History
Comments
Current Medications
Comments
Current Symptoms
Physical Examination
Comments
Laboratory Data
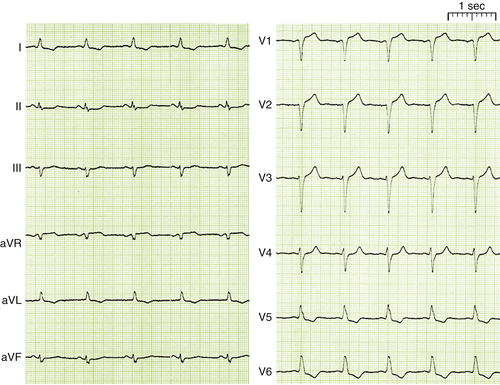
FIGURE 1-1 12 Lead ECG at admission showing sinus rhythm with a heart rate of 68 bpm and LBBB.
Comments
Electrocardiogram
Findings
Comments
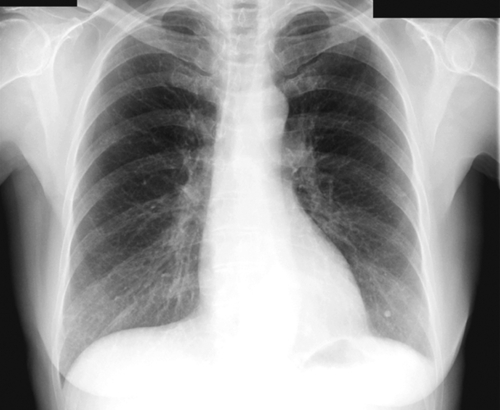
FIGURE 1-2 Chest radiograph at admission.
Chest Radiograph
Findings
Comments
Echocardiogram
Findings
Comments
Catheterization
Hemodynamics
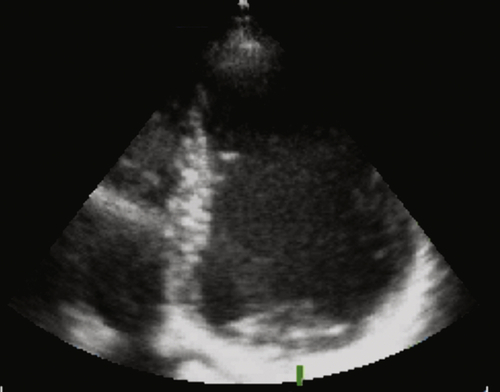
FIGURE 1-3 Echocardiography with an apical four-chamber view showing a severe dilatation of the left ventricle, highly reduced left ventricular function, and signs of dyssynchrony.
Findings
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
Final Diagnosis
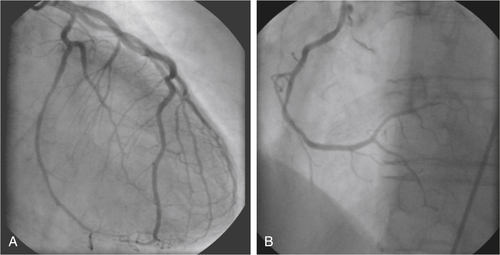
FIGURE 1-4 Coronary angiography of left (A) and right (B) coronary artery in a right anterior oblique 30-degree view (A) and a left anterior oblique 60-degree cranial 30-degree view (B).
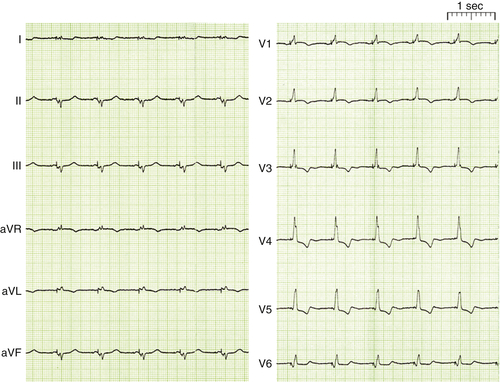
FIGURE 1-5 12-Lead electrocardiogram after implantation of a cardiac resynchronization therapy–implantable cardioverter-defibrillator device in sinus rhythm.
Plan of Action
Intervention
Postimplant Electrocardiogram
Findings
Comments
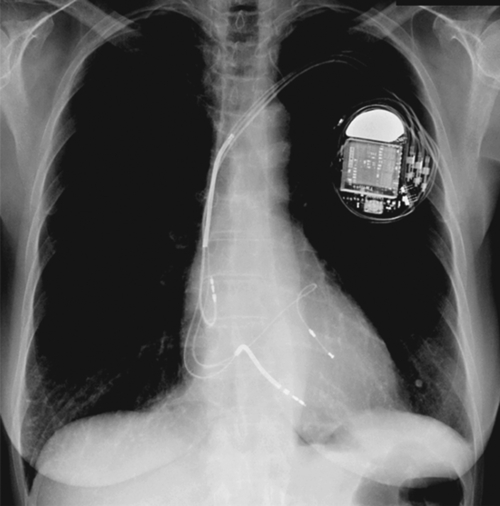
FIGURE 1-6 Chest radiograph after implantation of a CRT-ICD device showing the device, a bipolar atrial lead, a dual-coil right ventricular lead, and a bipolar left ventricular lead in a lateral branch of the great cardiac vein.
Postimplant Chest Radiograph
Findings
Comments
Echocardiogram (8 Weeks After Implantation)
Findings
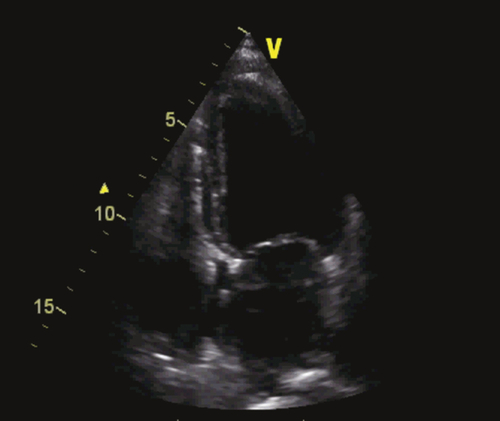
FIGURE 1-7 Echocardiography with apical four-chamber view 8 weeks after implantation with reduced left ventricular end-diastolic and end-systolic and slightly improved ejection fraction.
Comments
Outcome
Course
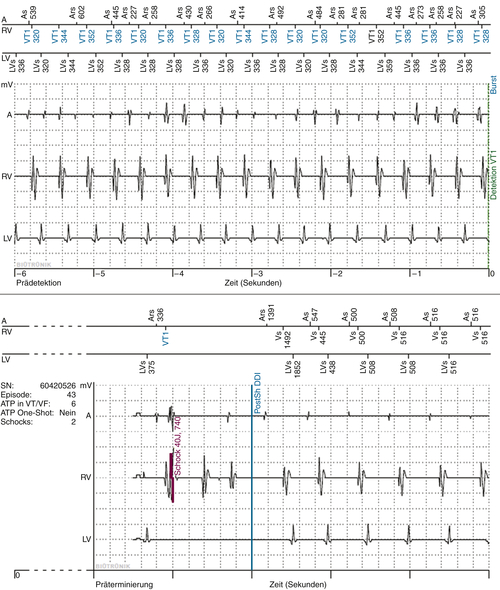
FIGURE 1-8 Intracardiac electrogram (EGM) of the atrial fibrillation episode via remote monitoring. A, Atrial EGM; Ars, atrial sense in refractory period; AS, atrial sense; LV, left ventricular EGM; LVS, left ventricular sense; RV, right ventricular EGM; VT1, right ventricular sense in VT1-zone; Zeit, time in seconds. After shock (40J) sinusrhythm was reestablished.
Findings
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Plan of Action
Intervention
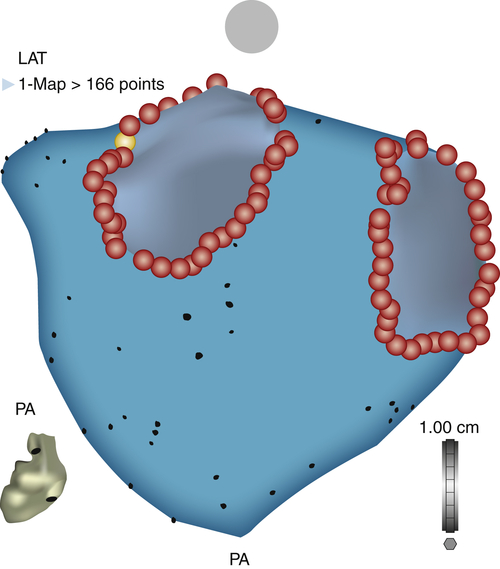
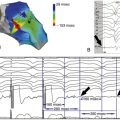
FIGURE 1-9 Posterior-anterior view of a three-dimensional CARTO reconstruction of the left atrium and circumferential ablation lines around the ipsilateral pulmonary veins.
Outcome
Selected References
1. Calkins H., Kuck K.H., Cappato R. et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design—a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2012;9:632–696.
2. Dickstein K., Vardas P.E., Auricchio A. et al. ESC Committee for Practice Guidelines: 2010 focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy—developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12:1526–1536.
3. Ouyang F., Bänsch D., Ernst S. et al. Complete isolation of the left atrium surrounding the pulmonary veins: new insights from the double-lasso technique in paroxysmal atrial fibrillation. Circulation. 2004;110:2090–2096.
4. Poole J.E., Johnson G.W., Hellkamp A.S. et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017.
5. Santini M., Gasparini M., Landolina M. et al. Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;7:167–172.
6. Wilton S.B., Leung A.A., Ghali W.A. et al. Outcomes of cardiac resynchronization therapy in patients with versus those without atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2011;8:1088–1094.