CHAPTER 10
Clinical biochemistry of nutrition
CHAPTER OUTLINE
INTRODUCTION
Food is essential for human life. At the extremes, too little leads to starvation and too much leads to obesity, each with its associated effects on morbidity and mortality. Between these extremes, different people across the world follow apparently very different diets with no obvious resulting differences in their day-to-day health. It is apparent that in the long term the composition of the diet has an effect on the incidence of certain diseases, for example ischaemic heart disease and certain types of cancer, but often these associations are difficult to tease out because of the many confounding factors involved. However, this does not prevent very firm opinions being formulated and held about what is and what is not ‘good nutrition’, based on a variety of influences from basic science to religion.
Biochemistry clearly has a role in establishing the way in which the body uses various nutrients and has been important in defining certain deficiency states. Clinical biochemistry is still important in diagnosing deficiencies of certain specific nutrients, but whether there is a good biochemical marker of overall nutritional status is less clear. This chapter begins with a consideration of the various nutrients, including the effects of their deficiency or excess, and then discusses nutritional assessment. The following chapter discusses nutritional disorders and their management, both from the point of view of nutrition as an aetiological factor in disease, and disorders that are not primarily nutritional but where dietary modification or nutritional support may be important in treatment.
NUTRITIONAL REQUIREMENTS
The ‘correct’ intake
Part of the explanation of the fact that apparently very different diets can sustain life equally well is the concept that individual foodstuffs actually comprise different combinations of certain basic nutrients, and that it is the supply of these basic nutrients that is important rather than their origin. However, even when individual nutrients are considered, the definition of the ‘correct’ intake is problematical since this may be taken, for example, to be any of the following: the intake that avoids clinical signs of deficiency; the intake that maintains a given circulating concentration or tissue content of the nutrient; the intake that cures symptoms or signs of clinical deficiency; the intake that maintains a balance between intake and consumption or loss from the body over a defined period, or any one of a variety of other definitions. Even if a suitable measure can be determined for a specific nutrient, there are further problems in setting the absolute figure, since within a population, requirements will vary between individuals (owing, for example to differences in age, gender size and body composition) and even in an individual, requirements may vary in the short term (owing, for example to pregnancy, illness or environmental stress).
It is thus difficult to accurately estimate average requirements for a nutrient, and most attempts to make recommendations about intakes have depended on setting levels where most of the population will not be deficient, although it is also possible to define intakes below which most of a population will develop deficiency, and in some cases intakes that are high enough to be toxic.
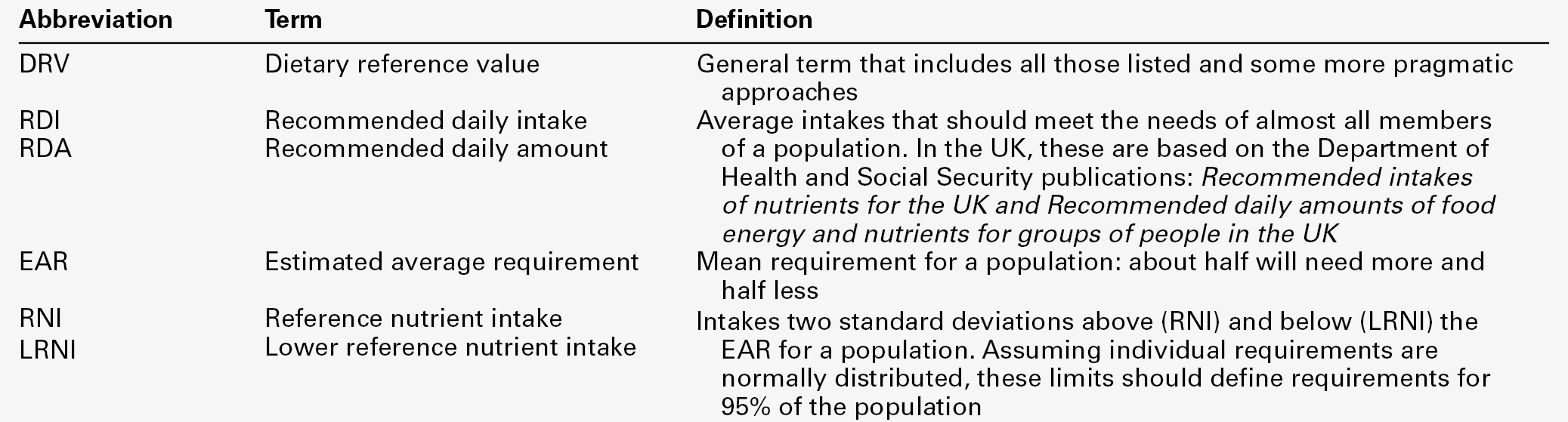
These points are covered in more detail in some of the texts listed under Further reading, below, and no attempt will be made here to define ideal intakes in absolute terms. Some of the terms used to describe nutrient requirements are defined in Table 10.1.
TABLE 10.1
Definitions of terms used in describing nutrient requirements
Adapted from Department of Health 1991.
Energy
The body uses energy in the maintenance of metabolic processes, in physical activity and in growth. In the resting individual, energy-requiring processes include the active pumping of ions across cell membranes, thermoregulation, cell division and basal function of all of the body’s systems. Resting energy expenditure appears to alter with energy intake to some extent. Physical activity, particularly if vigorous, can increase energy expenditure markedly, although such activity is not usually sustained for long enough to make the increase substantial when measured over a 24 h period. The laying down of new tissues represents an investment of energy, so it is not surprising that energy requirements, expressed in terms of body weight, are highest in infants and young children, with a gradual decline from the third decade into old age.
Thus, the energy requirement of an individual varies with body size and composition, gender, age, nutritional status and climate. In women, energy expenditure is increased during pregnancy and lactation, and in any individual, illness or response to trauma (e.g. systemic infection, burns) may cause a considerable increase.
Energy expenditure and energy intake do not always rise and fall in parallel. A sustained deficiency in energy intake generally leads to consumption of body stores of energy, including protein as well as glycogen and fat. Excessive energy intake (if sustained) results in obesity. Both of these conditions are considered further in the next chapter.
Dietary energy is mainly obtained from a combination of fat and carbohydrate. In a Western diet, each may account for 40–50% of total energy intake, although in developing countries the proportion from carbohydrate tends to be higher and that from fat, lower. Although dietary protein is generally thought of as a source of materials (amino acids) for endogenous protein production, it may also be used for energy production, for example if protein intake is adequate but non-protein energy intake is low. Ethanol also has to be considered as a significant energy source in certain populations; for example, in the UK, it forms 7% of average energy intake. (Interestingly, at higher ethanol intakes – 25–35% of dietary energy – ethanol appears not to be completely utilized as a source of energy, although the reason for this is not clear.)
Carbohydrate
Dietary carbohydrate provides 4.1 kcal/g and most is derived from sugars and starch. The sugars are mainly the monosaccharides glucose, fructose and galactose, and the disaccharides sucrose, lactose and maltose. Dietary sugars can be divided into those which are present in intact cells, such as in whole fruit (intrinsic sugars), and those which are free and readily absorbed as a result of having been added to food, usually as sucrose (extrinsic sugars). Milk sugars are usually considered as intrinsic. A high intake of extrinsic sugars is associated with an increased prevalence of dental caries. Sugar derivatives, such as the sugar alcohols sorbitol and xylitol, can be partially digested and can provide 2.4 kcal/g.
In the UK, it is recommended that in the adult, 40–50% of energy intake should be provided by carbohydrate, but non-milk extrinsic sugars should not exceed 11% of energy intake. There is considerable debate about whether a high intake of non-milk extrinsic sugars may have harmful effects in addition to the increased risk of dental caries. If such an intake results in an increased energy intake, then obesity is likely to be a problem, but in any case, ingestion of large amounts of extrinsic sugars can lead to raised plasma glucose, insulin and lipid concentrations, all of which are potentially harmful.
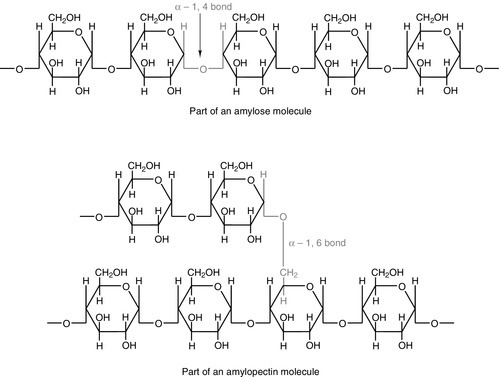
Large amounts of starch are not harmful: indeed, in developing countries, starch may form 75–80% of the total energy intake. Starches are α-glucan polysaccharides, of which there are two major forms, amylose and amylopectin (see Fig. 10.1). They are partially resistant to hydrolysis during digestion and are thus not totally available as an energy source. Certain other carbohydrates, that are not energy sources, but form part of ‘dietary fibre’, are considered later.
A deficiency in dietary carbohydrate will either lead to energy deficiency or result in potentially harmful amounts of other energy sources being required in the diet if energy intake is to be maintained. However, certain tissues have an obligatory requirement for glucose as an energy substrate (e.g. brain and nervous system (in the short term), red blood cells) and, while this need can be met by gluconeogenesis, some carbohydrate is necessary in the diet if ketosis is to be avoided.
Fat
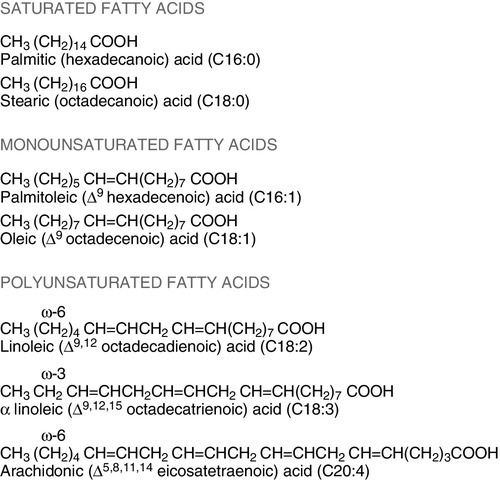
The term ‘fat’ includes a range of substances, including triacylglycerols (triglycerides), phospholipids and sterols (e.g. cholesterol). Triacylglycerols are the most common storage fats and are thus the most important fatty energy source in foods. They are a relatively ‘concentrated’ energy source, with a higher energy content per unit weight (9.3 kcal/g) than carbohydrate (4.1 kcal/g). Although all triacylglycerols can act in this way, there may be important long-term health differences between them, dependent on the structure of the fatty acid residues they contain. The main differences are in the length of the carbon chain, the presence (and number) of any unsaturated bonds and, if such bonds are present, the positional and geometric isomers present. Some examples of these differences are illustrated in Figure 10.2. The effects of excessive intake of triglycerides are discussed further in Chapter 11, and the differences in absorption and metabolism between long chain and medium chain triglycerides in Chapter 12.
Dietary fat deficiency does not generally seem to be a problem, as most fats necessary to the body can be endogenously synthesized when a high proportion of total energy intake is supplied by carbohydrate. However, there are certain fatty acids that appear to be essential, at least in small amounts. These are linoleic acid (C18:2, ω-6) (for nomenclature, see Fig. 10.2) and α-linolenic acid (C18:3, ω-3). They are important components of phospholipids, in which they help to maintain the function of cellular and subcellular membranes. They are also involved in the regulation of cholesterol transport, breakdown and excretion. They are the precursors of arachidonic (C20:4, ω-6), eicosapentaenoic (C20:5, ω-3) and docosahexaenoic (C22:6, ω-3) acids and are thus also important in the synthesis of prostaglandins, thromboxanes and leukotrienes. These three longer chain fatty acids are not strictly essential fatty acids (EFAs), but may become conditionally so in EFA deficiency. Adequate dietary supply of the longer chain fatty acids is probably also important during rapid brain growth in infancy, particularly in fast-growing premature infants.
The clinical effects of EFA deficiency include dermatitis, alopecia and fatty liver, but these are only seen when EFAs provide less than 1–2% of total dietary energy intake. In patients receiving long-term fat-free parenteral nutrition, cutaneous application of small amounts of appropriate oils has alleviated biochemical EFA deficiency.
Protein
Proteins have a key structural and functional role in virtually all bodily processes, and a supply of appropriate amino acids is necessary during normal turnover, with additional amounts if extra protein is being formed, for example during growth, pregnancy or lactation. This supply generally comes from dietary protein, of either animal or vegetable origin, but the actual amount of protein required depends on its amino acid content. Human protein contains 20 amino acids (Box 10.1), of which nine are recognized to be essential (or ‘indispensable’) in adults – the remaining 11 can be synthesized endogenously if the food protein is deficient in these but contains sufficient essential amino acids. Some amino acids, for example cysteine and glutamine, whilst not normally essential, may become so in conditions such as severe illness, trauma or burns, when requirements exceed the body’s ability to synthesize them. In children, due to the increased demands of growth, the threshold for an amino acid to become conditionally essential is lower. Only L-amino acids are incorporated into human proteins.
Some proteins, particularly those derived from animal sources such as eggs, milk, fish and meat, contain all the essential amino acids in sufficient amounts for protein synthesis and are said to be complete, or of high quality. Foods lacking one or more amino acids are termed incomplete proteins; these are often from plant sources such as pulses. In diets in which plant foods are the main source of protein, combining different types of food in appropriate quantities is required in order to obtain all the essential amino acids. However, the amino acid profile of dietary proteins does not completely predict the amounts of these amino acids that will be available for protein synthesis after digestion and absorption: some of them may be biologically unavailable. Lysine is particularly important in this regard, as its supply in many dietary proteins is relatively low in comparison to nutritional requirements. It becomes unavailable if its ε-amino group reacts with another molecule, because ε-amino bonds are not cleaved by digestive enzymes. Such bonds may form with other amino acids (e.g. the carboxyl side chains of glutamic or aspartic acids) or with reducing sugars (during cooking or storage of foods).
Deficiency of protein generally occurs as part of more generalized malnutrition and is discussed later. Excessive protein intake in renal disease accelerates deterioration in renal function (see Chapter 7) and it is possible that it may have effects in healthy people, for example on renal function and on bone mineral density.
Micronutrients
Vitamins and certain trace elements are essential components of the diet required in very small amounts. Deficiency of individual micronutrients classically results in typical symptoms and signs according to the vitamin or trace element involved; recognition and treatment of deficiency of a single micronutrient deficiency is relatively easy. However, milder forms of deficiency, particularly of multiple micronutrients concurrently, may be difficult to recognize clinically.
Micronutrient status is likely to be affected by acute illness, owing to a combination of reduced intake and increased demand. Such increased demand may be due to catabolism resulting from the illness, subsequent anabolism and from disease-specific increased losses, for example loss from fistulae, diarrhoea and dialysis. As laboratory concentration of micronutrients may be perturbed in the acute phase, deficiencies in this group of patients can be particularly difficult to assess.
It is increasingly being recognized that subclinical deficiencies of micronutrients may have detrimental effects, some of which are described in the following pages. As the assessment of micronutrients can be important in patients receiving supplementation, consideration is also given to aspects of micronutrient toxicity.
Vitamin D is considered with bone metabolism in Chapter 31. Cobalt as a constituent of vitamin B12 is discussed, together with this vitamin, folate and iron, in Chapter 27. Iodine is essential in the formation of thyroid hormones (see Chapter 19).
Vitamins
Fat-soluble vitamins
Vitamins are traditionally classified into those that are fat soluble (A, D, E and K) and the remainder, which are water soluble. This is probably still a useful distinction, as it predicts when deficiency is likely (e.g. fat-soluble vitamins in steatorrhoea), and is retained here.
The fat-soluble vitamins A, E and K are structurally different, but are all non-polar, water-insoluble lipids. Absorption of each of them requires micelle formation in the gut lumen, for which bile salts are necessary.
Vitamin A
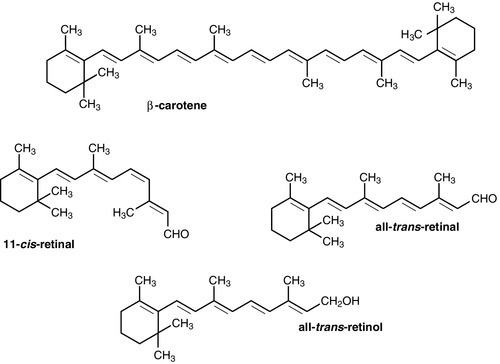
Dietary vitamin A occurs in a variety of forms which can be broadly divided into preformed vitamin A (retinol) and carotenoids, which are cleaved in the body to give vitamin A (see Fig. 10.3). In the UK, up to 75% of dietary vitamin A is preformed, derived from foods such as fortified cereals, margarine and dairy products, fish-liver oil and multivitamin preparations. Carotenoids are found in plants; the most important in the UK diet is β-carotene, which is found in carrots, dark green leafy vegetables, pumpkins and mangoes.
Retinol is transported in the bloodstream bound to retinol-binding protein (RBP), which in turn forms a complex with thyroid-binding pre-albumin. Protein-energy malnutrition can result in reduced synthesis of RBP and thus impaired retinol transport from the liver, producing a functional vitamin A deficiency, even in the presence of adequate liver reserves. β-Carotene undergoes oxidative fission in the intestine to give retinal (see Fig. 10.3), and hence retinol. The cleavage of provitamin A carotenoids appears to be inhibited when vitamin A stores are high, hence, toxicity from ingestion of plant sources is rare. Ingestion of excess preformed vitamin A leads to its absorption and hepatic storage.
The best understood of the actions of vitamin A is probably its role in vision. The 11-cis form of retinal is a component of the visual pigment rhodopsin, which is found in the rods of the retina. Light causes the retinal to change to the all-trans form (see Fig. 10.3) and this triggers a series of conformational and other changes, the end result being that an electrical signal is transmitted to the cortex of the brain and is perceived as light. A similar mechanism operates in the cones of the retina.
Vitamin A is also necessary for growth and for normal development and differentiation of tissues. These actions are mediated by retinoic acid, which is present in foods in only small amounts but can be manufactured in the body from retinol. It modulates gene expression by activation of nuclear receptors, of which there are two groups: retinoic acid receptors (RAR) bind all-trans-retinoic acid (and some other retinoids); retinoid X receptors (RXR) bind 9-cis-retinoic acid. The latter interact with other receptors (e.g. those for vitamin D and thyroid hormones), and in the absence of retinoic acid can also act as repressors of gene expression. Other metabolic roles of vitamin A include carrying mannosyl units in the synthesis of hydrophobic glycoproteins and the retinoylation of proteins, for example the regulatory subunits of cAMP protein kinases.
Vitamin A deficiency is common in some developing countries, particularly in children, and is the single most common preventable cause of blindness in the world. An early symptom is night blindness, because of inadequate amounts of 11-cis-retinal in the retina. However, deficiency also has important effects in mucus-secreting epithelia and so there are other consequences for the eyes. Keratinizing squamous metaplasia in the conjunctiva causes conjunctival xerosis and this may be followed by the appearance of white plaques of desquamated thickened epithelium, known as Bitot’s spots. Similar metaplasia in the cornea can lead to ulceration, which may progress to scarring and consequent blindness. Changes in other mucus-secreting epithelia, for example in the respiratory and gastrointestinal tracts, probably account in part for the lowered resistance to infections reported in vitamin A deficiency. In such areas of the world, public health programmes aimed at administering large doses of vitamin A have been successful in reducing morbidity and mortality associated with its deficiency, particularly in young children.
Acute ingestion of large amounts of preformed vitamin A can result in raised intracranial pressure, with headaches, nausea, vomiting and visual disturbances. Chronic overdosage is associated with bone damage including increased bone resorption, spontaneous fracture and sometimes raised plasma calcium concentration and alkaline phosphatase activity. Liver damage, hair loss and skin changes have also been reported.
Retinol is teratogenic (or, at least, the synthetic retinoids used in the treatment of certain dermatological conditions are) and so, in the UK, pregnant women are advised against self-medication with vitamin A and against consumption of liver and products made from it. A high intake of β-carotene results in an orange-yellow appearance of the plasma, body fat and skin, but is not usually regarded as harmful in the short term. However, there may be an increased incidence of malignancy in people taking β-carotene supplements, particularly among heavy smokers.
Vitamin E
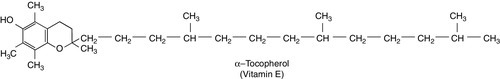
There are eight very similar compounds that have vitamin E activity, four tocopherols and four tocotrienols. The most active is the natural isomer of α-tocopherol, which accounts for about 90% of the vitamin E present in human tissues. Its structure is shown in Figure 10.4. It appears to be the major lipid-soluble antioxidant in cell membranes, acting to prevent the peroxidation of unsaturated fatty acids by free oxygen radicals. α-Tocopherol, but not its related tocopherols and tocotrienols, also has actions that are independent of its antioxidant properties. It modulates the transcription of certain genes, inhibits platelet aggregation and vascular smooth muscle proliferation and has an effect on cell signalling in the immune system.
The nutritional requirement for vitamin E is approximately proportional to the intake of polyunsaturated fatty acids. However, since foods rich in these (e.g. vegetable oils) also tend to contain large amounts of vitamin E, deficiency states are rare.
Vitamin E is not easily transported across the placenta and the first vitamin E deficiency state to be firmly established was in premature infants, who developed haemolytic anaemia, thrombocytosis and oedema. In children and adults who are unable to absorb or utilize vitamin E adequately (e.g. in cystic fibrosis or abetalipoproteinaemia), a progressive spinocerebellar degeneration may develop. The full syndrome consists of ataxia of the limbs, loss of position and vibration senses, absent deep tendon reflexes and pigmentary degeneration of the retina. Treatment with vitamin E supplements can prevent these neurological features or, if they are already present, arrest or even reverse them. For α-tocopherol to be made available to the tissues from very low density lipoprotein, it must first bind to hepatic α-tocopherol transfer protein. Individuals with a deficiency of this transfer protein develop a very similar clinical syndrome (ataxia with vitamin E deficiency, AVED).
There is some evidence that increased tissue concentrations of antioxidants, in particular vitamin E, may protect against conditions such as cancer and ischaemic heart disease, but this remains controversial.
Vitamin E supplements have also been taken to improve general well-being and sexual performance, but the only firm conclusion that can be drawn is that quite high intakes of vitamin E are non-toxic, although very high intakes may antagonize vitamin K and potentiate anticoagulant therapy.
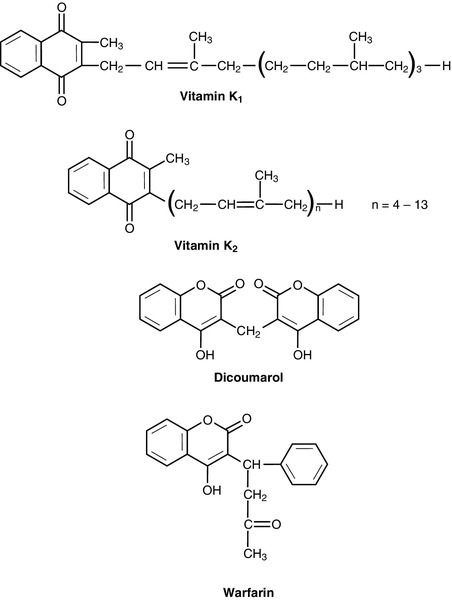
Vitamin K
The structures of the two forms of vitamin K and two vitamin K antagonists are shown in Figure 10.5. Vitamin K is involved in the post-translational modification of the blood coagulation factors II (prothrombin), VII, IX and X. A vitamin K-dependent enzyme system catalyses the carboxylation of the first ten amino-terminal glutamate residues in prothrombin to γ-carboxyglutamate (Gla). This carboxylation facilitates the binding of calcium, which is necessary for prothrombin to bind to platelet phospholipid, which is in turn necessary for the conversion of prothrombin to thrombin in the final common pathway of the clotting cascade. A similar process of γ-carboxylation occurs in factors VII, IX and X to form sites with a high affinity for calcium, and this is also known to occur in some clotting inhibitors (protein C and protein S). It also occurs in some proteins outside the clotting system, for example in osteocalcin and the matrix Gla protein in bone, in nephrocalcin in the renal cortex and in the product of the growth arrest specific gene (GAS6), which regulates differentiation and development in nervous tissue and apoptosis in other tissues.
The main dietary form of vitamin K is phytomenadione (K1), with leafy green vegetables being the richest source, although other vegetable sources are important in most diets. Menaquinones (K2) produced by the intestinal bacteria may also be important, since while circulating vitamin K is mainly phytomenadione, hepatic reserves are mainly menaquinones.
Vitamin K deficiency in its severest form leads to a bleeding syndrome, but dietary deficiency is rare after the first few months of life, unless there is an underlying disease affecting the absorption or utilization of the vitamin. However, vitamin K is undetectable in the blood of new-born babies, their hepatic reserves are low compared with adults and human breast milk contains barely adequate amounts. Thus, deficiency may present in infants as vitamin K deficiency bleeding (VKDB), within the first 24 h from the time of birth (early), between two and seven days (classic) or after seven days (late). Intracranial haemorrhage is rare in the early form, but occurs in more than 50% of babies with the late form and is likely to lead to death or severe neurological sequelae. The administration of vitamin K in the neonatal period abolishes the risk of deficiency. A single intramuscular injection of 1 mg of phytomenadione is highly effective for this purpose but suspicions, which now appear to be unfounded, were raised that its use led to an increased risk of childhood malignancy, particularly leukaemia. This adverse association has not been found for the administration of oral vitamin K, but the latter requires a multi-dose regimen. The current UK recommendation is that either intramuscular or oral vitamin K should be offered to all neonates after informed discussion with the parents.
Toxicity from naturally occurring K vitamins would appear to be rare, even in quite high doses. However, the use of synthetic preparations of menadione for nutritional purposes is best avoided, as serious unwanted effects have been recorded (e.g. haemolysis and liver damage in neonates).
Water-soluble vitamins
The water-soluble vitamins are polar compounds that, with the exception of vitamin B12, tend to be absorbed rapidly from the upper small intestine, mainly through either sodium-dependent active transport or carrier-mediated diffusion. Vitamin B12 and folate are discussed in Chapter 26.
Thiamin
The physiologically active form of thiamin (vitamin B1) is thiamin pyrophosphate (TPP). This functions as a cofactor in the conversion of pyruvate to acetyl-CoA by the pyruvate dehydrogenase complex and in the conversion of 2–oxoglutarate to succinyl-CoA by the 2–oxoglutarate dehydrogenase complex. It acts as a cofactor to the enzyme transketolase in the pentose phosphate pathway and plays a part in the metabolism of branched chain amino acids. Thiamin also has non-cofactor roles: thiamin triphosphate is found in nerve and muscle cells, where it activates membrane ion channels, possibly by phosphorylating them.
Thiamin is found in most foodstuffs, but wheatgerm, oatmeal and yeast are particularly rich sources. However, the body store of about 30 mg is only 30 times the daily requirement and so an inadequate diet is likely to lead to deficiency of thiamin sooner than of any other vitamin. Thiamin deficiency used to be widespread in areas where rice formed a major part of the diet, resulting from the consumption of polished rice (from which the husk had been removed). In present times, thiamin deficiency is seen more typically in alcoholics (alcohol decreases the absorption of thiamin). Diets high in carbohydrate require more thiamin for their assimilation than diets high in fat, and subclinical deficiency may be unmasked by refeeding with a carbohydrate-rich diet. Patients on long-term renal replacement treatment may become deficient in thiamin (and other water-soluble vitamins) unless given supplements.
Most of the clinical features of the disease beriberi respond to treatment with thiamin, suggesting that it is mainly due to thiamin deficiency. Two forms are described, one in which there is peripheral neuropathy, muscle weakness, general fatigue and reduced attention span (‘dry’ beriberi) and one in which there is also oedema and heart failure (‘wet’ beriberi). Some of these features may be the result of coexisting protein deficiency.
Patients with thiamin deficiency (commonly alcoholics) may present with a neuropathy and cardiomyopathy, but may also develop an encephalopathy, the Wernicke–Korsakoff syndrome. Wernicke encephalopathy and Korsakoff psychosis were originally described as two separate conditions, but now appear to be the acute and chronic manifestations, respectively, of a single condition. Wernicke encephalopathy has an acute onset, with ophthalmoplegia, nystagmus, ataxia and stupor or apathy. It is a medical emergency: the ophthalmoplegia, ataxia and lowered consciousness respond, in most cases within two days, to treatment with thiamin but, in up to 80% of cases, the mental changes fail to resolve completely and Korsakoff psychosis develops. Once established, this responds only slowly or not at all to thiamin treatment. The main features are retrograde amnesia, difficulty in assimilating new ideas and confabulation. Susceptibility to the development of this syndrome in thiamin deficiency may be greater in people who have a genetic variant of transketolase that binds thiamin less avidly than usual.
Thiamin is relatively non-toxic and can be given safely if deficiency is suspected. However, anaphylaxis has occurred after parenteral administration, and a wide variety of toxic effects has been described in adults with chronic intakes in excess of 50 mg/kg body weight or 3 g/24 h.
Riboflavin
Riboflavin (Vitamin B2) is a constituent of the two flavins found in flavoproteins, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), each of which acts as an electron carrier in many vital biological oxidoreduction systems. It is also a constituent of the cryptochromes, blue-light sensitive pigments in the eye that are important in the setting and maintenance of circadian rhythms. Clinical riboflavin deficiency is rare in countries where milk is a regular part of the diet; offal is another rich source. Even when dietary deficiency is present, clinical effects are rare because any riboflavin present in the body can be very efficiently conserved and reutilized.
The clinical features of riboflavin deficiency recorded in volunteers on a riboflavin-deficient diet include angular stomatitis, cheilosis, atrophic lingual papillae, glossitis, magenta tongue, seborrhoeic skin lesions, superficial lesions of the genitalia and vascularization of the cornea. The biochemical basis of these clinical manifestations is not immediately obvious and it may be that riboflavin deficiency has effects on the metabolism of other nutrients, for example pyridoxine, iron and folate.
Riboflavin absorption from the gastrointestinal tract is readily saturated, and any excess that does occur is rapidly excreted in the urine, so riboflavin does not accumulate in the body even when the oral intake is very high. This is probably just as well, since as well as reoxidation of reduced flavin coenzymes being a major source of oxygen radicals in the body, riboflavin is itself capable of non-enzymically generating reactive oxygen species.
Nicotinamide
Nicotinamide is a constituent of nicotinamide adenine dinucleotide (NAD+) and its phosphate (NADP+).
These are involved in a large number of oxidoreduction reactions in both cytosol and mitochondria, undergoing reversible reduction to NADH and NADPH, respectively. The major role of NADH is to transfer electrons from metabolic intermediates into the electron transfer chain, while NADPH acts as a reducing agent in a large number of biosynthetic processes. NAD+ and NADP+ are commonly referred to as coenzymes, but are probably better considered as true substrates.
Nicotinamide and its precursor nicotinic acid are both plentiful in animal and plant foods, although in some plant sources, for example maize, they are in a bound form and biologically unavailable. In man, a small amount is also formed in a minor pathway of tryptophan catabolism, via kynurenine to nicotinate, and it is usually accepted that 60 mg of tryptophan is equivalent to 1 mg of dietary nicotinamide or nicotinic acid. Both dietary and endogenous sources appear to be relatively important, since the deficiency state, pellagra, may occur when either is decreased. Simple dietary deficiency is now rare unless maize forms the main dietary constituent, but may be a complication of therapeutic low-protein diets. Decreased formation from tryptophan may occur where there is coexisting pyridoxine or riboflavin deficiency, since kynureninase and kynurenine hydroxylase are both dependent on these. Oestrogens also decrease the rate of tryptophan metabolism, so premenopausal women are more susceptible than men to borderline dietary deficiency. In Hartnup disease, there is decreased tryptophan absorption from the gut, and in carcinoid syndrome, there is increased usage of tryptophan in the synthesis of large amounts of 5-hydroxytryptamine – patients with either of these conditions are, therefore, particularly susceptible to decreases in nicotinamide and nicotinic acid intake.
The initial features of pellagra are non-specific (weakness, lassitude, anorexia and indigestion), but are followed by pigmented dermatitis in areas of skin exposed to sunlight, diarrhoea with widespread inflammation of epithelial surfaces and dementia, which may be preceded by irritability and depression.
Nicotinic acid is sometimes used in high doses for the treatment of combined hyperlipidaemia and the adverse effects are known to include flushing, hepatotoxicity, hyperuricaemia and impaired glucose tolerance.
‘Niacin’ is sometimes used to mean both nicotinic acid and nicotinamide and sometimes just one or other of them; because of this imprecision, the term has not been used here.
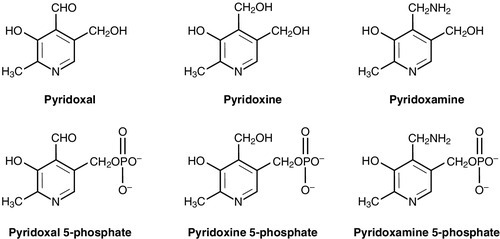
Vitamin B6
Pyridoxal, pyridoxine, pyridoxamine and their 5´-phosphates (Fig. 10.6) are interconvertible in the body and all have vitamin B6 activity. Pyridoxal phosphate is a cofactor for over 60 enzymes that catalyse reactions of amino acids, and so the absolute requirement is related to the rate of amino acid metabolism. It is also a cofactor for glycogen phosphorylase, and has roles in the modulation of steroid hormone action and the regulation of gene expression. Primary dietary deficiency of vitamin B6 is rare, since it is widely distributed in foods (although in some vegetable sources, it may be present in a biologically unavailable glycoside form), body stores of the vitamin are reasonable and it is synthesized by intestinal flora. The first definite cases of deficiency described were in infants fed a milk preparation that had been overheated during manufacture, destroying much of the vitamin B6. Some (but not all) of the infants developed neurological symptoms, including convulsions, which responded to vitamin B6 supplements. Human volunteers and animals fed a B6-deficient diet develop neurological symptoms together with changes in the mouth and skin. The changes in the nervous system are presumably due to the role of vitamin B6 in the metabolism of neurotransmitters. Certain drugs are known to interfere with vitamin B6 metabolism, for example isoniazid, which binds to pyrixoxal-5-phosphate reducing its availability, and penicillamine; vitamin B6 supplementation during drug treatment is advised.
Pharmacological doses of pyridoxine may be useful in certain rare inherited conditions, for example hypochromic sideroblastic anaemia, but in others (e.g. premenstrual tension), the evidence for benefit is less good, and in very high doses, pyridoxine may itself cause a severe peripheral sensory neuropathy.
Pantothenic acid
Pantothenic acid is a constituent of the coenzyme A molecule and is thus involved in many major metabolic pathways. It is also present (not as part of coenzyme A) in fatty acid synthase.
The name means ‘available everywhere’ and this reflects its distribution in foods. Particularly rich sources include liver, meat, cereals, milk, egg yolk and fresh vegetables. Spontaneous isolated deficiency in man has never been proven, but experimental deficiency in human volunteers leads to vomiting, malaise, abdominal distress and burning cramps, and later on, tenderness in the heels, fatigue and insomnia.
Biotin
Biotin acts as a coenzyme in a number of one-carbon transfer reactions. ATP hydrolysis is coupled to the carboxylation of the biotin to form N-1′-carboxybiotin, which then carboxylates the substrate of the enzyme. Examples include pyruvate carboxylase and acetyl-CoA carboxylase, so biotin is important in gluconeogenesis and lipogenesis and also plays a part in the catabolism of branched chain amino acids.
Biotin is widely distributed in foods, with offal, milk and eggs being particularly rich sources. Intestinal bacteria synthesize biotin, but it is not clear how much of this is available for absorption. Deficiency has been described in patients on parenteral nutrition deficient in biotin and also in people who consume large amounts of raw eggs: egg white contains a glycoprotein called avidin that binds biotin and prevents absorption. Such patients develop a fine scaly dermatitis and hair loss, but isolated biotin deficiency in man is otherwise unknown, apart from a suggestion that subclinical deficiency in pregnant women may be an aetiological factor in some birth defects. Even large doses of biotin appear to be non-toxic.
Vitamin C
Vitamin C comprises L-ascorbic acid and the oxidized derivative, L-dehydroascorbic acid. In animal tissues, 90% is in the form of ascorbic acid, but the two are interconvertible and both are biologically active. Man is unusual in that most other mammals can synthesize ascorbate from D-glucose or D-galactose. Guinea pigs cannot and so form a useful experimental model.
Vitamin C is an important aqueous antioxidant in the body, although its relationships with other antioxidants remain to be fully elucidated. Its best characterized role is in the post-translational reduction of proline to form hydroxyproline. This imino acid is relatively uncommon in the body apart from in collagen, where it is essential, and one of the effects of vitamin C deficiency is impaired collagen formation. Vitamin C also assists in the intestinal absorption of non-haem iron, by keeping it in the ferrous (Fe2 +) form.
The main dietary sources of vitamin C are citrus and soft fruits, together with the growing points of certain vegetables, although, in the UK, potatoes also make a significant contribution. It is one of the most labile of nutrients, being destroyed by oxygen, metal ions, alkaline conditions, heat and light: cooking reduces the amount of dietary ascorbic acid available for absorption.
Deficiency of vitamin C causes scurvy, with perifollicular (or more extensive) haemorrhages, bleeding gums, poor wound healing, failure of hair follicle eruption and anaemia. Factors assumed to contribute to the anaemia include blood loss, impaired iron absorption and a reduction in the capacity of ascorbic acid to protect folate from irreversible oxidation. Patients with scurvy may also experience changes in personality and psychomotor performance: it is thought that these are due to impaired synthesis of catecholamines by dopamine β-hydroxylase, a vitamin C-dependent enzyme. Dietary deficiency can occur, particularly if food is overcooked, and requirements are increased in certain patients, for example following trauma or surgery or in the presence of certain drugs, for example sulfasalazine, aspirin. Smokers also seem to have an increased turnover of vitamin C.
There have been many attempts to demonstrate benefits from an excess intake of vitamin C (i.e. higher than that required to prevent scurvy) in a diverse range of conditions. None of these has yet been proven, although as one of the proponents, Linus Pauling, was a double Nobel laureate, they remain widely held beliefs. Potential risks of very high doses include diarrhoea and increased formation of urinary oxalate, although the latter may be an in vitro effect in stored urine, and thus not a risk factor for renal stones.
Other organic substances
Food contains many other organic substances besides those described thus far. There is, however, no convincing evidence that any of these are essential nutrients in man, although some (e.g. choline, pangamic acid, laetrile) have been proposed as such, and choline is now regarded as an essential nutrient in the USA. Carnitine, which is involved in the transport of acyl-CoA across the mitochondrial membrane in fatty acid oxidation, is synthesized in the liver in adults, but there may be a dietary requirement in premature infants.
Some organic (and inorganic) substances are deliberately added to foods. They include preservatives, flavourings, colourings, sweeteners and processing aids. In the UK, there are strict legislative controls over the use of such additives.
There has been much interest in recent years in foods that contain nutrients that have benefits beyond their purely nutritional properties (sometimes known as functional foods), and some of these are considered further in Chapter 11.
Other organic compounds found in foods (e.g. alcohol, caffeine, solanine) have potentially toxic pharmacological effects. Solanine is a plant glycoalkaloid that occurs in small amounts in all potatoes, but at much higher concentrations in green potatoes. It acts as a cholinesterase inhibitor, and ingestion of large amounts causes abdominal pain followed by nausea, vomiting and respiratory problems that can be fatal. Cholinesterase activity returns to normal within a few hours of consumption of small amounts of solanine, so there is not usually any cumulative ill effect from repeat ingestion of small doses. Other toxins are formed in food as a result of processing or cooking it: for example 3-monochloropropane 1,3-diol (from industrial processing of vegetable protein), acrylamide 2-propenamide (from frying or baking foods) and polycyclic aromatic hydrocarbons (in foods exposed to wood smoke) are all carcinogenic, at least in animals. Trans fatty acids are formed during the hydrogenation of vegetable and fish oils, although they are also present naturally in meat and dairy products: an increased intake is associated with increased cardiovascular risk.
Contaminants in food have the potential to cause harm. Examples include bacteria, for example Campylobacter jejuni, which causes gastroenteritis; moulds, for example patulin, which can grow on apples, and aflatoxins from moulds found in foods, including peanuts and corn, which are carcinogens particularly associated with hepatic cell carcinoma.
Trace elements
Minerals are important nutrients, some being major body constituents while others (the trace elements) are required in much smaller amounts. The trace elements that are known to be essential (zinc, copper, selenium, molybdenum, manganese and chromium) are considered here, although there are a number of others (e.g. arsenic, cadmium, fluorine, lead, lithium, nickel, silicon, tin, vanadium) that have been suggested as having physiological roles, but which have yet to be proven to be essential.
Zinc
Zinc is an essential component of over 200 enzymes, having both catalytic and structural roles. Some enzymes appear to be more sensitive to zinc deficiency than others, and it is of relevance to the clinical biochemist that plasma alkaline phosphatase activity tends to decrease when zinc is deficient. Zinc is also incorporated into proteins that do not have enzyme activity; for example, it has a role in maintaining the aggregation of presecretory insulin granules and has direct and indirect roles in reducing free radical activity. Thus, zinc is important in most of the major metabolic processes in the body, including protein synthesis, cellular replication and collagen synthesis.
The best dietary sources of zinc are red meat, shellfish, nuts and cereals, although processing food tends to remove zinc (e.g. white flour is a poor source). Only about 30% of dietary zinc is absorbed and there is a significant enterohepatic circulation. Absorption occurs through both active and passive processes. Some zinc remains in enterocytes bound to a metallothionein, the proportion depending on the amount of metallothionein present. When the body content of zinc is high, metallothionein synthesis is stimulated, reducing the amount of zinc entering the circulation.
Although zinc is present in all body tissues, 60% is in skeletal muscle, 30% in bone and 4–6% in the skin. In the circulation, 80% is contained within red cells, while in the plasma, up to one-third is firmly bound to α2-macroglobulin and most of the remainder is loosely bound to albumin. The main route of excretion is in the faeces.
As might be expected from the diversity of its functions, deficiency of zinc tends to have a wide range of effects. Dermatitis and hair loss are frequent, together with growth retardation and poor wound healing. Anorexia, lethargy and impaired sensations of taste and smell may also occur. Immune function may be depressed, and since zinc has a role both in the hepatic synthesis of retinol-binding protein and in the conversion of retinol to retinal in the retina, night vision may be impaired.
Zinc deficiency can arise from inadequate intake (e.g. during parenteral feeding), impaired absorption or increased losses (e.g. through a gastrointestinal fistula). Zinc deficiency is an important part of protein–energy malnutrition and may limit weight gain during refeeding unless adequate amounts are provided. Requirements for zinc are increased in premature infants, during pregnancy and lactation and following trauma. The autosomal recessive disease acrodermatitis enteropathica is due to severely impaired intestinal absorption of zinc. Affected children have a scaly dermatitis (often secondarily infected), diarrhoea and impaired growth, all of which respond to oral zinc supplements in relatively high doses.
Chronic ingestion of high zinc intakes in otherwise healthy people may lead to features of copper deficiency (e.g. microcytic anaemia, neutropenia). This is because the induction of metallothionein synthesis in enterocytes by zinc results in increased binding of copper to metallothionein, which prevents its transfer to the circulation. (This effect is utilized in the treatment of some patients with Wilson disease; see Chapter 14.) Acute toxicity has been recorded following ingestion of water with a high zinc content, or the use of such water in renal dialysis.
Copper
The main role of copper is as a component of copper metalloenzymes, of which there are many. In the synthesis of collagen and elastin, the cross-linking reactions require various copper-containing amine oxidases, and copper is also involved in the oxidation of Fe2 + to Fe3 + during haemoglobin formation and the binding of iron to transferrin. Examples of other copper-containing enzymes include superoxide dismutase, tyrosinase and cytochrome c oxidase.
Copper is widely distributed in the diet, occurring, for example in shellfish, liver and kidney, with lesser amounts in milk, meat and cereals and very little in processed foods. Not all dietary copper is absorbed, the proportion varying from 35% to 70% for reasons that are still not fully understood, although an increased intake of zinc reduces absorption (see above). From the gut, copper is carried to the liver bound to albumin and there is incorporated into caeruloplasmin. Caeruloplasmin is then secreted into the blood and accounts for 80–90% of the circulating copper. The main route for excretion of copper is in the bile, with very little in the urine unless renal damage is present or copper-binding substances (e.g. penicillamine) have been given. Normal copper homoeostasis, therefore, depends on the balance between intestinal absorption and biliary excretion.
Overt copper deficiency is rare, since the liver contains substantial stores, but has been seen in malnourished children and in adults on long-term total parenteral nutrition (TPN). Features include neutropenia and skeletal fragility, with a microcytic hypochromic (iron-resistant) anaemia if the deficiency is prolonged. In adults, subclinical deficiency may be a risk factor for cardiovascular disease – in animals, copper deficiency can cause vascular and myocardial disease, together with hypercholesterolaemia. There is also a rare X-linked recessive disorder known as Menkes disease, in which there is impaired copper absorption and renal copper wasting. Affected boys show progressive developmental and mental retardation, their hair becomes depigmented and sparse and their bones are abnormal. Parenteral copper can restore both circulating and tissue concentrations, but is otherwise ineffective and death typically occurs by the age of three.
Excess copper in the body is toxic, as demonstrated by Wilson disease, which is discussed in Chapters 13 and 14. However, copper overload purely from chronic dietary excess is rare, although it has been described in association with contaminated water supplies. Although a problem of toxicology rather than nutrition, acute ingestion of copper salts can cause nausea, vomiting and diarrhoea, with intravascular haemolysis in severe cases.
Selenium
Selenium is essential to the activity of the enzyme glutathione peroxidase, which is an important component of cellular antioxidant capacity. Selenium deficiency results in both reduced enzyme activity and reduced amounts of the enzyme protein, although when the intake of selenium is increased, there is an increase in glutathione peroxidase activity only up to a plateau level. Other selenoproteins are known, including iodothyronine deiodinase, which catalyses the conversion of thyroxine to tri-iodothyronine, and thioredoxin reductase, which reduces nucleotides in DNA synthesis.
Cereals, meat and fish contribute most of the dietary selenium, mainly in the form of the amino acids selenomethionine and selenocysteine. About half of dietary selenium is absorbed, the main route of excretion being in the urine. Selenium deficiency, when it occurs, is generally the result of a low intake and has been described in patients on long-term parenteral nutrition and children with certain inherited metabolic diseases treated with very restrictive therapeutic diets, although such occurrences are rare. In certain areas of China, where the soil selenium is low, there is endemic deficiency of selenium and a selenium responsive cardiomyopathy (Keshan disease) has been described. However, a low average selenium intake has also been reported in other countries, with no specific selenium responsive diseases being described. Nonetheless, more recent epidemiological evidence has suggested an increased incidence of cancer in low selenium areas, and some countries (e.g. Finland) now add selenium to the fertilizers used for arable crops.
Excess dietary selenium causes toxicity in livestock, but it is only in selenium-rich areas of China that convincing endemic dietary selenosis has been described in man. Features include ‘garlic breath’ (due to dimethyl selenide), deformity and loss of hair and nails, skin and gastrointestinal symptoms and, in severe cases, neurological problems. Similar features have been seen in people taking selenium supplements that accidentally contained vastly more selenium than they were supposed to. In general, selenium toxicity seems to begin at intakes only ten times the normal intake, although the threshold may be even lower in the presence of certain diseases, for example cystic fibrosis.
Molybdenum
Molybdenum combines with molybdopterin to form molybdenum cofactor, essential for the activity of the enzymes xanthine oxidase, aldehyde oxidase and sulphite oxidase.
The dietary requirement for molybdenum is very small and it is present in most human diets in meats, legumes and grains, so that dietary deficiency is exceptionally rare. Molybdenum deficiency has been reported in a patient with Crohn disease on TPN, who developed fatigue, somnolence and amino acid intolerance that responded to molybdenum supplements. There is a rare, autosomal recessive, inherited deficiency of molybdenum cofactor, which results in severe neurological abnormalities, mental retardation, lens dislocation and xanthinuria (see Chapter 9) in affected children, but no clinical improvement results from dietary supplementation.
High dietary intakes of molybdenum may be associated with altered purine metabolism (an increased incidence of gout has been noted in some populations) and with poorly understood changes in copper metabolism.
Manganese
Manganese is a component of certain enzymes (e.g. pyruvate carboxylase, mitochondrial superoxide dismutase, arginase) and is also an activator of many others (e.g. hydrolases, glycosyl transferases, kinases, decarboxylases), so deficiency could potentially affect the metabolism of carbohydrates, glycosaminoglycans and cholesterol.
Good dietary sources of manganese include leafy vegetables, unrefined cereals and tea, although only a small proportion (3–4%) is actually absorbed. The body content of manganese is low, with about a quarter of this relatively fixed in bone and the highest tissue concentrations in the pancreas and liver. The usual route of excretion is in the bile.
Deficiency of manganese in animals causes poor growth, defective collagen formation, depressed reproductive function, impaired glucose tolerance and neurological deficits, but the effects of dietary deficiency in man are less well established. A case of probable manganese deficiency occurred in a man on long-term TPN (who had a decrease in vitamin K-dependent clotting factors, hypocholesterolaemia, mild dermatitis and a slight change in hair colour). Dietary toxicity of manganese is unlikely since absorption is low and excretion in the bile and urine efficient. Toxicity has been recorded in manganese miners, who develop a condition clinically similar to Parkinson’s disease, but this appears to be due to absorption from manganese ore dust in the lungs rather than from the intestine. High signals on T1-weighted magnetic resonance imaging (MRI) scans of the brain, consistent with manganese deposition, have been well documented in patients on long-term TPN in association with evidence of Parkinson disease and neuropsychiatric abnormalities.
Chromium
The main biological role of chromium in man appears to be to potentiate the action of insulin, as part of a low molecular weight chromium binding substance (chromodulin). Chromium may also be important in gene expression, lipoprotein metabolism and in maintaining nucleic acid structure.
Dietary sources of chromium include yeast, meat, whole grains, mushrooms and nuts. Absorption is very low and urinary excretion increases with intake. Human deficiency was first observed in long-term parenteral nutrition patients who developed insulin-resistant glucose intolerance and neuropathy. In animals, deficiency also causes impaired growth and fertility and hypercholesterolaemia, but these have not been seen in humans. In general, there is no conclusive evidence that diabetes and hypercholesterolaemia are usually associated with chromium deficiency, nor that supplementation ameliorates these conditions.
As a normal dietary constituent, chromium toxicity seems unlikely to occur. However, the absorption of chromium picolinate, which is marketed as a dietary substitute, is higher than from other dietary sources and there have been reports of adverse effects, including renal failure. Industrial toxicity of chromium salts is well established and, recently, local and systemic effects of chromium and cobalt from metallic joint prostheses have been recognized.
Fibre
Dietary fibre is a term used to describe carbohydrate polymers that are not hydrolysed by the endogenous enzymes of the gastrointestinal tract. These include edible carbohydrate polymers naturally occurring in the food, carbohydrate polymers that have been obtained from food raw materials by artificial means and certain synthetic polymers. Lignin (a complex carbohydrate polymer containing aromatic residues) is also included in the definition. Dietary fibre is, therefore, a mixture of substances, including non-starch polysaccharides (NSPs), and starch and oligosaccharides that are resistant to digestion. Most dietary fibre consists of NSPs, and consideration of the NSP content of foods is probably the most useful approach to fibre, particularly since NSPs can be measured with more specificity than total dietary fibre.
Non-starch polysaccharides are a complex group of polymers that can be classified in a number of ways, for example according to the constituent monomers or whether the resulting polymer is soluble or not. Cellulose is a polymer of glucose but, unlike starches, is an unbranched β-1,4 glucan. It constitutes about a quarter of the NSP intake of an average UK diet and about half of the insoluble NSP. Other, non-cellulosic, polysaccharides tend to be made up of hexoses (e.g. glucose in the soluble glucans), pentoses (e.g. arabinose and xylose in the partly soluble arabinoxylans) or uronic acids (e.g. galacturonic acid in the soluble pectins).
The exact composition of dietary NSPs depends on their source. The richest sources are whole grain cereals, with wheat, maize and rice containing mainly insoluble forms, and oats, barley and rye a significant proportion of soluble NSPs. Fruit and vegetables have a higher water content than cereals and so the proportional NSP content tends to be lower, with pulses and nuts probably being the next best sources after cereals. Vegetables tend to contain roughly equal proportions of the soluble and insoluble fractions, but in fruits, this varies widely, with uronic acid-derived NSPs being the main soluble form.
A low dietary fibre intake has been associated with an increased incidence of constipation, diverticular disease, appendicitis, gallbladder disease and carcinoma of the large bowel, although the precise basis of these associations is difficult to work out.
Analysing the benefits of dietary fibre is complicated by the fact that its exact quantification in food is technically difficult and that studies have tended to examine different types and combinations of fibre in different populations. A recent meta-analysis found an inverse association between the intake of dietary fibre, cereal fibre and whole grains and the risk of colorectal cancer, but no association with intake of fibre from fruit, vegetables or legumes. Possible mechanisms for this protective effect include an increase in stool bulk with dilution of faecal carcinogens and decrease in transit time with reduction in contact of carcinogens with the colorectal epithelium. Bacterial fermentation of fibre leads to the production of short chain fatty acids, which may have an additional protective effect by reducing intra-luminal pH. A similar inverse association between the intake of dietary fibre and breast cancer risk has been shown and inhibition of intestinal reabsorption of oestrogens with increased faecal excretion has been postulated as the basis of this.
Certain of the soluble NSPs have also been associated with short-term decreases in plasma glucose, insulin and cholesterol concentrations, but as yet there is no firm evidence either for a dietary lack of NSPs being implicated in the pathogenesis of type 2 diabetes or hyperlipidaemia, or for a role for dietary NSPs in the management of these conditions, other than the fact that a high dietary NSP intake tends to be a marker for a diet more in line with current ‘healthy eating’ concepts. Higher intake of dietary fibre is often associated with other lifestyle factors such as increased physical activity, lower alcohol and dietary fat consumption and lower prevalence of obesity, which may act as confounding factors in evaluating its role.
High dietary fibre intakes in infants and young children may displace energy-rich foods and restrict growth. In adults, high dietary intakes of NSP-rich foods are probably not harmful, although they may cause flatulence, and there is a possible increase in the risk of mechanical bowel problems such as colonic volvulus. Divalent cations can bind to certain constituents of NSPs, for example to uronic acid residues, or to associated phytates, but this does not seem to cause mineral deficiency on a mixed diet. However, caution should be exercised in recommending dietary fibre supplements, particularly those high in phytates, to populations with borderline mineral balance, for example unprocessed bran for the elderly.
ASSESSMENT OF NUTRITIONAL STATUS
General
It is important to be able to assess nutritional status, not only in large surveys attempting to define dietary reference values, but also to determine whether individual patients’ nutritional needs are being met, to identify patients likely to have increased morbidity and mortality without nutritional support, and to monitor progress with time.
However, the wide range of individual nutrients, and the fact that nutritional status is reflected in all body compartments, not all of which are easily accessible to measurement, means that precise assessments are difficult to make. The various techniques that are used include:
• dietary assessment
• measurement of anthropometric indices
• functional assessment
• laboratory-based techniques.
Clinical assessment
Evidence of nutritional disorders may become apparent during the normal clinical processes of history taking and physical examination. The history should ascertain normal food intake and dietary preferences, the patient’s usual weight and any unexplained weight changes. Any difficulties in chewing or swallowing are important, together with gastrointestinal symptoms such as anorexia, nausea, vomiting or altered bowel habit. In the past medical history, any chronic disease may have effects on nutritional status, and surgery involving the gastrointestinal tract or associated organs may also be of importance. Chronic use of alcohol or drugs (therapeutic or otherwise) may have nutritional implications, and socioeconomic factors are important in terms of availability of foodstuffs, adequacy of preparation and general support.
Physical examination may reveal clinical signs suggestive of generalized nutritional depletion (e.g. muscle wasting¸ oedema); evidence of deficiency of specific nutrients as described above may also be present.
Dietary assessment
Dietary assessment involves reviewing the intake of food, and its individual dietary components, and comparing the amount consumed with reference values to see whether deficiency or excess is likely. To be maximally accurate, this would involve weighing all food eaten and analysing its chemical composition, which is clearly impractical for clinical purposes. Hence, an approximation of food intake must be arrived at and reference then made to standard food tables to calculate nutrient content (see Ashwell, in Further reading, below).
Methods of dietary assessment can be divided into those that record current intake, recall past intake or estimate typical intake. Recording current intake can be achieved in a quantitative way in which all food is weighed or measured prior to being eaten. However, this is intrusive even for a short period and an alternative technique is for the patient to describe the amount of food eaten; photographic atlases are available to improve accuracy. Dietary recall methods involve asking the patient to recount everything consumed, usually in the previous 24 h. Whilst these methods lack accuracy and tend to underestimate food intake they can reveal major areas of deficiency, or excess, requiring further evaluation. Food frequency questionnaires evaluate typical food consumption over a longer period. They can be particularly useful for population studies, for example assessing vitamin D consumption in the elderly.
Anthropometric measurements
Clinical assessment can often indicate under- or overnutrition. However, a previously well nourished patient may be in a negative nutritional state for a long time before appearing clinically undernourished. Anthropometry comprises measurements of the human body and there are several anthropometric indices that are useful nutritional indicators as a baseline for initial assessment and as tools for ongoing monitoring.
Height and weight
In adults, body weight can be used as a measure of nutrition in various ways. An individual’s weight can be compared against ‘ideal’ or desirable weight using formulae or tables, although it is now usual to calculate the body mass index (BMI) (see Table 10.2) in this context. The BMI can be used to grade the severity of obesity or of chronic energy deficiency but as it cannot distinguish between fat mass and lean mass it can be unrepresentative in patients with a large muscle mass, for example athletes, or in patients with an increased weight due to fluid retention. Variations in BMI between ethnic groups are also documented. In situations when measurement of height is not possible (e.g. immobility, spinal disorder), alternative indices such as the demispan – the distance between the index/middle finger web and the sternal notch – can be used to calculate BMI.
TABLE 10.2
Body mass index (BMI)
BMI is calculated from the formula 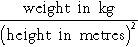
| BMI | Description | Grade of obesity or chronic energy deficiency |
| < 16 | Chronic energy deficiency | III |
| 16–16.9 | Chronic energy deficiency | II |
| 17–18.4 | Chronic energy deficiency | I |
| 18.5–24.9 | Desirable | |
| 25.0–29.9 | Overweight (or pre-obese) | |
| 30.0–34.9 | Obese | I |
| 35.0–39.9 | Obese | II |
| > 40.0 | Obese | III |
The ‘desirable’ and other ranges shown are for adult white individuals – the ranges are probably different for other racial groups, for example in Asians, the risks associated with being overweight begin to increase above 23 kg/m2.
In ill patients, calculation of weight lost as percentage of usual weight can be useful, weight loss of more than 10% being an indicator of poor clinical outcome. However, determination of weight loss by history has been proven to be inaccurate.
In the UK, it is recommended that all patients are screened for nutritional risk at presentation to a doctor or other healthcare professional, and, in in-patient settings (both in hospitals and in nursing homes), at regular intervals thereafter. A suitable tool for this purpose is the Malnutrition Universal Screening Tool (MUST), which considers weight and unintentional weight loss, the time over which nutrient intake has been reduced and/or the likelihood for impaired nutrient intake in the future (see Further reading, below, for more details).
In hospitalized patients, serial weight measurements are valuable in monitoring nutritional status, particularly where nutritional support is being provided. Acute changes are invariably due to changes in fluid balance (unless poor technique is responsible), but longer-term trends reflect tissue changes. As long as the patient is ambulant, weight measurements should be simple to obtain and record, although this may be surprisingly difficult to achieve on a busy hospital ward.
Nutrition is an important factor in growth and, in children, measurement of height and estimation of rate of growth is an important part of the assessment of nutritional status, in addition to measurement of weight.
Circumference measurements
In patients who are confined to bed, either because of debility or because movement is restricted by multiple fluid lines or splints, weight measurements are difficult to obtain unless the patient is in a special (and expensive) weighing bed. In these circumstances, some idea of tissue bulk may be obtained by a mid-arm circumference (MAC) measurement made with a tape measure. The measure should be placed round the non-dominant arm, at a point midway between the acromion (shoulder tip) and the olecranon process (point of the elbow). Age- and sex-related reference standards are available for the raw measurements, which include muscle, fat and bone or attempts can be made to refine the measurement to reflect muscle mass alone. The first stage is to correct for fat by including the triceps skinfold (TSF, see below) measurement and using the formula (MAC –(π × TSF)) to derive the mid-arm muscle circumference. This can be further manipulated to give uncorrected muscle area, and finally an adjustment made for the contribution of the humerus, to give corrected muscle area. The latter can then be used to estimate total body muscle mass, although given the difficulties in making the two original measurements accurately, the margin of error by this stage is quite high. In the research environment, dual-energy X-ray absorptiometry (DEXA) is being used to make similar measurements of body composition.
While these calculated values may be useful in epidemiological surveys, in nutritional support the initial MAC measurement alone may help to indicate whether support is required, and serial measurements help to assess the success of treatment, although short-term changes are more likely to be due to imprecision in measurement than changes in tissue bulk. Measurements of calf circumference can be used in a similar way.
Another ‘circumference’ parameter that may be useful in assessing the health risks of moderate obesity is to measure waist and hip circumferences and calculate the waist:hip ratio. This reflects the distribution of fat rather than the degree of obesity; the risk of ischaemic heart disease and stroke rises sharply with waist:hip ratios of more than 1.0 for men and 0.8 for women. Even more simply, measurement of waist circumference alone can be used in this context, with a significant increase in cardiovascular risk being associated with waist measurements of over 102 cm in men and 88 cm in women (in Caucasian populations).
Skinfold thickness
Measurement of skinfold thickness, obtained using calipers, is useful in assessing and monitoring nutritional status in patients who cannot be weighed, and also has a place in epidemiological surveys. However, the technique is prone to large variations, both within and between observers. The imprecision arises in identification of the exact location for measurement; the way the skinfold is picked up; the way the calipers are placed on the fold; the compression of the fold by the calipers and the exact timing of the reading. Some improvement in performance may be achieved by taking the mean of three readings, usually on the left (or non-dominant) side. As with circumference measurements, the presence of oedema at the measurement site may be a further confounding factor.
A variety of sites have been used for skinfold measurement, but the most common are triceps, biceps, subscapular and suprailiac. Equations are available for calculation of total body fat from these measurements (usually for research purposes), but this assumes that subcutaneous fat reflects total body fat, which is not always the case: obese men tend to lay down more intra-abdominal fat than women, and visceral fat and subcutaneous fat have been shown to be biologically distinct. In clinical practice, body weight is more useful than skinfold thickness in the management of obesity, but in undernourished patients the latter may be useful. Age- and sex-related reference standards are published (e.g. for triceps skinfold thickness) and so measurement at presentation may identify severe malnourishment: a triceps skinfold of < 5 mm almost always reflects low body fat stores. Serial measurements can help in monitoring nutritional support but, again, short-term changes are more likely to reflect imprecision in measurement than sudden changes in fat stores.
Functional assessment
The effects of nutritional status on certain aspects of bodily function can be used in the assessment of undernutrition, although with variable degrees of success. The best example is probably the effect of haematinic deficiency on red blood cell morphology where, for example a hypochromic, microcytic picture may be the first indication of iron deficiency and macrocytosis of vitamin B12 or folate deficiency (see Chapter 26).
Functional tests of muscle mass have also been used, for example grip strength, isometric knee extension and response to electrical stimulation. However, while muscle strength correlates with muscle mass in normal subjects, there are many non-nutritional factors that can cause weakness in sick patients, and malnutrition alone has to be quite severe before strength diminishes. There is also the possibility that repeated measurements may have a training effect on the muscles involved.
Visceral protein is sometimes disproportionately decreased in protein deficiency and various measures of visceral function have been used in nutritional assessment. Two of these are discussed below.
Hepatic secretory proteins
The liver synthesizes most of the circulating plasma proteins (apart from immunoglobulins) and, in epidemiological studies, there is a clear correlation between plasma concentrations of these proteins and other markers of malnutrition. For example, in adults who are otherwise well, a plasma albumin < 35 g/L and a plasma transferrin < 1.5 g/L usually indicate protein malnutrition. Since most of these proteins can be measured quite easily, there is a temptation to use them as nutritional markers in individual patients, but there are many pitfalls for the unwary.
Albumin is probably the most frequently measured plasma protein and low concentrations may reflect a deficiency in dietary protein intake. However, it must be remembered that decreased synthesis may be due to other factors, for example liver disease, and that the plasma concentration is also affected by fluid balance, loss of protein from the body, tissue catabolism and distribution of albumin across the various body compartments. It should also be remembered that, while the intravascular concentration of albumin is relatively high, more than half of the total mass of albumin is actually extravascular. It is noteworthy that in uncomplicated starvation, plasma albumin concentrations may remain normal for a relatively long period (the fractional catabolic rate decreases), whereas in sepsis, the concentration can fall significantly over a much shorter period.
Even in circumstances where the plasma albumin concentration does reflect nutritional status, the relatively long half-life (20 days) means that it does not respond to rapid changes and so measurements of other plasma proteins with shorter half-lives have been used. These include transferrin (9 days), prealbumin (1–2 days) and retinol-binding protein (10 h), but unfortunately, these all have similar drawbacks to albumin and are also affected by factors such as the acute phase response, oestrogen concentrations and factors relating to their own specific function (e.g. iron deficiency in the case of transferrin). Serial determinations may be of more use than single measurements, and measurement of a marker of the acute phase reaction (e.g. C-reactive protein) at the same time may help in interpretation. In clinical practice, measurement of these proteins rarely contributes to the management of individual patients, and failure to appreciate their limitations may lead to inappropriate decisions being made.
The immune response
Malnourished people are more susceptible to infections. Plasma immunoglobulin concentrations are generally maintained, but cell-mediated immunity may be impaired. The circulating absolute lymphocyte count is often low (< 2.0 × 109/L) in malnutrition, although this is a very non-specific finding.
Delayed cutaneous hypersensitivity testing against common allergens has been used to assess malnutrition, but many non-nutritional factors (e.g. infection, malignancy, radiation, surgery, drugs) affect the response and, in any case, the results may not be reproducible. In vitro tests of T cell function may be an alternative, but in clinical practice neither of these approaches is in common use.
Laboratory-based assessment of individual nutrients
Energy
Laboratory-based techniques are not in general use for the assessment of energy stores, whether of fat or carbohydrate. However, there are techniques for the measurement of energy expenditure that may be of use in assessing how much energy to provide as part of nutritional support.
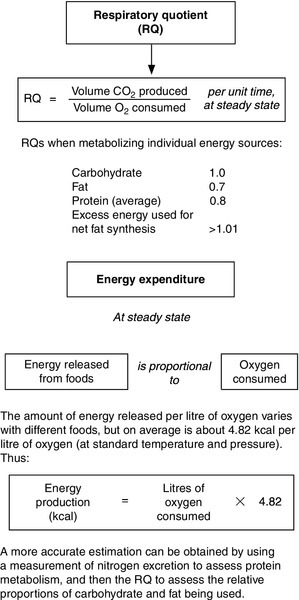
Direct calorimetry measures heat loss from the body, which can be used to derive the metabolic rate assuming that body temperature remains constant and no external work is performed. The technique involves the subject remaining in a special, insulated room equipped for heat exchange, so is not applicable in general clinical practice. Indirect calorimetry measures oxygen consumption and carbon dioxide production, and from these the respiratory quotient can be calculated, together with the energy expenditure (Fig. 10.7). This is the technique used by the ‘metabolic measurement carts’ that have been popular in some intensive care units.
The use of doubly-labelled water (containing the stable isotopes 2H and 18O) has produced new data on total energy expenditure in free-living individuals, but the technique is not suitable for the assessment of patients. Heart rate can also be used as an index of energy metabolism, but although the measurement is obviously very much easier, the relationship between heart rate and energy expenditure is not linear and there are many confounding factors, so it can be useful in studies of groups of people but not in individuals.
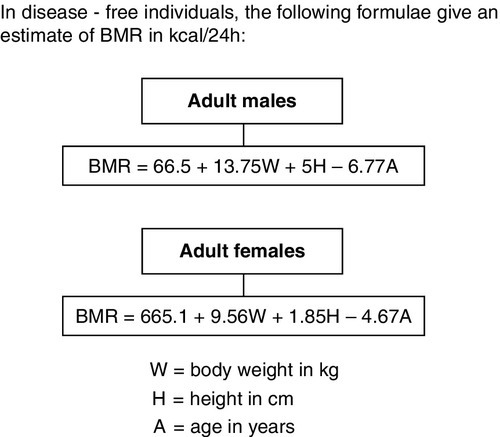
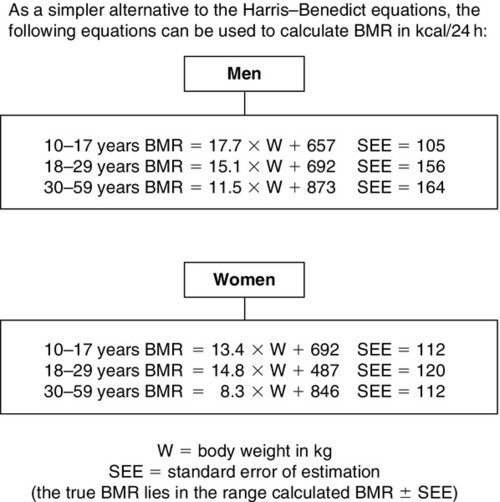
Basal metabolic rate (BMR) can be estimated for healthy individuals from knowledge of age, sex, height and weight using the Harris–Benedict (Fig. 10.8), Schofield (Fig. 10.9) or similar equations and, for sick patients, suitable adjustment can then be made for disease state, pyrexia, mobility etc. However, the more severely ill the patient, the less likely it is that the calculated figure truly reflects total energy expenditure.
Protein
Laboratory-based tests for visceral protein have already been discussed under functional tests of nutritional status. However, there are a number of other tests that assess total body protein (predominantly muscle).
Total body nitrogen can be measured by neutron activation (an estimate of total body protein is simply obtained by multiplying this figure by 6.25) and this can demonstrate changes over a period of only a few weeks. However, the technique is expensive and involves a significant dose of radiation, so it is only used as a research tool. Almost all body potassium is intracellular; there is virtually none in adipocytes and potassium in the skeleton and red blood cells is not readily exchangeable, so exchangeable potassium measured using dilution of 42K is a measure of fat-free mass. By monitoring both total body nitrogen and potassium, it can be shown that nitrogen losses in malnutrition are relatively higher from lean tissue (high potassium content) than from fibrous tissue (low potassium content).
Measurements of bioelectrical impedance have also been used to predict lean body mass. However, the technique actually measures total body water and the extension to lean body mass assumes a constant level of hydration in such tissue, which may not always be the case, particularly in sick individuals.
Outside the research environment, a direct measure of muscle mass may be obtained from the 24 h excretion of creatinine, a metabolite of muscle creatine. This is sometimes corrected for height (the creatinine:height ratio) and standard values are available. However, urinary creatinine excretion does fluctuate (e.g. with exercise, pyrexia, meat intake) and the technique depends on complete urine collections and normal renal function, so that in practice, it is not widely used.
It may sometimes be useful to assess nitrogen balance. Nitrogen intake may require dietary analysis or, for patients on enteral or parenteral formulations, it will already be known. Nitrogen output for research purposes involves analysing stool and urine for total nitrogen content, but in the past, for clinical purposes, urinary urea nitrogen has been measured and standard adjustments made for urinary non-urea nitrogen and faecal nitrogen. This approach was valid for reasonably well patients (assuming the urine was not infected with a urea-splitting organism), but diverges further from the true nitrogen output of the more severely ill the patient, so its use cannot be recommended.
Vitamins
It is sometimes useful to assess body stores of fat-soluble vitamins, as outlined below, but only rarely necessary to measure the water-soluble vitamins (with the exception of vitamin B12 and folate, see Chapter 26). This is because they are relatively non-toxic and, if deficiency is suspected, a trial of therapy is safe and simpler than trying to measure them. The interpretation of laboratory data is not without difficulty – some vitamins are affected by the acute phase response, for example vitamin D concentration may fall by up to 40% – so differentiation of true deficiency may be difficult. Measurement of concentrations of some vitamins in erythrocytes rather than plasma, during the acute phase, has been shown to be more representative of body stores.
Vitamin A
The standardized definition of vitamin A deficiency is in terms of liver stores of retinol, but this is not a practical indicator. By convention, a plasma retinol concentration < 0.7 μmol/L, is used to define deficiency. However, plasma retinol concentration tends to be maintained over a wide range of liver reserves, making it an insensitive detector of deficiency and as retinol is carried bound to retinol-binding protein (RBP) in the plasma, conditions reducing RBP concentration (e.g. liver disease) may lower plasma retinol concentration without reflecting decreased stores. The diagnosis of vitamin A deficiency is often made on clinical grounds. The response to a test dose of vitamin A may help to distinguish true deficiency from functional deficiency due to a lack of RBP. In the former, the plasma vitamin A concentration rises markedly within a few hours; in the latter it does not. Very high retinol intakes may increase the plasma concentration.
Plasma carotenes can also be measured and, in general, reflect dietary intake. Dietary assessment is usually all that is necessary for this, but measurement is occasionally helpful to establish whether an odd skin colouration is due to hypercarotenaemia or not.
Vitamin D
Patients with a nutritional deficiency of vitamin D may present with clinical symptoms and signs; suspicion may also be raised by the finding of an increase in plasma alkaline phosphatase activity. Plasma calcium may be low but is frequently within the reference range as a result of secondary hyperparathyroidism, which may result in a low plasma phosphate concentration.
The main storage form of vitamin D in the body is 25-hydroxycholecalciferol and this is also the main form in plasma, where it is bound to a specific binding protein. Measurement of plasma 25-hydroxycholecalciferol provides a useful indicator of vitamin D stores: the reference range for total 25-hydroxycholecalciferol is of the order of 50–140 nmol/L, although the result should be interpreted with regard to the time of year, as there can be a two-fold seasonal variation in temperate regions. Concentrations < 50 nmol/L indicate vitamin D insufficiency that may benefit from supplementation, and values < 25 nmol/L suggest deficiency.
It is also possible to measure the active metabolite of vitamin D (1,25-dihydroxycholecalciferol, calcitriol), although this is more difficult as the plasma concentrations are approximately 1000-fold lower than those of 25-hydroxycholecalciferol. Its synthesis is normally closely regulated and concentrations do not reflect vitamin D status. Such measurements tend only to be necessary in the investigation of rare, inherited disorders of vitamin D metabolism or action (see Chapter 30).
Vitamin D toxicity can occur if supplements are given, as the difference between appropriate and toxic intakes is fairly small. This is generally detected in the laboratory as hypercalcaemia that gets better when the supplements are reduced or stopped, but it is occasionally necessary to confirm the diagnosis with plasma vitamin D measurement.
Vitamin E
Vitamin E status is assessed by measurement of plasma tocopherol concentration, most commonly by high performance liquid chromatography. Increases in plasma lipoprotein concentrations tend to result in increases in plasma tocopherol, so it may be appropriate, in some circumstances (e.g. in patients with cholestasis or if the plasma tocopherol is found to be low in order to confirm deficiency) to express the result as a plasma tocopherol:cholesterol ratio.
Vitamin K
Although it is possible to measure vitamin K in plasma, the usual laboratory approach is to measure the plasma concentrations of the vitamin K-dependent clotting factors. In vitamin K deficiency, these are present in normal quantities, but in a non-functional state (proteins formed in vitamin K absence – PIVKA), and so a functional assay, conventionally the prothrombin time or international normalized ratio (INR), is used (although the activated partial thromboplastin time is also abnormal in vitamin K deficiency).
There is no generally available test of vitamin K excess, largely because its lack of toxicity makes it unnecessary.
Thiamin
The single most important factor in detecting thiamin deficiency is a high index of clinical suspicion, and the safest confirmatory test is a trial of thiamin supplements, since delay can be harmful in a deficient patient. However, assuming appropriate samples can be obtained without delaying treatment, there are laboratory tests that can be used to assess thiamin status.
Methods are now available for measurement of thiamine and its phosphate esters in whole blood, but because of the low concentration and consequent analytical problems, some laboratories still use functional tests of thiamin deficiency, the most common of which is probably measurement of red cell transketolase with and without the addition of thiamin pyrophosphate (TPP). In normal subjects, TPP enhancement of transketolase is up to 15%, but may be increased to as much as 25% in severe deficiency. Urinary thiamin measurement has been used, with low outputs being found in clinical deficiency states. Discrimination is said to be improved if the percentage excretion of a test dose is measured; a higher proportion is retained in deficiency.
Riboflavin
The laboratory approach to the assessment of riboflavin (vitamin B2) status is similar to that taken with thiamin. Urinary excretion can be measured and an output of 0.5–0.8 mg/24 h probably indicates an adequate intake, but does not necessarily reflect total body content. Factors such as age, physical activity and illness can also influence urinary excretion. Discrimination may be improved by measuring the percentage excretion of a loading dose of riboflavin, an increased proportion being retained in deficiency states.
In the blood, about 90% of riboflavin is present in the red cells. This can be measured directly, but measurement of red cell glutathione reductase activity is probably more useful. The enzyme is flavin adenine dinucleotide (FAD) dependent, so that activity falls in riboflavin deficiency, but is restored on addition of FAD. An increase in activity of 30% or more confirms riboflavin deficiency. The test assesses long-term tissue riboflavin status and is very sensitive. It cannot be used if there is coexistent red cell glucose 6-phosphatase deficiency.
Nicotinamide
Laboratory tests of nicotinamide status tend not to be entirely satisfactory, possibly because no specific functional test has yet been developed. Approaches used to date include measurement of whole blood NAD concentration and of urinary excretion of nicotinamide metabolites, in particular 1-methyl nicotinamide.
Vitamin B6
A variety of biochemical techniques have been described for the assessment of vitamin B6 status. Urinary excretion of 4-pyridoxic acid reflects immediate dietary intake and pyridoxal 5-phosphate in blood can also be measured, but indirect approaches are less technically demanding and have been more widely adopted.
In vitamin B6-deficient subjects, the urinary excretion of xanthurenic acid shows a marked increase following an L-tryptophan load, and this is the basis of the tryptophan load test. This is relatively easy to perform and has been widely used, but interpretation of the results requires caution as there are a number of other factors that can affect tryptophan metabolism. The most useful test currently available is probably the measurement of red cell aminotransferase (e.g. aspartate aminotransferase) activity, with and without the addition of pyridoxal 5-phosphate, in a technique analogous to that used for the functional assessment of thiamin (red cell transketolase) and riboflavin (red cell glutathione reductase). Serum aminotransferase activities are also affected by vitamin B6 deficiency, but the very wide fluctuations seen in disease states make them less useful than erythrocyte activities in this context.
Pantothenic acid
Tests of pantothenic acid status are rarely necessary and no biochemical test has become generally accepted. Pantothenic acid itself can be measured in urine and serum, the urinary excretion being more immediately affected by dietary intake. Assessment of the red cell content of coenzyme A may be a functional test of pantothenic acid status.
Biotin
Techniques have been described for the measurement of biotin in both urine and blood. However, these are not straightforward and, in practice, biotin status is rarely assessed biochemically.
Vitamin C
Vitamin C can be measured in plasma, white blood cells and urine. Unfortunately, none of these is entirely satisfactory in assessing vitamin C status. Plasma concentration tends to reflect recent intake and so will fall with decreased intake before body stores are depleted. Leukocyte (or buffy coat) vitamin C content probably reflects body stores better than the plasma concentration, but the measurement is technically difficult, requires large quantities of blood and, if the buffy coat content is measured, any abnormality in the relative proportions of white cells and platelets will introduce further error.
Urinary ascorbate can be measured relatively easily in a fresh sample, and output decreases quite markedly in deficiency. However, the technique is not very specific and various ascorbic acid saturation tests have been devised to improve on this. These all assume that if body stores are depleted, most of a standard daily dose of ascorbic acid will be retained until stores are repleted, after which relatively large amounts of ascorbate appear in the urine. These tests are not perfect, but are simple to carry out and appear to be satisfactory if a clinical suspicion of vitamin C deficiency needs to be confirmed. An added advantage is that any deficiency is actually treated by the test.
Trace elements
There is a general problem in assessing body stores of trace elements, in that plasma concentrations are not necessarily related to tissue content, as is apparent in the following discussion.
Zinc
Assessment of body zinc status is not easy. Measurement of serum zinc concentration is probably the simplest approach, but the result is influenced by many things other than total body zinc. There is a diurnal variation with a peak at about 10.00 h, fluctuation with recent dietary intake and a fall with hypoalbuminaemia. It also tends to fall in disease: for example, in acute inflammation, interleukin-1 stimulates zinc uptake by the liver, although in tissue destruction, the zinc may be maintained as zinc is released from the tissues, even though net balance is actually negative as losses in urine increase.
Urinary zinc excretion is also difficult to interpret. Low values may reflect deficiency, but, even if environmental contamination can be avoided, normal or raised excretion does not exclude deficiency; urinary losses may even be the cause of the deficiency.
Since serum and urine zinc measurements are generally unsatisfactory, a variety of other approaches have been tried. Hair zinc is liable to environmental contamination (e.g. from certain shampoos), turns over relatively slowly and depends on the rate of hair growth. Erythrocyte zinc content responds only slowly to changes in zinc status, which can be rapid. (However, the high zinc content of red cells, in the enzyme carbonate dehydratase, is important, since it means that in vitro haemolysis increases the measured serum zinc concentration.) Changes in the plasma and tissue activities of zinc-dependent enzymes like carbonate dehydratase and alkaline phosphatase have given varying results. Leukocyte zinc content correlates well with muscle content, but its measurement is not easy and requires large quantities of blood.
Serum zinc remains a common, albeit unsatisfactory, measure of zinc status, although some authorities state that a serum zinc concentration < 7 μmol/L is a clear indication of deficiency. When true deficiency is suspected, a therapeutic trial of zinc supplementation with careful clinical monitoring is probably the best approach.
Copper
The diagnosis of copper overload is discussed in Chapters 13 and 14. In the detection of copper deficiency, plasma copper concentration may be low, but deficiency may be masked because caeruloplasmin synthesis is increased in the acute phase response. Caeruloplasmin is also increased in pregnancy and by exogenous oestrogens. Measurement of caeruloplasmin before and after moderate copper supplementation may be a useful technique; an increase confirming deficiency. Interpretation of copper concentrations in other body fluids and tissues is difficult when looking for deficiency, although changes in the activities of copper-containing enzymes like superoxide dismutase may prove to be useful functional tests of copper deficiency in the future.
Selenium
Plasma selenium concentration is an indicator of recent selenium intake, rather than body stores. Measurement of whole blood selenium concentration has been used but may give a false picture if there has been recent blood transfusion. Selenium in plasma is bound to lipoproteins, which may decrease in concentration in malnutrition, causing a secondary decrease in plasma selenium not necessarily related to body content. The activity of the selenium-containing enzyme glutathione peroxidase tends to decrease in selenium deficiency and may be a better functional indicator, but it is not clear whether the measurement is best made in erythrocytes, platelets or even plasma. Excess selenium is excreted in the urine, so urinary measurements are useful in suspected toxicity, but are not helpful in deficiency.
Molybdenum
Plasma and urine concentrations of molybdenum are normally very low and measurements in these fluids are not useful in detecting deficiency. Increased urinary excretion of xanthine and sulphite, which decrease with molybdate supplementation, may be a useful approach in confirming suspected deficiency.
Manganese
Measurement of plasma and urine manganese is useful when investigating suspected toxicity, but since most manganese is intracellular, whole blood measurements are used in the detection of deficiency.
Chromium
Plasma and urine chromium can be measured by inductively coupled plasma mass spectrometry, but the limit of detection is such that they are more useful in detecting toxicity.
CONCLUSION
Clinical nutrition is a huge topic, with many different professions and specialties involved. Biochemical tests have their limitations in measuring nutritional status, and it is important for the clinical biochemist to be aware of these, in order that the tests are not used inappropriately.
ACKNOWLEDGEMENT
The author would like to thank Stephen K. Bangert who wrote the chapter in the previous edition of this book.
Further reading
Malnutrition Universal Screening Tool (MUST).
This is a five-step screening tool to identify adults who are malnourished, at risk of malnutrition, or obese. It can be downloaded from the website of the British Association for Parenteral and Enteral Nutrition:www.bapen.org.uk. Accessed November 2012.