CHAPTER 29 Wrist Arthritis
Arthroscopic Synovectomy, Abrasion Chondroplasty, and Radial Styloidectomy of the Wrist
The wrist, with its many articular surfaces, is a crucial anatomic link between the hand and forearm. When afflicted by arthritis and conditions limiting the range of motion, the wrist greatly affects the daily lives of patients. The advent of arthroscopy has revolutionized the practice of orthopedic and hand surgeons. Newer techniques and significant application of wrist and small joint arthroscopy can be attributed to many pioneers.1,2
Wrist arthroscopy has provided a tool with which to examine and treat intra-articular abnormalities. Early results for arthroscopic synovectomy of the wrist showed that it reduced pain and swelling and improved joint function.3–6 The transitory or permanent effects depend mainly on the activities of the patient and underlying cause of arthritis. Abrasion chondroplasty of the wrist has not been described at length, but it is known that “repair cartilage” (i.e., fibrocartilage) replaces articular cartilage, as demonstrated in several canine models.7 Abrasion chondroplasty appears to have a therapeutic role in patients with proximal pole hamate arthrosis or radiocarpal arthrosis. Preliminary results of this procedure have been excellent.8–10 This chapter surveys the indications and techniques for arthroscopic synovectomy, abrasion chondroplasty, and radial styloidectomy.
ANATOMY AND PHYSIOLOGY
Matrix metalloproteinases and proinflammatory cytokines (e.g., interleukin-1) are important mediators of cartilage destruction in patients with primary osteoarthritis. Interleukin-1 increases the synthesis of matrix metalloproteinases and thereby plays an important role in osteoarthritis.
Scaphoid fractures can result in osteoarthritis by three mechanisms:
PATIENT EVALUATION
Diagnostic Imaging
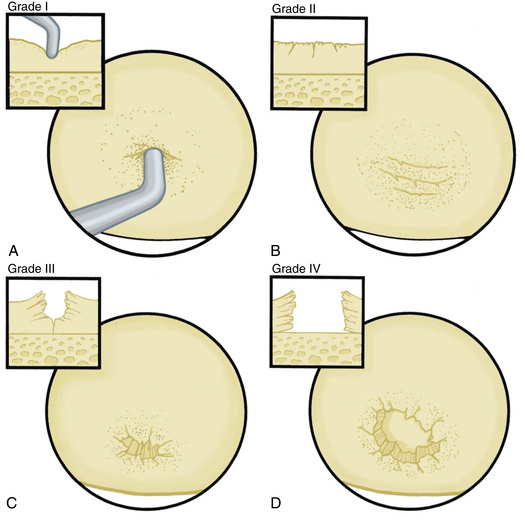
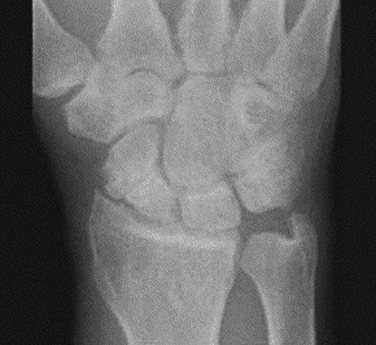
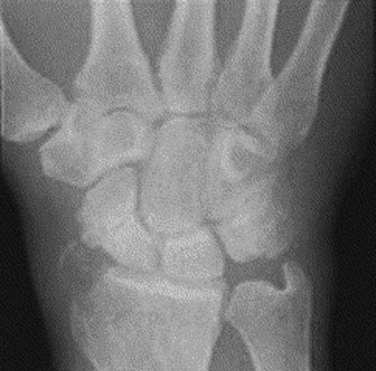
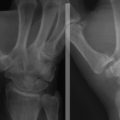
The radiographic evaluation system we use for wrist arthritis is the Outerbridge classification of cartilage defects (Fig. 29-1). We have found that with an appropriate clinical history, dedicated articular cartilage imaging has a sensitivity and specificity reaching the 95% confidence interval. This tool has been confirmed by arthroscopic findings and clinical correlations. However, Haims and colleagues think that magnetic resonance imaging (MRI) of the wrist (41 indirect MR arthrograms and 45 unenhanced [nonarthrographic] MR images) was not adequately sensitive or accurate for diagnosing cartilage defects in the distal radius, scaphoid, lunate, or triquetrum, as demonstrated by correlating MRI with arthroscopic findings.11 In cases of synovitis and ulnar-sided pathology, MRI results are a strong indicator of which areas need to be addressed with the arthroscope. Cartilage defects are often confirmed after diagnostic arthroscopy is completed.
Arthroscopic abrasion arthroplasty, subchondral drilling, and microfracture can be performed for focal chondral defects in patients with moderate degenerative wrist arthritis or when plain radiographs indicate vascular necrosis. MRI is a sensitive method for excluding the diagnosis of avascular necrosis and for evaluating the extent to which fibrocartilaginous repair tissue has formed postoperatively. These methods have been demonstrated for knee pathology, and we have established the same protocols for wrist defects.11.12
TREATMENT
Indications and Contraindications
Arthroscopic synovectomy provides effective treatment of patients with rheumatoid arthritis, juvenile rheumatoid arthritis, systemic lupus erythematosus, and postinfectious arthritis.3–5 Patients with post-traumatic joint contractures and septic arthritis of the wrist after failed systemic antibiotics and lavage also benefit from arthroscopic synovectomy. For rheumatoid patients, we follow the protocol established by Adolfsson.5 We treat those who with persistent joint synovitis have failed to respond to pharmacologic treatment for more than 6 months and who present with radiographic changes of grade 0, I, or II according to the staging system by Larsen and colleagues.13 For nonrheumatoid patients, the radiographic classification system used to evaluate the progression of disease is the Outerbridge classification system, which was originally developed for chondromalacia patellae.14 Patients with early presentation of systemic lupus erythematosus or reactive arthritis (bacterial or viral) and those with osteoarthritis with nominal radiographic changes and florid synovitis are also considered candidates for wrist synovectomy. Patients after intra-articular fractures or multiple previous wrist interventions also benefit from capsular release, removal of adhesions, and synovectomy.
Abrasion chondroplasty is effective in patients with proximal pole hamate arthrosis, which is a cause of ulnar-sided pain in wrists loaded during ulnar deviation; it is particularly effective in those with a type II lunate defect. When the arthrosis in this area is advanced and subchondral bone is exposed (Outerbridge grade IV), we follow the recommendation of Yao and coworkers: excision of the proximal pole.15 Lunate morphology plays a key role in this condition. The type II lunate and its medial facet during contact loading of the proximal pole of the hamate can lead to arthritis, with a reported occurrence of 44% for type II lunates but only 2% for type I lunates.9,15–21 Patients with this condition may have concomitant injuries that require treatment, such as triangular fibrocartilage complex (TFCC) tears, lunotriquetral interosseous ligament tears,9 ulnar impaction, and radial-sided pathology. A TFCC injury is almost always noted in the absence of any other structural problem when synovitis is noted on the ulnar side of the wrist.
Patients who require extensive open wrist procedures are not candidates for arthroscopic synovectomy. MRI in conjunction with clinical assessment of the patient aids decision making. Abrasion arthroplasty is contraindicated for patients with active rheumatoid disease, those who are medically unfit, and patients with active infections not located in the wrist.
TREATMENT
Traditional Arthroscopic Technique
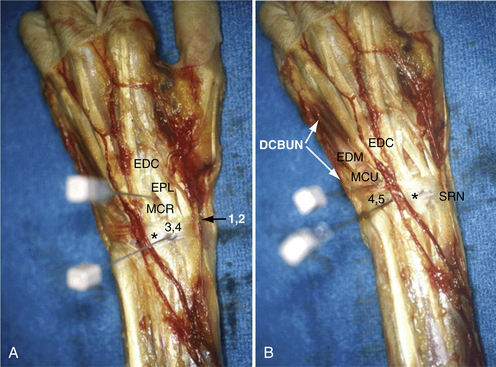
Arthroscopic synovectomy has become a well-described procedure.15 Aggressive arthroscopic débridement, including radial styloidectomy and partial resection of the scaphoid, has been reported. Resection of the lunate in patients with Kienböck disease may be performed arthroscopically. In the distal radioulnar joint, arthroscopy can be used for débridement of the TFCC and for a modified Darrach procedure that involves distal ulna resection. Arthroscopic reconstructive procedures have been described for repair of the lunate-triquetrum ligament and ulnocarpal ligament complex and for capsular placation. Arthroscopic wrist portals are described in Table 29-1.
| Dorsal Portals | Technique | Comment |
|---|---|---|
| 1-2 | Inserted in the extreme dorsum of the snuffbox just radial to the extensor pollicis longus tendon to avoid the radial artery | Gives access to the radial styloid, scaphoid, lunate, and articular surface of the distal radius |
| 3-4 | 1 cm distal to Lister’s tubercle, between the tendons of the third and fourth compartment | Main working portal; gives a wide range of movement and view |
| 4-5 | Between the common extensor fourth compartment and extensor indices in the fifth compartment | Main working portal |
| 6-R | Located distal to the ulna head and radial to the extensor carpi ulnaris tendon; established with a needle under direct vision through the arthroscope, avoiding damage to the triangular fibrocartilage complex | Alternate working portal allows visualization back to radial side of wrist |
| 6-U | Established under direct vision, similar to the 6-R portal; blunt dissection always used to avoid the dorsal branches of the ulnar nerve | Access to the ulnar-sided structures and visualization to radial side of wrist |
Arthroscopic Synovectomy and Abrasion Chondroplasty
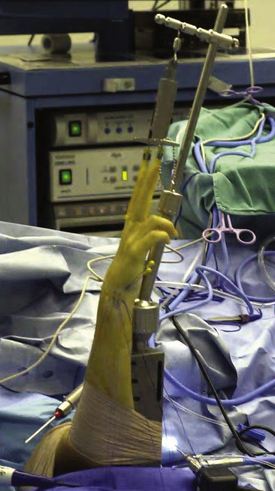
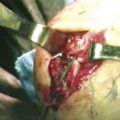
General or regional anesthesia is preferred. The patient is supine on the operating table with the shoulder along the edge of the table. A tourniquet is placed above the elbow and inflated to 250 mm Hg. The shoulder is abducted 70 to 90 degrees, and the forearm is suspended vertically by finger traps from an articulating arm attached to the operating table, and traction of 10 pounds is applied to help open the joint (Fig. 29-2).
The 3-4 portal is established in the soft spot 1 cm distal to Lister’s tubercle (Fig. 29-3). An 18-gauge needle is inserted first and angled 10 degrees volar to be parallel to the joint surface, thereby decreasing the risk of articular damage. The wrist is then distended with 5 to 7 mL of saline solution. A vertical stab incision is made with a no. 15 scalpel blade. A blunt trocar is then introduced into the joint. To maintain orientation, the thumb is kept on Lister’s tubercle until the arthroscope has been introduced. Inflow of lactated Ringer’s solution is gravity fed (i.e., no pump required). Subsequent portals are made with an outside-in technique, in which the needle is introduced in the joint. The 4.5 or maybe 6-R portal is used for a radiocarpal portal. They are identified by use of transillumination and introduction of the needle either radial to the extensor carpi ulnaris tendon or ulnar to the common extensor tendons and distal to the TFCC.
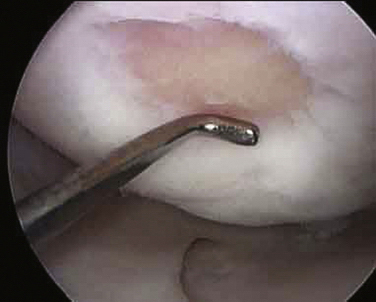
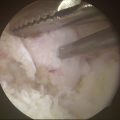
All of the inflamed tissue is removed using a motorized shaver system with a 3.5-mm-diameter synovial resector blade and 3.5-mm flexible shaver (Fig. 29-4). We have had good results with thermoregulation, which also decreases bleeding, but are careful not to touch the articular surfaces. Flow must be maintained within the wrist joint when using thermoregulation to avoid heat buildup. It is important to inspect the radial styloid, the radioscapholunate and radioscaphocapitate ligament, ulnar prestyloid recess, and the dorsoulnar region underneath the extensor carpi ulnaris subsheath. Midcarpal space synovitis is often found along the dorsoulnar region, volarly underneath the capitohamate joint, and in the scaphotrapeziotrapezoid joint. The distal radioulnar joint is inspected, and this can usually be done through a central defect in the horizontal portion of the TFCC, with synovectomy carried out through the 6-R portal. A separate distal radioulnar joint portal immediately proximal to the TFCC can be used for shaving while viewing through the radiocarpal joint if this defect is not present. Meticulous care must be used at all times to avoid chondral damage to the articular surfaces. Incisions are closed with Dermabond and augmented with Steri-Strips or with subcuticular Monocryl.
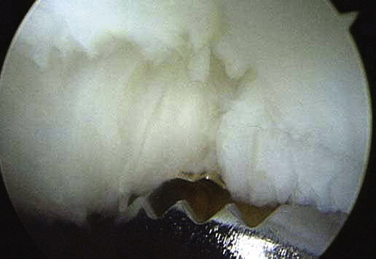
FIGURE 29-4 The rotator shaver is used to perform the synovectomy and remove all fibrillated cartilage.
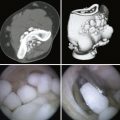
The skin incision for the 6-U portal is done with caution to avoid laceration or a painful neuroma of the dorsal sensory branch of the ulnar nerve. Light dressing is placed, and range of motion is tested immediately postoperatively under the supervision of a hand therapists. Radiocarpal arthritis treated with abrasion arthroplasty is carried out as described by Steadman and coworkers for the knee.10 Working portals in the wrist and the operative setup are established as previously described. Loose bodies are removed while doing the diagnostic part of the wrist arthroscopy. After a focal area of chondral damage is identified, specially designed awls (2.5 and 3.5 mm) are used to make multiple perforations, or microfractures, into the subchondral bone plate (Fig. 29-5). Perforations are made as close together as possible, but not so close that one breaks into another. They usually are approximately 1 to 2 mm apart (Fig. 29-6).
FIGURE 29-6 With the use of specially designed awls, microfractures are created in the subchondral bone 1 to 2 mm apart.
The integrity of the subchondral bone plate should be maintained. The released marrow elements (i.e., mesenchymal stem cells, growth factors, and other healing proteins) form a surgically induced superclot that provides an enriched environment for new tissue formation.10 The rehabilitation program is crucial to optimize the results of the surgery and early range of motion. Dressings and closure are carried out as previously described.
If indicated, we also perform a radial styloidectomy to alleviate the symptoms of wrist arthritis. The radial styloidectomy can be done arthroscopically.22
Arthroscopy aids in visualization to ensure complete resection of the arthritic portion of the styloid without sacrificing the ligamentous support of the wrist. A 3.5-mm burr is used by entering the 1-2 portal. The diameter of the burr is a good benchmark to gauge the amount of styloid removed, and ideally, this is less than 4 mm. An 18-gauge needle can be introduced into the bone to mark the end point of the styloid resection and verified by fluoroscopy. After we complete the procedure, we remove all debris and lose bodies from the wrist joint using the shaver (Figs. 29-7 and 29-8).
Interpositional arthroplasty on the wrist joint is another adjunct procedure performed by some surgeons. The technique was first attempted in the 1970s with the use of silicone sheets. The result did not last long, and it produced a serious inflammatory reaction in the wrist. More recently, tensor fascia lata allografts have been used for this purpose with good success. This procedure cannot be done arthroscopically. A 3-cm elongation of the 3-4 portal is done. The wrist capsule is opened, and the tensor fascia lata allograft is inserted into the joint. Care must be taken not to overstuff the joint. Long-term results of prospective, randomized studies are not available.
Wrist denervation by excising a 1-cm portion of the anterior and posterior interosseous nerve can be performed. We have found, along with studies, that this procedure decreases wrist pain significantly while improving range of motion and grip strength of the hand.23
Postoperative Rehabilitation
Postoperatively, patients are placed in a volar-based short arm splint for 7 to 10 days when a simple arthroscopy is performed, after which patients return for a wound check and suture removal at 2 weeks. Immediate wrist motion is encouraged, along with protection against vigorous activity for 6 weeks.
1. Ekman EF, Pochling GG. Principles of arthroscopy and wrist arthroscopy equipment. Hand Clin. 1994;10:557-566.
2. Gupta R, Bozentka DJ, Osterman AL. Wrist arthroscopy: principles and clinical applications. J Am Acad Orthop Surg. 2001;9:200-209.
3. Adolfsson L, Nylander G. Arthroscopic synovectomy of the rheumatoid wrist. J Hand Surg Br. 1993;18:92-96.
4. Adolfsson L, Frisen M. Arthroscopic synovectomy of the rheumatoid wrist. A 3.8 year follow-up. J Hand Surg Br. 1997;22:711-713.
5. Adolfsson L. Arthroscopic synovectomy in wrist arthritis. Hand Clin. 2005;21:527-530.
6. Roth JH, Poehling GG. Arthroscopic “ectomy” surgery of the wrist. Arthroscopy. 1990;6:141-147.
7. Altman RD, Kates J, Chun LE, et al. Preliminary observations of chondral abrasion in a canine model. Ann Rheum Dis. 1992;51:1056-1062.
8. Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfractures for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477-484.
9. Harley BJ, Werner FW, Boles SD, Palmer AK. Arthroscopic resection of arthrosis of the proximal hamate: a clinical and biomechanical study. J Hand Surg Am. 2004;29:661-667.
10. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391 Suppl:S362-S369.
11. Haims AH, Moore AE, Schweitzer ME, et al. MRI in the diagnosis of cartilage injury in the wrist. AJR Am J Roentgenol. 2004;182:1267-1270.
12. Amrami KK, Askari KS, Pagnano MW, Sundaram M. Radiologic case study. Abrasion chondroplasty mimicking avascular necrosis. Orthopedics. 2002;25:1018, 1107-1108.
13. Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol Diagn (Stockh). 1977;18:481-491.
14. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43:752-757.
15. Yao J, Osterman AL. Arthroscopic techniques for wrist arthritis (radial styloidectomy and proximal pole hamate excisions). Hand Clin. 2005;21:519-526.
16. Nakamura K, Patterson RM, Moritomo H, Viegas SF. Type I versus type II lunates: ligament anatomy and presence of arthrosis. J Hand Surg Am. 2001;26:428-436.
17. Nakamura K, Beppu M, Patterson RM, et al. Motion analysis in two dimensions of radial-ulnar deviation of type I versus type II lunates. J Hand Surg Am. 2000;25:877-888.
18. Malik AM, Schweitzer ME, Culp RW, et al. MR imaging of the type II lunate bone: frequency, extent, and associated findings. AJR Am J Roentgenol. 1999;173:335-338.
19. Dautel G, Merle M. Chondral lesions of the midcarpal joint. Arthroscopy. 1997;13:97-102.
20. Viegas SF, Wagner K, Patterson R, Peterson P. Medial (hamate) facet of the lunate. J Hand Surg Am. 1990;15:564-571.
21. Viegas SF. The lunatohamate articulation of the midcarpal joint. Arthroscopy. 1990;6:5-10.
22. Yao J, Osterman AL. Arthroscopic techniques for wrist arthritis (radial styloidectomy and proximal pole hamate excisions). Hand Clin. 2005;21:519-526.
23. Weinstein LP, Berger RA. Analgesic benefit, functional outcome, and patient satisfaction after partial wrist denervation. J Hand Surg Am. 2002;27:833-839.