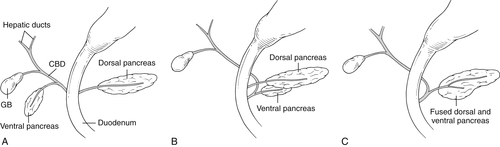
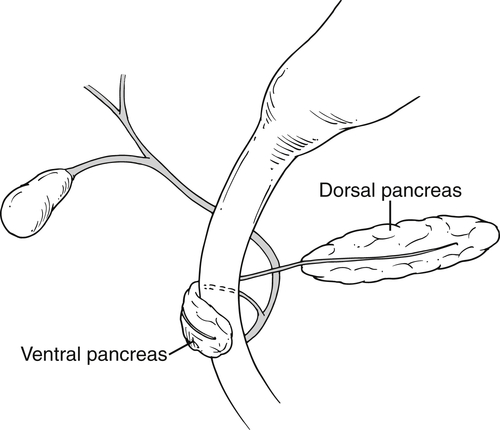
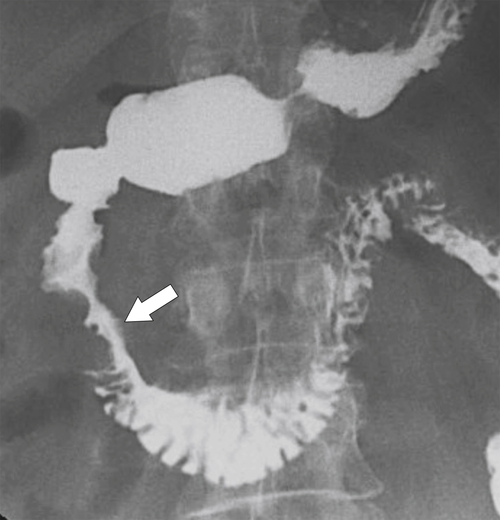
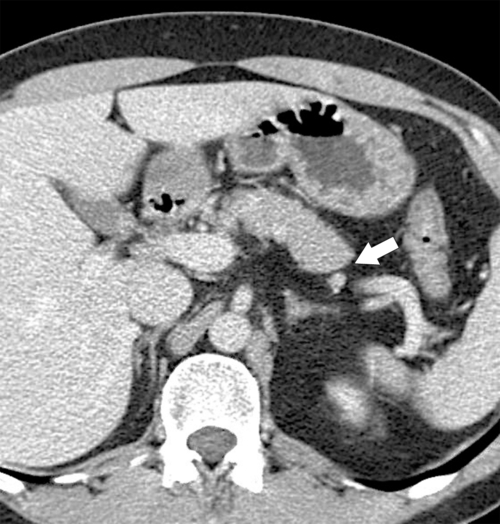
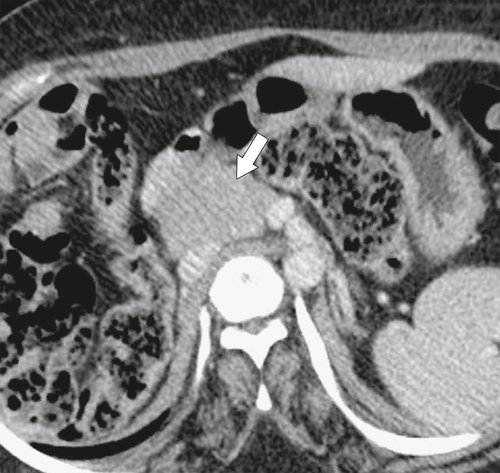
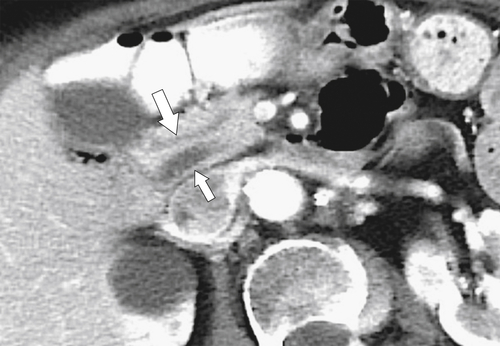
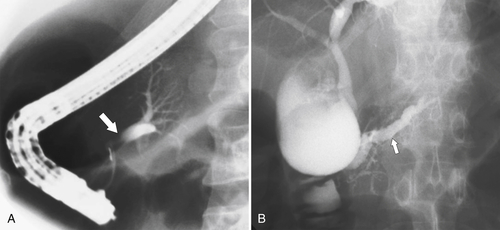
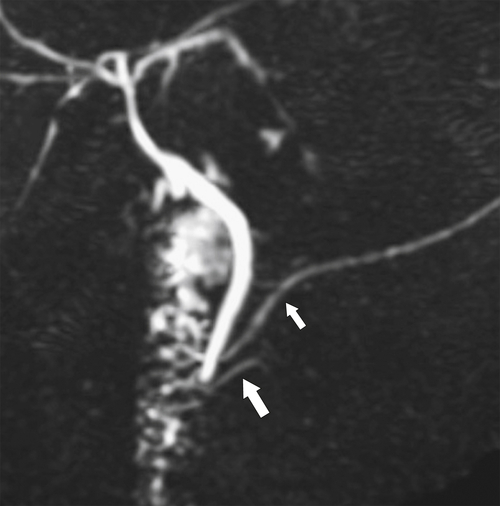
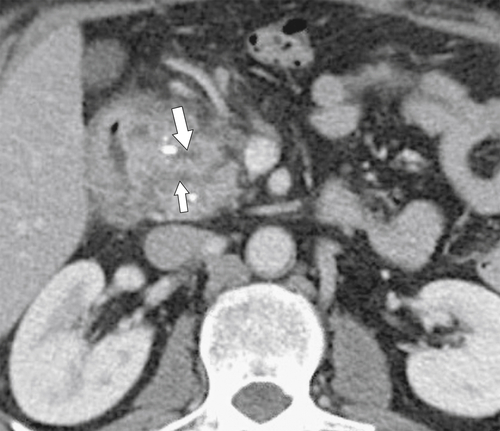
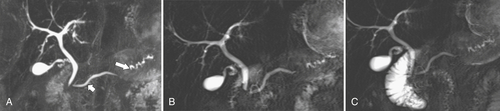
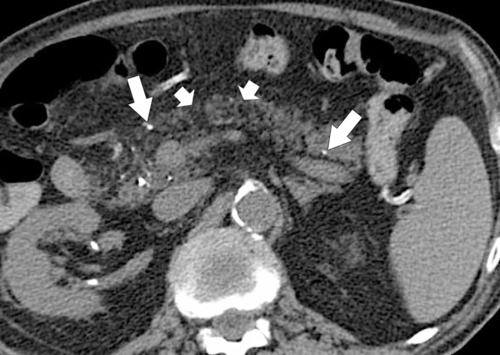
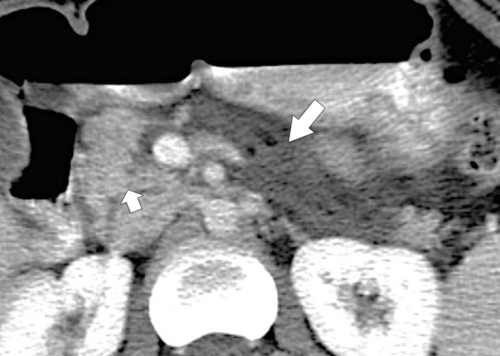
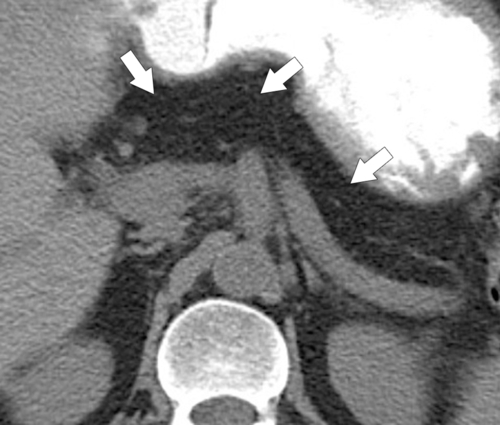
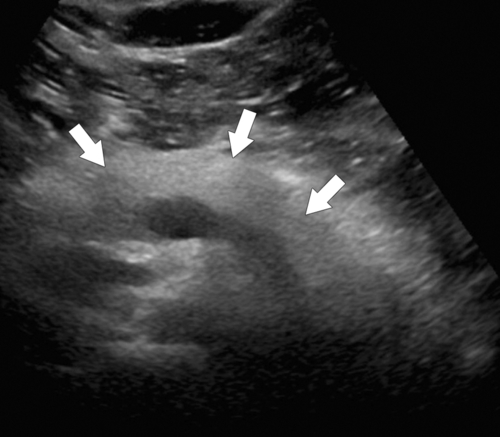
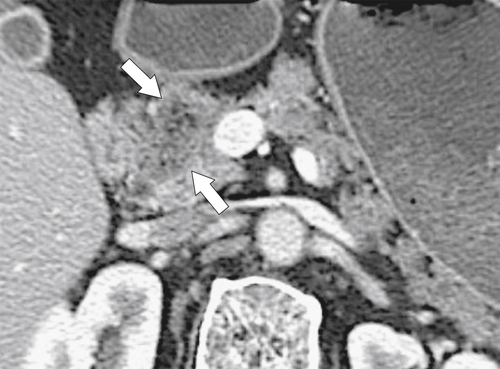
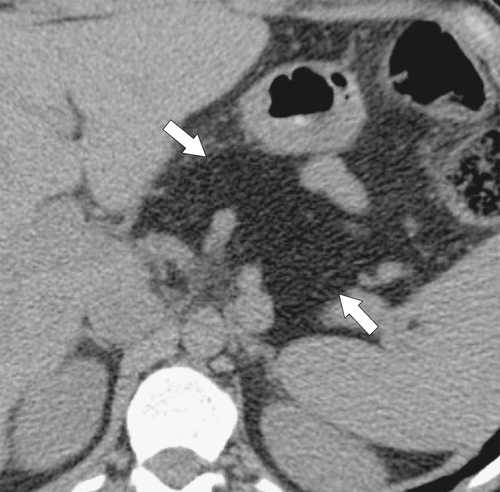
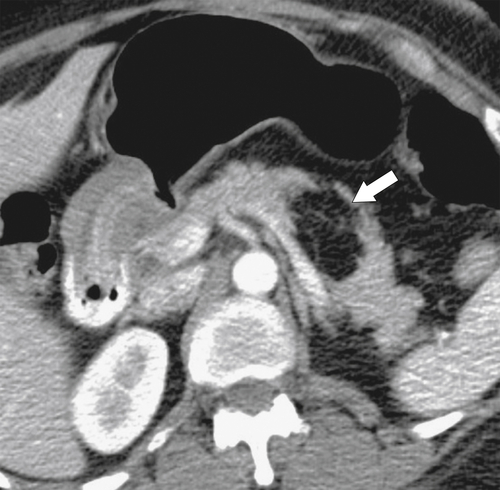
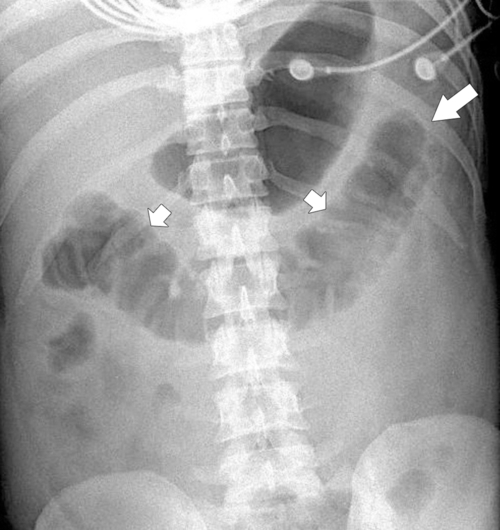
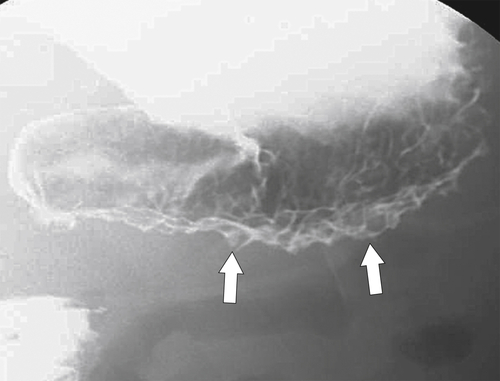
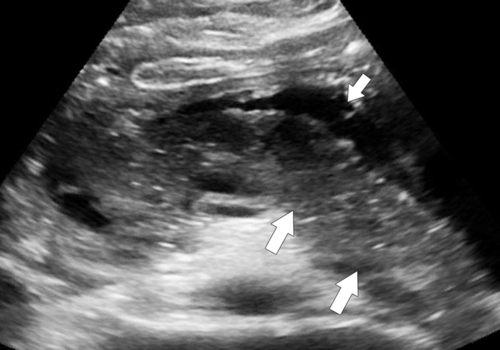
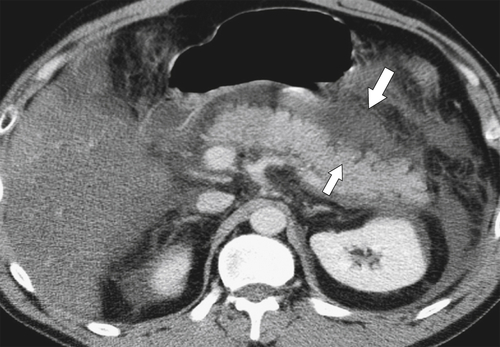
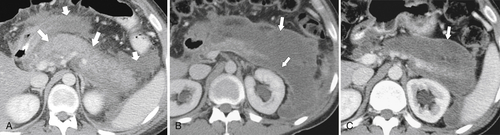
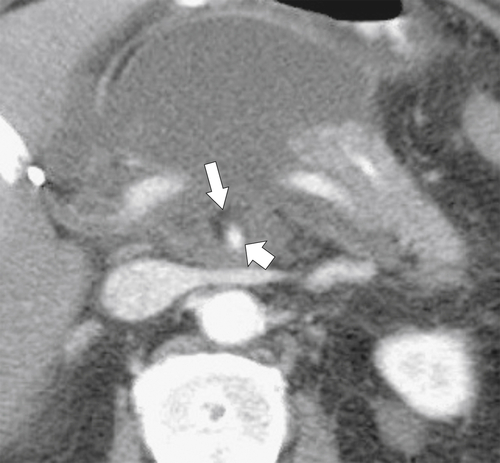
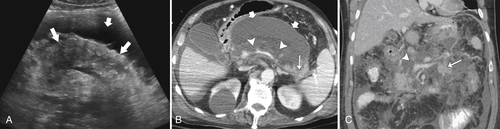
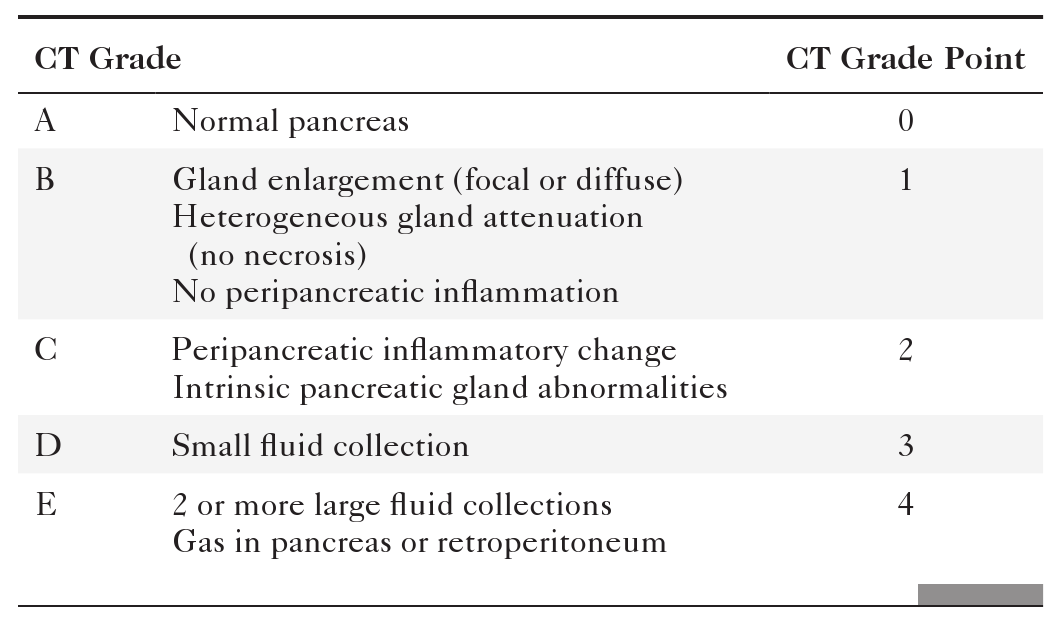
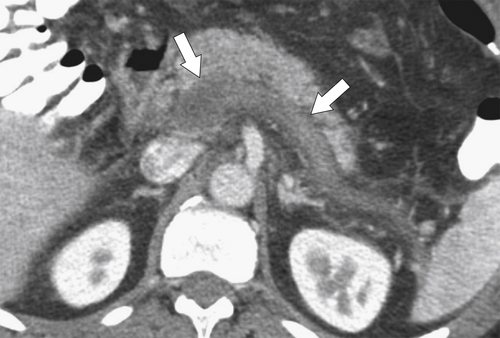
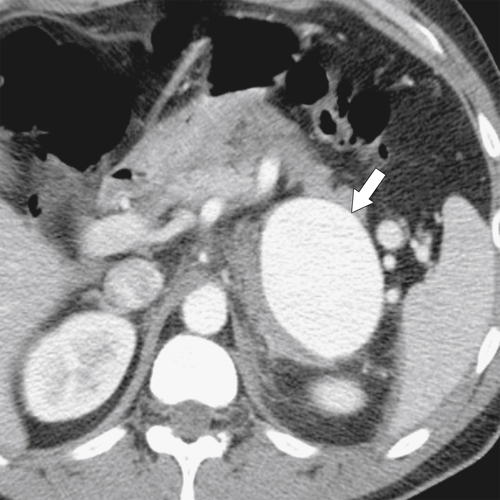
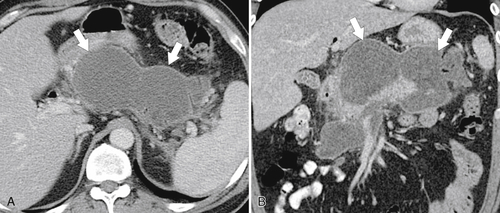
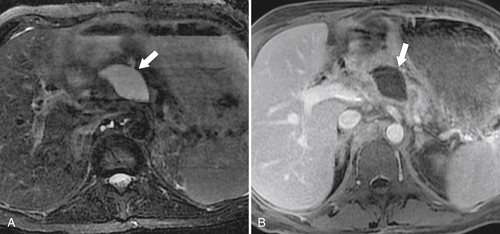
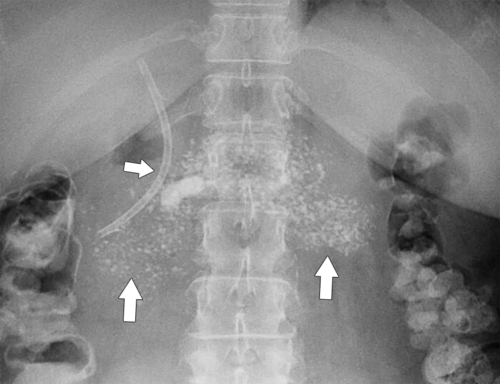
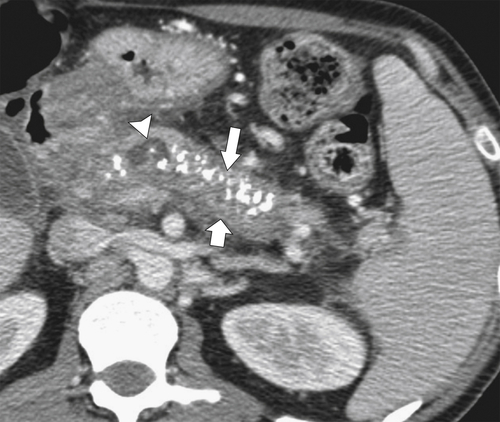
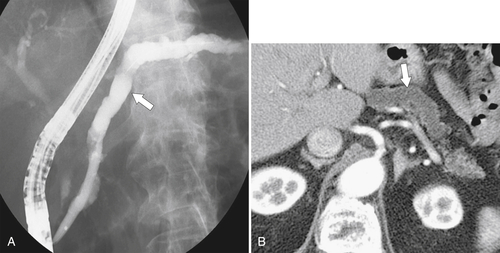
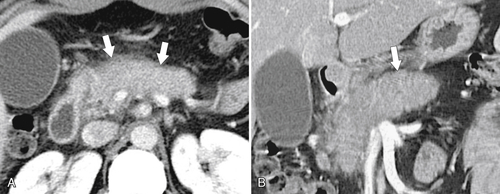
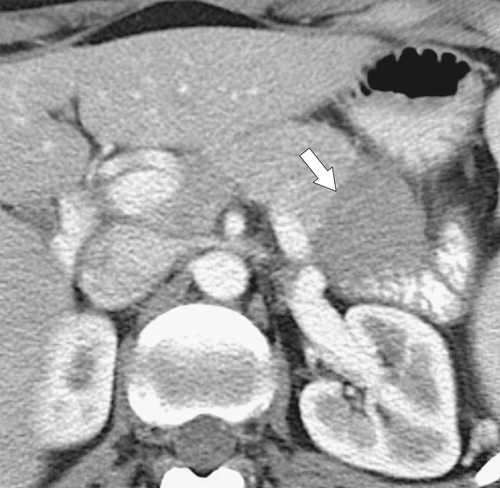
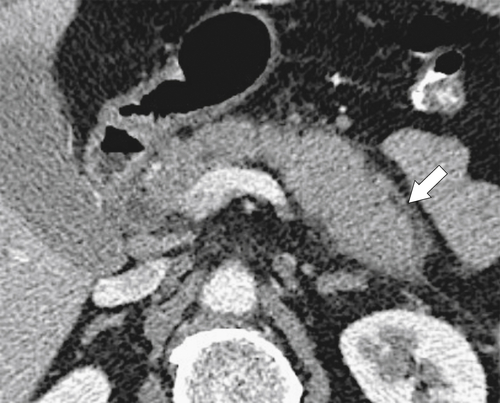
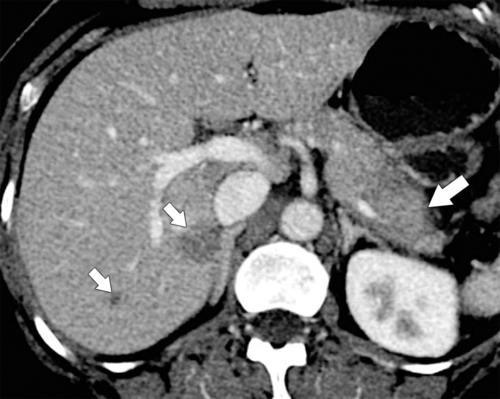
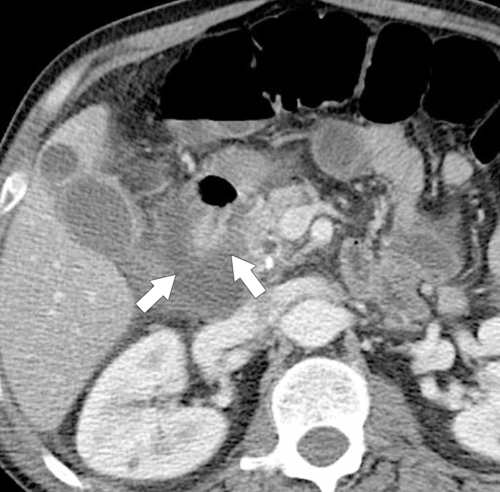
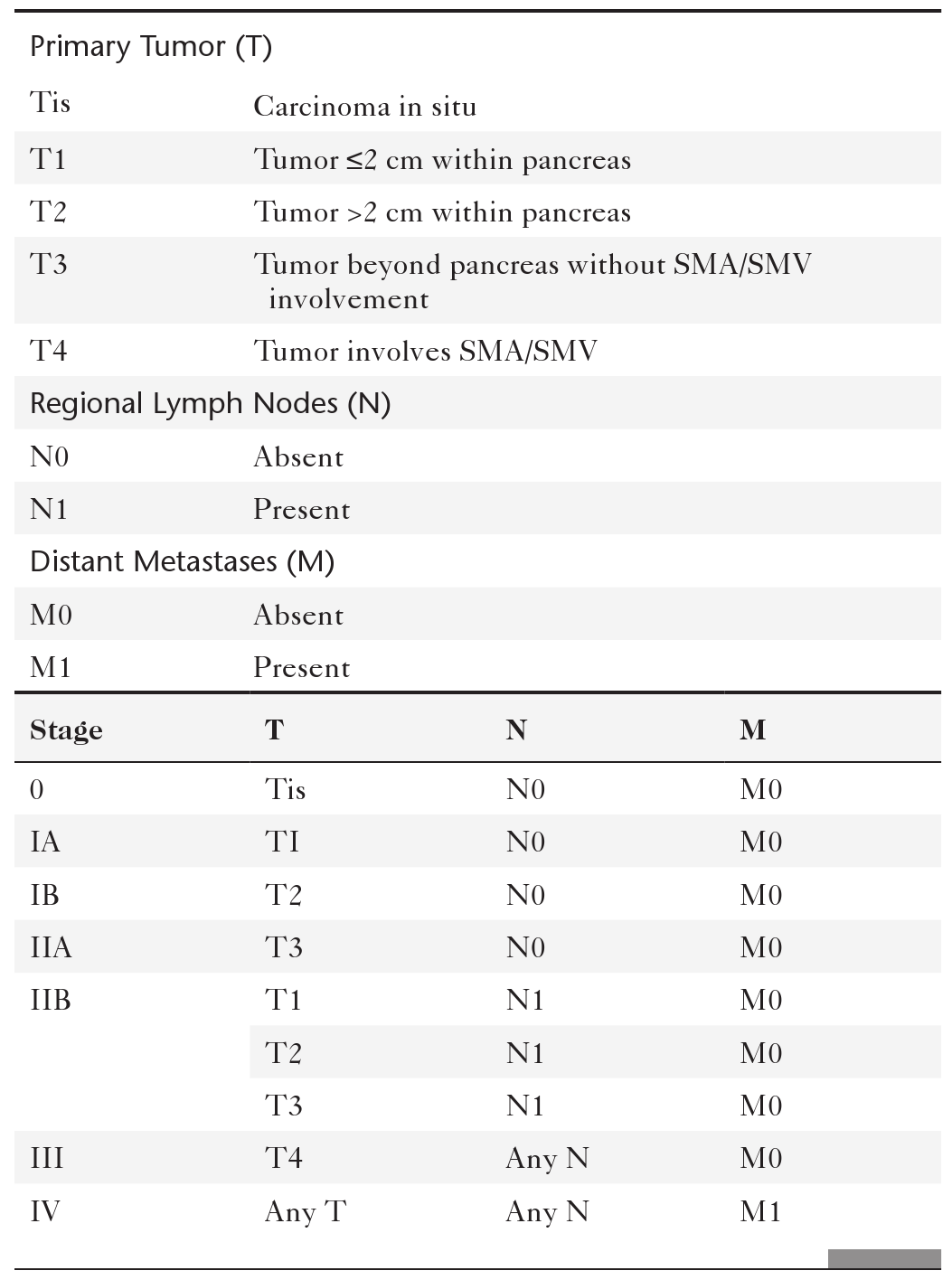
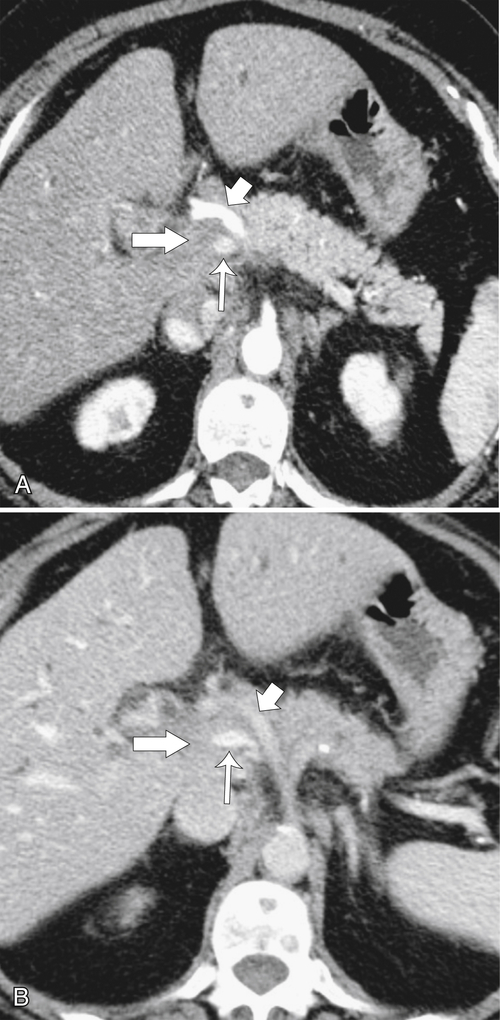
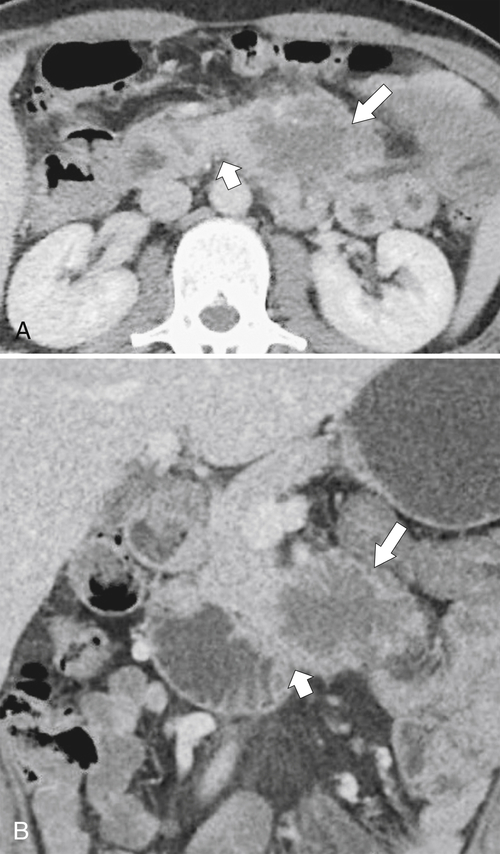
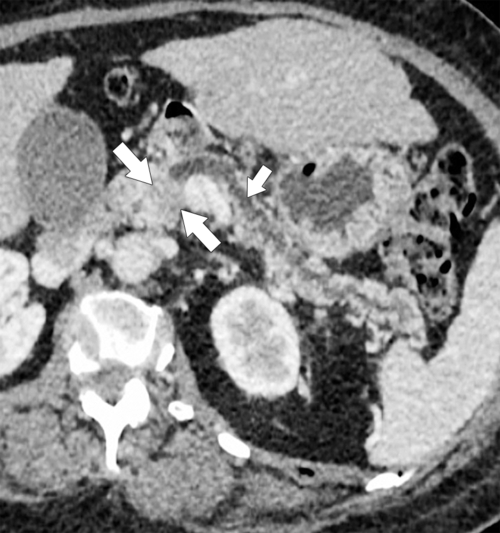
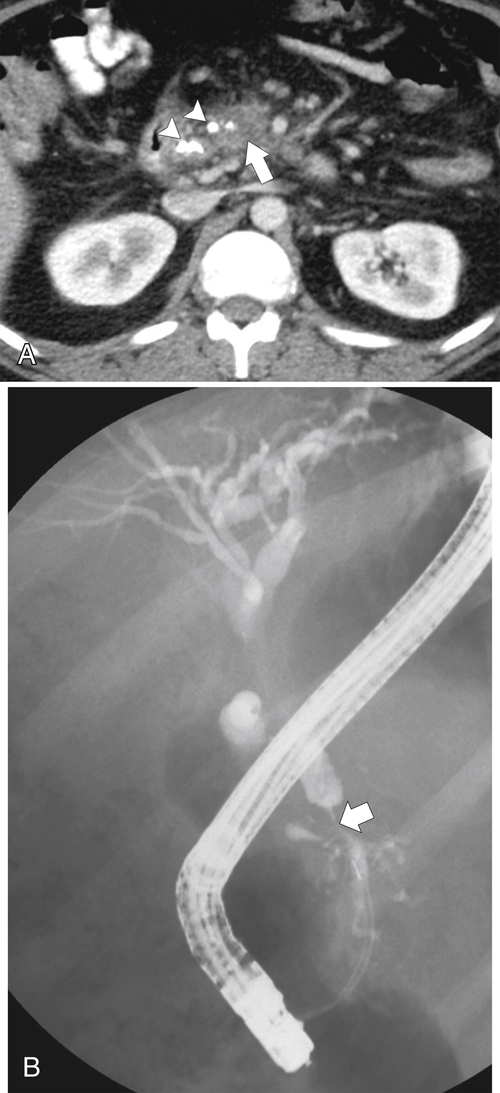
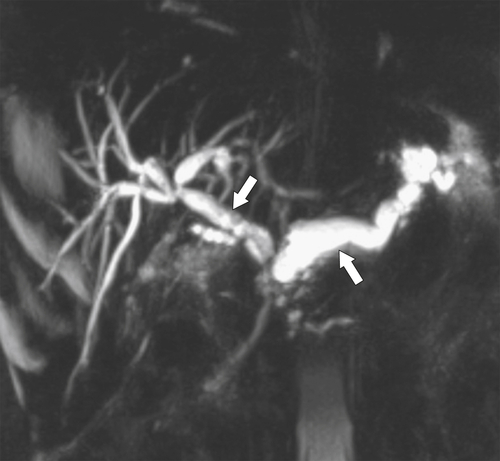
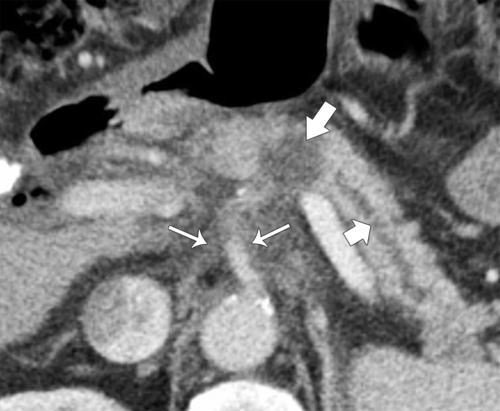
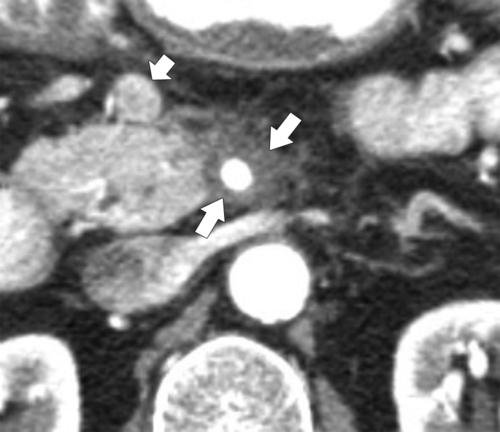
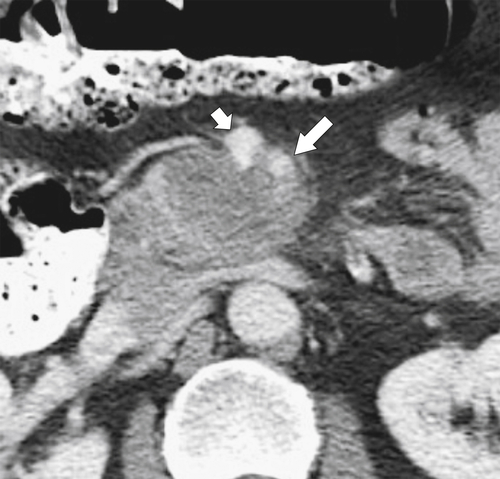
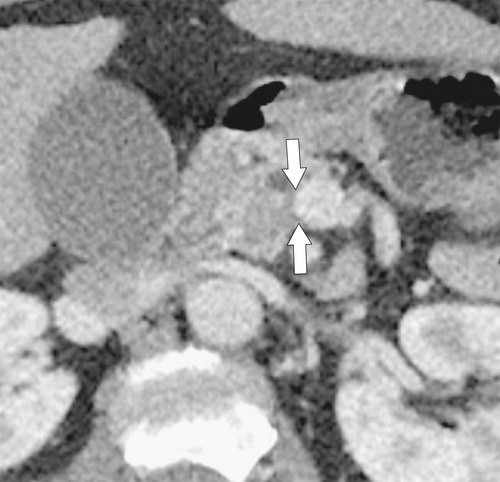
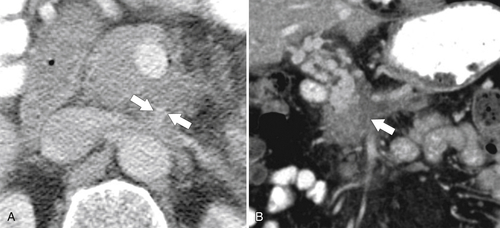
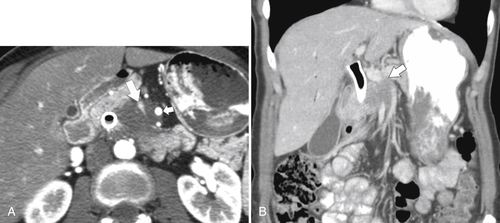
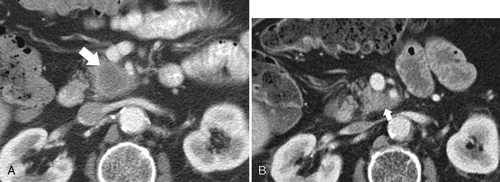
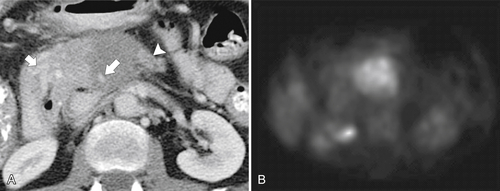
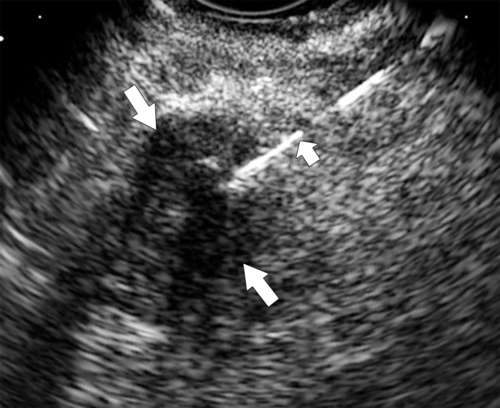
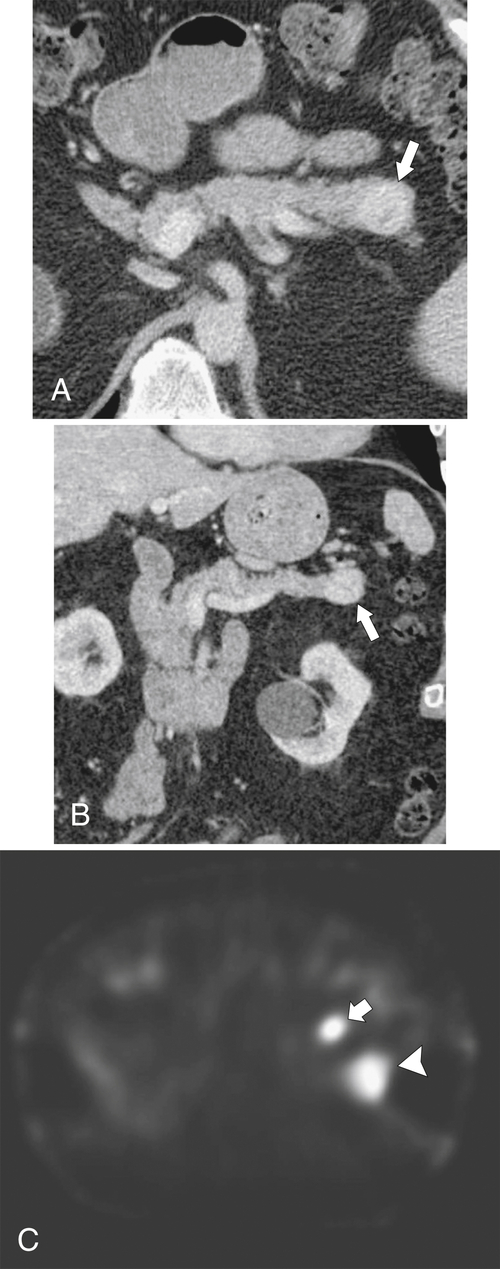
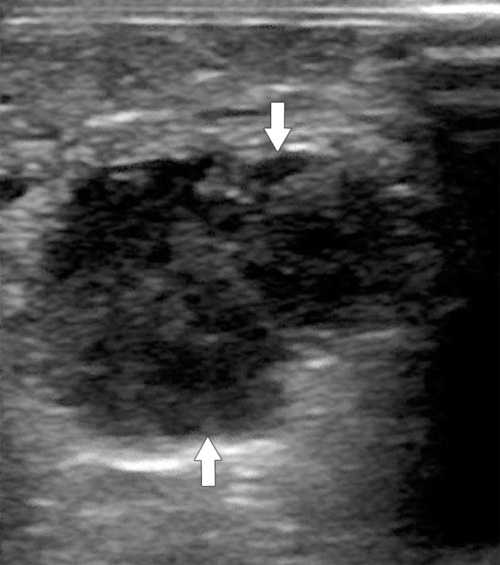
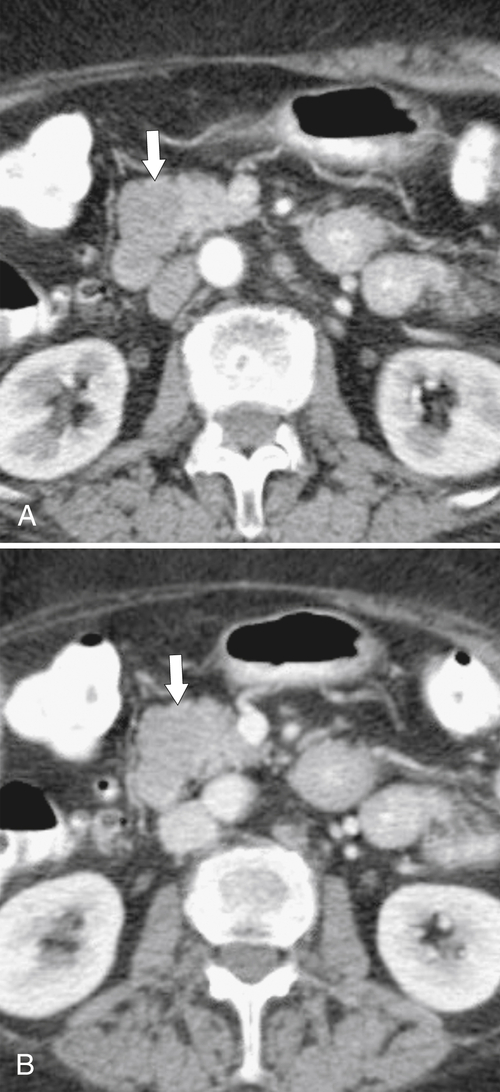
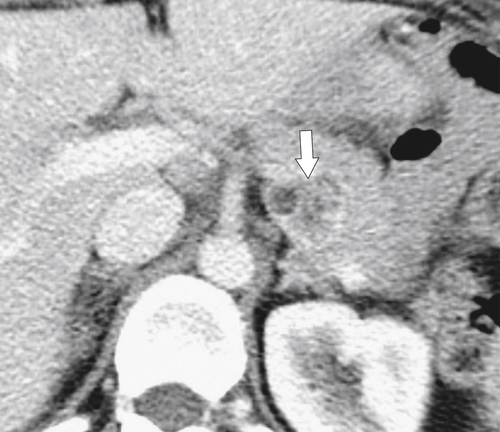
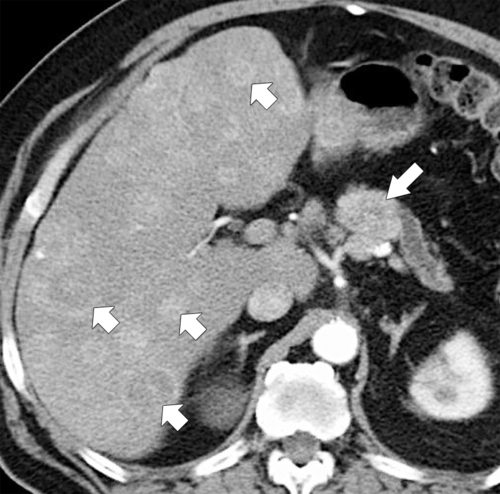
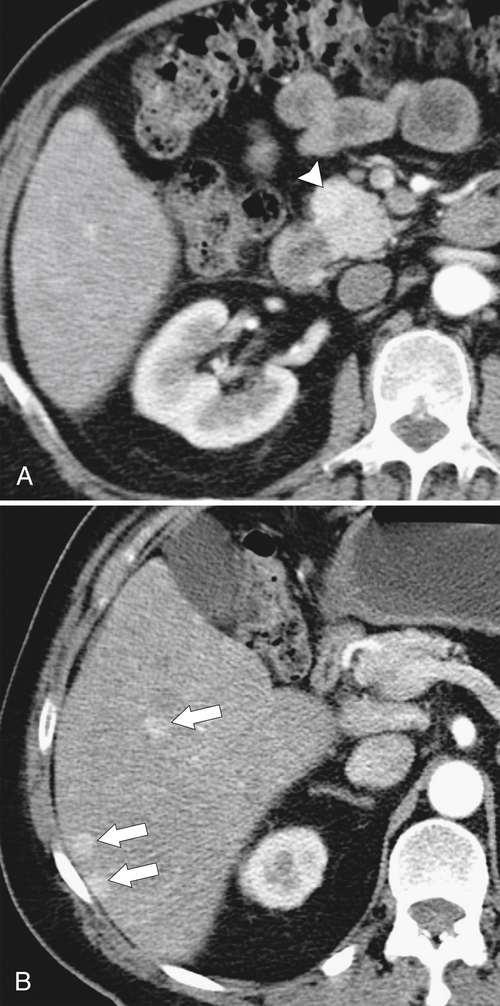
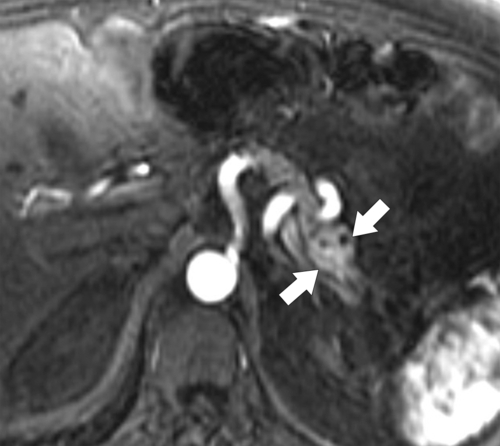
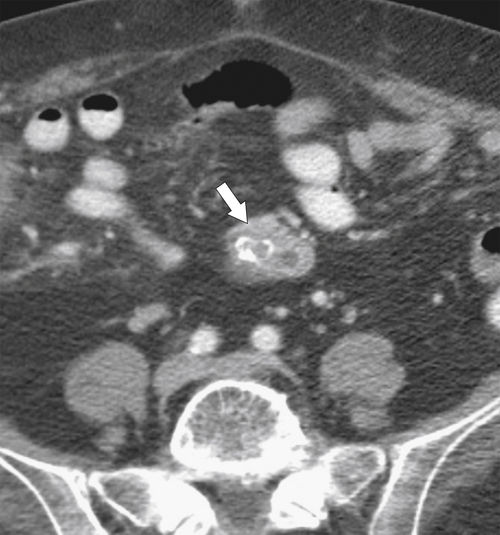
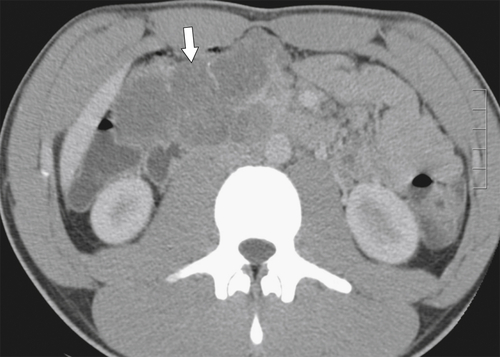
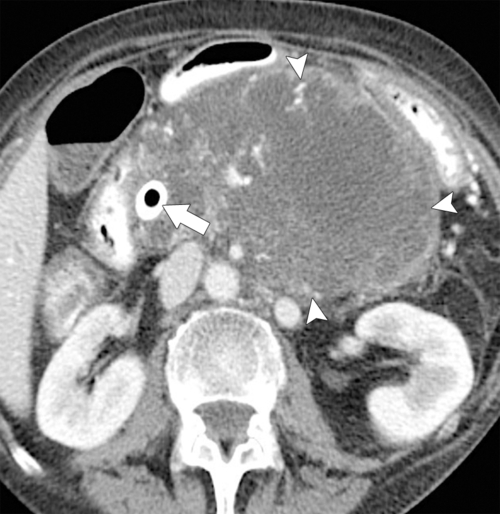
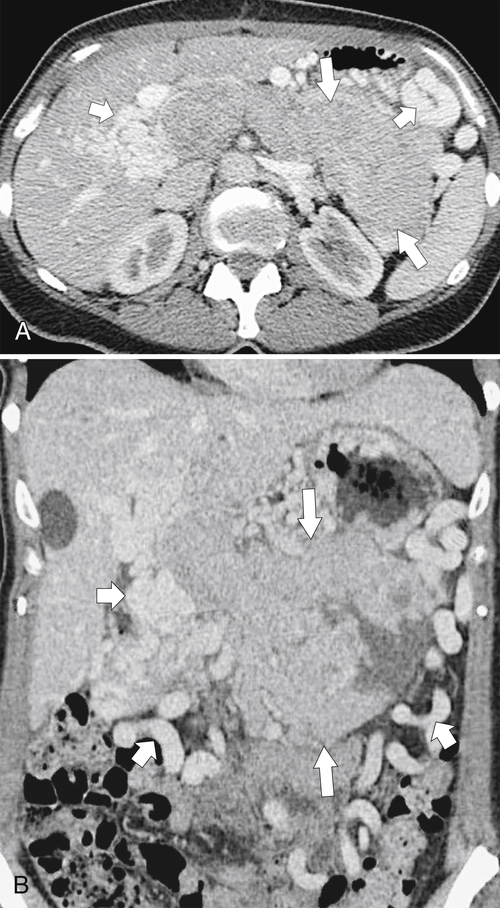
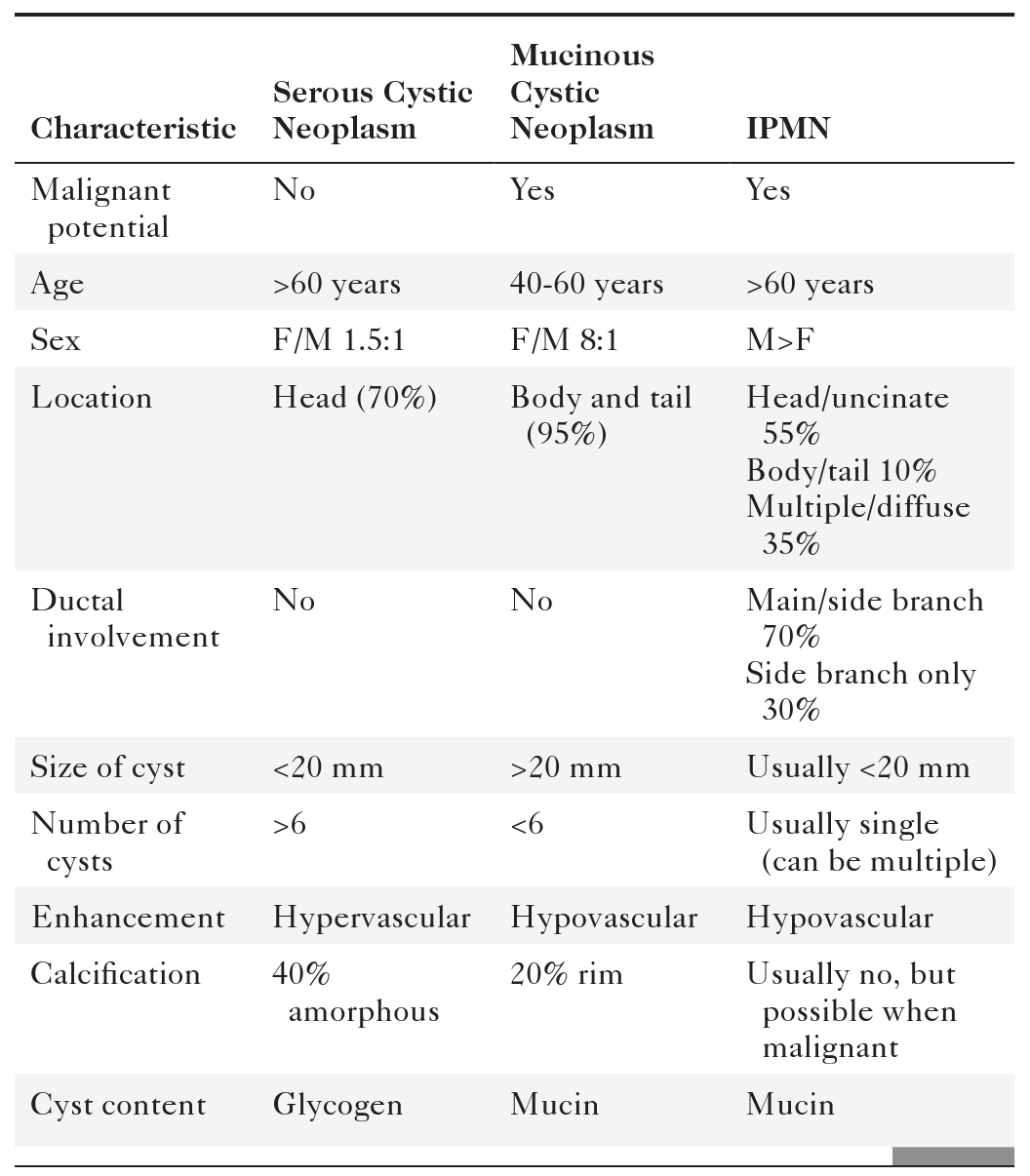
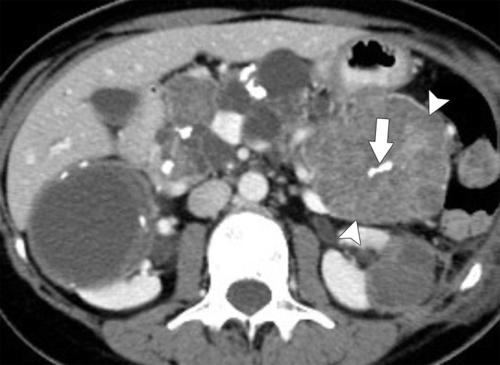
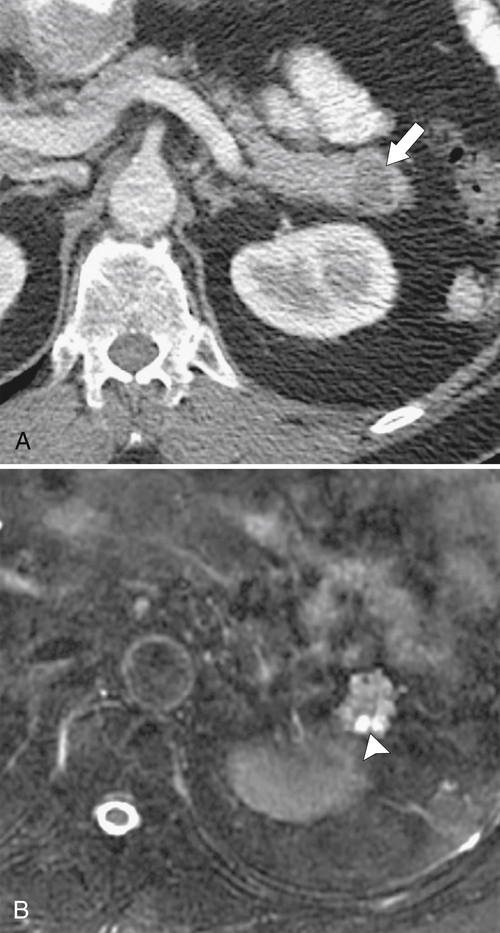
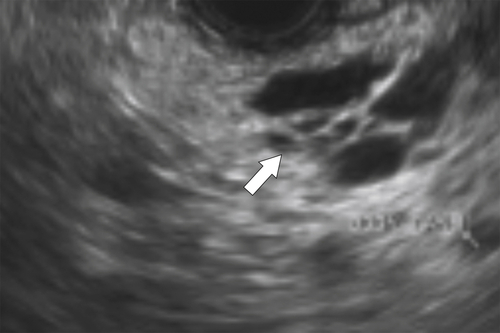
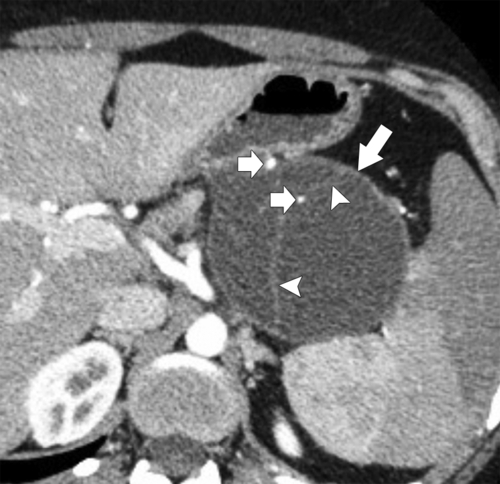
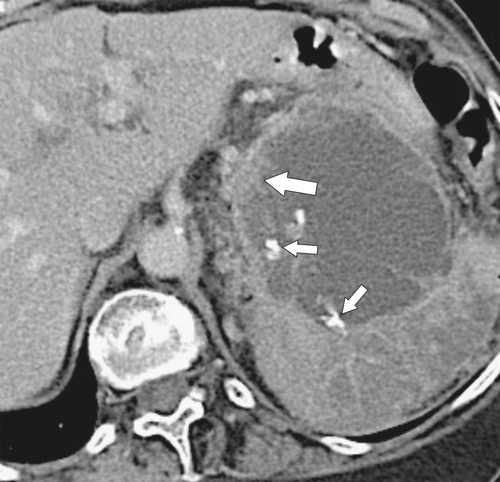
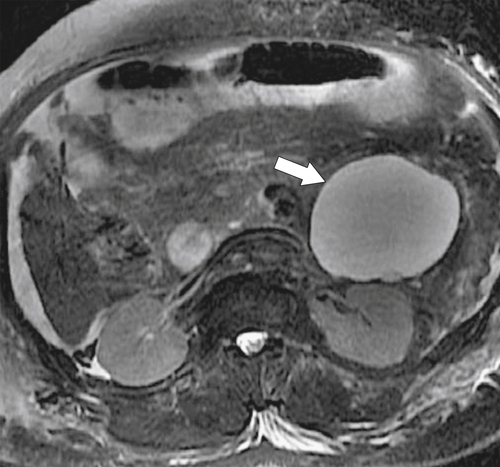
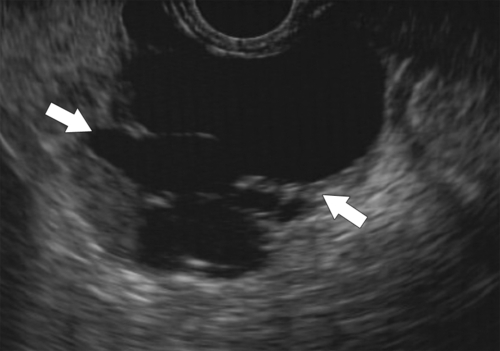
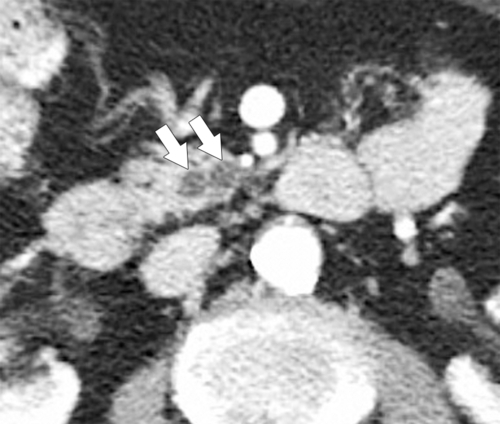
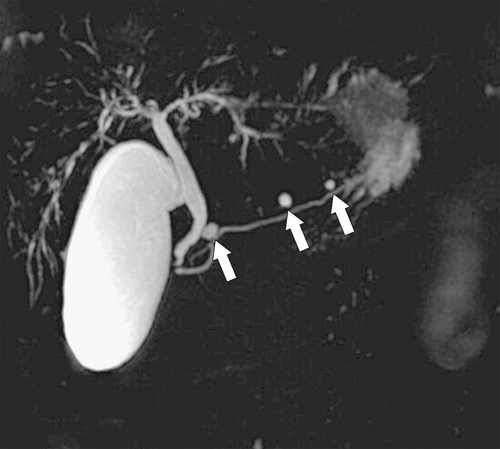
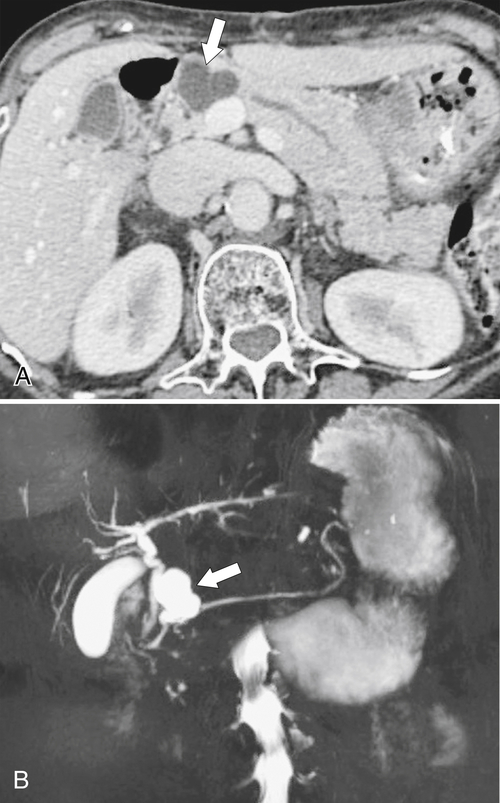
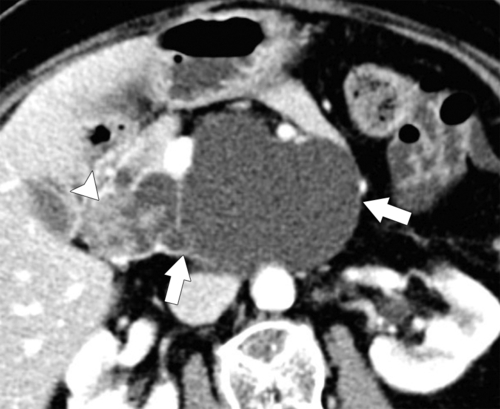
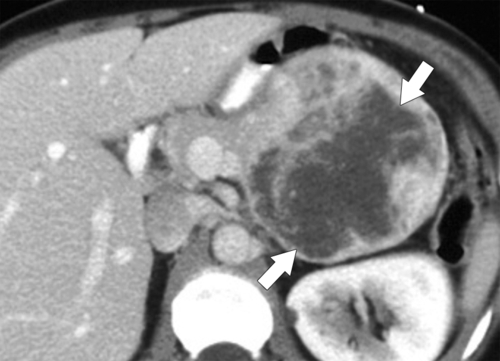
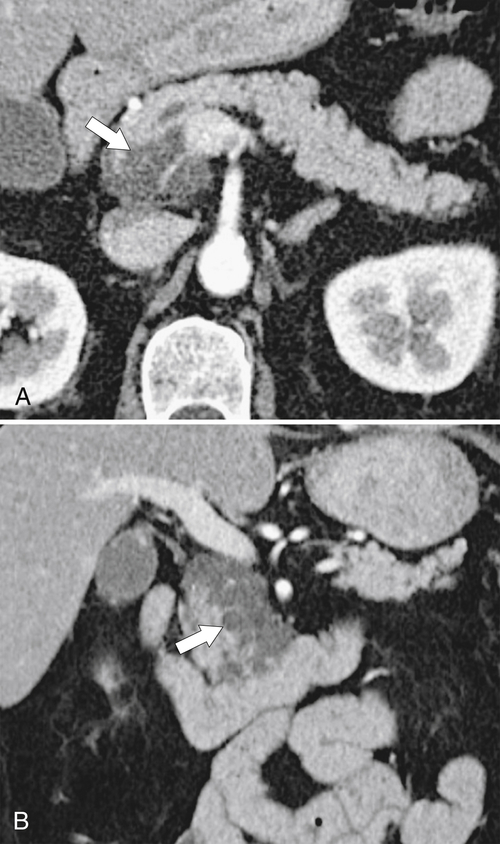
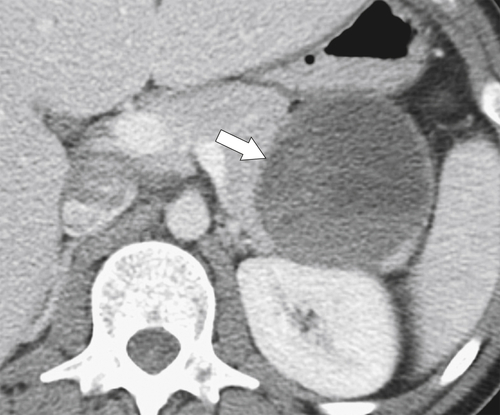
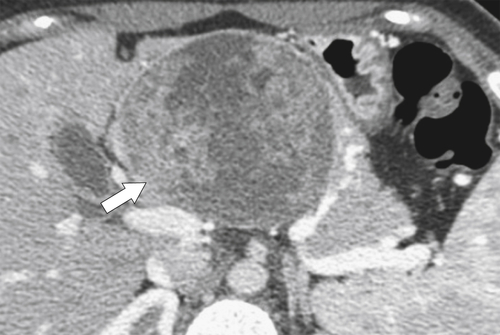
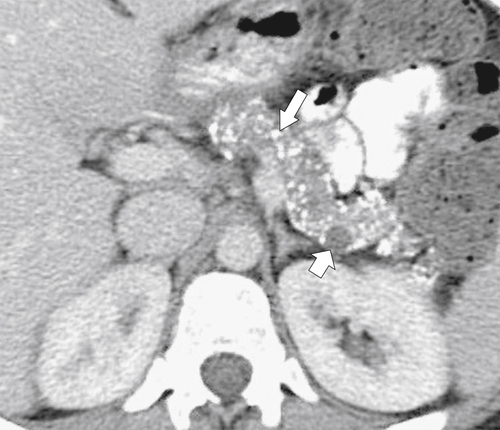
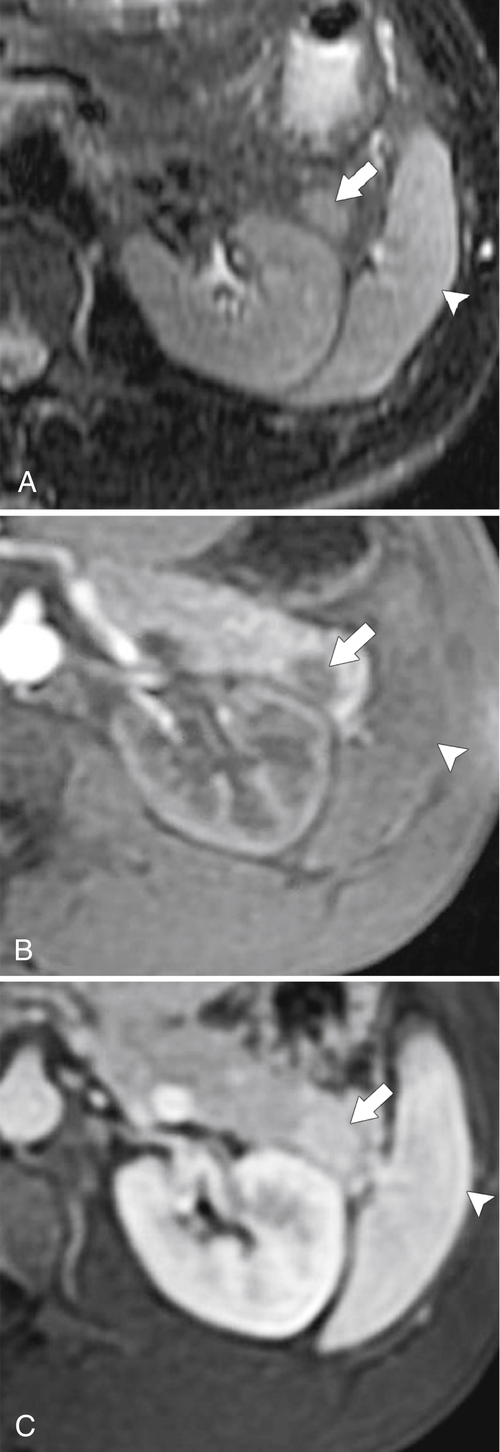
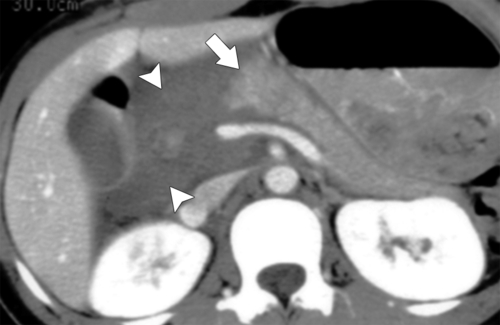
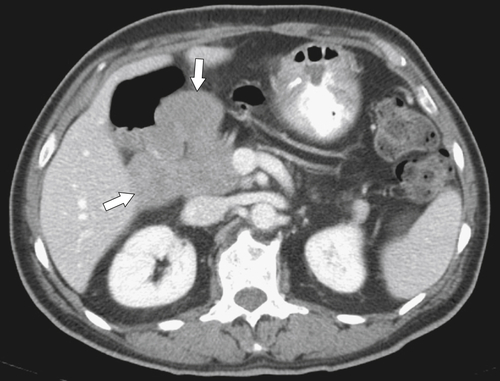
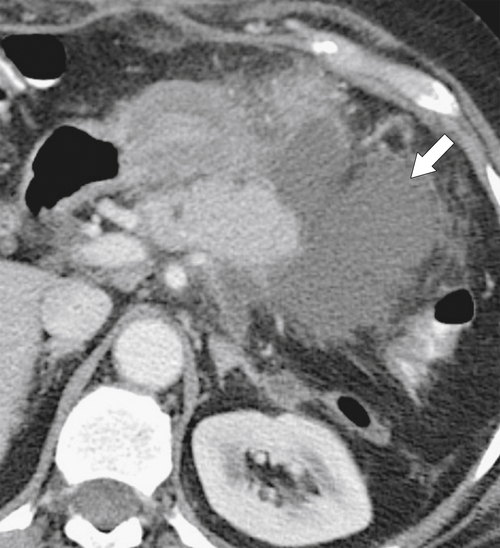
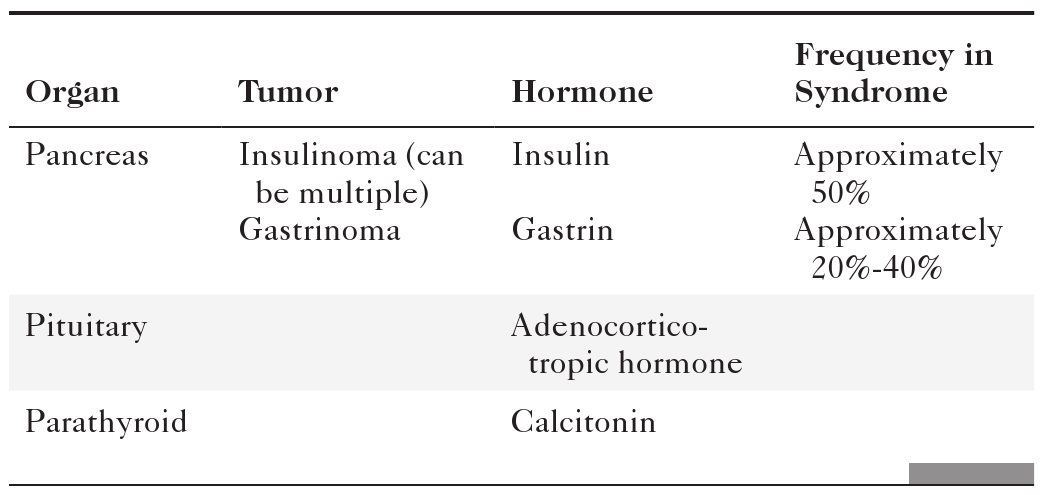
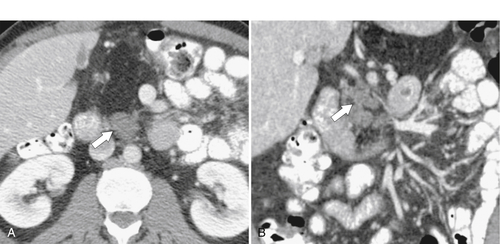
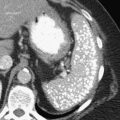
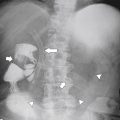
Anderson S.W. et al. Pancreatic duct evaluation: accuracy of portal venous phase 64 MDCT. Abdom Imaging 2009;34(1):55–63.
Axon A.T.R. Endoscopic retrograde cholangiopancreatography in chronic pancreatitis: Cambridge classification. Radiol Clin North Am 1989;27:39–50.
Balthazar E. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am 1989;27:19–37.
Balthazar E.J. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223(3):603–613.
Balthazar E.J. et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990;174:331–336.
Baudin G. et al. CT-guided percutaneous catheter drainage of acute infectious necrotizing pancreatitis: assessment of effectiveness and safety. AJR 2012;199:192–199. doi:10.2214/AJR.11.6984.
Blasbalg R. et al. MRI features of groove pancreatitis. AJR 2007;189(1):73–80.
Bodily K.D. et al. Autoimmune pancreatitis: pancreatic and extrapancreatic imaging findings. AJR 2009;192:431–437. doi:10.2214/AJR.07.295.
Bollen T.L. et al. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR 2011;197:386–392. doi:10.2214/AJR.09.4025.
Brennan D.D.D. et al. Comprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CT. Radiographics 2007;27(6):1653–1666. doi:10.1148/rg.276075034.
Bret P.M. et al. Pancreas divisum: evaluation with MR cholangiopancreatography. Radiology 1996;199:99.
Bronstein Y.L. et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR 2004;182:619–623.
Buccimazza I. et al. Isolated main pancreatic duct injuries spectrum and management. Am J Surg 2006;191(4):448–452.
Buetow P.C. et al. Islet cell tumors of the pancreas: clinical, radiologic, and pathologic correlation in diagnosis and localization. Radiographics 1997;17:453–472.
Buetow P.C. et al. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology 1996;199(3):707–711.
Choi B.I. et al. Solid and papillary epithelial neoplasms of the pancreas: CT findings. Radiology 1988;166(2):413–416.
Choi J.Y. et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR 2009;193(1):136–142.
Chung E.M. et al. From the archives of the AFIP: pancreatic tumors in children: radiologic-pathologic correlation. Radiographics 2006;26(4):1211–1238. doi:10.1148/rg.264065012.
Coakley F.V. et al. Pancreatic imaging mimics: part 1, imaging mimics of pancreatic adenocarcinoma. AJR 2012;199:301–308. doi:10.2214/AJR.11.7907.
Cohen-Scali F. et al. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology 2003;228(3):727–733.
Dodds W.J. et al. MEN I syndrome and islet cell tumors of the pancreas. Semin Roentgenol 1985;20:17–63.
Fletcher J.G. et al. Pancreatic malignancy: value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 2003;229(1):81–90. doi:10.1148/radiol.2291020582.
Friedman A.C. et al. Rare pancreatic malignancies. Radiol Clin North Am 1989;27:177–190.
Fugazzola C. et al. Cystic tumors of the pancreas: evaluation by ultrasonography and computed tomography. Gastrointest Radiol 1991;16:53–61.
Fukukura Y. et al. Pancreatic duct: morphologic evaluation with MR cholangiopancreatography after secretin stimulation. Radiology 2002;222(3):674–680: published online January 18, 2002; doi: 10.1148/radiol.2223010684.
Fukukura Y. et al. Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findings. Radiology 2012;263(3):732–740. doi:10.1148/radiol.12111222: published online April 24, 2012.
Graf O. et al. Arterial versus portal venous helical CT for revealing pancreatic adenocarcinoma: conspicuity of tumor and critical vascular anatomy. AJR 1997;169:119–123.
Gupta R. et al. Pancreatic intraductal papillary mucinous neoplasms: role of CT in predicting pathologic subtypes. AJR 2008;191(5):1458–1464: Erratum in AJR 191(6):1876, 2008.
Hagspiel K.D. et al. Evaluation of vascular complications of pancreas transplantation with high-spatial-resolution contrast-enhanced MR angiography. Radiology 2007;242(2):590–599.
Horton K.M. et al. Multi-detector row CT of pancreatic islet cell tumors. Radiographics 2006;26(2):53–464. doi:10.1148/rg.262055056.
Ichikawa T. et al. Atypical exocrine and endocrine pancreatic tumors (anaplastic, small cell, and giant cell types): CT and pathologic features in 14 patients. Abdom Imaging 2000;25(4):409–419.
Ichikawa T. et al. MDCT of pancreatic adenocarcinoma: optimal imaging phases and multiplanar reformatted imaging. AJR 2006;187:1513–1520. doi:10.2214/AJR.05.1031.
Kalb B. et al. MR imaging of cystic lesions of the pancreas. Radiographics 2009;29(6):1749–1765. doi:10.1148/rg.296095506.
Kawamoto S. et al. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics 2008;28(1):157–170. doi:10.1148/rg.281065188.
Kettritz U. et al. Contrast-enhanced MR imaging of the pancreas. MRI Clin North Am 1996;4:87.
Khrana B. et al. Macrocystic serous adenoma of the pancreas: radiologic-pathologic correlation. AJR 2003;181:119–123.
Kim J.H. et al. Metallic stent placement in the palliative treatment of malignant gastric outlet obstructions: primary gastric carcinoma versus pancreatic carcinoma. AJR 2009;193(1):241–247.
Kim J.H. et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010;257(1):87–96: published online August 9, 2010; doi: 10.1148/radiol.10100015.
Kim S.Y. et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR 2006;187(5):1192–1198.
Kim Y.H. et al. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics 2005;25(3):671–685. doi:10.1148/rg.253045104.
Lane M.J. et al. Diagnosis of pancreatic injury after blunt abdominal trauma. Semin Ultrasound CT MR 1996;17:177.
Lenhart D.K. et al. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR 2008;190:643–649. doi:10.2214/AJR.07.2761.
Lewis R.B. et al. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics 2012;30(6):1445–1464. doi:10.1148/rg.306105523.
Linsenmaier U. et al. Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics 2008;28(6):1591–1602. doi:10.1148/rg.286085524.
Macari M. et al. Differentiating pancreatic cystic neoplasms from pancreatic pseudocysts at MR imaging: value of perceived internal debris. Radiology 2009;251(1):77–84. doi:10.1148/radiol.2511081286.
Manfredi R. et al. Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging–MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology 2011;260(2):428–436: published online May 25, 2011; doi: 10.1148/radiol.11101729.
Manfredi R. et al. Idiopathic chronic pancreatitis in children: MR cholangiopancreatography after secretin administration. Radiology 2002;224(3):675–682.
Manfredi R. et al. Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology 2009;253(1):106–115: published online July 31, 2009; doi: 10.1148/radiol.2531080604.
Mathiu D. et al. Pancreatic cystic neoplasms. Radiol Clin North Am 1989;27:163–176.
Matos C. et al. MR imaging of the pancreas: a pictorial tour. Radiographics 2002;22:1: e2.
McNulty N.J. et al. Multi-detector row helical CT of the pancreas: effect of contrast-enhanced multiphasic imaging on enhancement of the pancreas, peripancreatic vasculature, and pancreatic adenocarcinoma. Radiology 2001;220:97–102.
Mergo P.J. et al. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics 1997;17:281–301.
Merkle E.M. et al. Imaging findings in pancreatic lymphoma: differential aspects. AJR 2000;174(3):671–675.
Mitchell D.G. MR imaging of the pancreas. MRI Clin North Am 1995;3:51.
Morgan D.E. et al. Resectability of pancreatic adenocarcinoma in patients with locally advanced disease downstaged by preoperative therapy: a challenge for MDCT. AJR 2010;194:668–674. doi:10.2214/AJR.09.3285.
Mortelé K.J. et al. CT-guided percutaneous catheter drainage of acute necrotizing pancreatitis: clinical experience and observations in patients with sterile and infected necrosis. AJR 2009;192:110–116. doi:10.2214/AJR.08.1116.
Nijs E. et al. Disorders of the pediatric pancreas: imaging features. Pediatr Radiol 2005;35(4):358–373: quiz 457..
Oshikawa O. et al. Dynamic sonography of pancreatic tumors: comparison with dynamic CT. AJR 2002;178(5):1133–1137.
Reinhold C. et al. MR cholangiopancreatography: potential clinical applications. Radiographics 1996;16:309–320.
Rizzo R.J. et al. Congenital abnormalities of the pancreas and biliary tree in adults. Radiographics 1995;15:49–68.
Roche C.J. et al. CT and pathologic assessment of prospective nodal staging in patients with ductal adenocarcinoma of the head of the pancreas. AJR 2003;180(2):475–480.
Ros P. et al. Cystic masses of the pancreas. Radiographics 1992;12:673–686.
Sahani D.V. et al. Autoimmune pancreatitis: disease evolution, staging, response assessment, and CT features that predict response to corticosteroid therapy. Radiology 2009;250(1):118–129: published online November 18, 2008; doi: 10.1148/radiol.2493080279.
Sahani D.V. et al. Autoimmune pancreatitis: imaging features. Radiology 2004;233(2):345–352: published online September 30, 2004; doi: 10.1148/radiol.2332031436.
Sahani D.V. et al. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics 2005;25(6):1471–1484. doi:10.1148/rg.256045161.
Sahani D.V. et al. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology 2006;238(3):912–919: published online January 26, 2006; doi: 10.1148/radiol.2382041806.
Sahani D.V. et al. State-of-the-art PET/CT of the pancreas: current role and emerging indications. Radiographics 2012;32(4):1133–1158. doi:10.1148/rg.324115143.
Sandrasegaran K. et al. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics 2007;27(5):1389–1400.
Scatarige J.C. et al. Pancreatic parenchymal metastases: observations on helical CT. AJR 2001;176(3):695–699.
Shanbhogue A.K.P. et al. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics 2009;29(4):1003–1026. doi:10.1148/rg.294085748.
Soto J.A. et al. Pancreas divisum: depiction with multidetector row CT. Radiology 2005;235(2):503–508.
Sun M.R.M. et al. Intraoperative ultrasonography of the pancreas. Radiographics 2010;30(7):1935–1953. doi:10.1148/rg.307105051.
Takahashi N. et al. Dual-phase CT of autoimmune pancreatitis: a multireader study. AJR 2008;190:280–286. doi:10.2214/AJR.07.2309.
Takahashi N. et al. Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR 2009;193:479–484. doi:10.2214/AJR.08.1883.
Takeshita K. et al. Differential diagnosis of benign or malignant intraductal papillary mucinous neoplasm of the pancreas by multidetector row helical computed tomography: evaluation of predictive factors by logistic regression analysis. J Comput Assist Tomogr 2008;32(2):191–197.
Tamura R. et al. Chronic pancreatitis: MRCP versus ERCP for quantitative caliber measurement and qualitative evaluation. Radiology 2006;238(3):920–928.
Taylor A.J. et al. Filling defects in the pancreatic duct on endoscopic retrograde pancreatography. AJR 1992;159:1203–1208.
Thoeni R.F. et al. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology 2012;262(3):751–764. doi:10.1148/radiol.11110947.
To’o K.J. et al. Pancreatic and peripancreatic diseases mimicking primary pancreatic neoplasia. Radiographics 2005;25(4):949–965. doi:10.1148/rg.254045167.
Triantopoulou C. et al. Groove pancreatitis: a diagnostic challenge. Eur Radiol 2009;19(7):1736–1743.
Vandermeer F.Q. et al. Imaging of whole-organ pancreas transplants. Radiographics 2012;32(2):411–435. doi:10.1148/rg.322115144.
Vlachou P.A. et al. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics 2011;31(5):1379–1402. doi:10.1148/rg.315105735.
Yamada Y. et al. Intraductal papillary mucinous neoplasms of the pancreas: correlation of helical CT and dynamic MR imaging features with pathologic findings. Abdom Imaging 2008;33(4):474–481.
Yamauchi F.I. et al. Multidetector CT evaluation of the postoperative pancreas. Radiographics 2012;32(3):743–764. doi:10.1148/rg.323105121.
Yoon S.H. et al. Small (≤20 mm) pancreatic adenocarcinomas: analysis of enhancement patterns and secondary signs with multiphasic multidetector CT. Radiology 2011;259(2):442–452: published online March 15, 2011; doi: 10.1148/radiol.11101133.
Yu J. et al. Normal anatomy and disease process of the pancreatic duodenal groove: imaging features. AJR 2004;183:839–846.
Zamboni G.A. et al. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology 2007;245(3):770–778: published online October 19, 2007; doi: 10.1148/radiol.2453061795.