Xanthines
After reading this chapter, the reader will be able to:
2. List all available xanthines used in respiratory therapy
3. Differentiate between the clinical indications of xanthines
4. Differentiate between the uses of xanthines
5. Discuss the proposed theories of activity for xanthines
Clinical indications for the use of xanthines
Use in asthma
Sustained-release theophylline is indicated as an alternative for maintenance (step 2) therapy of mild, persistent asthma and higher in patients older than 5 years of age and is listed as an alternative in step 3 and higher for patients older than 5 years of age in combination with an inhaled corticosteroid (ICS). Sustained-release theophylline is considered a less preferred alternative to low-dose ICS, cromolynlike agents, or antileukotrienes as second-line maintenance drug therapy in stable asthma. Theophylline is not recommended in the guidelines for children younger than 5 years or for any person with acute exacerbation of asthma.1
Use in chronic obstructive pulmonary disease
Current guidelines for the treatment of stable COPD state that bronchodilators are central to symptom management. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) states that inhaled bronchodilators are preferred when available. Theophylline is considered effective in COPD but, because of potential toxicity, is recommended as an alternative to inhaled bronchodilators such as β2 agonists or anticholinergic agents.2 Barr and associates3 conducted a meta-analysis on the use of xanthines for the treatment of COPD exacerbations. There findings suggest that xanthines should not be used in the treatment of COPD exacerbations. In randomized controlled trials, intravenous aminophylline has not shown any significant benefit over β2 agonists and anticholinergic therapy for COPD patients.4,5
Use in apnea of prematurity
If pharmacologic therapy is needed to stimulate breathing in apnea of prematurity, methylxanthines are considered the first-line agents of choice. Theophylline has been used most extensively, but Bhatia6 suggested that caffeine citrate may be the agent of choice. Caffeine citrate penetrates the cerebrospinal fluid better and has a higher therapeutic index with fewer side effects compared with theophylline. Caffeine citrate (Cafcit) has been approved for administration either intravenously or orally.
Specific xanthine agents
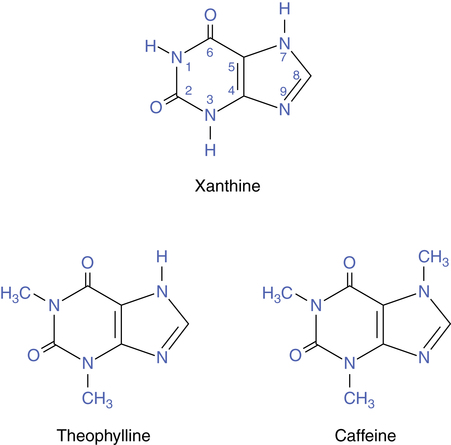
Theophylline is related chemically to the natural metabolite xanthine, which is a precursor of uric acid. Figure 8-1 shows the general xanthine structure and the structures of theophylline (1,3-dimethylxanthine) and caffeine (1,3,7-trimethylxanthine). Because of their methyl attachments, these agents are often referred to as methylxanthines. Another xanthine is theobromine. All three agents are found as alkaloids in plant species. Caffeine is found in coffee beans and kola nuts. Caffeine and theophylline are contained in tea leaves, and caffeine and theobromine are in cocoa seeds or beans. Historically, these natural plant substances have been used as brews for their stimulant effect.
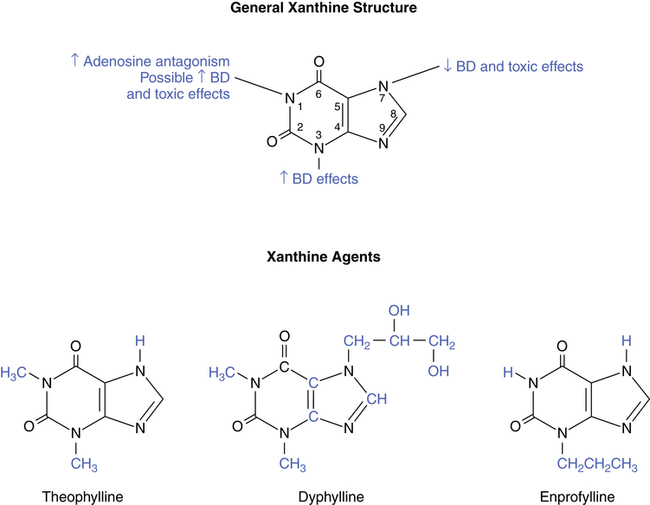
There are several synthetic modifications to the naturally occurring methylxanthines, including dyphylline (7-[2,3-dihydroxypropyl]theophylline), proxyphylline (7-[2-hydroxypropyl]theophylline), and enprofylline (3-propylxanthine). Table 8-1 lists xanthine derivatives, brand names, and available formulations. Theophylline is available in a variety of formulations, including sustained-release oral forms, as aminophylline for oral or intravenous administration, and rectal suppository forms.
TABLE 8-1
| XANTHINE DERIVATIVE | BRAND NAMES | FORMULATIONS |
| Theophylline | Theochron, Elixophyllin, Theo-24 | Tablets, capsules, syrup, elixir, extended-release tablets, capsules, injection |
| Oxtriphylline | Choledyl SA | Tablets, syrup, elixir, sustained-release tablets |
| Aminophylline | Aminophylline | Tablets, oral liquid, injection, suppositories |
| Dyphylline | Lufyllin | Tablets, elixir |
General pharmacologic properties
The xanthine group has the following general physiologic effects in humans:
• Central nervous system (CNS) stimulation
• Bronchial, uterine, and vascular smooth muscle relaxation
Caffeine and theophylline differ in the intensity of the effects listed previously. These differences are summarized in Table 8-2. Caffeine has more CNS-stimulating effect than theophylline, and this includes ventilatory stimulation. In clinical use, theophylline is generally classified as a bronchodilator because of the relaxing effect on bronchial smooth muscle.
TABLE 8-2
Differences in Intensity of Effects for Caffeine and Theophylline
| EFFECT | CAFFEINE | THEOPHYLLINE |
| Central nervous system stimulation | +++ | ++ |
| Cardiac stimulation | + | +++ |
| Smooth muscle relaxation | + | +++ |
| Skeletal muscle stimulation | +++ | ++ |
| Diuresis | + | +++ |
Structure-activity relationships
Figure 8-2 illustrates the general xanthine structure and the effect of attachments at various sites on the molecule. Also, the chemical structure of theophylline is shown in comparison with the theophylline derivatives dyphylline and enprofylline. The methyl attachments at the nitrogen-1 and nitrogen-3 positions for theophylline enhance its bronchodilating effect and its toxic side effects, which are discussed later in this chapter. In contrast, the structure of caffeine (see Figure 8-1) has an additional methyl group at the nitrogen-7 position, decreasing its bronchodilator effect in relation to theophylline. Dyphylline has the same methyl attachments at the nitrogen-1 and nitrogen-3 positions as theophylline but also has a large attachment at the nitrogen-7 position that decreases its bronchodilator potential. Enprofylline, which is not clinically available in the United States at this time, has potent bronchodilating effects, probably because of the large substitution at the nitrogen-3 position.
Proposed theories of activity
The exact mechanism of action of xanthines, and theophylline in particular, is unknown.7 It was originally thought that xanthines caused smooth muscle relaxation by inhibition of phosphodiesterase (PDE), leading to an increase in intracellular cyclic adenosine 3′,5′-monophosphate (cAMP). An increase in cAMP causes relaxation of bronchial smooth muscle. The effect of increased cAMP is described in Chapter 6 in the discussion of β-adrenergic agents. Use of this explanation to account for therapeutic xanthine actions has been questioned, however. Several alternative theories concerning the action of xanthines have been proposed in addition to PDE inhibition. Each of the proposed theories of activity for xanthines is briefly described and commented on in the following sections.
Inhibition of phosphodiesterase
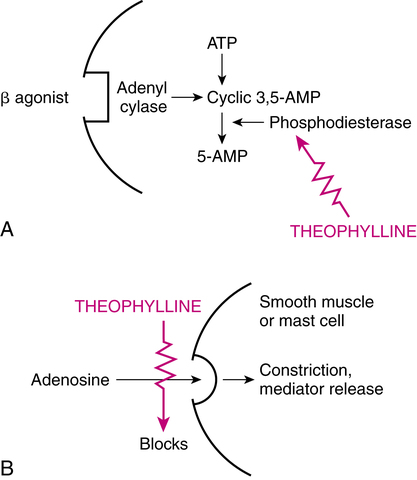
Theophylline is a weak and nonselective inhibitor of cAMP-specific PDE. The pathway by which this inhibition can lead to an increase in intracellular cAMP, with consequent bronchial relaxation or antiinflammatory effects, is illustrated in Figure 8-3, A. However, at the dosage levels used clinically in humans, theophylline is a poor inhibitor of the enzyme.8 As a result, this may not be the best theory on how xanthines exert a therapeutic effect. PDE is a generic term referring to at least 11 distinct families that have been identified as hydrolyzing cAMP or cyclic guanosine 3′,5′-monophosphate (cGMP) and that have unique tissue and subcellular distributions. The various PDE families differ in substrate specificity, inhibitor sensitivity, and cofactor requirements.7 There are two cAMP-hydrolyzing PDEs, referred to as PDE3 and PDE4, that may play a role in asthma. PDE4 is expressed in airway smooth muscle, pulmonary nerves, and many proinflammatory and immune cells. PDE4 inhibitors suppress processes thought to contribute to asthma inflammation by blocking the degradation of cAMP in target cells and tissue. The antiinflammatory effect of theophylline and xanthines is reviewed in the discussion of the clinical application of these drugs in COPD and asthma.
Antagonism of adenosine
An alternative explanation of bronchodilation is that theophylline acts by blocking the action of adenosine. This mechanism is illustrated in Figure 8-3, B. Adenosine is a purine nucleoside that can stimulate A1 and A2 receptors. A1-receptor stimulation inhibits cAMP, whereas A2-receptor stimulation increases cAMP. Inhaled adenosine has produced bronchoconstriction in asthmatic patients. Theophylline is a potent inhibitor of both A1 and A2 receptors and could block smooth muscle contraction mediated by A1 receptors.
This explanation is contradicted by the action of enprofylline, which is about five times more potent than theophylline for relaxing smooth muscle yet lacks a sufficient attachment at the nitrogen-1 position to provide adenosine antagonism.9 This can be seen in Figure 8-2 by comparing the structures of theophylline and enprofylline. In addition, A1 receptors are sparse in smooth muscle, and isolated animal tissue preparations have shown smooth muscle relaxation through adenosine stimulation of the A2 receptors.
Catecholamine release
A third explanation of xanthine action is that these agents cause the production and release of endogenous catecholamines, which could cause muscle tremor, tachycardia, and bronchial relaxation. Studies on plasma levels of catecholamines such as epinephrine have reported conflicting results, with both an increase and no change reported.10
Titrating theophylline doses
Equivalent doses of theophylline salts
The standard with which salts of theophylline are compared is anhydrous theophylline. Anhydrous theophylline is 100% theophylline. By contrast, salts of theophylline such as oxtriphylline (Choledyl SA) are not pure theophylline by weight. A 400-mg dose of Choledyl SA contains approximately 256 mg of theophylline, whereas Theo-24 (a brand of theophylline) is 100% anhydrous theophylline. A 200-mg dose of Choledyl SA does not give the same amount of theophylline as a 200-mg dose of Theo-24. Table 8-3 lists the equivalent dose of pure theophylline provided by the salts, such as oxtriphylline and aminophylline.
TABLE 8-3
| THEOPHYLLINE SALT | THEOPHYLLINE (%) | EQUIVALENT DOSE (mg) |
| Theophylline anhydrous | 100 | 100 |
| Theophylline monohydrate | 91 | 110 |
| Aminophylline anhydrous | 86 | 116 |
| Aminophylline dihydrate | 79 | 127 |
| Oxtriphylline | 64 | 156 |
Serum levels of theophylline
In 1972, Jenne and colleagues11 indicated that the optimal serum theophylline level for maximal bronchodilation in adults was 10 to 20 μg/mL. The effects associated with a range of serum levels are as follows:
• No effects seen: Less than 5 μg/mL
• Therapeutic range: 10 to 20 μg/mL
• Nausea: greater than 20 μg/mL
Since the originally proposed serum levels of 10 to 20 μg/mL, the recommended range has been changed to a more conservative 5 to 15 μg/mL for the management of asthma.12 GOLD recommendations for the use of theophylline in COPD suggest a target serum level of 5 to 10 μg/mL.2 Both of these ranges seek maximal therapeutic effect with minimal toxicity and side effects. It is stressed that the ranges listed for toxic effects are general. It is possible for an individual to bypass the nauseous phase of toxicity and immediately enter the seizure phase.
Although there is a dose-related response to higher serum levels of theophylline, there is evidence that the response does not continue at the same rate of increase as levels increase. The improvement in forced expiratory volume in 1 second (FEV1) tends to flatten above a serum level of 10 to 12 μg/mL, whereas the toxic effects of theophylline (discussed subsequently) tend to increase even within the therapeutic range of 10 to 20 μg/mL.13
Theophylline toxicity and side effects
An important problem with the use of theophylline is its narrow therapeutic margin; there is very little difference between the dose and serum level that give therapeutic benefit and that cause toxic side effects. Even within the therapeutic serum levels of 10 to 20 μg/mL, distressing side effects can be experienced. To reduce side effects, inhaled theophylline is being studied.14 The most common adverse reactions usually seen with theophylline are listed in Box 8-1.
Reactions to levels of theophylline also can be unpredictable from patient to patient. Studies are cited in which reported serum levels of 78.5 μg/mL and 104.8 μg/mL caused only gastrointestinal symptoms, whereas mean levels of 35 μg/mL caused cardiac arrhythmias or seizures.13 Also, minor side effects may provide little warning before serious toxic effects such as arrhythmias or seizures occur.
Factors affecting theophylline activity
Theophylline is metabolized in the liver and eliminated by the kidneys. Any condition that affects these organs can affect theophylline levels in the body. Interactions between other drugs and theophylline can affect serum levels of the drug. Some common drugs and conditions that increase or decrease theophylline levels are listed in Box 8-2.
Viral hepatitis or left ventricular failure can cause elevated serum levels of theophylline for a given dose because of decreased liver metabolism of the drug. An opposite effect—decreased serum levels—is caused by cigarette smoking, which stimulates the production of liver enzymes that inactivate methylxanthines15; this necessitates higher theophylline doses. Some drugs used for the treatment of tuberculosis, such as isoniazid, and the loop diuretics, such as furosemide (Lasix) or bumetanide (Bumex), are unpredictable in their effect and may either increase or decrease theophylline levels. Measurement of serum levels is extremely important when using these agents with theophylline.
Clinical uses of theophylline
More recent guidelines for the pharmacologic management of asthma and COPD do not indicate theophylline as first-line therapy.1,2 The disadvantages of theophylline are its narrow therapeutic margin, toxic effects, unpredictable blood levels and need for individual dosing, and numerous drug-drug and drug-condition interactions. The use of theophylline in asthma and COPD is described in the following sections.
Use in asthma
The role of theophylline preparations in the management of acute and stable asthma and COPD has been debated.16,17 In the treatment of asthma, theophylline is suggested for use after reliever agents, such as a β2 agonist, and other controller agents, such as ICS, or mediator antagonists (cromolyn-like drugs) targeting the underlying inflammation.1,2
Adachi and colleagues18 compared a combination of salmeterol and fluticasone propionate with a combination of fluticasone and sustained-release theophylline. Results favored the salmeterol/fluticasone combination in the treatment of moderate asthmatics.
Use in chronic obstructive pulmonary disease
Use as a maintenance agent in COPD is indicated if anticholinergics and β2 agonist fail to provide adequate control. Development of long-acting β2 agonists, such as salmeterol, formoterol, and arformoterol, offers an additional drug choice to preserve lung function in COPD before using theophylline, especially if theophylline was used to prevent nocturnal symptoms. The increased FEV1 with salmeterol gives more consistent improvement in lung function on a 12-hour basis and maintains a higher baseline of lung function.19 Theophylline and its salt, aminophylline, are listed as bronchoactive agents for managing an acute exacerbation of COPD in the GOLD guidelines; however, use of other bronchodilators is preferred.2
Nonbronchodilating effects of theophylline
Although theophylline is classified as a bronchodilator, it has a relatively weak bronchodilating action. The efficacy of theophylline in obstructive lung disease may be due to its nonbronchodilating effects on ventilation. This concept of the effectiveness of theophylline is consistent with the finding of significant clinical improvement despite little increase in expiratory flow rates in asthmatic patients.20 Mahler and colleagues21 documented the effect of theophylline in reducing dyspnea in patients with COPD when there was no reversibility of obstruction and no objective improvement in lung function, gas exchange, or exercise performance capability. The nonbronchodilating effects of theophylline are listed with a brief commentary.
Respiratory muscle strength
Theophylline can increase the force of respiratory muscle contractility, and this effect is thought to inhibit or reverse muscle fatigue and subsequent ventilatory failure. Theophylline can have the same effect on skeletal limb muscle. Aubier and associates22 showed increased diaphragmatic strength and transdiaphragmatic pressure generation by using electromyographic stimuli before and after theophylline administration.
Respiratory muscle endurance
Methylxanthines also show evidence of increasing respiratory muscle endurance and strength. Fatigue of the respiratory muscles can be prevented, especially with increased resistance. Xanthines have been shown to increase the time that an external inspiratory load could be sustained.20
Central ventilatory drive
Methylxanthines have also been shown to increase ventilatory drive at the level of the CNS. In particular, theophylline can increase phrenic nerve activity for a given level of chemical stimulus.21 This effect on ventilatory drive seems to occur at the level of the midbrain and may involve the neurotransmitter dopamine.
Antiinflammatory effects
Theophylline also has some antiinflammatory effects, which may explain its efficacy despite the fact that the drug is a relatively weak bronchodilator.16,24 Evidence indicates that theophylline can produce some degree of immunomodulation and an antiinflammatory and bronchoprotective effect through inhibition of cAMP-specific PDE enzymes, particularly PDE3 and PDE4, in proinflammatory cells and tissues. Theophylline has been shown to cause the following (see references for detailed antiinflammatory effects of theophylline16,24-28):
• Decreased migration of activated eosinophils into bronchial mucosa with allergen stimuli and reduced eosinophil survival
• Reduced T-cell proliferation and accumulation in atopic asthma with corresponding improvement in pulmonary function
• Inhibition of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α, and interferon-γ, and increased production of the antiinflammatory cytokine IL-10
• Attenuation of the late phase response to histamine in patients with allergic asthma
• Reduced airway responsiveness to stimuli such as histamine, methacholine, allergen, sulfur dioxide, distilled water, cyanates, and adenosine
• Increased activation of a group of enzymes, histone deacetylase, also known as lysine deacetylase, which are used by corticosteroids to reduce inflammatory response.
The antiinflammatory and immunomodulating effects of theophylline occur at lower plasma concentrations such as 9 to 10 μg/mL, in contrast to the concentrations usually recommended for bronchodilation, and may result in a steroid-sparing effect.25 Inhibitors of PDE4, with more selectivity than theophylline and reduced toxic effect profile, may offer a new class of antiinflammatory agents.7
Use in apnea of prematurity
When nonpharmacologic methods are unsuccessful in apnea of prematurity, xanthines such as theophylline and caffeine are still considered a first-line choice of drug therapy, as stated in the indications for this class of drug. Theophylline is biotransformed to caffeine in neonates.29 However, caffeine is preferable to theophylline for various pharmacologic reasons, as follows:6
• Caffeine penetrates more readily than theophylline into cerebrospinal fluid and can be effective in infants refractory to theophylline therapy.
• Caffeine is a more potent stimulant of the CNS and the respiratory system than theophylline.
• Dosing regimens are simpler and give more predictable results with caffeine than with theophylline, most likely because of smaller plasma fluctuations with caffeine.
• Caffeine has a wider therapeutic margin, with fewer side effects than theophylline.