Section 1 Resuscitation
1.1 Basic life support
Introduction
The patient with sudden out-of-hospital sudden cardiac arrest requires a bystander to initiate a number of actions in rapid sequence for any hope of a successful resuscitation. These steps are known as the ‘Chain of Survival’.1
Development of protocols
The guidelines for BLS must be evidence based and consistent across a wide range of providers. Many countries have established national committees to advise community groups, ambulance services and the medical profession on appropriate BLS guidelines. Table 1.1.1 shows the national associations that make up the International Liaison Committee on Resuscitation (ILCOR). This group meets every 5 years to review the BLS guidelines and to consider the scientific evidence that may lead to changes.
Table 1.1.1 Membership of the International Liaison Committee on Resuscitation (ILCOR)
| American Heart Association |
| Australian Resuscitation Council |
| European Resuscitation Council |
| Heart and Stroke Foundation of Canada |
| Inter-American Heart Foundation |
| New Zealand Resuscitation Council |
| Resuscitation Council of Southern Africa |
The most recent revision of the BLS guidelines occurred in 2005 and consisted of a comprehensive evaluation of the scientific literature for each aspect of BLS. Evidence evaluation worksheets were developed (available at www.c2005.org) and were then considered by ILCOR. The final recommendations were published in late 2005.2
Initial evaluation: DR ABCD approach
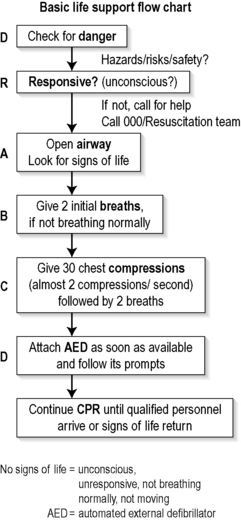
A flowchart for the initial evaluation of the collapsed patient is shown in Figure 1.1.1. It includes checking for danger, assessing responsiveness, then opening the airway, giving breaths and cardiac compressions, and attaching an automated defibrillator as soon as possible. This is known as the ‘DR ABCD’ approach. CPR is continued until qualified personnel arrive or signs of life return.6
Airway and breathing
Make an assessment of the airway and breathing if a patient has collapsed and is apparently unconscious.3,4 Place the patient supine, check the airway by visual inspection, and open the airway with a head-tilt and/or a chin-lift manoeuvre.
Circulation
It was traditionally recommended that a bystander should attempt to palpate a pulse in order to diagnose cardiac arrest and, if absent, commence external cardiac compressions (ECC). It is now currently recommended that untrained bystanders do not attempt to palpate for a pulse,5 as there is good evidence that the pulse check is inaccurate in this setting.7 Therefore, cardiac arrest may instead be presumed if breathing is absent, and is highly likely if breathing is inadequate.
Management
Airway obstruction
Make a careful sweep with a finger if inspection of the airway reveals visible foreign material or vomitus in the upper airway. Take particular care not to be bitten, not to cause pharyngeal trauma, and not to propel material down into the lower airway.
Cardiopulmonary resuscitation (CPR)
The bystander should immediately commence cardiopulmonary resuscitation (CPR) if cardiac arrest is diagnosed and the EMS has been summoned, using both expired air resuscitation (EAR) and ECC until a defibrillator arrives.6
Expired air resuscitation (EAR) or ‘rescue breathing’
Bystander ECC without EAR
Animal models show that some ventilation occurs during chest compressions, and it has been proposed that EAR may be withheld in adult patients who have a witnessed out-of-hospital cardiac arrest. A recent Japanese observational study (SOS-KANTO) compared the outcome of adult patients with a witnessed out-of-hospital cardiac arrest who received ECC only by bystanders with that of patients who received both EAR and ECC ‘conventional CPR’, as well as patients who received no bystander CPR.8 There was a favourable neurological outcome in 6.2% of patients who received ECC only, compared to a 3.1% favourable neurological outcome in the patients who received EAR plus ECC (P = 0.0195). Only 2.2% of patients who received no bystander CPR had a favourable neurological outcome. Therefore, a strong case may be made that bystanders perform only ECC and not EAR.9 However, this recommendation has not currently been endorsed by the Australian Resuscitation Council (ARC).
External cardiac compressions (ECC)
Place the patient supine on a firm surface such as a backboard, firm mattress or even the floor to optimize the effectiveness of chest compressions. Perform compressions that allow equal time for the compression and relaxation phases, with compression being approximately 50% of the cycle. Depress the lower sternum at least 4–5 cm in the adult, with complete recoil of the chest after each compression. Perform ECC at a rate of 100 compressions per minute, to ensure the delivery of a minimum of about 80 compressions per minute when accounting for the period spent on ventilations.5 Recommendations are essentially to ‘push hard, push fast, allow complete release and minimize interruptions’.
‘Cardiac pump’ mechanism
Whatever the predominant mechanism of blood flow, ECC results in only about 20% of cardiac output in the adult, mainly owing to the relative rigidity of the chest wall. Consequently, there is a progressive metabolic acidosis due to inadequate oxygen delivery during CPR. Few adults survive a cardiac arrest when ECC has been given for more than 30 minutes. Thus, most EMS allow paramedics to cease resuscitation if a patient in cardiac arrest has failed to respond to CPR and advanced life support measures after 30 minutes, provided there are no extraordinary circumstances such as hypothermia or drug overdose. See also Ch. 1.2on Advanced Life Support.
Defibrillation
Semi-automatic external defibrillation (SAED)
Most SAEDs have an algorithm that initially requests the delivery of three countershocks if ventricular fibrillation persists. As the recent ILCOR guidelines now recommend a single shock followed by 1 minute of CPR (except when using a manual defibrillator at a witnessed arrest), it is expected that SAEDs will progressively have their electronics upgraded to follow the new recommendations.2
Non-medical personnel and the SAED
Other first responders
A range of situations is proposed where non-medical personnel might use a SAED. Thus, the SAED may be used by first responders such as fire services, who co-respond with ambulance services. In Canada, the state of Ontario implemented an extensive programme to introduce rapid defibrillation across the state.10 The use of fire department first responders resulted in 92.5% of cardiac arrest patients being defibrillated in under 8 minutes, compared to 76.7% under the previous system (P<0.001). Survival to hospital discharge improved from 3.9% (183/4690 patients) to 5.2% (85/1614 patients) (P = 0.03). This study demonstrates that an inexpensive, multifaceted, optimized systems approach to rapid defibrillation can lead to significant improvements in survival after cardiac arrest.
A study of a fire-service first-responder programme in Melbourne, Australia, found that the time to defibrillation was reduced from a mean of 7.1 minutes for ambulance services to 6.0 minutes for a combined approach.11 However, this study was not powered to assess the impact on patient outcome.
Public area SAED
Alternatively, the SAED may be placed in a public area for use by designated personnel, such as security staff who undergo a short training programme. This approach has been shown to be effective in places with large at-risk populations, such as casinos.12
Public-access SAED
The SAED may be placed in a public area for use by personnel with no previous training at all in their use. At Chicago airport defibrillators were placed in strategic locations, with signs advising on their correct use.13 Over a 2-year period there were 21 patients with cardiac arrest, of whom 18 had an initial rhythm of ventricular fibrillation. A defibrillator was applied by a ‘good Samaritan’ bystander in 14 of these 18 patients and 11 were successfully resuscitated. Ten patients were alive and well 1 year later.
Shopping centres and apartment buildings
In a larger study,14 SAEDs were placed in 993 sites such as shopping centres and apartment buildings. More patients survived to hospital discharge when the units were assigned to volunteers trained in CPR plus using an AED (30 survivors among 128 arrests) than when the units were assigned to have volunteers trained in CPR only (15 among 107; P = 0.03). However, as most cardiac arrests occur at home or when ‘out and about’, the widespread implementation of this approach to all public areas would be costly and result in relatively few lives saved.15
Home SAED
Finally, a SAED may be placed in the home of a patient who is at increased risk of sudden cardiac arrest, for use by a relative who might witness the event. However, a recent study that enrolled 7001 patients concluded that survival rates from sudden cardiac arrest at home were not increased when a defibrillator was available in the home. 16
BLS summary
Changes to BLS
The main recent change to basic life support is the so-called DR ABCD approach: the performance of chest compressions at a rate of 100 per minute, with 30 compressions followed by two ventilations, or ‘30:2’, with no pause to determine the presence or absence of a pulse.16 The exceptions to this are resuscitation of the newborn (use a 3:1 ratio of 90 compressions and 30 inflations to achieve 120 ‘events’ per minute); and endotracheally intubated victims (use a ratio of 15:1 in adults and 15:2 in children). Also pulse checks and recovery checks are no longer performed, and CPR is only interrupted when signs of a return of spontaneous circulation are present.17
1 Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: The ‘chain of survival’ concept. A statement for health professionals from the advanced cardiac life-support subcommittee and the emergency cardiac care committee, American Heart Association. Circulation. 1991;83:1832-1847.
2 International Liaison Committee on Resuscitation 2005. International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2005;67:181-341.
3 Australian Resuscitation Council. Airway: Australian Resuscitation Council Guideline 2006. Emergency Medicine Australasia. 2006;18:325-327.
4 Australian Resuscitation Council. Breathing: Australian Resuscitation Council Guideline 2006. Emergency Medicine Australasia. 2006;18:328-329.
5 Australian Resuscitation Council. Compressions: Australian Resuscitation Council Guideline 2006. Emergency Medicine Australasia. 2006;18:330-331.
6 Australian Resuscitation Council. Cardiopulmonary resuscitation: Australian Resuscitation Council Guideline 2006. Emergency Medicine Australasia. 2006;18:332-334.
7 Bahr J, Klingler H, Panzer W, et al. Skills of lay people in checking the carotid pulse. Resuscitation. 1997;35:23-26.
8 SOS-KANTO study group. Cardiopulmonary resuscitation by bystanders with chest compression only (SOS-KANTO): an observational study. Lancet. 2007;369:920-926.
9 Ewy GA. Cardiac arrest – guideline changes urgently needed. Lancet. 2007;369:882-884.
10 Stiell IG, Wells GA, Field BJ, et al. Improved out-of-hospital cardiac arrest survival through the inexpensive optimization of an existing defibrillation program. Journal of the American Medical Association. 1999;281:1175-1181.
11 Smith KL, McNeill JJ. Emergency Medical Response Steering Committee. Cardiac arrests treated by ambulance paramedics and fire fighters. Medical Journal of Australia. 2002;177:305-309.
12 Valenzuela T, Roe TJ, Nichol G, et al. Outcomes of rapid defibrillation by security officers after cardiac arrests in casinos. New England Journal of Medicine. 2000;343:1206-1209.
13 Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. New England Journal of Medicine. 2002;347:1242-1247.
14 Hallstrom AP, Ornato JP, Weisfeldt M, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. New England Journal of Medicine. 2004;351:637-646.
15 Pell JP, Sirel JM, Marsden AK, et al. Potential impact of public access defibrillators on survival after out of hospital cardiopulmonary arrest: retrospective cohort study. British Medical Journal. 2002;325:515-520.
16 Bardy GH, Lee KL, Mark DB, et al. Home use of automated external defibrillators for sudden cardiac arrest. New England Journal of Medicine. 2008;358:1793-1804.
17 Wasserthiel J. Australian Resuscitation Guidelines: Applying the evidence and simplifying the process. Emergency Medicine Australasia. 2006;18:317-321.
1.2 Advanced life support
Introduction
Larsen et al., in 1993, calculated the time intervals from collapse to the initiation of BLS, defibrillation and other ALS treatments, and analysed their effect on survival from out-of-hospital cardiac arrest.3 When all three interventions were immediately available the survival rate was 67%. This figure declined by 2.3% per minute of delay to BLS, by a further 1.1% per minute of delay to defibrillation, and by 2.1% per minute to other ALS interventions. Without treatment, the decline in survival rate was the sum of the three coefficients, or 5.5% per minute.
Chain of survival
The importance of rapid treatment for cardiac arrest has led clinicians to develop a systems management approach, represented by the concept of the ‘Chain of Survival’, which has become the widely accepted model for the emergency medical services (EMS) systems.4 The Chain of Survival concept implies that more people survive sudden cardiac arrest when a cluster or sequence of events is set up as rapidly as possible. This Chain of Survival sequence includes:
Aetiology and incidence of cardiac arrest
Aetiology
The commonest cause of sudden cardiac arrest in adults is ischaemic heart disease.1,2,5 Other causes include respiratory failure, drug overdose, metabolic derangements, trauma, hypovolaemia, immersion and hypothermia.
Incidence
The population incidence of sudden cardiac death (within 24 hours of the onset of any symptoms) has been estimated as 1.24:1000/year in the USA.6 The incidence of cardiac arrest notified to ambulances in western metropolitan Melbourne, Australia, in 1995 was approximately 0.72:1000/year.7 From among 20 communities in developed nations worldwide a population average of 0.62:1000/year received attempted resuscitation after out-of-hospital cardiac arrest.6
Advanced life support guidelines and algorithms
International Liaison Committee on Resuscitation (ILCOR)
In 2000, the American Heart Association, in collaboration with the International Liaison Committee on Resuscitation (ILCOR), convened the International Guidelines 2000 Conference on CPR and Emergency Cardiac Care (ECC). This was the first international assembly gathered specifically to produce international resuscitation guidelines, where the International Guidelines 2000 for CPR and ECC were developed and then published.8 These guidelines represented a consensus of expert individuals and resuscitation councils and organizations across many countries, cultures and disciplines. The underlying principle guiding decision-making was that additions to existing guidelines had to pass a rigorous evidence-based review. Revisions or deletions occurred because of:
Researchers from the ILCOR member councils continued to apply and develop the above evidence evaluation process, which culminated in the publication in 2005 of the Consensus on Science and Treatment Recommendations (CoSTR).1
Consensus on Science and Treatment Recommendations (CoSTR)
Each ILCOR member body has used the CoSTR documents to develop its own guidelines for local use. Thus in 2006 both the Australian Resuscitation Council (ARC)5 and the New Zealand Resuscitation Council (NZRC)9 published their local guidelines. The ARC guideline on Adult Advanced Life Support2 includes an Adult Cardiorespiratory Arrest algorithm (Fig. 1.2.1). This is clear, concise, and easy to memorize and adapt into poster format, and is readily applied clinically.
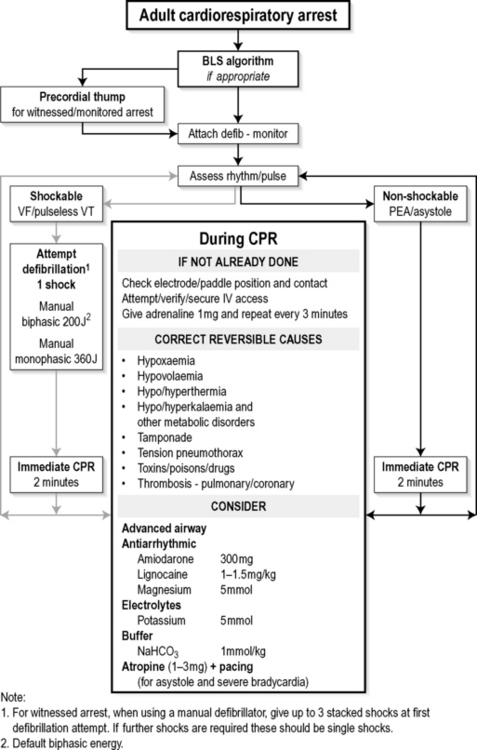
Fig. 1.2.1 Algorithm for management of adult cardiorespiratory arrest.
(Reproduced with permission from the Australian Resuscitation Council.)
However, resuscitation knowledge is still incomplete, and some of the ALS techniques currently in use are not supported by the highest levels of scientific rigour. Thus strict adherence to any guidelines should be guided by common sense. Individuals with specialist knowledge may modify them according to the level of their expertise and the specific clinical situation or environment in which they practise.10
Initiation of ALS
The ARC guidelines and algorithm qualify the commencement of BLS with the statement ‘if appropriate’.2 This is because BLS is only a temporary and inefficient substitute for normal cardiorespiratory function. ALS interventions are almost always necessary to produce the return of spontaneous circulation (ROSC).
Electrical defibrillation is the fundamental tenet of the treatment for VF and pulseless VT. However, the likelihood of defibrillation restoring a sustained, perfusing cardiac rhythm, and of a favourable long-term outcome, exists for as little as 90 seconds after the onset of cardiac arrest. The chances of survival to hospital discharge decline rapidly thereafter, as myocardial high-energy phosphate stores are consumed. Therefore, minimizing the time to defibrillation is the priority in resuscitation from sudden cardiac arrest. The purpose of BLS is to support the patient’s cardiorespiratory status as effectively as possible until equipment – particularly a defibrillator – and drugs become available.1,2
The point of entry into the ALS algorithm depends on the circumstances of the cardiac arrest. In many situations, such as out-of-hospital cardiac arrest, BLS will already have been initiated and should be continued while the defibrillator/monitor is being prepared. Diagnosis must be swift and the defibrillator attached without delay when the patient is being monitored at the time of a cardiac arrest.1,2
Attachment of the defibrillator/monitor and rhythm recogniton
Automated external defibrillator
Apply the self-adhesive pads in the standard anteroapical positions for defibrillation (see below) using an automated external defibrillator (AED). An internal microprocessor analyses the ECG signal and, if VF/VT are detected, it causes the AED to display a warning and then either deliver a shock (automatic) or advise the operator to do so (semi-automatic).2,11,12
Manual external defibrillator
The critical decision for the rescuer after applying the self-adhesive pads or paddles of a manual external defibrillator is whether or not the cardiac rhythm is VF/VT.1,2 Up to 70% of patients with an out-of-hospital cardiac arrest will be in VF/VT at the time of arrival of EMS personnel and a monitor/defibrillator.11 The vast majority of cardiac arrest survivors come from this group.1,2,4
Rhythm recognition
Ventricular fibrillation (VF)
VF is a pulseless, chaotic, disorganized rhythm characterized by an undulating, irregular pattern that varies in amplitude and morphology, with a ventricular waveform of more than 150/minute.1,2
Ventricular tachycardia (VT)
Pulseless VT is characterized by broad, bizarrely shaped ventricular complexes associated with no detectable cardiac output. The rate is more than 100/minute by definition, and is usually in excess of 150.1,2
Pulseless electrical activity (PEA) or electromechanical dissociation (EMD)
The absence of a detectable cardiac output in the presence of a coordinated electrical rhythm is called pulseless electrical activity (PEA), also known as electromechanical dissociation (EMD).1,2
Asystole
Defibrillation
The only proven effective treatment for VF and pulseless VT is electrical defibrillation.1,2,10,12 The defibrillator must be brought immediately to the side of the person in cardiac arrest and, if the rhythm is VF/VT, defibrillation attempted without delay.
Placement of pads or paddles
Pads or paddles are often identified as ‘sternum’ and ‘apex’, or ‘anterior’ and ‘posterior’, which is of no relevance for emergency transthoracic defibrillation. It simply allows detection by the pads/paddles of the correct orientation of certain perfusing cardiac rhythms prior to synchronized cardioversion.1,2,11,12,13
Anteroposterior pad or paddle position
An alternative is the anteroposterior position: the anterior pad/paddle is placed over the praecordium or apex, and the posterior pad/paddle is placed on the patient’s back in the left or right infrascapular region.
Do not attempt defibrillation over ECG electrodes or medicated patches, and avoid placing pad/paddles over significant breast tissue in females. Also the pad/paddles should be placed at least 12–15 cm away from the module and pulse generator, if the patient has respectively an implanted pacemaker or a cardioverter–defibrillator. Arrange to check the function of any pacemaker or cardioverter–defibrillator as soon as practicable after successful defibrillation.1,2,11,12
Timing of shocks
Three stacked shocks
The Australian Resuscitation Council (ARC) guidelines recommend that if the cardiac arrest is witnessed by a rescuer with a manual defibrillator, up to three stacked shocks should be used as the first defibrillation attempt. The pads or paddles should remain on the chest wall and the defibrillator immediately recharged after each shock, up to a total of three. Check the rhythm after each defibrillation attempt and repeat the shock if VF/VT persists. A total of three shocks should be delivered within 30–40 seconds. Any delay over 20 seconds between shocks is unacceptable and should lead to abandonment of the stacked shocks and immediate recommencement of CPR.2
Single shocks
Only deliver a single shock when the cardiac arrest has not been witnessed by the rescuer, or when additional shocks are required after the first three stacked shocks, followed immediately by CPR for 2 minutes. Do not delay recommencing CPR to assess the rhythm or to look for signs of life.2
The New Zealand Resuscitation Council (NZRC) guidelines and the ILCOR Universal Cardiac Arrest Algorithm do not recommend stacked shocks, but use the single shock regimen exclusively.1,9
Waveform and energy of shocks
Two main types of waveform are available from cardiac defibrillators.
Monophasic sinusoidal waveform
Older defibrillators use a damped monophasic sinusoidal waveform, which is a single pulse lasting for 3–4 ms. Set the energy level at the maximum when using a monophasic defibrillator in adults, which is usually 360 joules (J) for all shocks.1,2,9
Biphasic waveforms
Modern defibrillators using biphasic waveforms with impedance compensation should now be considered the ‘gold standard’. These biphasic (bidirectional) truncated transthoracic shock defibrillators are as effective at lower energies as standard damped sine-wave shock defibrillators, and result in fewer post-defibrillation ECG abnormalities.1,2,11–13
Set the level at 200 J for all shocks when using a biphasic defibrillator in adults. Other energy levels may be used if the relevant clinical data for that defibrillator suggest an alternative energy level provides comparable success to the ‘default’ energy level of 200 J.1,2,9
Optimizing transthoracic impedance
A critical myocardial mass must be depolarized synchronously for defibrillation to be successful. This interrupts the fibrillation and allows recapture by a single pacemaker. Thus the transthoracic impedance must be minimized in order to maximize the probability of success.1,2,11–13
Reduction of transthoracic impedance
Current-based defibrillation
Conventional defibrillators are designed to deliver a specified amount of energy measured in joules. Depolarization of myocardial tissue is accomplished by the passage of electrical current through the heart; clinical studies have determined that the optimal current is 30–40 amperes (A).11,13 The current delivered at a fixed energy is inversely related to the transthoracic impedance, so a standard energy dose of 200 J delivers about 30 A to the average patient. The current generated may be inadequate in patients with greater than average impedance, whereas patients with smaller transthoracic impedance may sustain myocardial damage from excessive current flow.11–13
Some newer current-based defibrillators automatically measure transthoracic impedance and then predict and adjust the energy delivered to avoid inappropriately high or low transmyocardial current. These devices have defibrillation success rates comparable to those of conventional defibrillators while cumulatively delivering less energy. The reduced energy should result in less myocardial damage and may reduce post-defibrillation complications.11–13
Automated external defibrillators (AED)
AEDs are highly accurate, some models demonstrating 100% specificity and 90–92% sensitivity in correctly identifying coarse VF.12 Their precision is less for fine VF and least for VT, but overall accuracy is comparable to that of an experienced cardiologist.11 EMS systems equipped with AEDs are able to deliver the first shock up to 1 minute faster than when using conventional defibrillators. Rates of survival to hospital discharge are equivalent to those achieved when more highly trained first responders use manual defibrillators.4
The major advantage of AEDs over manual defibrillators is their simplicity, which reduces the time and expense of initial training and continuing education, and increases the number of persons who can operate the device.4,11–12 Members of the public have been trained to use AEDs in a variety of community settings, and have demonstrated that they can retain skills for up to 1 year.4 Encouraging results have been produced when AEDs have been placed with community responders such as firefighters, police officers, casino staff, security guards at large public assemblies and public transport vehicle crews.4,11
The Australasian College for Emergency Medicine recommends that all clinical staff in healthcare settings should have rapid access to an AED or a defibrillator with AED capability.14
Technical problems
Whenever attempted defibrillation is not accompanied by skeletal muscle contraction take care to ensure good contact, and that the defibrillator is turned on, charged up, develops sufficient power and is not in synchronized mode. The operational status of defibrillators should be checked regularly, and a standby machine should be available at all times. The majority of defibrillator problems are due to operator error or faulty care and maintenance.12
Complications of defibrillation
The CPR ‘Code Blue’ process
Immediate defibrillation is of paramount importance for VF/pulseless VT, although periods of well-performed CPR help maintain myocardial and cerebral viability and may improve the likelihood of success with subsequent shocks.1,2 Current ALS guidelines recommend that, after delivering a single shock, CPR should be resumed immediately and continued for 2 minutes. Only after this period of CPR should the rhythm and pulse be reassessed and further treatment initiated as necessary.1,2,9
Rationale for resumption of CPR
The rationale for this is that after one defibrillator shock there is typically a delay of several seconds before a diagnostic-quality ECG trace is obtained. Additionally, even when defibrillation is successful, there is often a temporary impairment of cardiac function from seconds to minutes, associated with a weak or impalpable pulse. Thus waiting for a recognizable ECG rhythm or palpating for a pulse that may not be present anyway after successful defibrillation will unnecessarily delay the recommencement of CPR. This is detrimental to the patient who does not yet have ROSC.1,2
Algorithm loops
Pay attention to each of the following, either continuously or after each loop of the algorithm:1,2
PEA or asystole
Reassess the ECG rhythm after 2 minutes of CPR and, when appropriate, the pulse. Give a single shock without delay if VF/pulseless VT persist.1,2,9 However, when PEA or asystole are present on ECG rhythm and/or pulse assessment, do not defibrillate as it may be deleterious.1,2,11,12
The prognosis for PEA or asystole is much worse than for VF/VT, and unless there are potentially reversible causes (4Hs and 4Ts), the application of other ALS interventions (see below) is indicated, but seldom of value.1,2
Other ALS interventions
Not one ALS intervention other than defibrillation has been proved to improve patient outcome.1,2,10,15 Some clinicians maintain that ALS has an incremental benefit compared to defibrillation alone,4,15,16 but although some data support this, it remains impossible to prove.10 Cardiac pacing does not improve survival from asystole, either pre-hospital or in the emergency department (ED) setting.1,2
Advanced airway management
Endotracheal intubation is considered the best technique for airway management during cardiac arrest and is recommended in the ARC guidelines.2 However, no randomized controlled study exists that shows an improved outcome with endotracheal intubation compared to basic airway management.1,2,10,15,16 Other advanced airway devices studied during CPR as alternatives to the endotracheal tube include the laryngeal mask airway (LMA), and the oesophageal–tracheal combitube (Combitube). None is definitely superior to basic airway management during cardiac arrest in terms of consistently improved survival.1,2
Advanced airway techniques
The optimal technique for airway management depends on the equipment available, the circumstances of the cardiac arrest and the training and experience of the resuscitation team.1,2
Endotracheal intubation: advantages
One technical benefit of an advanced airway such as endotracheal intubation is that no interruption to chest compressions is necessary for ventilations during CPR. Also, an endotracheal tube isolates and protects the airway and allows suction, and the administration of drugs in the absence of vascular access.1,2,16,17
Endotracheal intubation: disadvantages
The main disadvantages of endotracheal intubation are the interruption to chest compressions during tube insertion, which should never exceed 20 seconds, and unrecognized oesophageal tube placement.1,2 Once an endotracheal tube has been inserted, correct placement must be verified by direct vision of the tube passing between the vocal cords, or by the success of chest ventilation as demonstrated by an oesophageal detector device (ODD) and/or by end-tidal CO2 recording, which will only register when there is ROSC.1,2
Ventilation and oxygenation
Cardiac arrest and CPR cause an increase in dead space and a reduction in lung compliance that compromise gas exchange. Therefore, a fractional inspired oxygen concentration (FIO2) of 1.0 (100% oxygen delivery system) is essential in cardiac arrest to optimize oxygen delivery.1,2,17
Minute volume
Carbon dioxide (CO2) production and delivery to the pulmonary circulation are limited by the markedly reduced cardiac output achieved during cardiopulmonary resuscitation. As a consequence, a relatively low minute volume of 3.5–5.0 L is sufficient to achieve adequate CO2 excretion and prevent hypercapnia. This situation will be altered if a CO2-producing buffer such as sodium bicarbonate is administered. A small increase in minute ventilation is then required to prevent the development of a respiratory acidosis.1,2
Ventilation rate and tidal volume
A ventilation rate of 8–10 per minute without pausing during chest compressions and a tidal volume of 400–500 mL (5–6 mL/kg) are sufficient to clear CO2 during most cardiac arrest situations, when an advanced airway is in place. This should cause a visible rise and fall of the patient’s chest.17 A self-inflating bag/valve/mask system and/or an airway intubation device remain the mainstay of advanced airway and ventilation management in ALS.1,2,17
Vascular access and drug delivery
Drug delivery
Give a 20–30 mL i.v. fluid flush following any administered drug and/or raise the limb to facilitate delivery to the central circulation.1,2 Central venous cannulae deliver drugs rapidly to the central circulation and should be used when already in place. Otherwise, as stated above, their insertion during CPR requires time and technical proficiency and interferes with defibrillation and the CPR process, which is unacceptable.1,2
Intratracheal route
The ideal dose and dilution of drugs given by this route are unknown, but recommendations include using 3–10 times the standard i.v. drug dose diluted in 10 mL of water or normal saline. The drug should be delivered via a catheter or quill placed beyond the tip of the endotracheal tube, and followed by ventilations to aid dispersion.1,2,18
Drug therapy in ALS
Not one drug used in resuscitation has been shown to improve long-term survival in humans after cardiac arrest.1,2,10,15 Despite this, a number of agents continue to be employed based on theoretical, retrospective or anecdotal evidence of their efficacy.1,2,10
Epinephrine (adrenaline)1,2,9,10,15,16
Indications
Lignocaine (lidocaine)1,2
Indications
Magnesium1,2,10
Indications
Vasopressin1,2,19
Vasopressin is an alternative vasopressor to epinephrine. There is currently insufficient evidence to support or refute its use either alone or in combination with adrenaline in any cardiac arrest rhythm.
Haemodynamic monitoring during CPR
When to discontinue ALS
The vast majority of patients who survive out-of-hospital cardiac arrest have ROSC before arrival at the ED. Only 33 of 5444 patients (0.6%) in 18 studies between 1981 and 1995, who were transported to an ED still in cardiac arrest after unsuccessful pre-hospital resuscitation, survived to hospital discharge.21 Twenty-four of the surviving patients arrived in the ED in VF, and 11 of these had their initial cardiac arrest in the ambulance en route to hospital, or had temporary ROSC before arrival. Thus virtually all patients arriving at an ED still in asystole from out-of-hospital cardiac arrest die without leaving hospital.
Ceasing CPR pre-hospital
A recommendation made in 1993 for out-of-hospital cardiac arrest in the normothermic patient was that resuscitation should cease if there was no ROSC after 25 minutes of ALS.22 Two important exceptions to this guide are:
These recommendations were applied and considered valid in a prospective study in that same year.23
In-hospital cardiac arrest outcomes
In-hospital cardiac arrest with a poor outcome
A poor outcome is linked to pre-existing conditions such as cardiogenic shock, metastatic cancer, renal failure, sepsis and an acute cerebrovascular accident. Age is not an independent predictor of outcome, either in hospital or for out-of-hospital cardiac arrest.24,25
Outcome of prolonged ALS
ALS resuscitation efforts lasting more than 30 minutes without ROSC at any stage are so uniformly unsuccessful that resuscitation should be abandoned, except in certain special circumstances such as hypothermia, possibly some drug overdoses, and following thrombolysis in suspected massive pulmonary embolism (PE).10,25 The return of spontaneous circulation at any time during the resuscitation process resets the clock to time zero.1,5
Prognosis for survival after cardiac arrest
The best prospect of neurologically intact long-term survival after a cardiac arrest occurs when:
Out-of-hospital cardiac arrest
Some variation in survival after an out-of-hospital cardiac arrest is due to differences in EMS systems, as well as to differing research methodology and data reporting. In a 1996 meta-analysis of 36 articles published between 1973 and 1992 describing 41 EMS systems in six countries,26 survival varied from 0% to 21%, with an overall mean survival of 8%.
In-hospital cardiac arrest
The prognosis for survival from in-hospital cardiac arrest is only marginally better, with survival to discharge averaging 13.8% of 12 961 patients described in reports published between 1961 and 1984.24 However, in a further seven reports published between 1978 and 1989 this dropped to 11% of 1804 patients.25
Uniform reporting in cardiac arrest research
Cardiac arrest research and the interpretation of available data have been hampered by inconsistent methodology and reporting. An important initiative is recognizing the need for uniform, internationally recognized definitions and guidelines for the reporting of cardiac arrest data. A number of templates have been developed that include the most relevant variables for describing and comparing cardiac arrest research results. These are referred to as Utstein-style guidelines or templates, after Utstein Abbey, near Stavanger, Norway, where expert researchers and clinicians gathered in 1990.27
1 International Liaison Committee on Resuscitation. 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiac Care. Science with Treatment Recommendations. Resuscitation. 2005;67:181-303.
2 Australian Resuscitation Council. Adult Advanced Life Support: Australian Resuscitation Guidelines 2006. Emergency Medicine Australasia. 2006;18:337-356. [Also online http://www.resus.org.au/ Accessed 26 Oct 2007]
3 Larsen MP, Eisenberg MS, Cummins RO, et al. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Annals of Emergency Medicine. 1993;22:1652-1658.
4 Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: the ‘chain of survival’ concept: a statement for health professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation. 1991;83:1832-1847.
5 Australian Resuscitation Council. ARC Guidelines 4–7, 9, 11–12. Emergency Medicine Australasia. 2006;18:325-371. [Also online http://www.resus.org.au/ Accessed 26 Oct 2007.]
6 Becker LB, Smith DW, Rhodes KV. Incidence of cardiac arrest: a neglected factor in evaluating survival rates. Annals of Emergency Medicine. 1993;22:86-91.
7 Bernard S. Outcome from prehospital cardiac arrest in Melbourne Australia. Emergency Medicine (Fremantle). 1998;10:25-29.
8 The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care – an international consensus on science. Resuscitation. 2000;46:1-447.
9 New Zealand Resuscitation Council. Level 1–7 Guidelines, 2006. Available online http://www.nzrc.org.nz/ Accessed 26 Oct 2007
10 Maguire JE. Advances in cardiac life support: sorting the science from the dogma. Emergency Medicine (Fremantle). 1997;9:1-21.
11 Truong JH, Rosen P. Current concepts in electrical defibrillation. Journal of Emergency Medicine. 1997;15:331-338.
12 Bossaert LL. Fibrillation and defibrillation of the heart. British Journal of Anaesthesia. 1997;79:172-177.
13 Kerber RE. Electrical treatment of cardiac arrhythmias: defibrillation and cardioversion. Annals of Emergency Medicine. 1993;22:296-301.
14 Australasian College for Emergency Medicine. Statement on early access, defibrillation. ACEM. July 2005;S1:1. Available online http://www.acem.org.au/ Accessed 26 Oct 2007
15 Pepe PE, Abramson NS, Brown CG. ACLS – Does it really work? Annals of Emergency Medicine. 1994;23:1037-1041.
16 Ornato JP, Paradis N, Bircher N, et al. Future directions for resuscitation research. III. External cardiopulmonary resuscitation advanced life support. Resuscitation. 1996;32:139-158.
17 Gabbott DA, Baskett PJF. Management of the airway and ventilation during resuscitation. British Journal of Anaesthesia. 1997;79:159-171.
18 Gonzalez ER. Pharmacologic controversies in CPR. Annals of Emergency Medicine. 1993;22:317-323.
19 Barlow M. Vasopressin. Emergency Medicine (Fremantle). 2002;14:304-314.
20 Ornato JP. Hemodynamic monitoring during CPR. Annals of Emergency Medicine. 1993;22:289-295.
21 Brennan RJ, Luke C. Failed prehospital resuscitation following out-of-hospital cardiac arrest: are further efforts in the emergency department warranted? Emergency Medicine (Fremantle). 1995;7:131-138.
22 Bonnin MJ, Pepe PE, Timball KT, et al. Distinct criteria for termination of resuscitation in the out-of-hospital setting. Journal of the American Medical Association. 1993;269:1457-1462.
23 Pepe PE, Brown CG, Bonnin MJ, et al. Prospective validation of criteria for on-scene termination of resuscitation efforts after out-of-hospital cardiac arrest. Annals of Emergency Medicine. 1993;22:884-885.
24 McGrath RB. In-house cardiopulmonary resuscitation after a quarter of a century. Annals of Emergency Medicine. 1987;16:1365-1368.
25 Jastremski MS. In-hospital cardiac arrest. Annals of Emergency Medicine. 1993;22:113-117.
26 Nichol G, Destsky AS, Stiell IG, et al. Effectiveness of emergency medical services for victims of out-of-hospital cardiac arrest: a meta-analysis. Annals of Emergency Medicine. 1995;27:700-710.
27 Dick WF. Uniform reporting in resuscitation. British Journal of Anaesthesia. 1997;79:241-252.
1.3 Ethics of resuscitation
Introduction
Ethics and the clinician
Some guidance about legal issues related to resuscitation in an Australasian context may be accessed in publications such as those from the Australian Resuscitation Council Guidelines1 and in the summary statement from the Medical Council of New Zealand.2
Philosophical models
The approach to ethical dilemmas may vary according to the philosophical perspective adopted.3 Although there are a variety of models describing moral decision making, only a pragmatic overview will be given here. In general terms, a utilitarian approach may be adopted which values the positive balance of good over bad brought about by any action; or, alternatively, a deontological approach, which values actions that adhere to overriding moral principles. However, moral philosophers have recognized that moral principles may compete against each other when specific actions are considered. Moral principles should be honoured, but when they are competing in a given circumstance, we should then consider the relative balance of good and bad that ensues from the application of each principle. Thus, we have a composite philosophy wherein both the principles and the consequences of their application may be considered.
The principles of Beauchamp and Childress
Beauchamp and Childress4 developed this further into a practical framework for medical ethical deliberation. They described four principles that should be honoured in medical decision making, and when these principles compete, the relative balance of good and bad should be considered. These four principles are:
Application of the principles of Beauchamp and Childress to resuscitation medicine
The harms of resuscitation
The benefits of resuscitation include the avoidance of death and the restoration of good health. The harms of resuscitation may be of the following five types:5
Advance directives
In the setting of some form of an advance directive, three questions should be considered:
Futility
The concept of futility has been widely discussed in the medical literature, with particular emphasis on resuscitation medicine.6 Regrettably, discussions of the harms of resuscitation have become stalled by failed attempts to define futility.
The balance of benefit and harm
Futility defines the absence of acceptable benefit for any given intervention, whereas reasonable ethical deliberation demands that we consider the ratio of benefit to harm. If there is no benefit, any harm at all would make the benefit:harm ratio unfavourable. However, even if the endeavour is not futile and brings about measurable benefit, this does not necessarily mean that the endeavour is the right thing to do, as the amount of harm that ensues, as defined above, may outweigh any benefit. It is the benefit:harm balance, as assessed by considering the four ethical principles and the five types of harm described above, that has the most relevance in determining whether to start or to stop a resuscitation.
Consent, withholding and withdrawing resuscitation
Presumed consent using professional substituted judgement
A modification of presumed consent is presumed consent using professional substituted judgement.7 This means the resuscitators gather as much information about the patient as they possibly can to attempt to understand how the patient would view this decision. This usually involves speaking with the patient’s loved ones. Then, with some knowledge of the likely outcome of the proposed resuscitation, based on previous experience and a knowledge of the medical literature, they can exercise their moral imagination by asking ‘Would I want this treatment if I was this patient?’ In this way the patient’s autonomy is as best respected as it can be under difficult circumstances, by combining a knowledge of the harms and benefits of the resuscitation with an appreciation of this balance from the patient’s perspective.
‘Not For Resuscitation’ (NFR) orders
A patient’s written predetermination of whether to consent to resuscitation, in the form of an ‘Advance Directive’ or ‘Living Will’, has already been discussed. However, most people who become our patients have not made explicit and accessable determinations of their wishes. Many who need resuscitation are already in hospital or another healthcare institution, or have been in the recent past. Consequently, there is great opportunity for decision making during resuscitation to be aided by careful, documented, prior consideration of whether the resuscitators have ‘permission to proceed’. Such documentation, when recorded in a patient’s notes, is called a Not For Resuscitation (NFR) or Do Not Resuscitate (DNR) order.
The content of NFR orders
Most large Australasian hospitals have a policy about NFR orders and an increasing number use standardized forms to record the resuscitation status of patients. In addition, many provide information leaflets for patients and relatives explaining the NFR process and status.8 Local policy, including the completion of standardized forms, should be followed.
Usually the NFR form will include the patient’s diagnosis, the reasons for an NFR order, the date of issue, the date when it should be reviewed, the nature of the discussion with the patient and/or the relatives, or reasons why such a discussion did not occur. In addition, it should record exactly what is intended by the NFR status (for example, not for cardiopulmonary resuscitation in the event of cardiac arrest, or not for intensive care), including clarification that all other care will be provided as required. There is some evidence that those who have NFR orders may be denied other care owing to a perception that there is ‘nothing more to offer them’.9 It is most important that an ethically sound decision about resuscitation not being what the patient wants does not lead to an immoral neglect to provide good care. The nature of what care can and should still be delivered – for example analgesia, intravenous fluids, non-invasive ventilation etc. – should be clearly documented.
Establishing an NFR order
There is some debate about having an NFR order without discussion with the patient, and some suggest that there should be a presumption of resuscitation for all who have not agreed to an NFR.10 This view is counter to the arguments discussed above, which propose that some form of consent needs to be obtained for resuscitation to proceed, just as it is needed for all other medical interventions. There will be occasions when discussion with the patient is not undertaken, particularly if the patient is without decision-making capacity, or resuscitation is clearly without benefit. No rational person would consent to an intervention that is clearly more harmful than beneficial, and so lack of consent can be presumed, as discussed above. These details should be recorded.
Practising resuscitation procedures on the newly dead
Practising resuscitation procedures – most commonly endotracheal intubation – on patients who have died after an unsuccessful resuscitation is common in many parts of the world.11 However, some view this with a repugnance that may be rationally argued. Others would propose that the benefit of this practice to subsequent patients outweighs any repugnance felt by others who witness it, or any harm done to the recently deceased.
Consent for practising resuscitation procedures on the newly dead
‘Construed consent’ is a modification of implied consent, suggesting that if consent was obtained for a certain procedure it can be construed for a related procedure. If it is conceded that a form of consent (presumed consent, as suggested above) is obtained to intubate a patient during resuscitation, can it be construed that consent also applies to intubation after death? There is a superficial logic to this, as to perform the same procedure on the same patient with the same equipment one minute before, and one minute after, death seems a continuum of the same therapeutic relationship. However, on close analysis there is a sufficiently significant difference as to render previous consent null and void. The consent to resuscitate is based on a contract between medical staff and the patient dedicated to helping the patient. When the objective is no longer to help the patient, the previous contract is irrelevant and a new one must be entered into. Intubating the deceased under the old contract is a violation of the trust inherent in the previously formed therapeutic relationship. An appreciation of this violation contributes to the repugnance towards the procedure.
Conclusions
Emergency medicine abounds with clinical dilemmas requiring ethical deliberation. Such deliberation may be influenced by theories regarding the consequences of action, theories based on moral principles, or some combination of these two. Beauchamp and Childress4 present a model for deliberation based on the principles of respect for autonomy, non-maleficence, beneficence and justice. Although this model frequently will not provide an answer that is beyond dispute, it does allow a rational examination of the important issues so that our subsequent actions will at least be better directed than they might otherwise have been.
1 Australian Resuscitation Council. Adult advanced life support: Australian Resuscitation Council Guidelines 2006. Emergency Medicine Australasia. 2006;18:337-356.
2 Medical Council of New Zealand. A doctor’s duty to help in a medical emergency, August 2006. http://www.mcnz.org.nz/portals/0/Guidance/Doctorsdutiesinanemergency.pdf. (Accessed, August 2007)
3 Beauchamp TL. Philosophical ethics. An introduction to moral philosophy, 2nd edn. McGrawHill, New York, 1991.
4 Beauchamp TL, Childress JF. Principles of biomedical ethics, 5th edn. Oxford: Oxford University Press, 2001.
5 Ardagh M. Preventing harm in resuscitation medicine. New Zealand Medical Journal. 1997;110:113-115.
6 Ardagh MW. Futility has no utility in resuscitation medicine. Journal of Medical Ethics. 2000;26:393-396.
7 Ardagh MW. Resurrecting autonomy during resuscitation: the concept of professional substituted judgement. Journal of Medical Ethics. 1999;25:375-378.
8 Sidhu NS, Dunkley ME, Egan MJ. ‘Not for resuscitation’ orders in Australian public hospitals: policies, standardized order forms and patient information leaflets. Medical Journal of Australia. 2007;186:725.
9 Shepardson LB, Younger SJ, Speroff T, Rosenthal GE. Increased risk of death in patients with do-not-resuscitate orders. Medical Care. 1999;37:727-737.
10 Ebrahim S. Do not resuscitate decisions: flogging dead horses or a dignified death? British Medical Journal. 2000;320:115-156.
11 Ardagh M. May we practise endotracheal intubation on the newly dead? Journal of Medical Ethics. 1997;23:289-294.