimia. Atypical eating disorders may be more common, with many patients going untreated.
Anorexia nervosa
Anorexia nervosa is characterized by weight loss (or in children lack of weight gain), leading to a body weight of at least 15% below the normal or expected weight for age and height. The weight loss is the result of patients perceiving themselves to be too fat and so setting a low target body weight that is achieved by food avoidance and, sometimes, obsessive exercise. Secondary to weight loss, patients with anorexia nervosa show evidence of endocrine disturbance involving the hypothalamo–gonadal axis with amenorrhoea in females and decreased libido or impotence in males.
Patients with anorexia nervosa may become severely emaciated and treatment includes nutrition support and psychological therapies. Such patients are at risk of refeeding syndrome (see p. 211), but may also exhibit other biochemical abnormalities prior to reintroduction of appropriate nutrition. Hypokalaemia is frequent in patients with eating disorders; causes include vomiting and loss resulting from misuse of laxatives and diuretics. Hypomagnesaemia is also common and may result from reduced intake and be secondary to laxative and diuretic abuse. Patients who vomit and abuse diuretics are also at risk of hypochloraemic metabolic alkalosis. Prolonged use of diuretics may lead to hyponatraemia. Patients who ‘water load’ prior to being weighed at clinic appointments may exhibit a dilutional hyponatraemia. Elevation of liver enzyme activities may be seen in anorexia. This is usually transient and relatively mild, although rarely, severe liver damage occurs; the abnormalities may occur before or during refeeding and suggested mechanisms include liver ischaemia, glutathione depletion and fatty change occurring on refeeding.
At low body weight, there are changes in the hypothalamo–pituitary–gonadal axis. Menstruation ceases and the endocrine changes are of hypogonadotrophic hypogonadism with low concentrations of follicle stimulating hormone, luteinizing hormone and oestradiol. These changes are thought to be related to low concentrations of leptin, which has a role in gonadotrophin releasing hormone secretion. In males, comparable changes occur with low concentrations of testosterone. Plasma concentrations of free thyroxine and free triiodothyronine tend to be low in anorexia, with increased concentrations of reverse triiodothyronine. The thyroid stimulating hormone response to thyroid releasing hormone is abnormal in some patients and it has been suggested that this also is secondary to reduced leptin secretion. In contrast, plasma cortisol concentrations tend to be increased. These and other endocrine changes in patients with anorexia nervosa are summarized in Box 11.1.
Bulimia nervosa
Bulimia nervosa is also characterized by a self-perception of being too fat. However, in contrast to anorexia, there are recurrent episodes of overeating. To attempt to counteract the fattening effects of the food consumed, the patient may alternate overeating with periods of starvation, or may induce vomiting and use drugs such as diuretics, laxatives and appetite suppressants to attempt weight reduction.
The endocrine disturbances in bulimia are milder than those in anorexia nervosa. Patients have mild hypercortisolism and basal thyroid hormone concentrations are normal. The secretion of cholecystokinin in response to a meal is impaired in bulimia. About 50% of patients have menstrual abnormalities, which may be one of two types. Patients with low body weight have low plasma oestradiol concentrations and failure of follicular development, while those with normal body weight have normal plasma oestradiol concentrations, but a failure of progesterone secretion in the luteal phase.
Again, the diagnosis is a clinical one, but biochemical tests are important in looking for deficiencies, for example of potassium. Interestingly, in view of the putative role of serotonin in feeding, monoamine oxidase inhibitors, tricyclic antidepressants and fluoxetine have been shown to have beneficial effects in bulimia. However, the effects are smaller than those produced by non-pharmacological therapies and are not maintained.
DIET IN THE AETIOLOGY OF DISEASE
Diet is known to modulate factors that are important in the aetiology of cardiovascular disease (CVD), and dietary change is an important element of CVD risk management. This topic is discussed in detail in Chapter 38. Diet is also important in the aetiology of some gastrointestinal diseases (e.g. coeliac disease), and this is discussed in Chapter 12. Other conditions in which diet plays a causative role include dental caries and some cancers.
Dental caries
The most common reasons for the loss of teeth are dental caries in children and periodontal disease in adults. Periodontal disease has only a minor association with nutrition, but there is a strong relationship between the formation of dental caries and diet.
The first stage of cariogenesis is the formation of plaque. Plaque begins to develop on the surface of a clean tooth by the adsorption of salivary proteins and glycoproteins to form the pellicle. Bacteria (e.g. Streptococcus mutans) then become attached to the pellicle and begin to reproduce, eventually forming a continuous layer. A polysaccharide coating then develops over them, formed from the action of bacterial enzymes on dietary sugars. This coating helps to maintain plaque integrity, but also acts as a source of energy for the bacteria during the gaps between the host’s meals. Dietary sugars diffuse into the plaque and are fermented by the plaque bacteria into mainly lactic acid, which then dissolves the mineral phase of tooth enamel, causing caries. Both the amount and frequency of sugars in the diet are positively correlated with caries formation. Some foods (e.g. milk, cheese and the sugar substitute xylitol) protect against dental caries, as does increasing salivary flow with sugar-free chewing gum.
Dietary fluoride, for example in fluoridated drinking water, is important in preventing the development of dental caries. A concentration of 1 mg/L increases the resistance of the enamel, particularly if the exposure occurs while it is being laid down prior to the tooth erupting, and reduces tooth decay in children by about 50%.
Cancer
Many associations have been noted between specific dietary components and various cancers (see Table 11.4). The overall risk of cancer appears to be highest in populations that consume diets high in fat and energy, in which there is a high prevalence of obesity and in which alcohol intake is high. Diets that are high in ‘fibre’, particularly those with a high fruit and vegetable content, appear to offer some protection from certain cancers. However, research in this area is difficult because, although dietary factors may predispose to or protect against cancer, the effect may be small compared with other known carcinogens (e.g. cigarette smoke), there may be interactions between different dietary constituents and methods for the measurement of dietary intakes are inherently inaccurate. Furthermore, altering the amount of any one component of the diet necessarily alters the relative amounts of the others.
TABLE 11.4
Some examples of associations between dietary factors and cancer
| Site of cancer | Associated dietary factors |
| Mouth, pharynx and larynx | Alcohol, very hot drinks, Chinese-style salted fish |
| Oesophagus | Alcohol, very hot drinks, nitrosamines (in preserved foods) |
| Stomach | Smoked fish, pickled foods, cured meats, salt; nitrosamines formed in the stomach from nitrites in the diet |
| Large intestine | High fat, high protein diet, low fibre intake |
| Liver | Aflatoxin-contaminated foods (aflatoxins are a product of the fungus Aspergillus flavus), alcohol |
| Breast | High fat diet, alcohol |
THERAPEUTIC DIETS, DIETARY SUPPLEMENTS AND NUTRACEUTICALS
Details of therapeutic diets can be found in textbooks of dietetics, but the main features of some of the more common ones are summarized in Table 11.5.
TABLE 11.5
The main features of some therapeutic diets
| Disease | Main features of dietetic management |
| Diabetes mellitus | Reduce risk of microvascular disease by achieving near normal glycaemia, while avoiding risk of hypoglycaemia |
| Reduce risk of macrovascular disease, including management of body weight, dyslipidaemia and hypertension | |
| 45–60% dietary energy from carbohydrate, especially from complex carbohydrates or those with low glycaemic index | |
| May have up to 10% energy from sucrose, provided it is eaten in the context of a healthy diet and spread throughout the day | |
| < 10% energy from saturated and trans fatty acids | |
| < 10% energy from polyunsaturated fatty acids, including ω3-polyunsaturated fatty acids from oily fish | |
| 10–20% energy from monounsaturated fatty acids | |
| Protein not more than 1 g/kg/day, i.e. not a high intake | |
| Obesity | Aim for 10% weight loss. Further targets can be set if appropriate |
| Reduce dietary energy by reducing fat and sugar intake | |
| Encourage a regular meal pattern, including complex carbohydrates at each meal | |
| Encourage physical activity | |
| Undernutrition, e.g. caused by dysphagia, cancer, loss of appetite | Increase protein and energy intake by food fortification measures, energy and nutrient-dense foods, and nourishing drinks |
| Prescribe nutritional supplements if measures above fail | |
| Hyperlipidaemia | Reduce saturated fat intake to 10% dietary energy |
| Partially replace saturated fats with monounsaturated fats and complex carbohydrates | |
| Increase ω3-polyunsaturated fatty acid intake | |
| At least five portions of fruit and vegetables per day | |
| Reduce salt intake | |
| Avoid excess alcohol | |
| Lose weight if obese | |
| Coeliac disease | Eliminate all gluten-containing foods (wheat, barley, rye and possibly oats) from the diet |
| Allergy | Exclusion diets, e.g. exclusion of wheat, milk, eggs or additives |
| Renal disease | Normal protein intake, 0.8–1.0 g/kg/day (low protein diets are now rarely used) |
| Maintain energy intake | |
| Adjust sodium, potassium and fluid intake depending on whether predominant retention or loss | |
| Inherited metabolic diseases, e.g. phenylketonuria, galactosaemia | Specialist dietary advice required |
Apart from the well-established benefits of therapeutic diets, claims are also made that some dietary constituents have health benefits beyond their purely nutritional role. Functional foods are defined as foods that provide a health benefit beyond basic nutrition, when consumed in normal amounts as part of a varied diet. Nutraceuticals are dietary supplements that provide a concentrated form of an agent from a food, in a dose exceeding that obtainable from a normal diet. All such products are sold as foods, or food supplements, rather than drugs, and so are not subject to pharmaceutical licensing regulations. There is, therefore, little evidence and there are few clinical trials to enable assessment of their efficacy and formal documentation of side-effects. It has been noted that kava (Piper methysticum), which is said to help in anxiety, is probably hepatotoxic and preparations of St John’s wort (Hypericum perforatum) are known to decrease the efficacy of antiretroviral drugs, ciclosporin, tacrolimus and some antiepilepsy drugs.
A probiotic is a preparation containing defined microorganisms in sufficient numbers to alter the microflora of the host and exert a beneficial health effect. Such preparations are commonly manufactured as a dairy product supplemented with species such as Bifidobacteria or Lactobacilli. They have been shown to be effective in reducing the duration of antibiotic-associated diarrhoea and alleviating symptoms and promoting digestion in lactose malabsorption.
Other dietary supplements for which there is less good evidence of benefit include coenzyme Q10 (for mitochondrial disorders, heart failure and ischaemia–reperfusion injury), saw palmetto (for benign prostatic hypertrophy) and taurine, carnitine, creatine, chondroitin and glucosamine (for osteoarthritis). The American Association of Clinical Endocrinologists has produced a useful summary of the available evidence (see Further reading, below).
PROVISION OF NUTRITION SUPPORT
Deficiencies of specific vitamins or minerals are treated by appropriate supplementation of individual nutrients, and this could be thought of as a form of nutritional support. However, the term is usually used to mean the provision of balanced mixtures of nutrients to replace all, or a major part, of normal food intake. It is recommended that nutrition support is provided by a multidisciplinary team (e.g. gastroenterologist, pharmacist, clinical biochemist, dietitian, nutrition nurse) that assesses patients on an individual basis and makes adjustments to their nutritional therapy as required. Such an approach has been shown to improve clinical outcomes and reduce morbidity and mortality.
Indications for nutrition support
The basic indications for nutrition support are intestinal failure (e.g. after massive bowel resection) or inability to eat (e.g. in neurological diseases that affect swallowing). However, it should be considered in any patient who is malnourished or likely to become so (see Box 11.2).
Simple measures to support nutritional intake include food enrichment, for example by the addition of cream to soup, or cheese to potato. Various proprietary preparations exist: modular supplements, typically in powder form, contain just one macronutrient, and a range of fortified puddings provide both protein and energy. Fortified juice-based products and nutritionally complete, milky-type drinks are also available.
For patients whose intake remains poor, despite use of such measures, or in whom their use is inappropriate, nutritional support via the enteral (gut) or parenteral (intravenous) route is required. Whenever possible, the gut, rather than the intravenous route, should be used to deliver nutritional support. There are several reasons for this. There appears to be no metabolic advantage in intravenous feeding and there are some positive advantages to enteral feeding. Nutrients are needed in the gut lumen to maintain the structural and functional integrity of the gastrointestinal tract. Enteral feeding also stimulates gallbladder motility and reduces the risk of cholelithiasis. Only a limited proportion of certain nutrients is actually absorbed from the gut and, in general, the enteral requirements for specific nutrients are better understood than parenteral requirements. Enteral feeding can supply the gut-specific substrates glutamine and short chain fatty acids, which are not usually present in commercial parenteral feeding solutions. Enteral feeding is also less hazardous than use of the intravenous route and, in financial terms, is much the cheaper of the two options.
In some situations, it may be difficult to decide which route is more appropriate. Particularly in postoperative patients, gut function tends to be assessed by clinical indicators of gut motility, for example the presence of bowel sounds and the passage of flatus or faeces. It is well-established that in such patients, intestinal function tends to return before motility. It may be safe, therefore, to commence enteral feeding cautiously before bowel sounds are heard.
Estimates can be made of the basal metabolic rate in order to tailor feeds to individual nutritional needs. However, guidelines produced by the UK National Institute for Health and Care Excellence (NICE) suggest that, for patients who are not severely ill or injured, or at increased risk of refeeding syndrome (see later), feeds should supply the following:
• 25–35 kcal/kg body weight/day total energy (including that derived from protein)
• 0.8–1.5 g protein (or equivalent as amino acids) (0.13–0.24 g nitrogen)/kg per day
• 30–35 mL fluid/kg (with allowance for extra losses, e.g. from drains or fistulae, and extra input, e.g. from intravenous drugs)
• adequate electrolytes, minerals and micronutrients and fibre if appropriate.
Enteral feeding
The route of administration of enteral feeding tends to be determined by the likely duration of feeding, although physical constraints, for example oral surgery, may need to be taken into consideration. For the majority of patients requiring short-term enteral feeding, the most appropriate method of delivery is via a fine-bore nasogastric feeding tube. Placement of the tube is relatively simple, although its correct positioning in the stomach should always be checked before use by ensuring aspiration of acidic gastric contents (using pH paper), lest inadvertent pulmonary (or, rarely, intracranial) misplacement has occurred.
There is a risk of gastric regurgitation and subsequent pulmonary aspiration during nasogastric feeding and, to minimize this, patients should be nursed at 45° wherever possible. For patients at increased risk of aspiration (e.g. those on ventilators or being nursed flat after certain neurosurgical procedures, diabetic patients with neuropathy) insertion of a feeding tube into the duodenum or jejunum may lessen the risk.
For long-term enteral feeding the preferred route is often a tube placed through the abdominal wall directly into the stomach – a gastrostomy. This avoids local problems such as rhinitis and nasal erosions, which can be associated with prolonged use of a nasogastric tube, and is more secure. This type of gastrostomy is usually inserted percutaneously under endoscopic control (PEG).
The exact nature of the enteral feed given is dependent upon the patient’s nutritional requirements, the indication for feeding and any comorbidity, for example renal failure. Whilst a normal diet could be liquefied and used for enteral feeding, this is not generally recommended owing to the risk of infection. Instead, proprietary feeds are preferred. These tend to be of three types: polymeric, pre-digested or disease-specific; they are typically nutritionally complete, may contain fibre and generally contain 1–1.5 kcal/mL.
In polymeric feeds, the nitrogen is in the form of whole protein, the carbohydrate source is partially hydrolysed starch and the fat contains long chain triglycerides. In patients with significant malabsorption, a pre-digested feed may be appropriate. In this, the nitrogen is supplied in the form of short peptides and the fat component contains both long and medium chain triglycerides. In severe malabsorption, elemental feeds providing nitrogen as amino acids and carbohydrate as glucose, with very little fat, can be used. Feeds can also be manufactured with their components adjusted to be suitable for the patient’s underlying condition, for example low sodium and volume in patients with liver failure.
Parenteral nutrition
In a patient whose gut is not functioning (or is not functioning sufficiently well to sustain adequate nutrition), parenteral feeding has to be used. Parenteral nutrition (PN) solutions are hyperosmolar and tend to be irritant when infused via peripheral cannulae. For short-term feeding, such administration is possible (particularly if hyperosmolarity is reduced by having fat as a major energy source), but infusion into a large vein is preferred. This is usually achieved via a midline, peripherally inserted central line (PICC) or a central venous line. For longer-term feeding, a tunneled line, such as a Hickman, is preferred, to reduce the possibility of infection. Ideally, lines should be used solely for PN. However, if vascular access is compromised, a line with multiple lumens may be provided, but one lumen should be specified and reserved for feeding.
Composition of parenteral nutrition fluids
The carbohydrate component of parenteral nutrition solutions is provided as glucose. Protein is given as a mixture of L-amino acids. There are relatively few data to enable characterization of the optimal quantity of total or individual amino acids. Parenteral glutamine has been shown to be of benefit in certain groups of patients, although the exact situations in which its use is appropriate, and the most appropriate dose, are not yet fully defined. There are, as yet, no definite clinical recommendations regarding supplementation of arginine, branched chain amino acids, cysteine or taurine in parenteral nutrition solutions. Lipid preparations for parenteral use consist of oil stabilized in an emulsion with egg yolk lecithin. The most widely available preparations worldwide are based on soybean oil, but newer products now exist including olive oil-soybean mixtures, fish oil emulsions and multiple lipids (e.g. ‘SMOF’ a mixture of soy, medium chain triglycerides, olive and fish oil). Fatty acids have a role in the immune response, for example via their incorporation into cell membranes and effects on membrane fluidity and receptor expression. Particularly in patients in whom a robust inflammatory response is required, or in whom there is a particular risk of exacerbation of oxidative stress, evidence is accumulating that the choice of lipid preparation may have an effect on outcome.
Parenteral nutrition should contain appropriate amounts of micronutrients. In the past, trace elements were added based on knowledge of the requirements of well patients eating a normal diet and instances of deficiency and toxicity occurred. Since then, the recommendations for the micronutrient component of commercial preparations used in PN have been regularly reviewed, the most recent modifications suggesting a decrease in the content of copper, manganese and chromium.
Premixed bags of PN solutions, with a variety of amino acid (or nitrogen), energy and electrolyte content, are readily available from commercial suppliers. The bag with contents most suitable for an individual patient’s requirements is chosen and variation in the rate of infusion allows some adjustment to the amount of nutrition administered. For patients whose requirements cannot be accommodated by such premixed bags, individually prescribed nutrition solutions can be prepared under sterile conditions in many hospital pharmacies.
Complications of parenteral nutritional support
Malnutrition is known to be associated with adverse effects that lead to complications and prolonged hospital stay. Nutrition support has been shown to improve quality of life and to reduce complication rates, infection, mortality and length of hospital stay. However, nutrition support, particularly when provided by the parenteral route, is itself associated with complications.
The main non-metabolic complications are related to the placement and presence of the feeding line. Complications associated with central venous catheterization include arterial puncture, air embolism and pneumothorax. Once inserted, good care of the line is essential, using sterile technique to minimize the major risk of catheter-related sepsis. Other possible complications include line blockage, line displacement and thromboembolism.
The aim of monitoring in patients receiving nutrition support is to ensure efficacy and to prevent or identify associated complications. It is important to keep careful records of the amount of nutrition that the patient has actually received and to chart any additional fluid input and losses. Regular weighing and measurement of skin fold thickness may provide an objective way of charting the effectiveness of nutrition support. A protocol for laboratory monitoring of nutritional support has been proposed by NICE (see Table 11.6). Other aspects to be monitored, particularly in patients on long-term feeding, include the course of the primary disease and the patients’ quality of life.
TABLE 11.6
Protocol for laboratory monitoring of nutrition support
| Parameter | Frequency |
| Full blood count | Baseline 1–2 times a week until stable Then weekly |
| Renal profile | Baseline Daily until stable Then 1–2 times a week |
| Liver profile (including prothrombin time) | Baseline Twice weekly until stable Then weekly |
| Glucose | Baseline 1–2 times a day (more if needed) until stable Then weekly |
| Calcium, albumin | Baseline Then weekly |
| Magnesium, phosphate | Baseline Daily if at risk of refeeding syndrome Three times a week until stable Then weekly |
| Iron, ferritin folate, vitamin B12 | Baseline Every 3–6 months |
| CRP | Baseline As required to assess presence of an acute phase response and assist interpretation of protein, trace element and vitamin results |
| Zinc, copper | Baseline Then every 2–4 weeks depending on result |
| Seleniuma | Baseline if risk of depletion Further testing dependent on baseline |
| Manganeseb | Every 3–6 months if on home PN |
| Vitamin Db | 6 monthly if on long-term support |
| Bone densitometryb | On starting home PN Then every two years |
a Needed primarily for patients having PN in the community.
b Rarely needed for patients having enteral feeding. PN, parenteral nutrition.
Electrolyte abnormalities are common in patients receiving PN and are most likely to occur soon after initiation of treatment. Abnormalities of sodium, potassium and magnesium are common and factors such as gut losses through vomiting, faecal or stomal loss, renal failure and body water excess, for example from ascites, may be contributory.
Hyperglycaemia is frequent in parenteral feeding, particularly in patients with coexisting sepsis and, if detected, should be controlled with a continuous peripheral insulin infusion: careful control of blood glucose has been shown to improve outcome in critically ill patients. Hypoglycaemia has been documented on abrupt cessation of total parenteral nutrition, but is not commonly encountered with modern feeding regimens.
Hypercalcaemia can occasionally occur in patients receiving parenteral nutrition that includes a vitamin D supplement, and hypocalcaemia can occur if insufficient supplements are given. Hypocalcaemia of unexplained origin should prompt measurement of the plasma magnesium concentration, as hypomagnesaemia may itself cause hypocalcaemia.
The refeeding syndrome comprises a group of abnormalities that may occur when nutrition is provided to those who are severely malnourished. The most well recognized feature of the syndrome is hypophosphataemia, but other electrolyte abnormalities can also occur together with cardiac, pulmonary, neuromuscular and haematological complications. It most commonly occurs in association with parenteral nutrition, although it has been documented as a complication of enteral nutrition. It should be anticipated in those patients at increased risk (see Box 11.3) in whom feeding should be commenced at a low rate and increased gradually.
The aetiology of the hypophosphataemia is probably multifactorial. One important factor is that, during prolonged starvation, energy production is derived principally from fatty acid oxidation rather than from glucose; however, on refeeding and reintroduction of carbohydrate, there is a rapid return to the use of glucose as the predominate substrate with a high requirement for phosphate to synthesize the phosphorylated intermediates and products of glycolysis (e.g. ATP and 2.3-DPG). This leads to hypophosphataemia. There is an intracellular shift of potassium and magnesium as a result of increased insulin release, which leads to hypokalaemia and hypomagnesaemia. Sodium and water retention may occur: an anti-natriuretic action of insulin has been suggested as a possible mechanism.
Thiamin deficiency is common in severely malnourished individuals. Thiamin is a cofactor in glycolysis and, when carbohydrate intake is restored after caloric deprivation, demands are increased. In thiamin deficiency, pyruvate is converted to lactate, which may lead to lactic acidosis. Precipitation of the complications of thiamin deficiency should be prevented in those at risk of refeeding syndrome by administration of thiamin (initially in amounts in excess of normal requirements) before commencing, and during, nutrition support.
The clinical features of refeeding syndrome are related to the electrolyte abnormalities and vitamin deficiency.
Mild elevation of plasma liver enzyme activities is common after several weeks of parenteral feeding. Liver histology reveals hepatic steatosis with accumulation of macro- and microvesicular fat. However, the histological abnormalities are more closely correlated with the presence of intra-abdominal sepsis, renal failure and pre- existing liver disease than with the duration of PN.
Liver disease is a significant complication of long-term PN, estimated to occur in 15–60% of patients. In adults, steatosis occurs, progressing to intrahepatic cholestasis and then cirrhosis. The incidence of liver disease is higher in children, in whom cholestasis occurs earlier and can be rapidly progressive. Aetiological factors include the nature of the primary disease and residual intestinal anatomy, the presence of sepsis, lack of enteral nutrition and possible nutrient deficiencies or toxicity. Management of PN-induced liver disease includes detection and treatment of sepsis and optimization of the feeding regimen, perhaps with consideration of reduction in energy content. Ursodeoxycholic acid has been used with some benefit, particularly in neonates; rarely, liver and small intestinal transplantation may be required.
The prevalence of metabolic bone disease, characterized by reduced bone mineral density (BMD), is high in patients on long-term PN. Possible contributing factors include abnormalities (insufficiency or excess) of vitamin D, calcium, vitamin K, copper and aluminium. In addition, inflammatory bowel disease, which is an aetiological factor in a number of the patients, is, itself associated with reduced BMD through the action of bone resorbing cytokines and corticosteroid use. The use of bisphosphonates may be associated with an increase in BMD (as assessed by dual-energy X-ray absorptiometry, DEXA) in patients on PN, but their effect on fracture prevention is unclear.
Short bowel syndrome
For the majority of patients, parenteral nutrition is required for a short period during intercurrent illness. For others, it is a long-term treatment and they incorporate its administration into their daily routine at home and are otherwise well. The majority of these patients will have a short bowel. A short bowel is defined as one that is of insufficient length to enable adequate absorption, so that supplementation of macronutrients, and/or water, and/or electrolytes is essential. This is likely to occur in patients left with < 200 cm of small bowel. The most common causes of short bowel syndrome are mesenteric artery thrombosis, Crohn disease and radiation damage. The majority of patients with a short bowel can be divided into two groups: those with a short bowel and a jejunocolic anastomosis, and those with a short bowel and a high output stoma. To facilitate management, the latter group can be divided into net ‘absorbers’ and net ‘secretors’. The absorbers generally have more than ~ 100 cm of jejunum remaining. They are able to absorb more sodium and water from the diet than they take orally and have low stomal output. Net secretors have very little residual jejunum; stomal output is high (4–8 L/24 h) and losses of sodium and water exceed oral input.
Management of a patient with a high output jejunostomy includes oral fluid restriction to reduce stomal output. Any fluid given should contain sodium in a concentration of at least 100 mmol/L, together with glucose, to minimize passive sodium loss and facilitate glucose-coupled absorption of sodium. Antimotility agents (e.g. loperamide and codeine) and antisecretory drugs (e.g. H2 antagonists, proton-pump inhibitors and somatostatin) may be prescribed to further reduce stomal output.
For some patients with short bowel syndrome, small bowel transplantation is an alternative to long-term parenteral nutrition. Indications for intestinal transplantation include PN-related liver disease, central venous catheter related thrombosis and frequent central line sepsis. Patients who experience frequent episodes of dehydration, despite intravenous fluids may also be eligible. Survival rates after transplantation have shown steady improvement and are currently about 80% at one year and 43% at ten years.
CONCLUSION
Nutritional disorders include a wide spectrum of conditions, including generalized undernutrition, overnutrition leading to obesity, the eating disorders and diseases where nutrition has a role in the aetiology. Globally, both undernutrition and obesity are important public health problems. The treatment of undernutrition is often complicated by factors such as war, famine and infectious diseases. Obesity remains difficult to treat once present, although the advances in the understanding of the physiology of feeding discussed in this chapter are leading to new pharmacological and surgical interventions.
Nutritional interventions in the treatment of diseases may involve the use of therapeutic diets, the administration of dietary supplements or the provision of nutritional support, either enteral or parenteral. Nutritional support is best delivered by a multidisciplinary team. Further research is required to establish the efficacy (or otherwise) of most dietary supplements.
ACKNOWLEDGEMENT
We would like to thank Stephen K. Bangert and Carel W. Le Roux who wrote the chapter in the previous edition of this book.