CHAPTER 35 Wrist Arthroscopy: The Future
Wrist arthroscopy, first described by Chen in 1979,1 was popularized by Roth and colleagues in an instructional course lecture of the American Academy of Orthopaedic Surgeons almost a decade later.2 Since then, it has undergone substantial developments and has become an essential diagnostic and therapeutic tool for surgeons addressing a multitude of conditions affecting the wrist.
With improved techniques and equipment, procedures that were once performed as open surgery can be undertaken arthroscopically. Although initial therapeutic wrist arthroscopy focused on basic reparative procedures, some of the more complex reconstructive and salvage procedures are being performed with this procedure (Table 35-1), and the indications for wrist arthroscopy continue to expand. This chapter reviews some of the latest advances and discusses future directions for wrist arthroscopy.
TABLE 35-1 Expanding Indications for Wrist Arthroscopy
| Procedures | Soft Tissue | Bone |
|---|---|---|
| Diagnostic | ||
| Removal (“ectomy”) | ||
| Stabilization | ||
| Reconstructive | ||
| Salvage |
DRUJ, distal radioulnar joint; STT, scaphotrapeziotrapezoid; TFCC, triangular fibrocartilage complex.
ASSESSMENT
Arthroscopic examination of the wrist should include the radiocarpal and midcarpal joints and, if indicated, the distal radioulnar joint (DRUJ). Visualization of the pisotriquetral joint can be achieved through a fenestration communicating with the radiocarpal joint (which is present in approximately 50% of the population).3 Traditionally, dorsal 3-4, 4-5, 6-R, and midcarpal portals have been used as diagnostic and working portals (Table 35-2). These portals limit access to the dorsal wrist capsule and impose restrictions on the surgeon’s ability to treat dorsal pathology. The development of volar portals4–6 has provided freedom to explore newer arthroscopic techniques to address certain pathologic conditions such as dorsal wrist ganglion7 or capsular restriction.8
TABLE 35-2 Arthroscopic Wrist Portals: Technique and Comment
| Portals | Technique | Comment |
|---|---|---|
| Dorsal Portals | ||
| 1-2 | Inserted in the extreme dorsum of the snuffbox just radial to the EPL tendon to avoid the radial artery48,49 | Gives access to the radial styloid, scaphoid, lunate, and articular surface of the distal radius |
| 3-4 | Portal is 1 cm distal to Lister’s tubercle between the tendons of the third and fourth compartment | Main working portal; gives a wide range of movement and view |
| 4-5 | Between the common extensor fourth compartment and the EI in the fifth compartment | Usually, the 6-R portal is preferred |
| 6-R | Located distal to the ulnar head and radial to the ECU tendon; portal is established under direct vision through the arthroscope with a needle and avoids damage to the TFCC | Main working portal |
| 6-U | Established under direct vision, similar to the 6-R portal; always use blunt dissection to avoid the dorsal branches of the ulnar nerve | 6-U and 6-R portals allow visualization back toward the radial side and access to the ulna-sided structures |
| DRUJ | Forearm supinated to relax the dorsal capsule; arthroscope is introduced into the axilla between radius and ulna underneath the TFCC | Gives a view of the DRUJ articulation |
| Midcarpal Portals | ||
| MCR | Soft depression palpated between proximal and distal carpal rows, 1 cm distal to the 3-4 portal along a line bordering the radial edge of the third metacarpal | Can be used to get across to the STT joint, scapholunate articulation, and distal pole of scaphoid |
| MCU | Soft depression palpated between proximal and distal carpal rows 1 cm distal to 4-5 portal and in line with the fourth metacarpal | Allows visualization of the distal lunate, lunotriquetral, and triquetral hamate articulation |
| STT | Between EPL and ECRB in the midcarpal row; on the ulnar margin of the EPL tendon; terminal branches of the radial sensory nerve are at risk | Used with the MCR portal for STT débridement |
| Volar Portals | ||
| VR | A 2-cm longitudinal incision is made over the FCR on radial side of volar proximal wrist crease, and the FCR is retracted ulnarly. Radiocarpal joint is identified with a needle, and the port is expanded with artery forceps.3 An inside-out technique also can be used. Work between the RSC and LR ligaments, staying to the radial side of the FCR tendon to avoid the median nerve.2 | Safe zone of 3 mm in all directions with respect to the palmar cutaneous branch of the median nerve (ulnarly) and radial artery (radially)2,3 |
| VU | A 2-cm longitudinal incision is made; the FCU is identified and retracted ulnarly with the ulnar nerve. Working in the interval between the FCU and common flexor tendons, inserting needle into the joint and then expanding the area with an artery forceps4 | Both volar portals are used to assist in reduction of distal radius fracture and to view the dorsal articular surface and dorsal ligaments |
| DRUJ | Use the same mini-open approach as for a VU portal; take care to stay below the TFCC | Gives a view of the DRUJ and deep-sided TFCC tears |
DRUJ, distal radioulnar joint; ECRB, extensor carpi radialis brevis; ECRL, extensor carpi radialis longus; ECU, extensor carpi ulnaris; EDC, extensor digitorum communis; EI, extensor indicis; EPB, extensor pollicis brevis; EPL, extensor pollicis longus; FCR, flexor carpi radialis; FCU, flexor carpi ulnaris; LR, long radiolunate; MCR, midcarpal radial; MCU, midcarpal ulnar; RSC, radioscaphocapitate ligament; STT, scaphotrapeziotrapezoid; TFCC, triangular fibrocartilage complex; VR, volar radial; VU, volar ulnar.
From Bain GI, Munt J, Turner PC. New advances in wrist arthroscopy. Arthroscopy. 2008;24:355-367.
The wrist can be visualized as a box with access through four sides (Fig. 35-1). The arthroscope can be introduced to provide the optimal view of pathology, and any number of portals can be used for instrumentation. The use of multiple portals does not increase the surgical insult significantly as long as standard precautions are taken to protect soft tissue structures, but it can ensure that the surgeon has optimal placement of instruments and the arthroscope.
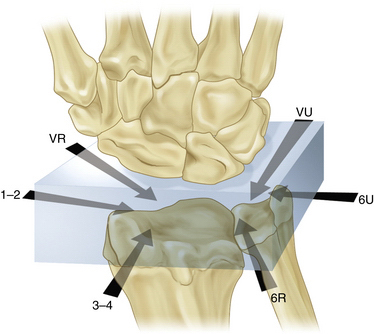
FIGURE 35-1 The box concept of wrist arthroscopy.
(Modified from Bain GI, Munt J, Turner PC. New advances in wrist arthroscopy. Arthroscopy. 2008; 24:355-367.)
Most investigators describe arthroscopy of the wrist in a manner similar to large joint arthroscopy, with infiltration of the joint with fluid (i.e., lactated Ringer’s solution). Dry arthroscopy of the wrist has not been associated with undue procedural difficulty.9 It provides a different perspective of tissue and chondral surfaces. Other benefits of the dry technique include a decreased risk of compartment syndrome and the availability of dry tissue planes in the event of conversion to open surgery. The main role of dry wrist arthroscopy is in the management of intra-articular distal radial fractures,10 but indications may broaden to include carpal fracture management and other reconstructive procedures.
The value of arthroscopy can be augmented by the use of fluoroscopy, which enables accurate intraosseous placement of drills and implants. It also can be used to confirm reduction of fractures or joint diastasis.11
SPECIFIC AREAS OF ADVANCEMENT
Carpal Instability
Assessment of Instability
Numerous radiologic investigations are used to assess carpal instability, including plain radiographs, fluoroscopy, arthrography, computed tomography (CT), and magnetic resonance imaging (MRI). Although three-compartment arthrography can identify intercarpal ligament perforations, it is inadequate for localizing these lesions.12 CT is less sensitive than MRI for detecting intercarpal ligament tears and triangular fibrocartilage complex (TFCC) injuries.13
Arthroscopy is the gold standard for the diagnosis of carpal instability. It has the benefit of giving surgeons direct visualization of the scapholunate and lunotriquetral ligaments and allows them to assess the state of the ligament, the extent of the ligament injury, and whether it is reparable. Associated problems such as hemorrhage, synovitis, chondral damage, and degenerative changes can be assessed at the time of arthroscopy.
Repair Procedures
Arthroscopic débridement alone has been used in the management of scapholunate interosseous ligament (SLIL) and lunotriquetral interosseous ligament (LTIL) injuries. Good results have been achieved from arthroscopic débridement of partial tears of the SLIL and LTIL.14,15 Weiss and colleagues described arthroscopic débridement as sole treatment for complete tears of the SLIL and LTIL.15
There has been interest in the use of ligament and capsular thermal shrinkage in the treatment of interosseous ligament injuries, and early results are promising. Good results have been achieved with arthroscopic débridement and thermal shrinkage of Geissler grade I or II injuries (Table 35-3).16–18 Battistella and coworkers compared outcomes of débridement alone and thermal shrinkage as a sole treatment of Geissler I SLIL injuries.19 They also compared the results of débridement and pinning with those of thermal shrinkage and pinning for Geissler II and III injuries. In both comparisons, superior outcomes were achieved with thermal shrinkage.19 However, the use of thermal shrinkage techniques remains controversial, and no long-term studies have been published to confirm its safety and efficacy.
TABLE 35-3 Geissler Arthroscopic Classification of Carpal Instability
| Grade | Description |
|---|---|
| I | Attenuation or hemorrhage of interosseous ligament as seen from the radiocarpal joint. No incongruence of carpal alignment in the midcarpal space. |
| II | Attenuation or hemorrhage of interosseous ligament as seen from the radiocarpal joint. Incongruence or step-off as seen from midcarpal space. A slight gap (less than width of a probe) between the carpal bones may be present. |
| III | Incongruence or step-off of carpal alignment is seen in the radiocarpal and midcarpal space. The probe may be passed through the gap between the carpal bones. |
| IV | Incongruence or step-off of carpal alignment is seen in the radiocarpal and midcarpal space. There is gross instability with manipulation. A 2.7-mm arthroscope may be passed through the gap between the carpal bones. |
From Geissler WB. Intra-articular distal radius fractures: The role of arthroscopy. Hand Clin. 2005;21:407-416.
In patients with significant instability (Geissler grades II through IV), arthroscopic débridement and percutaneous pinning have produced mixed results.20–22 Whipple’s series supported the concept that the chronicity of the lesion and the degree of instability affect the eventual outcomes.20 In that study, SLIL tears were treated with arthroscopically assisted reduction and percutaneous pinning, and outcomes were compared for patients with less than 3 months’ history and less than 3 mm of scapholunate displacement and for those with symptoms of more than 3 months’ duration and more than 3 mm of displacement. Eighty-five percent of patients in the first group maintained comfort and stability at 2 to 7 years, and 53% of patients in the second group remained symptom free at 1 to 3 years.
The reduction-association scapholunate (RASL) procedure is often performed as an open procedure for chronic scapholunate instability. An arthroscopically assisted method of RASL has been described, with arthroscopy facilitating anatomic reduction and precise placement of the cannulated screw, with the advantage of a three-portal rather than a two-incision approach.23
The future role of arthroscopy in treating carpal instability is likely to include arthroscopically assisted tendon graft reconstruction procedures, such as the modified Brunelli procedure.24
Preferred Technique
For advanced carpal collapse with degenerative changes, salvage procedures, including proximal row carpectomy, limited wrist fusion, or total wrist fusion, may be indicated. These procedures have been performed arthroscopically, although not routinely. Arthroscopic proximal row carpectomy was first described in 1997.25 Ho described techniques for arthroscopic limited wrist fusion and applied these techniques to scaphotrapeziotrapezoid (STT) fusion, scaphoidectomy and four-corner fusion, radioscapholunate fusion, radiolunate fusion, and lunotriquetral fusion.26 The common steps include assessment of radiocarpal and midcarpal articular surfaces to ensure that the joints to be preserved have articular cartilage that can maintain normal wrist movement and loading; cartilage denudation with an arthroscopic burr; correction of carpal deformity with joysticks; provisional fixation with Kirschner wires under fluoroscopic control; arthroscopic bone grafting using autogenous graft or bone graft substitutes introduced through the arthroscope cannula; and definitive fixation with cannulated compression screws. Ho described the use of Foley catheters at the time of grafting to contain graft in the joints where it is wanted. In the future, this may become mainstream practice.
PEARLS& PITFALLS
PITFALLS
Midcarpal Instability
Midcarpal instability (MCI) is a poorly understood entity, and there is no consensus on underlying pathomechanics, nomenclature, or optimal treatment.27 It is characterized by pain and clicking in the wrist, especially during ulnar deviation (i.e., dart thrower’s motion). This can be reproduced clinically under video fluoroscopy; abnormal movement at the midcarpal joint is observed and results in dynamic displacement of the distal carpal row (volarly or dorsally), as described by Lichtman and colleagues.28 The importance of the dorsal radiocarpal ligament and the ulnar arm of the volar arcuate ligament has been demonstrated in anatomic and biomechanical studies.
Arthroscopic thermal shrinkage “capsulorrhaphy” has been described.29 In Mason and Hargreave’s study, 13 patients (15 wrists) were treated for palmar midcarpal instability with arthroscopic thermal shrinkage of the ulnar and radial arms of the arcuate ligament and the accessible parts of the dorsal capsule at the radiocarpal and midcarpal levels.29 Clinical instability improved in all patients postoperatively, as did functional scores. These were early reports, and larger trials with consistent surgical techniques are needed before this technique can be adopted more widely.
Triangular Fibrocartilage Complex Lesions
Type 1b TFCC lesions are described as tears from the distal ulna attachment with or without ulnar styloid fracture. The dorsal and volar radioulnar ligaments have two elements, superficial (distal) and deep (proximal). Various arthroscopic techniques have been described for repair of a superficial tear by suture ligation to the extensor carpi ulnaris (ECU) subsheath and joint capsule. One study looked at the use of holmium:yttrium-aluminium-garnet (Ho:YAG) laser to treat traumatic TFCC lesions, primarily Palmer type 1a central disk rupture and meniscal homologue tears. The advantage of using the Ho:YAG laser for débridement in the wrist joint is that surrounding tissue does not become too hot. Heat is transmitted less than 3 mm in water, and flexible probes are available. The reported results for a cohort of 79 patients were promising, but the report provided no comparative sample and no preoperative functional or symptom data to validate the results.30 Further work is required in this interesting area, including comparison with conventional techniques of arthroscopic débridement.
Injury to the deep elements of the radioulnar ligaments most commonly occurs at the foveal insertion, although radial avulsion has been described. These elements form important primary ligamentous stabilizers of the DRUJ.31 Volar or dorsal dislocation of the ulnar head may be seen, depending on the additional stabilizers that are injured, and is likely to be related to the position of the wrist at the time of injury.
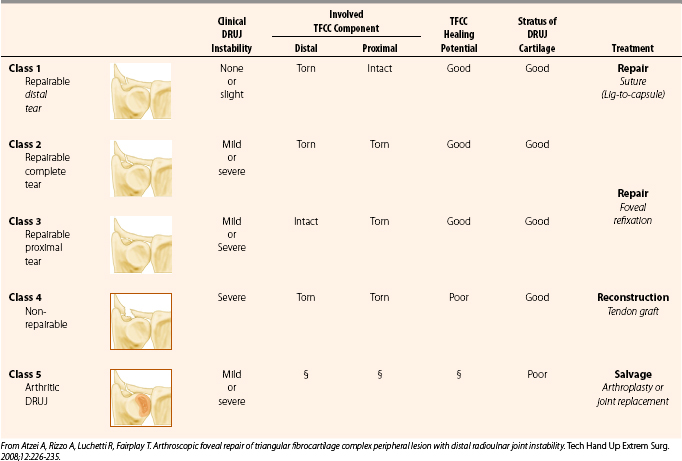
The patient typically complains of ulnar-sided wrist pain after a fall onto an outstretched hand or a violent twisting injury. Clicking may be experienced on forearm rotation. Tenderness may be elicited by palpation over the fovea with the arm in full supination. The deep TFCC lesion may be diagnosed on MRI with gadolinium contrast. In our practice, if clinical examination indicates instability of the DRUJ, a CT scan is performed with the wrist in full supination and in full pronation to assess the osseous architecture of the sigmoid notch and the instability of the ulnar head.32 Further assessment can be performed at the time of arthroscopy. With the arthroscope in the 3-4 portal, the trampoline test can be performed to assess the taughtness of the TFCC. Another useful test is to pass a hook probe through the 6-R portal and under the TFCC through the ulnar-sided tear; if the deep elements are disrupted, the TFCC can be pulled upward and radially.33 DRUJ arthroscopy can be used to directly assess the deep elements of the TFCC and to examine the chondral surface of the distal ulna. A classification specific to traumatic peripheral tears has been described that may guide treatment (Table 35-4).
The important elements that guide treatment decisions are the anatomic structures injured (i.e., distal or proximal elements, or both), the chronicity or retraction of the tear (i.e., how reparable it is), and the presence of arthrosis. For class 1 reparable distal element tears, arthroscopic suture ligation is acceptable. Atzei and colleagues described a technique for treatment of class 2 and 3 reparable complete and deep tears.33 It employed a suture anchor placed in the fovea through a small arthrotomy located 1 cm proximal to the 6-U portal. Positioning of the forearm in full supination moves the styloid dorsally and facilitates access. Curettage and drilling is performed as a mini-open procedure. With arthroscopic assistance, the sutures from the anchor are passed through the TFCC dorsally and palmarly with the aid of a Touhy needle and secured with the use of knot pushers. This technique has produced 95% good or excellent results, as measured with the Mayo wrist score, in 18 patients treated by the originator.33
An alternative technique for treatment of reparable Palmer 1b peripheral lesions is the suture welding technique described by Badia and coworkers,34 who have achieved excellent results in 23 patients. This knotless technique employs an ultrasonic device (Axya Suture Welding System, Axya Inc., Houston, TX) to weld prepositioned 2-0 nylon sutures through the periphery of the TFCC.
Preferred Technique
Our preferred technique for type 2 and 3 lesions, which is also suitable for treatment of type 4 nonreparable deep or combined tears, is a palmaris longus tendon autograft with an arthroscopically assisted mini-open technique that is an extension of the foveal repair concept. The indication for this procedure is DRUJ instability due to a foveal tear. If there is a radial-sided tear, this reconstruction is contraindicated, and an Adams procedure35 is the preferred reconstructive option.
Joint-Based Approach to Kienböck’s Disease
Lichtman’s radiologic classification system provides an assessment of the osseous structure of the lunate. It has been used historically to classify Kienböck’s disease, but it proved to have poor reliability.36 Arthroscopy has the benefit of direct visualization of the pathologic changes through the radiocarpal and midcarpal joints. It enables a joint-based approach to the assessment and management of Kienböck’s disease. As reported by Bain and coworkers, plain radiographs often underscore the severity of the articular surface involvement that can be seen arthroscopically.37
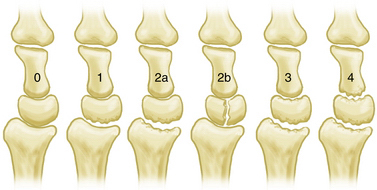
We developed an arthroscopic classification system for the assessment of Kienböck’s disease (Fig. 35-2). It is based on the number of nonfunctional articular surfaces that display extensive fibrillation, fissuring, articular cartilage loss, loose articular cartilage fragments, or osteochondral fractures. Although other changes such as synovitis are not included in the classification system, their presence can help indicate the severity of the chondral damage. This joint-based approach provides an algorithm for assessment of the nonfunctioning joints in a wrist with Kienböck’s disease, and it guides the reconstructive approach to removal of nonfunctioning articulations and restoration of function through mobilization of the unaffected carpal joints.
Preferred Technique
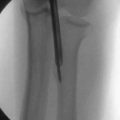
Our grading system for Kienböck’s disease can help guide the management of these cases. Grade 0 disease can be managed with extra-articular procedures such as joint leveling or a revascularization procedure of the lunate. Intraosseous hypertension is a putative cause of Kienböck’s disease. Alexander performed a technique of lunate forage (i.e., core biopsy) for the precollapse lunate, and early results were promising. Using a standard wrist arthroscopy setup and fluoroscopic control, the surgeon passes a 2-mm drill into the lunate through the 6-R portal to perform core decompression (Fig. 35-3). Further investigation of this technique is required to assess its efficacy in preventing progression to collapse and arthrosis.
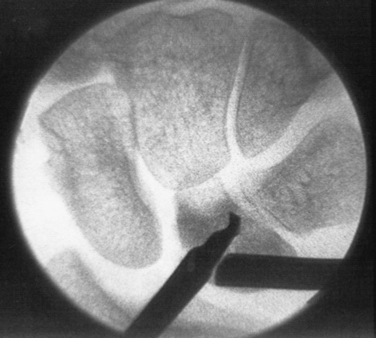
FIGURE 35-3 Core decompression of the lunate for precollapse Kienböck’s disease.
(From Bain GI, Smith ML, Watts AC. Arthroscopic assisted forage for Kienböck’s disease of the lunate. J Hand Surg Am. In press.)
Radioscapholunate fusion can be appropriate treatment for grade 1 or 2a changes, whereas grade 1 or 2b disease can be managed with a proximal row carpectomy. The higher grades of Kienböck’s disease (i.e., grades 3 and 4) require salvage procedures, such as wrist arthrodesis or arthroplasty. The use of arthroscopic débridement in the management of this condition was reported in one series to give excellent pain relief and improve the range of motion after 2 years of follow-up.38
The future role of arthroscopy in the management of Kienböck’s disease is encouraging. Leblebicioglu and colleagues39 reported in a small, prospective, randomized study that arthroscopic scaphocapitate fusion and capitate pole excision in stage IIIA and IIIB Kienböck’s disease resulted in shorter operating time, shorter hospital stay, earlier return to unrestricted daily activities, and equal range of motion and grip strength compared with open scaphocapitate fusion and lunate revascularization.
Ulnar Styloid Carpal Impaction
Ulnar styloid carpal impaction is an uncommon condition that is typically managed with open excision of the ulna styloid.40 Arthroscopic excision of the ulnar styloid can be successfully performed for stylocarpal impaction.41 Fluoroscopy can be used to confirm the correct positioning of the burr, which is then used to débride the ulnar styloid under arthroscopic vision. The TFCC is not violated, and recovery is rapid.
Wrist Ganglia
Ganglia can be excised arthroscopically to reduce scarring and to avoid the capsular wrist stiffness associated with an open resection.42 We have found that rehabilitation is more rapid after arthroscopic excision. Arthroscopy also provides the opportunity to assess any underlying instability of the intrinsic scapholunate ligament, which is implicated as a causative factor for dorsal and volar ganglia.
Initial cohort studies of ganglion excision have been encouraging. Osterman and Raphael reported one recurrence in their series of 150 patients. Rizzo and associates,43 in their series of 41 patients undergoing arthroscopic resection of a dorsal wrist ganglion, reported increased postoperative range of motion and grip strength with no intraoperative and postoperative complications. They had two recurrences requiring two attempts at open excision. They concluded that this was a safe and reliable procedure. Ho and associates44 reported successful evacuation of volar ganglion contents by means of wrist arthroscopy in five cases, with no complications or recurrences reported after 1.6 months of follow-up. A prospective, randomized study of arthroscopic versus open dorsal ganglion excision for 72 patients (41 arthroscopic, 31 open procedures) found no significant difference in rate of recurrence.45 The study suggests that additional long-term comparative studies are needed.
Painful intraosseous carpal ganglia can be treated arthroscopically. Ashwood and Bain reported results of arthroscopic débridement of intraosseous ganglia of the lunate using fluoroscopic guidance through a volar or dorsal portal, depending on the position identified by CT. This technique was found to be safe, with minimal morbidity and recurrence of symptoms during a follow-up period of 1 year.46
Preferred Technique
Our preferred method is that described by Osterman and Raphael.42 The ganglion and the portals are marked preoperatively. The arthroscope is placed in the 6-R portal, and the 1-2 portal is used for instrumentation. In approximately two thirds of patients, a pearl-like stalk can be visualized42; if this stalk is not seen, it is assumed that the origin is from the dorsal capsule, and these cases usually have associated synovitis. A needle is passed into the ganglion externally and advanced to the stalk.
Post-traumatic Contractures and Wrist Arthrofibrosis
Lee and Hausmann proposed a classification for wrist arthrofibrosis based on pathologic anatomic location, in which type I represents intrinsic adhesions and type II represents extrinsic contracture.29 The types are subdivided according to the location of the pathology (Box 35-1). The causes also can be considered as intra-articular, capsular, and extracapsular, and these designations can determine the appropriate method of surgical treatment. Intra-articular and capsular pathology may be best addressed with arthroscopic techniques, whereas extracapsular pathology mandates open surgical approaches.47
Box 35-1 Classification of Wrist Arthrofibrosis
From Geissler WB. Wrist arthrofibrosis. Hand Clin. 2006;22:529-538.
Arthroscopic release of dorsal8 and volar capsular47 contractures has been associated with good patient outcomes. However, the indications must be clear. The ideal patient has a post-traumatic contracture with intact joint surfaces and no carpal instability. Significant preexisting post-traumatic osteoarthritis limits any gains from this procedure and is considered a relative contraindication. Frank carpal instability pattern is considered an absolute contraindication to volar or dorsal release, because the procedure is likely to exacerbate the instability. Likewise, in patients who have conditions that predispose to ulnar translocation (e.g., rheumatoid arthritis, previous radial styloid resection), release of the radioscaphocapitate and the long and short radiolunate ligaments should be performed with caution.
Preferred Technique
When performing arthroscopic volar capsular release, the proximity of the major neurovascular structures must be considered because they are at risk for injury. The procedure is performed through the 3-4 and 6-R portals. At least part of the radioscaphocapitate ligament should be left intact to reduce the risk of ulnar translocation, and instruments should not be used through the volar periarticular fat to avoid neurovascular injury.47
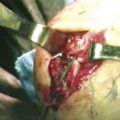
Dorsal capsular release is also performed through the 3-4 and 6-R portals. A nylon tape passed though the 3-4 portal can be railroaded between the extensor tendons, and the dorsal capsule can be used to retract the tendons dorsally (Fig. 35-4).8 This maneuver lessens the risk of injury to the extensor tendons as capsular excision is performed. Resection is carried out with basket forceps introduced through the 3-4 portal.
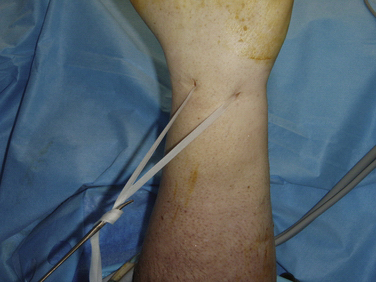
FIGURE 35-4 Dorsal capsulectomy may be assisted with tape to retract the tendons.
(From Bain GI, Munt J, Turner PC. New advances in wrist arthroscopy. Arthroscopy. 2008;24:355-367.)
PEARLS& PITFALLS
PEARLS
CONCLUSIONS
Wrist arthroscopic techniques are being expanded and refined. Many of the procedures discussed in this chapter require advanced techniques, which have evolved from those used in established open techniques. However, as enthusiasts, we must be prepared to examine the benefits of these new techniques with rigorous scientific study.
1. Chen YC. Arthroscopy of the wrist and finger joints. Orthop Clin North Am. 1979;10:723-733.
2. Roth JH, Poehling GG, Whipple TL. Arthroscopic surgery of the wrist. Instr Course Lect. 1988;37:183-194.
3. Arya AP, Kulshreshtha R, Kakarala GK, et al. Visualisation of the pisotriquetral joint through standard portals for arthroscopy of the wrist: a clinical and anatomical study. J Bone Joint Surg Br. 2007;89:202-205.
4. Abe Y, Doi K, Hattori Y, et al. A benefit of the volar approach for wrist arthroscopy. Arthroscopy. 2003;19:440-445.
5. Slutsky DJ. Wrist arthroscopy through a volar radial portal. Arthroscopy. 2002;18:624-630.
6. Tham S, Coleman S, Gilpin D. An anterior portal for wrist arthroscopy: anatomical study and case reports. J Hand Surg Br. 1999;24:445-447.
7. Edwards SG, Johansen JA. Prospective outcomes and associations of wrist ganglion cysts resected arthroscopically. J Hand Surg Am. 2009;34:395-400.
8. Bain GI, Munt J, Turner PC, Bergman J. Arthroscopic dorsal capsular release in the wrist: a new technique. Tech Hand Up Extrem Surg. 2008;12:191-194.
9. del Pinal F, Garcia-Bernal FJ, Pisani D, et al. Dry arthroscopy of the wrist: surgical technique. J Hand Surg Am. 2007;32:119-123.
10. del Pinal F. Dry arthroscopy of the wrist: its role in the management of articular distal radius fractures. Scand J Surg. 2008;97:298-304.
11. Bain GI, Hunt J, Mehta JA. Operative fluoroscopy in hand and upper limb surgery: one hundred cases. J Hand Surg Br. 1997;22:656-658.
12. Chung KC, Zimmerman NB, Travis MT. Wrist arthrography versus arthroscopy: a comparative study of 150 cases. J Hand Surg Am. 1996;21:591-594.
13. Morley J, Bidwell J, Bransby-Zachary M. A comparison of the findings of wrist arthroscopy and magnetic resonance imaging in the investigation of wrist pain. J Hand Surg Br. 2001;26:544-546.
14. Ruch DS, Poehling GG. Arthroscopic management of partial scapholunate and lunotriquetral injuries of the wrist. J Hand Surg Am. 1996;21:412-417.
15. Weiss AP, Sachar K, Glowacki KA. Arthroscopic débridement alone for intercarpal ligament tears. J Hand Surg Am. 1997;22:344-349.
16. Darlis NA, Weiser RW, Sotereanos DG. Partial scapholunate ligament injuries treated with arthroscopic débridement and thermal shrinkage. J Hand Surg Am. 2005;30:908-914.
17. Hirsh L, Sodha S, Bozentka D, et al. Arthroscopic electrothermal collagen shrinkage for symptomatic laxity of the scapholunate interosseous ligament. J Hand Surg Br. 2005;30:643-647.
18. Shih JT, Lee HM. Monopolar radiofrequency electrothermal shrinkage of the scapholunate ligament. Arthroscopy. 2006;22:553-557.
19. Battistella F, Golano P, Taverna E. Arthroscopic thermal shrinkage for scapholunate injuries. In: Slutsky DJ, Nagle D, editors. Techniques in Wrist and Hand Arthroscopy. Philadelphia, PA: Elsevier; 2007:86-92.
20. Whipple TL. The role of arthroscopy in the treatment of scapholunate instability. Hand Clin. 1995;11:37-40.
21. Osterman AL, Seidman GD. The role of arthroscopy in the treatment of lunatotriquetral ligament injuries. Hand Clin. 1995;11:41-50.
22. Darlis NA, Kaufmann RA, Giannoulis F, Sotereanos DG. Arthroscopic débridement and closed pinning for chronic dynamic scapholunate instability. J Hand Surg Am. 2006;31:418-424.
23. Aviles AJ, Lee SK, Hausman MR. Arthroscopic reduction-association of the scapholunate. Arthroscopy. 2007;23(105):e101-e105.
24. Van Den Abbeele KL, Loh YC, Stanley JK, Trail IA. Early results of a modified Brunelli procedure for scapholunate instability. J Hand Surg Br. 1998;23:258-261.
25. Culp RW, Lee Osterman A, Talsania JS. Arthroscopic proximal row carpectomy. Tech Hand Up Extrem Surg. 1997;1:116-119.
26. Ho PC. Arthroscopic partial wrist fusion. Tech Hand Up Extrem Surg. 2008;12:242-265.
27. Lichtman DM, Wroten ES. Understanding midcarpal instability. J Hand Surg Am. 2006;31:491-498.
28. Lichtman DM, Bruckner JD, Culp RW, Alexander CE. Palmar midcarpal instability: results of surgical reconstruction. J Hand Surg Am. 1993;18:307-315.
29. Mason WT, Hargreaves DG. Arthroscopic thermal capsulorrhaphy for palmar midcarpal instability. J Hand Surg Eur. 2007;32:411-416.
30. Infanger M, Grimm D. Meniscus and discus lesions of triangular fibrocartilage complex (TFCC): treatment by laser-assisted wrist arthroscopy. J Plast Reconstr Aesthet Surg. 2009;62:466-471.
31. Nakamura T, Makita A. The proximal ligamentous component of the triangular fibrocartilage complex. J Hand Surg Br. 2000;25:479-486.
32. Lo IK, MacDermid JC, Bennett JD, et al. The radioulnar ratio: a new method of quantifying distal radioulnar joint subluxation. J Hand Surg Am. 2001;26:236-243.
33. Atzei A, Rizzo A, Luchetti R, Fairplay T. Arthroscopic foveal repair of triangular fibrocartilage complex peripheral lesion with distal radioulnar joint instability. Tech Hand Up Extrem Surg. 2008;12:226-235.
34. Badia A, Khanchandani P. Suture welding for arthroscopic repair of peripheral triangular fibrocartilage complex tears. Tech Hand Up Extrem Surg. 2007;11:45-50.
35. Adams BD, Berger RA. An anatomic reconstruction of the distal radioulnar ligaments for posttraumatic distal radioulnar joint instability. J Hand Surg Am. 2002;27:243-251.
36. Goldfarb CA, Hsu J, Gelberman RH, Boyer MI. The Lichtman classification for Kienböck’s disease: an assessment of reliability. J Hand Surg Am. 2003;28:74-80.
37. Bain GI, Begg M. Arthroscopic assessment and classification of Kienböck’s disease. Tech Hand Up Extrem Surg. 2006;10:8-13.
38. Menth-Chiari WA, Poehling GG, Wiesler ER, Ruch DS. Arthroscopic débridement for the treatment of Kienböck’s disease. Arthroscopy. 1999;15:12-19.
39. Leblebicioglu G, Doral MN, Atay AA, et al. Open treatment of stage III Kienböck’s disease with lunate revascularization compared with arthroscopic treatment without revascularization. Arthroscopy. 2003;19:117-130.
40. Topper SM, Wood MB, Ruby LK. Ulnar styloid impaction syndrome. J Hand Surg Am. 1997;22:699-704.
41. Bain GI, Bidwell TA. Arthroscopic excision of ulnar styloid in stylocarpal impaction. Arthroscopy. 2006;22:677e1-677e3.
42. Osterman AL, Raphael J. Arthroscopic resection of dorsal ganglion of the wrist. Hand Clin. 1995;11:7-12.
43. Rizzo M, Berger RA, Steinmann SP, Bishop AT. Arthroscopic resection in the management of dorsal wrist ganglions: results with a minimum 2-year follow-up period. J Hand Surg Am. 2004;29:59-62.
44. Ho PC, Lo WN, Hung LK. Arthroscopic resection of volar ganglion of the wrist: a new technique. Arthroscopy. 2003;19:218-221.
45. Kang L, Akelman E, Weiss AP. Arthroscopic versus open dorsal ganglion excision: a prospective, randomized comparison of rates of recurrence and of residual pain. J Hand Surg Am. 2008;33:471-475.
46. Ashwood N, Bain GI. Arthroscopically assisted treatment of intraosseous ganglions of the lunate: a new technique. J Hand Surg Am. 2003;28:62-68.
47. Verhellen R, Bain GI. Arthroscopic capsular release for contracture of the wrist: a new technique. Arthroscopy. 2000;16:106-110.
48. Bain GI, Richards R, Roth J. Wrist arthroscopy: indications and technique. In: Peimer CA, ed. Surgery of the Hand and the Upper Extremity. New York, NY: McGraw-Hill; 1996:867-882.
49. Abrams RA, Petersen M, Botte MJ. Arthroscopic portals of the wrist: an anatomic study. J Hand Surg Am. 1994;19:940-944.