Chapter 109
Lower Extremity Arterial Disease
Decision Making and Medical Treatment
Jessica P. Simons, Andres Schanzer
Based on a chapter in the seventh edition by John W. York and Spence M. Taylor
The management of lower extremity peripheral artery disease (PAD) represents one of the most challenging problems for the vascular specialist. Although its worldwide prevalence is unknown, it is estimated that 8 to 12 million Americans are affected by lower extremity PAD.1–3 A clear association between the prevalence of PAD and increased age has been established.4,5 In an analysis of 2381 patients who participated in the United States National Health and Nutrition Examination Survey, the prevalence of PAD was found to be 4.3% overall, with prevalences of 0.9% in patients between the ages of 40 and 49 years, 2.5% in patients between the ages of 50 and 59 years, 4.7% in patients between the ages of 60 and 69 years, and 14.5% in patients more than age 69 years.4 The prevalence of PAD is expected to increase in the United States and worldwide as the population ages, cigarette smoking persists, and the epidemics of diabetes mellitus (DM), hypertension, and obesity grow.5
Each year, more than 100,000 of these patients will undergo some form of arterial revascularization. Decisions regarding the management of lower extremity PAD pose a unique challenge because of the complex interplay of factors that must be considered, including the underlying pathology, anatomic defects, degree of ischemia, availability of conduits, comorbid conditions, functional status, ambulation potential, and suitability of anatomy for successful revascularization. Appropriate management of lower extremity PAD requires a firm understanding of these factors for good decision making.
Patients with lower extremity ischemia are typically divided into two groups—those with intermittent claudication and those with critical limb ischemia (CLI)—depending on symptoms at presentation. Claudication and CLI are managed differently because of major differences in their natural histories and expected clinical outcomes after treatment. In general, there is more consensus among clinicians regarding decision making for CLI because the natural history of untreated CLI more frequently leads to limb loss than does claudication. Appropriate decision making requires a basic understanding of the systemic nature of the disease. Patients with CLI often have severe associated cardiovascular comorbidities and are generally older and in poorer health than those with claudication. Treatment must therefore be structured accordingly. In contrast, patients with claudication typically seek treatment for the relief of lifestyle limiting pain with ambulation. These patients exhibit a more benign natural history with respect to limb viability, with amputation rates of 1% to 7% at 5 years and clinical deterioration of the limb in only 25%.6–8 As with CLI, claudication is a marker of significant systemic atherosclerosis, with associated cardiovascular mortality rates at 1, 5, and 10 years as high as 12%, 42%, and 65%, respectively.6–8 All patients with PAD require medical management of their cardiovascular disease, and many also benefit from either endovascular or open revascularization, as discussed later.
Medical Management
Because atherosclerosis is a systemic disease, the initial treatment of lower extremity PAD should include risk factor modification in an effort to limit progression of the atherosclerotic process. Pharmacologic treatment geared toward the relief of symptoms and the stabilization of existing atherosclerosis is also essential. Patients presenting with PAD are at significantly increased risk for premature cardiovascular events, including myocardial infarction (MI), stroke, and death.9,10 Detection of occult PAD is an important indirect marker for systemic atherosclerosis.11 Any patient older than 40 years who has an ankle-brachial index (ABI) of less than 0.90 has significant PAD, even in the absence of symptoms.12 An ABI of less than 0.90 is 95% sensitive in identifying angiographically confirmed PAD (Fig. 109-1).5,13 Interestingly, more than 50% of patients with an abnormal ABI fail to show typical symptoms of claudication or CLI because of the coexistence of other major comorbidities, a condition sometimes referred to as “chronic subclinical lower extremity ischemia.” There is wide agreement that risk factor modification is indicated for any patient with lower extremity PAD, regardless of symptom presence or severity. Several guidelines have been published5,14–16,76 regarding the use of screening for PAD, including a recent systematic review.77 However, endovascular or surgical intervention for asymptomatic disease has not been shown to provide any benefit over waiting until the development of symptoms.14 The natural history of asymptomatic PAD is also not well studied. Citing population-based studies, the Trans Atlantic Inter Society Consensus (TASC II) authors5 concluded that the ratio of asymptomatic to symptomatic PAD patients is as high as 3 : 1 (Fig. 109-2). Unfortunately, there are currently no data available to accurately predict which patients will develop symptoms in the future.
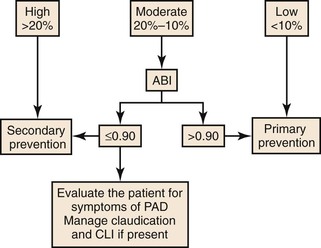
Figure 109-1 Cardiovascular 10-year risk score. Algorithm using the ankle-brachial index to assess systemic cardiovascular risk. CLI, Critical limb ischemia; PAD, peripheral arterial disease. (Redrawn from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease [TASC II]. J Vasc Surg 45[Suppl S]:S5-S61, 2007.)
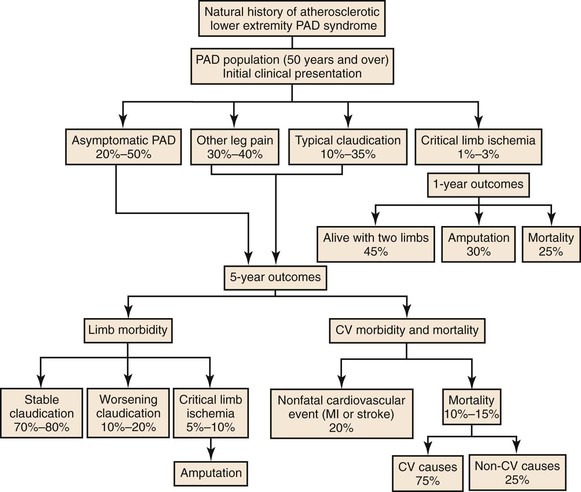
Figure 109-2 Future health state of the patient with claudication over 5 years. CV, Cardiovascular; MI, myocardial infarction; PAD, peripheral arterial disease. (Adapted from American College of Cardiology–American Heart Association guidelines; redrawn from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease [TASC II]. J Vasc Surg 45[Suppl S]:S5-S61, 2007.)
Risk Factor Modification
The risk factors associated with PAD are similar to those classically linked with coronary artery disease. Investigators from the Framingham Heart Study who analyzed “factors of risk” for coronary artery disease were the first to identify demographic and comorbid factors independently associated with systemic atherosclerosis.17,18 Numerous reports since then have confirmed that advanced age, tobacco use, diabetes, dyslipidemia, and hypertension are the primary risk factors associated with PAD (Fig. 109-3). More recent studies have identified non-Hispanic black race,4,19 chronic renal insufficiency,4,20 and elevated homocysteine levels21,22 as additional factors associated with the onset of PAD. All patients with the diagnosis of PAD require appropriate risk factor modification, regardless of whether more aggressive therapy is also being contemplated. Risk factor modification is discussed in detail in the section on Atherosclerotic Risk Factors and is summarized here.
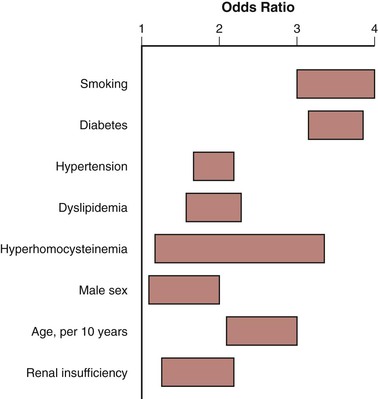
Figure 109-3 Odds ratios for risk factors for symptomatic peripheral artery disease. (Adapted from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease [TASC II]. J Vasc Surg 45[Suppl S]:S5-S61, 2007.)
Smoking
Smoking has been associated with lower extremity ischemia since the early 1900s and arguably remains the most important risk factor for its development. Smoking cessation has been shown to reduce the risk of MI and death in patients with PAD and to delay the progression of lower extremity symptoms from claudication to CLI and limb loss.23–25 The physiologic effects of smoking are incompletely understood; however, several pathologic processes have been implicated. Nicotine inhalation has been demonstrated to reduce high-density lipoprotein (HDL) levels, increase platelet aggregation, decrease prostacyclin, increase levels of thromboxane, and promote vasoconstriction.26 Each of these effects contributes to the development and progression of atherosclerotic disease.
Although the beneficial effects of smoking cessation have been clearly demonstrated,27 the role of smoking cessation in the treatment of intermittent claudication is less clear. Treadmill studies have demonstrated an increase in pain-free ambulation distances in some but not all patients after smoking cessation.5 Nonetheless, smoking cessation should be the goal in all patients with lower extremity ischemia to reduce their risk of cardiovascular events and limit the progression of lower extremity PAD. The importance of smoking cessation extends to patients who have undergone lower extremity revascularization because there is a threefold increased risk of graft failure in smokers compared with nonsmokers.28
The role of the physician is to educate patients about the consequences of this high-risk behavior, provide emotional support, and prescribe pharmacologic aids aimed at treating the addiction. Structured smoking cessation programs have demonstrated a 22% cessation rate at 5 years, compared with 5% in patients who attempt to stop smoking independently.27 The addition of pharmacologic agents, such as bupropion, have increased smoking cessation rates in randomized studies of patients with PAD, achieving 3-, 6-, and 12-month abstinence rates of 34%, 27%, and 22%, respectively, compared with 15%, 11%, and 9% in control groups.28 More recently, varenicline (Chantix, Pfizer Inc., New York, NY) has been approved for use in the United States, with remarkable early results. This pharmacologic agent acts as a partial agonist of the α4β2 nicotine acetylcholine receptor and was developed for the sole purpose of treating tobacco addiction. It stimulates the release of dopamine in sufficient quantities to reduce nicotine craving while minimizing the effects of withdrawal. Randomized studies of varenicline have shown continuous abstinence rates of 44% and 22% at 12 and 52 weeks, respectively, versus 17% and 8% in control groups.29 Despite the promise of these new pharmacologic agents, the prognosis for smoking cessation in most patients continues to be poor. Tobacco addiction tends to be inexorable and is characterized by frequent relapses and poor long-term cessation rates (see Chapter 27).
Diabetes Mellitus
The association between DM and atherosclerotic vascular disease is well documented.30–33 Diabetes is widely prevalent among patients with lower extremity ischemia. It has been estimated that each incremental 1% increase in glycosylated hemoglobin is associated with a 28% increase in risk for PAD.13 Atherosclerosis in individuals with DM occurs as a consequence of arterial wall degeneration due to alterations in nitric oxide availability to endothelial cells and the stimulation of proatherogenic activity in vascular smooth muscle cells by the reduction of phosphatidylinositol-3 kinase. Diabetes also alters blood component activity via enhanced platelet aggregation, increased blood viscosity, and elevation of fibrinogen levels.34
Although the microvascular complications of diabetic retinopathy and nephropathy appear to be a consequence of uncontrolled DM, the effect of intensive blood glucose control on macrovascular complications is unclear. The Diabetes Control and Complications Trial evaluated 1441 patients with type 1 diabetes and compared conventional therapy with intensive insulin therapy. Tighter glucose control regimens exhibited only a nonstatistically significant trend toward a reduction in cardiovascular events and had no demonstrable effect on the incidence of PAD.35 Additional studies have demonstrated similar findings; strict glucose control reduced microvascular complications but failed to significantly decrease macrovascular complications.36,37 Despite these findings, many experts continue to believe that strict glucose control in PAD patients is important to prevent further atherosclerotic complications. The current American Diabetes Association guidelines recommend hemoglobin A1c levels less than 7% as a treatment goal for all patients with DM. They further suggest that the goal of therapy should be to maintain glucose control as close to normal as possible (hemoglobin A1c <6%) without inducing significant hypoglycemia (see Chapter 28).5
Hypertension
The Framingham Offspring Study showed, and other studies confirmed, that hypertension is associated with a two- to threefold increased risk of PAD.38,39 Hypertension is also a risk factor for stroke, coronary artery disease, congestive heart failure, and chronic renal insufficiency. Current guidelines recommend a target blood pressure of less than 140/90 mm Hg in high-risk groups, such as those with documented PAD, and less than 130/80 mm Hg in patients who also have diabetes or renal insufficiency.40,41
The specific pharmacologic agent chosen for blood pressure control is less important than the maintenance of a normotensive state.42 All drugs that are effective at reducing systemic blood pressure decrease the risk of cardiovascular events. Most patients require multiple agents for adequate blood pressure control. Angiotensin-converting enzyme (ACE) inhibitors are particularly beneficial, as shown in the Heart Outcomes Prevention Evaluation (HOPE) study. Patients with adequate blood pressure control using the ACE inhibitor, ramipril, experienced a reduction in subsequent stroke, MI, and vascular-related mortality.43 Subgroup analysis of 4046 patients with PAD found a 22% risk reduction in patients randomized to ramipril compared with placebo. This finding was independent of the absolute reduction in blood pressure, leading the Food and Drug Administration (FDA) to approve ACE inhibitors as a cardioprotective drug in high-risk groups (see Chapter 30).
Dyslipidemia
Total serum cholesterol levels greater than 200 mg/dL (5.18 mmol/L) are associated with an increased risk of cardiac-related events, especially in combination with a low HDL fraction (<40 mg/dL [1.04 mmol/L] in men; <50 mg/dL [1.30 mmol/L] in women) and an elevated low-density lipoprotein (LDL) level (>130 mg/dL [3.37 mmol/L]).44–46 Lipid-lowering agents, specifically 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (“statins”), have been shown to decrease the risk of MI-related death in high-risk patients.47 The beneficial effects of statin therapy are pleomorphic and appear to be independent of their lipid-lowering properties; they work by stabilizing existing atherosclerotic plaques, decreasing oxidative stress, and reducing vascular inflammation.48 Statin therapy may also protect against thrombosis by altering the lipid content of platelets, thereby decreasing platelet aggregability.
The direct benefits of statin therapy on cardiac-related events have been demonstrated in multiple well-designed studies.49–51 Until recently, however, the effects of lipid management on PAD had been studied only in subgroup analyses of larger comprehensive coronary artery disease trials. For example, subgroup analysis of high-risk patients with PAD enrolled in the Heart Protection Study and treated with simvastatin found significant reductions in cardiovascular events (MI, stroke, vascular-related death).47 Perhaps more significantly, Feringa et al52 demonstrated in their cohort study of 1374 patients with lower extremity PAD that intense statin therapy (target LDL levels <70 mg/dL [1.81 mmol/L]) was independently associated with improved survival over a mean follow-up of 6 years. Benefits have also been seen in studies that have not evaluated a specific target LDL level; in the PREVENT III cohort183 of 1404 patients who underwent lower extremity bypass for CLI, use of any statin therapy (regardless of specific agent or specific dose) was associated with a significant 1-year survival benefit.184
The modulation of HDL cholesterol, although less well studied, is also believed to play an important role in dyslipidemia management in patients with PAD.46 Well-designed studies have found that adding niacin to conventional statin therapy results in regression of atherosclerotic plaques in patients with femoral artery stenosis and a decrease in the intima-media thickness of carotid artery plaques.53 These beneficial effects were observed in addition to a documented pronounced reduction in the progression of coronary artery disease. These PAD-specific benefits occur independently of lipid control. Improvements in leg function, ABI, walking performance, symptoms of claudication, and perioperative and long-term mortality have been demonstrated.54
Currently, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend an LDL cholesterol level of less than 100 mg/dL (2.59 mmol/L) in patients with PAD and an even lower level (<70 mg/dL [1.8 mmol/L]) in high-risk patients with more generalized atherosclerosis. In patients with PAD who have elevated triglycerides, precluding the accurate calculation of serum LDL levels, a non-LDL cholesterol level less than 130 mg/dL (3.37 mmol/L) is recommended (Table 109-1) (see Chapter 29).5,55–57
Table 109-1
TASC II Recommendations for Lipid Control in Patients With Peripheral Arterial Disease
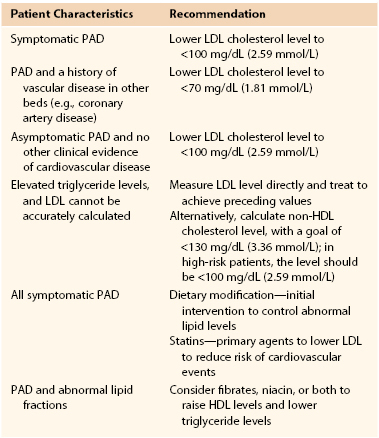
HDL, High-density lipoprotein; LDL, low-density lipoprotein; PAD, peripheral arterial disease; TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease.
Adapted from Norgren L, et al: TASC II Working Group. Inter-Society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45(Suppl S):S5-S61, 2007.
Homocysteinemia
The important influence of homocysteine metabolism on premature atherosclerosis was suspected in the 1990s when a distinct group of young patients with advanced atherosclerosis and no other established risk factors was investigated.58 Plasma levels of homocysteine are regulated in part by B vitamins, and vitamin supplementation lowers plasma homocysteine levels.59 Thus, low levels of folate and vitamin B6 are also associated with the risk of PAD, perhaps through the modulation of homocysteine levels.60 Elevated circulating homocysteine results in endothelial dysfunction and injury, followed by platelet activation and thrombus formation. Other effects of hyperhomocysteinemia include the production of hydrogen peroxide (which mediates endothelial injury), increases in factors XII and V, decreases in protein C, and inhibition of thrombomodulin and heparin sulfate. Early studies found elevated homocysteine to be an independent risk factor for coronary artery disease and stroke.61 More recently, however, studies, such as the Vitamin Intervention for Stroke Prevention Trial, have questioned the actual impact of abnormal homocysteine metabolism on premature atherosclerosis, because they failed to show a benefit of high-dose folic acid therapy on stroke prevention.62 Other randomized controlled trials by Liem et al63 and Wrone et al64 failed to show a beneficial impact of folic acid administration on cardiovascular endpoints in patients with stable coronary artery disease and end-stage renal disease.
Despite these recent reports, serologic evaluation for elevated homocysteine levels is still recommended for patients with family histories of multiple thrombotic events, patients with premature cardiovascular symptoms in the absence of conventional risk factors, and selected patients with coronary artery disease, PAD, stroke, deep venous thrombosis, and pulmonary embolism. Supplemental B vitamins or folic acid therapy may be worthwhile; however, level I data that show a benefit in the prevention of cardiovascular disease are lacking (see Chapter 26).5
Platelets and Thrombosis
Antiplatelet therapy is now widely accepted among physicians for the treatment of cardiovascular disease, and it has been shown to reduce the risk of nonfatal MI, ischemic stroke, and vascular-related death. It should be used in all patients with PAD. The Antiplatelet Trialists’ Collaboration included 102,459 patients with cardiovascular disease (MI, stroke, PAD, or other vascular disease) and concluded that the risk of fatal or nonfatal cardiovascular events in patients treated with antiplatelet therapy was 9.5%, compared with 11.9% in the untreated control group.65 Subgroup analysis of patients with claudication in this study revealed an 18% to 23% reduction in cardiovascular-related events.
Clopidogrel (Plavix, Bristol-Myers Squibb, New York, NY) is the only antiplatelet agent approved by the FDA for the secondary prevention of atherosclerotic vascular disease, including PAD. The primary supporting data were derived from the findings of the Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial, which randomized more than 19,000 patients with known cardiovascular disease to receive either daily clopidogrel (75 mg) or aspirin (325 mg).66 Endpoints of stroke, MI, or death were examined. Clopidogrel was associated with an overall 8.7% reduction in the composite endpoint. In a subgroup analysis of 6452 PAD patients, a relative cardiovascular risk reduction of 24% was found in the clopidogrel group compared with the aspirin group. Clopidogrel was well tolerated, with few adverse effects.66
The Clopidogrel for High Atherothrombotic Risk, Ischemic Stabilization, Management, and Avoidance (CHARISMA) investigators attempted to answer the question of whether dual antiplatelet therapy with aspirin and clopidogrel was superior to aspirin alone in preventing stroke, MI, and death in a broad population of patients at risk for cardiovascular events. In this randomized study of 15,603 patients, there was no significant difference in the composite outcome of MI, stroke, and death between the two groups.68 In the group that received dual antiplatelet therapy, there was a significantly greater rate of moderate bleeding (transfusion required) with a relative risk of 1.62 (2.1% compared with 1.3%).
A third antiplatelet drug, picotamide, was studied for the treatment of cardiovascular disease. Picotamide is an inhibitor of thromboxane A2 synthase and thromboxane-endoperoxide receptors. The Drug Evaluation in Atherosclerotic Vascular Disease in Diabetics (DAVID) study demonstrated that picotamide was more effective than aspirin alone in reducing overall mortality at 24 months in patients with PAD and type 2 diabetes.67
Two recent trials analyzed the benefit of aspirin for primary prevention in at-risk patients. The Antithrombotic Trialists’ Collaboration performed a meta-analysis of low-dose aspirin in the primary prevention of several cardiovascular endpoints and the incidence of major bleeding events. They found that aspirin therapy yielded a 12% proportional reduction in serious vascular events (0.51% aspirin versus 0.57% control per year; P = 0.0001), but also increased major gastrointestinal and extracranial bleeding events (0.10% versus 0.07% per year; P < .0001).69 The authors concluded that aspirin was of uncertain value for primary prevention when weighed against the risk of adverse bleeding events. Similarly, the Aspirin for Asymptomatic Atherosclerosis Trialists group conducted a randomized controlled trial on the use of low-dose aspirin for primary prevention of cardiovascular events in patients with diminished ABIs on screening examination.70 For the primary endpoint of fatal or nonfatal MI, stroke, or revascularization, they found no difference between groups (13.7 events per 1000 person-years in the aspirin group versus 13.3 in the placebo group; hazard ratio, 1.03; 95% confidence interval [CI], 0.84-1.27). There were no significant differences for any of the secondary endpoints, including major hemorrhage. They concluded there was no significant benefit to low-dose aspirin in the primary prevention of major vascular events in this at-risk, but clinically asymptomatic population.
Exercise Therapy for Claudication
Multiple reports have clearly demonstrated improvements in pain-free ambulation and overall walking performance with structured exercise training.5,71–73 Data from more than 20 randomized trials have confirmed that exercise therapy is the best initial treatment of intermittent claudication.73 The benefits of exercise extend beyond improvement in the symptoms of claudication. Regular aerobic exercise reduces cardiovascular risk by lowering cholesterol and blood pressure and by improving glycemic control. In most patients, claudication initiates a downward spiral of cardiovascular deconditioning that can result in an annual mortality rate as high as 12%.73 Ambulation distance can decline at a rate of 8.4 m/yr beginning with symptom onset.74 This cycle can be halted and even reversed with exercise training. In a recent report from Japan, Sakamoto et al75 showed that the implementation of structured exercise resulted in a 5-year cardiovascular event-free survival rate of 80.5% in patients with PAD, compared with 56.7% in untreated matched controls.
The current ACC/AHA guidelines support supervised exercise for the treatment of intermittent claudication as a level IA recommendation.76 The guidelines suggest that exercise training, in the form of walking, should be performed for a minimum of 30 to 45 minutes per session, three to four times per week, for a period not less than 12 weeks. During each session, the patient should be encouraged to walk until the limit of lower extremity pain tolerance is reached, followed by a short period of rest until pain relief is obtained, then a return to exercise. This cycle should be followed for the duration of the session. As the pain-free interval of ambulation increases, the level of exercise should be increased (Box 109-1).71
Although exercise therapy appears to be easy to implement, effectiveness is often limited by poor patient compliance. Studies have shown the superiority of clinic-based exercise programs over home-based programs.5 However, effective exercise training is not possible in up to 34% of patients because of comorbid medical conditions, and an additional 30% of patients simply refuse to participate in exercise training.5 In addition, supervised exercise training programs are usually not covered by third-party insurance plans, including Medicare. Therefore, although exercise therapy in motivated patients offers proven benefits, its effectiveness is applicable to only about one third of patients presenting with intermittent claudication.
Pharmacologic Treatment of Claudication
Pharmacologic therapy for intermittent claudication has been the subject of intense research for more than 30 years. To date, only two drugs (pentoxifylline and cilostazol) have achieved FDA approval for the treatment of intermittent claudication in the United States. However, a number of other medications have been investigated, with varying degrees of evidence supporting their efficacy (Table 109-2). These include a number of drugs and supplements with various reported mechanisms of action, such as changes in tissue metabolism (naftidrofuryl, levocarnitine), enhanced nitric oxide production (L-arginine), and vasodilatory effects (statins, buflomedil, prostaglandins, ACE inhibitors, K-134).
Table 109-2
Pharmacologic Treatment of Claudication
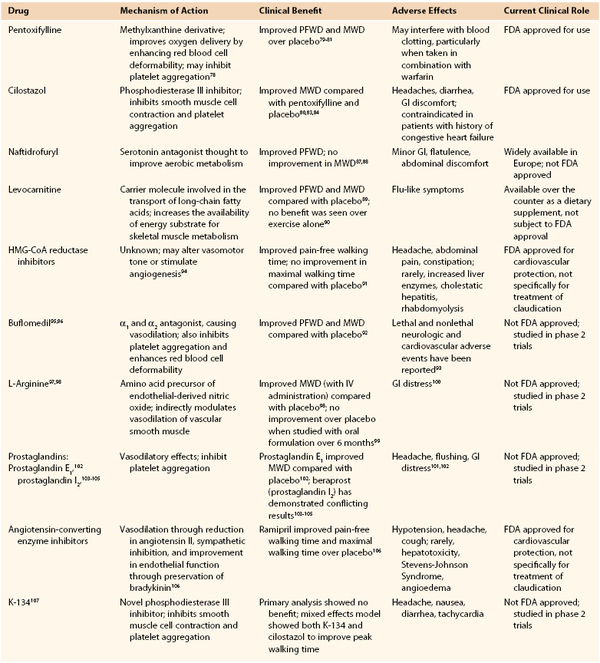
FDA, Food and Drug Administration; GI, gastrointestinal; HMG-CoA, hydroxy-3-methylglutaryl-coenzyme A; MWD, maximal walking distance; PFWD, pain-free walking distance.
Pentoxifylline
In 1984, pentoxifylline (Trental, sanofi-aventis, Paris, France) became the first drug approved by the FDA for the treatment of intermittent claudication. It is a methylxanthine derivative that is thought to improve oxygen delivery because of its rheolytic effect on red blood cell wall flexibility and deformability, ultimately reducing blood viscosity. Pentoxifylline is also believed to inhibit platelet aggregation and to increase fibrinogen levels.78 Early trials of pentoxifylline were promising and showed that maximal treadmill walking distances in patients with claudication were improved by 12% compared with placebo.79 Although walking distances improved, patient discomfort with walking typically persisted. Porter et al79 evaluated the efficacy of pentoxifylline in a multicenter double-blinded study and demonstrated modest improvements in pain-free ambulation distance and maximal walking distance after pentoxifylline administration in patients compared with the control group. These findings were reproduced in several well-designed studies.80,81
Although pentoxifylline has produced a very modest improvement in walking distance in clinical trials of patients with claudication, this improvement appears to be more statistically significant than clinically significant. In clinical practice, although some patients experience substantial long-term symptom relief with pentoxifylline, others do not, and it is impossible to predict patient response without a trial of the drug. Whether these observed improvements are a consequence of a placebo effect is unclear.
Pentoxifylline is well tolerated, safe, and relatively inexpensive. Although its clinical impact has been modest, pentoxifylline represents one of the earliest successful pharmacologic advances for the treatment of claudication. Dosing recommendations for pentoxifylline begin at 400 mg orally three times daily and can be increased as tolerated up to 1800 mg/day. Pentoxifylline can interfere with blood clotting, especially if taken with sodium warfarin. Pentoxifylline has rarely been associated with nausea, headache, anxiety, insomnia, drowsiness, and loss of appetite. Increased blood pressure can occur, so blood pressure should be monitored.79–81
Cilostazol
Cilostazol (Pletal, Otsuka Pharmaceutical Ltd., Tokyo, Japan) gained FDA approval in 1999 for the treatment of intermittent claudication. Oral administration of this phosphodiesterase III inhibitor increases cyclic adenosine monophosphate (cAMP) and results in a variety of physiologic effects, including the inhibition of smooth muscle cell contraction and platelet aggregation. Cilostazol is also thought to decrease smooth muscle cell proliferation, a process that has been implicated in coronary artery restenosis after percutaneous transluminal angioplasty.82 Finally, cilostazol has a beneficial effect on plasma lipid concentrations, resulting in a decrease in serum triglycerides and an increase in HDL. Although the precise mechanism by which cilostazol improves the symptoms of intermittent claudication is unknown, it is likely a combination of these effects.
Several controlled clinical trials, including a meta-analysis, have confirmed the efficacy of cilostazol.80,83,84 Results have shown increased maximal walking distances up to 50% and significant improvements in health-related quality of life (QoL) measures.83 There is also increasing evidence that cilostazol may modulate the synthesis of vascular endothelial growth factor (VEGF), potentially stimulating angiogenesis in patients with chronic lower extremity ischemia.85
The benefits of cilostazol in the treatment of intermittent claudication were compared with those of pentoxifylline in a randomized controlled trial performed by Dawson et al.80 They found that cilostazol therapy significantly increased maximal walking distance by 107 m (54% increase), compared with a 64-m improvement in the pentoxifylline group (30% increase). There was no difference in maximal walking distance improvement between the pentoxifylline and placebo groups. Regarding the durability of the effect, a recent pooled analysis of nine randomized controlled trials demonstrated a significant benefit in maximal walking distance compared with placebo at 6 months.86
Cilostazol has a moderate but notable adverse effect profile that includes headache, diarrhea, and gastrointestinal discomfort. Its use is contraindicated in patients with congestive heart failure, and high plasma drug levels may result when taken in combination with other medications metabolized by the liver via the cytochrome-P450 pathway: (CYP 3A4 and CYP 2C19).
The adverse effects of cilostazol can be minimized by initiating a progressive treatment regimen, starting at 50 mg/day for 1 week, increasing to 50 mg twice daily the following week, and finally achieving the standard dose of 100 mg twice daily in week 3. Of the pharmacologic agents used to treat claudication, cilostazol has the most data supporting its clinical use.
Intermittent Pneumatic Compression for Peripheral Artery Disease
Intermittent pneumatic compression (IPC) in combination with appropriate risk factor modification may be a viable method of treatment for patients with unreconstructable vascular disease, for those who are physiologically unfit for surgical intervention, or for patients with intermittent claudication who do not want invasive treatment. IPC involves sequential inflation and deflation of pneumatic pressure cuffs positioned at the foot or calf. Inflation-deflation rates vary according to the system used, each applying a pressure up to 120 mm Hg for 2 to 3 seconds before deflating. This sequence is continued at a rate of three cycles per minute throughout the treatment session. The physiologic effects of IPC are thought to be a consequence of three mechanisms: an increase in the arteriovenous pressure gradient; reversal of vasomotor paralysis; and enhanced release of nitric oxide.108
Sporadic favorable reports describing IPC for lower extremity ischemia, albeit in series with relatively small patient numbers, date from the 1960s. However, several recently published studies described the clinical benefit of IPC for the treatment of intermittent claudication and CLI.108–110 In one of these reports, 48 patients with CLI were randomized to receive IPC plus stringent wound care versus stringent wound care alone. With a follow-up of 18 months, the investigators observed limb salvage in 58% of those treated with IPC compared with 17% receiving stringent wound care alone (P < .01).110 These studies showed that although the role of IPC was not completely defined for the treatment of PAD, it might be beneficial in high-risk patients with limited treatment options.
Improvements in limb salvage have also been demonstrated using IPC. Kavros et al110 examined the effects of IPC on patients with chronic CLI or tissue loss in whom limb revascularization was thought to be impossible. These investigators achieved limb salvage with complete wound healing in 14 of 24 patients (54%) who underwent IPC, compared with 4 of 24 patients (17%) who received equivalent wound care but no IPC. Chang et al111 also demonstrated benefits on measures of health-related QoL. They randomized 31 patients with either disabling claudication or CLI and no reconstruction options to a 3-month course of IPC; among the treatment group, there were significant improvements in six of eight domains of health-related QoL, whereas the control group only experienced improvements in two domains. Although data are limited overall, this treatment method may have significant benefits in patients with CLI when combined with dedicated medical management and wound care, particularly in those with limited revascularization options.
Decision Making for Revascularization
Patients with lower extremity PAD present with a wide spectrum of symptoms. Patients may be asymptomatic, may experience only minor exertional leg pain, may experience significant walking impairment, or may present with ulceration or gangrene. The first critical step in decision making for the treatment of PAD, therefore, is to confirm that PAD is the responsible etiology of the patient’s symptoms (see Chapter 108). In brief, noninvasive vascular laboratory testing is indicated for patients with a history consistent with vasculogenic claudication, pain at rest or metatarsalgia, or tissue loss. Measurement of the ABI is the most commonly used PAD screening tool. An ABI of ≤0.90 has been demonstrated to have high sensitivity and specificity for the identification of PAD compared with the gold standard of invasive arteriography (evidence present of a peripheral hemodynamically significant arterial stenosis).5 Complete physical examination will often disclose pulse deficits, but intact pulses at rest do not rule out hemodynamically significant PAD. Once the symptom complex (either claudication or CLI) is determined to be secondary to PAD, decision making depends on symptom severity and whether the symptoms are from acute or chronic lower extremity ischemia. The following discussion relates to chronic lower extremity ischemia; acute ischemia is discussed in Chapters 161 and 162.
Decision making regarding revascularization is based first on symptom status and a sophisticated understanding of the natural history of the patient’s condition. As a result, treatment strategies may be very different for patients with disabling claudication compared with those with CLI because the risk of limb loss is dramatically different for the two conditions. Anatomic classification systems, such as the TASC classification system, may assist in gauging the extent of angiographic disease. Although the TASC II classification system can be helpful in the revascularization decision-making process (i.e., endovascular or open), atherosclerotic burden as measured by arteriography is not the sole factor upon which treatment decisions should be made. The TASC classification system lacks any features related to degree of ischemia, wounds, infection, functional status, and conduit availability, all of which are extremely important determinants of revascularization success. Clearly, angiographic anatomy alone cannot guide therapy.
Another critical element of decision making focuses on the determination of whether or not a patient will experience a meaningful benefit from a technically successful procedure. Technical success does not always equate directly with clinical success. As a result, it is important to assess baseline functional status and the burden of comorbid conditions. Patients who are either bedridden at baseline or who have prohibitive medical risks may incur significantly more benefit from a treatment that differs from the TASC II recommendations (based on lesion type alone), to more appropriately balance the chances for functional limb salvage with the risks of periprocedural morbidity. An assessment of the available conduit, if bypass is required, is included in this evaluation. The challenge of decision making in PAD is accurately assessing each of these factors and synthesizing a plan that optimizes the likelihood of a favorable outcome for each patient.
Defining Treatment Success
Optimal treatment must ultimately be tailored to each patient. The approach to decision making must involve careful discussions with the patient to identify what defines treatment “success.” This will not be the same for every patient.
Limb- and Patient-Centered Outcomes
Traditional definitions of treatment success include technical outcomes such as graft and/or stent patency. However, graft patency may not correlate well with limb preservation. Simons et al112 found that 10% of patients who underwent lower extremity bypass for CLI failed to achieve clinical improvement despite having a patent graft at 1 year postoperatively. Other outcome measures such as survival, or amputation-free survival (AFS) have also been widely used. Some have questioned the appropriateness of these endpoints. In a critique of the Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial,113 Conte114 noted that lower extremity bypass was generally not offered as a life-saving therapy, and therefore, survival was not an appropriate measure for comparisons between revascularization strategies. In addition, Conte stressed the importance of limb- and patient-centered outcomes, such as freedom from re-intervention. This shift towards more patient-centered outcomes is reflected in the Society for Vascular Surgery (SVS) objective performance goals (OPGs).115 These guidelines were developed specifically for comparative evaluations of treatments for CLI,116,117 but the endpoints chosen are key components of treatment success for PAD, namely, major adverse limb events, which included both freedom from major amputation and re-intervention. This inclusion shifts the focus of outcome measures from technical success rates, such as primary and secondary patency, to one that acknowledges a burden incurred by the patient with each intervention that is required to maintain that patency (Table 109-3). Goshima et al173 attempted to quantify the efforts required, by both the patient and the physician, to achieve functional limb salvage. They used nontraditional endpoints of hospital readmission within 6 months, index limb reoperation within 3 months, and wound healing time. They found that nearly 50% of patients required both readmission and reoperation, and time to wound healing exceeded 3 months in 54%.
Table 109-3
Critical Limb Ischemia Endpoint Definitions and Event Rates*
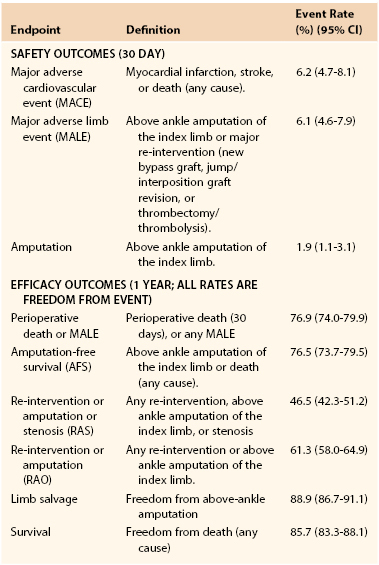
* As reported by the Society for Vascular Surgery Working Group for the development of objective performance goals for evaluating catheter-based treatment.
Data are pooled from prospective trials of vein bypass surgery in CLI.
(From Conte MS, et al: Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg 50:1462-1473, 2009.)
Functional Outcome
It has been recognized that traditional clinical measures of success—namely, reconstruction patency, limb salvage, and mortality—do not fully address the functional concerns of most patients who present with lower extremity PAD. Bypass patency after revascularization matters little to the patient or the patient’s family if progressive disability, limb loss, or death occurs over the ensuing months. A better understanding of such patient-oriented measures of success will have a profound impact on decision making for the treatment of lower extremity PAD.169 This departure from the traditional construct, which makes the implicit assumption that technical success, low morbidity and mortality, and anatomic patency are the criteria by which we should judge treatment effectiveness highlights a significant unmet need in PAD research. Considering procedurally oriented endpoints only, reconstruction patency rates of 60% to 80% and limb salvage rates of 70% to 90% have consistently been reported after the treatment of CLI, implying that with appropriate treatment methods, the problem of lower extremity PAD has largely been solved.5 However, in the late 1990s, the vascular surgery group from the Oregon Health Sciences Center published a series of studies that examined outcomes after lower extremity revascularization from the patient’s perspective. They concluded that superior results in terms of graft patency and limb salvage only partially defined success, and more often than not, patient function was not improved.170–172 Patients who present with CLI are often consumed by their disease and are condemned to a course of prolonged recovery with multiple re-operations and hospital admissions.173 The Oregon group reported that only 14% of patients who underwent revascularization had uncomplicated operations, symptom relief, wound healing, and no reoperation, and maintained their original level of function. In contrast, the remaining 86% required ongoing treatment in some fashion for the remainder of their lives. Based on such observations, it is apparent that clinically oriented endpoints, such as bypass graft patency and limb salvage, fail to accurately represent successful outcome after revascularization for CLI. However, the benefits of graft patency in terms of QoL, wound healing, and relief of chronic ischemic pain should not be discounted.
Claudication
Similarly for patients with claudication, surgeon-defined procedural endpoints may not accurately capture all relevant outcomes. Some have challenged the traditional approach to patients with claudication.174,175 Given the more benign natural history with respect to limb loss, nonoperative therapy is traditionally recommended as the first-line treatment; limb loss is therefore not a logical measure of treatment success. Kalbaugh et al,136 using the Short Form (36) Health Survey (SF-36), found that reassurances about the benign natural history of their condition did little to alleviate patients’ symptoms. Conversely, intervention for claudication improved QoL scores considerably. Improvements were similar to those found after coronary artery bypass surgery for angina and were half as beneficial as hip replacement surgery. In a more recent series, Taylor et al130 reported significant symptomatic improvement after intervention for claudication in nearly 80% of treated patients. Revascularization in this series was exceedingly safe, with no early amputations and a 99% limb salvage rate at 5 years.
Although it is accepted that revascularization is not appropriate in every case, there is clear evidence that QoL is improved by revascularization in most instances.176–179 The recent focus on patient-oriented endpoints has stimulated new research in the objective measurement of QoL for patients undergoing lower extremity revascularization. Various questionnaires have emerged as tools to measure QoL. Unfortunately, there is no consensus on the ideal questionnaire to use for the evaluation of patients with CLI. The two most commonly used surveys are the SF-36 and the Nottingham Health Profile.180,181 These questionnaires assess overall QoL and sometimes fail to consider disease-specific issues. Morgan et al182 developed the Vascular QoL Questionnaire as a disease-specific survey capable of assessing QoL in patients with CLI. To date, the largest study performed assessing QoL in patients with CLI is the Project of Ex-vivo Vein Graft Engineering via Transfection (PREVENT III) trial, which used the Vascular QoL Questionnaire to study QoL as a secondary endpoint in 1404 patients who underwent infrainguinal bypass.183 This study and others demonstrated significant improvement in QoL after bypass and also found that QoL correlated directly with bypass graft patency.178–181,183,211 In addition, prolonged patency is required in many patients to eventually achieve healing. A significant number of patients with CLI take longer than 6 months to heal175; nevertheless, they have their limbs and are at home, wound care is simplified, and ischemic pain is relieved. In addition, severe ischemia and extensive infection or soft tissue necrosis can be major factors in selecting tibial or pedal bypass over percutaneous transluminal angioplasty for patients with TASC type C and D disease (see Chapter 113); such patients are likely to require maximal and durable foot perfusion to achieve and maintain a healed foot.172,175,181
Critical Limb Ischemia
Taylor et al211 recently studied 331 consecutive patients treated for Rutherford class 5 and 6 ischemia (tissue loss). A bypass was deemed clinically successful if all four outcome criteria were met: (1) bypass patency until wound healing occurred; (2) limb salvage for at least 1 year; (3) maintenance of ambulatory status for at least 1 year; and (4) survival for at least 6 months. The authors found acceptable results when examining these components separately, including a graft patency rate of 72% and a limb salvage rate of 73% at 36 months. However, the clinical success rate, defined as the achievement of all four criteria, was only 44%. Furthermore, patients who presented with impaired ambulatory status, end-stage renal disease, gangrene, and infrainguinal disease (each independent statistical predictors) were especially prone to failure (Table 109-4).211 Prospects for a successful outcome became progressively dismal as the number of independent negative predictors increased. Patients harboring two of these independent predictors of failure experienced roughly a 33% probability of success; those with three predictors, a 10% probability of success; and those with all four independent predictors of failure, less than a 5% probability of success. Similar studies using consensus definitions of success are needed to help guide decision making regarding who should receive intervention and who should not.
Table 109-4
Probability of Failure after Bypass* When the Clinical Condition Is Present at Presentation
| Predictor Variable | Probability of Failure (%) | Odds Ratio (95% CI) |
| Impaired ambulation | 58 | 6.4 (2.9-14.4) |
| Infrainguinal disease | 46 | 3.9 (1.6-9.8) |
| ESRD | 35 | 2.5 (1.2-5.4) |
| Gangrene | 34 | 2.4 (1.5-4.0) |
| Hyperlipidemia | 11 | 0.6 (0.34-0.93) |
* Defined as patent bypass until healed, limb salvage for 1 year, maintenance of ambulation for 1 year, and survival for 6 months.
CI, Confidence interval; ESRD, end-stage renal disease.
(From Taylor SM, et al: Critical analysis of clinical success after surgical bypass for lower extremity ischemic tissue loss using a standardized definition combining multiple parameters: a new paradigm of outcome assessment. J Am Coll Surg 204:831-839, 2007.)
Treatment Guidelines According to Anatomic Disease Classification
Several classification systems have been developed that characterize PAD on the basis of anatomic descriptions of lesion type and location. The major goals included standardization of reporting disease burden, development of methods to correlate disease burden with clinical severity, and development of recommendations for method of intervention. Although these classification schemes provide some value, they notably fail to capture all the factors that determine the success of intervention.
Bollinger Classification
The Bollinger score, which was used by the BASIL trial, utilizes a scoring system to classify angiographic lesions in terms of pattern and severity.212 There is then an additive component that categorizes severity of lesions into four classes: plaques, stenoses <25%, stenoses <50%, stenoses >50%, or occlusions. The primary goal of this scoring system is to provide a semiquantitative method of evaluating atherosclerotic burden to facilitate comparisons either between patients or between time points (e.g., postinterventions) for the same patient.
Graziani Classification
The Graziani scoring system proposed a new morphologic categorization for disease severity among diabetic patients with CLI.213 Unlike the Bollinger score, this system described the frequency of various patterns of disease, and correlated angiographic findings with transcutaneous oxygen tension values.
Trans Atlantic Inter Society Consensus Classification
In January 2000, the TASC for the Management of Peripheral Arterial Disease published a document authored by a working group of representatives from 14 surgical vascular, cardiovascular, and radiologic societies.109 An updated document (TASC II) was published in January 2007.5 These important works went a step beyond the Bollinger and Graziani scoring systems, by not only classifying the lesions, but also by providing treatment recommendations according to lesion type. The authors compiled and interpreted evidence-based data concerning the treatment of lower extremity PAD and offered a series of treatment recommendations based on presentation.
Recognizing the importance of the pathologic anatomy for decision making, the TASC working group has classified anatomic patterns of disease involvement (types A through D) for both the aortoiliac (Fig. 109-4) and femoropopliteal (Fig. 109-5) segments, based on recommended treatment (endovascular versus open surgery). The TASC working group advocated endovascular treatment for TASC type A lesions and open surgical treatment for TASC type D lesions. For TASC type B and C lesions, the authors concluded that there was insufficient evidence to definitively recommend one modality over the other.5
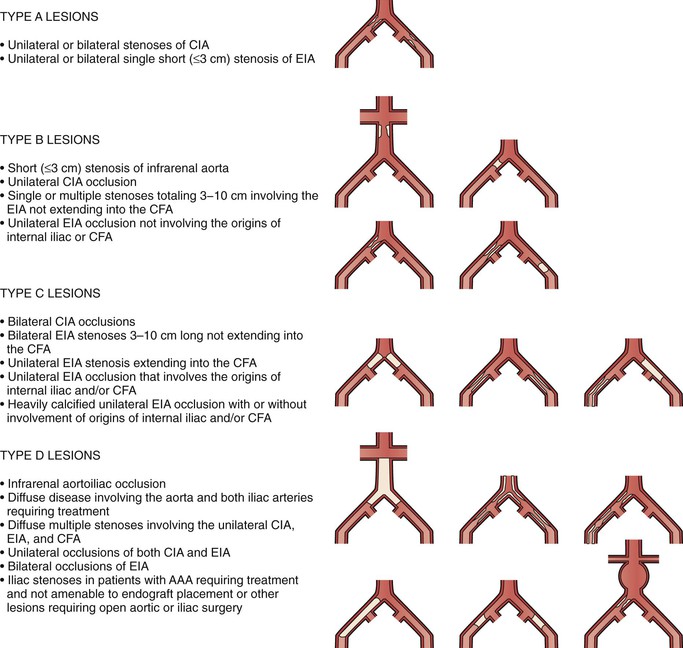
Figure 109-4 Trans Atlantic Inter Society Consensus classification of aortoiliac lesions. AAA, Abdominal aortic aneurysm; CFA, common femoral artery; CIA, common iliac artery; EIA, external iliac artery. (Redrawn from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45(Suppl S):S5-S61, 2007.)

Figure 109-5 Trans Atlantic Inter Society Consensus classification of femoropopliteal lesions. CFA, Common femoral artery; SFA, superficial femoral artery. (Redrawn from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 45(Suppl S):S5-S61, 2007.)
Future Classification Systems
Although the TASC guidelines are helpful, a grading system that is based on arterial anatomy alone is inadequate to guide therapy. As a result, the SVS commissioned the lower extremity guidelines working group to create a more comprehensive classification system to serve as a more robust decision-making aid. This new classification framework, entitled the SVS Threatened Limb Classification System, incorporates three major factors that affect amputation risk and clinical management: wound, ischemia, and foot infection (WIfI).230 The intent of this new SVS WIfI classification system is for it to be applied to patients across a broad spectrum of lower extremity arterial occlusive disease of varying severity and distribution. In the SVS WIfI system, wounds are classified from grade 0 to grade 3 based on size, depth, severity, and anticipated difficulty achieving wound healing. Ischemia is classified from grade 0 to grade 4 according to ABI, ankle systolic pressure, toe systolic pressure, or transcutaneous oximetry. Infection is classified from grade 0 to grade 3 based on simple objective clinical observations. Validation efforts are currently underway to evaluate whether the SVS WIfI system accurately stratifies patients to permit more meaningful analyses of outcomes for various forms of therapy in this challenging heterogeneous population.
Treatment Guidelines According to Presentation
Claudication
Traditional treatment recommendations for intermittent claudication have balanced the risk of intervention against the natural history of the disease. It has long been appreciated that claudication is a marker for more serious potential manifestations of systemic atherosclerosis. With the goal of preserving life and limb, many experts agree that the best strategy is to initiate systemic medical therapy aimed at reducing cardiac morbidity. This strategy is based on the low relative risk of limb loss in patients with claudication compared with the significant relative risks of stroke, MI, and death. The ACC/AHA guidelines suggest that the risk of major limb amputation for a patient with intermittent claudication is approximately 1% per year, whereas the risk of cardiac death is approximately 3% to 5% per year.5,76,125,126 Treatment strategies have therefore stressed cardiovascular risk factor modification and medical therapy as the best initial treatment for patients with PAD symptoms limited to intermittent claudication. Revascularization is recommended only in cases of severe claudication, and only after medical therapy has failed. Medical treatment for intermittent claudication consists of smoking cessation, exercise training, and pharmacologic therapy, as already described.
Medical Therapy versus Revascularization
The role of smoking cessation in treating the symptoms of claudication is unclear. Although studies have shown that smoking cessation can improve walking distance in some cases, these findings are not universal.5 The association between tobacco cessation and the reduction of subsequent cardiovascular events is undisputed, however. The rationale for smoking cessation is therefore based on reducing patient mortality and slowing the overall atherosclerotic disease process.
Currently available pharmacologic agents for claudication have already been discussed (see section on Pharmacologic Treatment of Claudication). The ACC/AHA guidelines recommend, in addition to routine antiplatelet therapy, a therapeutic trial of cilostazol (100 mg twice daily) as an effective method for increasing overall ambulation (class I recommendation). This agent is limited to patients with PAD and intermittent claudication and no history of congestive heart failure, because cilostazol is a phosphodiesterase-3 inhibitor capable of exacerbating ventricular dysfunction.76 Unfortunately, adverse effects prevent the routine use of cilostazol in up to 15% of patients.80,83 As an alternative to cilostazol, pentoxifylline (400 mg three times daily) may be of benefit in selected patients. Although this drug is well tolerated, data supporting its effectiveness are marginal.
When comparing medical to endovascular therapy, there are abundant data supporting the efficacy of medical therapy. For instance, the Edinburgh walking study consisted of a randomized trial to determine outcome differences in patients with intermittent claudication treated with angioplasty and stents versus medical management (daily low-dose aspirin, lifestyle modification) after 2 years. These investigators found no difference in maximal walking distance, treadmill distance until onset of claudication, and QoL measures between the two groups.127 Supervised exercise therapy has also been compared with primary stenting for disabling claudication due to aortoiliac occlusive disease in the Claudication: Exercise Versus Endoluminal Revascularization (CLEVER) trial.128 At 6-month follow-up, they reported that change in peak walking time was greatest for supervised exercise, intermediate for stenting, and least with pharmacologic therapy (mean change versus baseline, 5.8 ± 4.6, 3.7 ± 4.9, and 1.2 ± 2.6 minutes, respectively; P < .04 for the comparison of supervised exercise versus stenting). QoL evaluation revealed significant improvements in both the supervised exercise and the stenting groups compared with pharmacologic therapy, but the benefit was greater in the stenting group than in the exercise group.
In summary, when deciding between medical therapy and revascularization for the treatment of intermittent claudication, the risk-to-benefit ratio favors initial medical therapy in most cases. However, medical therapy may be effective in as few as 30% of patients because of noncompliance and drug intolerance. When revascularization is chosen, modern approaches have become predominantly endovascular because of its reduced procedural risks compared with open surgery. However, an analysis of practice patterns in New England between 2003 and 2009 demonstrated that an increasing proportion of lower extremity bypass procedures were performed for claudication in recent years (19%-31%; P < .0001). In addition, the percent of patients with a history of previous endovascular intervention steadily increased (13%-23%; P = .02).129 The authors suggested that the high rates of previous endovascular intervention seen might reflect a “treatment trap”; once the decision has been made to intervene procedurally for claudication, surgeons may feel obliged to perform an open revascularization if a previous endovascular approach failed to resolve symptoms. Clinical decision making ultimately must incorporate not only the risks and benefits of various treatment strategies, but also a discussion of realistic expectations as to the extent to which treatment will improve symptoms and improve QoL.
Endovascular Treatment versus Open Surgery
Ultimately, the selection of the best method of revascularization for an individual with claudication is based on a balance between the risks of the specific intervention and the degree and durability of improvement that can be expected from the intervention.5 Because the natural history of vasculogenic claudication is relatively benign, that balance usually does not favor open surgery. In contrast, its relatively low morbidity and mortality make endovascular therapy particularly attractive,5 and when it is anatomically feasible, endovascular therapy is generally preferred to open surgery for most cases of claudication.127 However, it is important to note that a growing body of evidence suggests that the concept that an endovascular option “does not burn any bridges” is false.131,132
Anatomy is a supremely important consideration when selecting the best interventional modality for patients with claudication and those with CLI. Prospective studies dating to the 1980s have characterized the arterial lesions and anatomy most conducive to long-term patency after angioplasty. Johnston et al133 demonstrated, in a prospective analysis, that the arterial anatomy and clinical presentation most amenable to long-term patency and success using angioplasty were focal arterial lesions in large-diameter vessels with adequate outflow. Outcomes were more favorable in nondiabetic patients presenting with claudication than in those with CLI. Thus, the arterial segment best managed with percutaneous transluminal angioplasty is the common iliac artery, a vessel with all the favorable anatomic characteristics identified by the Johnston et al133 study. Atherosclerotic lesions in this segment are usually focal and possess good outflow. Angioplasty patency rates at 5 years generally exceed 70%.130 Conversely, long-segment arterial disease, such as a long superficial femoral artery occlusion, is probably best treated with open bypass. Diffuse multisegmental disease, more common with CLI, can present a therapeutic dilemma.
Critical Limb Ischemia
CLI is defined as chronic lower extremity PAD with either ischemic rest pain or the tissue loss (nonhealing ulcers or gangrene) (see Chapter 108). Typically, symptoms have to be present for more than 2 weeks and associated with an ankle pressure of less than 50 mm Hg or a toe pressure of less than 30 mm Hg.127 Although far fewer patients present with CLI than with intermittent claudication, CLI patients consume the majority of treatment resources. A surprisingly small fraction of patients (<5%) with intermittent claudication progresses to CLI. Patients with “chronic subclinical ischemia”—those with low perfusion and ankle pressures but who are asymptomatic for a variety of reasons—are also at risk of developing of limb ischemia.127
Prognosis for CLI is generally considerably worse than for intermittent claudication; as many as 25% of CLI patients progress to major limb amputation within 1 year, and 25% die of cardiovascular complications within 1 year.134 However, CLI populations are a heterogeneous population, and it is therefore difficult to precisely define the natural history of CLI. For example, CLI patients in the placebo arm of the Circulase trial experienced an 87% limb salvage rate at 6 months,135 a limb salvage rate not dissimilar from the treatment arms in the BASIL trial157 or the PREVENT III trial.183 Decision making for CLI commonly poses three dilemmas: whether to treat medically or with intervention; if treating with intervention, whether to amputate or revascularize; and if revascularizing, whether to employ endovascular intervention or open surgery.
Medical Therapy versus Revascularization
The natural history of untreated CLI is poorly understood because most functional patients receive some type of revascularization. However, limb loss and cardiac death are common. One-year mortality ranges from 20% to 30%, with cardiac deaths outnumbering noncardiac deaths four to one.138 The best information regarding the natural history of nonrevascularized limbs in patients with CLI comes from the placebo arms of pharmacotherapy trials of patients with unreconstructable vascular disease. Results suggest that this subgroup has a dismal prognosis, with nearly 40% of limbs progressing to amputation at 6 months.139 Therefore, in functional patients, some type of revascularization is almost always preferable to medical therapy.
Medical therapy for CLI is not without some noteworthy successes, however. Wound care centers have become common adjuncts to many vascular surgical practices (see Chapter 83). Ischemic ulcer healing rates of 55% have been reported from dedicated centers using modern wound care methods, such as negative-pressure wound therapy, intense débridement, and antibiotic therapy.5 However, wound healing in such situations is often a slow, laborious, and unpredictable process. To date, pharmacotherapy for CLI has failed to yield any major breakthroughs. The routine use of prostanoids, vasodilators, antiplatelet agents, and even hyperbaric oxygen for the treatment of ischemic ulcers remains of unproven benefit.5
In summary, revascularization is an essential component in the relief of CLI. Although medical adjuncts geared at risk factor modification may be important to slow the progression of systemic atherosclerotic disease, they play a secondary role in the treatment of the severely ischemic limb. In those rare cases in which vascular disease is truly unreconstructable, a trial of intensive wound care, preferably at a dedicated wound care center, may yield satisfactory healing rates for motivated patients with superficial ulcerations, or it may avoid major limb amputation in high-risk patients who are approaching the end of life.
Limb Amputation versus Revascularization
For the majority of patients with CLI, revascularization is the interventional treatment of choice. However, primary limb amputation continues to be required in 10% to 40% of CLI patients because of overwhelming infection or unreconstructable vascular disease.5 Unreconstructable vascular disease accounts for nearly 60% of patients requiring secondary amputation.5 In many of these cases, revascularization has failed because of progression of disease, recurrent ischemia, or persistent infection or necrosis despite a patent revascularization.
Although counterintuitive, limb amputation and prosthetic rehabilitation can be an excellent option, offering an expedient return to a reasonable QoL in selected cases. Maintenance of ambulation can exceed 70%, and maintenance of independence can exceed 90% in young, good-risk patients after below-knee amputation.140 Clearly, amputation should be considered a tool capable of extending functionality and not a failure of treatment in these cases. If there is the potential for some degree of rehabilitation, the limb amputation should be performed at the lowest possible level at which healing can be expected, because the work of walking increases dramatically as the level of amputation becomes more proximal. Typically, patients with well-controlled medical comorbidities, a palpable femoral pulse, a warm calf, and no signs of infection are likely to heal after a below-knee amputation (see Chapter 118).
The use of an immediate postoperative prosthesis (IPOP) may also aid in expediting a patient’s recovery following below-knee amputation. This technique, first described in the 1960s, gained favor after publication of the Prosthetic Research Study by Burgess et al.141 They reported that the use of a rigid cast placed intraoperatively facilitated faster healing and return to ambulation. Folsom et al142 studied this technique in a population where the indication for amputation was not trauma, but rather severe infection or unreconstructable PAD. Of 65 patients, 86% returned to independent ambulation, with an average time to ambulation of 15.2 days after below-knee amputation. A more recent comparative analysis of IPOP compared with traditional soft dressings demonstrated no difference in complication rates and a lower incidence of revision (soft dressings 27.6% versus IPOP 5.4%; P = .021).143 Despite this, IPOP has not gained wide acceptance for unclear reasons.
Patients too sick or infirm to realize the benefit of limb revascularization should undergo palliative primary above-knee amputation. However, judging patients “too sick or infirm” can be difficult. Obviously, a nonambulatory, elderly, nursing-home patient with knee contractures and neuropathic heel ulcers would qualify for a palliative above-knee amputation. For patients who are minimally ambulatory, with multiple comorbidities, the decision is less clear cut. An individualized judgment is required to determine whether these patients will be better served by primary amputation or limb revascularization. In a recent single-institution study of 1000 consecutive revascularizations for CLI, preoperative functional performance status was the most important predictor of postoperative functional outcome—even more important than limb salvage itself.144 This finding strongly suggests that there is a definite subset of patients who are too sick or debilitated to realize the functional benefits of revascularization. Although more work is needed to better define such patients, this cohort is likely best suited for primary amputation.
Endovascular Treatment versus Open Surgery
For many years, the classic treatment approach for CLI has been open surgery. CLI is usually associated with multilevel arterial disease that is not ideally suited to percutaneous intervention. Diffuse, extensive PAD causing CLI in both aortoiliac and femoropopliteal locations (see Figs. 109-4 and 109-5) is best treated by surgical bypass according to TASC.5 However, the primacy of surgical bypass for CLI management has been challenged in recent years and has become the subject of intense debate. Those who favor open surgery for the treatment of CLI often cite superior reconstruction patency and increased durability.145–147 However, open surgery is usually associated with higher perioperative morbidity and longer hospitalization.148 Also, long-term postoperative graft surveillance is necessary to maintain a patent infrainguinal bypass, as has been shown in well-performed studies from both Europe and North America, suggesting that such surveillance is economically justified by preventing vein graft occlusion and late amputation.149,150 A re-intervention rate of 20% to 30% to treat failing grafts due to intrinsic vein graft stenoses is usually necessary to maintain the increased durability attributed to open surgery.149,151 Last, successful surgery depends on the presence of a suitable venous conduit for bypass.152,153 Those who favor interventional treatment cite the low morbidity and mortality associated with a procedure that is usually performed on an outpatient basis.154 Although proponents acknowledge the limited reconstruction patency rates associated with endovascular treatment, especially for the high-risk lesions often encountered in CLI, they argue that restenosis rarely jeopardizes subsequent surgery.154–156 In contrast, others have found that previous ipsilateral intervention has a negative influence on subsequent bypass. An analysis of the BASIL data by treatment received found 1-year AFS was 40% for bypass that followed a failed endovascular intervention, compared with 70% for the bypass-only group.132 The authors therefore do not endorse the concept of a “free shot” with an endovascular first approach. Nolan131 also found a correlation of graft failure and previous endovascular intervention; in a study of CLI patients who underwent lower extremity bypass in New England, those with a previous failed endovascular intervention had a higher incidence of major amputation (31% vs. 20%; P = .046) and graft occlusion (28% vs. 18%; P = .009) at 1 year. Although a causative relationship has not been established, the concept of “burning bridges” with an aggressive endovascular-first approach clearly deserves further study.
BASIL Trial.
There is a striking paucity of level I data to guide decision making for endovascular treatment versus open surgery. In the United Kingdom, the BASIL study represents the only randomized-controlled multicenter trial comparing angioplasty with open surgery for severe limb ischemia.157 In this study of nearly 450 patients randomized to bypass or balloon angioplasty for the initial treatment of infrainguinal disease, the findings support much of what is known about the two modalities and underscore several important caveats. Using AFS as the primary endpoint, the authors found that patients treated with bypass first had comparable outcomes to patients treated with balloon angioplasty first at 6 months (amputation or death: 21% with bypass first versus 26% with balloon angioplasty first; P = NS). Although early mortality was similar in both treatment groups, surgery was associated with higher morbidity. Crossover treatment after initial therapy (surgery to angioplasty or angioplasty to surgery) was common in both treatment groups, with more than half the angioplasty arm and approximately one third of the surgical arm requiring further intervention. At the end of 5 years, 55% of patients were alive without amputation, 8% were alive with amputation, 8% were dead after amputation, and 29% were dead without amputation. After 2 years, both AFS (hazard ratio, 0.37; P = .008) and overall survival (hazard ratio, 0.34; P = .004) were better in the surgical arm.
The BASIL trial reinforces several principles. It clearly supports the phenomenon of situational perfusion enhancement. Patients with lower extremity ulceration who would be expected to heal with conventional wound therapy and enhanced perfusion within 6 months are good angioplasty candidates. The TASC document currently recommends angioplasty over open surgery when the desired outcomes of the two modalities are comparable.5 However, angioplasty is probably not appropriate when recurrent ulceration and persistent ischemic symptoms are expected to exceed 6 months. The advantage of having surgery first becomes apparent at 2 years. If it appears that the patient’s life expectancy or the course of the disease will exceed 2 years, surgery is probably the more appropriate first intervention. Finally, the degree of treatment crossover in the BASIL trial is arguably its most remarkable finding. It stresses that angioplasty and open surgery are not “either/or” therapies, but are complementary. It underscores the importance of training surgeons who manage lower extremity ischemia so that they possess both open and endovascular skill sets.158
Impact of Conduit Availability, Preoperative Functional Status, and Comorbid Disease
Decision making in the treatment of lower extremity PAD has focused on how to treat patients. In the future, as the financial condition of the health care system continues to deteriorate, decision making will focus on whom to treat. It is naive to believe, for instance, that all patients who present with CLI will benefit from aggressive intervention. In the BASIL study, an independent data-monitoring committee that oversaw patient enrollment in this trial found that half the patients with severe limb ischemia were regarded as unsuitable or unfit for any form of revascularization. The authors concluded that patients eligible for revascularization represented the tip of an iceberg, the true dimensions of which remain incompletely defined.157 Interventional treatment cannot and should not be offered to everyone. The true task, then, is to refine definitions of success and construct tools using evidence-based data to help distinguish patients who will benefit from therapy from those who will not.
Conduit Availability
The availability of adequate conduit for open bypass plays a critical role in the decision of how best to treat a patient with lower extremity PAD, particularly CLI. A greater saphenous vein of adequate caliber, even if it must be harvested from the contralateral leg, remains the conduit of choice for open bypass.159 It has been shown to have superior durability compared with all other conduit choices (prosthetic grafts, short saphenous vein, spliced arm vein, and vein cuffs).160 In the absence of a good-caliber greater saphenous vein for bypass, endovascular revascularization becomes a more attractive option. Multiple authors have demonstrated excellent outcomes in large surgical bypass series using alternative autogenous vein options (cephalic vein, basilic vein, short saphenous vein).161–163 Performance of vein mapping before arteriography is essential because of the strong influence it can have on the decision of how aggressively to pursue an endovascular revascularization strategy.
Influence of Comorbid Conditions and Preoperative Functional Status
The ability of a patient to tolerate open revascularization must be assessed as part of the decision-making process. Several studies have sought to identify factors associated with perioperative morbidity and mortality. Using data on 9556 patients identified from the National Surgical Quality Improvement Program between 2007 and 2009, the 30-day mortality associated with lower extremity bypass was 1.8%.164 The incidence of nonfatal cardiac complications is higher. In a cohort of 2907 patients who underwent lower extremity bypass in New England between 2003 and 2007, cardiac complications occurred in 7.2%.129 Several other complications, both local and systemic, have been well described (Table 109-5). These are discussed, as well as recommendations for preoperative risk assessment, in Chapters 39 to 48.
Table 109-5
Morbidity after Infrainguinal Bypass for Claudication and Critical Limb Ischemia
| First Year | 3-5 Years | |
| Time for healing | 15-20 wk | — |
| Wound complications (%) | 15-25 | — |
| Persistent lymphedema (%) | 10-20 | Unknown |
| Graft stenosis (%) | 20 | 20-30 |
| Graft occlusion (%) | 10-20 | 20-40 |
| Major amputation (%) | 5-10 | 10-20 |
| Graft infection (%) | 1-3 | — |
| Perioperative death (%) | 1-2 | — |
| All death (%) | 10 | 30-50 |
(Adapted from Norgren L, et al: TASC II Working Group. Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45(Suppl S):S5-S61, 2007.)
Beyond the risk of perioperative complications, the likelihood a patient will derive mid- to long-term benefit from open revascularization must be included in the decision-making process. Several studies have sought to characterize risk factors for poor outcomes after open bypass. Several predictors of reduced AFS are well established in those with CLI (Table 109-6). Although many of these are comorbid conditions, such as advanced age and chronic kidney disease, others are elements of functional status, such as preoperative ambulation or independent living. In addition, previous contralateral major amputation has been shown to predict worse outcomes after open bypass.165 Although none of these studies identifies a single risk factor that is prohibitive for open bypass, for patients who present with many risk factors, including impaired functional status, the likelihood of long-term benefit from revascularization is reduced.
Table 109-6
Recent Studies Identifying Independent Predictors for Select Outcomes in Patients with Critical Limb Ischemia

BMI, Body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CLI, critical limb ischemia; GSV, great saphenous vein; JVS, Journal of Vascular Surgery; MI, myocardial infarction; PCI, percutaneous coronary intervention; RCT, randomized controlled trial.
From Schanzer and Conte: Critical limb ischemia. Curr Treat Options Cardiovasc Med 12:214-229, 2010.
Risk Prediction Models
Decision making in lower extremity PAD requires a highly individualized approach based on a number of preoperative factors. Several risk prediction models have been developed to aid in patient-specific risk stratification. These tools use statistical modeling based on patient data to identify predictors of a specific outcome, then apply a weighting system to them, such that a score may be given that corresponds to a likelihood of that outcome. For an individual patient, the score is based on the presence or absence of these factors. The sum then corresponds to a risk category, derived from the statistical modeling. The performance of the risk score can be assessed by tests of discrimination and calibration, and should ideally be validated using data from another cohort of patient data. An ideal risk score utilizes easily obtainable and clearly defined factors that are available preoperatively. This allows the surgeon to calculate the score, and offer a bedside risk prediction. It is important to note, however, that these risk scores are derived using a specific endpoint, at a specific point in time, and therefore, should similarly be narrowly applied. When used properly, risk scores provide evidence-based risk stratification, individualized to each patient. Several scores have been described; they differ slightly in the outcome they predict, and the time point at which the outcome is predicted. In addition, some have been externally validated in additional patient cohorts and have been tested using new endpoints. There is some commonality among the most widely recognized grading systems (Table 109-7), and some important differences are described in more detail in the following sections. It is important to note, however, that prediction models complement clinical judgment rather than replace it, and more research is needed to optimize these models. In particular, some have suggested that future study should incorporate clinical inputs beyond those used in the models described in the following, such as the degree of ischemia, extent of tissue loss, and presence of infection.166
TABLE 109-7
Comparison of the Grading Systems*

* Lower Extremity Grading System (LEGS), Finland National Vascular (Finnvasc), Project of Ex-vivo graft Engeneering by Transfection III (PREVENT III), and Bypass versus Angioplasty in Severe Limb Ischemia (BASIL) grading systems.
BASIL, Bypass versus Angioplasty in Severe Limb Ischemia; Finnvasc, Finland National Vascular; LEGS, Lower Extremity Grading System; PREVENT III, Project of Ex-vivo graft Engineering by Transfection III.
Lower Extremity Grading System
The Lower Extremity Grading System (LEGS) was proposed in 2002.134 The LEGS score is a standardization tool for decisions regarding revascularization strategies for PAD; it was created by a group of experienced surgeons using the best available data. The LEGS score, which is applicable to patients with either claudication or CLI, can be applied once the decision to intervene has been made. Although not a risk score in terms of statistical methodology, the score considers five objective criteria—angiographic pattern of disease, presenting complaints, functional status of the patient, medical comorbidities, and technical factors—to recommend the most appropriate interventional therapy (angioplasty, open surgery, or major limb amputation). The LEGS score has been studied prospectively, and outcomes in those treated according to and contrary to the LEGS algorithm have been compared retrospectively. Using arterial reconstruction patency, limb salvage, maintenance of ambulation, and maintenance of independent living as endpoints, patients treated according to LEGS have significantly better reconstruction patency, limb salvage, and maintenance of ambulation than those treated contrary to LEGS.136,137 Interestingly, the LEGS score is more applicable to decision making for the treatment of CLI than of claudication. Inspection of the algorithm shows that many of the principles reinforced in the BASIL trial are incorporated in the scoring system. Also, the TASC classifications (see Figs. 109-4 and 109-5) underscore the anatomic considerations that are important in decision making, something not considered in the BASIL trial. Other factors to be considered when deciding between endovascular treatment and open surgery are the degree of ischemia and the extent of tissue loss. Open surgery is preferred when ischemia and tissue loss are severe. Conventional wisdom has advocated the presence of in-line flow and a palpable pedal pulse for severe cases of foot ischemia. Unfortunately, objective data correlating the extent of revascularization required to heal specific degrees of tissue loss are lacking.
Finnvasc Risk Score
Data from the Finnvasc registry was used to develop a risk score that predicted 30-day outcomes (mortality and limb loss) following infrainguinal bypass for CLI.167 The score was derived from a cohort of nearly 4000 lower extremity open revascularizations performed in Finland between 1991 and 1999. A number of comorbid conditions and clinical factors were assessed for significance using univariate and multivariate analyses. The factors that ultimately remained in the model as significant predictors of 30-day limb loss and mortality were diabetes, coronary artery disease, foot gangrene, and urgent operation. The authors chose to assign one point to each of these factors, to produce a simple additive score. Using this adaptation of the score, the goodness of fit was adequate (c-statistic 0.605, 95% CI, 0.560-0.649, Hosmer and Lemeshow test; P < .0001). In the derivation set, patients were then stratified into five risk categories, with risk estimates for 30-day major limb loss and/or mortality ranging from 7.7% for those with a score of 0% to 27.3% for those with a score of 4. The authors applied the model to several other outcomes, including need for prolonged stay in an intensive care unit, and concluded it identified patients in whom a bypass might not be beneficial.
The Finnvasc score was also validated using long-term outcomes, rather than the original 30-day endpoint.168 Arvela et al168 tested the Finnvasc score using the Husvasc database (Helsinki University Central Hospital) of patients who underwent either open or endovascular lower extremity revascularization between 2000 and 2007, for the endpoint of AFS at 1 year postoperatively. They tested the model in a cohort of 1425 patients, with 747 (52.4%) treated with endovascular revascularization and 678 (47.6%) treated with open revascularization. They found that the Finnvasc score performed adequately both for the 30-day AFS endpoint as originally described and the 1-year endpoint (c-statistic, 0.63, 95% CI, 0.597-0.663; P < .0001). The authors noted that the Finnvasc score performed very similarly for both open and endovascular revascularizations, at both 30 days and 1 year; however, the authors cautioned that this performance was “far from optimal.” They suggested the best use of this tool was for identifying those patients at highest risk for poor outcomes, but it performed less well in risk stratifying the other groups.
PREVENT III Risk Score
Data from the PREVENT III randomized trial183 cohort was used to develop a risk score that predicted 1 year AFS (Fig. 109-6).185 This cohort was comprised of 1404 patients who underwent lower extremity bypass for CLI between 2001 and 2003, as part of a multicenter, randomized, controlled trial to evaluate the efficacy of edifoligide in preventing vein graft failure. This was divided into a derivation set (two thirds) and a validation set (one third). Schanzer et al185 derived a risk score that utilized factors that were available preoperatively; they found five significant predictors of 1-year AFS: dialysis-dependence, age ≥75 years, tissue loss, hematocrit ≤30%, and advanced coronary artery disease. A weighted integer score was then assigned to each of these based on the associated hazard ratios. The resulting integer score then corresponded to one of three strata of risk. The score was then internally validated using the remaining one third of the PREVENT III cohort and externally validated using data from 716 patients who underwent lower extremity vein bypass for CLI at one of three U.S. centers. The score performed similarly in all three sets. It demonstrated a range in AFS from 86% in the lowest risk group to 45% in the highest risk group. In addition to individualized patient counseling, the authors suggested that the PREVENT III score might be useful for identifying high-risk patients in whom therapies with less durability than vein bypass might be acceptable; conversely, for patients who were stratified as low risk, alternatives to traditional vein bypass must demonstrate at least comparable durability to be viable options.

Figure 109-6 PREVENT III (PIII) Critical Limb Ischemia Risk Score card. AMP, Amputation; CAD, coronary artery disease; HCT, hematocrit. (From Schanzer A, et al: Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 48:1464-1471, 2008.)
The authors subsequently validated the PREVENT III score in a regional quality improvement database, the Vascular Study Group of New England (VSGNE).186 They identified a cohort of 1166 patients who underwent infrainguinal vein bypass for CLI in New England between 2003 and 2007. The PREVENT III score was modified to exclude preoperative hematocrit because the VSGNE did not collect that variable throughout the study period. This modified PREVENT III (mPIII) score was validated for the original study endpoint of 1-year AFS and several other endpoints. These included 30-day limb salvage, mortality, and major adverse cardiac events; 1-year endpoints of graft patency and ability to ambulate were also tested. They found that the score was an effective tool for discriminating three strata of risk for AFS; in addition, when it was tested on perioperative major adverse cardiac events, perioperative and 1-year mortality, and 1-year ability to ambulate, it continued to discriminate well.
BASIL Survival Prediction Model
Data from the BASIL randomized trial,157 cohort was used to develop a risk score that predicted 1-year AFS.187 Between 1999 and 2004, 452 patients with severe limb ischemia were randomized to either bypass or balloon angioplasty. The authors concluded that those patients whose life expectancy exceeded 2 years would benefit from a bypass-first strategy, and conversely, that those patients with more limited life expectancy could achieve a similar outcome with an angioplasty-first strategy, with its attendant decreased morbidity and expense. Based on this interpretation, the authors constructed a prediction model for 2-year survival from time of randomization. They also modeled 1-year survival. The data were divided into a training set (75%) and a validation set (25%). They ultimately arrived at 10 factors that were significant on multivariable modeling: tissue loss, body mass index, serum creatinine, Bollinger score, age, smoking status, history of angina or MI, history of transient ischemic attack or stroke, number of measurable ankle pressures, and highest ankle pressure value. This tool is available online (http://www.basiltrial.com/survival_predictor.htm) and facilitates use of the model because the authors chose not to convert it to an additive score format. In addition, they used a statistical correction factor to acknowledge some of the limitations inherent in risk prediction, particularly in a data set of this size. When individual patient data are plugged into this tool, the output includes survival estimates for the time points of 6 months, 1 year, and 2 years.
The BASIL survival prediction model was validated in a cohort of 223 CLI patients from a university hospital setting between 2008 and 2010.188 The model was assessed for goodness of fit and was found to perform well for all three time points, but performed best for the 6-month endpoint (6 months: c-statistic, 0.700, 95% CI, 0.60-0.80; P < .001; 1 year: c-statistic, 0.651, 95% CI, 0.56-0.74, P < .003; 2 years: c-statistic, 0.681, 95% CI, 0.59-0.74; P < .001). The authors concluded that the BASIL survival prediction performed moderately well, but was limited by the requirement that the clinician calculate a Bollinger score.
Angiogenesis for Peripheral Arterial Disease
For patients with CLI who lack a revascularization option, novel alternative therapies may offer benefit. Angiogenesis is a naturally occurring phenomenon in response to tissue ischemia, and is promoted by proangiogenic factors, including VEGF, fibroblast growth factor, hypoxia-inducible factor-1α, and hepatocyte growth factor.189,190 Since the first case report using VEGF in a human patient by Isner et al in 1996,191 the modulation of growth factors to stimulate angiogenesis has become an area of interest in the treatment of lower extremity PAD, particularly in patients who are not candidates for surgical or interventional techniques. The concept of therapeutic angiogenesis entails efforts to increase the concentration of proangiogenic factors, thereby stimulating growth of new blood vessels from preexisting blood vessels to treat ischemic disease; this may be accomplished by administration of recombinant proteins or gene therapy that induces overexpression of these factors.189,192 Therapeutic angiogenesis may also be induced by implantation of endothelial progenitor cells. These modalities are under clinical investigation for the treatment of both ischemic heart disease and CLI. The putative concept by which angiogenesis improves limb perfusion is through the enlargement of collateral blood vessels and possible direct stimulation of wound healing by growth factors. Preclinical animal models, such as the rabbit hind limb ischemia model, have clearly shown that angiogenic gene therapy can improve limb perfusion compared with placebo-treated animals. Growth factors act as mitogens that stimulate endothelial cell proliferation in response to local tissue ischemia, but the mechanism has not been completely delineated.118 Several studies of therapeutic angiogenesis have now been conducted, including phase 3 clinical trials.120,121,193–196
Growth Factors
Many different growth factors involved in angiogenesis have been individually tested in clinical trials.119,120 Because the half-life of the recombinant protein is short, most trials have used gene therapy to allow for a more prolonged expression of the protein. Growth factors that have been studied include various VEGF isoforms, fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and the transcription factor, hypoxia-inducible factor-1α. Gene therapy has usually been delivered in either a plasmid or an adenovirus via intramuscular injection into the ischemic limb. Although several trials have evaluated the effect of gene therapy on claudication, the major emphasis has been on the CLI patient population. The Regional Angiogenesis with Vascular Endothelial Growth Factor Peripheral Arterial Disease (RAVE) study was a phase 2 trial of adenoviral delivery intramuscularly in patients with intermittent claudication120; there was no significant difference in peak walking time at 6 months.
Fibroblast Growth Factor
The Therapeutic Angiogenesis with Recombinant Fibroblast Growth Factor-2 for Intermittent Claudication (TRAFFIC) study was the first randomized clinical trial to show a positive effect of growth factor therapy in limb ischemia.121 In this study, patients with intermittent claudication were treated with 30 µg/kg of recombinant FGF-2 via bilateral femoral artery infusions; both maximal walking time and ABI increased at 90 days. Unfortunately, these encouraging results were offset by the adverse effects of proteinuria and lower extremity edema.
The Therapeutic Angiogenesis Leg Ischemia Study for the Management of Arteriopathy and Nonhealing Ulcer (TALISMAN) trial was a placebo-controlled randomized, prospective multicenter trial that demonstrated that intramuscular injection of FGF-1 resulted in a twofold decrease in major amputation compared with placebo.122 There was no difference in hemodynamics or wound healing between the groups. However, the phase 3 study that followed TALISMAN (TAMARIS)195 failed to show a significant difference at 1 year for the primary endpoint of time to major amputation or death (hazard ratio, 1.11, 95% CI, 0.83-1.49; P = 0.48).
Hepatocyte Growth Factor
The Study to Assess the Safety of Intramuscular Injection of Hepatocyte Growth Factor Plasmid to Improve Limb Perfusion in Patients with Critical Limb Ischemia (HGF-STAT) was a placebo-controlled randomized, prospective multicenter trial that demonstrated a doubling of the transcutaneous oxygen tension (tcPO2) from baseline in the HGF gene therapy group compared with the placebo group.123 In that trial, 80% of the gene therapy patients had a tcPO2 greater than 30 mm Hg at the 6-month endpoint, compared with 39% of the placebo-treated patients. However, there was no difference between groups for several clinical endpoints, including pain relief, wound healing, and major amputation. In a follow-up study, Powell198 evaluated the safety and efficacy of modified HGF gene delivery in CLI patients using endpoints of change in toe-brachial indices, pain at rest, wound healing, amputation, and death at 3 and 6 months. Complete ulcer healing at 12 months occurred in 31% of the HGF group and 0% of the placebo group (P = .28). There was no difference in major amputation of the treated limb (HGF 29% vs. placebo 33%) or mortality at 12 months (HGF 19% vs. placebo 17%) between groups.
Shigematsu et al196 performed a phase 3 randomized controlled trial of intramuscular HGF plasmid in patients with CLI. They found a significant improvement for the primary endpoint of resolution of rest pain and reduction of ulcer size at 12 weeks (70.4% in the treatment group vs. 30.8% in the placebo group; P = 0.014). They also demonstrated significant improvements on QoL measures among the treatment group, with no major adverse events recorded in 12 weeks of study.
Gene therapy to achieve therapeutic angiogenesis has now been investigated long enough that studies evaluating longer term outcomes are being reported. Makino et al197 reported 2-year outcomes for patients with CLI who were treated with HGF plasmid. Reduction in pain at rest was observed in 9 of 9 patients (100%) at 2 years; 9 of 10 patients (90%) experienced reduction in the size of ischemic ulcers by >25%. The authors noted some temporal trends: improvements in ABI and maximum walking distance peaked at approximately 3 to 6 months and then decreased slightly, whereas pain at rest and ulcer size improved continuously until 2 years after gene therapy. Tests of significance could not be conducted on any of these findings due to small sample size. This study was not randomized, and did not contain a control group.
Pooled Result Analysis
De Haro et al199 carried out a meta-analysis on the data presented in several of these studies; they applied a pooled analytic approach to evaluate the efficacy and safety of gene and cell angiogenic therapies. They found a significant improvement compared with placebo (odds ratio, 1.437, 95% CI, 1.03-2.0; P = .033) for all patients. Subgroup analysis did not show a significant difference in response rate among claudicants receiving gene therapy compared with placebo, but did find significant improvement in the CLI population. They also reported a significant difference for potential nonserious adverse events, with higher rates among the treatment group (edema, hypotension, proteinuria); there were no differences in mortality, malignancy, or retinopathy.
Because of the heterogeneous nature of the CLI population, these trials have demonstrated the necessity of incorporating a placebo control arm into the clinical trial design. These trials have also demonstrated that angiogenic gene therapy can improve perfusion in ischemic limbs; the extent to which there is clinical benefit has varied. Thus far, angiogenic gene therapy has proved to be safe. Potential side effects of gene therapy, such as the progression of diabetic retinopathy or the development of malignancy, have not been observed. There are currently several larger pivotal trials under way to assess the efficacy of angiogenic gene therapy on the clinically relevant endpoint of AFS.
Stem Cell Therapy
Stem cell therapy is another developing technique to induce therapeutic angiogenesis.
Autologous Cells
Autologous stem cell therapies have used bone marrow mononuclear cells or endothelial progenitor cells obtained from bone marrow harvest or, less frequently, from circulating peripheral blood stem cells. Endothelial progenitor cells can be identified by cell sorting for CD34-positive, VEGF receptor-2–positive cells. Once concentrated, the cells can be injected into the ischemic limb to induce angiogenesis.200 The mechanisms by which stem cell therapy induces angiogenesis are unclear. Tateishi-Yuyama et al,124 in a randomized prospective trial, showed that injection of bone marrow–derived mononuclear cell injections resulted in a significant increase in ABI and tcPO2 compared with controls. Other phase 2 trials of bone marrow–derived stem cells (derived from concentrated bone marrow aspirate) have also demonstrated significant benefits on clinical endpoints, including major amputation.201,202 The Use of Tissue Repair Cells (TRCs-Autologous Bone Marrow Cells) in Patients with Peripheral Arterial Disease to Treat Critical Limb Ischemia (RESTORE-CLI) trial also used bone marrow–derived stem cells, but utilized a technique of culturing and expanding the cells.203 They found a significant difference for the composite endpoint of death, major amputation, doubling of wound size, or new-onset gangrene (treatment group, 40% compared with placebo group, 67%) at 1 year (P = .003). A phase 3 trial was underway (REVIVE, NCT01483898, http://clinicaltrials.gov), but was halted due to low enrollment.
Major differences between the two techniques that utilize bone marrow–derived stem cells relate to the logistics of performing the therapy. The concentrated marrow technique requires removal of large quantities of bone marrow, but the treatment can be performed in that same setting. In contrast, the expansion technique involves harvest of a much smaller quantity of bone marrow, but requires a 2-week wait time before therapeutic injection can occur.204
Allogenic Cells
Allogenic stem cell therapies are also being studied. Placental stem cells are considered immune-privileged, and therefore, offer the advantage of off-the-shelf availability.204 Prather et al205 reported the use of placental-derived adherent stromal cells in a mouse model of hind-limb ischemia. They demonstrated significantly improved blood flow (P = .0008), increased capillary density (P = .021), reduced oxidative stress (P = 0.034), and reduced endothelial damage (P = 0.004) compared with placebo.
It is too early to determine what role, if any, therapeutic angiogenesis will have in the future care of patients with vascular disease. Early results from clinical trials are encouraging, but much work needs to be done to determine whether this will prove effective as a stand-alone therapy for patients with ischemic vascular disease, or will serve as an adjuvant treatment to current forms of revascularization.
Future Developments
Similar to other fields, such as oncology, the mapping of the human genome promises to advance therapeutic approaches to the treatment of lower extremity PAD. Future decision making will undoubtedly be affected by these advances. Alternative strategies for the pharmacologic treatment of vascular disease are under investigation. Strategies aimed at the neovascularization of ischemic tissue and the prevention of intimal hyperplasia are paramount. Stem cell transfer and implantation of progenitor bone marrow mononuclear cells are theorized to promote neovascularization.118 Such therapies may be particularly valuable for the treatment of CLI in patients with physiologic or anatomic contraindications for revascularization. Preliminary findings, to date, are encouraging.
The most promising area for substantial progress in the treatment of lower extremity PAD involves gene therapy. Several target genes and small molecules that are regulated by oxygen tension have been implicated in stimulating angiogenesis via the hypoxia-inducible factor pathway; research is ongoing to define the therapeutic potential of prolyl-4-hydroxylase inhibitors, mediators of the thioredoxin systems, the peroxisome proliferator-activated receptor–γ co-activator 1-α protein, micro-RNAs and oligonucleotides.206 Investigations of immune modulation therapy, targeting the inflammatory component of vascular disease in an effort to decrease the development and progression of atherosclerosis, and possibly, angioplasty-associated intimal hyperplasia, are encouraging. Other areas of research aimed at advancing our understanding of vascular disease include viral-directed gene transfer, targeted antibiotic therapy, and alternative circulating cell-free oxygen delivery vectors.
Future Trends
Decision Analysis
Decision analysis is a field of study that applies statistical methods and probabilities from existing literature to model various hypothetical outcomes for a given set of competing options.207 Decision analysis begins with identifying a problem for which there is more than one potential solution. A decision tree is then constructed that incorporates a valuation of any number of factors that could influence the decision, along with the likelihood of each branch along the decision tree occurring, such that the outcome associated with several different choices can be simulated. Rather than just imagining the outcome associated with different choices, decision analysis allows for predictions of outcomes, based on input data from the existing literature. Decision analysis is often applied to clinical scenarios where randomized controlled trials of competing therapies cannot be carried out. It often incorporates factors such as cost and QoL associated with various treatment options. One of the most important roles of model-based decision analysis is to identify which data parameters or assumptions can change policy decisions. This is done through sensitivity analysis, in which each parameter is varied through a wide range of possible values. If this variation does not lead to a change in policy conclusions, one can feel confident that the results of the analysis are likely to hold true in most clinical scenarios. Conversely, if an uncertain parameter is found to be influential, this may identify an important area for future research. Decision analysis can play an important role in clinical decision making in the treatment of PAD.
Brothers et al208 used decision analysis to assess three different strategies for the management of CLI: primary amputation, revascularization, or expectant management (Fig. 109-7). They found that revascularization was associated with a gain of 1.1 quality-adjusted life years (QALYs) compared with primary amputation, and 1.2 QALYs compared with expectant management. In terms of cost associated with the three treatment strategies, sensitivity analysis predicted revascularization to be the least costly treatment per QALY, as long as 1-month patency exceeded 11%. The authors concluded that revascularization offered the greatest benefit in terms of QALYs and cost in the management of CLI.

Figure 109-7 Decision tree of principal management options for patients with critical limb ischemia. Square nodes represent a decision point and round nodes represent a probability of an event occurring based on existing literature. The outcomes, which in this study are utility (U) values (translated into quality-adjusted life years), are designated with the following abbreviations. amb-amp, Ambulatory after amputation; amb-by, ambulatory after amputation following failed revascularization; amb-reamp, ambulatory after re-amputation; byp, success after revascularization; die, death occurs; mult, success after multiple revascularizations; non-amp, nonambulatory after amputation; non-byp-amp, nonambulatory after failed revascularization and amputation; non-reamp, nonambulatory after re-amputation; same, condition remains the same. (From Brothers TE, et al: Justification of intervention for limb-threatening ischemia: a surgical decision analysis. Cardiovasc Surg 7:62-69, 1999.)
Nolan et al209 applied decision analysis to treatment of claudicants with TASC B and C lesions of the superficial femoral artery. Using a hypothetical cohort of claudicants with either TASC B or C lesions, they modeled treatment with either angioplasty and stenting, or great saphenous vein bypass for the outcome measurement of QALYs. They found that TASC B lesions were best treated with angioplasty and stenting. However, for patients with TASC C lesions, angioplasty and stenting was preferred only under certain conditions. Using sensitivity analyses, they showed that stenting surpassed efficacy of open bypass for TASC C lesions if the primary patency of stenting was >32% at 5 years, if patient age was >80 years, or if operative mortality for open bypass exceeded 6%.
Cost-effectiveness analyses are another type of decision analysis with several applications in health care research, and clinical decision making in PAD. Barshes210 recently published a review of the methods of cost-effectiveness analysis, anticipating their increased utility as a health care financial crisis looms in the United States (Fig. 109-8). We believe that decision analysis and cost-effectiveness analyses will play a growing role in informing decision making in PAD in the coming years.

Figure 109-8 Two-dimensional plane demonstrating the relationship of incremental costs (vertical axis) and incremental effectiveness (horizontal axis) for two possible interventions. If a new intervention is both more costly and less effective than a current intervention (upper left quadrant), it is not economically preferred. If a new intervention is less costly and more effective (lower right quadrant), it is economically preferred. Formal cost-effectiveness analysis is useful for situations described by the upper right and lower left quadrants. Most frequently, a cost-effectiveness analysis focuses on the upper right quadrant to address the question of whether the more effective treatment is “worth” its greater cost. QALYs, Quality of life years. (From Barshes NR: A primer on cost-effectiveness analyses for vascular surgeons. J Vasc Surg 55:1794-1800, 2012.)
Cost Considerations
As health care costs continue to rise, decision making will increasingly be influenced by governmental and third-party payers. There is conflicting evidence regarding the most cost-effective methods of treating lower extremity PAD. Although there is evidence that revascularization is cost effective compared with primary amputation,152,214–217 the cost differential between strategies varies depending on the expense of rehabilitation after primary amputation in most series.215 Primary amputation for patients forgoing rehabilitation (as might occur in a nursing home patient) is cheaper than revascularization. Therefore, economic decision making depends on the patient’s postoperative rehabilitation potential, which is often determined by preoperative functional status.144 Despite the debate concerning the economic merits of one treatment over another, most would agree that the current financial system can ill afford to pay for multiple revascularizations of a single ischemic limb. The scenario becomes even more cost prohibitive, if after multiple revascularizations, major limb amputation results anyway. Future economic decision making will therefore require the identification of the most cost-effective single treatment for patients who present with PAD, and more specifically, CLI.
Unmet Needs
Definitive high-level evidence on which to base treatment decisions, with an emphasis on clinical and cost effectiveness, continues to be lacking. Treatment decisions in PAD are individualized, based on life expectancy, functional status, anatomy of the arterial occlusive disease, and surgical risk. For patients with aortoiliac disease, endovascular therapy has become the first-line therapy for all but the most severe patterns of occlusion, and aortofemoral bypass surgery is a highly effective and durable treatment for the latter group. For infrainguinal disease, available data suggest that surgical bypass with a vein is the preferred therapy for patients likely to survive 2 years or more, and for those with long segment occlusions or severe infrapopliteal disease who are acceptable surgical risks. Endovascular therapy may be preferred in patients with reduced life expectancy, those who lack usable vein for bypass, or who are at elevated risk for operation, and those with less severe arterial occlusions. Patients with unreconstructable disease, extensive necrosis involving weight-bearing areas, nonambulatory status, or other severe comorbidities may be considered for primary amputation or palliative measures.
Third-party payers in our current health care system will increasingly insist on the delivery of evidence-based care. Quality will be defined, increasingly monitored, and financially linked to compliance with Medicare All-Care measures. Care for lower extremity PAD will increasingly become protocol-driven, placing a premium on value and durability. For all of the preceding important reasons, future studies should continue to analyze the impact of comorbidities on patients with PAD. These studies need to be conducted in patients undergoing surgical bypass, endovascular repair, primary amputation, and conservative treatment because the effect of comorbidities can be very different based on the treatment type. A diverse set of standardized endpoints that attempt to capture the diverse experience relevant to patients with PAD needs to be defined. The time points at which these endpoints are studied also should be standardized. In this way, valid risk estimates can continue to inform the way we take care of patients, and in doing so, improve evidence-based patient care. Finally, the definitive treatment of lower extremity PAD has traditionally relied on procedural therapy, either open or endovascular revascularization. We are now at an exciting time, where mapping of the human genome, improved understanding of angiogenesis, and the growing ability to manipulate stem cell differentiation all hold great promise in providing meaningful medical solutions that may render many interventions unnecessary.
Selected Key References
Bradbury AW. BASIL Trial Participants. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicenter, randomized controlled trial. Lancet. 2005;366:1925–1934.
Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. [PREVENT III Investigators] J Vasc Surg. 2006;43:742–751.
Goodney PP, Schanzer A, Demartino RR, Nolan BW, Hevelone ND, Conte MS, Powell RJ, Cronenwett JL. Validation of the Society for Vascular Surgery’s objective performance goals for critical limb ischemia in everyday vascular surgery practice. [Vascular Study Group of New England] J Vasc Surg. 2011;54(1):100–108.e4.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Interventional Radiology, ACC/AHA Task Force on Practice Guidelines, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter-Society Consensus, Vascular Disease Foundation. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric and abdominal aortic): executive summary: a collaborative report from the American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society for Interventional Radiology and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312.
Nolan BW, De Martino RR, Stone DH, Schanzer A, Goodney PP, Walsh DW, Cronenwett JL. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. [Vascular Study Group of New England] J Vasc Surg. 2011;54(3):730–735.
Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society consensus for the management of peripheral arterial disease (TASC II). [TASC II Working Group] J Vasc Surg. 2007;45(Suppl S):S5–S67.
Simons JP, Schanzer A, Nolan BW, Stone DH, Kalish JA, Cronenwett JL, Goodney PP. Outcomes and practice patterns in patients undergoing lower extremity bypass. [Vascular Study Group of New England] J Vasc Surg. 2012;55(6):1629–1636.
Taylor SM, Kalbaugh CA, Blackhurst DW, Cass AL, Trent EA, Langan EM 3rd, Youkey JR. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–756.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Criqui MH, et al. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71:510–515.
2. Hirsch A, et al. Peripheral arterial disease detection, awareness and treatment in primary care. JAMA. 2001;286:1317–1324.
3. Hirsch AT, et al. National health care costs of peripheral arterial disease in the Medicare population. Vascular Medicine. 2008;13:209–215.
4. Selvin E, et al. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110:738–743.
5. Norgren L, et al. Inter-Society consensus for the management of peripheral arterial disease (TASC II). [TASC II Working Group] J Vasc Surg. 2007;45(Suppl S):S5–S67.
6. O’Riordan DS, et al. Realistic expectations for the patient with intermittent claudication. Br J Surg. 1991;78:861–863.
7. Cox GS, et al. Non-operative treatment of superficial femoral artery disease: long term follow-up. J Vasc Surg. 1993;17:172–181.
8. Muluk SC, et al. Outcome events in patients with claudication: a 15 year study in 2777 patients. J Vasc Surg. 2001;33:251–257.
9. Hirsch A, et al. Peripheral arterial disease detection, awareness and treatment in primary care. JAMA. 2001;286:1317–1324.
10. Bhatt D, et al. International prevalence, recognition, and treatment of cardiovascular risk factors and outpatients with atherothrombosis. JAMA. 2006;295:180–189.
11. Hirsch AT, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation. 2007;116:2086–2094.
12. Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621.
13. Hawkey GJ, et al. Medical treatment of peripheral arterial disease. JAMA. 2006;295:547–553.
14. U.S. Preventive Services Task Force (USPSTF). Screening for peripheral arterial disease: a brief evidence update. http://www.uspreventiveservicestaskforce.org/uspstf05/pad/padup.htm; 2005 [Accessed October 16, 2012] .
15. Moyer VA, U.S. Preventive Services Task Force. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults; U.S. Preventive Services Task Force recommendations statement. Ann Intern Med. 2013;159:342–348.
16. Greenland P, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2010;56:e50–e103.
17. Murabito JM, et al. Intermittent claudication: a risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49.
18. Dawber TR, et al. Some factors associated with the development of coronary heart disease: six years’ follow-up experience in the Framingham study. Am J Public Health Nations Health. 1959;49(10):1349–1356.
19. Kullo IJ, et al. Ethnic differences in peripheral arterial disease in the NHLBI Genetic Epidemiology Network of Arteriopathy (GENOA) study. Vasc Med. 2003;8(4):237–242.
20. O’Hare AM, et al. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation. 2004;109(3):320–323.
21. Graham IM, et al. Plasma homocysteine as a risk factor for vascular disease. The European Concerted Action Project. JAMA. 1997;277(22):1775–1781.
22. Welch GN, et al. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050.
23. Creager MA, et al. Atherosclerotic Vascular Disease Conference: Writing Group V: medical decision making and therapy. Circulation. 2004;109:2634–2642.
24. Jonason T, et al. Cessation of smoking in patients with intermittent claudication. Effects on the risk of peripheral vascular complications, myocardial infarction and mortality. Acta Med Scand. 1987;221:253–260.
25. Faulkner KW, et al. The effect of cessation of smoking on the accumulative survival rates of patients with symptomatic peripheral vascular disease. Med J Aust. 1983;1:217–219.
26. Couch NP. On the arterial consequences of smoking. J Vasc Surg. 1986;3:807–812.
27. Anthonisen NR, et al. The effects of smoking cessation intervention on 1, 4, 5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–239.
28. Tonstad S, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–955.
29. Gonzales D, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. [Varenicline Phase 3 Study Group] JAMA. 2006;296:47–55.
30. Kannel WB, et al. Diabetes and cardiovascular disease: the Framingham Study. JAMA. 1979;241:2035–2038.
31. Kannel WB, et al. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13–18.
32. Kannel WB, et al. Diabetes, fibrinogen, and risk of cardiovascular disease: the Framingham experience. Am Heart J. 1990;120:672–676.
33. Hooi JD, et al. Incidence of and risk factors for asymptomatic peripheral arterial occlusive disease: a longitudinal study. Am J Epidemiol. 2001;153:666–672.
34. American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341.
35. The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75:894–903.
36. Nathan DM, et al. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. [Diabetes Control and Complications Trial; Epidemiology of Diabetes Interventions and Complications Research Group] N Engl J Med. 2003;348:2294–2303.
37. Adler AI, et al. UKPDS 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25:894–899.
38. Murabito JM, et al. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J. 2002;143:961–965.
39. Diehm C, et al. High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: cross-sectional study. Atherosclerosis. 2004;172:95–105.
40. Chobanian AV, et al. Seventh report of the Joint National Committee on prevention, detection, valuation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252.
41. ESH/ESC European Society of Hypertension, European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1000–1053.
42. Mehler PS, et al. Intensive blood pressure control reduces the risk of cardiovascular events in patients with peripheral arterial disease and type 2 diabetes. Circulation. 2003;107:753–756.
43. Yusuf S, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153.
44. Martin MJ, et al. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet. 1986;2:933–936.
45. Pekkanen J, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990;322:1700–1707.
46. Gordon DJ, et al. High-density lipoprotein cholesterol and cardiovascular disease: four prospective American studies. Circulation. 1989;79:8–15.
48. Moreno PR, et al. The year in atherothrombosis. J Am Coll Cardiol. 2004;44:2099–2110.
49. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389.
50. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357.
51. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
52. Feringa HH, et al. The effect of intensified lipid-lowering therapy on long-term prognosis in patients with peripheral arterial disease. J Vasc Surg. 2007;45:936–943.
53. Taylor AJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517.
54. Mohler ER III, et al. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486.
55. De Backer G, et al. European guidelines on cardiovascular disease prevention and clinical practice. Third Joint Task Force of European and other Societies on Cardiovascular Disease Prevention in Clinical Practice. Atherosclerosis. 2004;173:381–391.
56. Grundy SM, et al. Applications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239.
57. Smith SC Jr, et al. AHA/ACC Scientific Statement: AHA/ACC guidelines for preventing heart attack and death in patients with atherosclerotic cardiovascular disease: 2001 update: a statement for healthcare professionals from the American Heart Association and American College of Cardiology. Circulation. 2001;104:1577–1579.
58. Boushey CJ, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057.
59. Wald DS, et al. Randomized trial of folic acid supplementation and serum homocysteine levels. Arch Intern Med. 2001;161:695–700.
60. Robinson K, et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation. 1998;97:437–443.
61. Refsum H, et al. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49:31–62.
62. Toole JF, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–575.
63. Liem A, et al. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol. 2003;41:2105–2113.
64. Wrone EM, et al. Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol. 2004;15:420–426.
65. ATC. Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81–106.
66. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–1339.
67. Neri Serneri GG, et al. Picotamide, a combined inhibitor of thromboxane A2 synthase and receptor, reduces 2-year mortality in diabetics with peripheral arterial disease: the DAVID study. [Drug Evaluation in Atherosclerotic Vascular Disease in Diabetics (DAVID) Study Group] Eur Heart J. 2004;25:1845–1852.
68. Bhatt DL, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717.
69. Antithrombotic Trialists’ Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860.
70. Fowkes FG, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303(9):841–848.
71. Stewart KJ, et al. Exercise training for claudication. N Engl J Med. 2002;347:1941–1951.
72. Laufs U, et al. Physical training increases collateral-dependent muscle flow in aged rats. Am J Physiol. 1990;258:H759–H765.
73. Gardner AW, et al. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980.
74. Aquino R, et al. Natural history of claudication: long term follow-up study of 1244 claudicants. J Vasc Surg. 2001;34:962–970.
75. Sakamoto S, et al. Long-term clinical outcome of supervised exercise rehabilitation among patients with peripheral arterial disease. J Am Coll Cardiol. 2007;49:351A.
76. Hirsch AT, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Intervention, Society for Vascular Medicine and Biology, Society for Interventional Radiology and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312.
77. Ferket BS, et al. Systemic review of guidelines on peripheral artery disease screening. Am J Med. 2012;125:198–208.
78. Samlaska CP, et al. Pentoxifylline. J Am Acad Dermatol. 1994;30:604–621.
79. Porter JM, et al. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am Heart J. 1982;104:66–72.
80. Dawson DL, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–530.
81. Hood SC, et al. Management of intermittent claudication with pentoxifylline: meta-analysis of randomized controlled trials. CMAJ. 1996;155:1053–1059.
82. Tsuchikane E, et al. Impact of cilostazol on restenosis after percutaneous coronary balloon angioplasty. Circulation. 1999;100:21–26.
83. Barnett AH, et al. The role of cilostazol in the treatment of intermittent claudication. Curr Med Res Opin. 2004;20:1661–1670.
84. Regensteiner J, et al. Effect of cilostazol on treadmill walking, community-based walking ability, and health-related quality of life in patients with intermittent claudication due to peripheral arterial disease: meta-analysis of six randomized controlled trials. J Am Geriatr Soc. 2002;50:1939–1946.
85. Lee TM, et al. Differential effects of cilostazol and pentoxifylline on vascular endothelial growth factor in patients with intermittent claudication. Clin Sci (Lond). 2001;101:305–311.
86. Pande RL, et al. A pooled analysis of the durability and predictors of treatment response of cilostazol in patients with intermitten claudication. Vasc Med. 2010;15(3):181–188.
88. Lehert P, et al. Naftidrofuryl in intermittent claudication: a retrospective analysis. J Cardiovasc Pharmacol. 1994;23:S48–S52.
89. Hiatt WR, et al. Propionyl-L-carnitine improves exercise performance and functional status in patients with claudication. Am J Med. 2001;110:616–622.
90. Hiatt WR. Effect of propionyl-L-carnitine on a background of monitored exercise in patients with claudication secondary to peripheral artery disease. J Cardiopulm Rehabil Prev. 2011;31(2):125–132.
91. Mondillo S, et al. Effects of simvastatin on walking performance and symptoms of intermittent claudication in hypercholesterolemic patients with peripheral vascular disease. Am J Med. 2003;114:359–364.
92. Leizorovicz A, et al. Oral buflomedil in the prevention of cardiovascular events in patients with peripheral arterial obstructive disease: a randomized, placebo controlled, 4-year study. [for the Limbs International Medicinal Buflomedil (LIMB) Study Group] Circulation. 2008;117:816–822.
93. Marimbert J. Lettre aux professionnels de santé: pharmacovigilancec et la sécurité d’emploi du buflomédil [letter to health professionals: pharmacovigilance and safety of use of buflomedil]. Agence française de sécurité sanitaire des produits de santé: Saint-Denis, France; 2006.
94. Mohler ER III, et al. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–1486.
95. De Backer TL, et al. Buflomedil for intermittent claudication. Cochrane Database Syst Rev. 2001 [CD000988] .
96. De Backer TL, et al. Oral vasoactive medication in intermittent claudication: utile or futile? Eur J Clin Pharmacol. 2000;56:199–206.
97. Maxwell AJ, et al. Nutritional therapy for peripheral arterial disease: a double-blind, placebo-controlled, randomized trial of HeartBar. Vasc Med. 2000;5:11–19.
98. Böger RH, et al. Restoring vascular nitric oxide formation by L-arginine improves the symptoms of intermittent claudication in patients with peripheral arterial occlusive disease. J Am Coll Cardiol. 1998;32:1336–1344.
99. Wilson AM, et al. L-Arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–195.
100. Dobesh PP, et al. Pharmacologic therapy for intermittent claudication. Pharmacotherapy. 2009;29(5):526–553.
101. Belch J, et al. Randomized, placebo-controlled, double-blind study of evaluating the efficacy and safety of AS-013, a prostaglandin E1 prodrug in patients with intermittent claudication. Circulation. 1997;95:2298–2302.
102. Diehm C, et al. Efficacy of a new prostaglandin E1 regimen in outpatients with severe intermittent claudication: results of a multicenter placebo-controlled double-blind trial. J Vasc Surg. 1997;25:537–544.
103. Lièvre M, et al. Oral beraprost sodium, a prostaglandin I(2) analogue, for intermittent claudication: a double-blind, randomized, multicenter controlled trial. Beraprost et Claudication Intermittente (BERCI) Research Group. Circulation. 2000;102:426–431.
104. Mohler ER III, et al. Treatment of intermittent claudication with beraprost sodium, an orally active prostaglandin I2 analogue: a double-blinded, randomized, controlled trial. J Am Coll Cardiol. 2003;41:1679–1686.
105. Lièvre M, et al. A dose effect study of beraprost sodium in intermittent claudication. J Cardiol Pharm. 1996;27:788–793.
106. Ahimastos AA, et al. Ramipril markedly improves walking ability in patients with peripheral arterial disease. Ann Intern Med. 2006;144:660–664.
107. Brass EP, et al. A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication. J Vasc Surg. 2012;55(2):381–389.e1.
108. Labropoulos N, et al. Hemodynamic effects of intermittent pneumatic compression in patients with critical limb ischemia. J Vasc Surg. 2005;42:710–716.
109. TASC. Management of Peripheral Arterial Disease (PAD) Transatlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31:S1–S287.
110. Kavros SJ, et al. Improving limb salvage in critical ischemia with intermittent pneumatic compression: a controlled study with 18-month follow-up. J Vasc Surg. 2008;47:543–549.
111. Chang ST, et al. Using intermittent pneumatic compression therapy to improve quality of life for symptomatic patients with infrapopliteal diffuse peripheral obstructive disease. Circ J. 2012;76:971–976.
112. Simons JP, et al. Failure to achieve clinical improvement despite graft patency in patients undergoing infrainguinal lower extremity bypass for critical limb ischemia. J Vasc Surg. 2010;51(6):1419–1424.
113. Adam DJ, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934.
114. Conte MS. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) and the (hoped for) dawn of evidence-based treatment for advanced limb ischemia. J Vasc Surg. 2010;51(5S):69S–75S.
115. Vascular Web. https://www.vascularweb.org/research/clinicalresearch/Pages/cli-objective-performance-goals.aspx [Accessed November 27, 2012] .
116. Conte MS, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–1473.
117. Geraghty PJ. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50(6):1462–1473.e3.
118. Opie SR, et al. Local endovascular delivery, gene therapy, and cell transplantation for peripheral arterial disease. J Endovasc Ther. 2004;11(Suppl 2):II151–II162.
119. Tongers J, et al. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16.
120. Rajagopalan S, et al. Regional angiogenesis with vascular endothelial growth factor peripheral arterial disease: a phase II randomized double-blind control study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938.
121. Lederman RJ, et al. therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. [TRAFFIC Investigators] Lancet. 2002;359:2053–2058.
122. Nikol S, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. [TALISMAN 201 Investigators] Mol Ther. 2008;16:972–978.
123. Powell RJ, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65.
124. Tateishi-Yuyama E, et al. Therapeutic angiogenesis using cell transplantation (TACT) study investigators. Therapeutic angiogenesis for patients with limb ischemia by autologous transplantation of bone marrow cells; a pilot study and a randomized controlled trial. Lancet. 2002;360:427–435.
125. Schadt DC, et al. Chronic atherosclerotic occlusion of the femoral artery. JAMA. 1961;175:937–940.
126. McAllister FF. The fate of patients with intermittent claudication managed nonoperatively. Am J Surg. 1976;132:593–595.
127. Whyman MR, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg. 1997;26:551–557.
128. Murphy TP, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–139.
129. Simons JP, et al. Outcomes and practice patterns in patients undergoing lower extremity bypass. J Vasc Surg. 2012;55(6):1629–1636.
130. Taylor SM, et al. Do current outcomes justify more liberal use of revascularization for vasculogenic claudication? A single center experience of 1000 consecutively treated limbs. J Am Coll Surg. 2008;6:1053–1064.
132. Bradbury AW, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. J Vasc Surg. 2010;51(5 Suppl):18S–31S.
133. Johnston KW, et al. 5-year results of a prospective study of percutaneous transluminal angioplasty. Ann Surg. 1987;206:403–413.
134. Taylor SM, et al. The LEGS Score: a proposed scoring system to direct treatment of chronic lower extremity ischemia. Ann Surg. 2003;237:812–819.
135. Brass EP. Parenteral therapy with lipo-ecraprost, a lipid-based formulation of a PGE1 analog, does not alter six-month outcomes in patients with critical leg ischemia. J Vasc Surg. 2006;43:752–759.
136. Kalbaugh CA, et al. Invasive treatment of chronic limb ischemia according to the Lower Extremity Grading System (LEGS) score: a 6-month report. J Vasc Surg. 2004;39:1268–1276.
137. Androes MP, et al. Does a standardization tool to direct invasive therapy for symptomatic lower extremity peripheral arterial disease improve outcomes? J Vasc Surg. 2004;40:907–915.
138. Norgren L, et al. TASC II Working Group. Inter society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S29–S30.
139. The I.C.A.I. Group. Long-term mortality as predictors in patients with critical leg ischemia. Eur J Vasc Endovasc Surg. 1997;14:91–95.
140. Taylor SM, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005;42:227–235.
141. Burgess EM, et al. JH. The management of lower extremity amputations surgery, immediate postsurgical prosthetic fitting: patient care. Superintendent of Documents, US Government Printing Office: Washington DC; 1969.
142. Folsom D, et al. Lower-extremity amputation with immediate postoperative prosthetic placement. Am J Surg. 1992;164:320–322.
143. Ali M, et al. A contemporary comparative analysis of immediate post-operative prosthesis placement following below-knee amputation. Ann Vasc Surg. 2013.
144. Taylor SM, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–756.
145. Alber M, et al. Meta-analysis of alternate autogenous vein bypass grafts to infrapopliteal arteries. J Vasc Surg. 2005;42:449–455.
146. Wolfle KD, et al. Graft patency and clinical outcome of femorodistal arterial reconstruction in diabetic and non-diabetic patients: results of a multi-centre comparative analysis. Eur J Vasc Endovasc Surg. 2003;25:229–234.
147. van der Zaag ES, et al. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. Eur J Vasc Endovasc Surg. 2004;28:32–37.
148. Hobbs SD, et al. Peri-operative myocardial injury in patients undergoing surgery for critical limb ischaemia. Eur J Vasc Endovasc Surg. 2005;29:301–304.
149. Visser K, et al. Duplex scan surveillance during the first year after infrainguinal autogenous vein bypass grafting surgery: cost and clinical outcomes compared with other surveillance programs. J Vasc Surg. 2001;33:123–130.
150. Wixon CL, et al. An economic appraisal of lower extremity bypass graft maintenance. J Vasc Surg. 2000;32:1–12.
151. Davies AH, et al. Is duplex scan surveillance of value after leg bypass grafting? Principal results of the Vein Graft Surveillance Randomized Trial (VGST). Circulation. 2005;112:1985–1991.
152. Giswold ME, et al. Modifiable patient factors are associated with reverse vein graft occlusion in the era of duplex scan surveillance. J Vasc Surg. 2003;37:47–53.
153. Nguyen LL, et al. Infrainguinal vein bypass graft revision: factors affecting long term outcome. J Vasc Surg. 2004;40:916–923.
154. Papavassiliou VG, et al. Techniques for the endovascular management of complications following lower limb percutaneous transluminal angioplasty. Eur J Vasc Endovasc Surg. 2003;25:125–130.
155. Lipsitz EC, et al. Fate of collateral vessels following subintimal angioplasty. J Endovasc Ther. 2004;11:269–273.
156. Lipsitz EC, et al. The value of subintimal angioplasty in the management of critical lower extremity ischemia: failure is not always associated with a rethreatened limb. J Cardiovasc Surg. 2004;45:231–237.
157. Bradbury AW. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicenter, randomized controlled trial. [BASIL Trial Participants] Lancet. 2005;366:1925–1934.
158. Mills JL Sr. Open bypass and endoluminal therapy: complementary techniques for revascularization in diabetic patients with critical limb ischemia. Diabetes Metab Res Rev. 2008;24(Suppl I):S34–S39.
159. Chew DK, et al. Bypass in the absence of ipsilateral greater saphenous vein: safety and superiority of the contralateral greater saphenous vein. J Vasc Surg. 2002;35:1085–1092.
160. Twine CP, et al. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2010;(5).
161. Belkin M. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21(2):282–293.
162. Gentile AT. Results of bypass to the popliteal and tibial arteries with alternative sources of autogenous vein. J Vasc Surg. 1996;23:272–279.
163. Calligaro KD. Use of arm and lesser saphenous vein compared with prosthetic grafts for infrapopliteal arterial bypass: are they worth the effort. J Vasc Surg. 1997;26:919–924.
164. Gupta PK. Development and validation of a risk calculator for prediction of mortality after infrainguinal bypass surgery. J Vasc Surg. 2012;56(2):372–379.
165. Baril DT, et al. Prior contralateral amputation predicts worse outcomes for lower extremity bypasses performed in the intact limb. J Vasc Surg. 2012;56:353–360.
166. Mills JL. Invited commentary. J Vasc Surg. 2013;57(1):7–8.
167. Biancari F, et al. Risk-scoring method for prediction of 30-day postoperative outcome after infrainguinal surgical revascularization for critical lower-limb ischemia: a Finnvasc registry study. World J Surg. 2007;31:217–225.
168. Arvela E, et al. Finnvasc score and modified Prevent III score predict long-term outcome after infrainguinal surgical and endovascular revascularization for critical limb ischemia. J Vasc Surg. 2010;52:1218–1225.
169. Landry GJ. Functional outcome of critical limb ischemia. J Vasc Surg. 2007;45:141A–148A.
170. Nehler MR, et al. Is revascularization and limb salvage always the best treatment for critical limb ischemia. J Vasc Surg. 2003;37:704–708.
171. Abou-Zamzam AM Jr, et al. Functional outcome after infrainguinal bypass for limb salvage. J Vasc Surg. 1997;25:287–297.
172. Nicoloff AD, et al. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998;27:256–266.
173. Goshima KR, et al. A new look at outcomes after infrainguinal bypass surgery: traditional reporting standards systematically underestimate the expenditure of effort required to attain limb salvage. J Vasc Surg. 2004;29:330–335.
174. Feinglass J, et al. Functional status and walking ability after lower extremity bypass grafting or angioplasty for intermittent claudication: results from a prospective outcomes study. J Vasc Surg. 2000;31:93–103.
175. Kalbaugh CA, et al. One year prospective quality-of-life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg. 2006;44:296–303.
176. Kukkonen T, et al. Functional outcome of distal bypasses for lower limb ischemia. Eur J Vasc Endovasc Surg. 2006;31:258–261.
177. Tangelder MJD, et al. Quality of life after infrainguinal bypass grafting surgery. J Vasc Surg. 2002;29:913–919.
178. Nguyen LL, et al. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical ischaemia. J Vasc Surg. 2006;44:977–984.
180. Litwin MS. Health-related quality of life. Penson DF, et al. Clinical research methods for surgeons. Humana Press: Totowa, NJ; 2006:237–253.
181. deVries M, et al. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg. 2005;41:261–268.
182. Morgan MBF, et al. Developing the vascular quality-of-life questionnaire: a new disease specific quality-of-life measure for the use of lower extremity ischemia. J Vasc Surg. 2001;33:679–687.
183. Conte MS, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751.
184. Schanzer A, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–781.
185. Schanzer A, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg. 2008;48(6):1464–1471.
186. Schanzer A, et al. Validation of the PIII CLI risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. J Vasc Surg. 2009;50:769–775.
187. Bradbury AW, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a survival prediction model to facilitate clinical decision making. J Vasc Surg. 2010;51:52S–68S.
188. Moxey PW, et al. The BASIL survival prediction model in patients with peripheral arterial disease undergoing revascularization in a university hospital setting and comparison with the FINNVASC and modified PREVENT scores. J Vasc Surg. 2013;57(1):1–7.
189. Collinson DJ, et al. Therapeutic angiogenesis in peripheral arterial disease: can biotechnology produce an effective collateral circulation. Eur J Vasc Endovasc Surg. 2004;28:9–23.
190. Gupta R, et al. Human studies of angiogenic gene therapy. Circ Res. 2009;105:724–736.
191. Isner JM, et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–374.
192. Fowkes FGR, et al. Gene therapy for critical limb ischaemia: the TAMARIS trial. Lancet. 2011;377:1894–1896.
193. Lazarous DF, et al. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol. 2000;36(4):1239–1244.
194. Grossman PM. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153(5):874–880.
195. Belch J, et al. Effect of fibroblast growth factor NV1FGF on amputation and death: a randomised placebo-controlled trial of gene therapy in critical limb ischaemia. Lancet. 2011;377(9781):1929–1937.
196. Shigematsu H, et al. Randomized, double-blind, placebo-controlled clinical trial of hepatocyte growth factor plasmid for critical limb ischemia. Gene Therapy. 2010;17:1152–1161.
197. Makino H, et al. Long-term follow-up evaluation of results from clinical trial using hepatocyte growth factor gene to treat severe peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2012;32(10):2503–2509.
198. Powell RJ. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg. 2010;6:1525–1530.
199. De Haro J, et al. Meta-analysis of randomized, controlled clinical trials in angiogenesis: gene and cell therapy in peripheral arterial disease. Heart Vessels. 2009;24:321–328.
200. Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967.
201. Iafrati MD, et al. Early results and lessons learned from a multicenter, randomized, double-blind trial of bone marrow aspirate concentrate in critical limb ischemia. J Vasc Surg. 2011;54:1650–1658.
202. Murphy MP, et al. Autologous bone marrow mononuclear cell therapy is safe and promotes amputation-free survival in patients with critical limb ischemia. J Vasc Surg. 2011;53:1565–1574.
203. Powell RJ, et al. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011;1032–1041.
204. Powell RJ. Update on clinical trials evaluating the effect of biologic therapy in patients with CLI. J Vasc Surg. 2012;56:264–266.
205. Prather WR, et al. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009;11(4):427–434.
206. Ouma G, et al. Targets and delivery methods for therapeutic angiogenesis in peripheral artery disease. Vasc Med. 2012;17(3):174–192.
207. Hunink MG, et al. Decision making in health and medicine: integrating evidence and values. Cambridge University Press: Cambridge, UK; 2001.
208. Brothers TE, et al. Justification of intervention for limb-threatening ischemia: a surgical decision analysis. Cardiovasc Surg. 1999;7:62–69.
209. Nolan B, et al. The treatment of disabling intermittent claudication in patients with superficial femoral artery occlusive disease–decision analysis. J Vasc Surg. 2007;45:1179–1184.
210. Barshes NR. A primer on cost-effectiveness analyses for vascular surgeons. J Vasc Surg. 2012;55(6):1794–1800.
211. Taylor SM, et al. Critical analysis of clinical success after surgical bypass for lower extremity ischemic tissue loss using a standardized definition combining multiple parameters: a new paradigm of outcome assessment. J Am Coll Surg. 2007;204:831–839.
212. Bollinger A, et al. Semiquantitative assessment of lower limb atherosclerosis from routine angiographic images. Atherosclerosis. 1981;38:339–346.
213. Graziani L, et al. Vascular involvement in diabetic subjects with ischemic foot ulcer: a new morphologic categorization of disease severity. Eur J Vasc Endovasc Surg. 2007;33:453–460.
214. Klevsgard R, et al. Quality of life associated with varying degrees of chronic lower limb ischaemia: comparison with a healthy sample. Eur J Vasc Endovasc Surg. 1999;17:319–325.
215. Johnson BF, et al. Surgery for limb threatening ischaemia: a reappraisal of the costs and benefits. Eur J Vasc Endovasc Surg. 1995;9:181–188.
216. Luther M. Surgical treatment for chronic critical leg ischaemia: a 5 year follow-up of socioeconomic outcome. Eur J Vasc Endovasc Surg. 1997;13:452–459.
217. Cheshire NJ, et al. The economics of femorocrural reconstruction for critical leg ischemia with and without autologous vein. J Vasc Surg. 1992;15:167–174.
218. Robinson WP, et al. Inferior outcomes of autogenous infrainguinal bypass in Hispanics: an analysis of ethnicity, graft function, and limb salvage. J Vasc Surg. 2009;49:1416–1425.
219. Schanzer A, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. J Vasc Surg. 2007;46:1180–1190.
220. Goodney PP, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg. 2010;51:71–78.
221. Schanzer A, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47:774–781.
222. Owens CD, et al. Refinement of survival prediction in patients undergoing lower extremity bypass surgery: stratification by chronic kidney disease classification. J Vasc Surg. 2007;45:944–952.
224. Rossi PJ, et al. Redo infrainguinal bypass: factors predicting patency and limb salvage. Ann Vasc Surg. 2003;17:492–502.
225. Toursarkissian B, et al. Angiographic scoring of vascular occlusive disease in the diabetic foot: relevance to bypass graft patency and limb salvage. J Vasc Surg. 2002;35:494–500.
226. Alback A, et al. Prediction of the immediate outcome of femoropopliteal saphenous vein bypass by angiographic runoff score. Eur J Vasc Endovasc Surg. 1998;15:220–224.
227. Simons J, et al. Failure to achieve clinical improvement despite graft patency in patients undergoing lower extremity bypass for critical limb ischemia. J Vasc Surg. 2010;51:1419–1424.
228. Taylor SM, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–755.
229. Nguyen LL, et al. Prospective multicenter study of quality of life before and after lower extremity vein bypass in 1404 patients with critical limb ischemia. J Vasc Surg. 2006;44:977–983.
230. Mills JL Sr, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk stratification based on Wound, Ischemia, and foot Infection (WIfI). [the Society for Vascular Surgery Lower Extremity Guidelines Committee] J Vasc Surg. 2013 [Oct 12. doi:pii: S0741-5214(13)01515-2. 10.1016/j.jvs.2013.08.003. [Epub ahead of print] PMID: 24126108] .