Chapter 22 Wound Management
For online-only figures, please go to www.expertconsult.com ![]()
In the care of acute injuries, attention is frequently focused on wound closure. In truth, most traumatic wounds would fare well if left open to heal by secondary intention.17 However, open wounds incur prolonged time to complete healing, discomfort of wound care, vulnerability to further injury, and larger scars. The critical goals of immediate wound care are to stop hemorrhage and limit morbidity related to infection. Secondary goals are to promote rapid healing and optimize cosmetic outcome.44
Types of Wounds and Definitions
Burns (see Chapter 13) may result from thermal or chemical insults. These injuries span a spectrum from trivial sunburn to deep tissue necrosis. The first principle of management is to limit further injury by extinguishing the source, seeking shade, washing out chemicals, and so on. For chemical exposures, voluminous irrigation is the key to limiting further injury. This should be done with clean (not necessarily sterile) water, and the run-off irrigant should be prevented from injuring more skin.7,15,34 Chemical quenchers (i.e., alkaline solution for an acid burn) should never be used because the resulting exothermic reaction will cause secondary injury. Once the burning process is arrested, burn care must focus on antisepsis because infection poses the single most important immediate threat.
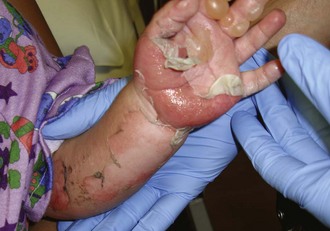
Burn wounds should be fastidiously cleaned to remove all foreign material and devitalized skin. Depth was formerly described as first, second, or third degree, but modern description relies on the descriptors partial thickness and full thickness (Figure 22-1). Blisters should be left intact unless they interfere with function. There is some debate about the management of blisters, but in a wilderness environment, the “biologic bandage” provided by an intact blister supersedes any academic debate about the presence of inflammatory mediators in the blister fluid. Blisters over the palms or joints are exceptions to this rule, because their presence will significantly limit motion and potentially engender contraction or decrement in function. One school of thought is that these blisters should be unroofed and gently scrubbed away with a moistened gauze or trimmed with scissors. Once cleaned, burns should be dressed and kept clean. Topical antiseptic agents should be applied to burns with invasion deep enough to result in blistering or erosion through the skin to white tissue or burned muscle underneath. Silver sulfadiazine is an excellent agent, and can be reapplied once or twice daily to prevent the wound from desiccating. In the absence of silver sulfadiazine (and for burns <1% of body surface area), antibiotic or antiseptic ointment (e.g., bacitracin), can be employed.
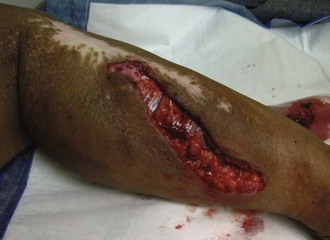
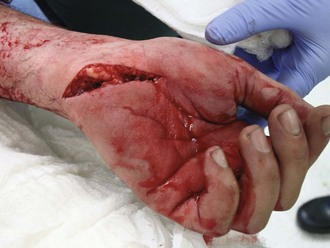
Lacerations can be deep or superficial, simple or irregular (Figure 22-2). Generally, lacerations are amenable to primary repair (i.e., closure at the time of injury). Closure simplifies wound care and optimizes cosmetic outcome. Much of the following discussion is dedicated to management of lacerations.
Clinical Presentation
Immediate evaluation of traumatic wounds should include determination of the type of injury. Focused assessment includes determination of surface area and apparent depth. Distal sensation and passive and active range of motion must be assessed to elucidate any associated nerve injury, tendon disruption, or fracture. Nerve and tendon injuries will not affect acute management but are important as a baseline examination. Fractures require immobilization, which is discussed in Chapter 27. Distal perfusion must be assessed to determine likelihood of vascular injury. If there is suspicion of vascular injury based on examination, depth of the wound, or blood at the scene, the wound should not be probed (nor blood clots dislodged) until one is prepared to place direct pressure to control hemorrhage and potentially ligate or suture the injured vessel.
Time since injury is very important when considering wound closure. Within several hours of injury, traumatic wounds become colonized with bacteria. Even without frank infection, heavy colonization impedes proper wound healing.17,23,45 A bacteria-laden wound that has its edges approximated will generally not heal and has increased risk for developing an abscess. Various investigators have tried to establish a firm time limit beyond which traumatic wounds should not be closed, but results are inconsistent.3,28,49
With copious irrigation and removal of foreign bodies, most wounds that are otherwise suitable for closure (see earlier discussion) can be closed if this is accomplished within 6 hours of injury.21 This deadline may be extended in the hospital setting, where thorough sharp debridement can be undertaken and the environment controlled. In some circumstances, wounds may even be closed days or even weeks after initially generated, but this involves sophisticated surgical care.29 This situation should not be confused with an acute wound in the wilderness, which in all circumstances should be thoroughly irrigated, foreign bodies removed, and dressings applied. Acute wounds in the wilderness should be closed only if they are clean, suitable materials and instruments are at hand, and less than 6 hours have passed since the injury.
Treatment
Personal protective equipment should be donned before interrogating, preparing, possibly closing, and dressing a wound. Eye shields and gloves are considered mandatory equipment. Eye shields are particularly important in preventing exposure of mucous membranes to bodily fluids. Gloves are essential and should be made of a nonlatex material and contain no talcum or other lubricant. Talcum, calcium carbonate, and cornstarch are common glove lubricants, and all have been shown to cause wound granulomas, foreign body reactions, adhesions, and chronic sinuses. Sterile gloves are less cumbersome to work with but are not required because they do not reduce the incidence of infection in an austere setting.37 Use of a mask has also not been demonstrated to reduce rates of acute wound infection but certainly provides a protective barrier for the care provider. Hair coverings are not necessary, because studies have failed to demonstrate reduction in wound infection rates.
Cleansing Techniques
Cleansing involves removing devitalized tissue, clearing debris, and copiously irrigating the area.21 This may involve vigorous manipulation of painful and sensitive tissues, so if oral analgesics are available, they should be used. Constricting jewelry and equipment should be removed from the affected area (e.g., rings, piercings). Ideally, hair around the wound is trimmed because the roots harbor bacteria. Hair should be trimmed to a few millimeters in height because shaving at the surface increases wound infection rates by causing superficial skin trauma.2 Eyebrow hair is an important exception, because trimming or shaving can result in permanent abnormal hair growth. If hair trimming is not possible, an antiseptic ointment can be used to paste the hair down away from the wound.
Water should be used to clean the wound. There is no demonstrated benefit to use of saline solutions, and there is no absolute need for the irrigant to be sterile.7,15,34 Although some persons mention use of dilute (1%) povidone-iodine as an irrigant, this is not proved to be more efficacious. Whatever method is available to generate potable water (e.g., iodine tablets, mechanical filter, irradiation, boiling) is sufficient to generate irrigation fluid for wound care. Hydrogen peroxide is injurious to deep tissues and should not be used as an irrigant.
The greatest impact on wound cleanliness is gained from volume of irrigant and simple mechanical debridement. Debridement is achieved by removal of obvious foreign material (as with forceps) and also by pulsing irrigant at the wound (the fluid agitation is an effective debrider) (Figure 22-3).30 The available volume should be used in successive bursts rather than a single “gush,” because dilution of wound contaminants is far superior with small serial volumes. Although the matter has not been systematically researched, a guideline that is often used is a minimum of 100 mL per centimeter of wound length. Optimal stream pressure has been determined to be 5 to 10 psi, but in a remote setting, use of pressure gauges or regulators is unnecessarily cumbersome; it is sufficient to realize that excessive pressures are not required.43,49 A large (20 to 60 mL) syringe attached to a 19- or 20-gauge needle or catheter is an effective instrument for this purpose. Alternatively, holes can be punched into the cap of a squeezable water bottle to generate the same effect. A splash shield at the tip of the instrument decreases personal exposure risk. If not available as a specific commercial product for this purpose, one can easily be fashioned from a paper or plastic plate, or base of a disposable water bottle or drinking cup.
Vascular Injuries
Bleeding from wounds can almost always be controlled with direct pressure. The most common reason for failure of direct pressure to control bleeding is too short a compression time. Initial pressure should be applied for 5 minutes (checked with a timepiece to ensure accurate timing). If bleeding persists, either pressure should be reapplied for 10 minutes, or pressure should be applied at both the wound and a proximal inflow point (e.g., popliteal, femoral, or brachial artery).24 The second most common reason for failure of direct pressure is that pressure is not applied in a focused manner over the site of bleeding. A broadly applied towel over a large wound will not be as effective at controlling bleeding as will finger-point pressure applied over the actual site of greatest bleeding.
In the face of profuse diffuse bleeding (as from a large raw wound), topical adjuncts are available to promote thrombosis.4 QuikClot is a granular coagulant that was originally designed to be poured onto wounds to stop diffuse bleeding. The mechanism of action of the original product is adsorption of water onto zeolites, which activates factor XII and catalyzes platelet aggregation. However, the granule form of this material is difficult to use for two reasons: the poured material is unwieldy and difficult to control in windy situations; and the reaction is exothermic, causing pain and risking burn injury. QuikClot has now been modified and integrated into a gauze dressing (also marketed as Combat Gauze). The active element is now kaolin, an alumina silicate that activates and accelerates the enzymatic clotting cascade. The solid consistency of Combat Gauze is more manageable and generates no heat on application. Celox gauze is based on chitosan, a natural product derived from shellfish. Initial versions of this product were granules to be poured into a wound, but it is now embedded into an actual gauze dressing and also has no exothermic properties. HemCon bandages are also chitosan based and not exothermic. These agents are all designed to be applied directly to bleeding wounds and compressed with direct pressure. Before wound closure, these dressings must be removed and bulky gelatinous deposits in the wound irrigated out (although mild residual coatings are reportedly safe to leave in place). All of these agents are supported by anecdotal reports of success and are clearly superior to simple gauze dressings, reducing bleeding by nearly an order of magnitude. However, head-to-head studies published to date have been underpowered and are difficult to interpret. Ongoing development of these products and comparative evaluation are the most active areas of acute wound care research and development.
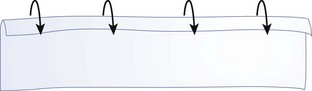
Tourniquets may be considered as a last resort to stop life-threatening hemorrhage. Great debate exists over the use of tourniquets and who should be allowed to apply them (laypersons, emergency first responders, midlevel health care providers, physicians, or even specialty-specific physicians). The more proximal a tourniquet is applied, the greater the risk for devastating tissue/limb loss. The best potential use of a tourniquet may be for a traumatic amputation in which there is copious diffuse bleeding at the open end. In this case, a tourniquet can be firmly applied just proximal to the open wound so that little distal ischemic tissue is created (Figure 22-4). Commercial tourniquets such as the Combat Action Tourniquet are available, but a blood pressure cuff is often an effective instrument. Whatever device is employed, it should be applied and intensified until pressure exceeds arterial pressure and then increased at least another 20 mm Hg or until bleeding stops. If transport to advanced surgical care is expected to be prolonged (more than 1 hour), the tourniquet should be slowly released every 45 to 60 minutes and allowed to gently bleed (i.e., so long as bleeding is not torrential) for 3 to 5 minutes. This allows intermittent perfusion of tissue and can gradually induce ischemic conditioning of the affected tissue to lessen the effects of ischemia and reperfusion. However, this is controversial, and some authorities do not advocate intermittent loosening to avoid dangerous hemorrhage. When a tourniquet is released, it should be done slowly over a minute or two, because recirculation of stagnant, acidic, and hyperkalemic blood from the nonperfused distal limb can lead to hemodynamic instability. If dysrhythmia is noted, the tourniquet should not be released further until the patient stabilizes. Hypotension and even cardiac arrest may result when a tourniquet is released; these dangers are frequently overlooked and can cause tragic outcomes.
Although profuse external hemorrhage is easy to localize, ischemic complications of vascular injuries are more subtle and sometimes overlooked. Examination findings are classically divided into “soft” and “hard” signs of vascular trauma, indicating the relative likelihood of a significant vascular injury (Table 22-1).24 Presence of a hard sign is more than 90% predictive of need for vascular intervention. Only about one-third of patients with a single soft sign have an abnormality on arteriography, and few of these require emergent intervention. Any patient with a hard sign of vascular injury requires immediate evaluation by a surgeon.
| “Hard Signs” | “Soft Signs” |
|---|---|
Anesthesia
Manipulation of traumatic wounds can be painful and anxiety provoking. Studies have demonstrated that music can reduce procedural anxiety.32,42 For most patients, explaining the steps involved can improve tolerance by guiding expectations.54 If vigorous debridement or suture closure is to be attempted, infiltration of local anesthetic can provide tremendous comfort for the patient and thus facilitate technically superior closure. Anesthetic solutions can be applied locally within tissue, as topical agents, or as regional nerve blocks.
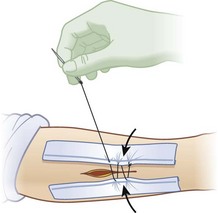
Direct intradermal or subcutaneous injection is the most common technique of local anesthetic use. These agents are synthesized in two related chemical classes: aminoamides (lidocaine, bupivacaine, dibucaine, etidocaine, mepivacaine, prilocaine, and ropivacaine) and aminoesters (chloroprocaine, procaine, tetracaine, and cocaine ). Characteristics of the most commonly used local agents are listed in Table 22-2. Inclusion of epinephrine in the solution effects local vasoconstriction by α1-adrenoceptor agonism and nearly doubles the duration of the local effect of the medication. By slowing systemic absorption of the anesthetic, it also increases the maximum allowable total dose. Inclusion of epinephrine in the anesthetic solution is believed by some to improve local hemostasis, but this is a minor contribution relative to the effect of increased tissue turgor from infiltration of the solution. Conventional teaching is that epinephrine-containing local anesthetic solutions should not be used in end-vascular beds such as the nose, fingers, toes, and penis because of concern for ischemia. However, there is literature to support safe use of these solutions for digital blocks.46,51 Using anesthetics without epinephrine allows maximum flexibility in terms of allowable location of use. Pain of injection for any local anesthetic can be attenuated by mixing 1 mEq of sodium bicarbonate with each 9 mL of anesthetic to buffer the solution. It should be noted that this delays the onset of action by approximately 5 minutes.
For all agents, intravascular administration or overdose will first cause agitation, tremor, and tinnitus, then seizures, and finally, cardiac arrest through aberrant conduction.19 These risks are particularly important to consider in children, in whom toxicity occurs at lower doses. Table 22-2 lists maximum doses of commonly employed local anesthetics, along with weight-based guidelines for children. Although ample anesthetic should be administered, caution must be exercised to ensure that no intravascular administration occurs and to avoid nearing the maximum dose. This principle is complicated by the fact that maximum doses are ill defined and are set at different levels in different nations.38 Of note, patients with liver disease and those taking medications (such as systemic antifungals) interfering with cytochrome P-450 metabolism will have decreased capacity to metabolize aminoesters. In patients with renal failure, the metabolism of bupivacaine is altered, which requires a dose reduction of 15% to 20%. Concern frequently arises over administration of epinephrine-containing local anesthetics to patients with heart disease. However, these dilute local/regional doses do not cause significant systemic hemodynamic effects.
Local anesthetics can be applied topically as well and have demonstrated efficacy in numbing the skin, although 20 to 30 minutes is required for onset. Topical anesthetics are particularly useful for superficial wounds over a large surface, such as burns and abrasions.26 A variety of formulations are available: TAC (0.5% tetracaine, 0.027 epinephrine, 11.8% cocaine), LET (4% lidocaine, 0.1% epinephrine, 0.5% tetracaine), and EMLA (2.5% lidocaine, 2.5% prilocaine in a eutectic mixture containing a thickener, emulsifier, and distilled water buffered to pH 9.4), and 5% lidocaine jelly. Characteristics of these agents are summarized in Table 22-3. Care must be taken to avoid application near mucous membranes because of the potential for systemic absorption and toxicity. Compared with TAC, LET has less potential for toxicity, is not a controlled substance, and is less expensive.1 LET should be stored in a light-resistant container and is stable for 6 months refrigerated and 4 weeks at room temperature. It should be discarded if the solution becomes discolored. Near mucous membranes, 3 mL of LET solution can be combined with 150 mg methylcellulose (4000 cps) by stirring for a few minutes, to create a gel preparation that should be used immediately. This lessens the likelihood of having the topical preparation run onto the mucous membranes.
True IgE-mediated allergic reactions to local anesthetics are very rare, particularly with aminoamide anesthetics.12,19,38 Typically these reactions are actually caused by preservatives such as methylparaben in multidose vials. One alternative is to use an aminoester anesthetic such as chloroprocaine. However, the principal metabolite of the aminoesters is para-aminobenzoic acid, which is chemically similar to methylparaben and can induce the same reactions. A reasonable approach to a reported allergy to a particular anesthetic is to use a preservative-free anesthetic of the other class.
Use of diphenhydramine has been described as an alternative to traditional local anesthetics.36 At 1% concentration, it can be injected subcutaneously or subdermally but causes more pain from injection than do the classic anesthetics, and this pain is not diminished by dilution or mixing with bicarbonate.39 More importantly, diphenhydramine injection can result in vesicle formation and tissue necrosis.18 Because of these characteristics, it is not recommended as a local anesthetic. In the absence of any pharmacologic anesthetics, ice may be applied around the wound edges for 5 minutes to diminish sensation.
Regional anesthetic techniques can be very useful for dealing with extremity wounds or fractures. This involves infiltrating the anesthetic around the nerve(s) innervating the affected region.52 These techniques rely on specific anatomic landmarks and are beyond the scope of this chapter.
Wound Closure Techniques
A variety of materials and approaches may be taken to close wounds. Sutures are generally required to approximate deep tissues, but the superficial aspects of wounds can be addressed with adhesive strips, sutures, glues, staples, or some combination thereof.22
Modern cyanoacrylate skin glues are effective, affordable, and more versatile than adhesive strips.8,22,40 They boast the added benefit of sealing the incision, which may be particularly useful in a wilderness setting involving exposure to the elements. Cyanoacrylate glues are popular. These include Dermabond, Indermil, and SurgiSeal. Each of these differs slightly in time to drying, maximum tensile strength, and viscosity, but the clinical variability is minor; selection should be based on availability and cost. Although many are stable at lower than 30° C (86° F), it should be noted that storage of Indermil for longer than 4 weeks requires refrigeration.
Another technique to close a wound if only tape, needle, and thread are available is accomplished as follows. Cut two strips of adhesive tape 2.5 cm (1 inch) longer than the wound. Fold one-quarter of each strip of tape over lengthwise (sticky to sticky) to create a long nonsticky edge on each piece (Figure 22-5). Attach one strip of the tape on each side of the wound, 0.6 to 1.3 cm (0.25 to 0.5 inch) from the wound, with the folded (nonsticky) edge toward the wound. Using a needle and thread, sew the folded edges together, cinching them tightly enough to bring the wound edges together properly (Figure 22-6). The tape will stick much better if you first apply a thin layer of benzoin to the skin.5
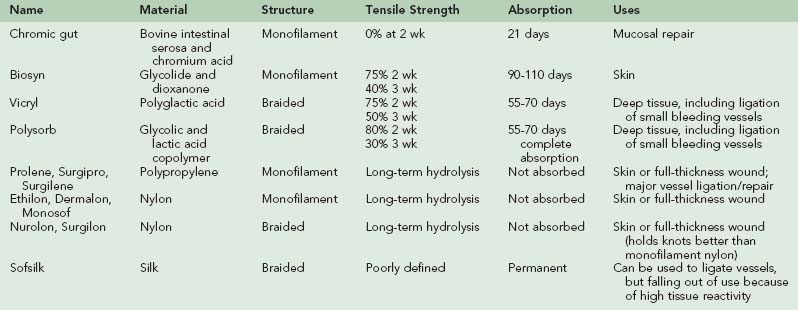
Sutures are the workhorse of wound closure because of durability and versatility; summary characteristics of the most versatile sutures for acute traumatic wounds are listed in Table 22-4.13,16,41 When suturing deep tissue, intermediate-durability absorbable materials such as polyglactin (e.g., Vicryl) or copolymeric glycolic and lactic acids (e.g., Polysorb) should be used. At 2 weeks, these materials retain approximately three-quarters of their original strength.11,14 Nondyed varieties are preferred within 5 mm (0.2 inch) of the skin surface to avoid wound tattooing. For mucosal lesions (such as within the mouth), rapid-absorbing gut suture is the most suitable.9 In all these cases, a tapered-tip needle should be used so that deep tissue is not lacerated. Ideally, skin suturing should be done with monofilament nonabsorbable material attached to a cutting needle. Nylon (e.g., Ethilon, Dermalon) and polypropylene (e.g., Prolene) are typical nonabsorbable sutures. Polypropylene is blue but does not tattoo skin as noted above for absorbable sutures. If proper materials are not available, nondyed absorbable suture with a tapered needle can be used on the skin, but nonabsorbable suture should not be buried within the wound; in this case, deep mattress sutures should be placed so that deep tissue is approximated but the suture can still be removed once healing is complete. Size selection is based on visibility and the degree of tension expected on the wound. For skin approximation, recommended sizes are: 5-0 or 6-0 for the face/neck/ears; 5-0 or 4-0 for fingers/hand; 4-0 or 3-0 for scalp/arm/leg/feet/chest/abdomen; and 3-0 or 2-0 on the back or over a major joint. Deeper layers (below the skin) generally require suture one size larger than or the same as is being used for the skin (e.g., deep upper arm laceration receives 4-0 absorbable suture to approximate deep layer, followed by 4-0 for skin).
The goals of deep tissue closure are to take tension off the skin closure and to obliterate the gap in deep tissue so that seroma or abscess is less likely to form.13,16,41 Muscle sheaths (i.e., fascia) should be reattached, and subcutaneous tissues should be sutured together in interrupted fashion. A deep wound may require multiple layers of suture before skin closure, but usually one or two suffice. Each “layer” of suture is indicated not by the absolute depth of tissue, but by the presence of distinct anatomic “planes” (e.g., separate fascial sheaths) that must be approximated. If significant muscle, tendon, or nerve injuries are recognized, suture repair should not be attempted in the acute setting, but written notes should be recorded for long-term follow-up (Figure 22-7). If required later, these repairs are performed in an elective setting after healing has occurred and functional deficits are delineated.
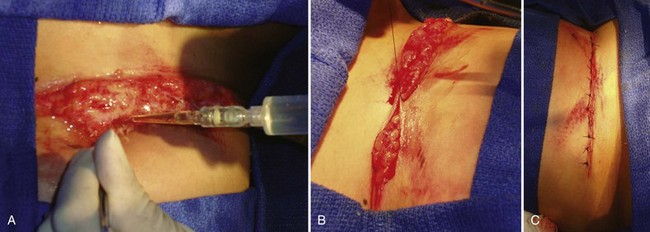
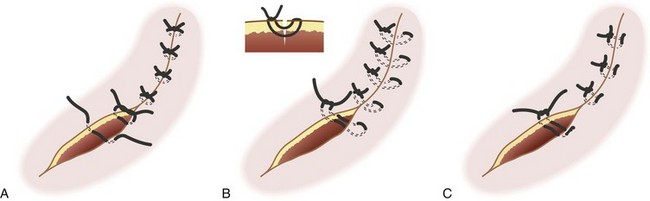
Traumatic wounds are typically irregular in shape and thus lend themselves to closure by interrupted technique. Running closure may be employed if there is a truly linear laceration. A straight or smoothly curved incision may be closed with a running suture to save time. An irregularly shaped wound should be closed with interrupted sutures to achieve optimal alignment (Figure 22-8, A; see Figure 22-3, B and C). Mattress techniques can be applied in deep wounds, thin skin, or those under tension.41,55 Vertical mattress sutures approximate the wound at two depths and evert wound edges for optimal healing (Figure 22-8, B). Horizontal mattress sutures close a single depth, evert wound edges, and distribute tension along the length of the incision (Figure 22-8, C). More complex wounds can benefit from application of advanced techniques, such as local rotational or advancement flaps.48,50
Mechanical clips can be applied more rapidly than can sutures. Ancient Hindus used ant mandibles to pinch skin edges together, then twisted off their bodies.53 Modern skin staplers are made by various manufacturers and are useful for rapid closure of wounds on thick skin such as the scalp or back. They can be particularly effective for rapidly achieving hemostasis in the scalp, which is richly perfused and frequently rebleeds after direct pressure is released.
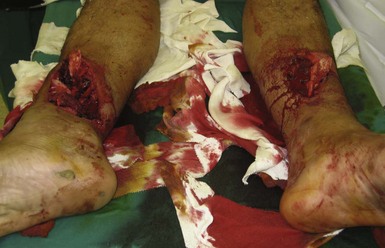
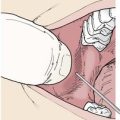
Wounds of the abdomen and chest have the potential to involve the viscera. If air is felt, heard, or seen emanating from a chest wound, or if the patient is complaining of shortness of breath, some form of pneumothorax must be present. The wound should not be closed tightly, but rather covered with a nonocclusive dressing. This will minimize contamination while allowing outflow of air so that tension pneumothorax does not develop. If intestine or omentum protrudes from an abdominal wound, there is considerable likelihood of visceral injury (Figure 22-9). Such a wound should be covered with a moist, secure dressing and not be sutured. Both of these conditions require prompt evaluation by a surgeon.
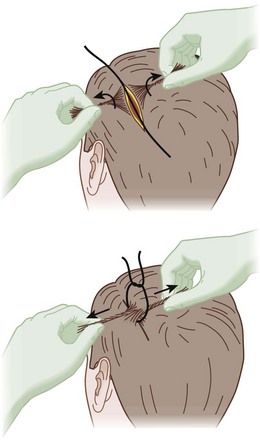
Scalp wounds occur frequently, can be very bloody, and have unique management options. The scalp is richly perfused, so even superficial lacerations can bleed profusely. Initial care should include firm direct pressure for several minutes. If bleeding does not abate, then secure scalp closure is necessary to assist in local pressure and minimize the potential space for hematoma formation. If the galea aponeurotica is lacerated and suture is available, it should be primarily repaired to further limit the space available for hematoma expansion. For skin closure, staplers are excellent in this circumstance because staples are rapidly applied and effective, and the scars are less noticeable on the scalp. Sutures may be used but are not necessary. If a scalp wound is bleeding and needs to be closed but no instruments are available, a hair tie may be performed. Hair is parted along the incision and twisted into cords on either side of the incision. Cords opposing one another across the laceration are then tied together to approximate the wound edges5,25 (Figure 22-10).
Timing of skin suture and staple removal depends on the individual wound. Healing and wound remodeling are gradual processes—at 1 week after injury, a typical wound has just 5% to 10% of the original tensile strength. The majority of healing takes place over the first 6 weeks, at which point tensile strength reaches 70% of baseline.17,44 Leaving skin closure material in place longer keeps tension off the wound but increases the likelihood of visible scar from the suture or staple itself. Therefore timing of removal varies based on durability and visibility of the affected area. On the face/neck, sutures are removed at 3 to 5 days, fingers/hand at 5 to 7 days, scalp/leg/chest/abdomen at 7 to 10 days, back/arm/feet at 10 to 14 days, and 14 days across any major joint. For each site, sutures should be left in place longer if scar integrity seems poor, malnutrition has been involved, or if the orientation of the wound subjects it to excessive tension. Sutures are removed with scissors, and staples are removed with a staple remover or by laterally spreading a hemostat between skin and staple. Once the sutures or staples are removed, the healing wound may be reinforced with adhesive strips or skin glue (as described earlier) to minimize tension while healing continues, especially in a wilderness environment where vigorous activity may strain the wound. Visibility of the resulting scar is minimized by keeping it out of direct sunlight, particularly for the first 3 months following injury.
Dressings and Aftercare
Wounds should be kept as clean as possible. Any wounds addressed in austere environments are at increased risk for infection and should be carefully monitored for cellulitis or abscess (see Complications). Incidence of infection obviously depends on the specific wound characteristics, anatomic location, and context of injury. Although up to 25% of acute wounds reveal Streptococcus, Staphylococcus, or Escherichia when cultured, most studies have revealed just a 5% rate of infection. Weighing this against the risk for adverse reactions and effect of selecting resistant species, antibiotics are not prescribed for all traumatic wounds.33 The decision to administer prophylactic or empiric antibiotics must be individualized to each situation. Patient factors that should encourage consideration of antibiotics include poorly controlled diabetes mellitus, malnutrition, recent chemotherapy, prosthetic valve or organ transplant recipient, peripheral vascular disease, renal failure, chronic liver disease, chronic corticosteroid use, or other immunocompromising condition. Wound factors include delay (more than 10 hours) to cleansing and repair, extensive devitalized tissue, heavy contamination or retained debris, bites, oral lacerations, foot wounds, open fractures, or injury to poorly perfused tissue (bone, joint, tendon).3,28,33
Tetanus is caused by Clostridium tetani, an organism found in soil and the excrement of humans and animals. The often fatal disease can be prevented by immunization, but 40% of Americans age 60 years or older lack seropositivity.31 Tetanus immunization status must be evaluated for all patients with traumatic wounds, and a booster dose of toxoid and/or immune globulin administered if the patient is at risk for infection (Table 22-5). The formulation of tetanus toxoid booster depends on the age of the patient and vaccination history (Table 22-6).10,27
| History of Immunization (Doses of Adsorbed Tetanus Toxoid Previously Received) | Clean and Minor Wound | All Other Wounds* |
|---|---|---|
| Unknown/less than series of three doses | Toxoid†,‡,§ | Toxoid and TIG‡,§,‖ |
| Three or more | ||
| Last within 5 yr | No prophylaxis | No prophylaxis |
| Last within 10 yr | No prophylaxis | Toxoid |
| Last over 10 yr | Toxoid | Toxoid and TIG‡,§,‖ |
TIG, Tetanus immune globulin.
* Such as contaminated with dirt, soil, saliva, or feces. Includes punctures, avulsions, missile injuries, crush, burns, and frostbite.
† Tetanus toxoid formulation depends on the age of the patient (see Table 22-6).
‡ No toxoid should be administered to infants younger than 6 weeks of age.
§ Patients with acquired immunodeficiency syndrome (AIDS) should receive TIG regardless of immunization history.
‖ TIG 250 units intramuscularly (for both adults and children). Toxoid should not be administered at the same site.
TABLE 22-6 Recommended Tetanus Toxoid Types
| Age | Tetanus Toxoid Formulation |
|---|---|
| <6 wk | No toxoid |
| >6 wk and <7 yr | DTaP |
| 7-10 yr | Td |
| 11-64 yr | Td if previously received Tdap or if pregnant Tdap if never received Tdap |
| 65 yr or older | Td |
DTaP, Pediatric diphtheria and tetanus toxoids and acellular pertussis; Td, tetanus and diphtheria toxoids; Tdap, tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis.
Complications
Cellulitis is diagnosed by the presence of blanching erythema around and spreading away from the edges of a wound and may demonstrate proximal streaking along lymphovascular channels. It is important to distinguish this from the hyperemia that commonly surrounds the edges of a healing wound. Erythema associated with normal wound healing is not as bright red as is cellulitis, has less dramatic blanching with pressure, is symmetric around the edges of the wound, and extends not more than a centimeter from the wound edges. If erythema is not limited by these characteristics, or it progresses in size, wound infection is present. The treatment for cellulitis is a systemic antibiotic. The chosen antibiotic (first-generation cephalosporin or antistaphylococcal penicillin) should cover typical skin flora but also take into consideration any particular exposures (e.g., open water injury, animal bite, soil contamination) that may be associated with the trauma and dictate coverage of different microbes.6 Cellulitis without abscess does not require suture removal. If cellulitis persists or progresses after 36 hours of the above treatments, the antibiotic spectrum should be broadened and the wound reevaluated to ensure that there is not an underlying abscess.
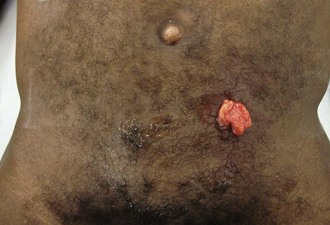
Abscess is a suppurative soft tissue infection. Pus is a mixture of dead organisms, leukocytes, and debris contained within a deep tissue space. An abscess will continue to expand, causing progressive pain, surrounding cellulitis, and potential systemic illness until drained (Figure 22-11, online). Antibiotics may stunt the growth or spread of associated cellulitis, but abscesses require drainage. This is accomplished by removing any sutures, staples, or tape keeping the wound closed. The abscess cavity is then copiously irrigated, and a soft probe is used to ensure that any loculated collections are properly drained. An excellent probe is a cotton-tipped swab dipped in antiseptic ointment to prevent deposition of cotton threads.
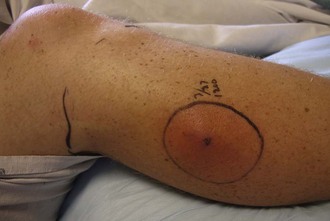
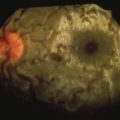
FIGURE 22-11 Minor puncture wound that has developed a secondary abscess and associated cellulitis.
(Courtesy Ramin Jamshidi, MD.)
Necrotizing soft tissue infections are true emergencies and require surgical intervention. These can take the form of superficial infections or deep fasciitis. The superficial variety can be easily recognized by the foul-smelling gray (“dirty dishwater”) drainage, but necrotizing fasciitis can develop surreptitiously because skin findings are often subtle and mistaken for simple cellulitis. The signs of deep infection include pain on passive motion, woody induration, and rapid spread.20 Antibiotics may stall the pace of progression, but these conditions are uniformly fatal if not addressed by a general surgeon. The definitive treatment is wide incision and drainage along with fluid resuscitation and intensive supportive care. This typically results in large, disfiguring wounds.
Compartment syndrome results from elevated pressures within a contained compartment; as pressure rises, it exceeds venous pressure, then eventually arterial pressure, resulting in tissue ischemia. In the wilderness setting, this is most likely to occur from blunt trauma, particularly with crush injury or a closed fracture. The classic evidence of increased compartment pressures are pulselessness, pallor, pain on passive motion, poikilothermia, and paresthesia.47 Pain on passive motion is the most sensitive and earliest sign to develop, but all are relatively late signs of compartment syndrome. Immediate surgical evaluation is needed because the definitive intervention is incision and release of the involved compartment. If signs of compartment syndrome develop from a penetrating injury, it suggests that there was an occult vascular injury and an expanding hematoma is trapped beneath the closure. The closure should be opened, blood/hematoma evacuated, and a fresh attempt made to control bleeding. If compartment syndrome is not addressed, irreversible tissue necrosis begins by 6 hours.
Persistent bleeding is a common concern of wilderness medical providers. Typically this indicates a missed injury or inadequate direct pressure. If a wound continues to bleed through a dressing, the bandage should be removed to allow direct inspection. Consider that a discrete vascular injury may have been missed, and an exposed vein or artery requires direct pressure or ligation.24 If a prolonged period of direct pressure does not stop hemorrhage, one may need to use hemostatic adjuncts or tourniquet application as discussed earlier.
Many wounds can be managed in a backcountry environment without interrupting the activities. However, some injuries mandate evacuation. These include open fractures, compartment syndrome, persistent bleeding, tourniquet use, progressive loss of sensation or motor function, progressive infection, and full-thickness burns that are circumferential or involve more than 5% of body surface area. Many wilderness-acquired wounds that do not necessitate evacuation still require formal medical evaluation upon return to civilization.35 These include any persistent detriment in motor function or sensation, ongoing drainage from the wound, persistent open wound, burns over the face or genitals, and burns over joints.
Wound Care Kit
Many adjuncts are available to facilitate wilderness wound care, but a kit with core supplies will suffice in the majority of circumstances. Table 22-7 lists suggested components of such a kit.
| Core Items | Optional Items | |
|---|---|---|
| Personal protection | Gloves (nonlatex) Eye shield Mask Headlamp |
Sterile gloves (nonlatex, nonpowdered) Surgical cap |
| Instruments | Needle driver (15.2 cm [6 inches]) Scissors (iris tip) Toothed forceps (Adson type) Nontoothed forceps (DeBakey type) |
Hemostats (curved) |
| Wound preparation | 60-mL syringe 18-guage needle or blunt cannula 21-guage needle Water filtration system Povidone-iodine swab packets |
Splash shield Chlorhexidine surgical scrub brush |
| Closure materials | 2-0 nylon (cutting needle) 4-0 nylon (cutting needle) 3-0 Vicryl (tapered needle) Skin stapler Dermabond skin glue vials Adhesive strips (1.3 cm [0.5 inch]) |
5-0 nylon (cutting needle) 6-0 nylon (cutting needle) 4-0 Vicryl (tapered needle) Mastisol (15 mL) |
| Dressings | Gauze (10 × 10 cm [4 × 4 inches]) Porous paper tape (1.3 cm [0.5 inch]) Gauze roll (11.4 cm × 3.7 m [4.5 inches × 4 yards]) Flexible, reusable splint |
Elastic roll (7.6 cm × 4.6 m [3 inches × 5 yards]) Mepilex (10 × 20.3 cm [4 × 8 inches]) Surgicel Nu-Knit (2.5 × 8.9 cm [1 inch × 3.5 inches]) QuikClot (10 × 10 cm [4 × 4 inches]) |
| Medications | 0.25% bupivacaine (10-mL vials) Antiseptic ointment (30 g [1 oz]) Cephalexin (500 mg) Ciprofloxacin (500 mg) |
Silver sulfadiazine 1% cream (50 g [1.8 oz]) 5% lidocaine jelly (35 g [1.2 oz]) |
1 Abraham MK, Oh JS. Recent advances in wound care. Trauma Rep. 2010;11:1.
2 Alexander JW, Fischer JE, Boyajian M, et al. The influence of hair-removal methods on wound infections. Arch Surg. 1983;118:347.
3 American College of Emergency Physicians. Clinical policy for the initial approach to patients presenting with penetrating extremity trauma. Ann Emerg Med. 1999;33:612.
4 Arnaud F, Teranishi K, Tomori T, et al. Comparison of 10 hemostatic dressings in a groin puncture model in swine. J Vasc Surg. 2009;50:632.
5 Auerbach PS. Minor bruises and wounds. In: Auerbach P, editor. Medicine for the outdoors. ed 5. Philadelphia: Elsevier; 2009:257-284.
6 Ball V, Younggren BN. Emergency management of difficult wounds: I. Emerg Med Clin North Am. 2007;25:101.
7 Bansal BC, Wiebe RA, Perkins SD, et al. Tap water for irrigation of lacerations. Am J Emerg Med. 2002;20:469.
8 Bozkurt MK, Saydam L. The use of cyanoacrylates for wound closure in head and neck surgery. Eur Arch Otorhinolaryngol. 2008;265:331.
9 Brown DJ, Jaffe JE, Henson JK. Advanced laceration management. Emerg Med Clin North Am. 2007;25:83.
10 Chapman LE, Sullivent EE, Grohskopf LA, et al. Recommendations for postexposure interventions to prevent infection with hepatitis B virus, hepatitis C virus, or human immunodeficiency virus, and tetanus in persons wounded during bombings and other mass-casualty events—United States, 2008. MMWR Recomm Rep. 2008;57:1.
11 Covidien suture product catalog. http://www.covidien.com/suturecatalog, 2010.
12 D’Eramo EM, Bookless SJ, Howard JB. Adverse events with outpatient anesthesia in Massachusetts. J Oral Maxillofac Surg. 2003;61:793.
13 Edlich RF, Long WB. Surgical knot tying manual, ed 3. Norwalk, Conn: Covidien; 2008.
14 Ethicon product catalog. http://www.ecatalog.ethicon.com, 2010.
15 Fernandez R, Griffiths R: Water for wound cleansing, Cochrane Database Syst Rev 23:CD003861, 2008.
16 Forsch RT. Essentials of skin laceration repair. Am Fam Phys. 2008;78:945.
17 Goldberg SR, Diegelmann RF. Wound healing primer. Surg Clin North Am. 2010;90:1133.
18 Green SM, Rothrock SG, Gorchynski J. Validation of diphenhydramine as a dermal local anesthetic. Ann Emerg Med. 1994;23:1284.
19 Harmatz A. Local anesthetics: Uses and toxicities. Surg Clin North Am. 2009;89:587.
20 Hasham S, Matteucci P, Stanley PR, et al. Necrotising fasciitis. BMJ. 2005;330:830.
21 Haury B, Rodeheaver G, Vensko J, et al. Debridement: An essential component of traumatic wound care. Am J Surg. 1978;135:238.
22 Hochberg J, Meyer KM, Marion MD. Suture choice and other methods of skin closure. Surg Clin North Am. 2009;89:627.
23 Hunt TK. Disorders of wound healing. World J Surg. 1980;4:271.
24 Jamshidi R, Lane J. Management principles of vascular trauma. In: Zelenock GB, Huber TS, Messina LM, et al, editors. Mastery of vascular and endovascular surgery. Philadelphia, Lippincott: Williams & Wilkins; 2006:611-617.
25 Karaduman S, Yürüktümen A, Güryay SM, et al. Modified hair apposition technique as the primary closure method for scalp lacerations. Am J Emerg Med. 2009;27:1050.
26 Kaweski S. Topical anesthetic creams. Plast Reconstr Surg. 2008;121:2161.
27 Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: Use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine. MMWR Recomm Rep. 2006;55:1.
28 Lammers RL, Hudson DL, Seaman LE. Prediction of traumatic wound infection with a neural network-derived decision model. Am J Emerg Med. 2003;21:1.
29 Lee CK, Hansen SL. Management of acute wounds. Surg Clin North Am. 2009;89:659.
30 Luedtke-Hoffmann KA, Schafer DS. Pulsed lavage in wound cleansing. Phys Ther. 2000;80:292.
31 McQuillan GM, Kruszon-Moran D, Deforest A, et al. Serologic immunity to diphtheria and tetanus in the United States. Ann Intern Med. 2002;136:660.
32 Menegazzi JJ, Paris PM, Kersteen CH, et al. A randomized, controlled trial of the use of music during laceration repair. Ann Emerg Med. 1991;20:348.
33 Moran GJ, Talan DA, Abrahamian FM. Antimicrobial prophylaxis for wounds and procedures in the emergency department. Infect Dis Clin North Am. 2008;22:117.
34 Moscati RM, Mayrose J, Reardon RF, et al. A multicenter comparison of tap water versus sterile saline for wound irrigation. Acad Emerg Med. 2007;14:404.
35 Patel PR, Miller MA. Postcare recommendations for emergency department wounds. Emerg Med Clin North Am. 2007;25:147.
36 Pavlidakey PG, Brodell EE, Helms SE. Diphenhydramine as an alternative local anesthetic agent. J Clin Aesthet Dermatol. 2009;2:37.
37 Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: A randomized controlled trial. Ann Emerg Med. 2004;43:362.
38 Rosenberg PH, Veering BT, Urmey WF. Maximum recommended doses of local anesthetics: A multifactorial concept. Reg Anesth Pain Med. 2004;29:564.
39 Singer AJ, Hollander JE. Infiltration pain and local anesthetic effects of buffered vs plain 1% diphenhydramine. Acad Emerg Med. 1995;2:884.
40 Singer AJ, Quinn JV, Clark RE, et al. Closure of lacerations and incisions with octylcyanoacrylate: A multi-center randomized controlled trial. Surgery. 2002;131:270.
41 Singer AJ, Rosenberg L. Basic suturing and tissue handling techniques. In: Singer AJ, Hollander JE, editors. Lacerations and acute wounds: An evidence-based guide. Philadelphia: FA Davis Company; 2003:108-132.
42 Sinha M, Christopher NC, Fenn R, et al. Evaluation of nonpharmacologic methods of pain and anxiety management for laceration repair in the pediatric emergency department. Pediatrics. 2006;117:1162.
43 Stevenson TR, Thacker JG, Rodeheaver GT, et al. Cleansing the traumatic wound by high pressure syringe irrigation. J Am Coll Emerg Physicians. 1976;5:17.
44 Sullivan SR, Engrav LH, Klein MB. Acute wound care. In: ACS surgery: Principles and practice. Chicago: American College of Surgeons; 2007:1.7.1-1.7.24.
45 Teller P, White TK. The physiology of wound healing: Injury through maturation. Surg Clin North Am. 2009;89:599.
46 Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260.
47 Tiwari A, Haq AI, Myint F, et al. Acute compartment syndromes. Br J Surg. 2002;89:397.
48 Trott AT. Complex wounds: Advanced repair techniques. In: Trott AT, editor. Wounds and lacerations: Emergency care and closure. Philadelphia: Elsevier; 2005:135-151.
49 Trott AT. Wound cleansing and irrigation. In: Trott AT, editor. Wounds and lacerations: Emergency care and closure. Philadelphia: Elsevier; 2005:83-91.
50 Tschoi M, Hoy EA, Granick MS. Skin flaps. Surg Clin North Am. 2009;89:643.
51 Waterbook AL, Germann CA, Southall JC. Is epinephrine harmful when used with anesthetics for digital nerve blocks? Ann Emerg Med. 2007;50:472.
52 Wedel DJ, Horlocker TT. Nerve blocks. In: Miller RD, editor. Miller’s anesthesia. Philadelphia: Elsevier; 2009:1639-1674.
53 Wheeler WM. Ants: Their structure and behavior. New York: Columbia University Press; 1960.
54 Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212.
55 Zuber TJ. The mattress sutures: Vertical, horizontal, and corner stitch. Am Fam Phys. 2002;66:2231.