Immunity, inflammation and infection
Inflammation
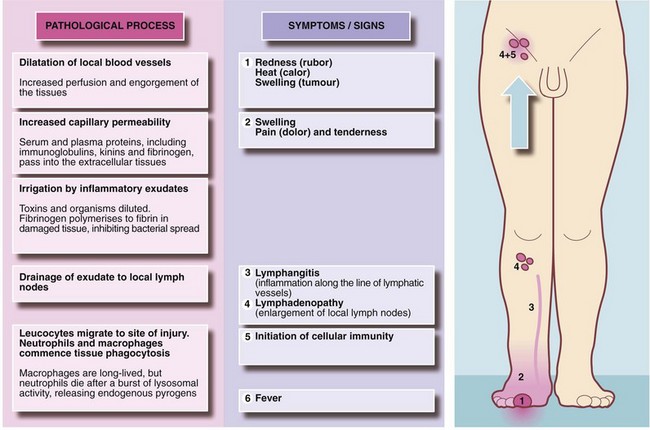
Introduction: Acute inflammation is the principal mechanism by which living tissues respond to injury. The purpose is to neutralise the injurious agent, to remove damaged or necrotic tissue and to restore the tissue to useful function. The central feature is formation of an inflammatory exudate with three principal components: serum, leucocytes (predominantly neutrophils) and fibrinogen.
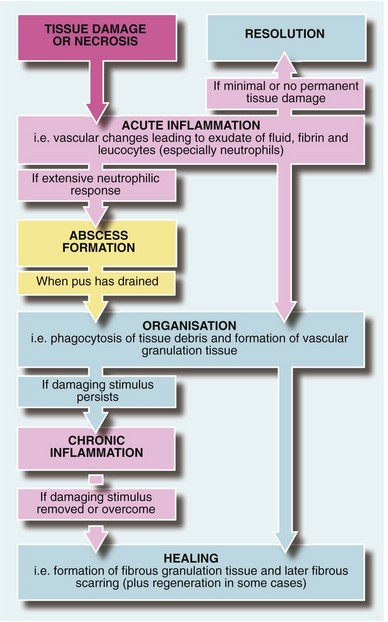
Formation of inflammatory exudate involves local vascular changes collectively responsible for the four ‘cardinal signs of Celsus’—rubor (redness), tumor (swelling), calor (heat) and dolor (pain)—as well as loss of function. These vascular phenomena are described in Figure 3.1. The outcomes of acute inflammation are summarised in Figure 3.2.
Resolution: If tissue damage is minimal and there is no actual tissue necrosis, the acute inflammatory response eventually settles and tissues return virtually to normal without evidence of scarring. A good example is the resolution of mild sunburn.
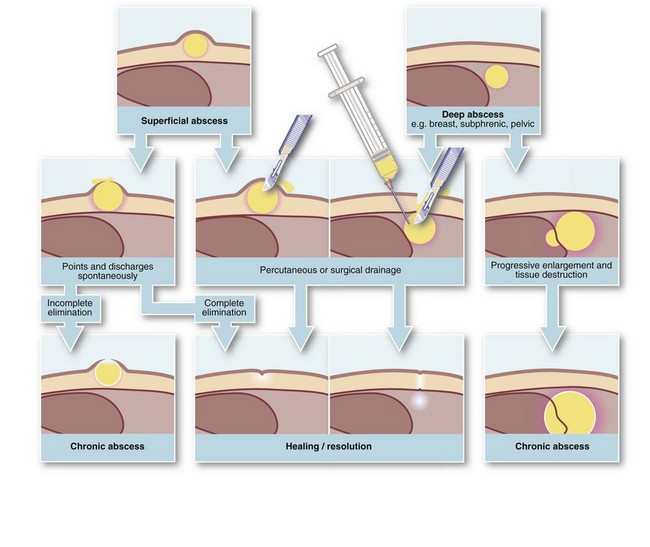
Abscess formation (Fig. 3.3): An abscess is a collection of pus (dead and dying neutrophils plus proteinaceous exudate) walled off by a zone of acute inflammation. Acute abscess formation particularly occurs in response to certain pyogenic microorganisms that attract neutrophils but are resistant to phagocytosis and lysosomal destruction. Abscesses also form in response to localised tissue necrosis and to some organic foreign bodies (e.g. wood splinters, linen suture material). The main pyogenic organisms of surgical importance are Staphylococcus aureus, some streptococci (particularly Strep. pyogenes), Escherichia coli and related Gram-negative bacilli (‘coliforms’), and Bacteroides species (spp.).
Even with small, well-localised abscesses, showers of bacteria may enter the general circulation (bacteraemia) but are mopped up by hepatic and splenic phagocytic cells before they can proliferate. This is responsible for the swinging pyrexia characteristic of an abscess. The abscess site may not be clinically evident if deep-seated (e.g. subphrenic or pelvic abscess) and the patient may be otherwise well. In the presence of an abscess, circulating neutrophils rise dramatically as they are released from the bone marrow; thus, a marked neutrophil leucocytosis (i.e. WBC greater than 15 × 109/L with more than 80% neutrophils) usually indicates a pyogenic infection. Severe infection causing excessive cytokine responses spilling over into the systemic circulation causes systemic sepsis and rapid clinical deterioration (see p. 51).
Antibiotics and abscesses: If appropriate antibiotics are given early enough, organisms can be eliminated before abscess formation. In surgical operations with a particular risk of infection, therefore, prophylactic antibiotics dramatically reduce abscess formation and other infective complications. However, once an abscess has fully formed, antibiotics seldom effect a cure because pus and necrotic material remain and the drug cannot gain access to the bacteria within. Nevertheless, antibiotics may halt expansion or even sterilise the pus; the residual sterile abscess is known as an antibioma.
Organisation and repair: The most common sequel to acute inflammation is organisation, in which dead tissue is removed by phagocytosis and the defect filled by vascular connective tissue known as granulation tissue. This tissue is gradually ‘repaired’ to form a fibrous scar. Sometimes the original tissue regenerates, i.e. rebuilds its specialised cells and structure.
Wound healing
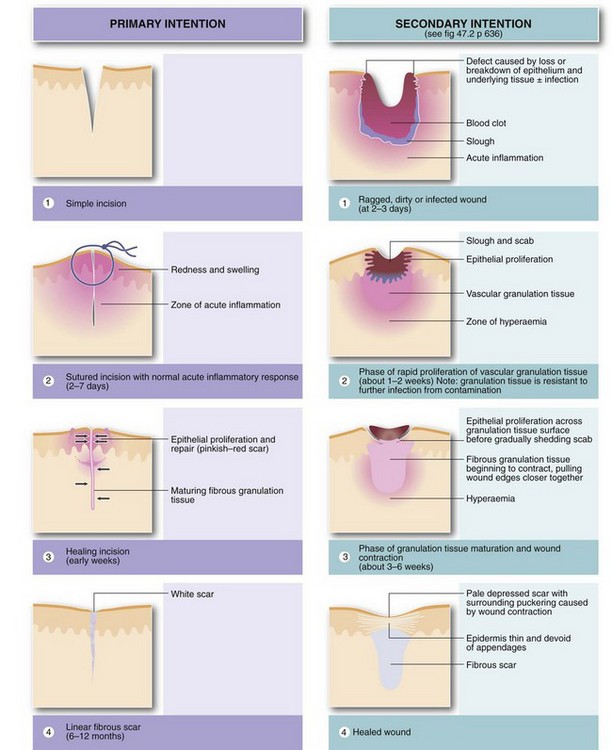
Healing by primary intention: The simplest example of organisation and repair is healing of an uncomplicated skin incision (see Fig. 3.4). There is no necrotic tissue and the wound margins are brought into apposition with sutures. An acute inflammatory response develops in the vicinity of the incision, and by the third day granulation tissue bridges the dermal defect. In the meantime, epithelium proliferating rapidly from the wound edges restores the epidermis. Fibroblasts invade the granulation tissue, laying down collagen so the repair is strong enough for suture removal after 5–10 days. The scar is still red but blood vessels gradually regress and it becomes a pale linear scar within a few months. This is known as healing by primary intention.
Healing by secondary intention: If tissue loss prevents the wound edges from coming together, healing has to bridge the defect, which is initially filled with blood clot. This later becomes infiltrated by vascular granulation tissue from the healthy wound base. Inflammatory exudate solidifies, forming a protective scab. Fibroblasts invade and lay down collagen in the extracellular spaces; after about a week, some fibroblasts differentiate into myofibroblasts and contraction of their myofibrils eventually shrinks the wound defect by 40–80%, beginning about 2 weeks after the injury. Over the weeks and months, blood vessels regress and more collagen is formed, leaving a relatively avascular scar; gradual contraction of the mature collagen (cicatrisation), combined with wound contraction, ensures the final scar is much smaller than the original defect. The epidermal defect is gradually bridged by epithelial proliferation from the wound margins. Epithelial cells slide over each other beneath the edges of the scab on the granulation tissue surface and the scab is eventually shed. This whole process is known as healing by secondary intention (see Fig. 3.4).
Chronic inflammation
Causes of chronic abscesses include:
• Infected foreign bodies are probably the most common cause in modern surgical practice. Foreign bodies implanted deliberately may become infected (e.g. synthetic mesh for inguinal hernia repair, prosthetic hip joint); others become embedded during trauma (e.g. glass fragments)
• Dead (necrotic) tissue can act as a foreign body, forming a nidus for infection. For example, diabetes may be complicated by deep foot infections with necrosis of tendon and bone leading to chronic abscesses and ulcers. Hairs deeply implanted in the natal cleft skin may cause a pilonidal sinus or abscess. An infected dead tooth or root fragment may intermittently discharge via an associated ‘gum boil’ (see Fig. 3.6). Chronic osteomyelitis is associated with remnants of dead bone known as sequestra
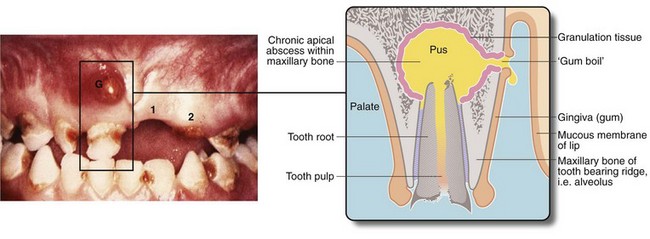
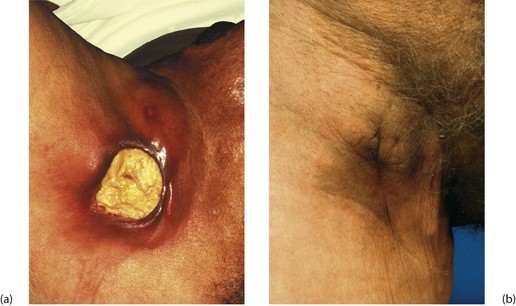
Fig. 3.6 ‘Gum boil’ as an example of a chronic abscess
Grossly neglected mouth showing widespread dental caries. There is an inflammatory swelling on the buccal (cheek) aspect of the alveolus G caused by a chronic apical dental abscess on the upper right incisor. Note the left central incisor 1 is missing and the left lateral incisor 2 has fractured at gum level because of caries. Sagittal section through gum boil of upper incisor tooth. The gum boil is in fact a sinus on the gum which discharges either chronically or intermittently. Exposed to infection, the tooth pulp has become necrotic while the apical abscess is slowly expanding due to the continued presence of infected necrotic tissue (i.e. the tooth pulp). The tooth root is all that remains after the crown has fractured due to dental caries
• Deep abscesses. A chronic abscess can arise without a foreign body if the acute abscess is so deep as to prevent spontaneous drainage. The best example is a subphrenic abscess
Specific granulomatous infections and inflammations
A tuberculous cold abscess, now rare in developed countries, is a pus-like accumulation of liquefied caseous material containing the occasional mycobacterium. In contrast to a pyogenic abscess, the lesion is cold to the touch since there is no acute inflammatory vascular response. Cervical lymph node tuberculosis (‘scrofula’) often produced a ‘collar-stud’ abscess, i.e. a superficial fluctuant abscess communicating with a deep (and often larger) lymph node abscess via a small fascial defect. Tuberculosis of the thoracolumbar spine causes local destruction and deformity and may track down beneath the inguinal ligament within the psoas sheath, presenting as a ‘psoas abscess’ in the groin. A tuberculous ulcer overlying tuberculous inguinal nodes is shown in Figure 3.7.
Infection
It is important to distinguish between colonisation, infection and sepsis:
• Colonisation is when bacteria are present in or on a host but do not cause an immune response or signs of disease
• Infection occurs when microorganisms provoke a sustained immune response and signs of disease, for example, when normal commensal bacteria in the colon such as E. coli contaminate the peritoneal cavity
• Sepsis (systemic sepsis) is the result of an excessive and inappropriate production of cytokines in response to severe infection or tissue necrosis (e.g. a gangrenous limb) that causes organ dysfunction and progressive organ failure
Clinically significant infection arises when the size of an inoculum or the virulence of a microorganism is sufficient to overcome the innate and adaptive immune responses and lead to symptoms. The virulence of an organism depends on its qualities of adherence and invasiveness and its ability to produce toxins. Tissue invasion of microorganisms may be enhanced by their secretion of enzymes (e.g. hyaluronidase and streptokinase), by mechanisms to avoid phagocytosis (e.g. encapsulation or spore formation), by inherent resistance to lysosomal destruction or by their ability to kill phagocytes. Toxins may be secreted by the organism (exotoxins) or released upon the death of the organism (endotoxins). In either case the toxin may produce local tissue damage (e.g. gas gangrene), cause distant toxic effects (e.g. tetanus), or activate cytokine systems to cause systemic sepsis, sometimes including disseminated intravascular coagulopathy.
Methods of control of nosocomial infection
Environment: Patient areas in hospitals must be clean and free from contaminating bacteria, including MRSA, especially where invasive procedures occur such as operating theatres and high-dependency units. Special precautions are taken in theatres (see Ch. 10, p. 124).
Staff: Staff must be vaccinated against hepatitis B. HIV-positive individuals should not undertake invasive procedures. Open wounds must be securely covered and staff with infective skin lesions should avoid patient contact. Universal blood and body fluid precautions should be taken to prevent viral transmission (see below) and guidelines followed for dealing with needle-stick injuries. Risk of MRSA and other infections can be drastically reduced by the simple measures such as washing hands with alcohol-based gel between every patient contact.
Patients: Bacterial swabs should be taken for MRSA from patients before admitting them to hospital and MRSA-positive patients should receive topical eradication therapy and have follow-up bacterial screening cultures to assess MRSA status before major surgery, e.g. hip replacements, arterial grafts. Patients known to have transmissible infections or to be carriers, e.g. of MRSA, should be nursed in isolation. Surgery should ideally be deferred on patients with acute respiratory or urinary tract infections.
Universal blood and body fluid precautions
Hepatitis B vaccination: Staff directly involved in patient care should be vaccinated against hepatitis B. Hepatitis B serology should be checked 2 months after completion of vaccination. Around 5% of healthy young people fail to seroconvert and should be revaccinated. Half of these will seroconvert and the remainder are genetic non-responders.
Needle-stick and other penetrating injuries: Sharps injury, especially from a contaminated hollow needle (needle-stick injury), may lead to transmission of infection if the patient carries a blood-borne virus. Such injuries are capable of transmitting hepatitis B and C but the risk for HIV is much lower because the viral concentration in HIV-positive fluids is much lower and the volume transmitted is small.
Viral infection following sharps injury: If a definite sharps injury has occurred that involves blood being transmitted from an infected person, the risk of hepatitis B infection to the recipient is about 25%. For hepatitis C the risk is 2% and for HIV 0.5%. There is also a very high risk after sharps injury in a recreational environment (e.g. needles left on the beach by intravenous drug users) since hepatitis B and HIV survive well in warm, moist conditions, especially in serum and tissue debris. Thus all sharps injuries should be treated with the utmost concern. A recommended protocol is shown in Box 3.1.
Principles of treatment of surgical infection
Removal of infected foci: A poorly vascularised infected area is effectively isolated from humoral and cellular defence mechanisms and from circulating antibiotics. Retained infected material may overactivate cytokine mechanisms and thus precipitate systemic sepsis. A vital first step is to remove infected necrotic tissue and drain collections of pus. This applies even if the patient appears too ill for operation because the very ill patient may recover dramatically after this type of surgery. Common examples include draining abscesses, amputating infected necrotic limbs, removing infected foreign bodies (e.g. cannulae, prostheses, trauma debris) and draining the infected contents of hollow viscera such as bile ducts, kidneys and ureters.
Antibiotic therapy (see Table 3.1):
Empirical antibiotic therapy: If treatment is urgent, antibiotics are chosen according to the most likely pathogens and local antibiotic sensitivity profiles. A Gram stain on material from a usually sterile site can guide initial therapy until detailed results are available. In abdominal wound infections where hollow viscera have not been opened, Staph. aureus is the likely organism and flucloxacillin can be commenced. If the patient is a known MRSA carrier, vancomycin may be indicated. If bowel has been opened, Gram-negative organisms including anaerobes are likely and an antibiotic regimen is chosen to include these.
Specific antibiotic therapy: Once microbiological results are available, therapy is modified to deal with the organisms and their sensitivities. ‘Narrow-spectrum’ therapy is more effective with fewer side-effects than broad-spectrum ‘empirical’ therapy. It also minimises superinfection with organisms such as Clostridium difficile and yeasts. C. difficile infections have become a major scourge of hospitals in North America and Europe, mainly due to an ageing population more susceptible to infection and the emergence of a highly virulent clone. Extreme measures to control its spread have been required including strict isolation of patients, high level environmental cleaning and strong antibiotic controls.
Bacteria of particular surgical importance
Pathophysiology: Staphylococci are Gram-positive cocci, and Staph. aureus is the main pathogenic species. It is part of normal human bacterial flora, with about 30% of the population being nasal carriers and 10% carrying it on perineal skin. Staph. aureus typically produces pustules, boils, breast abscesses, wound infections and osteomyelitis. Its virulence is partly due to the enzymes and toxins it produces. A few patients harbour virulent strains that produce the toxic shock syndrome toxin (TSST-1). Infection produces toxic shock syndrome with serious systemic effects such as hypotension, shock and multi-organ failure. Staph. epidermidis (formerly Staph. albus), a coagulase-negative staphylococcus, is a universal skin commensal rarely causing significant infection and meriting antibiotics only when it causes infections of exogenous materials such as prosthetic implants and intravenous cannulae.
Antibiotic sensitivities: Most strains were sensitive to penicillin but more than 85% are now resistant in family practice and hospital. Resistance is largely due to production of the enzyme beta-lactamase (also known as penicillinase). Most strains remain sensitive to a range of common antibiotics, e.g. flucloxacillin, erythromycin and some cephalosporins. Gentamicin is also active.
MRSA: Some strains of Staph. aureus are resistant to flucloxacillin, cephalosporins, gentamicin, erythromycin and chloramphenicol and are sensitive only to the glycopeptide antibiotics, vancomycin and teicoplanin, given parenterally; these are meticillin-resistant Staph. aureus (MRSA). Meticillin is employed in the lab to predict flucloxacillin and cephalosporin resistance. MRSA now accounts for about half of all S. aureus isolates in hospitals and is also a problem in the community with community-acquired MRSA (CA-MRSA). Ward areas at greatest risk are burns units, intensive care units and cardiothoracic, neonatal, orthopaedic and geriatric wards. It is often erroneously believed that MRSA is more pathogenic than other strains. In fact, the organisms excite similar inflammatory responses but MRSA infections are more difficult to treat. In some cases, oral treatment with tetracycline, co-trimoxazole or a combination of rifampicin and fusidic acid is appropriate; the combination prevents rapid development of resistance to each agent alone.
Streptococci
Pathophysiology: Streptococci are Gram-positive coccoid organisms first described in infected surgical wounds by Billroth in 1874. They are classified by their oxygen requirements into aerobic, anaerobic and microaerophilic and subdivided by their haemolysis patterns on blood agar culture plates. Alpha-haemolytic streptococci cause partial haemolysis with green discolouration; important pathogens include the viridans group and Strep. pneumoniae. Beta-haemolytic streptococci produce complete haemolysis and can be grouped serologically into Lancefield groups A to O. The important human pathogens are group A (Strep. pyogenes—of major surgical importance) and group B (Strep. agalactiae—a common cause of serious neonatal sepsis). Group C and G streptococci are occasional causes of cellulitis and bacteraemia. Microaerophilic streptococci such as Strep. milleri carry a group F antigen.
Streptococci of particular surgical significance:
Strep. pyogenes (group A Strep. and other beta-haemolytic streptococci): This is the main human pathogenic streptococcus and is carried in the upper respiratory tract by about 10% of children but less often by adults. It can cause cellulitis and is a common cause of sore throat as well as post-streptococcal syndromes such as rheumatic fever (which predisposes to cardiac valvular damage and risk of infective endocarditis).
Viridans streptococci: The viridans group are oral commensals of low virulence but are the most common organisms causing infective endocarditis. The subject is described in Chapter 8, p. 106.
Strep. pneumoniae (pneumococcus): This is the most common cause of lobar pneumonia and can cause bronchopneumonia in susceptible post-surgical patients, as well as middle ear infections (otitis media) and acute exacerbations of chronic bronchitis. Pneumococcal meningitis may occur in the young and elderly and may complicate head injury. Severe pneumococcal sepsis is particularly likely after splenectomy but may be prevented by vaccination and penicillin prophylaxis.
Anaerobic streptococci: These are bowel commensals and may form part of the mixed flora in intraperitoneal abscesses or infections associated with necrotic tissue, e.g. diabetic foot ulcers.
Antibiotic sensitivities: Penicillin is the drug of choice for most streptococcal infections. In seriously ill patients, benzylpenicillin is given parenterally. For less serious infections in patients able to tolerate oral therapy, penicillin V (phenoxymethylpenicillin), ampicillin or amoxicillin are the drugs of choice. Many streptococci are also sensitive to macrolides (e.g. erythromycin, clarithromycin). Some pneumococci are now partially resistant to penicillin. Most infections, however, are still cleared with high-dose penicillin although meningitis needs alternative antibiotics such as ceftriaxone or vancomycin.
Enterococci
Pathophysiology: The enterococci are Gram-positive cocci closely related to streptococci. E. faecalis (formerly Strep. faecalis) is the most common species. Enterococci are part of the normal bowel flora and may cause infection where bowel has been opened or else infect urinary or genital tracts.
Antibiotic sensitivities: Penicillin, ampicillin, amoxicillin or vancomycin are used for enterococcal infections. In serious infections such as endocarditis, a combination of high-dose penicillin and gentamicin ensures bactericidal activity. The cephalosporins are all ineffective. In hospitals where broad-spectrum cephalosporins are used empirically for bowel-related infections or septicaemia, enterococci are a frequent cause of nosocomial (hospital-acquired) infection. Vancomycin-resistant enterococci (VRE) are now being found. They are usually low-grade pathogens infecting intravascular lines in transplant and haematology patients and on ICUs. VRE endocarditis is difficult to treat, but newer antibiotics linezolid, daptomycin and quinupristin/dalfopristin are active against most strains.
Enterobacteriaceae
Pathophysiology: The Enterobacteriaceae are a large family of Gram-negative bacilli (rods) and usually make up about 1% of intestinal flora (see Table 3.2); they are known as coliforms and can be cultured under aerobic and anaerobic conditions and, like other bowel flora, grow in bile salt-containing media such as MacConkey or CLED agar; this helps identification.
Antibiotic sensitivities: Many coliforms are now resistant to ampicillin (and amoxicillin) and first-generation cephalosporins, e.g. cefalexin, but most are sensitive to second- and third-generation cephalosporins, e.g. cefuroxime, cefotaxime. Gentamicin is still a very effective and cheap agent. Many are sensitive to fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin) but resistance is emerging. For prophylaxis in bowel and biliary tract surgery and for related local and systemic infections, gentamicin or an amoxicillin–clavulanate combination (co-amoxiclav) is recommended for its additional anaerobe activity. Antibiotic and beta-lactamase inhibitor combination antibiotics such as co-amoxiclav or piperacillin–tazobactam are very active against bowel flora with good activity against anaerobes. Anaerobes are the main colonisers of the bowel and often accompany Enterobacteriaceae in infections.
‘Non-surgical’ Enterobacteriaceae: Other members of the family cause primary bowel infections. Salmonella typhi causes typhoid which may cause bowel perforations and Shigella causes bacillary dysentery. Rarely, Salmonella is incriminated in acute appendicitis and primary ‘mycotic’ aneurysms. An increasingly important cause of acute haemorrhagic colitis is E. coli O157:H7 and other verotoxin-producing E. coli. This is indistinguishable from acute haemorrhagic ulcerative colitis and should be sought bacteriologically in all cases. These strains have also caused large outbreaks of food-borne disease and produce a verotoxin (Shiga-like toxin) responsible for haemolytic uraemic syndrome (HUS) resulting in acute renal failure. Yersinia sometimes produces an acute ileal inflammation which may mimic acute appendicitis and has a similar appearance to Crohn’s disease at laparotomy. Campylobacter jejuni, the most common cause of food-borne infection, can also cause a pseudo-appendicitis by initiating terminal ileitis and mesenteric lymph node inflammation.
Pseudomonas
Pathophysiology: The main pathogen in this group of aerobic Gram-negative rods is Pseudomonas aeruginosa, an uncommon cause of surgical infection except in debilitated, hospitalised patients. It is found in a wide variety of habitats including soil, water, plants and animals, reflecting its predilection for moist environments. It is commonly found on hospital and cleaning equipment and even in chemical disinfectants and antiseptics. In about 10% of the population, Ps. aeruginosa is a normal intestinal commensal and is primarily an in-hospital (nosocomial) pathogen. The organism is resistant to many antibiotics and so tends to proliferate when other flora are suppressed by broad-spectrum antibiotics.
Antibiotic sensitivities: True infection must, as ever, be distinguished from colonisation where treatment is not indicated and would encourage development of resistance. Ps. aeruginosa is intrinsically resistant to most antibiotics; those that have activity include the aminoglycosides (gentamicin and tobramycin), some extended-spectrum beta-lactam antibiotics (ceftazidime or cefepime), the combination of piperacillin and tazobactam and the carbapenems (imipenem or meropenem). The quinolones, ciprofloxacin and ofloxacin, are the only orally effective anti-pseudomonal agents.
Anaerobes
• Gram-negative bacilli—Bacteroides fragilis and other Bacteroides species (spp.), Porphyromonas spp., Prevotella spp., Fusobacterium spp. and Bilophila wadsworthia
• Gram-positive cocci—Peptostreptococcus and microaerophilic streptococci
• Gram-positive bacilli—Clostridium spp. (spore forming), Actinomyces spp., Propionibacterium spp. and Bifidobacterium spp.
Antibiotic sensitivities: Most anaerobes are highly sensitive to metronidazole. Metronidazole can be given orally, intravenously or rectally, with the rectal route resulting in plasma levels equivalent to intravenous administration. Metronidazole is standard prophylaxis before appendicectomy and large bowel surgery, and has dramatically reduced peritoneal and wound infections. Other antibiotics with broad anaerobic activity are co-amoxiclav (Augmentin), piperacillin/tazobactam (Tazocin), the carbapenems (imipenem and meropenem) and chloramphenicol.
Pathophysiology: Bacteroides cause pyogenic infections after faecal contamination of the peritoneal cavity, along with other gut commensals, and occasionally cause sepsis in debilitated patients. Bacteroides were not identified as pathogens until the early 1970s because their strict anaerobic culture requirements were unrecognised. Indeed Bacteroides spp. were probably responsible for many so-called ‘sterile’ intra-abdominal abscesses. The importance of B. fragilis as a cause of surgical infection is probably still underestimated.
Clostridia
Gas gangrene: Gas gangrene results when Clostridium perfringens (formerly C. welchii) and other anaerobes (e.g. Bacteroides spp. and streptococci) proliferate in necrotic tissue, secreting powerful toxins. Toxins spread rapidly and destroy nearby tissues, generating gas which causes the characteristic sign of crepitus (‘crackling’) on palpation and the typical X-ray appearance (Fig. 3.8). Deep traumatic wounds involving muscle, and wounds contaminated with soil, clothes or faeces are most susceptible. The condition is very common in battle wounds—gas gangrene was responsible for vast numbers of deaths during the First World War.
Treatment of gas gangrene: Treatment of established gas gangrene is urgent and must proceed vigorously for any hope of survival. Treatment is with high doses of intravenous penicillin to kill organisms in viable and vascularised tissue, and emergency radical excision of all necrotic tissue. This involves carving back the necrotic muscle to healthy bleeding tissue; affected muscle is recognised by its brick-red colour and failure to contract on cutting. In the surgery of contaminated wounds, preventing clostridial infection requires meticulous excision of all necrotic tissue followed by wound packing rather than suturing. Further excisions are likely to be needed and delayed primary closure performed a few days later.
Tetanus: Tetanus is caused by Clostridium tetani, which also infects dirty wounds. The entry wound may be minute, perhaps caused by a rose thorn or splinter. The organism produces an exotoxin with little local effect but, even in minute quantities, with powerful neuromuscular effects causing widespread muscular spasm. The first signs are often acute muscle spasms and neck stiffness or trismus (‘lockjaw’). If untreated, these progress to opisthotonus (arching of the back due to extensor spasm), generalised convulsions and eventually death from exhaustion and respiratory failure several days later.
Pseudomembranous colitis: Pseudomembranous colitis can be the most serious form of antibiotic-associated diarrhoea (see Ch. 12) and is caused by overgrowth of a toxigenic Clostridium difficile. The organism gets its name from the difficulty of growing it in culture. Infection produces a thick fibrinous ‘membrane’ on large intestinal mucosa, within which the organism proliferates. Its toxins cause a profound watery and sometimes bloody diarrhoea, leading to dehydration and electrolyte loss.
Viruses of particular surgical importance
Human immunodeficiency virus (HIV)
Classification of HIV infections: The human immunodeficiency virus causes a chronic infection that usually progresses to the acquired immune deficiency syndrome (AIDS) over 7 or more years. The illness evolves through several stages or groups, classified by the US Centers for Disease Control, in 1986, as:
• (Group I) the acute seroconversion illness. Seroconversion occurs as long as 3 months after infection, and as many as 70% of infected patients are asymptomatic at the time of seroconversion. Patients usually test negative for antibodies against HIV before and during the seroconversion illness
• (Group II) the asymptomatic period during which patients usually feels completely well
• (Group III) as the disease progresses, the patient may develop generalised lymphadenopathy and wasting (AIDS-related complex)
• (Group IV) AIDS is manifest by development of unusual opportunistic infections (e.g. Pneumocystis pneumonia, cytomegalovirus (CMV) infections, cerebral toxoplasmosis, atypical mycobacterial infections), certain malignant diseases (Kaposi’s sarcoma, generalised or cerebral lymphoma, aggressive invasive uterine cervical cancer) and neurological disease (AIDS dementia complex)
The use of combinations of antiretroviral drugs (‘high activity antiretroviral therapy’) has dramatically affected the natural history, with a large sustained drop in mortality from opportunistic infections.
Surgical involvement in HIV cases: Surgeons may be involved in diagnosing bowel-related problems (e.g. oesophageal candidiasis) by oesophago-gastro-duodenoscopy (OGD). In late-stage disease, cytomegalovirus (CMV) infection may involve any part of the GI tract, ranging from mouth ulcers, to ulcers in the jejunum that may perforate, to colitis. For AIDS colitis, colonoscopy and biopsy are often required for diagnosis. Treatment involves intravenous antiviral drugs.
AIDS patients may develop severe perianal herpes with secondary anal fistula or abscess formation. In a patient with known AIDS, perianal lesions should be assumed to be herpes until proven otherwise as the presentation is often atypical. Kaposi’s sarcomas (see Ch. 46, p. 575) may require local excision. Other examples of surgical involvement with HIV-infected patients include insertion of long-term central venous catheters (e.g. Hickman line), insertion of percutaneous endoscopic gastrostomy (PEG) tubes for feeding, or joint replacements in HIV infected haemophiliacs.
Viral hepatitis
Hepatitis A: Hepatitis A is transmitted by the faecal–oral route and has an incubation period of 2–6 weeks. It rarely causes fulminating disease and never leads to chronic hepatitis or cirrhosis. Its only surgical importance is in the differential diagnosis of jaundice. A vaccine for hepatitis A is available.
Hepatitis B: Hepatitis B is transmitted by blood or body fluids, including sexual intercourse, or from mother to fetus or baby (termed vertical transmission). Incubation is 6 weeks to 6 months. Hepatitis B infection leads to chronic hepatitis and cirrhosis in 5–10%; this variety of cirrhosis commonly progresses to hepatocellular carcinoma (the most common cause world-wide). Hepatitis B is preventable by vaccination and this is indicated for neonates of mothers who carry the virus, all health care workers and people living in high-risk areas.
Exposure to hepatitis B virus has several possible outcomes:
• Acute fulminant hepatitis—rare but fatal
• Acute hepatitis—clearing of the virus leads to lifelong immunity
• Chronic infection—may lead to chronic hepatitis/cirrhosis (and hepatocellular carcinoma)
• Chronic carrier state—mainly due to infection at birth but can occur later with development of immune tolerance and no obvious active disease
Diagnosis of hepatitis B: The hepatitis B surface antigen (HBsAg) can be detected in blood in the early stages. Patients later develop antibodies to the viral core (anti-HBc) which is a marker of exposure to the virus but does not confer immunity. Later still, with clearance of the virus, patients develop surface antibodies (anti-HBs) which confer lifetime immunity. Patients who do not clear the virus remain surface antigen (HbsAg) positive and may become chronic carriers, i.e. remain infectious to other people and prone to risk of complications themselves.
Treatment of hepatitis B: Hepatitis B DNA can now be detected in blood, giving a definitive diagnosis and an estimate of the quantity of virus in the bloodstream (viral load) to guide treatment. Therapy with interferon and lamivudine has been partially successful in treating chronic infections, reducing infectivity and the risk of hepatocellular carcinoma. Chronic carriers failing therapy should be monitored for hepatocellular carcinoma by annual estimation of serum alpha-fetoprotein and undergo liver ultrasound scanning every 2 years, as partial hepatectomy can sometimes cure early cases.
Hepatitis C: Hepatitis C is transmitted via the same routes as hepatitis B but sexual transmission is believed to be less common. The incubation period is approximately 2 months. Chronic liver disease develops in a higher proportion of cases (30–50%) but is often of low grade. Hepatitis C is also an important cause of hepatocellular carcinoma world-wide.
Sepsis (see also http://www.survivesepsis.org/)
Multiple organ dysfunction and the systemic inflammatory response syndrome
Multiple organ dysfunction syndrome or MODS (multi-organ failure or MOF) was recognised as a clinical entity in the mid-1970s when it became recognised that any major physiological insult could lead to failure of organs remote from the initiating disease process. Later, the underlying condition was found to be an unrestrained systemic inflammatory response (systemic inflammatory response syndrome, SIRS), initiated by adverse events such as trauma, infection, inflammation, ischaemia or ischaemia–reperfusion injury. MODS is the most common reason for surgical patients to stay longer than 5 days in intensive care (see Box 3.2).
Pathophysiology of SIRS and MODS: SIRS involves widespread changes, including inflammatory cell activation leading to cytokine release, endothelial injury, disordered haemodynamics and impaired tissue oxygen extraction. It thus represents grossly exaggerated activation of innate immune responses intended as host defences. An unregulated release of inflammatory mediators causes widespread microvascular, haemodynamic and mitochondrial changes that eventually lead to organ failure.
Mediators of SIRS and MODS: SIRS and MODS involve complex interactions of endogenous and sometimes exogenous mediators. A range of cytokines is released from activated macrophages and from endothelial and other reticulo-endothelial cells. The suite of cytokines released depends on the nature of the provoking agent and is governed by the specific type of Toll-like receptor that recognises the aggressor. Cytokines released include tumour necrosis factor-alpha (TNF-alpha), the interleukins (particularly IL1 but also IL2, IL6) and platelet activating factor. Pharmacological attempts to suppress excess cytokine responses have thus far been ineffective.
Sepsis: The classic septic response, with a hyperdynamic circulation, systemic signs of inflammation and disrupted intermediary metabolism, can be induced in healthy volunteers by injecting lipopolysaccharide (from the cell walls of Gram-negative bacteria) or the cytokines TNF-alpha or IL1. In Gram-negative sepsis, the lipopolysaccharide component of the organisms powerfully activates Toll-like receptors on dendritic cells and hence the whole inflammatory cascade. This results in endothelial activation which increases vascular permeability and neutrophil–endothelial interaction. The final common pathway may involve activated neutrophils migrating into the interstitial space of the affected organ and tissue hypoxia. In sepsis, these and other circulating factors working in synergy bring about the devastating effects of MODS.
Clinical conditions leading to sirs and mods
• Inducing excessive release of endogenous cytokines
• Disrupting oxygen delivery to the tissues
• Impairing intestinal barrier function allowing translocation of intestinal bacteria and endotoxin to the portal and systemic circulations
Organ failure induced by acute pancreatitis is caused by a combination of these factors.
Infection: The infection source that leads to MODS may be acquired (e.g. intra-abdominal abscess) or endogenous, i.e. from the patient’s bowel. Local infection, especially with Gram-negative bacteria, stimulates the release of inflammatory cytokines. A similar response is provoked by a substantial volume of necrotic tissue, e.g. gangrenous leg, and is worse if the tissue is infected. These paracrine responses are beneficial in a local sense, combating infection by increasing blood flow and vascular permeability to allow influx of dendritic cells and macrophages, and activating neutrophils to degranulate and release cytotoxic oxygen radicals. If the stimulating factor is great, a cascade is initiated which leads to sepsis and MODS. Superoxide radicals and other circulating factors then damage cells elsewhere, causing widespread vasodilatation and increased vascular permeability leading to hypotension and circulatory collapse. The myocardium is depressed and cellular metabolic functions are disrupted.
Endogenous sources of infection: Large bowel is a reservoir for bacteria and endotoxin which are normally safely contained. If the intestinal barrier is breached by splanchnic ischaemia, impoverished luminal nutrition of enterocytes or altered intestinal flora, translocation of bacteria into the portal circulation can occur in as little as 30 minutes. If the liver Kupffer cells are also impaired, intestinal bacteria and endotoxin are not prevented from reaching the systemic circulation in the normal way. This may explain the potential for renal failure in jaundiced patients undergoing operation (hepatorenal syndrome). This endogenous source probably explains the 30% of patients who suffer organ dysfunction without an obvious source of infection. Typically, such patients become affected after prolonged hypotension (hypovolaemic or cardiogenic shock) or hypoxaemia (e.g. multiple trauma victims), or as a result of direct visceral ischaemia (e.g. prolonged aortic clamping and hypotension in a patient with a ruptured aortic aneurysm).
Surgical aspects: In surgical patients, multiple organ dysfunction often results from a complication such as a bowel anastomotic leak or from severe acute pancreatitis (which may be sterile but becomes devastating if infected). Tissue necrosis following trauma, or death of an ischaemic limb may also precipitate the syndrome.
Other preventive factors in at-risk patients: Adequate and early fluid resuscitation is vital in patients with hypovolaemia, including in trauma victims, acute pancreatitis or bowel obstruction, because the loss of intravascular volume leads to deficient tissue perfusion (i.e. shock) and splanchnic ischaemia. Maintaining tissue oxygenation is also vital; at-risk patients must have arterial blood gases and pH estimated and receive supplemental oxygen or assisted ventilation as required. To help prevent intestinal bacterial translocation, enterocytes and colonocytes are best supported by enteral feeding, if necessary by a feeding jejunostomy or a fine-bore nasogastric tube. Glutamine, arginine and omega-3 fatty acids are believed to be important.