Chapter 59 Vascular Infection
Primary Arterial Infections
The first published experience with primary arterial infection is generally credited to Koch, who reported a patient with a ruptured superior mesenteric artery (SMA) aneurysm in 1851.1 Arterial infections were not well appreciated before this time because the basic tenets of bacteriology and infection were not yet developed. Breakthroughs in human bacteriological research by Pasteur and others in the mid-19th century set the stage for a basic understanding of the pathophysiology and classification of vascular infections.
Classification and Etiology
Nearly all primary arterial infections result in the formation of aneurysms or pseudoaneurysms. Osler coined the term mycotic aneurysm in 1885 to describe infected aortic arch aneurysms containing “fresh fungal vegetation” in a patient who had concomitant aortic valve vegetations.2 While “mycotic” aneurysms apply only to the subset of individuals with infected aneurysms caused by septic emboli, the term has been loosely applied to include all infected aneurysms, regardless of etiology. This practice is confusing and prevents meaningful comparison of the numerous small series of infected aneurysms reported in the modern literature.
The most widely accepted classification of arterial infections was introduced by Wilson et al. in 1978.3 In keeping with the traditional definition introduced by Osler, the authors classified a mycotic aneurysm as one that occurred in an otherwise normal, nonaneurysmal artery as a result of septic emboli of endocardial origin. A preestablished aneurysm that became infected as a result of bacteremia is classified as an infected aneurysm. Microbial arteritis refers to infection of a normal or atherosclerotic (i.e., nonaneurysmal) artery that has become infected as a result of bacteremia. This most often results in rupture with formation of a pseudoaneurysm. Traumatic infected aneurysms include infected aneurysms due to trauma or iatrogenic injury (e.g., complications of arteriography). Contiguous arterial infection is due to direct extension of an adjacent infection into the wall of the artery, such as infected aortitis associated with vertebral osteomyelitis. The specific classification based on etiology will be acknowledged wherever appropriate in this chapter. However, for purposes of clarity and simplification, the generic term infected aneurysm will be applied to include all arterial infections discussed.
The etiology of infected aneurysms has changed in the past 150 years. In the pre-antibiotic era, 86% of patients with arterial infections had evidence of endocarditis.4 Following widespread use of antibiotics, the incidence of infective endocarditis has relegated the true mycotic aneurysm to a rare entity. In 1984, Brown and associates reported a collective series of infected aneurysms based on a search of the English literature.5 The etiology could be determined in 75% of the 180 subjects. The authors separated the reported experience into cases occurring before 1965 and those occurring since that time. Accuracy of the results suffers because of important differences in diagnosis and reporting frequency between the two periods. Nevertheless, this collective experience remains the largest comparative analysis of infected aneurysms to date. In the earlier part of the series, endocarditis was still the leading cause of infected aneurysms, but arterial trauma of all types became the leading cause after 1965. The authors attributed this etiological shift to a substantial change in the pattern of antibiotic use for treatment of sepsis and trauma.5 Other explanations include an increased prevalence of intravenous drug abuse and the widespread application of transarterial interventional procedures. The increase in interventional procedures was particularly notable after the pioneering work of Grunzig and others in the 1970s. The enthusiasm for endovascular technology seen in recent years suggests that arterial trauma may soon become an even more important cause of arterial infection.
Pathogenesis of Infected Aneurysms
The basic mechanisms leading to formation of infected aneurysms have been studied most extensively in the abdominal aorta. Chronic uninfected abdominal aortic aneurysms (AAAs) are thought to form as a result of both destruction of medial elastin and adventitial collagen (see Chapter 37).6 Elastolytic matrix metalloproteinases (MMPs) play a central role, leading to initial aortic dilation.6 Collagenase MMP leads to collagen failure that eventually leads to gross enlargement and eventual rupture of the AAA. The extensive transmural infiltration typically seen in noninfected AAAs suggests that inflammation and immune responses play a significant role in AAA formation.7 Interestingly, infectious causes have also been postulated: histochemical studies have demonstrated that as many as 55% of AAAs harbor Chlamydia pneumoniae.7 Thus, it appears that infected and noninfected AAAs may represent two ends of the same spectrum, with progression to infection depending on organism virulence and host resistance.
A number of important clinical differences have been noted between infected and uninfected aneurysms of the abdominal aorta. In contrast to chronic AAA, infected AAAs follow a more rapid course, have a predilection for the suprarenal aorta, and may be isolated to a small segment of an otherwise normal aorta. Recent studies have revealed a number of interesting findings related to the etiology of infected AAA. First, Buckmaster et al. have shown that elastolytic activity is derived from host leukocytes, not from the infectious organisms.8 However, infectious organisms play a central role in collagen degradation. Many bacterial isolates produce collagenases,9 and bacteria are capable of activating the collagenase promoter in macrophage-like cells.10 Furthermore, a variety of bacterial proteases activate MMP-1, MMP-8, and MMP-9.9 Therefore, infected AAAs appear be the consequence of bacterial proteases causing rapid collagen breakdown in a previously normal aorta.9 The collagenase activity may be relatively localized, leading to formation of a saccular AAA or pseudoaneurysm in an otherwise normal appearing vessel. Collagenase activity may also be intensive, which may explain the rapid course associated with infected AAAs. The reason infected AAAs are predisposed to the suprarenal aorta has not been elucidated.
Pathological findings of infected aortic aneurysms have been described by Hsu and Lin.11 Typical findings included aortic atherosclerosis, acute suppurative inflammation, neutrophil infiltration, and bacterial clumps. Two thirds of patients in their series showed acute inflammation superimposed on severe chronic atherosclerosis; the remainder showed atherosclerosis with chronic inflammation or pseudoaneurysms.
Anatomical Location
In their classic 1984 study, Brown and associates documented 243 infected aneurysms in 180 patients in the following distribution: 38% femoral artery, 31% abdominal aorta, 8% SMA, 7% brachial artery, 6% iliac artery, 5% carotid artery, 3% ulnar/radial arteries, 1% hepatic artery, less than 1% subclavian artery, and less than 1% popliteal artery.5 Notably, there were no cases involving the suprarenal or thoracoabdominal aorta in this series. More recent reports have documented a much higher prevalence of infected aortic aneurysms involving the segments proximal to the renal arteries,12–15 suggesting the aorta is the most frequent site of involvement.
Bacteriology
Approximately 75% of clinically infected aneurysms are associated with positive culture results.5 There have been significant shifts in the bacteriological patterns of infected aneurysms that have paralleled the changes in etiology. Prior to 1960, gram-positive organisms predominated, particularly Streptococcus pneumoniae, Streptococcus, and Staphylococcus aureus.3 More recent series have reported a higher prevalence of gram-negative organisms, paralleling the increasing number of arterial infections due to bacteremia, particularly Salmonella species.13,16 Gram-negative sepsis in elderly patients is a frequent clinical scenario in these circumstances.17 It appears that the bacteriological pattern is continuing to evolve: several reports have identified S. pneumoniae as an increasingly frequent cause of infected aortic aneurysms.14,18,19 The prevalence of organisms associated with opportunistic infections such as fungus and Mycobacterium species may also be on the rise owing to the increasing prevalence of chronic diseases associated with impaired immunity.
Salmonella infections deserve special emphasis. For uncertain reasons, Salmonella organisms have a tendency to infect the abdominal aorta. Up to 36% of all infected aneurysms of the aorta are due to Salmonella species; conversely, 65% of Salmonella vascular infections are localized to the aorta.20 Humans become infected by ingestion of contaminated food or water. Of the patients who develop Salmonella gastroenteritis, a small proportion will develop bacteremia that may result in extraintestinal seeding and infection. The interval between the onset of gastrointestinal symptoms and development of aortic infection may be several weeks.20 Diagnosis can be difficult because the signs and symptoms are nonspecific: more than half of patients have ruptured aneurysms before the diagnosis is made.20 Common isolates from aortic wall specimens have included Salmonella choleraesuis, Salmonella typhimurium, and Salmonella enteritidis. Salmonella aortitis is an extremely morbid condition that has historically been associated with mortality rates of 50% and a high rate of reinfection after revascularization.20 However, this trend appears to be reversing: in a more recent series, Hsu et al. documented an 11% mortality rate and showed that Salmonella was a predictor of survival compared to other microorganisms.21 Much of this effect may have been due to the fact that the Salmonella infected patients in this series were significantly younger than patients who died with other infections.
Syphilitic aneurysms, once a common cause of death due to aortic arch rupture, are now very rare. In a review of the literature on infected aortic aneurysms, Leon and Mills reported that syphilitic aneurysms occur most commonly in the ascending and arch aorta; they are uncommon below the sixth vertebral body.22 These aneurysms result from the intense inflammatory response associated with the treponemes that lodge in vasa vasorum.
Infected Aortic Aneurysms
The definition of aortic aneurysm infection has been confounded by the fact that 10% to 15% of patients with uninfected chronic AAA will have positive culture results from intraluminal thrombus removed at the time of aneurysm repair. Fortunately, positive culture results are not associated with an increased risk of late graft infection.23,24 However, this underscores the fact that the definition of infected AAA relies on other criteria such as operative findings (inflammation and purulence), clinical symptoms (fever, pain, leukocytosis), aneurysm architecture (saccular or localized), and positive aneurysm wall culture.13
Modern series of infected aortic aneurysms reveal that the majority of affected patients have comorbid conditions associated with immunosuppression such as diabetes, chronic renal failure, chronic steroid use, human immunodeficiency virus (HIV), or cancer.13,25 Nearly 50% of patients have had a recent documented infection such as pneumonia or urinary tract infection,13 and several reports have documented direct extension of vertebral osteomyelitis.14,26,27
Features
Infected aneurysms of the aorta may involve any segment from the ascending arch to the distal infrarenal area. In a series of 43 patients with infected aortic aneurysms treated at the Mayo Clinic over a 25-year period, Oderich et al. documented a wide distribution of lesions.13 No segment of the aorta was spared. As can be seen in Figure 59-1, 40% of infected aneurysms were localized to the infrarenal aorta, and the remaining lesions were almost evenly distributed in the juxta/pararenal, paravisceral, thoracoabdominal, and descending thoracic segments. A similar distribution has been observed by others.14,28,30
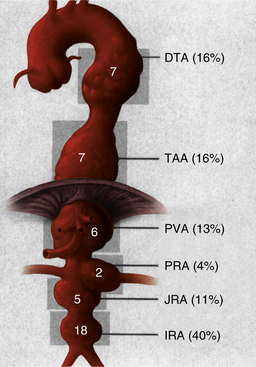
Figure 59-1 Distribution of infected aortic aneurysms in a series of 43 patients treated at the Mayo Clinic.
(From Oderich GS, Panneton JM, Bower TC, et al: Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg 34:900, 2001, with permission from the Society for Vascular Surgery and the American Association for Vascular Surgery.)13
The majority of aortic infections can be classified as bacterial aortitis.11 Most lesions are saccular and well localized in an otherwise normal-appearing aorta (Fig. 59-2). This appearance is highly suggestive of a ruptured aortic pseudoaneurysm, which is pathognomonic for aortic infection. In a minority of cases, infection of a preexisting aortic aneurysm may make diagnosis difficult. In these circumstances, suspicion of infection is completely reliant on clinical information.
Diagnosis
The majority of patients with infected aortic aneurysms are symptomatic. In the series by Oderich et al., 93% of patients had symptoms, the most common of which were fever, back pain, and leukocytosis (Table 59-1).13 Blood cultures are positive in approximately 75% of cases.13 The degree of symptomatology may be an important indicator of prognosis. Recent data suggest that the systemic inflammatory response syndrome (SIRS), a marker for sepsis, is associated with increased morbidity and mortality in patients with infected aortic aneurysms.14,29 Diagnostic criteria for SIRS include the presence of two or more of the following: body temperature above 38 °C or below 36 °C; heart rate over 90 beats/min; respiratory rate over 20 breaths/min or a partial pressure of carbon dioxide in arterial blood (PaCO2) less than 32 torr; and white blood cell (WBC) over 12,000 cells/mm3, less than 4000 cells/ mm3, or 10% immature (band) forms.29
Table 59-1 Clinical Presentation and Laboratory Findings in 43 Patients Treated at the Mayo Clinic for Infected Aortic Aneurysms
| No. Patients (%) | |
|---|---|
| Symptomatic | 40 (93) |
| Fever | 33 (77) |
| Pain (abdominal or back) | 28 (65) |
| Leukocyte count >12,000/mm3 | 23 (54) |
| Chills | 22 (51) |
| Sweats | 12 (28) |
| Enlarging aneurysm | 12 (28) |
| Nausea/vomiting or diarrhea | 10 (25) |
| Pulsatile mass | 7 (16) |
| Hemodynamic instability | 3 (7) |
Adapted from Oderich GS, Panneton JM, Bower TC, et al: Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg 34:900, 2001.13
Diagnosis of infected aortic aneurysms relies on a number of imaging techniques. The presence of a saccular aneurysm in a patient with typical symptoms is pathognomonic, and the diagnosis is confirmed if blood cultures are positive. Gas bubbles and periaortic fat stranding on magnetic resonance imaging (MRI) or enhanced computed tomography (CT) scans are also diagnostic for infection (Fig. 59-3) but are not universally present. More subtle signs include periaortic stranding, proximity to abnormal fluid collections or nearby infections such as vertebral osteomyelitis, and rapid aneurysm expansion over several days.15 Indium-111-labeled WBC scans have been used in some patients, but the 80% sensitivity and specificity of this test suggest that it has limited usefulness. Angiography has been recommended to localize the infection and plan appropriate operative treatment in all patients. As an alternative, newer imaging techniques such as CT angiography (CTA) can be used to assess periaortic tissues, localize the infectious process, and evaluate vascular anatomy. This technique has the advantage of being minimally invasive compared to standard angiography, and it essentially combines two tests into one.
Treatment
Treatment of infected aortic aneurysms is surgical. Although treatment with broad-spectrum antibiotics may “sterilize” an infected aneurysm, the aneurysm still requires treatment because of the significant risk of rupture. In a series of 22 high-risk patients treated with antibiotics but without resection, Hsu et al. reported that 50% had in-hospital mortality, and 59% of those who survived to leave the hospital died of aneurysm rupture in late follow-up.30
The urgent nature of infected aortic aneurysms cannot be overstated because of the potential for rupture with exsanguination. In a recent series, nearly half of all patients with infected AAAs had already ruptured at the time of surgery, including free ruptures in 20%.31 Once the diagnosis is confirmed with appropriate imaging, preoperative preparation should be completed as rapidly as possible—preferably within 2 or 3 hours. Patients with hemodynamic instability should be transported immediately to the operating room. Stable patients should be admitted to an intensive care unit for rapid fluid repletion, institution of broad-spectrum intravenous antibiotics, and placement of appropriate monitoring devices. Blood should be typed and crossmatched, and at least four units of packed red blood cells should be available in the operating room.
Surgical treatment of infected aortic aneurysms depends on the location and extent of the infection. The most common operation is ligation and débridement of the infected arterial segment, with revascularization of the lower extremities using grafts brought through uninfected tissues remote from the infected site (extra-anatomical bypass).28,32 For example, infected aneurysms of the infrarenal aorta can be treated with ligation of the abdominal aorta distal to the renal arteries and revascularization of the lower extremities using axillofemoral bypass grafts. However, proximal aortic aneurysms involving the renal arteries, visceral aorta, or descending thoracic aorta are much more complicated. These aortic segments require direct revascularization to preserve blood flow to the kidneys, intraabdominal organs, and the spinal cord. In these cases, aortic débridement with direct revascularization of the affected segment within the infected bed (in situ reconstruction) is appropriate.
In situ reconstruction has been performed with prosthetic grafts, but the reinfection rate of up to 20% makes this option unattractive except in patients who are unstable at the time of operation.33,34 Prolonged administration of antibiotics is generally recommended in these patients.35 As an alternative, a rifampin-bonded gelatin-impregnated Dacron has been recommended in patients with arterial infections caused by susceptible organisms such as S. aureus36; however, these grafts are not effective against methicillin-resistant S. aureus or Escherichia coli infections.37 Additional alternatives include human allografts and autogenous vein grafts. The midterm results of cryopreserved human allografts are encouraging: Brown et al. reported a series of 52 patients with infected aortas or aortic grafts who underwent replacement with cryopreserved allografts.38 At 20 months follow-up, three patients had graft thrombosis or stenosis, and one developed a recurrent ilioenteric fistula; the remainder had no evidence of aneurysmal change or reinfection. The experience with autogenous superficial femoral-popliteal vein (SFPV) grafts is also encouraging. Several small series attest to the negligible reinfection rates and excellent durability associated with in situ SFPV reconstruction.14,39 The early enthusiasm for this option is buoyed by excellent experience using the SFPV to replace infected prosthetic grafts of the aorta (see later discussion).
The modern enthusiasm for endovascular therapy has extended to infected aortic aneurysms. Several small series have suggested that placement of endografts in combination with long-term antibiotics represent definitive treatment of infected aortic aneurysms with similar outcomes to open aortic repair.40,41 However, these data are countered by reports of a high rate of graft infection requiring removal, or extension of the aortic infection to more proximal segments.42,43 This appears to be a particular risk in patients with Salmonella infections. Therefore, we suggest that endovascular repair might be a good option to treat acute complications from infected aortic aneurysms such as aerodigestive fistulas. However, these grafts should not be considered definitive treatment, and additional surgical therapy is indicated after resolution of the acute problem.42
Infected Femoral Artery Aneurysms
Femoral Artery Infections Associated with Invasive Procedures
Compared to alternative approaches involving direct puncture of the axillary artery or the abdominal aorta, the transfemoral approach is associated with the lowest risk of complications. Modern results indicate that the overall arterial complication rate of transfemoral catheterizations is less than 1%; the risk of femoral artery infection is exceedingly rare.44,45 However, the increasing popularity of transcatheter techniques suggests that the absolute number of patients with catheter-related complications is likely to rise.
In an effort to reduce the incidence of pseudoaneurysms, many interventionalists use percutaneous closure devices to mechanically seal the arterial puncture site. Compared to manual compression, these devices have resulted in earlier mobilization and discharge of patients after arterial catheterization. However, these devices have been associated with a slightly higher risk of femoral artery infection. In a recent meta-analysis, Biancari et al. reported that the risk of access site infection is 0.6% after closure devices, compared to 0.2% without closure devices.46 Risk factors for infection include diabetes, obesity, therapeutic intervention, and groin hematoma.
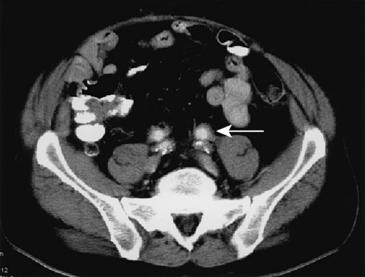
Primary femoral artery infections present as pseudoaneurysms. Common findings include pain at the puncture site, fever with chills, and a pulsatile groin mass. The onset of symptoms may be delayed up to several weeks after the original puncture. Gram-positive organisms predominate, especially S. aureus, but gram-negative bacteria are common isolates. Treatment involves institution of broad-spectrum antibiotics, débridement of the infected arterial segment, removal of all closure device material, and excision of grossly infected adjacent tissues. Direct revascularization with autogenous saphenous vein has been associated with a high risk of reinfection and vein graft blowout.47 Alternatively, use of SFPV has been associated with excellent durability and resistance to infection in two small series.48,49 Simple ligation of the common femoral artery (CFA) may be preferable if the femoral bifurcation is uninvolved.50 If revascularization is necessary, the bypass should be routed through extra-anatomical tissues to avoid infected areas. A transobturator bypass is ideally suited to this situation.51
Femoral Artery Infections in Intravenous Drug Abusers
Approximately three fourths of all admissions for accidental intravenous drug injections involve the lower extremity, and the femoral artery is the most common site of involvement.52 Most patients present with a painful, pulsatile groin mass, often associated with overlying cellulitis. The most commonly cultured organism is S. aureus. Appropriate treatment involves ligation of the affected arterial segment to reduce the risk of hemorrhage and débridement of all grossly contaminated tissue to remove the septic focus. The advisability of subsequent revascularization remains controversial.
Avoidance of revascularization rarely leads to amputation. Earlier reports documented an 11% amputation rate when one artery was ligated and a 33% amputation rate after triple-vessel ligation.53 More recent reports suggest that the incidence of amputation is much lower. Ting and Cheng performed routine ligation in 34 infected femoral pseudoaneurysms, including 24 that involved the femoral bifurcation.54 The mean postoperative ankle-brachial index was .43 after triple ligation and .52 with single-vessel ligation. Although 88% of patients had some degree of intermittent claudication after discharge, there were no instances of delayed limb loss. Cheng and colleagues reported a similar rate of claudication after single- or triple-vessel ligation, but one patient (5%) required above-knee amputation.55 Mousavi et al. reported the results of femoral artery ligation for infected pseudoaneurysms in 134 illicit drug users. There were no amputations in this series.56
Modern consensus is that infected femoral aneurysms in drug addicts are best treated with ligation alone. Most patients will suffer some degree of claudication, but the risk of early and late amputation is low. Immediate revascularization should be limited to cases in which no Doppler signal is detected at the ankle after femoral artery ligation.57 In the vast majority, staged revascularization should be considered in patients with limiting claudication after the infection has been completely cleared. The known propensity for prosthetic graft infection from a remote injection site suggests that autogenous tissue is preferable in these circumstances. In the absence of usable saphenous vein, the SFPV represents an excellent alternative.48
Infected Aneurysms of the Superior Mesenteric Artery
The SMA is the third most common site of visceral aneurysms from all causes, but it is the most common site for infected aneurysms in the splanchnic circulation.58,59 Original studies from more than 20 years ago reported that approximately 60% of SMA aneurysms had an infectious etiology,60 but this proportion appears to be decreasing. More recent series have reported an infectious etiology in 5% to 33% of reported cases.58,59,61 Infected SMA aneurysms usually occur secondary to subacute bacterial endocarditis, and the most commonly isolated organism is nonhemolytic Streptococcus.58,62
Most infected SMA aneurysms occur in patients younger than 50 years; men and women are equally affected.58,60 Only 10% of patients are completely asymptomatic.58 Some degree of abdominal discomfort is present in two thirds, and up to half have a tender, mobile, pulsatile mass.60 Fever, nausea, vomiting, gastrointestinal hemorrhage, and jaundice may also be present.
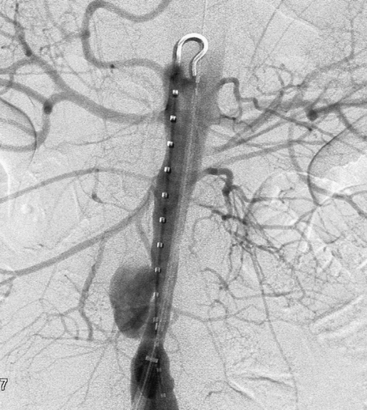
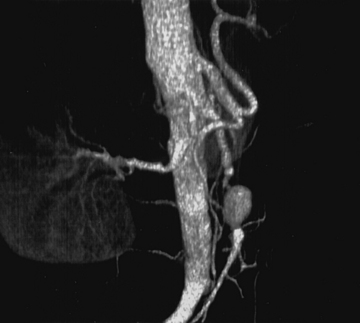
Superior mesenteric artery aneurysms tend to occur within 5 cm of the SMA origin, but any segment may be affected. Aneurysms may be suspected when vascular calcifications are seen on plain radiographs of the abdomen. Diagnosis should be confirmed with appropriate imaging studies that also localize the extent of the aneurysm such as standard mesenteric angiography or CTA (Fig. 59-4). Most infected SMA aneurysms are single, with variable involvement of visceral branches.
The natural history of infected SMA aneurysms is one of progression and eventual rupture; in fact, rupture has occurred at the time of presentation in 38% to 50% of patients.58 Reported mortality rate after rupture approaches 30%.61 Treatment includes transabdominal exploration and ligation of the arterial segments proximal and distal to the aneurysm. Complete excision is hazardous due to the proximity of the superior mesenteric vein and pancreas; therefore, débridement should be limited to exposed portions of the aneurysm wall and to the aneurysm sac contents. In the vast majority of cases, extensive mesenteric collateralization preserves bowel viability after SMA ligation. Therefore, ligation with resection of short segments of nonviable intestine is almost always appropriate. Direct revascularization is necessary in approximately 15% of cases. Bypass should be performed with autogenous tissue; we have found the SFPV to be an excellent alternative with superior patency compared to saphenous vein.63
Infected Carotid Artery Aneurysms
Infected aneurysms are rare in the extracranial carotid circulation. In a series of 67 carotid artery aneurysms treated over a 35-year period at the Texas Heart Institute, only one was infected.64 Most patients present with fever and a tender pulsatile neck mass. Medial deviation of the pseudoaneurysm may lead to clinical findings suggestive of a parapharyngeal mass. Before the antibiotic era, most carotid artery infections were the consequence of direct spread from pharyngeal infections. Most lesions are currently due to septicemia from bacterial endocarditis.
Gram-positive organisms, especially S. aureus and S. pyogenes, are common isolates from infected carotid aneurysms, but Salmonella infections have also been reported.65,66 Treatment involves ligation of all infected segments, even if this requires ligation of the internal and external carotid branches. Owing to the potential for graft disruption and exsanguinating hemorrhage, revascularization is rarely indicated. To prevent propagation of the internal carotid thrombus into the middle cerebral artery circulation, systemic anticoagulation with warfarin is recommended. Anticoagulation should theoretically be continued until the thrombus becomes stable, a period not longer than 6 weeks. Although most patients can be expected to tolerate internal carotid ligation without sequelae, temporary occlusion with a balloon catheter should be performed in the preoperative period. Patients who develop neurological deficits during balloon occlusion should be considered for prophylactic extracranial-intracranial bypass through remote uninfected tissues. As an alternative, some patients may benefit from hypertensive therapy combined with hypervolemia and hemodilution.66 To decrease the risk of exsanguination during carotid exposure, Wales et al. have advocated staged repair using a covered endovascular stent to temporarily exclude the pseudoaneurysm before proceeding to early definitive surgical management.67
Other Infected Aneurysms
Aside from femoral artery aneurysms, infected aneurysms of the lower extremity are exceedingly rare. The vast majority of infected popliteal artery aneurysms are a consequence of septic embolization from infective endocarditis.68 Mycotic aneurysms of the tibioperoneal trunk and tibial vessels have also been reported. Most infections involve gram-positive organisms such as Streptococcus, but Salmonella species have been recovered in a significant number of recently reported cases. The most common presentation is rupture, although thrombosis with foot ischemia has also been described. Treatment involves excision of the infected arterial segment and revascularization using autogenous bypass grafts.
Prosthetic Graft Infections
Risk Factors and Pathogenesis
The pathogenesis of graft infections is multifactorial and partly related to the site of implantation. Contamination prior to implantation due to failed sterilization techniques or breaks in packaging is thought to occur very infrequently. Likewise, gross breaks in sterile technique are rare. Most graft infections occur as a result of unrecognized bacterial contamination at the time of implantation. Exposure of the graft material to surrounding skin is a likely source; viable bacteria remain in the dermis of the skin despite antiseptic preparation.69 Graft contamination can also occur from remote infections such as cellulitis or pyelonephritis. Wet gangrene of a toe can increase the risk of infection in a prosthetic femoropopliteal bypass graft. Similarly, concurrent intraabdominal procedures such as cholecystectomy or appendectomy can expose an aortic graft to the patient’s enteric flora, thereby increasing the risk of graft infection.
A number of specific risk factors have been associated with aortic graft infections. Colonic ischemia following the repair of ruptured and nonruptured aortic aneurysms is associated with a high risk of graft infection due either to direct contamination or to hematogenous seeding from bacterial translocation.70 It has long been recognized that graft infections are more common after emergency repair of ruptured aortic aneurysms compared to elective operations.71 The emergent nature of ruptured AAA repair likely leads to inadvertent breaks in sterile techniques as the surgical team rushes to gain vascular control. In addition, patients presenting with either acute occlusion of the aorta or rupture of an aortic aneurysm are at high risk to develop postoperative SIRS. This SIRS response leads to an initial production of a proinflammatory cytokine response followed by a compensatory antiinflammatory cytokine response. This response renders the patient immunocompromised and at risk for nosocomial infections. Theoretically, this immunocompromised state may contribute to the increased risk of graft infection by hematogenous seeding during episodes of bacteremia.
Aortic grafts are uniquely prone to primary bacterial colonization at the time of aortic aneurysm repair. As already noted, many studies have demonstrated that the mural thrombus found in aneurysms is frequently colonized with bacteria. Macbeth et al. found that up to 43% of arterial walls were culture-positive for bacteria at the time of surgery.72 In this series, all of the aortic graft infections (.9%) occurred exclusively in patients with positive aortic wall cultures. The most common isolate was Staphylococcus epidermidis (71%) followed by Streptococcus species (13%) and other isolates (16%).72 Similarly, Buckels et al. in 1985 found that graft infection occurred more frequently in patients with positive cultures of aortic contents compared to those with negative cultures.73 More contemporary studies have failed to confirm an absolute association between positive aortic cultures and subsequent graft infections. Farkas et al. reported positive cultures in 37% of 500 aortic aneurysms.24 However, only one patient with a positive culture developed a graft infection. In contrast, 6 of 296 patients with negative cultures developed aortic graft infections during follow-up.24 Based on these observations, it is clear that colonization of the mural thrombus by bacteria plays, at most, a minor role in the pathogenesis of aortic graft infection. Results from more contemporary experiences may be due to the consistent use of perioperative antibiotic therapy.
Local and regional factors may also play a role in development of graft infections. Use of groin incisions (e.g., aortobifemoral bypass) can more than double the risk of graft infection compared to aortic grafts that remain completely intraabdominal (e.g., aortobiiliac bypass or aortic tube graft repairs). This may be related to the local environment of the groin, an area associated with one of the highest concentrations of Staphylococcus species found on the body.69 In addition, dissection in the groin disrupts abundant femoral lymphatics, leading to risk of lymph leak, groin wound breakdown, and direct graft contamination. Furthermore, the lymphatic system transports bacteria from distal sites of infection to the groin lymph nodes; opening these channels exposes the graft to potential contamination. Translocated bacteria have been demonstrated in animals and humans. In patients presenting with complex foot infections, positive lymphatic cultures at the time of amputation have been demonstrated in up to 20% of patients.74 Cultures of groin lymph nodes at the time of vascular reconstruction have revealed bacteria in 11%. However, these cultures do not correlate with subsequent groin wound infections.75
Graft material also plays a role in the pathogenesis of graft infections. The immune system responds to the foreign body by walling off the offending agent. The initial response is an acute inflammation, with influx of neutrophils followed by macrophages. These inflammatory cells produce cytokines and release proteases in an attempt to eliminate the foreign body. This initial response has a negative effect on bacterial survival; however, if the inoculum is large, some bacteria may survive. The graft interstices may offer a safe haven for bacteria and allow them to survive the initial inflammatory phase. After the acute inflammatory response, a reparative phase begins. This stage is characterized by fibroblasts depositing collagen in response to locally secreted cytokines. A resulting connective tissue barrier shields bacterium from detection and obliteration by immune competent cells. This results in a closed space for the bacteria to thrive and grow on exudative proteins existing in an acidic and ischemic environment. In the absence of infection, the reparative phase culminates in tissue ingrowth and incorporation of the graft. However, if bacterial colonization is present, the graft fails to incorporate, and chronic inflammation continues. Failure to incorporate may be due to fibroblast inhibition by the bacterial components found in the perigraft fluid.76 This results in the failure to obliterate the closed space around the graft and failure of incorporation. Bacteria are left to thrive in this closed space, eventually becoming an abscess. This can manifest as perigraft fluid that may express through incisions with sinus tract formation. In addition, the artery may be degraded at suture lines, resulting in pseudoaneurysm formation.
Aortic Graft Infection
Over the last 2 decades, refinements in diagnosis and operative management of aortic graft infections have resulted in improved mortality and morbidity. Mortality during the early experience of aortic graft infection approached 50%, and limb loss rates were as high as 75%. With refinement in technique, the respective mortality and limb loss rates have decreased to 20% or less in many series.77
Incidence
The exact incidence of graft infection following aortic reconstruction is not precisely known because most series are retrospective and suffer from lack of inclusive follow-up. The best estimates suggested that the incidence of graft infection following aortic reconstructions ranges from 1% to 5%.77–79 More contemporary data can be abstracted from the U.K. Small Aneurysm Trial, a randomized study of AAA repair in patients with small aneurysms. Although this study did not directly report the incidence of aortic graft infection, a number of graft-related complications were documented. Three patients in the trial had late aortic rupture following AAA repair, and four patients died following development of aortoenteric fistula. Most of these complications can be assumed to represent complications from aortic graft infection and represent 2% of the total patients.80 The true incidence of aortic graft infection remains inadequately described, but most authorities agree the incidence is quite low and certainly less than 2% in the contemporary experience.
Classification
To better understand the pathogenesis and natural history of graft infections, classification systems have been developed. Wound complications following vascular reconstruction were first classified by Szilagyi et al. in 1972.81 The authors characterized wound complications following prosthetic graft placement in terms of anatomical involvement. Grade I lesions involved only the dermis. Grade II lesions extended into the subcutaneous tissue without involvement of the graft, while grade III lesions involved the graft by direct extension.81 Grade III lesions can be considered technical problems associated with wound closure. Certainly, local factors such as ischemic tissue play a significant role in wound infections, but they may be avoidable. This grading scheme is important only for early graft infections, which account for less than 1% of all graft infections.82 It does not take into account graft infections that present during late follow-up after aortic reconstruction. This grading scheme is more relevant to infections that follow infrainguinal arterial reconstructions, which result most frequently from complications of wound infections.
More relevant to the understanding of aortic graft infection is the scheme developed by Bandyk, who categorized aortic graft infections according to time of presentation after graft implantation.82 This classification scheme offers better insight into the origin of graft infections and is a better predictor of the type of bacteria that will be found infecting the graft. Bandyk defined an early graft infection as one that occurred less than 4 months after implantation, and late graft infections manifested after 4 months. Graft infections are subcategorized in terms of presentation: perigraft infection, graft-enteric erosion, and graft-enteric fistula. Most early perigraft infections represent the sequela of Szilagyi grade III wound infection; that is, extension of local wound infections to involve the graft. Remote sites of infection (e.g., wet gangrene) or immunosuppression are thought to play a minor role in early graft infections.82 The diagnosis of an early perigraft infection is usually obvious: most patients present with purulent drainage from the wound, signs of sepsis with bacteremia, acute pseudoaneurysm formation, or anastomotic disruption with hemorrhage. Early infections will be manifest almost exclusively in wounds located at or below the femoral level. These infections can be expected to contain any one of a variety of organisms including gram-positive and gram-negative organisms. In contrast, late perigraft infections tend to occur months to years following implantation. Most will present more than 1 year following implantation, and the majority result from S. epidermidis infection with biofilm production.
Etiology
Most aortic graft infections are thought to occur by direct contamination or extension of adjacent infections. No good evidence exists confirming that bacteremia contributes to aortic graft infection in humans. However, there is ample evidence from animal studies that bacteremia can result in prosthetic graft infections.83,84 Antibiotic therapy during bacteremia in dog models prevents prosthetic graft infections.84 All these models consist of bacteremia immediately following graft implantation. In humans, bacteremia frequently occurs after aortic surgery, yet the rate of acute graft infection is very low. Bacteremia likely plays only a minor role in acute aortic graft infections. The role of bacteremia in late-occurring graft infection is not known, but it is thought to be a rare cause of late graft infection.
Should patients with vascular grafts receive prophylactic antibiotics before procedures associated with bacteremia, such as dental or genitourinary instrumentation? Although past case reports have suggested a possible association, there is little evidence to indicate that these procedures lead to graft infection. Late-occurring graft infections are caused only rarely by organisms found in mouth flora.85 Graft incorporation may act as a barrier to bacteremia and prevent late graft infections. In contrast to patients with prosthetic cardiac valves, current American Heart Association (AHA) guidelines do not recommend antibiotic prophylaxis after vascular graft placement for patients undergoing dental, respiratory, gastrointestinal, or genitourological procedures.86
Clinical Presentation
The clinical presentation of late graft infection can be subtle. Latent graft infections can present as chronic femoral pseudoaneurysms. At repair, the graft will be poorly incorporated, with surrounding perigraft fluid. These findings are considered diagnostic for graft infection and have been associated with positive cultures in 71% of cases.87 Lack of graft incorporation is more sensitive for graft infection; Padberg et al. found that 97% of incorporated grafts were not infected.87 Infection may be a primary process for the formation of anastomotic femoral pseudoaneurysm. Up to 60% of clinically uninfected femoral pseudoaneurysms will be culture positive at repair.88 The presence of a latent graft infection should be considered in all cases of femoral pseudoaneurysms. Another subtle sign of latent graft infection is graft thrombosis. This is particularly true if the graft has failed in the past, requiring revision either surgically or by thrombolysis. All patients with graft limb thrombosis or femoral pseudoaneurysms should have CT scan imaging of the graft to rule out infection prior to repair or revision. Hydronephrosis may also herald a latent graft infection.89 All patients with prior aortic surgery should have a CT to evaluate for latent graft infection when hydronephrosis is encountered.
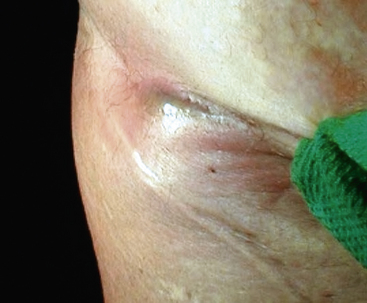
Less subtle presentations of latent graft infections include draining sinuses from groin wounds (Fig. 59-5) and a history of bleeding from a groin pseudoaneurysm. Bleeding from the groin in the presence of a pseudoaneurysm is a surgical emergency and must be addressed immediately. Such bleeding episodes are herald bleeds and portend life-threatening hemorrhage if not dealt with expeditiously. Graft infection may also present as gastrointestinal bleeding in a patient with prior aortic surgery. All such patients should be considered to have an aortoenteric fistula until proven otherwise. Rupture of a previously repaired aneurysm or pseudoaneurysm at the proximal anastomosis should be considered the sequelae of graft infection. An updated summary of the clinical manifestations of aortic graft infections in 187 patients with aortic graft infections treated at University of Texas Southwestern Medical Center is found in Table 59-2. This experience is similar to other published series.85,90
Table 59-2 Clinical Manifestations of Aortic Graft Infections* in 187 Patients: University of Texas Southwestern Medical Center Experience
| Clinical Manifestation | No. Patients(%) |
|---|---|
| Open groin sinus | 81 (43) |
| Femoral pseudoaneurysm | 68 (36) |
| Constitutional/weight loss | 61 (32) |
| Ischemia | 52 (29) |
| Sepsis | 40 (21) |
| AE erosion/fistula | 26 (14) |
| Bleeding | 23 (12) |
AE, aortoenteric.
* Many patients had more than one sign.
Adapted from Ali AT, Modrall JG, Hocking J, et al: Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg 50:30, 2009.124
Diagnosis
Computed tomography scan
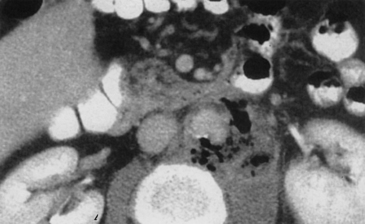
The CT scan remains the gold standard for diagnosis of aortic graft infections. Findings on CT that are suggestive of graft infections include loss of continuity of the aortic wrap (i.e., the residual native aortic wall closed over the graft at the time of repair), pseudoaneurysms, perigraft fluid, perigraft inflammation with loss of tissue planes, perigraft air, and focal bowel wall thickening. Presence of any of these findings is highly suggestive of aortic graft infection. Figure 59-6 demonstrates the presence of perigraft fluid in a patient with an infected aortic graft. The same patient was found to have bilateral femoral artery pseudoaneurysms (Fig. 59-7). The reported sensitivity and specificity of CT scans to diagnose all graft infections is 95% and 85%, respectively.91 Sensitivity and specificity approaches 100% when findings of perigraft fluid, perigraft inflammation, or ectopic gas are present.89,91 However, CT scanning may not be able to accurately diagnose subtle graft infections manifest solely by the presence of perigraft fluid. While CT imaging of low-grade aortic graft infection has a specificity of 100%, the sensitivity is only 55%.89 The other disadvantage of CT scanning is the requirement for intravenous contrast. This may be contraindicated in patients with chronic renal insufficiency or dye allergies. However, the CT modality is readily available, safe, inexpensive, and familiar to clinicians; it remains the imaging modality of choice for initial evaluation.
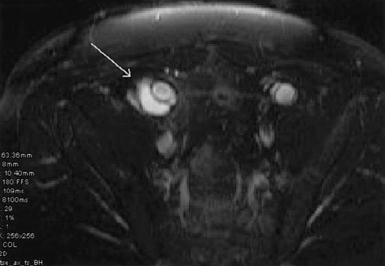
Magnetic resonance imaging
The major advantage of MRI, in comparison to CT scanning, is the ability to diagnose small fluid collections and differentiate inflammatory changes from chronic hematomas. Perigraft fluid has low to medium signal intensity on T1-weighted images and high intensity on T2-weighted images. Noninfected aortic grafts will have perigraft fibrosis without the characteristic bright fluid “halo” seen surrounding infected grafts on heavily T2-weighted images. Tissue surrounding infected aortic grafts frequently exhibits heterogeneous increased signal intensity that is not seen in association with sterile grafts.92 Olofsson et al. found the sensitivity and specificity of MRI for diagnosing graft infections to be 85% and 100%, respectively.93 The ability of MRI to detect small fluid collections on T2-weighted images gives this modality a distinct advantage over CT scanning to diagnose low-grade S. epidermidis graft infections. The authors use CT scans as the initial imaging modality, and MRI is used to find latent graft infections not detected by CT.
Radionuclide scanning
Radionuclide scanning relies on labeled WBCs to localize areas of infection and inflammation. A variety of scanning techniques have been developed to aid in diagnosis. The major pitfall of such imaging techniques is false-positive results. Initially, gallium-67 and indium-111 were used without WBC labeling. The sensitivities and specificities of imaging with gallium and indium were quite high, but these tracers have been largely abandoned owing to uptake by the gastrointestinal tract and kidneys that obscures the aorta and makes analysis difficult. More recently, WBC labeling techniques have become the norm. Indium-111-oxine–labeled WBC scans have been found to be very sensitive (82%-100%) but have a lower specificity (80%-83%).94–96 The low specificity of this technique is due to co-labeling of platelets that can deposit on noninfected graft surfaces, resulting in an unacceptably high false-positive rate.
Other techniques have been employed in an attempt to increase the sensitivity of radionuclide scanning. These include labeling WBC with technetium-99 m hexametazime or technetium-99 m D,L-hexamethylpropylene amine oxide (Tc-99 m-HMPAO). These techniques are less expensive and do not suffer from the co-labeling problems seen with indium. Liberatore et al. reported a sensitivity for Tc-99 m-HMPAO of 100% and a specificity of 92%.97 Other techniques included indium-111-labeled immunoglobulin (Ig)G and avidin/indium-111-labeled biotin scintigraphy.92 Both of these techniques appear to have increased specificity compared to indium labeled WBCs, but the published experience has been limited. The role of radionuclide scanning is not entirely clear; some centers use radionuclide scanning as the primary mode of imaging for graft infections. Perera et al. have suggested that these scans are most useful when diagnosis by CT is equivocal or there is a low-grade infection.77 Like all nuclear medicine imaging techniques, the results can be dependent on the skill and experience of the interpreting radiologist. The most rational approach is to use such scanning techniques as an adjunct to both CT scanning and MRI.
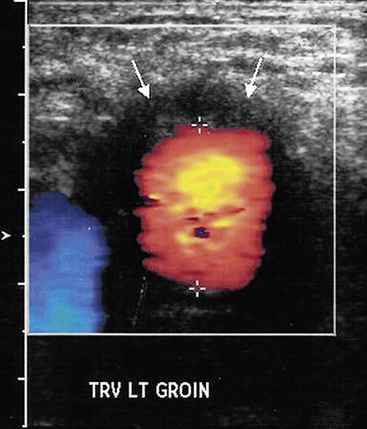
Ultrasound
Ultrasonography has a limited role in the diagnosis of aortic graft infection. Although ultrasound can accurately diagnose the presence of perigraft fluid (Fig. 59-8), the intraabdominal portion of grafts is not readily imaged. The primary utility of ultrasound is to diagnose femoral pseudoaneurysms and perigraft fluid around infrainguinal grafts or aortic grafts that extend to the groin.
Percutaneous aspiration
Some authors have advocated percutaneous aspiration to confirm the diagnosis of a suspected graft infection.98,99 In the routine diagnosis of graft infection, this modality offers little additional information beyond conventional noninvasive imaging techniques, and percutaneous aspiration can lead to introduction of bacteria into an otherwise sterile fluid collection. Percutaneous aspiration may offer some assistance in the high-risk patient to confirm the diagnosis prior to embarking on surgical repair. Concurrent placement of an external drainage catheter can be used as a therapeutic measure. Belair et al. reported a series of 11 patients treated with percutaneous drainage and antibiotic therapy.98 In this retrospective series, four patients were successfully treated with percutaneous drainage and antibiotic therapy. The remaining patients required adjunctive procedures (two surgical drainage, four graft excision), and one patient died as a complication of hemorrhage following drainage.98 Percutaneous drainage with lifelong antibiotic therapy may be an option for the very high-risk patient who is not anticipated to survive graft excision.
Operative exploration
In rare circumstances, there may be a high index of suspicion for graft infection, with no supporting evidence on imaging of graft infection. In such situations, operative exploration may be the only way to determine the presence of a latent graft infection.78 As noted earlier, the finding of a nonincorporated graft is not necessarily diagnostic for graft infection, but the finding of a well-incorporated graft does rule out the diagnosis of infection. Operative exploration may most helpful in determining the extent of graft infection. If preservation of a portion of a graft is entertained, operative exploration is often the only mechanism to determine whether a graft infection is isolated to a segment of the graft or the entire graft is infected. Careful preoperative planning is required before such operative explorations to ensure that noninvolved graft is not inadvertently contaminated.
Diagnostic Pitfalls with Early Graft Infection
Although most early graft infections are obvious, normal findings in the immediate postoperative period may be misconstrued as signs of graft infection. There is no good diagnostic solution in such cases. Fluid and air surrounding the graft are common findings in the early postoperative period and are not necessarily indicative of infection. The finding of air around the graft is seen routinely until 1 week following implantation; air is not considered to be pathognomonic for infection until 4 to 7 weeks have elapsed.100,101 Likewise, fluid around the graft is a common finding. Virtually all patients will have some degree of hematoma around the graft in the early postoperative period, but fluid persisting past 3 months is abnormal and highly suspicious for graft infection.89
Magnetic resonance imaging evaluation for early graft infection suffers from the same pitfalls as CT scanning. Magnetic resonance imaging cannot distinguish between air and calcium in the wall aortic wall remnant and thus depends on the finding of perigraft fluid. Labeling of WBCs for scintigraphy is also unreliable in the early postoperative period. Ramo et al. found that 29% of Tc-99 m-HMPAO–labeled WBC scans were positive in 24 patients examined 2 weeks following surgery.102 At 3 months, 4 of 24 studies continued to be positive. Only one patient was ultimately found to have an infected graft. Sedwitz et al. found a similar lack of specificity for the diagnosis of early graft infection (< 3 months) using indium-labeled WBC scintigraphy.103
Direct aspiration of perigraft fluid is not helpful in diagnosis of early graft infection and is not recommended in the early postoperative period owing to the potential for introducing bacteria into an otherwise sterile fluid collection.92 Any of these imaging modalities may be helpful in the early postoperative period if they are negative. A negative study will lead the clinician to entertain other diagnoses to explain the clinical findings that have raised the suspicion of an early graft infection. However, a positive study is not useful; the clinician will have to rely on judgment, and operative exploration may be the only solution to this vexing clinical dilemma.
Diagnosis of Aortoenteric Fistula
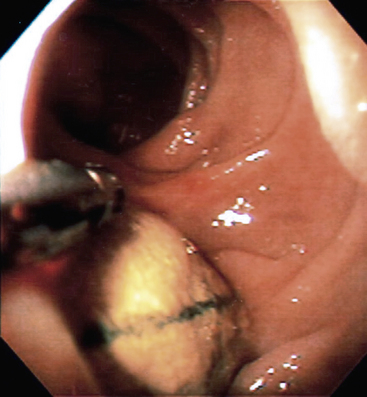
The diagnosis of aortoenteric fistula can be as challenging as diagnosing an early graft infection. Any patient presenting with gastrointestinal hemorrhage and a history of aortic reconstruction should be considered to have an aortoenteric fistula until proven otherwise. Both MRI and CT can fail to diagnose graft enteric fistulas. Magnetic resonance imaging can fail to clearly demonstrate ectopic air that may be misinterpreted as aortic wall calcifications. Computed tomography scanning may fail to diagnose fistulas because of limited inflammation or fluid around the graft, resulting in misinterpretation.92 If the patient is stable, either MRI or CT should be obtained. Concerning findings are absence of a soft-tissue plane between adjacent bowel and the graft, as well as ectopic air or perigraft fluid. Extravasation of contrast into the bowel is virtually never seen. All stable patients should undergo upper endoscopy to include the fourth portion of the duodenum. Endoscopy rarely demonstrates visible graft, but endoscopy should be performed to rule out other causes of upper gastrointestinal hemorrhage, such as peptic ulcer disease or gastroesophageal varices. Colonoscopy may be indicated in selected patients suspected of having graft erosions into the colon (Fig. 59-9). In unstable patients or patients in whom no other source of bleeding is found, operative exploration is needed to rule out graft-enteric fistula. During exploration, the entire duodenum and any other adherent bowel must be entirely dissected free to rule out a fistula.
Bacteriology
A wide variety of bacterial pathogens can be cultured from infected grafts. The type of pathogen that will be isolated can be anticipated from the timing of presentation. The culture results from Yeager and colleagues’ experience treating 60 infected grafts104 are typical and presented in Table 59-3. In late-occurring graft infections, staphylococcal species are predominant, with S. epidermidis found most frequently. Bandyk et al. reported that 60% of late-occurring graft infections were culture positive for S. epidermidis.105 Although the most commonly cultured organism responsible for early graft infection is S. aureus,106 early graft infections will have a higher frequency of gram-negative rods and atypical organisms such as anaerobes and yeast. Aortoenteric fistulas and graft erosions are characterized by gram-negative rod and yeast infections. Pseudomonal infections are notorious for their virulent course. Graft and arterial disruption are common. This is related to production of elastase and alkaline proteases by Pseudomonas, leading to arterial degradation and eventual disruption.69 Time of presentation of the graft infection and the pathogens found at the time of therapy will guide antibiotic therapy.
Table 59-3 Cultures from Infected Aortic Grafts in 32 Patients
| Organism | No. Cases |
|---|---|
| Staphylococcus aureus | 14 (44) |
| Staphylococcus epidermidis | 14 (44) |
| Bacteroides | 3 (9) |
| Escherichia coli | 2 (6) |
| Streptococcus | 2 (6) |
| Klebsiella | 1 (3) |
| Pseudomonas | 1 (3) |
| Enterococcus | 1 (3) |
| Clostridium | 1 (3) |
| Serratia | 1 (3) |
| Candida | 1 (3) |
| Corynebacterium | 1 (3) |
| Propionibacterium | 1 (3) |
| No growth | 2 (6) |
| Multiple organisms | 10 (31) |
Adapted from Yeager RA, Taylor LM Jr, Moneta GL, et al: Improved results with conventional management of infrarenal aortic infection. J Vasc Surg 30:76–83, 1999.104
Optimal length of treatment with antibiotics after graft excision is not known. Some authorities have recommended a 6-week course of parenteral antibiotics followed by 6 months of suppressive therapy to treat concurrent arterial wall infection.72 Macbeth et al. based his treatment decision on cultures from pathological specimens and aortic biopsies at the time of infected graft excision.72 Patients with positive arterial wall cultures who were treated with only minimal débridement and short-term antibiotics all suffered aortic stump disruption or other arterial wall disruption. Consequent to these findings, the authors advocated 6 weeks of parenteral antibiotics, followed by 6 months of oral suppressive therapy in patients with positive arterial wall cultures.
Treatment of Aortic Graft Infections
Antibiotic Therapy
Limited treatment using lifelong antibiotic therapy or percutaneous drainage and antibiotic therapy are options for the most high-risk patient with a limited life expectancy. There are little data to support such treatment regimens. Roy and Grove reported a series of high-risk patients treated with antibiotic therapy alone for proven or suspected graft infections.106 Only two of the patients had proven infections of preexisting grafts. All patients were alive at the median follow-up of 36 months without systemic symptoms of infection. Although this appears to be a reasonable option, it should be stressed that this therapy is appropriate only for latent graft infections due to low-virulence organisms, particularly S. epidermidis. This therapy should be condemned in all but the most high-risk patients. Infection by more virulent bacteria has a grave prognosis, and antibiotic therapy alone will have little impact on the natural history of the infection.
Total Graft Excision and Extra-Anatomical Revascularization
After completing the extra-anatomical bypass, the infected graft is removed. Timing of the two stages is controversial. The extra-anatomical bypass can be performed just prior to removal of the infected graft during the same operative procedure, or the procedures can be staged with extra-anatomical bypass performed several days prior to removal of the infected graft. Staging procedures give the patient time to recover from the initial bypass and avoid a long procedure. Proponents of the staged procedure purport a lower operative morality and increased limb salvage than the single-stage operative strategy.107,108
There are several disadvantages to extra-anatomical bypass with total graft excision. The most worrisome complication is infection of the new bypass graft. Reinfection rates can be as high as 20%, but most series report a low reinfection rate of less than 10%.67,68,108 The notoriously poor durability of the extra-anatomical bypasses is also a concern. The highest reported primary patency rate for axillary-femoral-femoral bypasses is over 75%, but the reported primary patency rates of unilateral axillary-femoral bypasses average approximately 60% at 3.5 years.107,109 The most devastating complication of this procedure is disruption of the oversewn aortic stump (aortic stump blowout). Fortunately, this lethal complication is rare. Because of these disadvantages, a number of other options for graft excision and revascularization have been introduced.
Total Graft Excision with In Situ Replacement Using Prosthetic Graft
In situ replacement of an infected graft with a new prosthetic graft is technically the simplest method of revascularization and avoids the potential for aortic stump blowout. However, replacing an infected prosthetic graft with a new prosthesis poses the very real potential for recurrent graft infection. In situ prosthetic replacement may be best used as a salvage operation for unstable patients with either aortoenteric fistula or ruptured proximal pseudoaneurysms. Fortunately, the reported rates of clinically apparent reinfection following in situ replacement for aortoenteric fistula is surprisingly low (< 15%).110,111 Replacement with new graft may be appropriate for localized graft infections such as those found in the setting of aortoenteric fistula. Recurrent infection in the setting of gross graft infection has been disappointing. The authors use in situ replacement with prostheses as a bridge to definitive therapy with autogenous replacement at a later operation.
The advent of antibiotic-bonded Dacron grafts appeared to offer improved results for in situ prosthetic replacement. This modality seems to be most appropriate for treatment of graft infections with biofilm-producing S. epidermidis or S. aureus. Young et al. reported a series of nine patients treated with rifampin-soaked grafts and found that the reinfection rate was 11%.111 Bandyk et al. and Hayes et al. both concluded that rifampin-bonded grafts are acceptable for treatment of low-grade biofilm graft infections by S. epidermidis and S. aureus.112,113 Bandyk et al. found that recurrent infection occurred in less than 10% of patients.112 Hayes et al. only reported reinfection in patients with meticillin-resistant S. aureus (MRSA) infections.113 It appears that antibiotic-bonded grafts may offer improved results, but only in selected patients with low-grade S. epidermidis and possibly S. aureus infections. They are usually not appropriate in cases involving more virulent organisms. Similar findings have recently been reported using silver-coated polyester grafts for in situ replacement. In a series of 24 patients with a variety of polymicrobial graft infections, Batt et al. documented a 40% prevalence of graft reinfections.114
Total Graft Excision with In Situ Replacement Using Arterial Allograft
An alternative to in situ replacement with prosthetic graft is replacement with arterial allograft. Animal studies have demonstrated the allograft is relatively resistant to infection when antibiotic loaded.115 The experience in humans has confirmed this finding. Leseche et al. reported a series of 28 patients treated with allografts for graft infection or infected aortic aneurysms.116 They reported no recurrent infection. In a series of 49 patients, Vogt et al. reported two patients with recurrent infections that resulted in death.117 However, in this series there were four deaths related to allograft technical complications. In three patients, allograft side branch rupture resulted in three aortoenteric fistulas that were uniformly fatal. A fourth patient died intraoperatively from rupture of a friable allograft. In a recent study of 110 consecutive patients with aortic infections, Bisdas et al. reported a 9% overall operative mortality. During a mean follow-up of 36 months, 6% required reoperation for graft deterioration, but no recurrence of infection was noted.118 Others have noted that reoperation for reinfection or degenerative changes in allografts is common (9%-17%) and that pathological changes are seen in up to 26% of patients.38,116 In situ allograft and prosthetic graft replacement may be best used a temporizing technique until more definitive therapy can be undertaken.
Total Graft Excision with In Situ Replacement Using Autogenous Veins
Perhaps the best solution for management of prosthetic graft infection is in situ reconstruction with SFPV. This conduit has proven to be the most resistant conduit to infection, has unchallenged patency rates, avoids the risk of aortic stump blowout, and rarely degenerates. Operative mortality following aortic reconstruction with SFPV has been reported as less than 10%.119–123 Reinfection is very rare. In our experience, only one patient suffered infection of an SFPV graft, and the offending organism was Candida. Franke noted two cases of SFPV infection that occurred in the face of overwhelming Pseudomonas infection.123 In our experience, all patients with pseudomonal infections have been treated successfully, but it should be pointed out that none of our patients presented with overwhelming sepsis. Patients with overwhelming sepsis may be better served by extra-anatomical bypass and graft excision. SFPV is very durable, with primary patency rates of greater than 80% and less than 5% of grafts requiring revision.122,124 Venous morbidity is minimal.125
Partial Graft Excision
With the improvement in imaging techniques, some authors have advocated partial graft excision if the aortic graft infection can be localized to the femoral portion on preoperative imaging. Reconstruction can be carried out by extra-anatomical bypass or in situ grafting. This technique is most appropriate for patients with late S. epidermidis infections. It is not appropriate for patients with early graft infections because the entire graft is almost invariably involved.82 Towne et al. described treating 14 patients with confirmed S. epidermidis or S. aureus infection using partial graft excision, wide local débridement, and in situ replacement with PTFE graft.126 Only 2 of the 14 patients ultimately developed infection in the remaining graft, but the new PTFE grafts remained uninfected. Calligaro et al. demonstrated that graft patch remnants on infrainguinal vessels can be safely left in situ at the time of graft excision in over 92% of patients.127 Reilly et al. have described good results with partial graft excision and extra-anatomical bypass.77 In the authors’ most recent experience, only 63% of patients require total graft excision. In our experience, subtotal graft excision can be attempted if the body of the graft is found to be incorporated at the time of surgical exploration. In these situations, planned reconstruction with SFPV has a real advantage. The body of the graft can be explored prior to violating the clearly infected portion. If the entire graft is infected, total graft excision and reconstruction with SFPV can be undertaken. If the body of the graft is not infected, the graft can be divided and sewn to the SFPV. The wound is closed, the infected portion of the graft is removed, and the reconstruction completed. Subtotal graft excision should only be considered in high-risk patients with late-occurring graft infections. Such patients should be followed closely, and infection of the residual graft should be anticipated.
Graft Infection Following Endovascular Repair
Graft infection following stent graft repair of aneurysm is becoming more frequently reported. As of 2010, there have been 102 reports of abdominal endograft infections in the literature.128 In a series of 494 consecutive stent grafts (389 abdominal aorta and 105 thoracic aorta), Heyer et al. reported a prevalence of abdominal endograft and thoracic endograft infection of 0.26% and 4.8%, respectively.129 All affected patients suffered significant morbidity. While rare, endograft infections are extremely difficult to diagnose. The typical clinical presentation and radiographic finding associated with stent graft infections are not well described and await a more mature experience with these endovascular techniques. Infected grafts have been associated with highly virulent organisms including Propionobacterium, Staphylococcus, Streptococcus, and Enterobacter.129 Treatment for infected abdominal aortic endografts includes complete graft excision and extra-anatomical or in situ bypass. We have used the SFPV for in situ replacement with gratifying results in these circumstances. On the other hand, treatment of infected thoracic endografts should include graft excision and bypass with antibiotic-soaked prosthetic graft or cryopreserved allograft if more virulent organisms are present.
Treatment of Peripheral Graft Infections
The incidence of graft infection following infrainguinal peripheral arterial reconstruction ranges from 2%-5%.130 Most peripheral graft infections occur subsequent to extension of local wound infections. Late infections of autogenous grafts are very rare and most frequently occur in thrombosed grafts. Late infections occur more commonly in prosthetic grafts. Since most infections result from extension of a local wound infections (Szilagyi grade III), the microbiology can be varied, with gram-negative rods playing a prominent role. In a series of 68 patients with infected infrainguinal autogenous grafts, Treiman et al. found S. aureus and S. epidermidis, most commonly followed closely by Pseudomonas.131
In patients who will require concurrent revascularization at the time of infected graft excision, the management options are different for prosthetic grafts versus autogenous graft. In virtually all cases of prosthetic graft infection, the entire graft is involved in the infectious process. This requires total graft excision with revascularization through uninfected tissues. Revascularization is performed first with autogenous conduit if possible. Careful planning and inventive tunneling are required if cross-contamination is to be avoided. If the graft originates from the femoral artery, the profunda femoris artery, approached lateral to the sartorius muscle, can be used as the site of the proximal anastomosis. If the profunda femoris artery is diseased, the iliac vessels can be used, and the graft can be tunneled through the obturator foramen into the thigh. The recipient artery or run off artery must be one level below the infective process. If the popliteal artery above the knee is involved in the infective process, the new runoff vessel will have to be the below-the-knee popliteal artery or the tibial vessels. More distal reconstruction to uninvolved tibial vessels will be required if the infected graft terminates at the popliteal vessel below the knee or more distal. Often the graft will have to be tunneled through the lateral thigh to avoid the previously violated medial thigh. The below-the-knee popliteal artery, peroneal artery, and the anterior tibial artery can all be approached through lateral leg incisions. After reestablishing flow and closing the wounds, the prosthetic graft is excised through a separate incision. If possible, the entire graft should be excised with autogenous patching of the donor and recipient arteries to avoid late complications from infected graft remnants. Well-incorporated remnants of prosthetic grafts can be left in place with successful healing and few late complications, but these patients will require close observation.127
In a series of 16 patients with autogenous graft infections without disruption, Calligaro et al. were able to successfully salvage 11 grafts.132 Of these patients, six were treated with muscle flap coverage, with only one failure and no graft associated mortality. Patients treated with operative débridement and antibiotic-soaked dressing changes had more complications and higher mortality from graft complications. Limb salvage was obtained in 6 of 10 patients. In this series, the overall operative mortality was 19%, and the amputation rate was 8%.132 Tukiainen et al. reported improved results with graft preservation, aggressive débridement, and muscle flap coverage in a series of 14 patients with autogenous graft infections.133 One patient required a late amputation because of ongoing graft infection, and four patients had late graft occlusions. All four patients with late graft occlusions had in situ replacement with new graft for graft disruption and hemorrhage. There were no graft-related mortalities and no graft disruptions from recurrent infection.133 Treiman identified graft disruption with bleeding, elevated WBCs, fever, and renal insufficiency as the only predictors for graft failure and limb loss following selective graft preservation.131
In the authors’ experience, wound infections that involve autogenous grafts without graft disruption represent graft contamination rather than graft infections. Such grafts can be treated by graft preservation and muscle flap coverage. It is imperative that well- vascularized muscle be used to cover such grafts. If the graft is not covered by muscle, continued wound sepsis with progression to frank graft infection and disruption can be anticipated. True graft infections manifest as graft degradation and hemorrhage. Invariably, pathological examination of such grafts will reveal pathogens in the wall of the graft. Such grafts should be treated by graft ligation with revascularization through uninfected tissue planes; uninfected portions of the graft may be left place. In situ reconstruction with new autogenous graft such as contralateral saphenous vein or arm vein134 has been reported. In the face of graft degradation and hemorrhage, muscle flap coverage is not a good option and should be reserved only for patients with limited autogenous conduit. In these rare circumstances, close observation with prolonged antibiotic therapy is needed. Such patients should be observed in the intensive care unit setting until wound healing and absence of recurrent graft infection is assured. Graft ligation with or without primary amputation may be the safest course in such patients.
Suppurative Thrombophlebitis
Widespread use of intravenous therapy in hospital patients has made primary venous infections more common than their arterial counterparts. The term suppurative thrombophlebitis implies a localized infection of the vein wall associated with intraluminal thrombosis, which should be differentiated from catheter-related sepsis. While the two may be temporally related in the same patient, they can usually be distinguished by the following characteristics: (1) catheter sepsis is not usually associated with vein wall suppuration, and (2) in catheter sepsis, the intraluminal thrombus is adherent to the catheter, not the vein wall. The following discussion will focus on diagnosis and management of suppurative thrombophlebitis; more complete information on catheter sepsis is available elsewhere.135
Peripheral Vein Suppurative Thrombophlebitis
Thrombophlebitis is the most common complication of peripheral vein infusion, occurring in up to a fourth of hospitalized patients receiving intravenous therapy via veins of the forearm or hand.136 Pathogenesis has been related to irritation of the vein from the catheter material, infusate, or bacteria. Thrombosis occurs as a result of localized stasis and prostaglandin-mediated activation of the coagulation cascade.137 Suppurative superficial thrombophlebitis results from infection of the thrombus, which is estimated to occur in 0.2% to 2% of peripheral vein catheter insertions.138 Onset of infection is a serious development, resulting in significant morbidity and prolonged hospital stay. Development of life-threatening infections such as osteomyelitis or endocarditis may occur after a single episode of superficial suppurative thrombophlebitis. This complication is more common with plastic catheters than with steel (“scalp vein”) cannulas and is related to duration of intravenous catheterization.139 Prolonged catheterization is the most important predictor of peripheral vein infusion thrombophlebitis; this has led to the recommendation by the Centers for Disease Control and Prevention (CDC) that short peripheral intravenous catheters should be changed every 72 hours.
Although there is a higher risk of suppurative superficial thrombophlebitis from catheters inserted in the lower extremity, upper-extremity involvement is the more common presentation. Affected patients have signs of local inflammation, including tenderness, erythema, induration, and warmth over the involved superficial vein. Differentiation between noninfected and suppurative thrombophlebitis may be difficult. Systemic signs of infection such as fever, tachycardia, and leukocytosis are not universally present. Bacteremia occurs in the majority of patients, and gross pus within the vein lumen may be found in up to half the cases.139 Diagnosis often relies on a positive culture of the indwelling catheter tip. S. aureus is the most common isolate, but gram-negative organisms are becoming more frequent. Antibiotic resistance is common.
Central Vein Suppurative Thrombophlebitis
Central suppurative thrombophlebitis following intravenous line sepsis
Suppurative thrombophlebitis often follows prolonged intravenous therapy in immunocompromised patients. The condition is most common in patients receiving total parenteral nutrition, in critically ill patients receiving intravenous therapy through central venous catheters, and in those with long-term cannulation devices such as Hickman or Broviac catheters. Central suppurative thrombophlebitis may also be the consequence of intravenous drug abuse (see earlier discussion). Catheter infections are usually due to microorganisms that migrate from the skin entry site, but hematogenous seeding and contaminated fluids have also been implicated.135 Suppurative thrombophlebitis occurs subsequent to infection of the thrombus that typically develops around the intravenous device. The thrombus becomes attached to the central vein wall and causes localized inflammation.
Central suppurative thrombophlebitis should be suspected in any patient who fails to improve after removal of an infected central venous catheter. Systemic signs of infection are more common than venous obstructive symptoms such as arm edema. Diagnosis can be made by demonstrating a deep vein thrombosis (DVT) (by duplex ultrasonography, venography, or MRI) in a septic patient with positive blood cultures who does not have other sources of primary infection.140 Computed tomography scans may be useful to demonstrate a vein thrombosis, especially if gas is detected in the vein lumen.139
Treatment of central suppurative thrombophlebitis involves removal of central catheters, use of broad-spectrum antibiotics, and anticoagulation with heparin. In some cases, fibrinolytic therapy141 or surgical thrombectomy142 may be required. Long-term anticoagulation with warfarin is recommended to reduce the risk of embolization and recurrent thrombosis. A 2- to 3-week course of culture-directed antibiotics is usually appropriate.
Pelvic suppurative thrombophlebitis
Infection of the deep pelvic veins usually develops 2 to 3 weeks postpartum or after gynecological complications such as criminal abortions or other severe pelvic infections. Diagnosis of pelvic suppurative thrombophlebitis should be suspected in a postpartum woman with high fevers, chills, and abdominal pain. Large veins may cause ureteral obstruction, resulting in severe flank pain.139 Nearly 80% of cases are on the right side due to compression of the right ovarian vein at the pelvic brim by the gravid uterus.139 Although a tender vein can be palpated on pelvic examination in up to 30% of women, physical examination may be normal.139 Computed tomography scanning and MRI have both been useful to confirm deep pelvic vein thrombosis; a positive test in the clinical setting of sepsis confirms the diagnosis. Pelvic suppurative thrombophlebitis usually responds to broad-spectrum intravenous antibiotics. It remains controversial whether patients benefit from anticoagulation with heparin. Occasionally, patients do not respond to conservative treatment, and drainage of a pelvic abscess or ligation of the affected vein has been required.
Suppurative Thrombophlebitis of the Portal Vein (Pylephlebitis)
Suppurative thrombophlebitis of the portal vein usually follows infection of an organ drained by the portal vein or a contiguous structure. Historically, pylephlebitis was most commonly due to appendicitis.143 Widespread use of antibiotics and early diagnosis and intervention for abdominal infections have made the condition rare. Most modern cases occur as a complication of diverticulitis, but it has also followed other intraabdominal infections such as appendicitis, acute cholecystitis, and foreign body perforation.144 However, some are due to secondary infection of portal vein thrombosis associated with a hypercoagulable disorder such as cirrhosis or malignancy.145
The clinical presentation of patients with pylephlebitis is nonspecific: most have fevers of unknown origin with variable signs of systemic toxicity. Abdominal pain occurs in about three fourths of affected patients, and up to 20% have severe sepsis.144 Ultrasound may be helpful to detect thrombus within the lumen of the portal vein, but CT is the more common test to confirm the diagnosis. Magnetic resonance imaging with angiography may be able to discern acute from chronic thrombus.144,145 Many patients have other intraabdominal processes such as abscesses or common bile duct stones. Small intrahepatic liver abscesses may also be present.
Treatment of pylephlebitis involves use of broad-spectrum intravenous antibiotics and eradication of the underlying infection. Early treatment is critical to reduce the risk of ischemic bowel infarction from mesenteric vein thrombosis. Although systemic administration of broad-spectrum antibiotics is usually adequate, catheter infusion of antibiotics directly into the portal vein may result in more prompt improvement.146 Intraabdominal infections should be treated promptly by percutaneous drainage or surgical intervention. In rare cases, laparotomy and thrombectomy of the portal system have been used in severely ill patients.144 In all cases, antibiotics should be continued until complete resolution or cavernous transformation of the thrombus, as confirmed with CT or MRI.144 This usually requires at least 4 to 6 weeks. Fortunately, development of acute portal hypertension with variceal hemorrhage is uncommon.
Septic Thrombosis of the Cavernous Sinuses
Widespread use of antibiotics has dramatically reduced the incidence of cavernous sinus thrombophlebitis, but it is still occasionally seen in patients with severe head and neck infections. Infection of the ethmoid and sphenoid sinuses is the most common primary source that leads to cavernous sinus thrombophlebitis.147 S. aureus is isolated in approximately 60% to 70% of cases, followed by S. pneumoniae, gram-negative bacilli, and anaerobes.147 Early diagnosis and treatment are extremely important; mortality rates of 20% to 30% are still reported in the modern era.147
Patients with cavernous sinus thrombophlebitis typically present with fever, ptosis, proptosis, chemosis, and external ophthalmoplegia.147 Ocular signs are due to damage of nerves that traverse the cavernous sinus. Headache, papilledema, and periorbital swelling are present in more than half of affected patients. Diagnosis may be established by high-resolution CT scans or by MRI. Management includes treatment of the underlying infection (sinusitis, dental abscess, tonsillitis) and use of broad-spectrum antibiotics. Cavernous sinus drainage is almost never performed.147 At least one study148 suggests that anticoagulation with heparin may reduce the mortality rate in survivors. Severe sequelae have been reported in most survivors, including lung, brain, and orbital abscesses and prolonged cranial nerve dysfunction. Early diagnosis and treatment remain the keys to reducing potential disasters in these patients.
Septic Thrombophlebitis of the Internal Jugular Vein
This disorder was first described in 1936 by Lemierre, who reported a 90% mortality rate in his patients.149 Widespread use of antibiotics has reduced the prevalence and mortality of septic thrombophlebitis of the internal jugular vein (also known as postanginal septicemia or Lemierre’s syndrome), but the rarity of the syndrome also means that it is often overlooked. Several stages have been described.144 Septic thrombophlebitis of the internal jugular vein usually follows an acute oropharyngeal infection; a sore throat is the most common presentation. In the second stage, local invasion of the parapharyngeal space leads to septic thrombophlebitis of the internal jugular vein. This most commonly presents as swelling and tenderness of the neck, which should be considered a serious development in a patient with pharyngitis. Metastatic infections occur in the third stage, most commonly the lungs and joints.
Diagnosis of Lemierre’s syndrome should be considered in any patient with current or recent pharyngitis who presents with tenderness in the anterior cervical triangle. Clinical signs during the course of disease include fever, abdominal pain, and hyperbilirubinemia.144 Most patients have already developed metastatic infections at the time of diagnosis. A triad of pharyngitis, tender/swollen neck, and noncavitating pulmonary infiltrates has been described.150 The most common isolate is Fusobacterium necrophorum, an anaerobic gram-negative rod that is a normal inhabitant of the oral cavity.144,150 Diagnostic tests such as ultrasonography, CT, or MRI should be used to confirm the presence of internal jugular vein thrombosis. Computed tomography or MRI may be helpful to delineate the presence of neck abscesses that require drainage; these tests are also useful to follow the local anatomy once treatment has been instituted. Treatment involves a 3- to 6-week course of intravenous antibiotics; surgical excision of the internal jugular vein is only necessary in patients with uncontrolled sepsis or recurrent septic emboli despite appropriate antibiotic therapy (< 10% of cases).
1 Koch L. Ueber aneurysma der arterial mesenterichae superioris. Inag Di Erlangen, 1851.
2 Osler W. The Gulstonian lectures on malignant endocarditis. Br Med J. 1885;1:467.
3 Wilson S.E., Van Wagenen P., Passaro E.Jr. Arterial infection. Curr Probl Surg. 1978;15:1.
4 Stengel A., Wolferth C.C. Mycotic (bacterial) aneurysms of intravascular origin. Arch Intern Med. 1923;31:527.
5 Brown S.L., Busuttil R.W., Baker J.D., et al. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg. 1984;1:541.
6 Thompson R.W. Reflections on the pathogenesis of abdominal aortic aneurysms. Cardiovasc Surg. 2002;10:389.
7 Ailawadi G., Eliason J.L., Upchurch G.R. Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584.
8 Buckmaster M.J., Curci J.A., Murray P.R., et al. Source of elastin-degrading enzymes in mycotic aortic aneurysms: bacteria or host inflammatory response? Cardiovasc Surg. 1999;7:16.
9 Tilson M.D. Pathogenesis of mycotic aneurysms. Cardiovasc Surg. 1999;7:1.
10 Pierce R.A., Sandefur S., Doyle G.A., et al. Monocytic cell type-specific transcriptional induction of collagenase. J Clin Invest. 1996;97:1890.
11 Hsu R.B., Lin F.Y. Surgical pathology of infected aortic aneurysm and its clinical correlation. Ann Vasc Surg. 2007;21:742.
12 Gomes M.N., Choyke P.L., Wallace R.B. Infected aortic aneurysms. A changing entity. Ann Surg. 1992;215:435.
13 Oderich G.S., Panneton J.M., Bower T.C., et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34:900.
14 Fillmore A.J., Valentine R.J. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg. 2003;196:435.
15 Macedo T.A., Stanson A.W., Oderich G.S., et al. Infected aortic aneurysms: imaging findings. Radiology. 2004;231:250.
16 Oz M.C., Brener B.J., Buda J.A., et al. A ten-year experience with bacterial aortitis. J Vasc Surg. 1989;10:439.
17 McNamara M.F., Roberts A.B., Bakshi K.R. Gram-negative bacterial infection of aortic aneurysms. J Cardiovasc Surg (Torino). 1987;28:453.
18 Brouwer R.E., van Bockel J.H., van Dissel J.T. Streptococcus pneumoniae, an emerging pathogen in mycotic aneurysms? Neth J Med. 1998;52:16.
19 Goswami R., Cleveland K.O., Gelfand M.S. Evolving infectious aortitis caused by Streptococcus pneumoniae. South Med J. 2004;97:1004.
20 Katz S.G., Andros G., Kohl R.D. Salmonella infections of the abdominal aorta. Surg Gynecol Obstet. 1992;175:102.
21 Hsu R.B., Chen R.J., Wang S.S., et al. Infected aortic aneurysms: clinical outcome and risk factor analysis. J Vasc Surg. 2004;40:30.
22 Leon L.R.Jr, Mills J.L.Sr. Diagnosis and management of aortic mycotic aneurysms. Vasc Endovasc Surg. 2010;44:5.
23 van der Vliet J.A., Kouwenberg P.P., Muytjens H.L., et al. Relevance of bacterial cultures of abdominal aortic aneurysm contents. Surgery. 1996;119:129.
24 Farkas J.C., Fichelle J.M., Laurian C., et al. Long-term follow-up of positive cultures in 500 abdominal aortic aneurysms. Arch Surg. 1993;128:284.
25 Gouny P., Valverde A., Vincent D., et al. Human immunodeficiency virus and infected aneurysm of the abdominal aorta: report of three cases. Ann Vasc Surg. 1992;6:239.
26 McHenry M.C., Rehm S.J., Krajewski L.P., et al. Vertebral osteomyelitis and aortic lesions: case report and review. Rev Infect Dis. 1991;13:1184.
27 Hagino R.T., Clagett G.P., Valentine R.J. A case of Pott’s disease of the spine eroding into the suprarenal aorta. J Vasc Surg. 1996;24:482.
28 Muller B.T., Wegener O.R., Grabitz K., et al. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra-anatomic repair in 33 cases. J Vasc Surg. 2001;33:106.
29 Ihaya A., Chiba Y., Kimura T., et al. Surgical outcome of infectious aneurysm of the abdominal aorta with or without SIRS. Cardiovasc Surg. 2001;9:436.
30 Hsu R.B., Chang C.I., Wu I.H., et al. Selective medical treatment of infected aneurysms of the aorta in high risk patients. J Vasc Surg. 2009;49:66.
31 Dubois M., Daenens K., Houthoofd S., et al. Treatment of mycotic aneurysms with involvement of the abdominal aorta: single-centre experience in 44 consecutive cases. Eur J Vasc Surg. 2010. Aug 16 (Epub ahead of print)
32 Woon C.Y., Sebastian M.G., Tay K.H., et al. Extra-anatomic revascularization and aortic exclusion for mycotic aneurysms of the infrarenal aorta and iliac arteries in an Asian population. Am J Surg. 2008;195:66.
33 Robinson J.A., Johansen K. Aortic sepsis: is there a role for in situ graft reconstruction? J Vasc Surg. 1991;13:677.
34 Hollier L.H., Money S.R., Creely B., et al. Direct replacement of mycotic thoracoabdominal aneurysms. J Vasc Surg. 1993;18:477.
35 Fichelle J.M., Tabet G., Cormier P., et al. Infected infrarenal aortic aneurysms: when is in situ reconstruction safe? J Vasc Surg. 1993;17:635.
36 Gupta A.K., Bandyk D.F., Johnson B.L. In situ repair of mycotic abdominal aortic aneurysms with rifampin-bonded gelatin-impregnated Dacron grafts: a preliminary case report. J Vasc Surg. 1996;24:472.
37 Koshiko S., Sasajima T., Muraki S., et al. Limitations in the use of rifampicin-gelatin grafts against virulent organisms. J Vasc Surg. 2002;35:779.
38 Brown K.E., Heyer K., Rodriguez H., et al. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J Vasc Surg. 2009;49:660.
39 Benjamin M.E., Cohn E.J.Jr, Purtill W.A., et al. Arterial reconstruction with deep leg veins for the treatment of mycotic aneurysms. J Vasc Surg. 1999;30:1004.
40 Berchtold C., Eibl C., Seelig M.H., et al. Endovascular treatment and complete regression of an infected abdominal aortic aneurysm. J Endovasc Ther. 2002;4:543.
41 Kan C.D., Lee H., Luo C.Y., et al. The efficacy of aortic stent grafts in the management of mycotic abdominal aortic aneurysm–institute case management with systemic literature comparison. Ann Vasc Surg. 2010;24:433.
42 Lew W.K., Rowe V.L., Cunningham M.J., et al. Endovascular management of mycotic aortic aneurysms and associated aortoaerodigestive fistula. Ann Vasc Surg. 2009;23:81.
43 Forbes T.L., Harding G.J. Endovascular repair of Salmonella infected abdominal aortic aneurysms: a word of caution. J Vasc Surg. 2006;44:198.
44 Oweida S.W., Roubin G.S., Smith R.B.III, et al. Postcatheterization vascular complications associated with percutaneous transluminal coronary angioplasty. J Vasc Surg. 1990;12:310.
45 McCann R.L., Schwartz L.B., Pieper K.S. Vascular complications of cardiac catheterization. J Vasc Surg. 1991;14:375.
46 Biancari F., D’Andrea V., Di Marco C., et al. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159:518.
47 Whitton H.H.Jr, Rehring T.F. Femoral endarteritis associated with percutaneous suture closure: new technology, challenging complications. J Vasc Surg. 2003;38:83.
48 Bell C.L., Ali A.T., Brawley J.G., et al. Arterial reconstruction of infected femoral artery pseudoaneurysms using superficial femoropopliteal vein. J Am Coll Surg. 2005;200:831.
49 Schneider J.R., Oskin S.I., Verta M.J.Jr. Superficial femoral vein graft interposition in situ repair for femoral mycotic aneurysm. Ann Vasc Surg. 2009;23:147.
50 Arora S., Weber M.A., Fox C.J., et al. Common femoral artery ligation and local débridement: a safe treatment for infected femoral artery pseudoaneurysms. J Vasc Surg. 2001;33:990.
51 Patel A., Taylor S.M., Langan E.M.III, et al. Obturator bypass: a classic approach for the treatment of contemporary groin infection. Am Surg. 2002;68:653.
52 Buerger R., Benitez P. Surgical emergencies from intravascular injection of drugs. In: Bergan J.J., Yao J.S.T. Vascular surgical emergencies. Orlando: Grune & Stratton; 1987:309–318.
53 Reddy D.J., Smith R.F., Elliott J.P.Jr, et al. Infected femoral artery false aneurysms in drug addicts: evolution of selective vascular reconstruction. J Vasc Surg. 1986;3:718.
54 Ting A.C., Cheng S.W. Femoral pseudoaneurysms in drug addicts. World J Surg. 1997;21:783.
55 Cheng S.W., Fok M., Wong J. Infected femoral pseudoaneurysm in intravenous drug abusers. Br J Surg. 1992;79:510.
56 Mousavi S.R., Saberi A., Tadayon N., et al. Femoral artery ligation as treatment for infected for pseudoaneurysms secondary to drug injection. Acta Chir Belg. 2010;110:200.
57 Padberg F.Jr, Hobson R., Lee B., et al. Femoral pseudoaneurysm from drugs of abuse: ligation or reconstruction? J Vasc Surg. 1992;15:642.
58 Lorelli D.R., Cambria R.A., Seabrook G.R., et al. Diagnosis and management of aneurysms involving the superior mesenteric artery and its branches–a report of four cases. Vasc Endovascular Surg. 2003;37:59.
59 Stone W.M., Abbas M., Cherry K.J., et al. Superior mesenteric artery aneurysms: is presence an indication for intervention? J Vasc Surg. 2002;36:234.
60 Stanley J.C., Wakefield T.W., Graham L.M., et al. Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg. 1986;3:836.
61 Messina L.M., Shanley C.J. Visceral artery aneurysms. Surg Clin North Am. 1997;77:425.
62 Zimmerman-Klima P.M., Wixon C.L., Bogey W.M.Jr, et al. Considerations in the management of aneurysms of the superior mesenteric artery. Ann Vasc Surg. 2000;14:410.
63 Modrall J.G., Sadjadi J., Joiner D.R., et al. Comparison of superficial femoral vein and saphenous vein as conduits for mesenteric arterial bypass. J Vasc Surg. 2003;37:362.
64 El Sabrout R., Cooley D.A. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg. 2000;31:702.
65 Grossi R.J., Onofrey D., Tvetenstrand C., et al. Mycotic carotid aneurysm. J Vasc Surg. 1987;6:81.
66 Nader R., Mohr G., Sheiner N.M., et al. Mycotic aneurysm of the carotid bifurcation in the neck: case report and review of the literature. Neurosurgery. 2001;48:1152.
67 Wales L., Kruger A.J., Jenkins J.S., et al. Mycotic carotid pseudoaneurysm: staged endovascular and surgical repair. Eur J Vasc Endovasc Surg. 2010;39:23.
68 Safar H.A., Cina C.S. Ruptured mycotic aneurysm of the popliteal artery. A case report and review of the literature. J Cardiovasc Surg (Torino). 2001;42:237.
69 Seabrook G.R. Pathobiology of graft infections. Semin Vasc Surg. 1990;3:81.
70 Woodcock N.P., el Barghouti N., Perry E.P., et al. Is bacterial translocation a cause of aortic graft sepsis? Eur J Vasc Endovasc Surg. 2000;19:433.
71 Reilly L.M., Altman H., Lusby R.J., et al. Late results following surgical management of vascular graft infection. J Vasc Surg. 1984;1:36.
72 Macbeth G.A., Rubin J.R., McIntyre K.E.Jr, et al. The relevance of arterial wall microbiology to the treatment of prosthetic graft infections: graft infection vs. arterial infection. J Vasc Surg. 1984;1:750.
73 Buckels J.A., Fielding J.W., Black J., et al. Significance of positive bacterial cultures from aortic aneurysm contents. Br J Surg. 1985;72:440.
74 Fisher D.F.Jr, Clagett G.P., Fry R.E., et al. One-stage versus two-stage amputation for wet gangrene of the lower extremity: a randomized study. J Vasc Surg. 1988;8:428.
75 Josephs L.G., Cordts P.R., DiEdwardo C.L., et al. Do infected inguinal lymph nodes increase the incidence of postoperative groin wound infection? J Vasc Surg. 1993;17:1077.
76 Henke P.K., Bergamini T.M., Watson A.L., et al. Bacterial products primarily mediate fibroblast inhibition in biomaterial infection. J Surg Res. 1998;74:17.
77 Perera G.B., Fujitani R.M., Kubaska M. Aortic graft infection: update on management and treatment options. Vasc Endovasc Surg. 2006;40:1.
78 Valentine R.J. Diagnosis and management of aortic graft infection. Semin Vasc Surg. 2001;14:292.
79 Hallett J.W.Jr, Marshall D.M., Petterson T.M., Gray D.T., et al. Graft-related complications after abdominal aortic aneurysm repair: reassurance from a 36-year population-based experience. J Vasc Surg. 1997;25:277.
80 The United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445.
81 Szilagyi D.E., Smith R.F., Elliott J.P., et al. Infection in arterial reconstruction with synthetic grafts. Ann Surg. 1972;176:321.
82 Bandyk D.F. Aortic graft infection. Semin Vasc Surg. 1990;3:122.
83 White J.V., Freda J., Kozar R., et al. Does bacteremia pose a direct threat to synthetic vascular grafts? Surgery. 1987;102:402.
84 Goeau-Brissonniere O., Leport C., Lebrault C., et al. Antibiotic prophylaxis of late bacteremic vascular graft infection in a dog model. Ann Vasc Surg. 1990;4:528.
85 Jones L., Braithwaite B.D., Davies B., et al. Mechanism of late prosthetic vascular graft infection. Cardiovasc Surg. 1997;5:486.
86 Baddour L.M., Bettman M.A., Bolger A.F., et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015.
87 Padberg F.T.Jr, Smith S.M., Eng R.H. Accuracy of disincorporation for identification of vascular graft infection. Arch Surg. 1995;130:183.
88 Seabrook G.R., Schmitt D.D., Bandyk D.F., et al. Anastomotic femoral pseudoaneurysm: an investigation of occult infection as an etiologic factor. J Vasc Surg. 1990;11:629.
89 Orton D.F., LeVeen R.F., Saigh J.A., et al. Aortic prosthetic graft infections: radiologic manifestations and implications for management. Radiographics. 2000;20:977.
90 Sharp W.J., Hoballah J.J., Mohan C.R., et al. The management of the infected aortic prosthesis: a current decade of experience. J Vasc Surg. 1994;19:844.
91 Low R.N., Wall S.D., Jeffrey R.B.Jr, et al. Aortoenteric fistula and perigraft infection: evaluation with CT. Radiology. 1990;175:157.
92 Modrall J.G., Clagett G.P. The role of imaging techniques in evaluating possible graft infections. Semin Vasc Surg. 1999;12:339.
93 Olofsson P.A., Auffermann W., Higgins C.B., et al. Diagnosis of prosthetic aortic graft infection by magnetic resonance imaging. J Vasc Surg. 1988;8:99.
94 Brunner M.C., Mitchell R.S., Baldwin J.C., et al. Prosthetic graft infection: limitations of indium white blood cell scanning. J Vasc Surg. 1986;3:42.
95 Reilly D.T., Grigg M.J., Cunningham D.A., et al. Vascular graft infection: the role of indium scanning. Eur J Vasc Surg. 1989;3:393.
96 Lawrence P.F., Dries D.J., Alazraki N., et al. Indium 111-labeled leukocyte scanning for detection of prosthetic vascular graft infection. J Vasc Surg. 1985;2:165.
97 Liberatore M., Iurilli A.P., Ponzo F., et al. Clinical usefulness of technetium-99m-HMPAO-labeled leukocyte scan in prosthetic vascular graft infection. J Nucl Med. 1998;39:875.
98 Belair M., Soulez G., Oliva V.L., et al. Aortic graft infection: the value of percutaneous drainage. AJR Am J Roentgenol. 1998;171:119.
99 Harris K.A., Kozak R., Carroll S.E., et al. Confirmation of infection of an aortic graft. J Cardiovasc Surg (Torino). 1989;30:230.
100 Qvarfordt P.G., Reilly L.M., Mark A.S., et al. Computerized tomographic assessment of graft incorporation after aortic reconstruction. Am J Surg. 1985;150:227.
101 O’Hara P.J., Borkowski G.P., Hertzer N.R., et al. Natural history of periprosthetic air on computerized axial tomographic examination of the abdomen following abdominal aortic aneurysm repair. J Vasc Surg. 1984;1:429.
102 Ramo O.J., Vorne M., Lantto E., et al. Postoperative graft incorporation after aortic reconstruction–comparison between computerised tomography and Tc-99m-HMPAO labelled leucocyte imaging. Eur J Vasc Surg. 1993;7:122.
103 Sedwitz M.M., Davies R.J., Pretorius H.T., et al. Indium 111-labeled white blood cell scans after vascular prosthetic reconstruction. J Vasc Surg. 1987;6:476.
104 Yeager R.A., Taylor L.M.Jr, Moneta G.L., et al. Improved results with conventional management of infrarenal aortic infection. J Vasc Surg. 1999;30:76.
105 Bandyk D.F., Berni G.A., Thiele B.L., et al. Aortofemoral graft infection due to Staphylococcus epidermidis. Arch Surg. 1984;119:102.
106 Roy D., Grove D.I. Efficacy of long-term antibiotic suppressive therapy in proven or suspected infected abdominal aortic grafts. J Infect. 2000;40:184.
107 Seeger J.M., Pretus H.A., Welborn M.B., et al. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg. 2000;32:451.
108 Reilly L.M., Stoney R.J., Goldstone J., et al. Improved management of aortic graft infection: the influence of operation sequence and staging. J Vasc Surg. 1987;5:421.
109 Yeager R.A., Moneta G.L., Taylor L.M.Jr, et al. Improving survival and limb salvage in patients with aortic graft infection. Am J Surg. 1990;159:466.
110 Walker W.E., Cooley D.A., Duncan J.M., et al. The management of aortoduodenal fistula by in situ replacement of the infected abdominal aortic graft. Ann Surg. 1987;205:727.
111 Young R.M., Cherry K.J.Jr, Davis P.M., et al. The results of in situ prosthetic replacement for infected aortic grafts. Am J Surg. 1999;178:136.
112 Bandyk D.F., Novotney M.L., Back M.R., et al. Expanded application of in situ replacement for prosthetic graft infection. J Vasc Surg. 2001;34:411.
113 Hayes P.D., Nasim A., London N.J., Sayers R.D., Barrie W.W., Bell P.R., et al. In situ replacement of infected aortic grafts with rifampicin-bonded prostheses: the Leicester experience (1992 to 1998). J Vasc Surg. 1999;30:92.
114 Batt M., Jean-Baptiste E., O’Connor S., et al. In situ revascularization for patients with aortic graft infections: a single centre experience with silver coated polyester grafts. Eur J Vasc Endovasc Surg. 2008;236:182.
115 Knosalla C., Goeau-Brissonniere O., Leflon V., et al. Treatment of vascular graft infection by in situ replacement with cryopreserved aortic allografts: an experimental study. J Vasc Surg. 1998;27:689.
116 Leseche G., Castier Y., Petit M.D., et al. Long-term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J Vasc Surg. 2001;34:616.
117 Vogt P.R., Brunner-LaRocca H.P., Lachat M., et al. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:80.
118 Bisdas T., Bredt M., Pichlmaier M., et al. Eight-year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;2010:323.
119 Valentine R.J., Clagett G.P. Aortic graft infections: replacement with autogenous vein. Cardiovasc Surg. 2001;9:419.
120 Cardozo M.A., Frankini A.D., Bonamigo T.P. Use of superficial femoral vein in the treatment of infected aortoiliofemoral prosthetic grafts. Cardiovasc Surg. 2002;10:304.
121 Daenens K., Fourneau I., Nevelsteen A. Ten-year experience in autogenous reconstruction with the femoral vein in the treatment of aortofemoral prosthetic infection. Eur J Vasc Endovasc Surg. 2003;25:240.
122 Ehsan O., Gibbons C.P. A 10-year experience of using femoro-popliteal vein for revascularization in graft and arterial infections. Eur J Vasc Endovasc Surg. 2009;38:172.
123 Franke S., Voit R. The superficial femoral vein as arterial substitute in infections of the aortoiliac region. Ann Vasc Surg. 1997;11:406.
124 Ali A.T., Modrall J.G., Hocking J., et al. Long-term results of the treatment of aortic graft infection by in situ replacement with femoral popliteal vein grafts. J Vasc Surg. 2009;50:30.
125 Modrall J.G., Hocking J.A., Rosero E., et al. Late incidence of chronic venous insufficiency after deep vein harvest. J Vasc Surg. 2007;46:520.
126 Towne J.B., Seabrook G.R., Bandyk D., et al. In situ replacement of arterial prosthesis infected by bacterial biofilms: long-term follow-up. J Vasc Surg. 1994;19:226.
127 Calligaro K.D., Veith F.J., Valladares J.A., et al. Prosthetic patch remnants to treat infected arterial grafts. J Vasc Surg. 2000;31:245.
128 Setacci C., De Donato G., Setacci F., et al. Management of abdominal endograft infection. J Cardiovasc Surg. 2010;51:33.
129 Heyer K.S., Modi P., Morasch M.D., et al. Secondary infections of thoracic and abdominal aortic endografts. J Vasc Interv Radiol. 2009;20:173.
130 Chang J.K., Calligaro K.D., Ryan S., et al. Risk factors associated with infection of lower extremity revascularization: analysis of 365 procedures performed at a teaching hospital. Ann Vasc Surg. 2003;17:91.
131 Treiman G.S., Copland S., Yellin A.E., et al. Wound infections involving infrainguinal autogenous vein grafts: a current evaluation of factors determining successful graft preservation. J Vasc Surg. 2001;33:948.
132 Calligaro K.D., Veith F.J., Schwartz M.L., et al. Management of infected lower extremity autologous vein grafts by selective graft preservation. Am J Surg. 1992;164:291.
133 Tukiainen E., Biancari F., Lepantalo M. Deep infection of infrapopliteal autogenous vein grafts–immediate use of muscle flaps in leg salvage. J Vasc Surg. 1998;28:611.
134 Parmar C.D., Kumar S., Torella F. Autogenous basilic vein for in situ replacement of infected prosthetic vascular grafts: initial experience. J Vasc Surg. 2009;17:158.
135 Chitticki P., Sherertz R.J. Recognition and prevention of nosocomial vascular device and related bloodstream infections in the intensive care unit. Crit Care Med. 2010;38:S363.
136 Tagalakis V., Kahn S.R., Libman M., et al. The epidemiology of peripheral vein infusion thrombophlebitis: a critical review. Am J Med. 2002;113:146.
137 Lewis G.B.H., Hecker J.F. Infusion thrombophlebitis. Br J Anaesth. 1985;57:220.
138 Stratton C.W. Infection related to intravenous infusions. Heart Lung. 1982;11:123.
139 Bayer A.S., Scheld W.M. Endocarditis and intravascular infections. In: Mandell G.L., Bennett J.E., Dolen R. Principles and practice of Infectious Diseases. ed 5. New York: Churchill Livingstone; 2000:857–902.
140 Miceli M., Atoui R., Thertulien R., et al. Deep septic thrombophlebitis: an unrecognized cause of relapsing bacteremia in patients with cancer. J Clin Oncol. 2004;22:1529.
141 Andes D.R., Urban A.W., Archer C.W., et al. Septic thrombosis of the basilic, axillary, and subclavian veins caused by a peripherally inserted central venous catheter. Am J Med. 1998;105:446.
142 Kuiemeyer H.W., Grabitz K., Buhl R., et al. Surgical treatment of septic deep venous thrombosis. Surgery. 1995;118:49.
143 Plemmons R.M., Dooley D.P., Longfield R.N. Septic thrombophlebitis of the portal vein (pyelophlebitis): diagnosis and management in the modern era. Clin Infect Dis. 1995;2:1114.
144 Chirinos J.A., Garcia J., Alcaide M.L., et al. Septic thrombophlebitis. diagnosis and management, Am J Cardiovasc Drugs. 2006;6:9.
145 Singh P., Yadav N., Visvalingam V., et al. Pyelophlebitis – diagnosis and management. Am J Gastroenterol. 2001;96:1312.
146 Pelsang R.E., Johlin F., Dhada R., et al. Management of suppurative pyelophlebitis by percutaneous drainage: placing a drainage catheter into the portal vein. Am J Gastroenterol. 2001;96:3192.
147 Ebright J.R., Pace M.T., Niazi A.F. Septic thrombosis of the cavernous sinuses. Arch Intern Med. 2001;161:2671.
148 Levine S.R., Twyman R.E., Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988;28:517.
149 Lemierre A. On certain septicemias due to anaerobic organisms. Lancet. 1936;1:701.
150 Chirinos J.A., Lichtstein D.M., Garcia J., et al. The evolution of Lemierre syndrome: report of two cases and review of the literature. Medicine. 2002;81:458.