Chapter 48 Raynaud’s Phenomenon
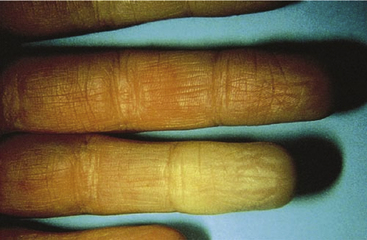
Episodic vasospastic ischemia of the digits was first described by Maurice Raynaud in the quotation above1 (Fig. 48-1). Raynaud’s phenomenon comprises sequential development of digital blanching, cyanosis, and rubor following cold exposure and subsequent rewarming2 (Fig. 48-2). Emotional stress also precipitates Raynaud’s phenomenon. The color changes are usually well demarcated and primarily confined to fingers or toes. Blanching, or pallor, occurs during the ischemic phase of the phenomenon and is secondary to digital vasospasm. During ischemia, arterioles, capillaries, and venules dilate. Cyanosis results from the deoxygenated blood in these vessels. Cold, numbness, or paresthesias of the digits often accompany the phases of pallor and cyanosis. With rewarming, digital vasospasm resolves, and blood flow dramatically increases into the dilated arterioles and capillaries. This “reactive hyperemia” imparts a bright red color to the digits. In addition to rubor and warmth, patients often experience a throbbing sensation during the hyperemic phase. Thereafter, the color of the digits gradually returns to normal. Although the triphasic color response is typical of Raynaud’s phenomenon, some patients may develop only pallor and cyanosis. Others may experience only cyanosis.
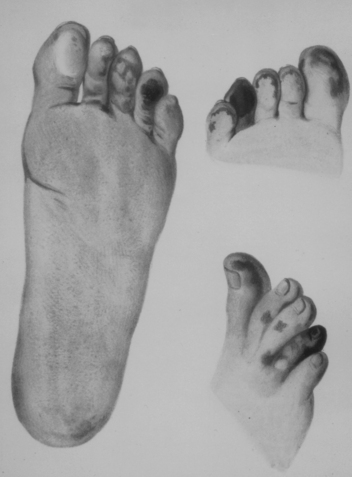
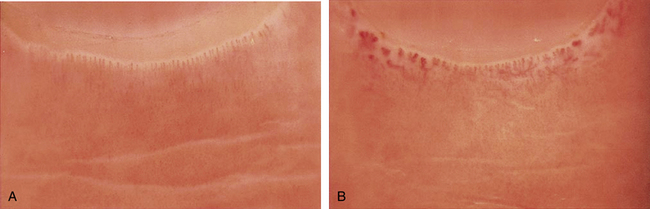
Figure 48-1 A patient with Raynaud’s phenomenon.
(From Raynaud M: Local asphyxia and symmetrical gangrene of the extremities, London, 1862, New Sydenham Society. Courtesy Boston Medical Library in the Francis A. Countway Library of Medicine.)
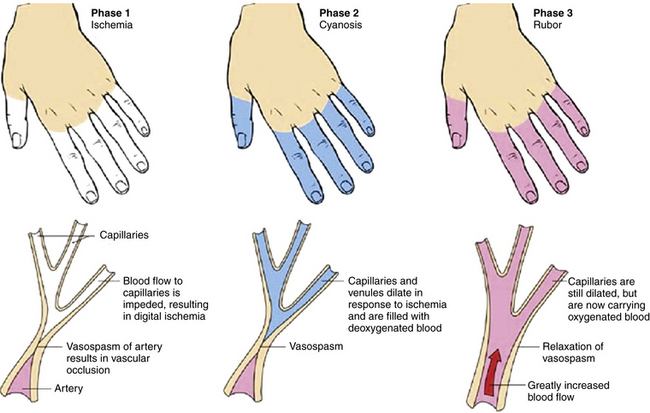
Figure 48-2 Raynaud’s phenomenon may have three color phases: blanching, cyanosis, and rubor.
(From Creager MA: Raynaud’s phenomenon. Med Illus 2:84, 1983.)
The classification of Raynaud’s phenomenon is broadly separated into two categories: (1) the idiopathic variety, termed primary Raynaud’s phenomenon, and (2) the secondary variety, associated with other disease states or known causes of vasospasm (Box 48-1). Secondary causes of Raynaud’s phenomenon include collagen vascular diseases, arterial occlusive disease, thoracic outlet syndrome, several neurological disorders, blood dyscrasias, trauma, and several drugs.
Overview of Primary Raynaud’s Phenomenon
Primary Raynaud’s phenomenon, or idiopathic episodic digital vasospasm, is the most common diagnosis of patients who present with Raynaud’s phenomenon.2 The diagnosis is based on criteria originally established by Allen and Brown,3 including (1) intermittent attacks of ischemic discoloration of the extremities, (2) absence of organic arterial occlusions, (3) bilateral distribution, (4) trophic changes—when present, limited to the skin and never consisting of gross gangrene, (5) absence of any symptoms or signs of systemic disease that might account for the occurrence of Raynaud’s phenomenon, and (6) symptom duration for 2 years or longer. If a normal erythrocyte sedimentation rate (ESR), normal nailfold capillary examination, and negative test for antinuclear antibodies (ANAs) are added to these criteria, the diagnosis is more secure.
Women are affected approximately five times more frequently than men. In one large study, the average age of onset of Raynaud’s phenomenon was 31 years; 78% of the patients were younger than 40 when symptoms began.4 Onset of symptoms in women may occur between menarche and menopause. Raynaud’s phenomenon is also known to occur in young children.5,6 Prevalence of primary Raynaud’s phenomenon varies with climate, with 4.6% of the population affected in warm climates, compared with 17% in cooler climates.6 There is a significant familial aggregation of primary Raynaud’s phenomenon. Approximately 26% of patients may know of one or more relatives who have the phenomenon, suggesting a genetic predisposition.7
In the vast majority of patients, the fingers are the initial sites of involvement.2 At first, blanching or cyanosis may involve only one or two fingers (Fig. 48-3). Later, color changes may develop in additional fingers, and symptoms occur bilaterally. In about 40% of patients, Raynaud’s phenomenon involves the toes as well as the fingers. Isolated Raynaud’s phenomenon of the toes occurs in only 1% to 2% of patients. Rarely, the ear lobes, tip of the nose, or tongue are affected.
Several studies have correlated Raynaud’s phenomenon with migraine headaches and variant angina, suggesting a common mechanism for vasospasm.8–10 An association with vasospasm in the kidney,11 retina,12 and pulmonary13 vessels has also been described. Further evidence is the report of a family with three generations of systemic arterial vasospastic disease involving Raynaud’s phenomenon, variant angina, and migraine headaches.14 Differences in the responses of pharmacological intervention make the hypothesis of a common mechanism less appealing.15 Propranolol has been successfully used to prevent migraine headaches.16 In contrast, β-adrenoceptor blockers are not beneficial in variant angina and may cause Raynaud’s phenomenon.17,18 Similarly, nitrates are used for variant angina but are not beneficial in Raynaud’s phenomenon and often cause headaches. Ergot alkaloids are effective for treating migraine headaches but can cause coronary and digital vasospasm.19,20
Of all the forms of Raynaud’s phenomenon, primary Raynaud’s phenomenon has the most benign prognosis. In the group of patients identified by Gifford and Hines4 followed for a period of 1 to 32 years (average 12 years), 16% reported worsening of their symptoms, and 38%, 36%, and 10%, respectively, reported no change, improvement, or disappearance of symptoms. Sclerodactyly or trophic changes of the digits occurred in approximately 3% of patients during follow-up, and less than 1% of patients lost part of a digit. In some patients, scleroderma may develop after Raynaud’s phenomenon has been present as the only symptom for more than 20 years. Wollersheim et al.21 reported that measuring ANAs by immunofluorescence and immunoblotting in patients with Raynaud’s phenomenon had a positive predictive value of 65% and 71%, and a negative predictive value of 93% and 83%, respectively, for development of a connective tissue disease.
Pathophysiology
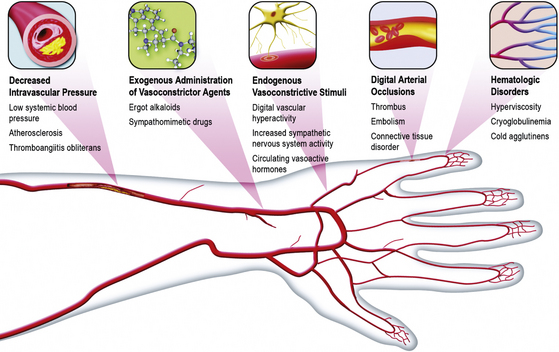
The precise cause of Raynaud’s phenomenon has not been clearly identified. It is quite likely that a variety of physiological and pathological conditions may contribute to or cause digital vasospasm2 (Box 48-2 and Fig. 48-4).
![]() Box 48-2 Possible Pathophysiological Mechanisms of Raynaud’s Phenomenon
Box 48-2 Possible Pathophysiological Mechanisms of Raynaud’s Phenomenon
Local Vascular Hyperreactivity
The observation that episodic digital vasospasm occurs during cold exposure has led several investigators to consider the possibility that Raynaud’s phenomenon occurs as a result of a local vascular hyperreactivity. In 1929, Sir Thomas Lewis observed that following exposure of the finger to cold, vasospasm could be produced even after nerve blockade or sympathectomy.22 These experiments were repeated and confirmed 60 years later.23 Therefore, the vasospastic response of the Raynaud’s phenomenon may occur in the absence of efferent digital nerves. The possibility of local vascular hyperreactivity was examined by Jamieson et al.24 They compared the magnitude of reflex vasoconstriction in each hand following application of ice to the neck while one hand was kept at 26 °C and the other at 36 °C.9 At 36 °C, the reflex vasoconstrictor response was comparable in normal subjects and patients with primary Raynaud’s phenomenon. In the hand cooled to 26 °C, however, reflex vasoconstriction was exaggerated in patients with Raynaud’s phenomenon. This response led these investigators to hypothesize that digital α1 adrenoceptors were sensitized by cold exposure.
A series of studies by Vanhoutte et al.25 have supported the hypothesis that cooling potentiates the vascular response to sympathetic nerve activation. Vasoconstriction, in response to exogenous norepinephrine, also is increased by cooling. Augmentation of adrenergic-mediated vasoconstriction by cooling occurs despite generalized depression of contractile machinery and diminished release of norepinephrine from sympathetic nerve endings in the vessel wall. The most likely hypothesis is that cold causes changes at the level of the adrenoceptor, such as an increase in the affinity for norepinephrine or greater efficacy of the agonist/receptor complex. Vanhoutte et al.25 have reported that α2 adrenoceptors are more sensitive than α1 adrenoceptors to temperature change. Whereas cooling slightly depresses α1 adrenergic–mediated vasoconstriction, it markedly augments α2 adrenergic–mediated responses. Conversely, warming augments α1-adrenergic vasoconstriction and depresses α2-adrenergic vasoconstriction.26
These experimental observations may have important implications regarding the pathophysiology of Raynaud’s phenomenon. Flavahan et al.27 examined the distribution of α1 and α2 adrenoceptors in arterial tissue from amputated limbs of patients who did not have vascular disease. They reported that α2 adrenoceptors were more prominent in digital arteries. Chotani et al.28 found that human dermal arterioles selectively expressed α2C adrenoceptors. Jeyaraj et al.29 observed that cooling redistributed α2C adrenoceptors from the Golgi to the plasma membrane in human embryonic kidney cells. It is therefore an intriguing observation by Keenan and Porter that the density of α2 adrenoreceptors is increased in platelets from patients with Raynaud’s disease.30
In support of these findings, Coffman and Cohen reported that α2 adrenoceptors were more important than α1 adrenoceptors in mediating sympathetic nerve–induced vasoconstriction in the fingers.31 They administered the α1-antagonist prazosin and the α2-antagonist yohimbine to patients with Raynaud’s phenomenon during reflex sympathetic vasoconstriction caused by body cooling. Whereas prazosin caused no significant change in finger blood flow or finger vascular resistance, yohimbine significantly increased finger blood flow and decreased finger vascular resistance. This study confirmed that postjunctional α2 adrenoceptors are present in human digits and strongly suggested that these receptors contribute to digital vasoconstriction during environmental cooling in patients with Raynaud’s phenomenon.
Thereafter, Coffman and Cohen demonstrated that compared to normal subjects, patients with Raynaud’s phenomenon were hypersensitive to the vasoconstrictor effects of clonidine, an α2-adrenoceptor agonist, but not to phenylephrine, an α1-adrenoceptor agonist.31 Cooke et al.32 found that both α1– and α2-adrenoceptor antagonists induced digital vasodilation in patients with acute Raynaud’s phenomenon, yet did not inhibit digital vasoconstriction caused by local digital cooling. Although still speculative, these studies suggest that episodic digital vasospasm may be secondary to a predominance of postjunctional α2 adrenoceptors in digits of patients with primary Raynaud’s phenomenon.
Increased Sympathetic Nervous System Activity
Although appealing as a potential mechanism for digital vasospasm, the concept of exaggerated reflex sympathetic vasoconstrictor responses to cold environment has not been convincingly demonstrated. Increased concentrations of epinephrine and norepinephrine in peripheral venous blood at the wrist were found to be higher in patients with primary Raynaud’s phenomenon than in normal subjects by one investigator,33 but others found normal local levels of norepinephrine in brachial arterial and venous blood samples.34 The latter group of investigators reported that the reflex vasoconstrictor response of the hand to a cold stimulus in affected patients is similar to that in a control group, and there were comparable vasoconstrictor responses to the intraarterial infusion of tyramine, a drug that causes vasoconstriction by releasing norepinephrine from sympathetic nerve terminals. Central thermoregulatory control of skin temperature has also been reported to be comparable in normal individuals and patients with primary Raynaud’s phenomenon.35 Finally, microelectrode recordings of skin sympathetic nerve activity do not demonstrate an abnormality in patients with primary Raynaud’s phenomenon.36 There was no hypersensitivity of the vessels to strong sympathetic stimuli or abnormal increase in sympathetic outflow.
β-Adrenergic Blockade
Raynaud’s phenomenon is observed frequently in individuals treated with β-adrenoceptor antagonists.37–39 It may be inferred from this observation that β-adrenergic vasodilation normally attenuates digital vasoconstrictor tone. Cohen and Coffman40 examined the effect of isoproterenol and propranolol on fingertip blood flow after vasoconstriction had been induced by a brachial artery infusion of norepinephrine or angiotensin, or reflexly by environmental cooling. Intraarterial isoproterenol administration increased fingertip blood flow during infusions of norepinephrine and angiotensin, but not during reflex sympathetic vasoconstriction. Conversely, propranolol served to potentiate vasoconstriction caused by intraarterial norepinephrine, but not that caused by reflex sympathetic vasoconstriction. These investigators concluded that a β-adrenergic vasodilator mechanism may be active in human digits, but does not modulate sympathetic vasoconstriction. There is no evidence to support the contention that decreased sensitivity or number of β adrenoceptors contributes to the pathophysiology of Raynaud’s phenomenon in the absence of pharmacological blockade of β adrenoceptors.
Vasoconstriction Caused by Circulating Vascular Smooth Muscle Agonists
The possibility that vasoconstrictors released during platelet aggregation may be pertinent to the pathophysiology of Raynaud’s phenomenon has been further evaluated by studies that have either measured levels of TxA2 or administered a thromboxane synthetase inhibitor.41,42 Coffman and Rasmussen compared the thromboxane synthetase inhibitor dazoxiben to placebo in patients with either primary or secondary Raynaud’s phenomenon.41 Dazoxiben did not affect total fingertip blood flow or fingertip capillary blood flow, whether measured in a warm (28.3 °C) or cool (20 °C) environment. With chronic treatment, there was a small decrease in frequency of vasospastic episodes in patients with primary Raynaud’s phenomenon. To date, however, there is insufficient evidence to support a role for TxA2 in digital vasospasm.
Endothelin-1 is an endothelium-derived, powerful, and prolonged-acting vasoconstrictor agent suggested to play a part in the pathogenesis of Raynaud’s phenomenon. It rises in response to a cold pressor test and constricts cutaneous blood vessels.43 Studies measuring ET-1 in primary or secondary Raynaud’s phenomenon have been conflicting.44 Controlled clinical trials of ET-1 receptor antagonism in the treatment of Raynaud’s disease have achieved little success.45,46 It is therefore doubtful that it plays a role in Raynaud’s phenomenon.
Decreased Intravascular Pressure
Patency of a blood vessel requires balance between arterial wall tension (favoring closure of the vessel) and intravascular distending pressure. Landis measured intravascular pressure in patients with Raynaud’s phenomenon by introducing a micropipette into a large digital capillary.47 During cyanosis, capillary pressure fell to approximately 5 mmHg, and flow ceased. These findings suggested that the site of closure was proximal to the capillaries at the arterial level. Interestingly, Thulesius reported that brachial artery blood pressure in patients with primary Raynaud’s phenomenon was significantly lower than that in a normal control population.48 Cohen and Coffman also found that blood pressure was lower in patients with primary Raynaud’s phenomenon compared with normal subjects.49 In addition to lower brachial blood pressure, systolic blood pressure (SBP) measured at the proximal and distal digital arteries averaged 18 mmHg less than that in normal digits.
Hyperviscosity may reduce blood flow velocity in digital vessels, leading to a decrease in intravascular pressure. Indeed, Raynaud’s phenomenon occurs in patients with hyperviscosity due to polycythemia vera or Waldenström macroglobulinemia.50,51 In patients with Raynaud’s phenomenon secondary to disorders such as cryoglobulinemia and cold agglutinin disease, hyperviscosity caused by cooling may contribute to digital vasospasm.52–54 Indeed, cooling has been shown to abolish hand blood flow in patients with cold agglutinins, possibly because the vessels become occluded by agglutinated red cells.54 Data invoking hyperviscosity as a cause of Raynaud’s phenomenon in patients who do not have an established blood dyscrasia, however, are less compelling.
Secondary Causes of Raynaud’s Phenomenon
The secondary causes of Raynaud’s phenomenon include collagen vascular diseases, arterial occlusive disorders, thoracic outlet syndrome, several neurological disorders, blood dyscrasias, trauma, and several drugs (see Box 48-1).
Collagen Vascular Diseases
Systemic sclerosis (scleroderma)
Several serological studies are consistent with the diagnosis of scleroderma. Erythrocyte sedimentation rate may be elevated, and ANAs are present in the majority of individuals with this disorder. Patients may have antibodies to nucleolar antigens, nuclear ribonucleoprotein, and to the centromeric region of metaphase chromosomes. In patients with systemic sclerosis and Raynaud’s phenomenon, capillary microscopy often demonstrates enlarged and deformed capillary loops surrounded by relatively avascular areas, particularly in the nailfolds.55 Angiography frequently demonstrates digital vascular obstruction.
Systemic lupus erythematosus
6. Serositis, including pleuritis or pericarditis.
7. Renal disorders, including persistent proteinuria or cellular casts.
8. Neurological disorders, such as seizures and psychosis.
9. Hematological disorders, including hemolytic anemia, leukopenia, lymphopenia, or thrombocytopenia.
11. Abnormal titers of antinuclear antibody, especially anti- deoxyribonucleic acid (DNA)56
Rheumatoid arthritis
Raynaud’s phenomenon also occurs in patients with rheumatoid arthritis. These patients may have vasculitis of medium-sized vessels, as well as proliferative endarteritis of small vessels. Crops of small brown spots may be observed in the nail beds and digital pulp. Digital blood flow is often reduced in patients with rheumatoid arthritis, and angiography frequently reveals occlusions of one or more digital arteries.57 The diagnosis is suggested in patients who have at least one joint with synovitis, with the synovitis not explained by another disease, and who score 6/10 points or more on the following consensus diagnostic criteria58:
1. Involvement of 2 to 10 large joints (1 point).
2. 1 to 3 small joints (2 points).
3. 4 to 10 small joints (3 points).
4. More than 10 joints (5 points).
5. Low-positive rheumatoid factor (RF) or low-positive anticitrullinated protein/peptide (ACPA) (2 points).
6. High-positive RF or high-positive ACPA (3 points).
7. Abnormal C-reactive protein (CRP) or abnormal ESR (1 point).
Primary sjögren’s syndrome
Sjögren’s syndrome is an autoimmune disease that mainly affects exocrine glands and leads to dryness of the eyes and mouth, but it can have extraglandular manifestations. Raynaud’s phenomenon has been reported in 13% to 33% of patients and may precede the sicca symptomatology in many.59 Presence of anticentromere antibody increases the likelihood of associated Raynaud’s phenomenon, possibly because of an association with an increase in fibrous tissues.60 The clinical course is usually milder than in patients with systemic sclerosis. Antinuclear antibody tests are often positive, but the diagnosis is usually made by the clinical picture.
Mixed connective tissue disease
Mixed connective tissue disease (MCTD) is a disorder with overlapping clinical features of SLE, scleroderma, and myositis, and presence of a distinctive antibody against U1-ribonucleoprotein (RNP). Raynaud’s phenomenon is the main symptom in mixed connective tissue disease, and trophic abnormalities of the fingers are frequently observed. Elevated anti-U1-RNP antibody titers and an abnormal capillaroscopic pattern are specific for the condition.61
Arterial Occlusive Disease
Thromboangiitis obliterans (or Buerger’s disease) is an inflammatory occlusive vascular disorder involving small- and medium-sized arteries and veins, often accompanied by Raynaud’s phenomenon (see Chapter 44). In addition to Raynaud’s phenomenon, clinical features of TAO include claudication of the affected extremity and migratory superficial vein thrombosis in a young male.
Thoracic Outlet Syndrome
Compression of the neurovascular bundle as it courses through the neck and shoulder can result in a symptom complex that includes Raynaud’s phenomenon as well as shoulder and arm pain, weakness, paresthesias, and claudication of the affected upper extremity (see Chapter 62). Raynaud’s phenomenon may result from the decreased intravascular pressure caused by extrinsic compression of the subclavian artery. Whether compression of the brachial plexus alters sympathetic nervous system activity is unknown.
Neurological Disorders
Raynaud’s phenomenon has been reported in approximately 10% of patients with carpal tunnel syndrome.62 This entrapment neuropathy is due to compression of the median nerve as it passes through the carpal tunnel. It may result from pregnancy, localized tenosynovitis, trauma, hypothyroidism, amyloidosis, or activities associated with repeated motion of the wrist. Patients usually experience paresthesias or weakness in the distribution of the median nerve. The diagnosis is suggested when symptoms are reproduced by tapping the volar surface of the wrist (Tinel sign) or by maintaining flexion of the wrist (Phalen maneuver). Nerve conduction tests usually demonstrate abnormalities of the median nerve at the wrist. Supportive treatment includes splints and antiinflammatory drugs. With severe persistent symptoms, surgical release of the carpal ligament may be beneficial.
Complex regional pain syndrome, previously known as reflex sympathetic dystrophy or causalgia, is another neurological disorder associated with cyanotic extremities and involves pain and tenderness of a distal extremity, with accompanying vasomotor instability (see Chapter 52).
Blood Dyscrasias
Patients with cold agglutinins occasionally develop Raynaud’s phenomenon. It is generally thought that Raynaud’s phenomenon develops when proteins precipitate on red blood cells and agglutinate within the digital vessel during exposure to cold. Prolonged exposure may cause thrombosis and subsequent digital gangrene. Cold agglutinin disease usually involves immunoglobulin (Ig)M antibodies that are reactive with I antigen.63 The antibody titer is high at 4 °C and low at 37 °C. These antibodies also may cause cold-induced hemolysis. Agglutination usually does not occur in temperatures above 32 °C. Cold agglutinins may arise spontaneously or occur in patients with mycoplasma pneumonia, infectious mononucleosis, or lymphoproliferative disorders. Cold agglutinin disease may be short lived in patients with infectious causes but is often persistent in patients with lymphoproliferative disease.
Cryoglobulins are a group of proteins that precipitate in cold serum and may cause Raynaud’s phenomenon.64 Cryoglobulins are associated with monoclonal and polyclonal gammopathies in disorders such as Waldenström macroglobulinemia, SLE, and rheumatoid arthritis. Cryoglobulinemia has been categorized into three subtypes. Type I cryoglobulins include monoclonal immunoglobulins of a single class, usually associated with lymphoproliferative disorders such as multiple myeloma. Type II encompasses mixed cryoglobulins containing monoclonal IgM or rheumatoid factor and polyclonal IgG. This may occur in patients with Waldenström macroglobulinemia or chronic active hepatitis. In patients with Waldenström macroglobulinemia, about 10% of macroglobulins are cryoglobulins. Type III cryoglobulinemia includes polyclonal IgM and IgG immunoglobulins, as may occur in SLE. Indeed, approximately 80% of patients with lupus have cold-insoluble precipitates. In these patients, there is a significantly higher level of cryoprecipitating IgM class rheumatoid factors than in other patients. Most patients with mixed cryoglobulinemia have a chronic hepatitis C infection.
Cryofibrinogenemia is a rare condition that may be associated with digital vasospasm.65 The plasma, but not the serum, of patients with cryofibrinogenemia forms a gelatinous precipitate at 4 °C. Disorders associated with cryofibrinogenemia include disseminated intravascular coagulation, collagen vascular diseases, thromboembolism, and diabetes mellitus.
Trauma
Various traumatic injuries are associated with Raynaud’s phenomenon and have been designated traumatic vasospastic diseases. Causes of traumatic vasospastic disease include electric shock injury, thermal injuries such as frostbite, and mechanical percussive injury associated with piano playing and typing. The most common traumatic cause of Raynaud’s phenomenon is repeated exposure to vibrating tools. This has occasionally been referred to as vibration white finger syndrome. It has been reported in lumberjacks and other users of chainsaws, stonecutters who use air hammers, operators of pneumatic hand grinders and impact wrenches in the engine manufacturing industry, and road drillers. Prevalence of Raynaud’s phenomenon induced by vibration ranges from 33% to 71% among members of populations at risk and increases with exposure time.66,67 It has been suggested that the combination of vibration and cold exposure in many of these workers is responsible for development of Raynaud’s phenomenon.68 Pathophysiological changes may involve both the vascular and neurological systems in these individuals and may contribute to digital vasospasm. Intimal thickening of peripheral arteries has been reported in animals exposed to repeated vibration, but pathological changes of the blood vessels have not consistently been demonstrated.69 Medial muscular hypertrophy and subintimal fibrosis have been found in biopsy specimens of digital arteries of patients in one study, but not in others. Nailbed capillaroscopy has shown a reduction in the number of capillaries. Arteriograms of these patients have shown arterial occlusion of the distal radial and ulnar arteries and frequently of the palmar arch.
Neurophysiological abnormalities have not been consistently demonstrated in patients with Raynaud’s phenomenon secondary to vibration. Although some have found a high instance of abnormal electromyograms, others have found that episodes of Raynaud phenomena occur independently of electromyographic abnormalities.70 Peripheral nerve conduction velocities are often abnormal in vibrating tool operators, and pathological changes have also been reported in the nerves of patients with vibration white finger, including axonal degeneration, demyelination, and collagenization of perineurium and endoperineurium. Thus the precise pathophysiology of Raynaud’s phenomenon in patients repeatedly exposed to vibratory stimuli is unclear. Some have suggested that overexcitation of the Pacinian corpuscles causes reflex efferent sympathetic nerve activity. Others have suggested that following vibration, cutaneous vessels become more reactive to sympathetic stimuli.
Drugs and Toxins
Various drugs have been implicated in producing Raynaud’s phenomenon or digital vasospasm (see Box 48-1). Although some of these drugs act by directly causing vasoconstriction, the mechanism whereby others cause Raynaud’s phenomenon is not known.
Raynaud’s phenomenon has also been associated with use of at least three chemotherapeutic agents: vinblastine, gemcitabine, and bleomycin. Although it is unknown how these compounds cause Raynaud’s phenomenon, it has been reported that bleomycin causes pathological changes in small blood vessels. Vinblastine can induce peripheral neuropathy and perhaps interfere with the autonomic reflexes. Gemcitabine therapy has been associated with Raynaud’s phenomenon and digital ischemia due to endothelial damage secondary to inflammatory changes, and from thrombotic microangiopathy.71
Industrial exposure to vinyl chloride polymerization processes may cause acro-osteolysis of the distal phalanges of the fingers, changes that are occasionally associated with Raynaud’s phenomenon, but one recent study found the phenomenon less frequent among workers than in the population.72
β-Adrenoceptor blocking drugs may cause Raynaud’s phenomenon. Although the mechanism of action is unknown, possibilities include unopposed stimulation of vascular α adrenoceptors or reflex sympathetic vasoconstriction initiated by the central cardiovascular depressant effect of β-adrenergic blockade. It remains controversial whether cardioselective β-adrenoceptor blocking drugs cause Raynaud’s phenomenon less frequently than nonselective drugs, and whether there is less digital vasospasm with drugs that also have α-adrenoceptor blocking properties or intrinsic sympathomimetic activity. One placebo-controlled study examined both cardioselective and nonselective β-adrenoceptor blockers in patients who already had Raynaud’s phenomenon.73 Compared with placebo, neither metoprolol nor propranolol decreased fingertip blood flow, despite exposure to a cool environment. Furthermore, chronic treatment did not increase the number of vasospastic attacks in patients receiving either drug compared with placebo. One might conclude from these observations that β-adrenoceptor blocking drugs may cause Raynaud’s phenomenon in some individuals, but these drugs do not seem to adversely affect frequency of vasospastic attacks, nor do they decrease finger blood flow in patients with Raynaud’s phenomenon.
Miscellaneous Causes
Hypothyroidism may be associated with Raynaud’s phenomenon.74 In these cases, thyroid replacement alleviates the episodes of digital vasospasm. Although the mechanism is unknown, peripheral vasoconstriction may occur in hypothyroid patients to conserve heat. Alternatively, edematous thickening of the vascular wall could predispose to vessel closure during normal sympathetic stimuli.
Patients with arteriovenous fistula (AVF) may develop Raynaud’s phenomenon; it is particularly prevalent in patients undergoing hemodialysis.75 This may be secondary to decreased blood flow and blood pressure in the digits of the limb with the fistula.
Pulmonary hypertension and Raynaud’s phenomenon may occur in the same patients. Some of these may have a connective tissue disorder such as scleroderma.76 Approximately 30% of patients with pulmonary arterial hypertension (PAH) have elevated titers of antinuclear antibody.77,78 Ten percent of women with PAH have Raynaud’s phenomenon.78 This frequency is not different from that occurring in the general population, so it is not clear whether the association of primary pulmonary hypertension and Raynaud’s phenomenon is coincidence or related to a common neurohumoral or immunological mechanism.
Paraneoplastic Raynaud’s phenomenon is a rare complication of a number of different malignancies (e.g., carcinomas, sarcomas, lymphomas, leukemias), at times accompanied by paraneoplastic dermatomyositis and characterized by capillaroscopic findings similar to scleroderma.79,80
Diagnostic Tests
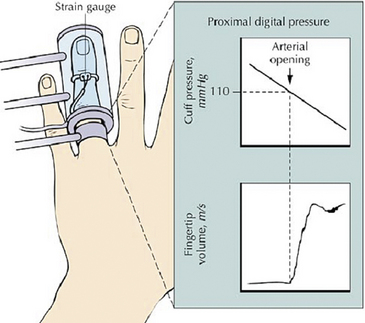
Noninvasive vascular tests may be employed to evaluate patients with Raynaud’s phenomenon (see Chapter 12). The effect on finger systolic pressure of local cooling with ischemia is an objective test for Raynaud’s phenomenon,81 but this is too cumbersome a test for routine use because it involves measuring digital systolic pressure with cooling plus 5 minutes of ischemia at four different temperatures (Fig. 48-5). Patients with Raynaud’s phenomenon have a greater reduction or loss of finger systolic pressure with cooling compared with normal subjects, who show a gradual decrease.
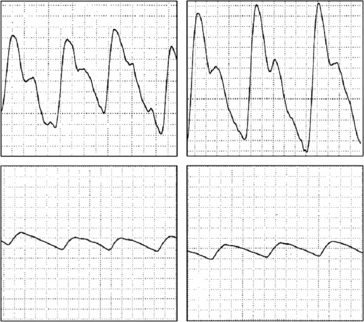
The pulse volume waveform may distinguish patients with Raynaud’s phenomenon who have digital ischemia secondary to vascular occlusive lesions (Fig. 48-6). During local digital warming, the vessels dilate. The pulse waveform is usually normal during warming in patients with Raynaud’s phenomenon if obstructive lesions are not present, and may be abnormal if atherosclerosis, TAO, or other fixed obstructive digital vascular pathology impairs digital blood flow.
Various serological studies such as for collagen vascular disorders or blood dyscrasias are useful to screen for secondary causes of Raynaud’s phenomenon. These tests include ESR, serum protein electrophoresis, and assays for antinuclear antibody, rheumatoid factor, cryoglobulins, and cold agglutinins. Indications for these and other serological studies are usually suggested by the history and physical examination. Nailfold capillary microscopy may be used to detect the deformed capillary loops and avascular areas typical of collagen vascular disorders55 (Fig. 48-7). Roentgenography of the cervical spine is used to detect cervical ribs.
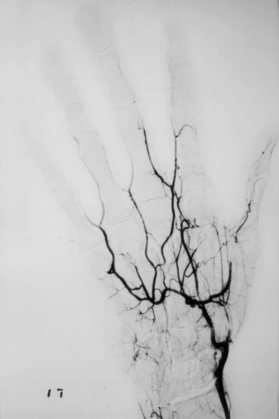
Angiography is rarely indicated, since Raynaud’s phenomenon is a diagnosis based primarily on history. Angiography may be indicated, however, in patients with persistent digital ischemia secondary to atherosclerosis, TAO, or emboli from a subclavian artery aneurysm, in order to plan revascularization procedures (Fig. 48-8).
Treatment
Treatment programs must be individualized and designed according to the underlying cause of Raynaud’s phenomenon and severity of symptoms. Therapy directed specifically at the symptoms of Raynaud’s phenomenon can be categorized as (1) conservative measures, (2) pharmacological intervention, and (3) surgical sympathectomy (Box 48-3). In individuals with well-defined secondary causes of Raynaud’s phenomenon, treatment should also be directed specifically at the underlying cause. For example, if a patient has been taking a vasoactive medication, such as an ergot alkaloid, or has been treated with a β-adrenergic blocking drug for hypertension, removal of the offending agent may reduce or eliminate the Raynaud’s phenomenon. Similarly, specific treatment may be directed at other secondary causes such as arterial occlusive disorders, connective tissue diseases, and blood dyscrasias. The following discussion focuses on treatment designed to palliate Raynaud’s phenomenon.
Conservative Measures
Patients with primary Raynaud’s phenomenon often benefit from reassurance. An explanation describing the frequency of the disease in the general population, its precipitating factors, and its benign prognosis is reassuring and allays fears of amputation. Patients should avoid unnecessary cold exposure and should wear loose, warm clothing. In addition to gloves and adequate foot protection, the trunk and head should be kept warm to avoid reflex vasoconstriction. Moving to a warmer climate is rarely feasible; furthermore, vasospasm may be induced after a move by even small changes in environmental temperature. Patients should use a moisturizing cream on their digits to prevent drying and cracking. Cigarette smoking should be avoided, since nicotine causes cutaneous vasoconstriction. Because the sympathetic nervous system mediates the vasoconstrictor response to cold and emotional distress, behavioral therapy has been proposed as a means of ameliorating the symptoms of Raynaud’s phenomenon. Studies have been reported both supporting and refuting the efficacy of biofeedback training in this disorder.78,82–87 The techniques used for biofeedback training in most of these studies were different. Furthermore, objective means of assessing responses varied from determination of digital temperature during cold exposure to queries regarding symptomatic improvement. Several uncontrolled studies indicated that following biofeedback training, patients were able to increase digital temperature and possibly decrease the frequency of vasospastic attacks.78,82–85 Patients usually trained to increase their digital temperature during either local or environmental cold exposure. In one controlled study that compared temperature biofeedback with nifedipine for treatment of primary Raynaud’s phenomenon, biofeedback was not better than control treatment, and inferior to the drug.88 Another form of behavioral therapy, Pavlovian conditioning, has been shown to increase digital temperature during cold exposure.89 Although behavioral therapy may be effective in some individuals, there are insufficient data to support its routine use for patients with Raynaud’s phenomenon.
Pharmacological Intervention
Two classes of drugs are effective for treatment of Raynaud’s phenomenon: calcium channel blockers and sympathetic nervous system inhibitors.2,90,91 Other classes of drugs for which efficacy has not been firmly established include serotonin antagonists, angiotensin-converting enzyme inhibitors (ACEIs), and direct-acting smooth muscle relaxants such as nitrates, endothelin antagonists, phosphodiesterase type 5 (PDE5) inhibitors, and vasodilator prostaglandins (PGs).
Calcium channel blockers
Calcium entry blockers are the most effective drugs for treating Raynaud’s phenomenon. Most of the evidence accumulated to date involves nifedipine, which interferes with vascular smooth muscle contraction by antagonizing calcium influx. This drug decreases digital vascular resistance in patients with Raynaud’s phenomenon during environmental cold exposure and increases digital SBP and digital skin temperature during local cold exposure.92,93 In multiple placebo-controlled trials, nifedipine decreased frequency and severity of Raynaud’s phenomenon.2,90 It has also been reported to be effective treatment in children.94 Although nifedipine is not of benefit in all patients, symptoms improve in about two thirds of individuals. Patients with both primary and secondary Raynaud’s phenomenon have shown improvement. Extended-action preparations should be used, starting with 30 mg daily and increasing to 60 mg, 90 mg, and 120 mg if necessary.2,95 Side effects include hypotension, lightheadedness, indigestion, and peripheral edema.
Less information is available about the other calcium channel blocking drugs. Diltiazem, however, has been reported in several studies to improve symptoms of Raynaud’s phenomenon.96,97 One preliminary report also indicated that it was effective in patients with Raynaud’s phenomenon secondary to vibration injury.98 Felodipine, at a dose of 2.5 or 5 mg, may be as effective as nifedipine but has not been as extensively studied.99,100 Isradipine and amlodipine have been reported beneficial in small studies. In contrast, verapamil has not been shown to be effective in patients with Raynaud’s phenomenon.101
Sympathetic nervous system inhibitors
Prazosin hydrochloride is an α1-adrenoceptor blocker. Since postsynaptic α1 adrenoceptors are found on digital vessels, clinical improvement following administration of this drug might be anticipated.2,91 However, one study reported only modest increases in digital blood flow during intraarterial infusion of prazosin. Uncontrolled observations have suggested that prazosin is effective in roughly 50% of patients with Raynaud’s phenomenon. A few small placebo-controlled double-blind studies suggest that prazosin in doses ranging from 1 to 4 mg twice daily reduce the frequency of Raynaud’s phenomenon.102–104 Tachyphylaxis may occur with prazosin, often necessitating dosage increments up to 10 mg three times daily. Side effects of prazosin include hypotension, particularly after the first few doses, leading to lightheadedness or syncope. In addition, patients may develop headache, drowsiness, or fatigue. Long acting α1-adrenoceptor blockers, such as doxazosin and terazosin, have not been studied in patients with Raynaud’s phenomenon, but would probably have effects similar to prazosin.
Selective serotonin reuptake inhibitors
One small study found that fluoxetine, a selective serotonin reuptake inhibitor (SSRI), reduced the number and severity of vasospastic attacks in 53 patients with primary or secondary Raynaud’s phenomenon compared with nifedipine; more clinical trial data are needed.105 Erythromelalgia has been reported as a complication of SSRI therapy for Raynaud’s phenomenon.106
Renin-angiotensin system inhibitors
Angiotensin II is unlikely to mediate digital vasospasm in most patients with Raynaud’s phenomenon.107 If elevation of this hormone is due to other pathological conditions, it could conceivably contribute to digital vasospasm in patients already predisposed to Raynaud’s phenomenon. One such population is patients with systemic sclerosis and malignant hypertension. One report described two patients with systemic sclerosis, malignant hypertension, renal failure, and digital ischemia who were treated with the ACEI captopril. Hypertension, renal insufficiency, and digital ischemia all improved following drug administration.108 However, another study reported that captopril decreased vasospastic attacks in patients with primary Raynaud’s phenomenon, but not in patients with Raynaud’s phenomenon secondary to scleroderma.109 Other uncontrolled studies have suggested that captopril reduces symptoms in Raynaud’s disease. The angiotensin receptor antagonist losartan has been reported to decrease the frequency and severity of vasospastic attacks in 15 patients with primary Raynaud’s phenomenon.110 More studies of this class of drug are necessary.
Endothelin receptor antagonists
Case reports and case series have described success in the use of endothelin receptor antagonists in the treatment of refractory Raynaud’s phenomenon.111–113 In a small placebo-controlled trial of patients with Raynaud’s phenomenon secondary to systemic sclerosis, bosentan, a dual endothelin receptor antagonist, did not lessen frequency, duration, pain or severity of attacks.45 In a large multicenter double-blind, placebo-controlled trial of 24 weeks of therapy in 188 patients with systemic sclerosis, bosentan reduced occurrence of new digital ulcers but had no effect on ulcer healing; other secondary endpoints such as pain and disability were negative.46
Phosphodiesterase type 5 inhibitors
The PDE5 inhibitors prevent breakdown of cyclic guanosine monophosphate (cGMP), causing relaxation of vascular smooth muscle cells (VSMCs) and vasodilation. Accumulating evidence supports use of these agents in patients with Raynaud’s phenomenon.114 In a placebo-controlled crossover trial in patients with secondary Raynaud’s phenomenon resistant to vasodilator therapy, the PDE5 inhibitor sildenafil reduced frequency of vasospasm and shortened attack duration while improving mean capillary flow velocity.115 In a trial of similar design, the PDE5 inhibitor tadalafil reduced frequency and severity of vasospastic attacks and caused resolution of digital ulcers.116 Sildenafil also reduced frequency of vasospastic attacks in a placebo-controlled study of 57 patients with Raynaud’s phenomenon secondary to limited cutaneous systemic sclerosis.117
Direct vascular smooth muscle relaxants
Organic nitrate preparations including nitropaste are often used in patients with Raynaud’s phenomenon. One study reported that topical glyceryl trinitrate was an effective therapy for patients with Raynaud’s phenomenon, but limited in utility because of headache.118 A novel formulation of topical nitroglycerin, MQX-503 gel, may offer relief in Raynaud’s phenomenon and be better tolerated than nitropaste.119 Nitroprusside has been used to treat severe ergotism-caused vasospasm. No other convincing evidence exists that chronic treatment with nitrate preparations ameliorates Raynaud’s phenomenon.90
Prostaglandins
Prostaglandins inhibit platelet aggregation and are vasodilators. Several uncontrolled studies have suggested that intravenous (IV) infusions of prostaglandin E1 (PGE1) promote healing of digital ulcers in patients with scleroderma.2,90 In a placebo-controlled multicenter study, however, IV PGE1 was no more effective than placebo in reducing symptoms of Raynaud’s phenomenon or healing digital ulcers. Several placebo-controlled studies, however, have reported long-term benefit in the treatment of severe Raynaud’s phenomenon with IV iloprost, a prostacyclin (PGI2) analog, for 3 to 5 days, or with prostacyclin for 3 weeks.2,90 There were significant decreases in frequency, duration, and severity of vasospastic attacks, or increases in indices of finger blood flow for up to 9 weeks. In one study that compared IV iloprost with oral nifedipine, both drugs were of benefit subjectively, but parameters of finger blood flow were only increased with iloprost. Side effects of iloprost depend on dosage and include headaches, flushing, nausea, vomiting, and jaw pain. Oral prostacyclin preparations, unfortunately, have not proven of value in the treatment of Raynaud’s phenomenon.
Statins
HMG-CoA reductase inhibitors (“statins”) exhibit pleiotropic effects on endothelial function and therefore might be anticipated to retard the vascular pathology of Raynaud’s phenomenon. One prospective placebo-controlled clinical trial of atorvastatin 40 mg daily demonstrated improvement in digital ulcers in patients with Raynaud’s phenomenon secondary to systemic sclerosis.120
N-acetylcysteine
N-acetylcysteine, a powerful antioxidant, is postulated to decrease free radical injury of the endothelium. A pilot study of IV N-acetylcysteine for 5 days in 22 patients with Raynaud’s phenomenon due to systemic sclerosis decreased vasospastic attack frequency and severity, compared with pretreatment values.121 Controlled studies are needed with this interesting compound, which is used safely in other disorders.
Sympathectomy
The success rate of sympathectomy for episodic digital vasospasm of the upper extremity is not as good as might be anticipated. Raynaud’s phenomenon recurs in the majority of patients.2,122,123 Successful relief of digital ischemia is less in patients with secondary forms of Raynaud’s phenomenon than in patients with primary Raynaud’s phenomenon. In contrast, the majority of patients experience improvement in symptoms in their lower extremities following lumbar sympathectomy. Possibly, lumbar sympathectomy is more complete than thoracodorsal sympathectomy, in which residual sympathetic pathways may develop following surgery.
Digital sympathectomy, also referred to as adventitial stripping, has been advocated by some surgeons for treatment of digital ischemia, particularly that secondary to severe Raynaud’s phenomenon.124 This technique may improve digital blood flow and allow healing of digital ulcerations.2,88,90
Botulinum Injection
Botulinum toxin type A is a neurotoxin that results in flaccid muscle paralysis. Injection of botulinum toxin into the perineurovascular tissue of the wrist or the distal palm, or along the digits, has been reported to relieve recalcitrant Raynaud’s phenomenon in several case series, but no controlled clinical trial has been reported.125,126
1 Raynaud M. L’Asphyxie Locale et de la Gangrene Symmetrique des Extremities. London: New Sydenham Society; 1862.
2 Wigley F.M. Clinical practice. Raynaud’s Phenomenon. N Engl J Med. 2002;347:1001–1008.
3 Allen E.V., Brown G.E. Raynaud’s disease: a critical review of minimal requisites for diagnosis. Am J Med Sci. 183, 1932.
4 Gifford R.W.Jr, Hines E.A.Jr. Raynaud’s disease among women and girls. Circulation. 1957;16:1012–1021.
5 Guntheroth W.G., Morgan B.C., Harbinson J.A., et al. Raynaud’s disease in children. Circulation. 1967;36:724–729.
6 Maricq H.R., Carpentier P.H., Weinrich M.C., et al. Geographic variation in the prevalence of Raynaud’s phenomenon: Charleston, SC, USA, vs Tarentaise, Savoie, France. J Rheumatol. 1993;20:70–76.
7 Freedman R.R., Mayes M.D. Familial aggregation of primary Raynaud’s disease. Arthritis Rheum. 1996;39:1189–1191.
8 O’Keeffe S.T., Tsapatsaris N.P., Beetham W.P.Jr. Increased prevalence of migraine and chest pain in patients with primary Raynaud disease. Ann Intern Med. 1992;116:985–989.
9 Miller D., Waters D.D., Warnica W., et al. Is variant angina the coronary manifestation of a generalized vasospastic disorder? N Engl J Med. 1981;304:763–766.
10 Zahavi I., Chagnac A., Hering R., et al. Prevalence of Raynaud’s phenomenon in patients with migraine. Arch Intern Med. 1984;144:742–744.
11 Cannon P.J., Hassar M., Case D.B., et al. The relationship of hypertension and renal failure in scleroderma (progressive systemic sclerosis) to structural and functional abnormalities of the renal cortical circulation. Medicine (Baltimore). 1974;53:1–46.
12 Salmenson B.D., Reisman J., Sinclair S.H., et al. Macular capillary hemodynamic changes associated with Raynaud’s phenomenon. Ophthalmology. 1992;99:914–919.
13 Vergnon J.M., Barthelemy J.C., Riffat J., et al. Raynaud’s phenomenon of the lung. A reality both in primary and secondary Raynaud syndrome. Chest. 1992;101:1312–1317.
14 Krumholz H.M., Goldberger A.L. Systemic arterial vasospastic syndrome: familial occurrence with variant angina. Am J Med. 1992;92:334–335.
15 Coffman J.D., Cohen R.A. Vasospasm–ubiquitous? N Engl J Med. 1981;304:780–782.
16 Tokola R., Hokkanen E. Propranolol for acute migraine. Br Med J. 1978;2:1089.
17 Marshall A.J., Roberts C.J., Barritt D.W. Raynaud’s phenomenon as side effect of beta-blockers in hypertension. BMJ. 1976;1:1498–1499.
18 Yasue H., Touyama M., Shimamoto M., et al. Role of autonomic nervous system in the pathogenesis of Prinzmetal’s variant form of angina. Circulation. 1974;50:534–539.
19 Curry R.C.Jr, Pepine C.J., Sabom M.B., et al. Effects of ergonovine in patients with and without coronary artery disease. Circulation. 1977;56:803–809.
20 Schroeder J.S., Bolen J.L., Quint R.A., et al. Provocation of coronary spasm with ergonovine maleate. New test with results in 57 patients undergoing coronary arteriography. Am J Cardiol. 1977;40:487–491.
21 Wollersheim H., Thien T., Hoet M.H., et al. The diagnostic value of several immunological tests for anti-nuclear antibody in predicting the development of connective tissue disease in patients presenting with Raynaud’s phenomenon. Eur J Clin Invest. 1989;19:535–541.
22 Lewis T. Experiments relating to the peripheral mechanism involved in spasmodic arrest of the circulation in the fingers, a variety of Raynaud’s disease. Heart. 1929;15:7–101.
23 Freedman R.R., Mayes M.D., Sabharwal S.C. Induction of vasospastic attacks despite digital nerve block in Raynaud’s disease and phenomenon. Circulation. 1989;80:859–862.
24 Jamieson G.G., Ludbrook J., Wilson A. Cold hypersensitivity in Raynaud’s phenomenon. Circulation. 1971;44:254–264.
25 Vanhoutte P.M., Cooke J.P., Lindblad L.E., et al. Modulation of postjunctional alpha-adrenergic responsiveness by local changes in temperature. Clin Sci (Lond). 1985;68(Suppl 10):121s–123s.
26 Cooke J.P., Shepherd J.T., Vanhoutte P.M. The effect of warming on adrenergic neurotransmission in canine cutaneous vein. Circ Res. 1984;54:547–553.
27 Flavahan N.A., Cooke J.P., Shepherd J.T., et al. Human postjunctional alpha-1 and alpha-2 adrenoceptors: differential distribution in arteries of the limbs. J Pharmacol Exp Ther. 1987;241:361–365.
28 Chotani M.A., Mitra S., Su B.Y., et al. Regulation of alpha(2)-adrenoceptors in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;286:H59–H67.
29 Jeyaraj S.C., Chotani M.A., Mitra S., et al. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1195–1200.
30 Keenan E.J., Porter J.M. Alpha-adrenergic receptors in platelets from patients with Raynaud’s syndrome. Surgery. 1983;94:204–209.
31 Coffman J.D., Cohen R.A. Role of alpha-adrenoceptor subtypes mediating sympathetic vasoconstriction in human digits. Eur J Clin Invest. 1988;18:309–313.
32 Cooke J.P., Creager S.J., Scales K.M., et al. Role of digital artery adrenoceptors in Raynaud’s disease. Vasc Med. 1997;2:1–7.
33 Peacock J.H. Peripheral venous blood concentrations of epinephrine and norepinephrine in primary Raynaud’s disease. Circ Res. 1959;7:821–827.
34 Kontos H.A., Wasserman A.J. Effect of reserpine in Raynaud’s phenomenon. Circulation. 1969;39:259–266.
35 Downey J.A., LeRoy E.C., Miller J.M.3rd, et al. Thermoregulation and Raynaud’s phenomenon. Clin Sci. 1971;40:211–219.
36 Fagius J., Blumberg H. Sympathetic outflow to the hand in patients with Raynaud’s phenomenon. Cardiovasc Res. 1985;19:249–253.
37 Greenblatt D.J., Koch-Weser J. Adverse reactions to beta-adrenergic receptor blocking drugs: a report from the Boston collaborative drug surveillance program. Drugs. 1974;7:118–129.
38 Marshall A., Barritt D.W., Roberts C.J. Letter: Raynaud’s phenomenon as side effect of beta-blockers. Br Med J. 1976;2:301.
39 Eliasson K., Lins L.E., Sundqvist K. Peripheral vasospasm during beta-receptor blockade – a comparison between metoprolol and pindolol. Acta Med Scand Suppl. 1982;665:109–112.
40 Cohen R.A., Coffman J.D. Beta-adrenergic vasodilator mechanism in the finger. Circ Res. 1981;49:1196–1201.
41 Coffman J.D., Rasmussen H.M. Effect of thromboxane synthetase inhibition in Raynaud’s phenomenon. Clin Pharmacol Ther. 1984;36:369–373.
42 Luderer J.R., Nicholas G.G., Neumyer M.M., et al. Dazoxiben, a thromboxane synthetase inhibitor, in Raynaud’s phenomenon. Clin Pharmacol Ther. 1984;36:105–115.
43 Zamora M.R., O’Brien R.F., Rutherford R.B., et al. Serum endothelin-1 concentrations and cold provocation in primary Raynaud’s phenomenon. Lancet. 1990;336:1144–1147.
44 Smyth A.E., Bell A.L., Bruce I.N., et al. Digital vascular responses and serum endothelin-1 concentrations in primary and secondary Raynaud’s phenomenon. Ann Rheum Dis. 2000;59:870–874.
45 Nguyen V.A., Eisendle K., Gruber I., et al. Effect of the dual endothelin receptor antagonist bosentan on Raynaud’s phenomenon secondary to systemic sclerosis: a double-blind prospective, randomized, placebo-controlled pilot study. Rheumatology (Oxford). 2010;49:583–587.
46 Matucci-Cerinic M., Denton C.P., Furst D.E., et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2011;70:32–38.
47 Landis E.M. Micro-injection studies of capillary blood pressure in Raynaud’s disease. Heart. 1930;15:247.
48 Thulesius O. Methods for the evaluation of peripheral vascular function in the upper extremities. Acta Chir Scand Suppl. 1976;465:53–54.
49 Cohen R.A., Coffman J.D. Reduced fingertip arterial pressure in Raynaud’s disease. J Vasc Med Biol. 1989;1:21.
50 Imhof J.W., Baars H., Verloop M.C. Clinical and haematological aspects of macroglobulinaemia Waldenstrom. Acta Med Scand. 1959;163:349–366.
51 Jahnsen T., Nielsen S.L., Skovborg F. Blood viscosity and local response to cold in primary Raynaud’s phenomenon. Lancet. 1977;2:1001–1002.
52 Marshall R.J., Shepherd J.T., Thompson I.D. Vascular responses in patients with high serum titres of cold agglutinins. Clin Sci (Lond). 1953;12:255–264.
53 McGrath M.A., Penny R. Blood hyperviscosity in cryoglobulinaemia: temperature sensitivity and correlation with reduced skin blood flow. Aust J Exp Biol Med Sci. 1978;56:127–137.
54 Hillestad L.K. The peripheral circulation during exposure to cold in normals and in patients with the syndrome of high-titre cold haemagglutination. I. The vascular response to cold exposure in normal subjects. Acta Med Scand. 1959;164:203–229.
55 Maricq H.R., LeRoy E.C., D’Angelo W.A., et al. Diagnostic potential of in vivo capillary microscopy in scleroderma and related disorders. Arthritis Rheum. 1980;23:183–189.
56 Tan E.M., Cohen A.S., Fries J.F., et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277.
57 Fischer M., Mielke H., Glaefke S., et al. Generalized vasculopathy and finger blood flow abnormalities in rheumatoid arthritis. J Rheumatol. 1984;11:33–37.
58 Aletaha D., Neogi T., Silman A.J., et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581.
59 Garcia-Carrasco M., Siso A., Ramos-Casals M., et al. Raynaud’s phenomenon in primary Sjogren’s syndrome. Prevalence and clinical characteristics in a series of 320 patients. J Rheumatol. 2002;29:726–730.
60 Nakamura H., Kawakami A., Hayashi T., et al. Anti-centromere antibody-seropositive Sjogren’s syndrome differs from conventional subgroup in clinical and pathological study. BMC Musculoskelet Disord. 2010;11:140.
61 Lambova S.N., Kuzmanova S.I. Raynaud’s phenomenon in common rheumatic diseases. Folia Med (Plovdiv). 2006;48:22–28.
62 Waller D.G., Dathan J.R. Raynaud’s syndrome and carpal tunnel syndrome. Postgrad Med J. 1985;61:161–162.
63 Cooper R.A., Bunn H.F. Hemolytic anemia. In: Braunwald E., et al. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill, 1987.
64 Trejo O., Ramos-Casals M., Garcia-Carrasco M., et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001;80:252–262.
65 Jager B.V. Cryofibrinogenemia. N Engl J Med. 1962;266:579–583.
66 Letz R., Cherniack M.G., Gerr F., et al. A cross sectional epidemiological survey of shipyard workers exposed to hand-arm vibration. Br J Ind Med. 1992;49:53–62.
67 Mirbod S.M., Yoshida H., Nagata C., et al. Hand-arm vibration syndrome and its prevalence in the present status of private forestry enterprises in Japan. Int Arch Occup Environ Health. 1992;64:93–99.
68 Davies T.A., Glaser E.M., Collins C.P. Absence of Raynaud’s phenomenon in workers using vibratory tools in a warm climate. Lancet. 1957;272:1014–1016.
69 Okada A., Inaba R., Furuno T. Occurrence of intimal thickening of the peripheral arteries in response to local vibration. Br J Ind Med. 1987;44:470–475.
70 Hisanaga H. Studies of peripheral nerve conduction velocities in vibrating tool operators. Sangyo Igaku. 1982;24:284–293.
71 Kuhar C.G., Mesti T., Zakotnik B. Digital ischemic events related to gemcitabine: report of two cases and a systematic review. Radiol Oncol. 2010;44:257–261.
72 Laplanche A., Clavel-Chapelon F., Contassot J.C., et al. Exposure to vinyl chloride monomer: results of a cohort study after a seven-year follow up. The French VCM Group. Br J Ind Med. 1992;49:134–137.
73 Coffman J.D., Rasmussen H.M. Effects of beta-adrenoreceptor-blocking drugs in patients with Raynaud’s phenomenon. Circulation. 1985;72:466–470.
74 Nielsen S.L., Parving H.H., Hansen J.E. Myxoedema and Raynaud’s phenomenon. Acta Endocrinol (Copenh). 1982;101:32–34.
75 Nielsen S.L., Lokkegaard H. Cold hypersensitivity and finger systolic blood pressure in hemodialysis patients. Scand J Urol Nephrol. 1981;15:319–322.
76 Preston I.R., Hill N.S. Evaluation and management of pulmonary hypertension in systemic sclerosis. Curr Opin Rheumatol. 2003;15:761–765.
77 Fuster V., Steele P.M., Edwards W.D., et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580–587.
78 Rich S., Dantzker D.R., Ayres S.M., et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–223.
79 Schildmann E.K., Davies A.N. Paraneoplastic Raynaud’s phenomenon–good palliation after a multidisciplinary approach. J Pain Symptom Manage. 2010;39:779–783.
80 Lambova S., Muller-Ladner U. Capillaroscopic pattern in paraneoplastic Raynaud’s phenomenon. Rheumatol Int. 2011. published online 21 Jan (DOI: 10.1007/s00296-010-1715-8)
81 Nielsen S.L. Raynaud phenomena and finger systolic pressure during cooling. Scand J Clin Lab Invest. 1978;38:765–770.
82 Keefe F.J., Surwit R.S., Pilon R.N. A 1-year follow-up of Raynaud’s patients treated with behavioral therapy techniques. J Behav Med. 1979;2:385–391.
83 Keefe F.J., Surwit R.S., Pilon R.N. Biofeedback, autogenic training, and progressive relaxation in the treatment of Raynaud’s disease: a comparative study. J Appl Behav Anal. 1980;13:3–11.
84 Yocum D.E., Hodes R., Sundstrom W.R., et al. Use of biofeedback training in treatment of Raynaud’s disease and phenomenon. J Rheumatol. 1985;12:90–93.
85 Stambrook M., Hamel E.R., Carter S.A. Training to vasodilate in a cooling environment: a valid treatment for Raynaud’s phenomenon? Biofeedback Self Regul. 1988;13:9–23.
86 Guglielmi R.S., Roberts A.H., Patterson R. Skin temperature biofeedback for Raynaud’s disease: a double-blind study. Biofeedback Self Regul. 1982;7:99–120.
87 Freedman R.R., Ianni P., Wenig P. Behavioral treatment of Raynaud’s phenomenon in scleroderma. J Behav Med. 1984;7:343–353.
88 Comparison of sustained-release nifedipine and temperature biofeedback for treatment of primary Raynaud phenomenon. Results from a randomized clinical trial with 1-year follow-up. Arch Intern Med. 2000;160:1101–1108.
89 Jobe J.B., Sampson J.B., Roberts D.E., et al. Induced vasodilation as treatment for Raynaud’s disease. Ann Intern Med. 1982;97:706–709.
90 Coffman J.D. Raynaud’s phenomenon. Curr Treat Options Cardiovasc Med. 2000;2:219–226.
91 Belch J.J., Ho M. Pharmacotherapy of Raynaud’s phenomenon. Drugs. 1996;52:682–695.
92 Creager M.A., Pariser K.M., Winston E.M., et al. Nifedipine-induced fingertip vasodilation in patients with Raynaud’s phenomenon. Am Heart J. 1984;108:370–373.
93 Nilsson H., Jonason T., Leppert J., et al. The effect of the calcium-entry blocker nifedipine on cold-induced digital vasospasm. A double-blind crossover study versus placebo. Acta Med Scand. 1987;221:53–60.
94 Matucci Cerinic M., Falcini F., Bartolozzi G., et al. Nifedipine treatment of Raynaud’s phenomenon in a paediatric age. Int J Clin Pharmacol Res. 1985;5:67–69.
95 Jobe J.B., Beetham W.P.Jr, Roberts D.E., et al. Induced vasodilation as a home treatment for Raynaud’s disease. J Rheumatol. 1985;12:953–956.
96 Kahan A., Amor B., Menkes C.J. A randomised double-blind trial of diltiazem in the treatment of Raynaud’s phenomenon. Ann Rheum Dis. 1985;44:30–33.
97 Rhedda A., McCans J., Willan A.R., et al. A double blind placebo controlled crossover randomized trial of diltiazem in Raynaud’s phenomenon. J Rheumatol. 1985;12:724–727.
98 Matoba T., Chiba M. Effects of diltiazem on occupational Raynaud’s syndrome (vibration disease). Angiology. 1985;36:850–856.
99 Schmidt J.F., Valentin N., Nielsen S.L. The clinical effect of felodipine and nifedipine in Raynaud’s phenomenon. Eur J Clin Pharmacol. 1989;37:191–192.
100 Kallenberg C.G., Wouda A.A., Meems L., et al. Once daily felodipine in patients with primary Raynaud’s phenomenon. Eur J Clin Pharmacol. 1991;40:313–315.
101 Kinney E.L., Nicholas G.G., Gallo J., et al. The treatment of severe Raynaud’s phenomenon with verapamil. J Clin Pharmacol. 1982;22:74–76.
102 Nielsen S.L., Vitting K., Rasmussen K. Prazosin treatment of primary Raynaud’s phenomenon. Eur J Clin Pharmacol. 1983;24:421–423.
103 Wollersheim H., Thien T., Fennis J., et al. Double-blind, placebo-controlled study of prazosin in Raynaud’s phenomenon. Clin Pharmacol Ther. 1986;40:219–225.
104 Russell I.J., Lessard J.A. Prazosin treatment of Raynaud’s phenomenon: a double blind single crossover study. J Rheumatol. 1985;12:94–98.
105 Coleiro B., Marshall S.E., Denton C.P., et al. Treatment of Raynaud’s phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology (Oxford). 2001;40:1038–1043.
106 Rey J., Cretel E., Jean R., et al. Serotonin reuptake inhibitors, Raynaud’s phenomenon and erythromelalgia. Rheumatology (Oxford). 2003;42:601–602.
107 Challenor V.F. Angiotensin converting enzyme inhibitors in Raynaud’s phenomenon. Drugs. 1994;48:864–867.
108 Lopez-Ovejero J.A., Saal S.D., D’Angelo W.A., et al. Reversal of vascular and renal crises of scleroderma by oral angiotensin-converting-enzyme blockade. N Engl J Med. 1979;300:1417–1419.
109 Tosi S., Marchesoni A., Messina K., et al. Treatment of Raynaud’s phenomenon with captopril. Drugs Exp Clin Res. 1987;13:37–42.
110 Dziadzio M., Denton C.P., Smith R., et al. Losartan therapy for Raynaud’s phenomenon and scleroderma: clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial. Arthritis Rheum. 1999;42:2646–2655.
111 Selenko-Gebauer N., Duschek N., Minimair G., et al. Successful treatment of patients with severe secondary Raynaud’s phenomenon with the endothelin receptor antagonist bosentan. Rheumatology (Oxford). 2006;45(Suppl 3):iii45–iii48.
112 Ramos-Casals M., Brito-Zeron P., Nardi N., et al. Successful treatment of severe Raynaud’s phenomenon with bosentan in four patients with systemic sclerosis. Rheumatology (Oxford). 2004;43:1454–1456.
113 Tsifetaki N., Botzoris V., Alamanos Y., et al. Bosentan for digital ulcers in patients with systemic sclerosis: a prospective 3-year followup study. J Rheumatol. 2009;36:1550–1552.
114 Levien T.L. Phosphodiesterase inhibitors in Raynaud’s phenomenon. Ann Pharmacother. 2006;40:1388–1393.
115 Fries R., Shariat K., von Wilmowsky H., et al. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–2985.
116 Shenoy P.D., Kumar S., Jha L.K., et al. Efficacy of tadalafil in secondary Raynaud’s phenomenon resistant to vasodilator therapy: a double-blind randomized cross-over trial. Rheumatology (Oxford). 2010;49:2420–2428.
117 Herrick A.L., van den Hoogen F., Gabrielli A., et al. Modified-release sildenafil reduces Raynaud’s phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. 2011;63:775–782.
118 Teh L.S., Manning J., Moore T., et al. Sustained-release transdermal glyceryl trinitrate patches as a treatment for primary and secondary Raynaud’s phenomenon. Br J Rheumatol. 1995;34:636–641.
119 Chung L., Shapiro L., Fiorentino D., et al. MQX-503, a novel formulation of nitroglycerin, improves the severity of Raynaud’s phenomenon: a randomized, controlled trial. Arthritis Rheum. 2009;60:870–877.
120 Abou-Raya A., Abou-Raya S., Helmii M. Statins: potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35:1801–1808.
121 Sambo P., Amico D., Giacomelli R., et al. Intravenous N-acetylcysteine for treatment of Raynaud’s phenomenon secondary to systemic sclerosis: a pilot study. J Rheumatol. 2001;28:2257–2262.
122 Coffman J.D. Raynaud phenomenon. New York: Oxford University Press; 1989.
123 de Trafford J.C., Lafferty K., Potter C.E., et al. An epidemiological survey of Raynaud’s phenomenon. Eur J Vasc Surg. 1988;2:167–170.
124 Yee A.M., Hotchkiss R.N., Paget S.A. Adventitial stripping: a digit saving procedure in refractory Raynaud’s phenomenon. J Rheumatol. 1998;25:269–276.
125 Fregene A., Ditmars D., Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446–452.
126 Neumeister M.W. Botulinum toxin type A in the treatment of Raynaud’s phenomenon. J Hand Surg Am. 2010;35:2085–2092.