33 Cancer of the Nasal Cavity and Paranasal Sinuses
Epidemiology, Etiology, and Pathogenesis
Malignancies arising from the nasal cavity and paranasal sinuses are relatively rare tumors of the head and neck. They account for only 3% of all upper respiratory tract cancers, with a yearly incidence of 1 per 100,000 people.1 Because of their rarity, these sites are grouped together in most published reports. It is often difficult to determine the exact site of origin, because most of these tumors present at an advanced stage and extensively involve adjacent sites. Among the tumors arising in this anatomic region, 60% to 90% involve the paranasal sinuses, the majority being in the maxillary antrum. There is a 2 : 1 male predominance for these tumors.2,3 Most patients with carcinomas arising in the sinonasal region are older than 40 years of age.2,4 Esthesioneuroblastoma may occur in much younger patients as well.
Unlike other upper and lower respiratory tract carcinomas, nasal cavity and paranasal sinus cancers have not been associated with cigarette smoking.5 Chronic sinusitis, although frequently coexistent with malignant tumors in this region, is not a causative agent.6 However, an increased risk of adenocarcinoma of the nasal cavity and ethmoid sinus has been associated with wood dust exposure.7–9 A meta-analysis of 11 published studies of men with wood-related occupations showed that the odds ratio for developing adenocarcinoma was 13.5, with the risk correlative with the quantity and duration of exposure.10 An increased risk (odds ratio 2.4) of developing squamous cell carcinomas of the sinonasal region was seen only among those employed for 30 or more years in jobs with exposure to fresh wood. Other industrial risk groups include leather tanners11 and nickel refinery workers (250-fold risk for developing squamous cell carcinoma of the maxillary antrum12 and more than 40-fold risk for developing squamous cell carcinoma of the nasal cavity13). Thorotrast, a radioactive contrast medium used in the 1960s for radiographic studies of the maxillary sinus, is an established carcinogenic agent for maxillary sinus carcinoma.
Because of the relative rarity of sinonasal cancer, there are a lack of studies analyzing the underlying cytogenetic and molecular findings. In one of the few published reports, overexpression of p53 was found in 60% of sinonasal carcinomas, including 42 squamous cell carcinomas and 10 adenocarcinomas, in a Taiwanese study on Asian patients.14
Anatomy
Nasal Cavity
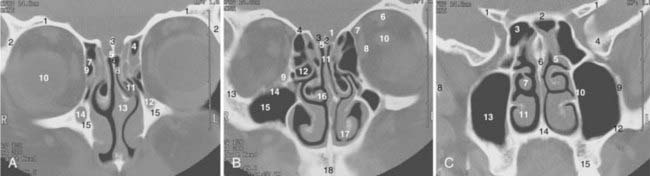
The coronal and transverse sections of the nasal cavity are illustrated in Fig. 33-1, Fig. 33-2, and Fig. 33-3. Anteriorly, the nasal cavity begins from the limen nasi, the line of transition from skin to mucous membrane. The nasopharynx is situated directly behind the nasal cavity and communicates with it by the posterior nasal aperture. Inferiorly, the floor is composed of the hard palate. Superiorly, the nasal cavity borders the base of the skull (frontal sinuses, cribriform plate of the ethmoid bone, and ethmoid air cells). The medial walls of the maxillary sinuses define the lateral extent of the nasal cavity. The midline septum divides the nasal cavity into two halves. Three turbinates (or conchae)—superior, middle, and inferior—protrude downward and medially from the lateral wall into the nasal cavity, forming three meatus. The superior turbinate is much smaller than the middle and inferior turbinates, and is situated directly in front of the sphenoidal sinus. The nasolacrimal duct drains into the nasal cavity below the inferior turbinate.
Maxillary Sinus
The maxillary sinus (also called the maxillary antrum) is a pyramidal cavity (see Figs. 33-1C, 33-2D, and 33-3A) of approximately 15 cm3 volume (3.7 × 2.5 × 3.0 cm). The base of the pyramid is composed of the medial wall, which separates the maxillary sinus from the nasal cavity, and the apex is in the zygomatic process. Superiorly, the floor of the orbit forms the roof of the antrum. Anteriorly, the facial wall is located behind the cheek and curves inward into the sinus. Posteriorly, the infratemporal wall borders infratemporal and pterygopalatine fossae. The floor of the maxillary sinus lies inferior to the floor of the nasal cavity, especially in edentulous patients.15 Secretion from the maxillary sinus is drained into the nasal cavity via openings in the middle meatus. The medial wall of the sinus, of all its confines, is the most complex. It forms the inferior aspect of the lateral wall of the nose. Contained within it is the nasolacrimal duct. The exit of this duct is approximately 1 cm from the pyriform rim. The ostium of the maxillary antrum is traditionally described emptying into the posterior aspect of the hiatus semilunaris. The roots of the second premolar and first two molars penetrate the bony floor of the maxillary sinus.
Ethmoid Sinuses
The ethmoid cells, collectively called a sinus, lie between the nasal cavity and the orbit (see Figs. 33-1B, 33-2C, and 33-3B). The air cells, like a honeycomb, have the thin orbital plate of the frontal bone of the anterior cranial fossa for a roof (fovea ethmoidalis). They are grouped into anterior, middle, and posterior air cells on each side. The anterior cell is closely related to the frontal sinus and connects to the nasal cavity via the middle meatus. The middle ethmoid cell makes a bulge into the lateral wall of the nasal cavity (bulla ethmoidalis) and also communicates with the middle meatus. The posterior ethmoid cells are closely related to the optic canal and nerve, and open into the superior meatus. These openings between the nasal cavity and the ethmoid cells are an obvious route of tumor extension. The fragile medial wall of the orbit, formed by the lamina papyracea of the ethmoid bone, is extremely porous and is an easy conduit for tumor spread from the ethmoid sinus into the orbit. The superior portion of the nasal septum separates the right and left ethmoid cells. Most anterior ethmoid air cells extend within 1 cm of the anterior skin surface between the medial canthi. The orbits are conical and the ethmoid sinuses expand posteriorly and inferiorly to form the medial walls of the orbit. The optic nerves lie at about the same level as the roof of the ethmoid cells.16 The floor of the orbit rises posteriorly; thus the orbital apex lies superior to the inferior rim of the orbit.
Sphenoidal Sinus
The sphenoidal sinus is an air cavity within the body of the sphenoid bone. It is a midline structure located anterior to the clivus, posterior to the superior meatus of the nasal cavity (see Figs. 33-2B, 33-2C, and 33-3C). The lateral sphenoid sinus wall has a series of bulges and grooves corresponding to a number of vital structures that traverse the cranial side of its lateral walls. The cavernous sinuses lie lateral to the sphenoidal sinus with all their vessels (internal carotid artery) and cranial nerves (II, III, IV, V1, V2). The pituitary fossa and the optic chiasm lie above and the nasopharynx is located below the sphenoidal sinus. The sphenoidal sinus can be very extensive and may extend laterally between the maxillary nerve and the nerve of the pterygoid canal, inflating the greater wing of the sphenoid bone and pterygoid process. The sphenoidal sinus opens into the nasal cavity via the sphenoethmoid recess.
Frontal Sinus
The pair of frontal sinuses, located within the frontal bone, is irregular in size and shape and often represents an extension of an anterior ethmoid cell (see Figs. 33-1A and 33-2A). The sinuses lie above the orbits. Lined with respiratory epithelium, the frontal sinus drains into the maxillary sinus via the frontonasal duct.
Pathologic Conditions
The most common benign tumors arising in the sinonasal region are inflammatory polyps and benign mixed minor salivary gland tumors. Other tumors are histologically benign but behave in a locally aggressive and destructive manner. These tumors include inverted papillomas and midline granulomas. Inverted papillomas arise from squamous or schneiderian epithelium and most often involve the lateral nasal wall. They may destroy bone and tend to recur if not excised completely. From 10% to 15% of inverted papillomas are associated with malignant squamous degeneration.17,18 Inverted papillomas are best treated with en bloc resection with medial maxillectomy (recurrence rate <10%).18,19 Midline granuloma syndrome describes a process of progressive midline facial destruction from various causes including an immunologic or rheumatoid process and lymphomatous proliferation. Often a definitive diagnosis cannot be made on the basis of a biopsy. If the biopsy suggests Wegener granulomatosis, the treatment consists of systemic steroids or cytotoxic drugs or both. If the biopsy suggests lymphomatosis or reticulosis, the patient should have a systemic workup for lymphoma and be treated with localized radiation if no other disease is found. Midline lethal granuloma is a diagnosis of exclusion and describes progressive, painful destruction of the nasal cavity, paranasal sinuses, and hard palate. Death may eventually result from massive hemorrhage or infection once the base of the skull is eroded. The treatment for this condition is radiation therapy.
Melanoma and olfactory neuroblastoma, also known as esthesioneuroblastoma, are rare epithelial malignancies arising in the nasal cavity. Esthesioneuroblastoma originates from olfactory nerves and is considered a neuroendocrine tumor. It occurs predominantly in young patients between 10 and 20 years old, although a second peak is observed in an older group between the ages of 50 and 60 years.20–22 Olfactory neuroblastomas have a wide spectrum of clinical behavior. Some are slow-growing and tend to be localized, whereas others may be highly aggressive with local destruction and spread as well as distant metastasis. The incidence of cervical nodal involvement is 20%. The most common presenting symptoms are epistaxis, nasal obstruction, and a loss of the sense of smell. Mucosal melanomas are most often found in the nasal cavity and can be primary or metastatic. Overall, less than 1% of melanomas arise from the sinonasal tract. Nasal melanomas can often be amelanotic and may require immunohistochemical and electron-microscopic examination for definitive histologic diagnosis. Much more so than the cutaneous melanomas, nasal melanomas have a high incidence of local recurrence,23,24 and the patient may benefit from postoperative radiation therapy.
Undifferentiated carcinomas have been reported to represent a distinctive, rare, and highly aggressive neoplasm. They are composed of small- and medium-sized cells and have to be distinguished from melanoma, lymphoma, olfactory neuroblastoma, rhabdomyosarcoma, neuroendocrine carcinoma, and poorly differentiated squamous cell carcinoma.25 They present at an advanced stage widely involving the nasal, maxillary, and ethmoid complexes. Orbital and intracranial extension is seen in the majority of cases. Prognosis is extremely poor, with 80% to 90% of patients dying within 1 year of extensive local and metastatic disease.26 The role of systemic chemotherapy as an adjunct to aggressive local therapy needs to be investigated.
Nonepithelial tumors include lymphoma, plasmacytoma, and sarcoma.
Clinical Presentation
Cervical lymph node metastases on initial presentation are uncommon; most large series report an incidence of less than 10% to 15%.27–29 Distant metastases on initial presentation are even less frequent, with a reported incidence of less than 5%.30
Routes of Spread
Perineural Spread
The sensory nerve supply of the maxillary, sphenoidal, and ethmoid sinuses; the nasal cavity and palate mucous membrane; the upper teeth and gums; and the adjacent facial skin extending from the lower lid to the upper lip, including the nasal vestibule, derives from the maxillary branch of the trigeminal nerve (cranial nerve V2). The anterior-superior alveolar branch of the infraorbital nerve runs in the facial wall of the maxillary sinus to the upper incisor and canine teeth. The posterior superior alveolar branch (dental nerve) pierces the infratemporal wall and supplies the mucosa of the maxillary antrum. The zygomatic nerve supplies sensory fibers to the lacrimal gland.31 Involvement of the nerve branches of the maxillary nerve by the tumor often leads to numbness and paresthesias in the skin and mucous membrane of this region.
Perineural extension into the central nervous system is more commonly associated with minor salivary gland tumors, especially with adenoid cystic carcinomas; however, it may occur also with other histologic types, especially in the setting of recurrence after surgery. Commonly involved nerves for perineural spread include olfactory nerves (from the cribriform plate into the anterior cranial fossa), the infraorbital nerve, and nerves that run through the superior orbital fissure (into the cavernous sinus or middle cranial fossa).32 Also commonly involved is the foramen rotundum, which transmits the maxillary nerve (cranial nerve V2) and carries sensory information from the lower eyelid and cheek into the trigeminal nucleus.
Diagnosis and Staging
Diagnosis
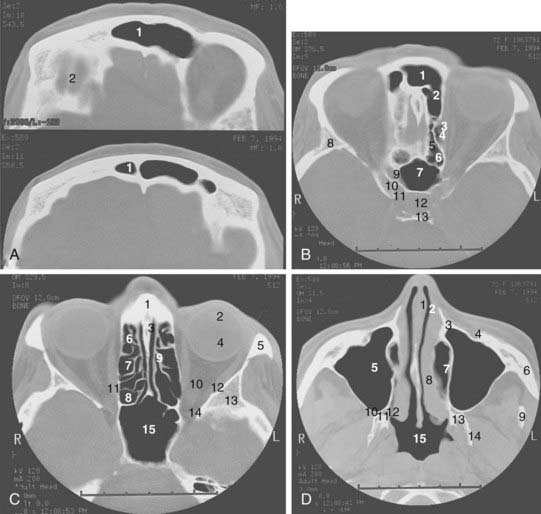
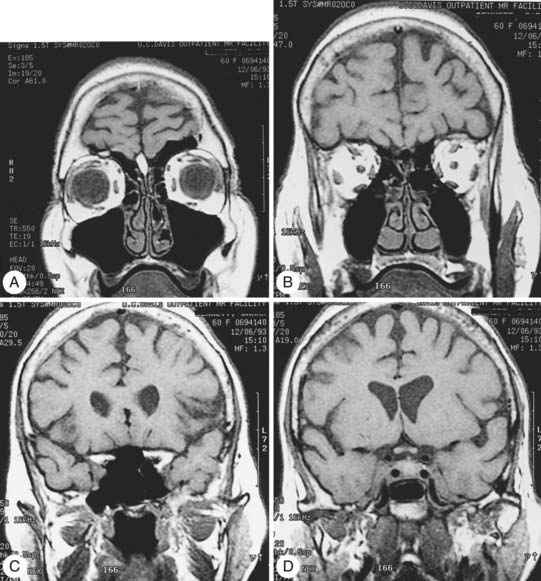
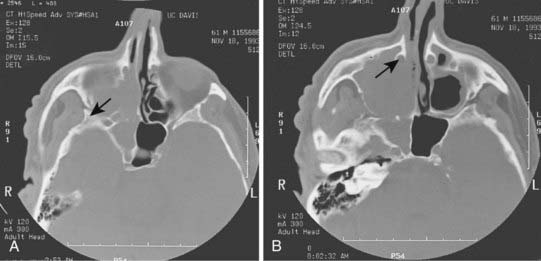
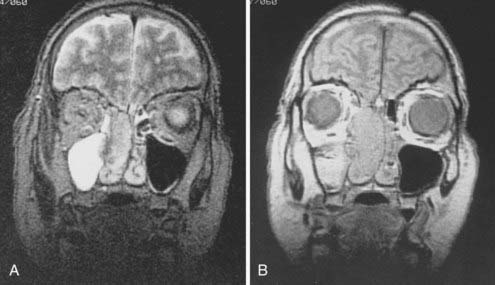
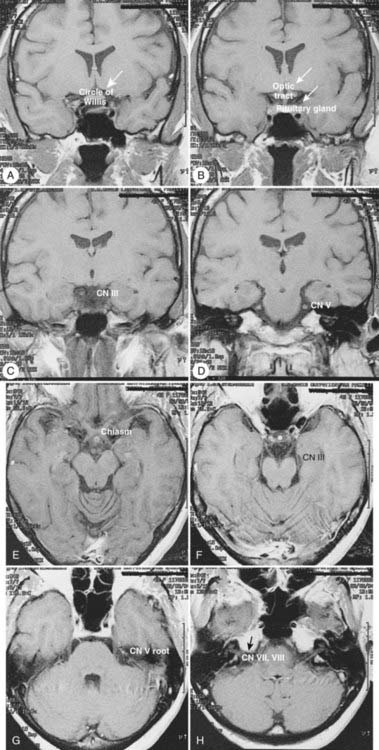
Radiologic evaluation is of paramount importance in the diagnosis and staging of nasal cavity and paranasal sinus tumors. Imaging has essentially replaced surgical exploration for staging and tumor mapping in this region. The most useful studies are computed tomography (CT) and magnetic resonance imaging (MRI). CT defines early cortical bone erosion more clearly (Fig. 33-4), whereas MRI better delineates soft tissue. MRI can also differentiate among opacification of the sinuses resulting from fluid, inflammation, or tumor (Fig. 33-5).32 CT performs better than MRI in evaluating thin bony structures, such as paranasal sinuses and orbita. MRI may demonstrate subtle perineural spread and involvement of the cranial nerve foramen and canals (Fig. 33-6 and Fig. 33-7).33 MRI is better than CT in evaluating intracranial or leptomeningeal spread. MRI is also more useful in assessing skull-base erosion. Sagittal and coronal MRI sections better visualize lesions involving the cribriform plate, basisphenoid, and floor of the middle cranial fossa.34 Thus, as a single modality, MRI may confer more information than CT.
Staging
The staging classification for the epithelial tumors of the nasal cavity and paranasal sinuses has been extensively revised in the sixth edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis staging system (Table 33-1).35 In addition to the maxillary sinus, the nasoethmoid complex has been added as a second site with two regions within the site: the nasal cavity and ethmoid sinuses. The nasal cavity is further divided into four subsites: septum, floor, lateral wall, and vestibule. The ethmoid sinus region is subdivided into two subsites: right and left. For the maxillary sinus, T4 lesions have been divided into T4a (resectable) and T4b (unresectable), leading to the division of stage IV into stages IVA, IVB, and IVC. No widely accepted staging classification exists for frontal and sphenoidal tumors, as they are rare.
Treatment
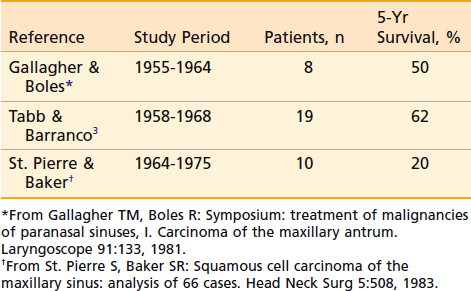
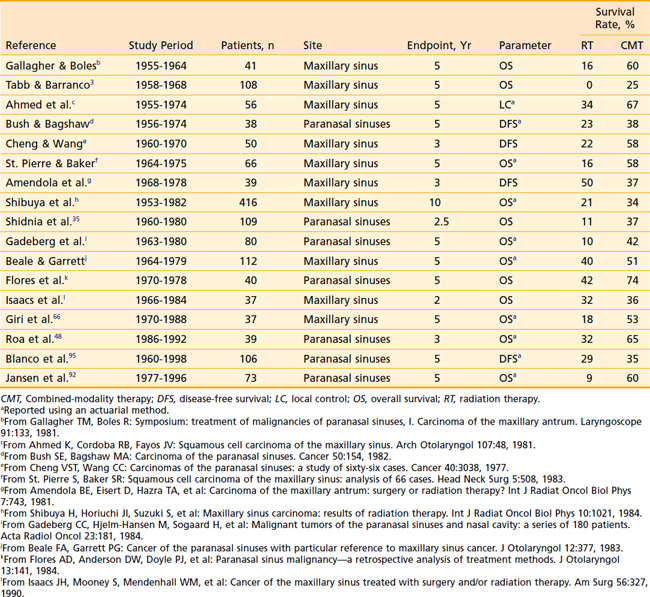
Although surgery alone or radiation therapy alone has been used with curative intent in the treatment of select nasal cavity or paranasal sinus carcinomas, most cases warrant combined-modality therapy (Table 33-2 and Table 33-3). In recent years, surgery followed by postoperative radiation therapy has been the mainstay of therapy for resectable lesions. Surgery is considered superior to radiation as a single modality for control of small lesions of the nasal septum or those limited to the infrastructure of the maxillary sinus.3 Although primary radiation therapy has a high cure rate for small squamous carcinomas of the nasal cavity, the potential for optic nerve injury from the high-dose radiation therapy required to achieve a good control rate must be considered. Massive tumors with extensive involvement of the nasopharynx, base of skull, sphenoidal sinuses, brain, or optic chiasm are considered unresectable. Some institutions have been studying the efficacy of combined radiation and radiosensitizing chemotherapy for unresectable squamous cell carcinoma of the nasal cavity and paranasal sinuses. Early results of this approach have been promising.36 If radiation therapy alone is to be used for large lesions, a hyperfractionated regimen may allow the delivery of higher doses than conventional radiation.
Table 33-2 Results of Treatment: Combined-Modality Therapy of Surgery and Radiation Therapy Compared With Definitive Radiation
Surgery
Surgical Procedures
The goal of surgery for nasal cavity and paranasal sinus tumors is to achieve en bloc resection of all involved bone and soft tissue with clear margins while maximizing the cosmetic and functional outcome. The extent and site of the incision depend on the location of the lesion. Limited nasal cavity lesions may be resected with medial maxillectomy. Ethmoid lesions usually require medial maxillectomy and en bloc ethmoidectomy. This is the most common surgical approach for inverted papillomas. The development of a combined craniofacial procedure for lesions involving the inferior surface of the cribriform plate and the roof of the ethmoid bone offers access to the anterior cranial fossa, orbit, and pterygopalatine fossa, and allows a rational oncologic resection, depending on anatomic considerations. In addition, this approach results in excellent cosmesis and improvement in the cure of lesions associated with extremely poor prognosis otherwise.37 The bony defect in the anterior cranial floor is closed with a vascularized pericranial or temporal muscle flap.
Primary surgery for maxillary antral cancers is radical maxillectomy that removes en bloc the entire maxilla and ethmoid sinus via a Weber-Fergusson incision. Patients with tumors limited to the infrastructure do well after surgery alone as long as the margins of resection are adequate. Suprastructure lesions may involve the orbit, necessitating orbital exenteration. Resection of involved periosteum and frozen-section control of adjacent orbital contents with preservation of the eye may be possible in select lesions with involvement of the periorbita without intraorbital extension. Orbital preservation surgery in select patients with involvement of the bony orbit but not soft tissue does not appear to result in poorer survival or local control than those undergoing exenteration.38,39 The radical maxillectomy defect is covered with a split-thickness skin graft. As a general rule, the surgical defect should not be obliterated during the initial surgery. An open cavity allows cleansing and direct visual inspection during follow-up.
Skull-Base Surgery
Base-of-skull surgery has been growing as a discipline of head and neck surgery, addressing the need for more radical resection of extensive tumors involving the frontal brain, cavernous sinus, sphenoidal sinus, clivus, pterygoid space, and petrous bone. The classic criteria for inoperability include (1) superior extension of the tumor through the dura into the frontal lobes; (2) posterior extension of the tumor beyond the cribriform plate and fovea ethmoidalis to a point at which there is excessive traction on the optic chiasm or invasion of the prevertebral fascia or both; (3) involvement of both optic nerves; and (4) lateral extension into the region of the superior orbital fissure and cavernous sinus.4 In a combined-team approach with neurosurgery, many previously unresectable sinonasal tract tumors are successfully resected at some centers. This technique is evolving, and outcomes of such aggressive surgery in those lesions with otherwise dismal prognosis need to be validated.
Postsurgical Rehabilitation
The primary consideration for rehabilitation after radical surgery is function. Preoperative evaluation by a prosthodontist is necessary to obtain dental impressions and to assess the dentition that will remain after surgery. A surgical splint prepared preoperatively is used to fill the defect during the immediate postoperative period. Use of the splint facilitates immediate speech and swallowing. A temporary obturator is then fitted until the cavity completely heals several months later, at which time a permanent obturator can be made.40
Radiation Therapy
Radiation therapy was more often administered preoperatively in the 1960s and 1970s; however, during the previous decades, most centers have been using radiation therapy in the postoperative adjuvant setting after radical surgery for squamous cell carcinomas of the nasal cavity and paranasal sinuses. Although pre- and postoperative radiation may result in similar control rates (Table 33-4), there are definite advantages to surgery before radiation. Preoperative radiation may obscure the initial extent of disease and erroneously lead to a more conservative resection; thus surgery may not quite encompass the microscopic disease. Preoperative radiation also increases the infection rate and the risk of postoperative wound complications. Radiation therapy in the postoperative setting has the advantage of accurate pathologic review of all structures at risk, and the radiation portals can then be designed to encompass the entire extent of disease with adequate margins. Upfront surgery also allows drainage of infected sinuses before radiation. Postoperative radiation therapy is started 4 to 6 weeks after surgery. In those patients who are deemed medically inoperable or who refuse radical surgery, primary radiation therapy has been employed with differing success (10% to 70% 5-year survival) depending on the stage and extent of the tumor.41,42
Table 33-4 Results of Treatment in Maxillary Sinus Tumors: Preoperative and Postoperative Radiation Therapy

Minor salivary gland carcinomas are resected first and irradiated postoperatively if the histologic examination reveals a high-grade or adenoid cystic variety, extensive perineural spread, positive or close margins of resection, or extensive primary tumor (T3 or T4). The radiation portals should include neural pathways up to the cranial nerve ganglion at the base of the skull in adenoid cystic carcinomas and high-grade lesions with extensive perineural invasion. There is sparse literature available regarding the outcome of definitive irradiation for unresectable salivary gland tumors of the nasal cavity and paranasal sinuses. The results of definitive conventional photon irradiation of unresectable salivary gland tumors in general were poor (17% local control at 2 years) in at least one randomized trial of photon versus neutron therapy43; however, whether neutron therapy offers any advantage over photons is controversial, and this controversy is discussed in Chapter 32. Notably, a recent study from investigators at the University of California, San Francisco, reported 5- and 10-year local control of 70% and 57% among patients treated with photon irradiation alone for unresectable or medically inoperable salivary gland carcinomas, with the rates depending on T stage and radiation dose.44
Radiotherapeutic Technique: External Beam
It is most advantageous to base the treatment volume on treatment-planning CT with MRI correlation, if available. MRI-derived gross tumor volumes (GTVs) may be smaller and have less interobserver variation than CT-derived GTVs. CT and MRI are complementary in delineating the GTV.45 The complex anatomy of this region and the presence of numerous critical, dose-limiting organs such as optic nerves, chiasm, eyes, lacrimal gland, auditory apparatus, pituitary, brainstem, and spinal cord, render tumors of the sinonasal tract ideal candidates for sophisticated treatment planning. Since the introduction of CT-based treatment planning and immobilization devices in the 1980s, improvement in survival rates while reducing the incidence of eye complications has been reported.46,47 A three-dimensional (3-D) system allows comprehensive visualization of the tumor volume and adjacent normal anatomy through “beam’s-eye view” displays (Fig. 33-8 and Fig. 33-9). Careful definition of the anatomic structures of interest and of the extent of the tumor, together with immobilization devices for precise setup, permits accurate targeting of the tumor. The ability of a 3-D system to use nonaxial and noncoplanar fields allows greater flexibility in treatment planning, so that the dose distribution conforms to the tumor volume in 3-D space, thus sparing the surrounding normal tissue to a greater extent. Dose-volume histograms (Fig. 33-10) are able to record accurately the doses delivered to differing volumes of adjacent dose-limiting structures. This technology has great potential for improving local control, while decreasing the risk of long-term sequelae of therapy and allowing dose escalation that has not been possible with conventional two-dimensional treatment-planning systems. Thus, the role of a conformal 3-D treatment system will become even more important in the setting of primary radiation therapy that requires high doses beyond 70 Gy. Although 3-D conformal plans can provide bilateral sparing of the globe for most patients, it may be more difficult to spare optic nerves, especially on the ipsilateral side, when prescription dose exceeds the normal tissue-tolerance doses.

FIGURE 33-9 • Three-dimensional beam’s-eye views: wedged-pair portals. A, Anterior view. B, Lateral view. C, Oblique view.
During the initial setup, the patient’s head is placed in a neutral supine position (Fig. 33-11). A tongue depressor is placed in the mouth to displace the tongue from the treatment area. In a postoperative setting, the patient wears the obturator for the maxillary defect during the simulation. A CT-compatible thermoplastic facial mask is made to immobilize the head. An anterior portal is set up with the inferior border splitting the tongue blade (near the commissure of the lips), and the upper border is determined per the superior extension of the tumor. The ipsilateral border should include the entire maxillary sinus and the contralateral border should cover the medial wall of the orbit and medial maxillary wall (just medial to the limbus of the contralateral eye). For massive lesions involving the contralateral maxillary sinus, the lateral border should be extended to include entire contralateral sinuses. The lateral portal is set up with the anterior border flashing the skin of the cheek and the posterior border at the posterior aspect of the clivus, splitting the vertebral bodies. The patient is scanned in the treatment position in the facial mask. For primary radiation therapy of unresectable tumors, CT with intravenous contrast material is recommended to take advantage of the enhancing characteristics of neoplasms. In a postoperative setting, CT without contrast suffices. Thin-cut CT with 3- to 5-mm spacing is recommended through the tumor volume, whereas outside the immediate tumor volume region, 1-cm cuts are obtained from the top of the skull through the mid-neck.
After the tumor volume and normal soft tissue and bony anatomy (e.g., sinuses, skull, base of skull, brain) and critical structures (e.g., eyes, optic nerve, chiasm, brainstem, and spinal cord) are contoured on the CT axial images, beams are placed with a 1.5- to 2-cm margin. The contralateral eye is blocked, and greater than two thirds of the ipsilateral eye48 are also blocked unless there is intraorbital infiltration by the tumor. Most of these cases have had orbital exenteration during surgery, and the entire orbital defect is then included in the tumor volume (Fig. 33-12). Compared with a conventional plan, which routinely includes one half to one third of the ipsilateral eye,15 greater sparing of the ipsilateral eye is possible without sacrificing tumor control by using a 3-D conformal plan (see Fig. 33-10).

FIGURE 33-12 • Intensity-modulated radiation therapy isodose plans in sagittal and coronal planes: A, coronal view; B, sagittal view.
In general, four fields, using an anterior and two lateral wedged portals plus an intraorbital electron portal, are used to treat the target volume (Fig. 33-13). Less commonly, three-field techniques (without the anterior electron portal) are used, and for small lesions confined to the ipsilateral maxillary sinus, a wedged pair of anterior and lateral portals is used (Fig. 33-14). With the four-field technique, the eyes are blocked from the anterior and lateral photon portals. The interorbital electron portal makes up the dose to the posterior nasal cavity, ethmoid sinus, and medial orbit. If the three-field technique is used, the anterior border of the lateral portal is placed at the bony canthus and the anterior portal is weighted more heavily (2 : 1 to 3 : 1).15
Most commonly, 6-mV photons are used; however, a higher-energy beam may be used in conjunction with low-energy electrons (9 or 12 meV) in the anterior portals. Lower-energy photons result in a greater dose gradient and a less homogeneous distribution. A typical loading favors the anterior portal by 2 : 1. The generated isodose curves should reflect the effect of inhomogeneity corrections, although the dose is calculated at the central axis without inhomogeneity corrections (Fig. 33-15 and Fig. 33-16). In a postoperative setting, 60 to 63 Gy are prescribed to the target volume at a 1.8 to 2 Gy daily fraction, and an additional cone-down boost may be delivered to the areas of involved margin or gross residual tumor. For unresectable tumors, doses in excess of 70 Gy are recommended. If the tumor involves critical structures or if they cannot be excluded from the high-dose volume by using tight margins, a hyperfractionated regimen (1 to 1.2 Gy twice-daily fractionation) or concurrent delivery of radiosensitizing chemotherapy should be considered. However, it is yet unknown whether optic nerves and chiasm can be differentially spared from the late effects relative to acute effects on the tissue and tumor by the use of a hyperfractionation scheme.
Normal-tissue complication probability calculations may be useful in assessing complication risk better than point dose tolerance criteria for the chiasm, optic nerve, and retina.49 It is important to assess the overall risk of blindness for the patients in addition to the risk for the individual visual pathway structures. Patients should be informed of the risk of radiation-induced damage to the chiasm or eye, or both, and eventual blindness. Preradiation ophthalmologic examination is prudent in all patients undergoing sinus radiation therapy to establish baseline acuity and to detect any preexisting abnormalities.
Intensity-Modulated Radiation Therapy
Given the irregular contours of the tumors arising in the paranasal sinuses and nasal cavity and the presence of vital structures in this region, intensity-modulated radiation therapy (IMRT) using inverse treatment planning systems and computer optimization may render a greater therapeutic ratio for tumors of the paranasal sinuses compared with the more standard forward planning 3-D conformal therapy. IMRT can result in better sparing of the optic apparatus, especially in definitive cases in which high doses of radiation are necessary for gross tumor eradication. IMRT strategies for paranasal sinus malignancies can be strikingly different in various aspects, such as beam setup; total number of segments; GTV, clinical tumor volume, and planning tumor volume (PTV) dose coverage; and dose statistics for organs at risk (Fig. 33-17, Fig. 33-18, and Fig. 33-19).47 Although several different treatment planning systems are available, the basic principles underlying this technology are similar.
Multiple dosimetric studies have shown that improved dose distributions can be achieved with the use of IMRT compared with conventional and 3-D conformal radiation planning.50–54 From a practical standpoint, the dose delivered to the optic pathways can be selectively reduced by IMRT, which has the potential to preserve binocular vision, particularly for patients who have extensive and large-volume disease in the paranasal sinuses. Tsien and colleagues retrospectively replanned 13 patients with locally advanced paranasal sinus cancer who were initially treated via conformal techniques. Using a priorities-and-tradeoffs model, the authors illustrated the flexibility and utility of IMRT to selectively save normal critical structures while maintaining dose to the target.55
Evidence is starting to emerge that the theoretical benefits from sophisticated planing techniques such as IMRT are indeed translating into clinical advantages. In a longitudinal analysis of 127 patients treated with radiation therapy from 1960 to 2005 at the University of California, San Francisco, the incidence of grade 3 or greater late ocular toxicity among patients treated with conventional, 3-D conformal, and IMRT was 20%, 9%, and 0%, respectively.56 In another series from Memorial Sloan-Kettering, none of the 85 patients who underwent postoperative radiotherapy treated with CT simulation developed grade 3 or greater late complications of the eye with a median follow-up of 60 months among surviving patients.57 Other series have reported similar findings in terms of the reduction of late toxicity with IMRT.58,59
Image-Guided Radiation Therapy
Evidence is starting to emerge that improvements in setup accuracy and precision can be achieved with the use of image-guided radiation therapy (IGRT) techniques for head and neck cancer.60,61 This is particularly relevant for patients treated using IMRT because the distance between very high- and low-dose regions can often be a matter of millimeters. Using volumetric CT data acquired at the time of each daily treatment to guide radiation delivery may be especially useful for tumors of the paranasal sinuses and nasal cavity because they lie in close proximity to vital organs such as the brain, ears, and optic pathways. The use of daily IGRT, however, is associated with an increased peripheral dose to the patient and adds to the machine time for each case.62 How and whether continuing advances in targeting will lead to improvements in the therapeutic ratio remain an area of active debate.
Fig. 33-20 illustrates the use of megavoltage CT images obtained on the treatment couch for a patient treated by IMRT (Fig. 33-21) with a large, unresectable squamous cell carcinoma of the maxillary sinus. As illustrated in the axial images obtained at day 1 of treatment (see Fig. 33-20B), the large tumor occupies most of the maxillary sinus, has infiltrated into the soft tissue, and has destroyed most of the zygomatic bone.

FIGURE 33-21 • Intensity-modulated radiotherapy treatment plan demonstrating dose distribution for the patient described in Fig. 33-20. A, Axial image. B, Coronal image. C, Sagittal image. The patient was treated with a simultaneous integrated (dose painting) technique with the orange colorwash representing 70 Gy; the yellow colorwash representing 63 Gy; and the green colorwash 56 Gy. The purple denotes the 45 Gy distribution. Radiation therapy was delivered in 35 daily fractions.
Proton Beam Radiation Therapy
Proton therapy may be particularly advantageous in the treatment of paranasal sinus and nasal cavity cancers, given the deep-seated locations requiring high doses of radiation. Given their unique physical characteristics, protons may provide improved sparing of normal critical organs in the region by taking advantage of the Bragg peak.63 Mock and colleagues performed a treatment planning comparison of proton therapy versus photon therapy with IMRT and 3-D conformal methods for five patients with paranasal sinus cancer.64 The authors showed that proton therapy reduced the amount of normal, uninvolved tissue exposed to radiation, most dramatically at low- and mid-isodose levels. Proton therapy also significantly reduced doses to selective organs at risk, including the optic pathways and brain. To date, there is limited data reporting on clinical outcomes with the use of proton therapy for this treatment site. In one of the few published reports to date, investigators from Massachusetts General Hospital demonstrated encouraging outcomes among 102 patients with locally advanced sinonasal malignancy is treated by proton-beam radiation therapy, with or without surgery.65 The observed 5-year local control rates were 95%, 82%, and 87%, among patients who underwent complete resection, partial resection, and biopsy, only, respectively. Notably, however, long-term toxicity was not reported.
Stereotactic Radiosurgery
More recently there has been an interest in the use of stereotactic radiosurgery for the nasal cavity and paranasal sinus tumors with skull-base involvement. Haberman and colleagues66 reported their experience at the University of Graz, Austria, treating eight patients who underwent primary surgery and postoperative gamma knife radiosurgery. At 3 years, six patients were alive (all without local recurrence, four without evidence of disease) with no adverse effects. There is a potential for using this technique or a fractionated stereotactic radiotherapy technique as a boost for gross residual disease in addition to conventional image-based radiotherapy in select patients who have small residual tumor volume at the skull base. These new techniques should be investigated further in a prospective trial. Intensity-modulated radiosurgery/radiotherapy using a micro-multileaf collimator was compared against forward-planning techniques using beam modification by enhanced dynamic wedge.67 In this report, dose-volume histogram analysis demonstrated that a significant reduction in dose to neighboring critical structures could be achieved through intensity modulation patterns determined from inverse planning, while a marginal change was achieved in the target volume dose uniformity.
Chemotherapy
Although numerous publications have reported a high percentage of good initial responses and some evidence of improved survival with cytotoxic chemotherapy, the efficacy of such therapy as a part of combined treatment for advanced sinonasal carcinoma has yet to be determined in a large clinical trial. The most commonly used regimen has been cisplatin-based.68,69 Overall response rates ranging from 80% to 90% have been reported in previously untreated patients with paranasal sinus malignancies. Japanese groups reported on the use of intra-arterial 5-fluorouracil (5-FU) chemotherapy as a radiosensitizing agent in an effort to reduce the extent of required surgery.70,71 Others employed sequential intra-arterial bleomycin and methotrexate followed by preoperative radiation therapy and subsequent radical surgery for advanced maxillary sinus carcinomas with good local control.72 Lee and colleagues73 reported an excellent immediate tumor response rate (>90%) using a highly selective intra-arterial infusion of cisplatin-based, multiagent induction chemotherapy. More recently, combined radiation therapy and cisplatin-based chemotherapy for radiation sensitization were studied for the definitive management of unresectable base-of-skull carcinomas with encouraging early results.36 There is, however, no established role for adjuvant chemotherapy in the management of sinonasal malignancies.
Treatment of Rare Epithelial Tumors
Olfactory neuroblastoma is an extremely rare entity arising in the nasal cavity olfactory epithelium. The commonly used Kadish staging system is based on the extent of invasion of adjacent structures (Table 33-5). Prognosis is determined on the basis of stage and resectability. The primary treatment is craniofacial resection. The role of postoperative radiation therapy is controversial. Although some attribute improved local control and survival to aggressive surgical resection via a craniofacial approach combined with radiation therapy,21,22 others argue that patients treated with surgery alone have better results than with combined surgery and radiation therapy.74 This may reflect adverse patient selection for the combined-modality therapy. Earlier-stage olfactory neuroblastoma patients enjoy excellent survival with surgery and postoperative radiation therapy: stage A at 96% and stage B at 83% at 5 years.75 Stage C, however, has a much worse prognosis (53%) because of greater local as well as distant failure. The overall 5-year survival rate is approximately 50%. Distant metastases develop in 25% to 30% of patients.76 Chemotherapy has been employed in these advanced cases with limited success.77–79 A prospective study of 19 patients (4 stage B and 15 stage C) with malignant neuroendocrine tumors of the sinonasal tract conducted at the Massachusetts General Hospital between 1992 and 199880 reported an improved outcome for patients with olfactory neuroblastoma and neuroendocrine carcinoma treated with aggressive multimodality therapy. Patients received neoadjuvant cisplatin-etoposide chemotherapy for two cycles and high-dose proton-photon radiotherapy (69.2 cobalt gray equivalents [CGE] in 1.6 to 1.8 CGE per fraction twice daily in a concomitant boost schedule) with radical surgery reserved for nonresponders. Responders received two more cycles of adjuvant chemotherapy. With the median follow-up of 45 months, 5-year survival and local control rates were 74% and 88%. No radiation-induced visual loss was observed because of the precision delivery of radiation with stereotactic setup and protons; however, four patients developed asymptomatic radiation-induced damage to the frontal or temporal lobe by MRI criteria and two patients showed soft-tissue and bone necrosis. Despite sensitivity to platinum-based chemotherapy, patients with high-grade tumors tend to have a more aggressive course than those with lower-grade tumors.81
Table 33-5 Kadish Staging System for Olfactory Neuroblastoma
| Stage A | Tumor confined to the nasal cavity |
| Stage B | Tumor in nasal cavity that extends to paranasal sinus |
| Stage C | Tumor that extends to the orbit, base of skull, or cranial cavity or with cervical/distant metastases |
Treatment of Benign and Nonepithelial Tumors
Lethal midline granuloma is a highly destructive process. In the process of ruling out Wegener granulomatosis, most of the patients with lethal midline granuloma syndrome have failed a trial of systemic steroids. The primary treatment is radiotherapy. All nasal cavity and paranasal sinuses should be included in the treatment portals for the initial 40 Gy, as marginal recurrences have been observed. The final cone-down boost is delivered to the gross areas of destruction at a total dose of 45 to 50 Gy. The local failure rate even at this dose level approaches 30%.82
Although sinonasal lymphomas are relatively rare in Western countries, in Asian populations they represent the second most frequent group of extranodal lymphomas after gastrointestinal lymphomas. The B-cell phenotype is typically located in the paranasal sinuses and has a slight predominance in Western countries, whereas the T/NK-cell phenotype is most common in Asian and South American countries and is typically located in the nasal cavity. The T/NK-cell lymphomas have an aggressive, angioinvasive growth pattern that often results in necrosis and bony erosion.83 Patients with T-lineage disease appear to have a particularly poor outcome.84 Sinonasal lymphomas tend to present as localized disease (stages I and II) but often relapse in the abdomen. Thus staging should include endoscopic examination of the gastrointestinal tract. The role of surgery in the management of non-Hodgkin lymphoma of the paranasal sinuses is limited to biopsy for pathologic diagnosis. Treatment of sinonasal lymphoma depends on the grade and stage of the tumor, and follows the general guidelines for the treatment of malignant lymphomas. It may involve local radiation therapy, single-agent or combination anthracycline-based chemotherapy, or combined-modality therapy with chemotherapy followed by consolidated radiation therapy. Aggressive lymphomas involving the base of the skull may require systemic chemotherapy as well as central nervous system prophylaxis.
Rhabdomyosarcoma is the most commonly found soft-tissue sarcoma in the sinonasal tract. These primitive tumors have the morphology of developing striated muscle and constitute one of the “small blue round tumors” of childhood, or peripheral neuroectocrine tumors. Eight percent of head and neck rhabdomyosarcomas arise in this region,85 and they constitute one of the five parameningeal sites of rhabdomyosarcoma (orbit, infratemporal fossa, middle ear, and nasopharynx are the other sites). These are predominantly tumors of the pediatric population and are treated according to the guidelines of the Intergroup Rhabdomyosarcoma Study (IRS). Treatment involves a combined modality including chemotherapy, radiation therapy, and surgery. Unlike rhabdomyosarcomas at other sites, parameningeal rhabdomyosarcomas are less amenable to surgical extirpation; thus treatment consists predominantly of chemotherapy and local radiotherapy. Hyperfractionated radiotherapy did not result in improvement in a randomized study by IRS-IV. Other soft-tissue sarcomas are treated with wide local excision with pre- or postoperative radiation therapy.
Complications of Therapy
For patients treated with radiation therapy for malignancies of the paranasal sinuses and nasal cavity, the high doses required to achieve local control coupled with the proximity of disease sites to sensitive normal tissue have historically been associated with a high incidence of treatment-induced morbidity. Katz and colleagues86 reported a high rate of visual complications for radiation therapy in their series of tumors of the nasal cavity and ethmoid/sphenoid/frontal sinuses. Of 78 patients, 21 (27%) developed unilateral blindness resulting from radiation retinopathy or optic neuropathy; however, most of these complications were anticipated because the ipsilateral eye was irradiated to a high dose. Four patients (5%) unexpectedly developed bilateral blindness caused by optic nerve injury. All four of these patients received irradiation alone and were treated before 1985. The authors suggest that a combination of surgery and radiation therapy be given in an effort to reduce the total dose needed to achieve local control, and they also suggest improving dose homogeneity within the treatment volume to avoid overdosing the optic nerve.
Late retinal complications of radiation therapy for advanced nasal and paranasal malignancies were retrospectively studied by Takeda and colleagues.87 Between 1982 and 1996, 43 eyes of 25 patients were exposed to radio therapy. None of the patients had tumor invasion into the eyes. The patients were followed ophthalmologically for a minimum of 2 years. Radiation retinopathy was observed in 7 eyes, with a cumulative incidence of 25% and median interval before the onset of symptoms of 32 months (range 16 to 60). Neovascular glaucoma developed in 3 eyes, with the cumulative incidence of 7% and median period to the onset of symptoms of 22 months (range 16 to 26). Obstruction of the central retinal artery was observed in one eye. No patients who received less than 50 Gy developed retinal complications. The eyes exposed to greater than 50 Gy with more than 60% retinal area irradiated resulted in a 62% rate of severe retinal complications.
The series of 3-D conformal therapy of paranasal sinus malignant tumors reported by Roa and associates48 revealed more encouraging results with respect to preservation of critical structures. There was only one case of limited optic neuropathy and one case of possible radiation-induced cataract. There was no blindness related to irradiation. Another report of 3-D conformal radiotherapy to median PTV doses of 60 Gy for 40 patients with locally advanced paranasal sinuses and nasal cavity tumors suggests an improved visual pathway complication rate. With a median follow-up of 19 months, two patients developed cataracts and one patient developed ipsilateral blindness caused by vascular glaucoma.88
Neurocognitive effects of therapeutic irradiation for skull-base tumors were reported by Meyers and colleagues from the University of Texas M.D. Anderson Cancer Center.89 Nineteen patients who received paranasal sinus irradiation at least 20 months and up to 20 years before assessment were given a battery of neuropsychologic tests of cognitive function. Radiation was delivered by a three-field technique with the median dose of 60 Gy (range 50 to 68 Gy). Memory impairment was found in 80% of the patients. One third of the patients manifested difficulty with visual-motor speed, frontal lobe executive functions, and fine motor coordination. Two patients had frank brain necrosis with resultant dementia and blindness, and three had evidence of brain atrophy. Three patients experienced pituitary dysfunction. Neurocognitive symptoms were related to the total dose of radiation delivered, but not to the volume of brain irradiated. Improvement in dose distribution using image-based conformal technique or IMRT should decrease the incidence of these significant late brain injuries.
Other major complications of combined surgery and radiation therapy reported in the past include osteomyelitis or radionecrosis of bone at the base of the skull, or both; meningitis; hemorrhage; and aseptic brain necrosis.90 With advancement in surgical techniques, antibiotic coverage, and radiotherapeutic techniques, these sometimes fatal complications are seen much less commonly.
It is important to recognize that most publications that have reported high rates of complications included patients treated using non-IMRT techniques. As data begins to emerge for patients treated using IMRT, the benefits of this technology with respect to preserving normal tissue should become better appreciated. In the preliminary University of California, San Francisco, experience reporting on 36 patients treated using IMRT, observed complications included mucositis, conjunctivitis, keratitis, cellulitis, dacryocystitis, and parotiditis—all of which resolved with conservative medical management. In the late setting, no patients experienced a complete loss of vision as a result of treatment, with reported side effects including chronic xerophthalmia, chronic lacrimal stenosis, cataract formation, eustachian tube dysfunction, and radiation necrosis of the gyrus rector muscle resulting in gaze limitation.91 A longitudinal analysis of the University of California, San Francisco, experience showed that the incidence of grade 3 or higher late toxicity differed significantly among patients treated with conventional techniques compared with 3-D radiation therapy and IMRT. For instance, 13% of patients treated with IMRT developed any grade 3 or higher late complication compared with 22% and 54%, respectively, of patients treated using 3-D and conventional radiation therapy, respectively.56
Results of Therapy
The literature of nasal cavity and paranasal sinus carcinomas is difficult to interpret because of their relatively infrequent incidence and because of the wide variability in surgical and radiotherapeutic techniques employed during the long period during which the reported cases were accumulated. Compounding the rarity of these tumors, there are a variety of histologic types of cancers that develop in this anatomic region, all with different biologic behavior. Most reports show overall local control rates from 40% to 60%. Wang42 reported Massachusetts General Hospital’s experience of nasal cavity squamous cell carcinomas from 1960 to 1985 (Table 33-6). Although the numbers of patients were small, a combination of radiation and surgery appeared to produce better 3-year disease-free survival than radiation alone (78% versus 50%). He reported similar results in favor of combined surgery and radiation for squamous cell carcinoma of the maxillary and ethmoid sinuses (55% versus 38%, and 55% versus 33%, respectively).42
Table 33-6 Three-Year Disease-Free Survival After Radiation Therapy Alone or Combined Radiation Therapy and Surgery for Squamous Cell Carcinoma of the Nasal Cavity and Paranasal Sinuses: Massachusetts General Hospital Experience
| Site | Survival, n (%) | |
|---|---|---|
| RT Alone | RT and Surgery | |
| Nasal cavity | 10 (50) | 9 (78) |
| Maxillary sinus | 35 (38) | 44 (55) |
| Ethmoidal sinus | 12 (33) | 22 (55) |
RT, Radiation therapy.
The most consistently identified factor predicting for improved survival among those treated for carcinomas of the nasal cavity and paranasal sinuses is gross total tumor resection. Among 127 patients treated at the University of California, San Francisco, the 5-year local control was 65% for those treated with radiation therapy postoperatively after gross total resection compared with 44% among those who underwent radiation therapy in the presence of macroscopic disease.56 In another study from the Netherlands,92 the addition of debulking surgery prior to radiation therapy dramatically improved 5-year overall survival from 9% to 60%. The other variable that has consistently been demonstrated to correlate with outcome is tumor extent, with most studies confirming that patients with T1 and T2 tumors having superior rates of local control and overall survival compared with those with T3 and T4 tumors.93,94 Because the vast majority of patients present with locally advanced cancers, additional data analyzing prognostic factors such as intracranial invasion, cranial nerve involvement, dural attachment, and orbital infiltration—all of which would currently categorize a tumor as T4 in the current AJCC staging system—is urgently needed. Although the most recent version of the AJCC staging system published in 2002 attempted to address this by dividing cases into T4a (resectable) and T4b (unresectable), it is still unclear how this distinction may influence prognosis.
Despite the biases discussed previously, several large series reporting treatment results are particularly instructive. Investigators from Memorial Sloan-Kettering Cancer Center recently analyzed 85 patients with carcinomas of the nasal cavity and paranasal sinuses uniformly treated by gross total resection followed by postoperative radiation therapy and reported a 5-year local control of 62%.57 On multivariate analysis, squamous cell histology and involvement of the cribriform plate predicted for an increased likelihood of local recurrence. In another series from Washington University, the 5-year local control was 58% among 106 patients treated by radiation therapy alone, or combined with surgery for paranasal sinus cancer.95 Likewise, investigators from the University of California, San Francisco, reported a 5-year local control rate of 62% among 127 patients treated by radiation therapy with or without surgery from 1960 to 2005.56 Although the majority of the patients included in the University of California series had maxillary sinus cancers and squamous cell histology, the respective proportions nevertheless ranged from 43% to 76% and 53% to 82%, respectively. These differences are potentially significant because other studies have suggested that patients with maxillary sinus tumors have better outcomes than those whose primary tumors arise from the ethmoid, frontal, and sphenoid sinuses, although the infrequency of the latter as well as the difficulty in determining the exact site of origin make drawing definitive conclusions problematic.86,94,96
Similarly, others have shown that patients with nonsquamous cell histologies have improved outcomes compared with those with squamous cell carcinoma of the nasal cavity and paranasal sinuses.97,98 Although a study from the University of Florida reported a very encouraging 5-year local control rate of 70% among 78 patients treated with radiation therapy, this study was notable for its large proportion of patients with adenocarcinoma.86 Furthermore, patients were managed with radiation therapy alone (47 patients) or in conjunction with surgery (27 patients). Four patients also received chemotherapy in addition to radiation with or without surgery. The more common histologies included 25 squamous cell carcinomas, 31 minor salivary gland tumors, 14 undifferentiated carcinomas, and 8 esthesioneuroblastomas. The 5-year local control rate for stage I (limited to the site of origin) was 86%; stage II (extension to adjacent sites), 65%; and stage III (destruction of skull base or pterygoid plates, or intracranial extension), 34%. The 5-year actuarial local control rate for patients receiving postoperative irradiation was 79%, and for those receiving irradiation alone was 48% (p = 0.05). Of the 39 patients who received no elective neck treatment, 33 (85%) did not experience recurrence in the neck compared with 25 (89%) of 28 patients who received elective neck irradiation (ENI). They concluded that surgery and postoperative radiation therapy may result in improved local control, absolute survival, and complications when compared with radiation therapy alone. ENI was felt to be unnecessary for patients with early-stage disease.
Lee and Ogura99 reported on 96 patients with maxillary sinus carcinoma treated at Washington University in St. Louis between 1969 and 1976. A combination of preoperative radiation and surgery produced 5-year overall survival rates of 60%, 45%, 28%, and 38% for T1, T2, T3, and T4 lesions, respectively. None of the 23 patients treated with radiation alone survived 5 years.
A retrospective analysis of 60 cases (46 maxillary antrum and 14 ethmoid sinus) of paranasal sinus cancer treated at Northwestern University between 1970 and 1985 was reported by Sisson and associates.4 The most common histologic type was squamous cell carcinoma (53%) followed by adenoid cystic carcinoma (17%). The 5-year survival probability for antral and ethmoid cancer was 48% and 68%, respectively. Survival did not differ significantly whether radiation therapy was administered preoperatively or postoperatively (65% versus 63%, respectively), although there were more advanced (T3 and T4) tumors in the preoperative radiotherapy group. Seven patients with small antral tumors were treated with surgery alone, with an 86% 5-year survival rate.
A large retrospective review of 220 patients with nasal cavity and paranasal sinus carcinoma treated at the University of California, Los Angeles, between 1975 and 1994 was reported by Dulguerov and colleagues.100 With a minimum follow-up of 4 years, the 5-year actuarial survival rate was 63% and the local control rate was 57%. The factors associated with a worse prognosis included squamous or undifferentiated histologies; advancing T classification; ethmoid location; treatment by radiotherapy alone; and tumor extension to pterygomaxillary fossa, frontal and sphenoid sinuses, cribriform plate, and dura. In multivariate analysis, histology, extension to the pterygomaxillary fossa, and dural invasion remained significant. The authors also performed a systematic review of published articles on patients with malignancies of the nasal and paranasal sinuses during the preceding 40 years and concluded that a progressive improvement in outcome had been made for patients with squamous cell and glandular carcinoma, maxillary and ethmoid sinus primary tumors, and most treatment modalities.
Myers and colleagues101 reported 5-year and 10-year actuarial disease-specific survival of 52% and 35%, respectively, for patients with paranasal sinus malignancies treated at the University of Texas Southwestern Medical Center between 1980 and 1997. Most patients presented with locally advanced disease (88%) and without nodal involvement (96%) or distant metastasis (96%). Approximately half of the patients presented with squamous cell histology. Of the patients in this study group, 62% underwent surgery as part of a multimodality curative treatment plan or alone as curative treatment. Of these, 13% had unresectable local disease and received nonsurgical palliative therapy.
The risk of lymph node metastasis and the controversy surrounding the elective nodal irradiation in patients with maxillary sinus carcinoma was addressed in a report by Le and colleagues.102 In this retrospective review of 97 patients treated at Stanford University and the University of California, San Francisco between 1959 and 1996, the overall incidence of lymph node involvement at diagnosis was 9%, with the most common sites of nodal involvement at levels 1 and 2. Of 36 patients who had neck irradiation, 25 received ENI for N0 necks. With the median follow-up of 22 months, the 5- and 10-year actuarial survival rates were 34% and 31%, respectively. Following treatment, the 5-year risk of nodal relapse was 12%. Squamous cell histology was associated with a high incidence of initial nodal involvement and subsequent nodal relapse. Elective nodal irradiation effectively prevented nodal relapse in patients with squamous cell histology. There was no nodal relapse in 13 patients who received ENI while 6 of 26 patients (20%) without ENI relapsed in the neck. Nodal relapse was associated with a high rate of distant metastasis and poorer survival. The authors advocate the use of ENI in patients with T3 and T4 squamous cell carcinoma of the maxillary sinus. Results of this study are corroborated by a series reported by Paulino and colleagues.103 Of 42 patients with squamous cell carcinoma of the maxillary sinus in this series, 9.5% initially presented with cervical lymphadenopathy and 29% later developed recurrence in the neck after undergoing surgical resection of the primary tumor and postoperative irradiation to sinuses without ENI. More than one third of the neck recurrence (10.5% of total) represented isolated neck failure. The authors advocate ENI in all stages. However, Dirix and colleagues reported on 127 patients with sinonasal cancers treated by radiation therapy, none of whom received ENI. With a median follow-up of 5.6 years, only 6 patients (5%) developed a regional failure in the neck.104
Table 33-7 summarizes several series of results of radiation therapy alone, surgery alone, or combined surgery and radiation for maxillary sinus carcinomas. Overall, it is reasonable to expect 70% to 80% primary-site tumor control for early maxillary sinus carcinomas (T1 and T2) and 50% to 60% for more advanced tumors (T3 and T4) after surgery and radiation compared with 50% and 20% to 25%, respectively, after radiation therapy alone. Definitive irradiation of unresectable lesions can achieve 10% to 15% 5-year survival.36
Table 33-7 Primary Tumor Control According to T Stage and Treatment Modality for Maxillary Sinus Carcinoma

The success of therapy for malignant minor salivary gland tumors of the paranasal sinuses and nasal cavity appears to depend on the combination of radiation therapy and surgery and the histologic grade of these tumors.105 Of 66 patients, 36 with adenoid cystic carcinoma and 30 with adenocarcinoma were treated at the University of Texas M.D. Anderson Cancer Center between 1951 and 1980. Most patients were treated with surgery with or without radiation therapy. The local control rates with a minimum follow-up of 2 years were 47% for surgery alone and 76% for planned surgery and irradiation. Patients with high-grade adenoid cystic carcinomas fared significantly worse than those with the low-grade variety. Of the low-grade patients, 47% remained disease free after 5 to 21 years, whereas only 12% of the high-grade group remained disease free between 2 and 3 years after treatment. Although the difference was not as pronounced as in adenoid cystic carcinomas, there was a trend toward better survival among patients with low-grade adenocarcinomas compared with the high-grade group.
In a small retrospective analysis, Naficy and colleagues106 reported a 6-year survival rate of 50% for 7 patients with adenoid cystic carcinoma of the paranasal sinuses treated by radiation alone, compared with 73% for 10 patients treated by combined surgery and radiation. Overall local control rate was poor at 24%.
Claus and colleagues107 reported treatment outcome of 47 patients with adenocarcinoma of the ethmoid sinuses treated with surgery and high-dose postoperative radiation therapy between 1985 and 2001 at the Ghent University Hospital in Belgium. Of these cases, 60% were locally advanced with T3 and T4 disease. With the median follow-up of 32 months, the 3-year and 5-year disease-free survival rates were 62% and 36%, respectively, and local-regional tumor control rates were 70% and 59%, respectively. Patients presenting with intracranial tumor invasion relapsed within 7 months after the end of radiotherapy. Radiation-induced dry-eye syndrome and optic neuropathy was observed in 7 and 2 patients, respectively, of the 47 cases.
The results of a 3-D conformal series of predominantly advanced paranasal sinus malignancies from the University of Michigan compare favorably with older series using conventional planning systems.48 Between 1986 to 1992, 15 patients were treated with primary radiotherapy to a median prescribed dose of 68.4 Gy for unresectable paranasal sinus malignancies. Postoperative radiation therapy was administered to 24 patients for close margins or microscopic or gross residual disease. The median prescribed doses were 55.8, 59.4, and 67.8 Gy, respectively. With the median follow-up of 4.5 years, the local control at 3 years and actuarial overall survival rate at 4 years for the definitive radiation group were both 32%. The local control rates at 3 and 5 years for the adjuvant group were 75% and 65%, respectively. The actuarial overall survival rates at 3 and 5 years were 65% and 60%, respectively.
Radiation therapy alone has yielded suboptimal results for advanced, resectable nasal cavity and paranasal sinus cancer. Hoppe and colleagues108 recently published the Memorial-Sloan Kettering experience with radiation therapy for unresectable cancers. Of the 39 patients included in this series, 32 received concurrent platinum-based chemotherapy. With a median follow-up of 90 months, the 5-year progression-free survival was only 21%. Notably, an improvement in local-regional control and overall survival was observed when doses of greater than 65 Gy were used. Promising results had been previously reported by Harrison and colleagues36 using concomitant chemotherapy and accelerated radiotherapy in 20 patients with malignant unresectable skull-base tumors, including 11 T4 paranasal sinus/cavity and 9 T4 nasopharynx cancers. Of these, 15 had squamous cell carcinoma and 5 had minor salivary gland histologic types. All patients received accelerated fractionated radiation using a concomitant boost technique to 70 Gy in 6 weeks. Radiotherapy was given once a day at 1.8 Gy per fraction during the first 4 weeks. During the last 2 weeks, the course was accelerated to twice daily with a 1.8 Gy fraction in the morning given to a larger field, and a 1.6 Gy fraction in the afternoon given to a smaller boost field. Cisplatin (100 mg/m2) was given concurrently on days 1 and 22 of radiation. Although the follow-up is relatively short (median follow-up, 11 months), local progression-free survival was 94% at 2 years, and distant metastases-free survival, 57%. The overall survival was 80%. An updated report of this study at minimum follow-up of 3 years demonstrated a continued excellent local control rate of 78% for paranasal sinus tumors.109 Another report of a prospective trial of multimodality therapy in advanced paranasal sinus carcinoma from the University of Chicago suggests encouraging long-term outcome using neoadjuvant chemotherapy followed by surgery and postoperative concomitant chemoradiotherapy.110 Fifteen patients with stage III or IV paranasal sinus carcinoma underwent three cycles of cisplatin and 5-FU induction chemotherapy followed by surgery and postoperative concomitant chemoradiotherapy with hydroxyurea and 5-FU in a week-on/week-off sequence, to a median tumor dose of 60 Gy. Five patients achieved pathologic complete response. The 10-year overall survival, disease-free survival, and local control rates were 56%, 73%, and 79%, respectively.
1. Grant RN, Silverberg E. Cancer statistics 1970. New York: American Cancer Society; 1970. pp 8
2. Lewis JS, Castro EB. Cancer of the nasal cavity and paranasal sinuses. J Laryngol Otol. 1972;86:255.
3. Tabb HG, Barranco SJ. Cancer of the maxillary sinus. Laryngoscope. 1971;81:818.
4. Sisson GA, Toriumi DM, Atiyah RA. Paranasal sinus malignancy: a comprehensive update. Laryngoscope. 1989;99:143.
5. Preston-Martin S, Henderson BE, Pike MC. Descriptive epidemiology of cancers of the upper respiratory tract in Los Angeles. Cancer. 1982;49:2201.
6. Hyams VJ, Batsakis JG, Michaels L. Tumors of the respiratory tract and ear. In: Hartmann WH, editor. Atlas of tumor pathology, Series 2. Washington DC: Armed Forces Institute of Pathology; 1988:58.
7. Acheson ED, Cowdell RH, Hadfield E, et al. Nasal cancer in woodworkers in the furniture industry. Br Med J. 1968;2:587.
8. Acheson ED, Pippard EC, Winter PD. Mortality of English furniture makers. Scand J Work Environ Health. 1984;10:211.
9. Hernberg S, Westerholm P, Schultz-Larsen, et al. Nasal and sinonasal cancer. Connection with occupational exposures in Denmark, Finland, and Sweden. Scand J Work Environ Health. 1983;9:315.
10. Gordon I, Boffetta P, Demers PA. A case study comparing a meta-analysis and a pooled analysis of studies of sinonasal cancer among wood workers. Epidemiology. 1998;9:518-524.
11. Acheson ED, Cowdell RH, Jolles B. Nasal cancer in the Northamptonshire boot and shoe industry. Br Med J. 1970;1:385.
12. Enterline PE, March GM. Mortality among workers in a nickel refinery and alloy manufacturing plant in West Virginia. J Natl Cancer Inst. 1982;68:925.
13. Torjussen W, Solberg LA, Hgetweit AC. Histopathologic changes of nasal mucosa in nickel workers: A pilot study. Cancer. 1979;44:963.
14. Fang SY, Yang JJ, Ohyama M. Assessment of p53 protein expression in normal mucosa and benign and malignant lesions of the nasal cavity. Oncology. 1998;55:168-173.
15. Parsons JT, Stringer SP, Mancuso AA, et al. Nasal vestibule, nasal cavity, and paranasal sinuses. In: Million RR, Cassisi NJ, editors. Management of head and neck cancer: A Multimodality approach. 2nd ed. Philadelphia: JB Lippincott; 1994:551.
16. Bridger MWM, van Norstrand AWP. The nose and paranasal sinuses: applied surgical anatomy. J Otolaryngol. 1978;7(suppl 6):1.
17. Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathologic study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192.
18. Myers EN, Fernau JL, Johnson JT, et al. Management of inverted papilloma. Laryngoscope. 1990;100:481.
19. Lawson W, LeBenger J, Som P, et al. Inverted papilloma: an analysis of 87 cases. Laryngoscope. 1989;99:1117.
20. Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma: a clinical analysis of 17 cases. Cancer. 1976;37:1571.
21. Levine PA, McLeon WC, Cantrell RL. Olfactory esthesioneuroma blastoma: the University of Virginia experience 1960–1985. Laryngoscope. 1990;100:1199.
22. Schwaab G, Micheau C, Pacheco L, et al. Olfactory esthesioneuroma: a report of 40 cases. Laryngoscope. 1988;98:872.
23. Batsakis JG, Regezi JA, Solomon AR, et al. The pathology of head and neck tumors: mucosal melanomas, part 13. Head Neck Surg. 1982;4:404.
24. Trapp TK, Fu YS, Calcaterra TC. Melanoma of the nasal and paranasal sinus mucosa. Arch Otolaryngol Head Neck Surg. 1987;113:1086.
25. Rosenthal DI, Barker JL, El-Naggar AK, et al. Sinonasal malignancies with neuroendocrine differentiation: patterns of failure according to histologic phenotype. Cancer. 2004;101:2567-2573.
26. Levine PA, Frierson HFJr, Stewart FM, et al. Sinonasal undifferentiated carcinoma: a distinctive and highly aggressive neoplasm. Laryngoscope. 1987;97:905.
27. Cantu G, Bimbi G, Miceli R, et al. Lymph node metastasis in malignant tumors of the paranasal sinuses: prognostic value and treatment. Arch Otolaryngol Head Neck Surg. 2008;2:170-177.
28. Kim GE, Chung EJ, Lim JJ, et al. Clinical significance of neck node metastasis in squamous cell carcinoma of the maxillary antrum. Am J Otolaryngol. 1999;20:383-390.
29. Porceddu S, Martin J, Shanker G, et al. Paranasal sinus tumors: Peter Maccallum Cancer Institute Experience. Head Neck. 2004;26:322-330.
30. Myers LL, Nussenbaum B, Bradford CR, et al. Paranasal sinus malignancies: an 18-year single institution experience. Laryngoscope. 2002;112:1964-1969.
31. Anderson JE. The cranial nerves. In Anderson JE, editor: Grant’s Atlas of Anatomy, 7th ed, Baltimore: Williams & Wilkins, 1978. Section 8–6
32. Som PM, Shapiro MD, Biller HF, et al. Sinonasal tumors and inflammatory tissues: differentiation with MR imaging. Radiology. 1988;167:803.
33. Daniels DL, Pech P, Pojunas KW, et al. Trigeminal nerve: anatomical correlation with MR imaging. Radiology. 1986;159:577.
34. Virapongse C, Mancuso A, Fitzsimmons J. Value of magnetic resonance imaging in assessing bone destruction in head and neck lesions. Laryngoscope. 1986;96:284.
35. Chapter 6: Nasal cavity and paranasal sinuses. In Greene FL, Page DL, Fleming ID, et al, editors: AJCC Cancer Staging Manual, 6th ed, New York: Springer, 2002.
36. Harrison LB, Pfister DG, Kraus D, et al. Management of unresectable malignant tumors at the skull base using concomitant chemotherapy and radiotherapy with accelerated fractionation. Skull Base Surg. 1994;4:127.
37. Cheesman AD, Lund VJ, Howard DJ. Craniofacial resection for tumors of the nasal cavity and paranasal sinuses. Head Neck Surg. 1986;8:429.
38. Imola MJ, Schramm VLJr. Orbital preservation in surgical management of sinonasal malignancy. Laryngoscope. 2002;112:1357.
39. Carrau RL, Segas J, Nuss DW, et al. Squamous cell carcinoma of the sinonasal tract invading the orbit. Laryngoscope. 1999;109:230.
40. Carrau RL, Myers EN, Johnson JT. Paranasal sinus carcinoma—diagnosis, treatment and prognosis. Oncology. 1992;6:43.
41. Shidnia H, Horseback NB, Saghafi N, et al. The role of radiation therapy in treatment of malignant tumors of the paranasal sinuses. Laryngoscope. 1984;94:102.
42. Wang CC. Carcinoma of the paranasal sinuses. In: Wang CC, editor. Radiation therapy for head and neck neoplasms: indications, techniques, and results. 2nd ed. Littleton, Mass: Year Book Medical; 1990:294.
43. Griffin TW, Pajak TF, Laramore GE, et al. Neutron vs photon irradiation of inoperable salivary gland tumors: results of an RTOG-MRC cooperative randomized study. Int J Radiat Oncol Biol Phys. 1988;15:1085.
44. Chen AM, Bucci MK, Quivey JM, et al. Long-term outcome of patients treated by radiation therapy alone for salivary gland carcinomas. Int J Radiat Oncol Biol Phys. 2006;66:1044-1050.
45. Rasch C, Keus R, Pameijer FA, et al. The potential impact of CT-MRI matching on tumor volume delineation in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 1997;39:841.
46. Tsujii H, Kamada T, Arimoto T, et al. The role of radiotherapy in the management of maxillary sinus carcinoma. Cancer. 1986;57:2261.
47. Claus F, Mijnheer B, Rasch C, et al. Report of a study of IMRT planning strategies for ethmoid sinus cancer. Strahlenther Onkol. 2002;178:572.
48. Roa WH, Hazuka MB, Sandler HM, et al. Results of primary and adjuvant CT-based three-dimensional radiotherapy for malignant tumors of the paranasal sinuses. Int J Radiat Oncol Biol Phys. 1994;28:857.
49. Martel MK, Sandler HM, Cornblath WT, et al. Dose-volume complication analysis for visual pathway structures of patients with advanced paranasal sinus tumors. Int J Radiat Oncol Biol Phys. 1997;38:273.
50. Huang D, Xia P, Akazawa P, et al. Comparison of treatment plans using intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:158-168.
51. Pacholke HD, Amdur RJ, Louis DA, et al. The role of intensity modulated radiation therapy for favorable stage tumor of the nasal cavity or ethmoid sinus. Am J Clin Oncol. 2005;28:474-478.
52. Claus F, de Gersem W, de Wagter C, et al. An implementation strategy for IMRT of ethmoid sinus cancer with bilateral sparing of the optic pathways. Int J Radiat Oncol Biol Phys. 2001;51:318-331.
53. Adams EJ, Nutting CM, Convery DJ, et al. Potential role of intensity-modulated radiotherapy in the treatment of tumors of the maxillary sinus. Int J Radiat Oncol Biol Phys. 2001;51:579-588.
54. Wang Z, Zhang Z, Dong L, et al. Effectiveness of noncoplanar IMRT planning using a parallelized multiresolution beam angle optimization method for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2005;63:594-601.
55. Tsien C, Eisbruch A, McShan D, et al. Intensity-modulated radiation therapy (IMRT) for locally advanced paranasal sinus tumors: incorporating clinical decisions in the optimization process. Int J Radiat Oncol Biol Phys. 2003;55:776-784.
56. Chen AM, Daly ME, Bucci MK, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys. 2007;69:141-147.
57. Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int J Radiat Oncol Biol Phys. 2007;67:691-702.
58. Ernst-Stecken A, Lambrecht U. Dose escalation in large anterior skull-base tumors by means of IMRT. First experience with the Novalis system. Strehlenther Onkol. 2006;182:183-189.
59. Hoppe BS, Wolden SL, Zelefsky MJ, et al. Postoperative intensity-modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands: technique, early outcomes, and toxicity. Head Neck. 2008;30:925-932.
60. Balter JM, Kessler ML. Imaging and alignment for image-guided radiation therapy. J Clin Oncol. 2007;25:931-937.
61. Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67:670-677.
62. Purdy JA. Dose to normal tissues outside the radiation therapy patient’s treated volume: a review of different radiation therapy techniques. Health Phys. 2008;95:666-676.
63. Chen AW, Liebsch NJ. Proton radiation therapy for head and neck cancer. J Surg Oncol. 2008;97:697-700.
64. Mock U, Georg D, Bogner J, et al. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147-154.
65. Resto VA, Chan AW, Deschler DG, et al. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2007;30:222-229.
66. Habermann W, Zanarotti U, Groell R, et al. Combination of surgery and gamma knife radiosurgery—a therapeutic option for patients with tumors of nasal cavity or paranasal sinuses infiltrating the skull base. Acta Otorhinolaryngol Ital. 2002;22:74.
67. Leavitt DD, Watson G, Tobler M, et al. Intensity-modulated radiosurgery/radiotherapy using a micromultileaf collimator. Med Dosim. 2001;26:143.
68. LoRusso P, Tapazoglou E, Kish J, et al. Chemotherapy for paranasal sinus carcinoma. Cancer. 1988;62:1.
69. Vokes EE, Moran WJ, Mick R, et al. Neoadjuvant and adjuvant methotrexate, cisplatin, and fluorouracil in multimodal therapy of head and neck cancer. J Clin Oncol. 1989;7:838.
70. Sakai SM, Hohki A, Fuchihata H, et al. Multidisciplinary treatment of maxillary sinus carcinoma. Cancer. 1983;52:1360.
71. Sato Y, Morita M, Takahashi HO, et al. Combined surgery, radiotherapy, and regional chemotherapy in carcinoma of the paranasal sinuses. Cancer. 1970;25:571.
72. Moseley HS, Thomas LR, Everts EC, et al. Advanced squamous cell carcinoma of the maxillary sinus. Am J Surg. 1981;141:522.
73. Lee YY, Dimery IW, Van Tassel P, et al. Superselective intra-arterial chemotherapy of advanced paranasal sinus tumors. Arch Otolaryngol Head Neck Surg. 1989;115:503.
74. Biller HF, Lawson W, Sachdev VP, et al. Esthesioneuroblastoma: surgical treatment without radiation. Laryngoscope. 1990;100:1199.
75. Elkon D, Hightower SI, Lim ML, et al. Esthesioneuroblastoma. Cancer. 1979;44:1087.
76. Olsen KD, DeSanto LW. Olfactory neuroblastoma: biologic and clinical behavior. Arch Otolaryngol. 1983;109:797.
77. Goldsweig HG, Sundaresan N. Chemotherapy of recurrent esthesioneuroblastoma. Case report and review of the literature. Am J Clin Oncol. 1990;13:139.
78. Spaulding CA, Krayak MS, Constable WC, et al. Esthesioneuroblastoma: a comparison of two treatment eras. Int J Radiat Oncol Biol Phys. 1988;15:581.
79. Wade PMJr, Smith RE, Johns ME. Response of esthesioneuro blastoma to chemotherapy: Report of five cases and review of the literature. Cancer. 1984;53:1036.
80. Fitzek MM, Thronton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;15:2623.
81. McElroy EAJr, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: The Mayo Clinic experience. Neurosurgery. 1998;42:1023.
82. Smalley SR, Cupps RE, Anderson JA, et al. Polymorphic reticulosis limited to the upper digestive tract. Natural history and radiotherapeutic considerations. Int J Radiat Oncol Biol Phys. 1988;15:581.
83. Vidal RW, Devaney K, Ferlito A, et al. Sinonasal malignant lymphomas: a distinct clinopathological category. Ann Otol Rhinol Laryngol. 1999;108:411.
84. Hausdorff J, Davis E, Long G, et al. Non-Hodgkin’s lymphomas of the paranasal sinuses: clinical and pathological features, and response to combined-modality therapy. Cancer J Sci Am. 1997;3:303.
85. Berry MP, Jenkin RDT. Parameningeal rhabdomyosarcoma in the young. Cancer. 1981;48:281.
86. Katz TS, Mendenhall WM, Morris CG, et al. Malignant tumors of the nasal cavity and paranasal sinuses. Head and Neck. 2002;24:821.
87. Takeda A, Shigematsu N, Suzuki S, et al. Late retinal complications of radiation therapy for nasal and paranasal malignancies: relationship between irradiated-dose area and severity. Int J Radiat Oncol Biol Phys. 1999;44:599.
88. Pommier P, Ginestet C, Sunyach M, et al. Conformal radiotherapy for paranasal sinus and nasal cavity tumors: three-dimensional treatment planning and preliminary results in 40 patients. Int J Radiat Oncol Biol Phys. 2000;48:485.
89. Meyers CA, Geara F, Wong PF, et al. Neurocognitive effects of therapeutic irradiation for skull base tumors. Int J Radiat Oncol Biol Phys. 2000;46:51.
90. Giri SPG, Reddy EK, Gemer LS, et al. Management of advanced squamous cell carcinoma of the maxillary sinus. Cancer. 1992;69:657.
91. Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007;67:151-157.
92. Jansen EP, Keus RB, Hilgers FJ, et al. Does the combination of radiotherapy and debulking surgery favor survival in paranasal sinus carcinoma? Int J Radiat Oncol Biol Phys. 2000;48:27-35.
93. Cheng VS, Wang CC. Carcinomas of the paranasal sinuses: a study of sixty-six cases. Cancer. 1977;40:3038-3041.
94. Carinci F, Curioni C, Padula E, et al. Cancer of the nasal cavity and paranasal sinuses: a new staging system. Int J Oral Maxillofac Surg. 1996;25:34-39.
95. Blanco AI, Chao KS, Ozyigit G, et al. Carcinoma of the paranasal sinuses: long-term outcomes with radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:51-58.
96. Waldron JN, O’Sullivan B, Warde P, et al. Ethmoid sinus cancer: twenty-nine cases managed with primary radiation therapy. Int J Radiat Oncol Biol Phys. 1994;28:857-865.
97. Orvidas LJ, Lewis JE, Weaver AL, et al. Adenocarcinoma of the nose and paranasal sinuses: a retrospective study of diagnosis, histologic characteristics, and outcome in 24 patients. Head Neck. 2005;27:370-375.
98. Spiro JD, Soo KC, Spiro RH. Nonsquamous cell malignant neoplasms of the nasal cavities and paranasal sinuses. Head Neck. 1995;17:114-118.
99. Lee F, Ogura JH. Maxillary sinus carcinoma. Laryngoscope. 1981;91:133.
100. Dulguerov P, Jacobden MS, Allal AS, et al. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92(12):3012.
101. Myers LL, Nussenbaum B, Bradford CR, et al. Paranasal sinus malignancies: an 18-year single institution experience. Laryngoscope. 2002;112:1964.
102. Le QT, Fu KK, Kaplan MJ, et al. Lymph node metastasis in maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys. 2000;46:541.
103. Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys. 1997;39:283.
104. Dirix P, Nuyts S, Geussens Y, et al. Malignancies of the nasal cavity and paranasal sinuses: long-term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:1042-1050.
105. Goepfert H, Luna MA, Lindberg RD, et al. Malignant salivary gland tumors of the paranasal sinuses and nasal cavity. Arch Otolaryngol. 1983;109:662.
106. Naficy S, Disher MJ, Esclamdo RM. Adenoid cystic carcinoma of the paranasal sinuses. Am J Rhinol. 1999;14:311.
107. Claus F, Boterberg T, Ost P, et al. Postoperative radiotherapy for adenocarcinoma of the ethmoid sinuses: treatment results for 47 patients. Int J Radiat Oncol Biol Phys. 2002;54:1089.
108. Hoppe BS, Nelson CJ, Gomez DR, et al. Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2008;72:763-769.
109. Harrison LB, Raben A, Pfister DG, et al. A prospective phase II trial of concomitant chemotherapy and radiotherapy with delayed accelerated fractionation in unresectable tumors of the head and neck. Head Neck. 1998;20:497.
110. Lee MM, Vokes EE, Rosen A, et al. Multimodality therapy in advanced paranasal sinus carcinoma: superior long-term results. Cancer J Sci Am. 1999;5:219.
111. Bristol IJ, Ahamad A, Garden AS, et al. Postoperative radiotherapy for maxillary sinus cancer: long-term outcomes and toxicities of treatment. Int J Radiat Oncol Biol Phys. 2007;68:719-730.
112. Hu YH, Tu GY, Qi YQ, et al. Comparison of pre- and postoperative radiation in the combined treatment of carcinoma of maxillary sinus. Int J Radiat Oncol Biol Phys. 1982;8:1045-1049.
113. Issacs JH, Mooney S, Mendenhall WM, et al. Cancer of the maxillary sinus treated with surgery and/or radiation therapy. Am Surg. 1990;56:327-330.
114. Zaharia M, Salem LE, Travezan R, et al. Postoperative radiotherapy in the management of cancer of the maxillary sinus. Int J Radiat Oncol Biol Phys. 1989;17:967-971.