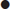Resuscitation
Edited by Anthony Brown
1.1 Basic Life Support
Stephen A Bernard and Sameer Pathan
Introduction
Basic Life Support (BLS) aims to maintain respirations and circulation in the cardiac arrest victim. BLS involves a major focus on cardiopulmonary resuscitation (CPR) with minimal use of ancillary equipment. It includes chest compressions with or without rescue breathing and defibrillation with a manual or semiautomated external defibrillator (SAED). BLS can be successfully performed immediately by any rescuer with little or no training and, in the out-of-hospital cardiac arrest (OHCA), BLS has proven value in the survival of neurologically intact victims [1–3].
This chapter outlines an approach to BLS that can be delivered by any rescuer, while awaiting the arrival of emergency medical services (EMS) or medical expertise able to provide Advanced Life Support (ALS) (see Chapter 1.2).
Chain of Survival
The series of linked actions for a victim of sudden cardiac arrest is known as the ‘Chain of Survival’ [4]. The first steps are early recognition of those at risk of or in active cardiac arrest and an immediate call to activate help from the emergency medical services. This is followed by early commencement of CPR with an emphasis on chest compressions and rapid defibrillation, which significantly improves the chances of survival from ventricular fibrillation (VF) in OHCA [1–3]. Cardiopulmonary resuscitation plus defibrillation within 3–5 min of collapse following VF in OHCA can produce survival rates as high as 49–75% [5–7]. Each minute of delay before defibrillation reduces the probability of survival to hospital discharge by 10–12% [2,3]. The final links in the Chain of Survival are effective Advanced Life Support and a new focus (5th link) on integrated post-resuscitation care, targeted at optimizing and preserving cardiac and cerebral function [8–10].
Development of protocols
Any guidelines for BLS must be evidence based and consistent across a wide range of providers. Many countries have established national committees to advise community groups, ambulance services and the medical profession on appropriate BLS guidelines. Table 1.1.1 shows the national associations that made up the International Liaison Committee on Resuscitation (ILCOR) in 2010. The ILCOR group meets every 5 years to review the BLS and ALS guidelines and to evaluate the scientific evidence that may lead to changes. The next scheduled update is in 2015.
Table 1.1.1
Membership of the International Liaison Committee on Resuscitation (ILCOR) 2010
American Heart Association (AHA)
European Resuscitation Council (ERC)
Heart and Stroke Foundation of Canada (HSFC)
Resuscitation Council of Southern Africa (RCSA)
Australian and New Zealand Committee on Resuscitation (ANZCOR)
InterAmerican Heart Foundation (IAHF)
Resuscitation Council of Asia (RCA)
Revision of the BLS guidelines, 2010
The most recent revision of the BLS guidelines occurred in 2010 and followed a comprehensive evaluation of the scientific literature for each aspect of BLS. Evidence evaluation worksheets were developed and were then considered by ILCOR (available at http://circ.ahajournals.org/content/122/16_suppl_2/S606.full.pdf). The final recommendations were published in late 2010 [11].
Australian Resuscitation Council (ARC) and New Zealand Resuscitation Council (NZRC) BLS guidelines
Subsequently, each national committee endorsed the guidelines with minor regional variations to take into account local practices. The recommendations of the Australian Resuscitation Council (ARC) combined with the New Zealand Resuscitation Council (NZRC) on BLS were co-published in 2010 (available at http://www.resus.org.au/policy/guidelines/ and http://www.nzrc.org.nz/ respectively) [12].
DRSABCD approach to Basic Life Support
A flowchart for the initial evaluation and provision of BLS for the collapsed patient is shown in Figure 1.1.1. This is based on a DRSABCD approach that includes Dangers?; Responsive?; Send for help; open Airway; normal Breathing? start CPR; and attach Defibrillator. This process therefore covers the recognition that a patient has collapsed and is unresponsive, with a safe approach checking for danger and immediately sending for help to activate the emergency medical response team. This is followed by opening the airway and briefly checking for abnormal or absent breathing, with rapid commencement of chest compressions with breaths if the pulse is absent. A defibrillator is attached as soon as it is available and prompts followed if it is automatic or semiautomatic.
Change to the adult BLS in 2010
A significant change to the adult BLS in the ILCOR 2010 resuscitation guidelines was the recommendation for a CAB (Compressions, Airway, Breathing) sequence instead of an ABC (Airway, Breathing, Compressions) sequence. This was aimed at minimizing any delay to initiate chest compressions, particularly when sudden collapse is witnessed and of likely cardiac origin. Thus, rescuers of adult cardiac arrest victims should begin resuscitation with 30 compressions followed by two breaths, rather than opening the airway and delivering breaths first (that wastes valuable time) [11]. ILCOR 2010 also mentions that, for unresponsive adults and children, the airway may be opened using the head tilt–chin lift manoeuvre when assessing breathing or giving ventilations.
Regional variations
There are, however, regional variations in the interpretation and incorporation of opening the airway within the BLS algorithm. In the European Resuscitation Council (ERC) and the Australian Resuscitation Council (ARC) with the New Zealand Resuscitation Council (NZRC) algorithm, opening the airway comes before assessment of breathing followed by compression if required. This effectively preserves the ABC sequence to avoid confusion, whereas the ILCOR 2010 guidelines and the American Heart Association (AHA) Resuscitation Guidelines 2010 recommend following a CAB sequence.
The ILCOR 2010 universal BLS algorithm with ARC and NZRC considerations is discussed in the remainder of this chapter.
Check for dangers
As the patient is being approached, the rescuer should immediately consider any dangers that may be associated with the collapse of the patient. For example, the patient may have been electrocuted and there is a substantial risk of death to the rescuer if the power source is not switched off prior to patient contact.
There may also be significant danger from injury from a passing vehicle in the case of a motor vehicle collision where a patient is unconscious, as well as the potential for fire. Therefore, unless the patient is entrapped, an unconscious patient should be carefully extricated from a vehicle prior to the arrival of emergency medical services, taking care to minimize movement of the spine. The risk of injury from fire or explosion is considered to exceed the risk of moving an unconscious patient prior to immobilization of the cervical spine with a collar.
Collapse in a confined space
A patient who has collapsed in a confined space raises the possibility of poisoning with a toxic gas such as carbon monoxide. Do not enter the scene until it is declared safe by emergency services, usually the fire brigade. Likewise, the potential for hazardous agent release must be considered if multiple victims are present, such as an organophosphate causing multiple collapses and cardiac arrest. In this setting, rescuers must not enter the area and should await the arrival of EMS with a specialist Hazmat team to declare the area safe.
Check for response and send for help
The patient who has collapsed is rapidly assessed to determine whether there is unconsciousness and absence of normal breathing, indicating possible cardiorespiratory arrest. This is assessed by a gentle ‘shake and shout’ and observation of the patient’s response, rather than looking specifically for signs of life (that was deemed potentially confusing). Unresponsive patients should then be assessed for absent or inadequate breathing.
Lay rescuers should suspect cardiac arrest if the patient is unresponsive to ‘shake and shout’ and immediately telephone the EMS (‘call first’). Lay rescuers should then follow the advice given by a dispatcher to provide BLS care. A trained rescuer or healthcare providers may check for unresponsiveness and abnormal breathing at the same time and then activate the EMS or cardiac arrest team.
Alternatively, healthcare rescuers may commence resuscitation focusing on the airway for approximately 2 minutes before calling the EMS (‘CPR first’), when the collapse is due to suspected airway obstruction (choking) or inadequate ventilation (drowning, hanging, etc.).
Assessment of airway, breathing and circulation
Make an assessment of breathing if a patient has collapsed and is apparently unconscious [12]. Place the patient supine if face down. A trained lay rescuer or healthcare rescuer may open the airway using the head tilt–chin lift manoeuvre when assessing breathing or giving ventilations, taking care not to move the neck in a suspected trauma case.
Adequate respiration is assessed by visually inspecting the movement of the chest wall rise and listening for upper airway sounds. Occasional deep (agonal gasps) respirations may continue for a few minutes after the initial collapse in cases of cardiac arrest. These respirations are not considered to represent normal breathing.
Cardiopulmonary resuscitation
Cardiopulmonary resuscitation (CPR) is required if the patient is found to have inadequate or absent breathing on initial assessment. If the initial assessment of an unconscious patient reveals adequate respiration, turn the victim on his/her side and maintain in the semi-prone recovery position. Make constant checks to ensure continued respiration while awaiting the arrival of the EMS.
Current recommendations for the untrained lay rescuer is that he or she should not attempt to palpate for a pulse, as a pulse check is inaccurate in this setting [11]. A healthcare provider should take no more than 10 seconds to check for a pulse. If the rescuer does not definitely feel a pulse within 10 seconds, chest compressions should be started immediately.
The prior ABC sequence of BLS meant that chest compressions were often delayed while the rescuer opened the airway, positioned the patient, retrieved a barrier device or gave mouth-to-mouth expired air resuscitation (EAR) breaths as two initial ‘rescue breaths’. These are difficult and challenging to an untrained lay rescuer and result in significant delay in starting chest compressions, or worse still, not attempting CPR at all. Therefore, in the CAB sequence of BLS, the assessment is limited to checking for response and breathing and management starts with delivering 30 chest compressions.
Management
Chest compressions
All lay rescuers (trained or untrained) and healthcare rescuers should begin CPR if the victim is unresponsive and not breathing (ignoring occasional agonal gasps). Place the patient supine on a firm surface, such as a backboard, hard mattress or even the floor, to optimize the effectiveness of chest compressions. The emphasis is on delivering high-quality chest compressions: rescuers should push hard to a depth of at least 5 cm (or 2 inches) at a rate of at least 100 compressions per minute, allowing full chest recoil and minimizing interruptions in chest compressions [11]. Hence the maxim: ‘push hard, push fast, allow complete recoil and minimize interruptions’.
‘Cardiac pump’ or a ‘thoracic pump’ mechanism
There is still ongoing debate as to whether external chest compressions generate blood flow via a ‘cardiac pump’ mechanism or a ‘thoracic pump’ mechanism. Whatever the predominant mechanism of blood flow, owing to the relative rigidity of the chest wall, chest compressions result in around 20% of normal cardiac output in the adult.
Chest compressions with ventilation
Rescuers trained to provide ventilation should give two rescue breaths after each 30 compressions, for a compression–ventilation ratio of 30:2.
Chest compression only CPR
In untrained rescuers, ‘compression-only’ CPR is recommended where rescuers are unable or unwilling to perform mouth-to-mouth breaths (‘standard’ CPR). This ‘compression-only’ CPR technique is also recommended when EMS dispatchers are providing telephone advice to the untrained rescuer.
Healthcare professionals as well as lay rescuers are often uncomfortable doing mouth-to-mouth ventilations with an unknown victim of cardiac arrest. This should not, however, prevent them from carrying out ‘Hands only’ or ‘Chest compression only’ CPR. Compression only CPR by lay rescuers still improves survival over no CPR at all. In addition, there is growing evidence to support compression only CPR being as effective as conventional CPR, particularly when sudden collapse is witnessed and of likely cardiac origin [13–18].
Passive chest recoil
In support of ‘Hands only’ or ‘Chest compression only’ CPR is that if the airway is open, passive chest recoil during the relaxation phase of the chest compressions does also provide some air exchange. However, during prolonged CPR supplementary oxygen with assisted ventilation will be necessary. The precise interval during which the performance of compression only CPR is acceptable is currently unknown. Interestingly, no prospective adult cardiac arrest study has demonstrated that lay rescuer conventional CPR provides a better outcome than compression only CPR prior to EMS arrival.
Airway and breathing
It is reasonable to open the airway using the head tilt–chin lift manoeuvre when assessing breathing or giving ventilations in an unresponsive adult or child. Solid material in the oropharynx should be removed with a careful sweep of a finger if inspection of the airway reveals visible foreign material or vomitus in the upper airway. Take particular care not to cause pharyngeal trauma or propel material further down into the lower airway and not to be bitten!
Foreign body airway obstruction
If a victim suspected of a foreign body airway obstruction (FBAO) can cough, encourage him or her to cough and expel it out. If the cough is ineffective and the patient is conscious, give him/her up to 5 back blows with the heel of the hand and then up to 5 chest thrusts at the same compression point as in CPR, but sharper and slower. These techniques may be alternated, but make certain to call for EMS.
Airway obstruction manoeuvres
A num- ber of manoeuvres have been proposed to clear the airway if it is completely obstructed by a foreign body. In many countries, abdominal thrusts are still endorsed as the technique of choice (Heimlich manoeuvre). However, as this technique is associated with life-threatening complications, such as intra-abdominal injury, it is no longer recommended by the ARC or NZRC. Instead, the preferred technique for clearing an obstructed airway is by alternating back blows and/or chest thrusts.
Airway equipment
Simple airway equipment may be used as an adjunct to EAR, when cardiac arrest occurs in a medical facility. These include a simple face-mask, or bag/valve/mask ventilation, with or without an oropharyngeal Guedel airway. This equipment has the advantage of familiarity, decreases the risk of cross-infection, is aesthetically more appealing and may deliver additional oxygen, but does require prior training [19].
Whichever technique of assisted ventilation is used, the adequacy of the tidal volume is assessed by a rise of the victim’s chest, delivered over one second. There is insufficient evidence to support or refute the use of a titrated oxygen concentration or constant 21% oxygen (room air), compared with 100% oxygen during adult cardiac arrest. Current guidelines during adult cardiac arrest therefore support the use of any of expired air ventilation, bag/valve/mask using air, or up to 100% oxygen if available [11].
Defibrillation
As soon as a defibrillator arrives, the electrode pads are attached to the victim and the device switched on. Self-adhesive defibrillation pads have practical benefits over paddles for routine monitoring and defibrillation. They are safe, effective and now preferred to standard defibrillation paddles but, in all cases, the safety of rescuers and other team members is still paramount on shock delivery.
Shock delivery
When using a semiautomatic external defibrillator, the rescuer follows the voice instruction ‘stand clear’ and ‘press the button’ when asked to deliver the shock, if this is indicated. When using a manual defibrillator, the healthcare rescuer must personally select the desired energy level and deliver a shock after recognizing a shockable rhythm (VF or pulseless VT).
Minimizing interruptions to chest compressions
Irrespective of the resultant rhythm, chest compressions must be resumed immediately after each shock to minimize the ‘no-flow’ time; that is the time during which compressions are not delivered, for example during any rhythm analysis. Strategies to reduce the delay between stopping chest compressions and the delivery of a shock, the ‘pre-shock pause’, must also be kept to an absolute minimum to improve the chances of shock success [20,21]. Even a 5–10 second delay will reduce the chances of the shock being successful [22–24].
All modern defibrillators are now biphasic rather than monophasic and are more effective in terminating ventricular arrhythmias at lower energy levels [25,26]. However, there is still no randomized study that shows superiority in terms of neurological survival or survival to hospital discharge.
If a shock is not indicated, the rescuer should immediately resume CPR at a 30:2 compression–ventilation ratio and wait for EMS arrival or for the victim to start to recover.
Semiautomatic external defibrillator
The semiautomatic external defibrillator is now considered part of BLS. SAED devices are extremely accurate in diagnosing ventricular fibrillation or ventricular tachycardia. SAEDs are simple, safe and effective when used by either lay rescuers or healthcare professionals (in or out of hospital) [27]. A systematic review assessing the risk of SAED use to the rescuers reported 29 adverse events associated with defibrillation [28], but none have been published since 1997 [29].
Lay rescuer/non-medical personnel and public access SAED
SAEDs have been shown to be an effective part of the BLS programme in public places, such as airports [6], sport facilities, offices, aircraft [30,31], and in casinos [7], where minimally trained rescuers are on the scene quickly for a witnessed cardiac arrest. Lay rescuer AED programmes by police officers as first responders have achieved reported survival rates as high as 49–74% [32,33].
Recent data from national studies in Japan and the USA [34,35] showed that where an AED was available, victims were defibrillated sooner and had a better chance of survival.
Home access SAED
Finally, an SAED may be placed in the home of a patient who is at increased risk of sudden cardiac arrest, for use by a relative who might witness the event. However, a recent study that enrolled 7001 patients concluded that survival rates from sudden cardiac arrest at home were not increased, even when a defibrillator was available in the home [36].
Implantable cardioverter defibrillator and CPR
Patients at highest immediate risk of unexpected cardiac arrest may have an implantable cardioverter defibrillator (ICD) inserted which, on sensing a shockable rhythm, will discharge approximately 40 J through an internal pacing wire embedded in the right ventricle. Although most patients with an implanted defibrillator remain conscious during defibrillation, CPR should be commenced if the patient fails to respond to the ICD counter shocks and becomes unconscious. In such cases, any intermittent firing of the implanted defibrillator presents no additional risk to bystanders or medical personnel. However, wearing gloves and minimizing contact with the patient while the device is discharging is prudent.
Basic Life Support (BLS) summary
The five links in the ‘Chain of Survival’ BLS for a patient with sudden cardiac arrest include the following:
 Immediate recognition of the emergency and activation of help/the EMS system
Immediate recognition of the emergency and activation of help/the EMS system
 Early CPR with an emphasis on chest compressions
Early CPR with an emphasis on chest compressions
 Earliest use of defibrillation
Earliest use of defibrillation
Cardiac arrest may be presumed if the adult victim is unresponsive and not breathing normally (ignoring occasional gasps) without assessing for a pulse. A trained rescuer may open the airway using the head tilt–chin lift manoeuvre as part of the breathing assessment, but the lay/untrained rescuer should waste no time to initiate chest compressions. Rescuers should activate help/the EMS system and start chest compressions immediately. If a lone healthcare rescuer responds to suspected asphyxia or respiratory-related cardiac arrest (e.g. immersion or drowning), it is still reasonable for the healthcare rescuer to provide 2 minutes CPR before leaving the victim alone to activate EMS.
All rescuers, whether trained or not, should at least provide chest compressions to a victim of cardiac arrest, with a strong emphasis on delivering high quality chest compressions. Trained rescuers should also provide 2 rescue breath ventilations after each 30 chest compressions at a ratio of 30:2, that is to deliver 5 cycles each 2 minutes. The compression rate should be at least 100 per minute and a depth of at least 5 cm (or 2 inches). All BLS guidelines encourage the use of an SAED by lay rescuers in cardiac arrest, maintaining chest compressions while charging the defibrillator to minimize any pre-shock pause.
Basic Life Support care should be continued until advanced help arrives, the victim starts to wake or the rescuer becomes exhausted and the situation is considered hopeless.
1.2 Advanced Life Support
John E Maguire
Introduction
A patient in cardiac arrest is the most time-critical medical crisis an emergency physician manages. The interventions of Basic Life Support (BLS) and Advanced Life Support (ALS) have the highest probability of success when applied immediately, become less effective with the passage of time and, after only a short interval without treatment, are ineffectual [1,2].
Larsen et al., in 1993, calculated the time intervals from collapse to the initiation of BLS, defibrillation and other ALS treatments and analysed their effect on survival from out-of-hospital cardiac arrest [3]. When all three interventions were immediately available, the survival rate was 67%. This figure declined by 2.3% per minute of delay to BLS, by a further 1.1% per minute of delay to defibrillation and by 2.1% per minute to other ALS interventions. Without treatment, the decline in survival rate is the sum of the three, or 5.5% per minute.
Chain of Survival
The importance of rapid treatment for cardiac arrest led to the development of a systems management approach, represented by the concept of a ‘Chain of Survival’, which has become the accepted model for emergency medical services (EMS) [4]. The Chain of Survival concept implies that more people survive sudden cardiac arrest when a cluster or sequence of events is activated as rapidly as possible. This Chain of Survival sequence includes:
All the links in the chain must connect, as weakness in any one reduces the probability of patient survival. ALS involves the continuation of BLS as necessary, but with the addition of manual defibrillation, advanced invasive airway and vascular access techniques and the administration of pharmacological agents.
Aetiology and incidence of cardiac arrest
The commonest cause of sudden cardiac arrest in adults is ischaemic heart disease [1,2]. Other causes include respiratory failure, drug overdose, metabolic derangements, trauma, hypovolaemia, immersion and hypothermia.
The population incidence of sudden cardiac death (within 24 hours of the onset of any symptoms) is estimated as 1.24:1000/year in the USA [5]. The incidence of cardiac arrest notified to ambulances in western metropolitan Melbourne, Australia in 1995 was approximately 0.72:1000/year [6]. Among 20 communities in developed nations worldwide a population average of 0.62:1000/year received attempted resuscitation after out-of-hospital cardiac arrest [5].
Advanced Life Support guidelines and algorithms
The most clinically relevant advance in ALS, over the last two decades, is the substantial simplification of the management of cardiac arrest by the development of widely accepted universal guidelines and algorithms that include scientifically proven therapies.
International Liaison Committee on Resuscitation (ILCOR)
The American Heart Association, in collaboration with ILCOR, convened the International Guidelines 2000 Conference on CPR and Emergency Cardiac Care (ECC) in 2000. This was the first international assembly gathered specifically to produce international resuscitation guidelines and the International Guidelines 2000 for CPR and ECC were developed and then published [7]. These guidelines represented a consensus of expert individuals and resuscitation councils and organizations across many countries, cultures and disciplines. The underlying principle guiding decision making was that additions to existing guidelines had to pass a rigorous evidence-based review. Revisions or deletions occurred because of:
 lack of evidence to confirm effectiveness, and/or
lack of evidence to confirm effectiveness, and/or
 additional evidence to suggest harm or ineffectiveness, and/or
additional evidence to suggest harm or ineffectiveness, and/or
 evidence that superior therapies had become available [7].
evidence that superior therapies had become available [7].
The ILCOR member councils committed to holding an International Consensus Conference and releasing guidelines every 5 years to maintain and develop the level of scientific rigour and cooperation. The most recent conference was held in Dallas in February 2010 and the Consensus on Science and Treatment Recommendations (CoSTR) documents arising from that conference were published later that year [1].
Australasian guidelines and algorithms
Each ILCOR member body is expected to use the CoSTR documents to develop its own guidelines for local use. The Australian Resuscitation Council (ARC) and the New Zealand Resuscitation Council (NZRC) released joint Australasian guidelines in December 2010 [2]. These Australasian guidelines include an Adult Cardiorespiratory Arrest algorithm (Fig. 1.2.1) that is clear, concise and easy to memorize and adapt into poster format and is readily applied clinically. This algorithm provides the framework used throughout this chapter to discuss ALS interventions.
However, resuscitation knowledge is still incomplete and some ALS techniques currently in use are not supported by the highest levels of scientific rigour. Thus, strict adherence to any guideline should be informed by common sense. Individuals with specialist knowledge may modify practice according to the level of their expertise and the specific clinical situation or environment in which they practise.
Initiation of ALS
The Australasian guidelines and algorithm qualify the commencement of BLS with the statement ‘if appropriate’ [2]. This is because BLS is only a temporary and inefficient substitute for normal cardiorespiratory function. ALS interventions are almost always necessary to produce the return of spontaneous circulation (ROSC).
The purpose of BLS is to support the patient’s cardiorespiratory status as effectively as possible until equipment – particularly a defibrillator – and drugs become available [1,2]. Electrical defibrillation is fundamental to successful treatment for VF and pulseless VT. However, the likelihood of defibrillation restoring a sustained, perfusing cardiac rhythm and of a favourable long-term outcome is greatest for as little as 90 seconds after the onset of cardiac arrest. The chances of survival to hospital discharge decline rapidly thereafter, so minimizing time to defibrillation is the priority in resuscitation from sudden cardiac arrest. There is some evidence indicating that a brief period (1.5–3 min) of CPR before defibrillation may improve survival in patients where the EMS response is greater than 4–5 minutes, but this is not strong enough to make a practice recommendation.
The point of entry into the ALS algorithm depends on the circumstances of the cardiac arrest. In situations where there are multiple rescuers, BLS should be initiated or continued while the defibrillator-monitor is being prepared. When a defibrillator-monitor is readily available it should be obtained and attached immediately and a single rescuer should do this without commencing BLS. Diagnosis must be swift and the defibrillator attached without delay when the patient is being monitored at the time of a cardiac arrest [1,2].
Attachment of the defibrillator-monitor and rhythm recognition
Automated external defibrillator
Apply the self-adhesive pads in the standard anteroapical positions for defibrillation (see below) when using an automated external defibrillator (AED). An internal microprocessor analyses the ECG signal and, if VF/VT is detected, the AED displays a warning and then either delivers a shock (automatic) or advises the operator to do so (semiautomatic) [1,2,8,9].
Manual external defibrillator
The critical decision for a rescuer, after applying the self-adhesive pads or paddles of a manual external defibrillator, is whether or not the cardiac rhythm is VF/VT [1,2]. Up to 70% of patients with an out-of-hospital cardiac arrest will be in VF/VT at the time of arrival of the EMS personnel and a defibrillator-monitor [8]. The vast majority of cardiac arrest survivors come from this group [1,2,4].
Rhythm recognition
Ventricular fibrillation
VF is a pulseless, chaotic, disorganized rhythm characterized by an undulating, irregular pattern that varies in amplitude and morphology, with a ventricular waveform of more than 150/minute [1,2].
Pulseless ventricular tachycardia
Pulseless VT is characterized by broad, bizarrely shaped ventricular complexes associated with no detectable cardiac output. The rate is more than 100/minute by definition and is usually in excess of 150 [1,2].
Asystole
Asystole is identified by the absence of any electrical cardiac activity on the monitor. Occasionally, it is incorrectly diagnosed (‘apparent asystole’) on the ECG monitor because:
Pulseless electrical activity/electromechanical dissociation
The absence of a detectable cardiac output in the presence of a coordinated electrical rhythm is called pulseless electrical activity (PEA), also known as electromechanical dissociation (EMD) [1,2].
Defibrillation
The only proven effective treatment for VF and pulseless VT is electrical defibrillation [1,2,9]. The defibrillator must be brought immediately to the person in cardiac arrest and, if the rhythm is VF/VT, defibrillation delivered without delay.
Placement of pads or paddles
Pads or paddles are often identified as ‘sternum’ and ‘apex’, or ‘anterior’ and ‘posterior’, which is of no relevance for emergency transthoracic defibrillation. It simply allows detection by the pads/paddles of the correct orientation of certain perfusing cardiac rhythms prior to synchronized cardioversion [1,2,8–10].
Anteroapical pad or paddle position
There are two accepted positions for the defibrillation pads or paddles to optimize current delivery to the heart. The most common is the anteroapical position: one pad/paddle is placed to the right of the sternum just below the clavicle, and the other is centred lateral to the normal cardiac apex in the anterior or midaxillary line (V5–6 position).
Anteroposterior pad or paddle position
An alternative is the anteroposterior position: the anterior pad/paddle is placed over the praecordium or apex and the posterior pad/paddle is placed on the patient’s back to the left or right of the spine at the level of the lower scapula, or even in the interscapular region.
Do not attempt defibrillation over ECG electrodes or medicated patches and avoid placing pads/paddles over significant breast tissue in females. Also the pads/paddles should be placed at least 8 cm away from the module and pulse generator, if the patient has an implanted pacemaker or a cardioverter–defibrillator, respectively. Arrange to check the function of any pacemaker or cardioverter–defibrillator as soon as practicable after successful defibrillation [1,2,8,9].
Waveform and energy of shocks
Two main types of waveform are available from cardiac defibrillators.
Biphasic waveforms
All modern defibrillators use biphasic waveforms with impedance compensation now considered the ‘gold standard’. Biphasic (bidirectional) truncated transthoracic shock defibrillators are effective at lower energies and result in fewer post-defibrillation ECG abnormalities [1,2,8–10].
Set the level at 200 J for all shocks when using a biphasic defibrillator in adults. Other energy levels may be used if the relevant clinical data for that defibrillator suggest an alternative energy level provides comparable success to the ‘default’ energy level of 200 J [1,2].
Monophasic sinusoidal waveform
Old defibrillators use a damped monophasic sinusoidal waveform, which is a single pulse lasting for 3–4 ms. Set the energy level at the maximum when using a monophasic defibrillator in adults, which is usually 360 J for all shocks [1,2].
Optimizing transthoracic impedance
A critical myocardial mass must be depolarized synchronously for defibrillation to be successful. This interrupts the fibrillation and allows recapture by a single pacemaker. The transthoracic impedance must be minimized to maximize the probability of success [1,2,8–10].
Reduction of transthoracic impedance
 Use pads/paddles of 10–13 cm in diameter for adults. Smaller paddles/pads allow too concentrated a discharge of energy that may cause focal myocardial damage [8,9]. Larger pads/paddles do not make good chest contact over their entire area and/or may allow current to be conducted through non-myocardial tissue [8,10].
Use pads/paddles of 10–13 cm in diameter for adults. Smaller paddles/pads allow too concentrated a discharge of energy that may cause focal myocardial damage [8,9]. Larger pads/paddles do not make good chest contact over their entire area and/or may allow current to be conducted through non-myocardial tissue [8,10].
 Use conductive pads or electrode paste/gel. This reduces impedance by 30% [8]. Take care to ensure that there is no electrical contact between the pads or paddles, either directly or through electrode paste, as this results in current arcing across the chest wall [8–10].
Use conductive pads or electrode paste/gel. This reduces impedance by 30% [8]. Take care to ensure that there is no electrical contact between the pads or paddles, either directly or through electrode paste, as this results in current arcing across the chest wall [8–10].
 Apply a pressure of 5–8 kg to the paddle when adhesive pads are not being used [1,2,8].
Apply a pressure of 5–8 kg to the paddle when adhesive pads are not being used [1,2,8].
 Perform defibrillation when the chest is deflated, i.e. in expiration [10].
Perform defibrillation when the chest is deflated, i.e. in expiration [10].
Current-based defibrillation
Conventional defibrillators are designed to deliver a specified amount of energy measured in joules. Depolarization of myocardial tissue is accomplished by the passage of electrical current through the heart; clinical studies have determined that the optimal current is 30–40 amps (A) [8,10]. The current delivered at a fixed energy is inversely related to the transthoracic impedance, so a standard energy dose of 200 J delivers about 30 A to the average patient.
The current generated may be inadequate in patients with greater than average impedance, whereas patients with smaller transthoracic impedance may sustain myocardial damage from excessive current flow [8–10].
Some newer current-based defibrillators automatically measure transthoracic impedance and then predict and adjust the energy delivered to avoid an inappropriately high or low transmyocardial current. These devices have defibrillation success rates comparable to those of conventional defibrillators, while cumulatively delivering less energy. The reduced energy should result in less myocardial damage and may reduce post-defibrillation complications [8–10].
Automated external defibrillators (AED)
AEDs were first introduced in 1979 and have become standard equipment in EMS systems for use outside hospital, as well as in many areas within hospital.
AEDs are highly accurate, some models demonstrating 100% specificity and 90–92% sensitivity in correctly identifying coarse VF [9]. Their precision is less for fine VF and least for VT, but overall accuracy is comparable to that of an experienced cardiologist [8]. EMS systems equipped with AEDs are able to deliver the first shock up to 1 minute faster than when using a conventional defibrillator. Rates of survival to hospital discharge are equivalent to those achieved when more highly trained first responders use manual defibrillators [4].
The major advantage of AEDs over manual defibrillators is their simplicity, which reduces the time and expense of initial training and continuing education and increases the number of persons who can operate the device [4,8,9]. Members of the public have been trained to use AEDs in a variety of community settings and have demonstrated that they can retain skills for up to one year [4]. Encouraging results have been produced when AEDs have been placed with community responders, such as firefighters, police officers, casino staff, security guards at large public assemblies and public transport vehicle crews [4,8].
The Australasian College for Emergency Medicine recommends that all clinical staff in healthcare settings should have rapid access to an AED or a defibrillator with AED capability [11].
Delivering a shock
If the rhythm is assessed as shockable (VF or pulseless VT), the defibrillator should be charged while CPR continues. Then, after healthcare personnel are clear of the patient, a single shock is delivered [1,2]. Following this shock, CPR should be recommenced immediately, without delaying to assess or analyse either the pulse or the rhythm.
If the resuscitation team leader is uncertain whether the rhythm is shockable or non-shockable, no shock should be given.
Three stacked shocks
Previous ARC guidelines have recommended up to three stacked shocks as initial management when the arrest is witnessed and a defibrillator is immediately available. This is no longer included in the algorithm but can be considered if the delay before and between each shock will be less than 10 seconds, or during cardiac catheterization and/or early post-cardiac surgery.
The pads or paddles should remain on the chest wall and the defibrillator immediately recharged after each shock. The rhythm is checked while the defibrillator is recharging and the shock is repeated up to a total of three if VF/VT persists. Any delay over 10 seconds between shocks is unacceptable and should lead to abandonment of the stacked shocks and immediate commencement of CPR [2].
Technical problems
Whenever attempted defibrillation is not accompanied by skeletal muscle contraction, take care to ensure good contact and that the defibrillator is turned on, charged up, develops sufficient power and is not in synchronized mode [1,2,9]. The operational status of defibrillators should be checked regularly and a standby machine should be available at all times. The majority of defibrillator problems are due to operator error or faulty care and maintenance.
Complications of defibrillation
 Myocardial injury and post-defibrillation dysrhythmias may occur with cumulative high-energy shocks.
Myocardial injury and post-defibrillation dysrhythmias may occur with cumulative high-energy shocks.
 Skeletal muscle injury or thoracic vertebral fractures are possible, albeit rare.
Skeletal muscle injury or thoracic vertebral fractures are possible, albeit rare.
 Also ensure that the patient, rescuers and equipment are dry before defibrillation is ever attempted in wet conditions, such as outdoors or around a swimming pool area [1,2,8,9].
Also ensure that the patient, rescuers and equipment are dry before defibrillation is ever attempted in wet conditions, such as outdoors or around a swimming pool area [1,2,8,9].
CPR ‘Code Blue’ process
Shockable rhythms
Immediate defibrillation is essential for VF/pulseless VT, although periods of well-performed CPR help maintain myocardial and cerebral viability and may improve the likelihood of success with subsequent shocks [1,2]. After delivering a single shock, CPR should be resumed immediately and continued for 2 minutes or until the patient becomes responsive or resumes normal breathing.
The rationale for this is that after one defibrillator shock there is typically a delay of several seconds before a diagnostic-quality ECG trace is obtained. Additionally, even when defibrillation is successful, there is temporary impairment of cardiac function from seconds to minutes, associated with a weak or impalpable pulse. Thus, waiting for a recognizable ECG rhythm or palpating for a pulse that may not be present even after successful defibrillation unnecessarily delays the recommencement of CPR. This is detrimental to the patient who does not yet have ROSC [1,2].
At the conclusion of this period of CPR, reassess the ECG rhythm and, when appropriate, the pulse. Give a single shock without delay if VF/pulseless VT persists [1,2].
Non-shockable rhythms
When PEA or asystole are present on ECG rhythm and/or pulse assessment, do not defibrillate as this may be deleterious. The prognosis for these conditions is much worse than for VF/VT and, unless there is potentially a reversible cause, the application of other ALS interventions (see below) is indicated, but seldom of value [1,2].
Cardiac pacing does not improve survival from asystole, either pre-hospital or in the emergency department (ED) setting [1,2].
Algorithm loops
Either continuously or during each 2-minute CPR cycle of the algorithm, give attention to the following [1,2]:
 administer 100% oxygen when available
administer 100% oxygen when available
 use waveform capnography to confirm airway placement and monitor the adequacy of CPR
use waveform capnography to confirm airway placement and monitor the adequacy of CPR
 administer adrenaline every second loop, i.e. every 4–5 minutes
administer adrenaline every second loop, i.e. every 4–5 minutes
 administer other drugs or electrolytes as indicated for individual circumstances
administer other drugs or electrolytes as indicated for individual circumstances
 correct potentially reversible conditions that may have precipitated the cardiac arrest and/or reduced the chances of successful resuscitation. These are listed in Figure 1.2.1 and are conveniently remembered under the headings of the ‘4Hs and 4Ts’.
correct potentially reversible conditions that may have precipitated the cardiac arrest and/or reduced the chances of successful resuscitation. These are listed in Figure 1.2.1 and are conveniently remembered under the headings of the ‘4Hs and 4Ts’.
‘4Hs’
‘4Ts’
Even the best-trained team will be unable to complete all of these management aspects within a single loop of the algorithm, but further opportunity will present if subsequent cycles are necessary [1,2].
Other ALS interventions
Not one ALS intervention other than defibrillation has been proven to improve patient outcome [1,2]. Some clinicians maintain that ALS has an incremental benefit compared to defibrillation alone [4], but although some data support this, it remains impossible to prove.
Advanced airway management
Endotracheal intubation is considered the technique of choice for airway management during cardiac arrest and is recommended in the Australasian guidelines [2]. However, no randomized controlled study exists that shows an improved outcome with endotracheal intubation compared to basic airway management [1,2]. Other alternative advanced airway devices studied during CPR include the laryngeal mask airway (LMA), and the oesophageal–tracheal combitube (Combitube). None is definitely superior to basic airway management during cardiac arrest in terms of consistently improved survival [1,2].
Endotracheal intubation
The optimal technique for airway management depends on the equipment available, the circumstances of the cardiac arrest and the training and experience of the resuscitation team [1,2]. A self-inflating bag/valve/mask system and/or an airway intubation device remain the mainstay of advanced airway and ventilation management in ALS.
When a sufficiently experienced person is available tracheal intubation should be performed, providing it does not interfere with or impede the CPR process. Laryngoscopy should be carried out during chest compressions with a strong recommendation that only a short interruption in chest compressions, not exceeding 20 seconds, should be permitted when the tracheal tube is inserted between the cords.
Once the tube has been inserted, correct placement must be verified by seeing the tube pass between the cords, clinical observation of chest rise/fall and auscultation and, importantly, an exhaled carbon dioxide detector, such as a waveform capnograph.
The main benefit of an advanced airway, such as endotracheal intubation, is that no interruption to chest compressions is then necessary for ventilations during CPR. Also, an endotracheal tube isolates and protects the airway, allows suction and facilitates ventilation [1,2].
Ventilation and oxygenation
Cardiac arrest and CPR cause an increase in dead space and a reduction in lung compliance that compromise gas exchange. Therefore, a fractional inspired oxygen concentration (FIO2) of 1.0 (100% oxygen delivery system) is essential in cardiac arrest to maximize oxygen delivery [1,2].
Minute volume
Carbon dioxide (CO2) production and delivery to the pulmonary circulation are limited by the markedly reduced cardiac output achieved during CPR. As a consequence, a relatively low minute volume of 3.5–5.0 L is sufficient to achieve adequate CO2 excretion and prevent hypercapnia. This situation will be altered if a CO2-producing buffer, such as sodium bicarbonate, is administered. A small increase in minute ventilation is then required to prevent the development of a respiratory acidosis [1,2].
Ventilation rate and tidal volume
A ventilation rate of 8–10 per minute without pausing during chest compressions and a tidal volume of 400–500 mL (5–6 mL/kg) are sufficient to clear CO2 during most cardiac arrest situations when an advanced airway is in place. This should cause a visible rise and fall of the patient’s chest [1,2].
Vascular access and drug delivery
Intravenous route
The ideal route of drug delivery should combine rapid and easy vascular access with quick delivery to the central circulation. The intravenous (IV) route is preferred. This is most easily performed by inserting a cannula into a large vein in the upper limb or into the external jugular vein. Avoid lower limb veins because of their poor venous return from below the diaphragm during CPR, as well as immediate or inexperienced central line insertion which can have fatal consequences, such as pneumothorax or arterial laceration.
Drug delivery
Give a 20–30 mL IV fluid flush following any drug administered and/or raise the limb to facilitate delivery to the central circulation [1,2]. A central venous cannula delivers drugs rapidly to the central circulation and should be used when already in place. Otherwise, their insertion during CPR requires time and technical proficiency and interferes with defibrillation and the CPR process, which is unacceptable [1,2].
Intraosseous (IO) route
The intraosseous (IO) route is also acceptable for drug delivery in adults as well as children [1,2]. Suitable sites of insertion include above the medial malleolus or the proximal tibia. Practice is needed to perfect the technique, usually with a semiautomatic, hand-held drill device.
Intratracheal route
The intratracheal instillation of drugs is an alternative during CPR, especially when tracheal intubation precedes venous access. Adrenaline, lignocaine and atropine may be safely administered through the endotracheal tube if there is a delay in achieving vascular access, although their efficacy is unproven (as it is for all ALS drugs by any route).
The ideal dose and dilution of drugs given by this route are unknown, but using 3–10 times the standard IV drug dose diluted in 10 mL of water or normal saline is recommended. The drug should be delivered via a catheter or quill placed beyond the tip of the endotracheal tube and followed by ventilations to aid dispersion [1,2].
Fluid therapy
Crystalloid solutions are used for the IV delivery of drugs during CPR. Glucose-containing solutions are avoided during CPR as they may contribute to post-arrest hyperglycaemia, which reduces or impairs cerebral recovery [1,2].
Drug therapy in ALS
Not one drug used in resuscitation has been shown to improve long-term survival in humans after cardiac arrest [1,2]. Despite this, a number of agents are employed based on theoretical, retrospective or anecdotal evidence of their efficacy [1,2].
Adrenaline (epinephrine)
The putative beneficial actions of adrenaline in cardiac arrest relate to its α-adrenergic effects, which result in an increased aortic blood pressure with increased perfusion of the cerebral and coronary vascular beds and reduced blood flow to splanchnic and limb vessels. Adrenaline is considered the ‘standard’ vasopressor in cardiac arrest [1,2].
Indications
Adverse effects
Dosage
The standard adult dose is 1 mg IV every 4–5 minutes. Higher doses have not been shown to improve long-term outcome.
Amiodarone
Amiodarone has some benefit in refractory VF/VT in the setting of out-of-hospital cardiac arrest [1,2]. Additionally, studies show an improvement in defibrillation response when amiodarone is given in VF or haemodynamically unstable VT.
Indications
Adverse effects
Dosage
The initial bolus of amiodarone is 300 mg or 5 mg/kg, followed by a further 150 mg if necessary.
Atropine
Atropine has no consistent benefits in cardiac arrest and is no longer recommended for routine use in asystole/PEA [1,2].
Calcium
Calcium is only indicated when the cardiac arrest is caused or exacerbated by the conditions listed below [1,2].
Indications
Adverse effects
Dosage
The initial dose is 5–10 mL of 10% calcium chloride, or 15–30 mL of 10% calcium gluconate (three times the volume of calcium chloride for the equivalent cation dose).
Lignocaine (lidocaine)
The antiarrhythmic properties of lignocaine in cardiac arrest are inconsistent [1,2]. Its continued use is based purely on familiarity and historical precedent. The role of lignocaine in the prophylaxis of VF/VT is also unclear and at best equivocal.
Indications
Adverse effects
Dosage
The initial dose is 1–1.5 mg/kg, with an additional bolus of 0.5 mg/kg after 5–10 minutes if indicated.
Magnesium
Magnesium is indicated when the cardiac arrest is caused or exacerbated by the conditions listed below [1,2]. There is no support for routine use at present.
Indications
Adverse effects
Dosage
The initial dose is a 5 mmol bolus (1.25 g or 2.5 mL of a 49.3% solution) repeated if indicated and followed by an infusion of 20 mmol (5 g or 10 mL of a 49.3% solution) over 4 h.
Potassium
Potassium is only indicated when the cardiac arrest is caused or exacerbated by the condition listed below [1,2]. There is no support for its routine use in cardiac arrest.
Indication
Adverse effects
Dosage
A bolus of 5 mmol of potassium is given IV.
Sodium bicarbonate
Sodium bicarbonate is only indicated when the cardiac arrest is caused or exacerbated by the conditions listed below [1,2]. There is no support for its routine use in cardiac arrest.
Indications
Adverse effects
Dosage
The initial dose is 1 mmol/kg (1 mL/kg of 8.4% sodium bicarbonate) over 2-3 minutes, then as guided by the arterial blood gases.
Vasopressin
Vasopressin is an alternative vasopressor to adrenaline. There is currently insufficient evidence to support or refute its use either alone or in combination with adrenaline in any cardiac arrest rhythm [1,2].
Consider administration for:
Adverse effects
Dosage
The dose is a single IV bolus of 40 U administered once during the episode of cardiac arrest.
Haemodynamic monitoring during CPR
End-tidal CO2 (ETCO2)
Animal and clinical studies indicate that measuring ETCO2 is effective and informative for determining progress during CPR, particularly if there is ROSC [1,2].
ETCO2 typically falls to less than 10 mmHg at the onset of cardiac arrest. It can rise to between one-quarter and one-third of the normal level with effective CPR, and rises to normal or supranormal levels over the next minute following ROSC. The changes in ETCO2 parallel similar proportionate increases in cardiac output.
Changes in ETCO2
An ETCO2 of less than 10 mmHg during attempted resuscitation from cardiac arrest is an indication of ineffective CPR. This may be as a result of inadequate ventilation due to airway obstruction or even oesophageal intubation; or due to a minimal cardiac output because of poor technique or underlying causes, such as hypovolaemia, pulmonary embolism or pericardial tamponade (part of the 4Hs and 4Ts). Conversely, a sharp rise in ETCO2 may be the first indication of ROSC.
ETCO2 may also have a prognostic value, as patients who are eventually successfully resuscitated have higher ETCO2 values during CPR than those who never have ROSC. Exercise caution when interpreting ETCO2 following the administration of adrenaline, as this causes a decrease in ETCO2 which does not necessarily indicate a poorer prognosis, unless it simply reflects a prolonged resuscitation attempt.
Australasian guidelines advise that ETCO2 monitoring is a safe and effective non-invasive indicator of cardiac output during CPR and is an early indicator of ROSC in an intubated patient.
Arterial blood gases
Arterial blood gas (ABG) monitoring during cardiac arrest is used as an indicator of oxygenation and the adequacy of ventilation, but is not an accurate measure of tissue acidosis [1,2]. An increase in PaCO2 may indicate improved tissue perfusion during CPR or with ROSC, if ventilation is constant. The measurement of ABGs should never interfere with the overall performance of good CPR.
Post-resuscitation care
This is covered in detail elsewhere but is mentioned here as successful ROSC is only the first step in recovery from cardiac arrest [1,2]. The post-cardiac arrest syndrome comprising brain injury, myocardial dysfunction, a systemic ischaemia/reperfusion response and persistence of the causative pathology often complicate the post-resuscitation phase. Among patients surviving to ICU admission but subsequently dying in hospital, brain injury is the cause of death in 68% after out-of-hospital cardiac arrest and in 23% after in-hospital cardiac arrest.
Implementation of a comprehensive, structured post-resuscitation treatment protocol may improve survival in cardiac arrest victims after ROSC. The most important elements of such a protocol are summarized in the algorithm for adult ALS management (see Fig 1.2.1):
When to discontinue ALS
The vast majority of patients who survive out-of-hospital cardiac arrest have ROSC before arrival at the ED. Only 33 of 5444 patients (0.6%) in 18 studies between 1981 and 1995, who were transported to an ED still in cardiac arrest after unsuccessful pre-hospital resuscitation survived to hospital discharge [12]. Twenty-four of the surviving patients arrived in the ED in VF and 11 of these had their initial cardiac arrest in the ambulance en route to hospital or had temporary ROSC before arrival. Thus, virtually every patient arriving in an ED still in asystole from out-of-hospital cardiac arrest will die without leaving hospital.
Ceasing CPR pre-hospital
A recommendation made in 1993 for out-of-hospital cardiac arrest in the normothermic patient was that resuscitation should cease if there was no ROSC after 25 minutes of ALS [13]. Two important exceptions to this guide are:
These recommendations were applied and considered valid in a prospective study in that same year [14].
In-hospital cardiac arrest outcome
There are no early absolute predictors of futility in the resuscitation of patients with in-hospital cardiac arrest. Some variables are, however, associated with a greater or lesser chance of survival to discharge. Better outcomes are linked to ventricular tachyarrhythmias, the commencement of resuscitation within 5 minutes of collapse and ROSC within 15 minutes of CPR.
In-hospital cardiac arrest with a poor outcome
A poor outcome is linked to pre-existing conditions, such as cardiogenic shock, metastatic cancer, renal failure, sepsis and an acute cerebrovascular accident. Age alone is not an independent predictor of outcome, either in hospital or for out-of-hospital cardiac arrest [15,16].
Outcome of prolonged ALS
ALS resuscitation efforts lasting more than 30 minutes without ROSC at any stage are so uniformly unsuccessful that resuscitation should be abandoned; except in certain special circumstances, such as hypothermia, possibly some drug overdoses and following thrombolysis in suspected massive pulmonary embolism (PE) [16]. The return of spontaneous circulation at any time during the resuscitation process resets the clock time to zero [1,2,16].
Prognosis for survival after cardiac arrest
The best prospect of neurologically intact long-term survival after a cardiac arrest occurs when:
Out-of-hospital cardiac arrest
Some variation in survival after an out-of-hospital cardiac arrest is due to differences in EMS systems, as well as differing research methodology and data reporting. A 1996 meta-analysis of 36 articles published between 1973 and 1992 from 41 EMS systems in six countries showed survival varied from 0 to 21%, with an overall mean survival of 8% [17].
In-hospital cardiac arrest
The prognosis for survival from in-hospital cardiac arrest is only marginally better, with survival to discharge averaging 13.8% of 12 961 patients in reports published between 1961 and 1984 [15]. However, in a further seven reports published between 1978 and 1989 this dropped to 11% of 1804 patients [16].