Chapter 55 Graft Remodeling and Ligamentization After Anterior Cruciate Ligament Reconstruction
Several studies have analyzed the various changes that occur during graft healing.1–24 Two main sites of healing exist, which should be separately assessed because their biological processes vary substantially: the intraarticular graft remodeling, often referred to as ligamentization, and the intratunnel graft incorporation, which develops either by bone–bone or tendon–bone healing.
In the beginning of the 20th century, Wilhelm Roux described the “law of functional adaptation,” elucidating on the fact that “an organ will adapt itself structurally to an alteration, quantitative or qualitative in function,”25 laying groundwork for later research on ligamentization. He observed that soft tissue structures such as ligaments and tendons undergo specific changes in their mechanical and biological properties when they are exposed to a different mechanical loading and biological environment. Amiel et al were among the first authors1,26 to analyze the specific functional adaptation of an ACL replacement graft and postulate the term ligamentization. They found a continuous development of a patellar tendon graft with biological and mechanical properties different from the ACL into a structure that closely resembled these properties of the intact ACL. They showed that the patellar tendon underwent several phases of remodeling: an early phase with central graft necrosis and hypocellularity and no detectable revascularization of the graft tissue. This was followed by a phase of proliferation—the time of most intensive remodeling and revascularization—and finally a ligamentization phase that provided characteristic restructuring of the graft toward the properties of the intact ACL. Amiel described this process as a transformation, not a restoration, of the native ACL because characteristic differences remained compared with its replacement grafts. This study laid the foundation for increased research efforts to improve the understanding of the basic science of intraarticular ACL graft healing or ligamentization. With the evolution of ACL reconstruction techniques to graft fixation in bone tunnels, it was not until the beginning of the past decade that the first studies were published on the biological processes during osseous graft incorporation.* It was recognized that the combined healing of the intraarticular remodeling and the intraosseous graft incorporation was dictating the mechanical function of the joint after ACL reconstruction.
Early Graft-Healing Phase
In comparison to studies of the subsequent proliferation and ligamentization phases, significantly less studies exist with analyses of the biological events of this early graft healing phase. Most authors agree, using different in vivo animal models,1,2,18,30,31 that this time period is marked by increasing graft necrosis, mainly in its center, and hypocellularity. Ultrastructural cell changes such as mitochondrial swelling, dilatation of the endoplasmatic retinaculum, and intracytoplasmatic deposition of lipids, as well as macroscopic swelling and increased cross-sectional area, illustrate the increasing graft necrosis and degradation.30 During this time, no graft revascularization can be observed.2,9,32,33 The graft necrosis leads to a release of a number of cytokines, such as tumor necrosis factor (TNF)–a, interleukin (IL)–1ß, and IL-6, in addition to chemokines that trigger a cascade of growth factors expression, which in turn result in cell migration and proliferation as well as extracellular matrix synthesis and revascularization.11,34 This remodeling activity becomes more pronounced during the latter proliferative phase. However, already between the first and second weeks, an influx of cells can be seen into the graft’s periphery.30,31 Kleiner et al9 and later Yoshikawa et al33 were able to demonstrate that these cells were originating from tissue other than the graft itself and that all original graft cells were completely replaced by 2 to 4 weeks. They hypothesized that the source of cells was either the synovial fluid, cells from the stump of the native ACL, or bone marrow elements originating from drilling maneuvers. Therefore Arnoczky2 suggested that preservation of the ACL stump and the Hoffa fat pad might be beneficial, especially for the early healing period.
During the first postoperative weeks, the graft’s overall collagen structure and its crimp pattern are still maintained,1 even though the beginning disintegration of the collagen fibrils and their orientation can be observed as early as 3 weeks after reconstruction.30,35 This explains the slow decrease in the mechanical properties of the graft at this early healing phase.4,15,20
Very little is known about the healing processes in the osseous tunnels, which will be described in more detail in the following chapter of this book. In summary, only little graft incorporation can be seen during this early stage of healing, such that the mechanical properties of the freshly ACL reconstructed knee joint are primarily relying on the mechanical fixation of the graft. Biomechanical testing of intraarticular ACL reconstructions between 2 and 4 weeks4,5,13,20 shows consistent failure by graft pullout from the tunnel, indicating insufficient anchorage of the graft to the tunnel wall. The mechanical strength of the ACL reconstruction at this time is significantly lower than that at the time of implantation. However, it continues to decrease until around 6 weeks, when a further increase in graft remodeling activity can be found and the failure site shifts to the intraarticular graft region.4,15,20
The decrease in mechanical strength might lead to the conclusion that early graft loading (i.e., immediate loading of the freshly reconstructed knee joint) should be avoided. However, several studies have pointed out the importance of adequate mechanical loading for the healing graft. Ohno et al36 stress-deprived the patellar tendon in vivo and found a significant loss of tensile strength as early as 1 week with further deterioration until 6 weeks of healing. This loss in tensile strength was accompanied by splitting and defragmentation of collagen bundles as early as 2 weeks. Similar findings were reported by Majima et al,37 who examined differences in complete and partial stress-shielding of a soft tissue graft, detecting a significantly higher loss in tensile strength from the first to third week of healing for the complete stress-shielded group. In another study38 the authors explained this observation with ultrastructural changes in the collagen composition that shifted to small-diameter fibrils, which were shown to provide less mechanical strength than the large-diameter fibrils found in the intact ACL.39,40 However, overloading of the graft can also lead to impaired graft healing. Tohyama and Yasuda showed in their model using an in situ frozen patellar tendon that a reduction of the cross-sectional area of the tendon by half (thereby doubling the tendon stress during loading) resulted in substantially reduced tensile strength as early as 3 weeks, contrary to only a slight increase in tendon stress (when the cross-sectional area was reduced by only one-third), which did not significantly impair the mechanical strength.41
Proliferation Phase of Graft Healing
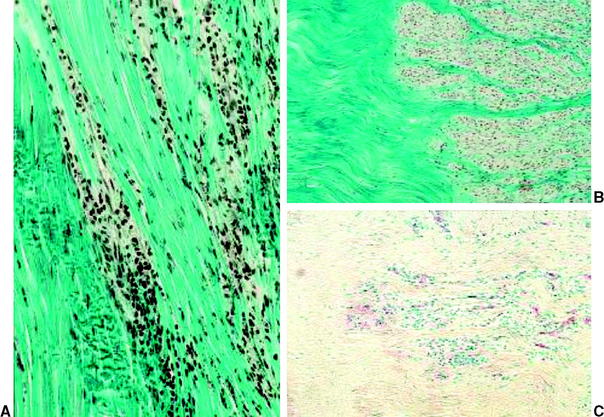
During this phase, cellularity constantly increases and substantially surpasses that of the intact ACL, as was observed in various in vivo animal models.3,8,18,20,42 Cell clusters are found at the perimeter of the graft around 6 weeks, with large acellular areas remaining in the graft’s center (Fig. 55-1). These hypercellular regions were shown to consist of mesenchymal stem cells18 and activated fibroblasts11 that are actively secreting several growth factors such as basic fibroblast growth factor (bFGF), TGF-ß1, and isoforms of platelet-derived growth factor (PDGF) to initiate and maintain graft remodeling. Kuroda et al11 found that the release of these growth factors peaks between the third and sixth week and almost completely ceases at 12 weeks of healing, which lends further explanation for the maximum remodeling activity during this proliferation phase. A more even distribution of cells throughout the graft slowly develops thereafter. Cell numbers are still increased but recede toward the intact ACL cellularity at the end of the proliferation phase.17,20 An increased number of specific fibroblasts, so-called myofibroblasts, are also found during this healing phase.43,44 These fibroblasts have the ability to exert isometric tension on the surrounding cellular and extracellular matrix. In the intact ACL they seem to be responsible for the crimping structure of the collagen fibers.45 These contractile fibroblasts are progressively expressed during the first three postoperative months17,44 in the healing ACL graft, when they seem to be responsible for the restoration of the in situ tension that is required for the later ligamentization process.
At the same time of increased cellular proliferation and intense revascularization of the graft tissue, Yoshikawa et al24 found upregulated expression of vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis, already at 2 to 3 weeks postreconstruction, which is triggered by hypoxia during the avascular necrosis of the early healing phase (Fig. 55-2).47 However, they did not find a significant increase in vascular outgrowth before the fourth and eighth week, confirming the descriptive findings of other previously published studies. Petersen et al14 and Unterhauser et al42 independently showed that revascularization progresses from the periphery of the graft toward the entire graft diameter at the end of the proliferation phase around 12 weeks of healing (see Fig. 55-2). Vascular density then returns to values of the intact ACL during the phase of ligamentization by 6 months.14,42 It is assumed that this intense revascularization triggers and retains the maximal remodeling activity. It has been a matter of debate whether such increased revascularization is beneficial to the healing of the graft. Recent studies found that upregulation of revascularization (e.g., by exogenous application of VEGF) enhanced cellular infiltration and fibroblast expression during the proliferation phase of healing, but this also included a significant deterioration of the graft’s mechanical properties.33 Weiler et al were able to relate the vascularity of the healing ACL graft in sheep to its mechanical properties using gadolinium-enhanced magnetic resonance imaging (MRI).23 They found that the time of maximal revascularization coincides with the lowest mechanical properties of the healing graft tissue, which was seen around 6 weeks. Tohyama and Yasuda were able to show that increased remodeling activity in terms of extracellular infiltration and revascularization was directly related to the decline in the graft’s mechanical properties.19 These findings support the reports of numerous other studies that all found the mechanical properties to be at their minimum around the proliferation phase of healing at 6 to 8 weeks.*. Graft failure at this time point occurs either by midsubstance tear20 or graft pullout due to stripping of the graft tissue out of its bone tunnels.3,13 This illustrates that the graft tissue has become the weak link in the reconstruction compared with the graft–bone interface (due to the lack of graft incorporation) during the early healing phase. Another factor that has been identified to play a role in the reduced mechanical properties is the loss of regular collagen orientation and crimp pattern, which has progressed since the early healing phase. It is not until the ligamentization phase that a slow restoration of the collagen orientation and crimp pattern progresses1,8,17,20 (Fig. 55-3). At the beginning of the proliferation phase, a significant decrease in collagen fibril density is demonstrated, which is followed by increased collagen synthesis48 and a subsequent return to values of the intact ACL at 12 weeks, as shown in an electron microscopy study by Weiler et al.20 During this time of new collagen formation, a shift can be observed from large-diameter collagen fibrils, which are dominant in the intact ACL or patellar or hamstring tendon graft, to small-diameter fibrils.9,20,49,50 It has been hypothesized that this shift in collagen diameter and the increased expression of collagen type III in the healing graft51 might explain why a full restoration of the mechanical strength of the intact ACL has not been observed even after 2 years of healing.
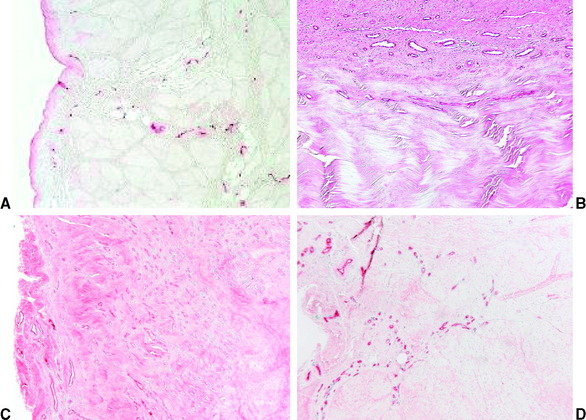
Fig. 55-2 Revascularization during graft healing.17 A, Intact anterior cruciate ligament (ACL); B, 6 weeks; C, 12 weeks. D, 52 weeks.
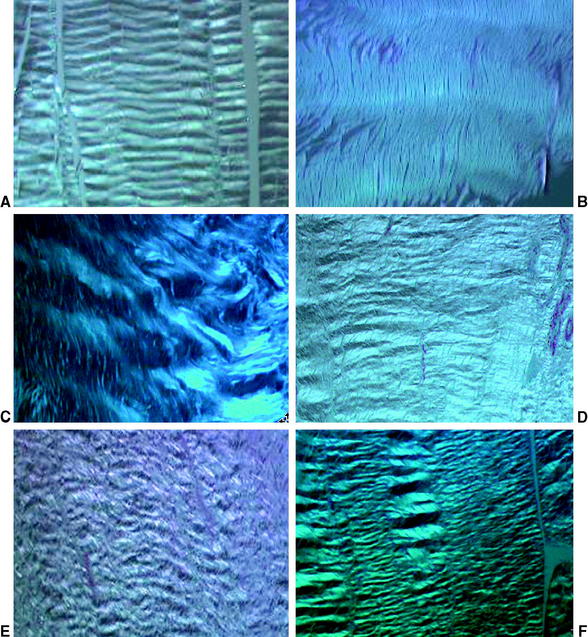
Fig. 55-3 Change in collagen crimp during graft healing (polarized light microscopy ×200; sheep model17,44). A, Intact anterior cruciate ligament (ACL); B, flexor tendon graft; C, 6 weeks; D, 12 weeks; E, 24 weeks; F, 52 weeks.
Although a substantial deterioration of the mechanical properties of the healing graft during the proliferation phase has been reported in animal models, clinical outcomes after ACL reconstruction with immediate aggressive rehabilitation have been more successful. Several human biopsy studies found significant differences between the remodeling activity of human ACL grafts during the first 3 months and the healing graft in animal models. Although the previously described healing phases of animal models (graft necrosis, recellularization, revascularization) are also found in human ACL graft biopsies,16,52 the remodeling activity of human ACL grafts seems to be reduced. The complete replacement of all intrinsic graft cells by extrinsic cells, as in animal models, has not been shown in the human healing ACL graft.16,52 Rougraff and Shelbourne16 found viable intrinsic graft cells in human biopsy specimens at all time points between 3 and 8 weeks after ACL reconstruction. Also, the excessive graft necrosis observed in animal studies could not be found in humans, in whom necrosis or degeneration never involved more than 30% of the graft’s biopsies. Large areas of the human ACL graft seem to stay unchanged, displaying tendinous structure with normal collagen alignment and crimp pattern.52 These areas were histologically identical to the native patellar tendon, suggesting survival of portions of the original graft. Neovascularization was also found but did not seem to be as excessive as in the animal model.52 Loss of collagen organization was only detected in areas of neovascularization in human biopsies, which corresponds with the findings in animal models. These findings might explain why early loading and aggressive rehabilitation during the first 3 postoperative months after human ACL reconstruction did not result in a significant increase in failure rates. However, human biopsy studies confirm the remodeling cascade of (limited) graft necrosis, recellularization, revascularization, and changes in collagen crimp and composition during the early healing and proliferation phases, also suggesting that the human ACL graft might have its lowest mechanical strength around 6 to 8 weeks postoperatively. The most appropriate loading for optimization of this phase of graft healing will have to be determined. It must be high enough to stimulate graft cells to produce cellular and extracellular components for preservation of graft stability without compromising graft integrity, which might result in early stretch-out of the ACL reconstruction.
Ligamentization Phase of Graft Healing
It has been shown in animal studies that cellularity slowly returns to values of the intact ACL between 3 and 6 months after reconstruction.17,23,42,46 The typical ovoid shape of metabolically active fibroblasts with its increased cytoplasm/nucleus ratio of the proliferation phase slowly changes into the less metabolically active shape of linear, spindle-like fusiform cells that are normally seen in the intact ACL. Vascularity throughout the graft decreases and returns to values of the intact ACL, and vessels become evenly distributed throughout the entire graft between 6 and 12 months2,17,23,24,42,46 (see Fig. 55-3). It has also been shown in rabbit, dog, and sheep models1,21,23,53 for certain extracellular matrix proteins such as glycosaminoglycans and collagen cross-links that the healing graft undergoes a transformation from its initial tissue properties (e.g., a patellar tendon of free soft tissue tendon graft) to properties of the intact ACL during this ligamentization phase1,53 as early as 6 months. Although certain biological features of the healing graft have been reported to return to the morphology of the intact ACL, several differences remain, especially regarding the extracellular matrix. Collagen fibers regain their organization into fascicles after complete loss of alignment and initial dense packaging during the ligamentization phase, which microscopically resembles the appearance of the intact ACL around 6 to 12 months after reconstruction.17,44 However, their initial loss in collagen crimp and strict parallel alignment during the proliferation phase is only partially restored. A regular crimp of the collagen fibers can be seen as early as 6 months, but even after 2 years its frequency stays increased compared with the intact ACL, as shown in sheep.17,44 The change from a bimodal distribution of small and dominating large collagen fibers of the patellar or hamstring tendon graft to a unimodal pattern of only small collagen fibers of the healing graft does not change during the phase of ligamentization8,20,28 (Fig. 55-4). The heterogenous composition of collagen fibers of varying diameter of the intact ACL is never restored.54 The increased synthesis of collagen type III of the proliferation phase decreases during the ligamentization phase but continues to be sustained in significantly higher concentrations than in the intact ACL even at 2 years.30,55 Ng et al found in a dog model of ACL reconstruction that type III collagen also remained increased in the remodeling graft at 1 year but returned to values of the intact ACL by 3 years, suggesting that the ligamentization process might continue longer than previously expected.53 Type III collagen is normally found in scar or early ligamentous repair tissue and has a lower mechanical strength than type I collagen. The findings of persistent small-diameter collagen fibrils and increased type III collagen content are therefore especially important to understand why all animal models demonstrated significantly lower mechanical properties of the healing graft than that of the intact ACL even after long-term healing of up to 2 years.* It has been shown that the mechanical properties of ACL reconstructed knee joints improve substantially during the phase of ligamentization and reach their final maximal properties at around 1 year. But until now there has not been a single animal study demonstrating that the structural properties (e.g., failure load, stiffness) of the healing graft could surpass 50% to 60% of the intact ACL.† Some studies were able to show that these compromised mechanical properties would still allow for restoration of anteroposterior (AP) laxity to the laxity of the contralateral intact ACL,22 but others observed significant lower AP laxity even 3 years after reconstruction.56 In summary, in animal models overall restoration of graft integrity and histological appearance is completed between 6 and 12 months of healing, acquiring morphology similar to that of the intact ACL. This is also substantiated by the mechanical properties that reach their maximal strength around 12 months without any further significant changes thereafter. However, characteristic differences, especially in extracellular matrix composition, remain, and the initial mechanical strength of the intact ACL is not restored.
Although human biopsy studies showed substantial differences from animal models for the proliferation phase, the ligamentization phase seems to be rather similar in both models in terms of biological progression. However, the timeline of these biological changes appears to be different in human versus animal models. Rougraff et al57 analyzed 23 biopsies of human patellar tendon ACL reconstruction between 3 weeks and 6.5 years postoperatively. They found that necrosis took place in much smaller areas of the graft at 3- and 6-week biopsies than was shown in animal models. However, they found overall degeneration (albeit limited compared with animal models) to increase until 6 to 10 months and only slowly disappear between 1 and 3 years postoperatively. Neovascularity and hypercellularity only slowly appeared and carried on until 10 months, which differs from observations in animal models. Some nonbiopsy studies that evaluated graft revascularization using gadolinium-enhanced MRI during the course of healing for 2 years6 could not detect any revascularization except from the peri-ligamentous ACL graft tissue, which is in contrast to the findings of Weiler et al,23 who analyzed sheep ACL reconstruction (also using gadolinium-enhanced MRI) and could detect significantly upregulated neovascularization during the first 3 postoperative months. This underlines the differences in remodeling activity between humans and animal models, even though all human biopsy studies have shown that neovascularization does occur but that the extent of vascularity might be below the threshold detectable with gadolinium-enhanced MRI. Overall, Rougraff et al57 concluded that the proliferation phase seemed to be delayed compared with animal models, with the highest remodeling activity occurring between 3 and 10 months. Identical findings were made by Falconiero et al58 using patellar tendon and hamstring tendon ACL reconstruction. They found that hypercellularity and hypervascularity had not returned to control intact ACL values before 6 to 12 months, with fiber alignment being restored around 6 months. No details are given on ultrastructural differences between the healing graft and the intact ACL in this study. Full histological maturity was not found prior to 12 months of healing. Other studies54,57 even found increased cell counts and differing fiber alignment beyond 3 years, with graft being indistinguishable from the intact ACL as late as 3-year biopsies. Human biopsy studies that analyzed changes of the extracellular matrix observed changes that are in line with the findings of animal models. Marumo et al59 found that the collagen cross-links (dihydroxylysinonorleucine/hydroxylysinonorleucine ratios) of patellar tendon and hamstring tendon autografts had changed from time zero, when they were significantly different from the intact ACL, to 1 year postoperatively, when both grafts had acquired cross-link ratios that were identical to the intact ACL, confirming the ligamentization process found in animal models. Interestingly, biopsy specimens taken at 6 months still showed significantly different cross-link ratios of the healing grafts compared with the intact ACL, which is different from the earlier cross-link restoration found in animal models. This also confirms the differing timeline of the remodeling of human ACL grafts. Regarding collagen remodeling, Cho et al60 and Abe et al54 confirmed the findings of Weiler et al20 and others8,28 that patellar tendon54 and hamstring tendon60 ACL grafts showed a replacement of large-diameter fibrils by small-diameter fibrils, which did not change even after more than 2 years after reconstruction, confirming the observations made in animal models.
1 Amiel D, Kleiner JB, Roux RD, et al. The phenomenon of “ligamentization”: anterior cruciate ligament reconstruction with autogenous patellar tendon. J Orthop Res. 1986;4:162-172.
2 Arnoczky SP, Tarvin GB, Marshall JL. Anterior cruciate ligament replacement using patellar tendon. An evaluation of graft revascularization in the dog. J Bone Joint Surg. 1982;64A:217-224.
3 Ballock RT, Woo SL, Lyon RM, et al. Use of patellar tendon autograft for anterior cruciate ligament reconstruction in the rabbit: a long-term histologic and biomechanical study. J Orthop Res. 1989;7:474-485.
4 Goradia VK, Rochat MC, Grana WA, et al. Tendon-to-bone healing of a semitendinosus tendon autograft used for ACL reconstruction in a sheep model. Am J Knee Surg. 2000;13:143-151.
5 Grana WA, Egle DM, Mahnken R, et al. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344-351.
6 Howell SM, Knox KE, Farley TE, Taylor MA. Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med. 1995;23:42-49.
7 Itoh S, Muneta T, Shinomiya K, et al. Electron microscopic evaluation of the effects of stress-shielding on maturation of the mid-substance and ligament-bone junction of the reconstructed anterior cruciate ligament in rabbits. J Mater Sci Mater Med. 1999;10:185-190.
8 Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21:176-185.
9 Kleiner JB, Amiel D, Harwood FL, et al. Early histologic, metabolic, and vascular assessment of anterior cruciate ligament autografts. J Orthop Res. 1989;7:235-242.
10 Kobayashi M, Watanabe N, Oshima Y, et al. The fate of host and graft cells in early healing of bone tunnel after tendon graft. Am J Sports Med. 2005;33:1892-1897.
11 Kuroda R, Kurosaka M, Yoshiya S, et al. Localization of growth factors in the reconstructed anterior cruciate ligament: immunohistological study in dogs. Knee Surg Sports Traumatol Arthrosc. 2000;8:120-126.
12 Liu SH, Panossian V, al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997;339:253-260.
13 Papageorgiou CD, Ma CB, Abramowitch SD, et al. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sports Med. 2001;29:620-626.
14 Petersen W, Unterhauser F, Pufe T, et al. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch Orthop Trauma Surg. 2003;123:168-174.
15 Rodeo SA, Arnoczky SP, Torzilli PA, et al. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg. 1993;75:1795-1803.
16 Rougraff BT, Shelbourne KD. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1999;7:9-14.
17 Scheffler SU, Dustmann M, Gangéy I, et al. The biological healing and restoration of the mechanical properties of free soft-tissue allografts lag behind autologous ACL reconstruction in the sheep model. Trans Orthop Res. 2005. abstract #0236, Washington, DC
18 Shino K, Kawasaki T, Hirose H, et al. Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg. 1984;66B:672-681.
19 Tohyama H, Yasuda K. Extrinsic cell infiltration and revascularization accelerate mechanical deterioration of the patellar tendon after fibroblast necrosis. J Biomech Eng. 2000;122:594-599.
20 Weiler A, Forster C, Hunt P, et al. The influence of locally applied platelet-derived growth factor-BB on free tendon graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:881-891.
21 Weiler A, Hoffmann RF, Bail HJ, et al. Tendon healing in a bone tunnel. Part II: histologic analysis after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:124-135.
22 Weiler A, Peine R, Pashmineh-Azar A, et al. Tendon healing in a bone tunnel. Part I: biomechanical results after biodegradable interference fit fixation in a model of anterior cruciate ligament reconstruction in sheep. Arthroscopy. 2002;18:113-123.
23 Weiler A, Peters G, Maurer J, et al. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. A two-year study in sheep. Am J Sports Med. 2001;29:751-761.
24 Yoshikawa T, Tohyama H, Enomoto H, et al. Temporal changes in relationships between fibroblast repopulation, VEGF expression and angiogenesis in the patellar tendon graft after anterior cruciate ligament reconstruction. Trans Orthop Res. 2004. abstract #0236, San Francisco
25 Roux W. Die Entwicklungsmechanik; ein neuer Zweig der biologischen Wissenschaft. Leipzig: Wilhelm Engelmann, 1905.
26 Amiel D, Frank C, Harwood F, et al. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1:257-265.
27 Blickenstaff KR, Grana WA, Egle D. Analysis of a semitendinosus autograft in a rabbit model. Am J Sports Med. 1997;25:554-559.
28 Liu SH, Yang RS, al-Shaikh R, et al. Collagen in tendon, ligament, and bone healing. A current review. Clin Orthop Relat Res. 1995;318:265-278.
29 Tomita F, Yasuda K, Mikami S, et al. Comparisons of intraosseous graft healing between the doubled flexor tendon graft and the bone-patellar tendon-bone graft in anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:461-476.
30 Bosch U, Kasperczyk WJ. Healing of the patellar tendon autograft after posterior cruciate ligament reconstruction–a process of ligamentization? An experimental study in a sheep model. Am J Sports Med. 1992;20:558-566.
31 Kleiner JB, Amiel D, Roux RD, et al. Origin of replacement cells for the anterior cruciate ligament autograft. J Orthop Res. 1986;4:466-474.
32 Shino K, Horibe S. Experimental ligament reconstruction by allogenic tendon graft in a canine model. Acta Orthop Belg. 1991;57:44-53.
33 Yoshikawa T, Tohyama H, Katsura T, et al. Local administration of VEGF enhances mechanical deterioration of the tendon grafted to reconstruct the ACL, although it accelerates angionesis and cellularity and infiltration: a sheep model study. Trans Orthop Res. 2005. abstract #0320, Washington, DC
34 Kawamura S, Ying L, Kim HJ, et al. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425-1432.
35 Goradia VK, Rochat MC, Kida M, et al. Natural history of a hamstring tendon autograft used for anterior cruciate ligament reconstruction in a sheep model. Am J Sports Med. 2000;28:40-46.
36 Ohno K, Yasuda K, Yamamoto N, et al. Effects of complete stress-shielding on the mechanical properties and histology of in situ frozen patellar tendon. J Orthop Res. 1993;11:592-602.
37 Majima T, Yasuda K, Yamamoto N, et al. Deterioration of mechanical properties of the autograft in controlled stress-shielded augmentation procedures. An experimental study with rabbit patellar tendon. Am J Sports Med. 1994;22:821-829.
38 Majima T, Yasuda K, Tsuchida T, et al. Stress shielding of patellar tendon: effect on small-diameter collagen fibrils in a rabbit model. J Orthop Sci. 2003;8:836-841.
39 Flint MH, Craig AS, Reilly HC, et al. Collagen fibril diameters and glycosaminoglycan content of skins–indices of tissue maturity and function. Connect Tissue Res. 1984;13:69-81.
40 Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc Roy Soc Lond B Biol Sci. 1978;203:305-321.
41 Tohyama H, Yasuda K. The effect of increased stress on the patellar tendon. J Bone Joint Surg. 2002;84B:440-446.
42 Unterhauser FN, Bail HJ, Hoher J, et al. Endoligamentous revascularization of an anterior cruciate ligament graft. Clin Orthop Relat Res. 2003;414:276-288.
43 Unterhauser FN, Bosch U, Zeichen J, et al. Alpha-smooth muscle actin containing contractile fibroblastic cells in human knee arthrofibrosis tissue. Winner of the AGA-DonJoy Award 2003. Arch Orthop Trauma Surg. 2004;124:585-591.
44 Weiler A, Unterhauser FN, Bail HJ, et al. Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res. 2002;20:310-317.
45 Murray MM, Martin SD, Martin TL, et al. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg. 2000;82A:1387-1397.
46 Clancy WGJr, Narechania RG, Rosenberg TD, et al. Anterior and posterior cruciate ligament reconstruction in rhesus monkeys. J Bone Joint Surg. 1981;63A:1270-1284.
47 Jackson JR, Minton JA, Ho ML, et al. Expression of vascular endothelial growth factor in synovial fibroblasts is induced by hypoxia and interleukin 1beta. J Rheumatol. 1997;24:1253-1259.
48 Spindler KP, Andrish JT, Miller RR, et al. Distribution of cellular repopulation and collagen synthesis in a canine anterior cruciate ligament autograft. J Orthop Res. 1996;14:384-389.
49 Jackson DW, Grood ES, Cohn BT, et al. The effects of in situ freezing on the anterior cruciate ligament. An experimental study in goats. J Bone Joint Surg. 1991;73:201-213.
50 Tsuchida T, Yasuda K, Kaneda K, et al. Effects of in situ freezing and stress-shielding on the ultrastructure of rabbit patellar tendons. J Orthop Res. 1997;15:904-910.
51 Bosch U, Kasperczyk WJ, Oestern HJ, et al. The patellar tendon graft for PCL reconstruction. Morphological aspects in a sheep model. Acta Orthop Belg. 1994;60:57-61.
52 Johnson LL. The outcome of a free autogenous semitendinosus tendon graft in human anterior cruciate reconstructive surgery: a histological study. Arthroscopy. 1993;9:131-142.
53 Ng GY, Oakes BW, Deacon OW, et al. Long-term study of the biochemistry and biomechanics of anterior cruciate ligament-patellar tendon autografts in goats. J Orthop Res. 1996;14:851-856.
54 Abe S, Kurosaka M, Iguchi T, et al. Light and electron microscopic study of remodeling and maturation process in autogenous graft for anterior cruciate ligament reconstruction. Arthroscopy. 1993;9:394-405.
55 Petersen W, Laprell H. Insertion of autologous tendon grafts to the bone: a histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc. 2000;8:26-31.
56 Ng GY, Oakes BW, Deacon OW, et al. Biomechanics of patellar tendon autograft for reconstruction of the anterior cruciate ligament in the goat: three-year study. J Orthop Res. 1995;13:602-608.
57 Rougraff B, Shelbourne KD, Gerth PK, et al. Arthroscopic and histologic analysis of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:277-284.
58 Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197-205.
59 Marumo K, Saito M, Yamagishi T, et al. The “ligamentization” process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: a biochemical study. Am J Sports Med. 2005;33:1166-1173.
60 Cho S, Muneta T, Ito S, et al. Electron microscopic evaluation of two-bundle anatomically reconstructed anterior cruciate ligament graft. J Orthop Sci. 2004;9:296-301.