Wound Closure Techniques
Wound Healing
The inflammatory phase (days 0-5) begins immediately after wounding and within 1 to 2 hours; there is a release of vasoactive substances including histamine, serotonin, and cytokines. These substances cause an increase in local vessel permeability leading to an increased exudation of plasma. The leaky capillaries allow cells such as T lymphocytes, leukocytes, neutrophil granulocytes, monocytes, and macrophages to reach the wound area. Neutrophils (polymorphonuclear leukocytes) predominate in the wound during the first 48 hours after wounding. The polymorphonuclear leukocytes provide a nonspecific cellular defense that is the initial protection against wound infection. The nuclei of neutrophils contain proteolytic enzymes that facilitate cleaning of the wound and phagocytosis of bacteria. Following the neutrophils, macrophages start to migrate into the wounded area and predominate by day 4. The macrophages’ main role is to phagocytose debris and to digest bacteria. At the fourth day of wound healing, fibroblasts begin to appear. During this early phase of wound healing, the tensile strength of the wound is minimal and is the result of the fibrin coagulum in the wound bed.1
In the absence of significant infection or wound contamination, the second stage of wound healing begins. The proliferative or granulation phase (days 6-14) is characterized by the formation of granulation tissue in the wound. Granulation tissue consists of a mixture of cellular elements, including fibroblasts and inflammatory cells, along with newly formed capillaries contained within a matrix of collagen, fibronectin, and hyaluronic acid. Early in the second phase, there is a rapid increase in the numbers of fibroblasts. The increase is secondary to an influx as well as in situ production of fibroblasts.2 Structural proteins required for wound repair are primarily synthesized by fibroblasts. Most important, fibroblasts produce large quantities of collagen, which makes up the majority of the extracellular wound matrix. It is this matrix that is responsible for the tensile strength of scar tissue. The collagen is initially deposited in a disorganized fashion. Through cross-linking, the individual collagen fibrils are subsequently reorganized into regularly organized bundles. The new collagen array is oriented along the lines of mechanical stress in the healing wound. The process of fibroblast proliferation and synthetic activity is termed fibroplasia. Revascularization of the wound proceeds in parallel with fibroplasia.3 Capillary buds sprout from blood vessels adjacent to the wound and extend into the wound space. With continued growth, the new vessels eventually branch at their tips and join to form multiple capillary loops. With formation of capillary loops, blood flow recommences. New sprouts then extend from these loops to form an active capillary plexus.
The differentiation or maturation phase is the third segment of wound healing. This occurs from day 15 to 1 year postoperatively and beyond. During this period, there is a gradual egress of fibroblasts and macrophages. Overall, the wound becomes less vascular. Collagen fibers progress on the continuum from disarray to organization. As the collagen becomes more organized, wound strength continues to improve; 45% of normal wound strength is achieved by day 70 and 50% by day 120.4 In general, with optimal wound healing, scar tissue strength approaches 80% of the original tissue strength before wounding. Many factors contribute to normal wound healing; these are listed in Table 4-1. An understanding of these factors assists successful primary wound closure.
TABLE 4-1
Factors Affecting Wound Healing
| Local Factors | General Factors |
| Blood supply | Age |
| Denervation | Endocrine function (pancreas, thyroid) |
| Fluid collection | Drug therapy (anti-inflammatory, cytotoxic) |
| Infection | Sepsis |
| Previous or concurrent irradiation | Major organ failure (pulmonary, cardial, hepatic, renal) |
| Mechanical stress | Obesity |
| Surgical technique | Malignant disease |
Investigators have used their understanding of wound healing science to guide novel therapies. Lee et al5 showed that the use of laser therapy (intense pulsed light) or microneedle techniques could increase collagen deposition during the wound healing response. An increase in collagen deposition tended to result in improved scar appearance and overall wound healing. Other investigators have shown a decrease in the incidence of scar hypertrophy and an improvement in wound healing by use of lasers to treat scars after surgery.6 Kim et al5 showed that three postoperative treatments of suture line scars with the erbium : glass laser (1550 nm) dramatically improved the appearance of thyroidectomy scars.
Surgical Technique
Local Anesthesia
The pharmacologic action of local anesthetics is to block nerve impulses by disrupting the permeability to sodium during an action potential. The interval between an anesthetic injection and its influence on the action potential is dependent on the pharmacokinetics of the drug used and the dosage administered. There are significant differences in potency and duration between various anesthetic agents. An agent’s potency and length of action depend on its level of hydrophobicity.7 When the agent is mixed with epinephrine, the resulting vasoconstriction increases the time required for clearance of the agent. This reduction in clearance rate increases the agent’s duration of action and decreases the total dosage required to achieve an effective nerve block. Many surgeons use anesthetics containing epinephrine for the added benefit of improved hemostasis.
The core chemical structure of local anesthetics is an amine connected to an aromatic end. The amine end is hydrophilic, and the aromatic end is lipophilic. Changing the amine or aromatic end alters the pharmacokinetics of the drug. There are two classes of local anesthetics based on variations at the amino end: amino amides and amino esters.8 The class of amino amides includes lidocaine, mepivacaine, and bupivacaine. The amino esters group includes tetracaine, cocaine, and benzocaine.
Lidocaine is the most frequently used infiltrative local anesthetic. It is available in a variety of concentrations. A 1% solution is commonly used with or without epinephrine. With a rapid onset of action, it is an ideal choice for simple soft tissue surgery. Bupivacaine has a longer duration of action than lidocaine and thus may be more appropriate for complex wounds requiring prolonged repair times. The author (B.C.M.) prefers a 1 : 1 mixture of 0.5% bupivacaine (Marcaine) and 1% lidocaine with a total concentration of 1 : 100,000 epinephrine. If a surgical procedure is to occur without general anesthesia, 10% by volume of an 8.4% bicarbonate solution is included in the anesthetic mixture. This mixture produces an excellent rapid-onset anesthesia with long-term analgesia. The addition of bicarbonate buffers the solution and decreases injection discomfort.9
During infiltration, the surgeon must avoid intravascular injection. This complication can be minimized by frequent aspiration during introduction of the local anesthetic. Inadvertent intravascular administration can lead to lidocaine toxicity. Central nervous system lidocaine toxicity occurs first. The initial manifestations are excitatory, such as tingling, numbness, mental status changes, and eventually seizures. As lidocaine blood levels increase, there is a progression to central nervous system depression with somnolence and increasing respiratory depression. Higher serum concentrations have cardiovascular effects, such as myocardial depression and arrhythmia.
Incisions
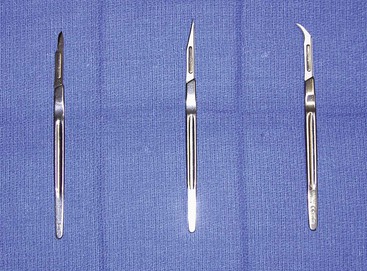
A wide variety of scalpel blades are commercially available for performing skin incisions (Fig. 4-1). For soft tissue surgery, most surgeons prefer the No. 15 blade. This scalpel blade provides a sharp tip for precise angulation and a moderately rounded belly for an efficient cutting surface. A No. 11 blade is often helpful for complex incisions, such as those required for a geometric broken-line scar revision. This blade has an elongated tip with a straight belly that allows an accurate incision of acute angles with short sides. A newer scalpel blade, the 15C, is a modification of the traditional No. 15 blade. This design is a hybrid of the No. 11 and No. 15 designs. An elongated tip with a rounded but low-profile belly allows traditional scalpel use with improved accuracy for complex geometric incisions.
Soft Tissue Technique
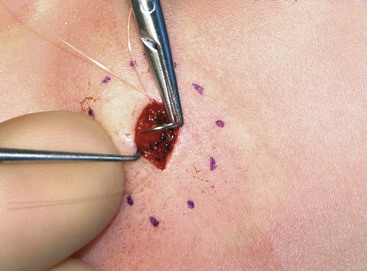
The proper handling of soft tissues is the foundation of successful wound closure and healing. Whether the surgeon is completing a primary wound closure or transferring a local flap, attention should be directed toward the details of wound approximation. Wound closure often begins with the placement of subcutaneous sutures. Proper eversion of the skin at the margin of the wound is required for accurate placement of these sutures. The use of tissue graspers, such as Adson or Adson-Brown forceps, is favored by many surgeons. Although these instruments make tissue handling facile, the operator must be careful not to induce a soft tissue crush injury. An alternative is to use a single-prong skin hook (Fig. 4-2). In this technique, the tissue edge is everted with the skin hook and the operator’s middle finger. The suture needle is then stabilized with the skin hook. After reloading of the needle, the contralateral suture is placed in a similar fashion. Once this technique is mastered, it allows skin edge eversion with less soft tissue trauma compared with the use of tissue forceps for wound repair.
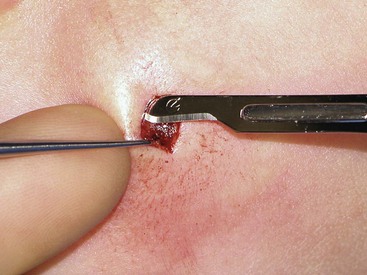
Soft tissue dissection is an essential component of wound closure. Proper technique includes a uniform undermining of the skin for primary wound closure. Whereas there are some tissue planes that lend themselves to blunt dissection (e.g., subgaleal, sub–superficial musculoaponeurotic system), sharp dissection is the general rule. Dissection at the proper depth is facilitated by the use of a single-prong tissue hook. The operator should use the nondominant hand to engage the skin hook while the dominant hand holds the scalpel. The middle finger of the nondominant hand then relays tactile information while maintaining traction to aid the dissection (Fig. 4-3). Although this two-handed technique can be technically challenging, its use results in a more consistent plane of dissection.
Various methods of wound closure are detailed later in this chapter. Each of these methods relies on the fundamental principle of a tension-free wound repair. Achieving a tension-free wound closure is important in the short-term to prevent wound dehiscence and to promote tissue viability. Wound closure tension also plays a role in the long-term appearance of the scar.10 In soft tissue surgery, a number of techniques should be used to decrease wound closure tension. Undermining of the soft tissue at the wound edge will allow easier approximation. Depending on the anatomic location and depth of dissection, a layered closure with deep buried sutures will effectively diminish wound strain. For some procedures, a series of relaxing incisions, such as galeatomies beneath the forehead skin, allow the recruitment and advancement of adjacent tissue and reduction of wound closure tension.
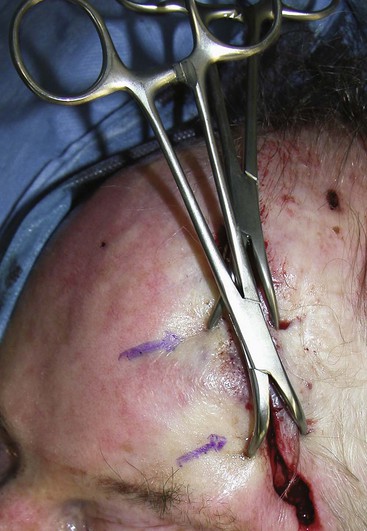
When undermining and layered wound closure fail to minimize wound closure tension, the surgeon may elect to alter the mechanical properties of the skin itself. Because skin is viscoelastic, its intrinsic shape can be altered by mechanical creep. Mechanical creep is defined as the elongation of skin beyond the skin’s intrinsic extensibility by a constant load over time. The stretching is usually performed intraoperatively, and tissue lengthening is due to collagen realignment, displacement of fluids, and fragmenting of elastin fibers. The force used to elicit mechanical creep may be applied with standard instruments, such as towel clamps applied to the skin (Fig. 4-4), or with a number of commercially available devices. Disadvantages of rapid intraoperative skin expansion include tissue damage and problems with extrusion of the expanding device. A promising technique has been the use of external tissue expanders. These devices work more slowly than intraoperative devices. They apply steady and significant tissue traction around an open wound without causing tissue trauma. They are effective in assisting in closure of large wounds that cannot be repaired with conventional techniques.11
A large variety of needle drivers are available to surgeons. The delicate nature of soft tissue work creates a number of requirements for these instruments. The jaws of the driver must be able to effectively grasp the small-caliber needles commonly used for facial skin closure. In addition, the instrument should accommodate the wrist pronation required for proper suture placement. The small-caliber Castroviejo needle driver is an excellent choice for soft tissue work (Fig. 4-5). With its unique design, the locking version of the Castroviejo provides excellent needle traction while allowing more ergonomic wrist pronation. The result is a more accurate and consistently placed suture.
Wound Apposition
Wound repair should be performed with the least wound closure tension necessary to approximate the wound margins. Wound closure tension can be minimized with the placement of proper subcutaneous sutures. The addition of dermal sutures will also assist with reduction of dead space and skin edge eversion. The path of such sutures should start and end at a point farthest from the surface of the skin. On placement of dermal sutures, care must be taken to match the entry and exit points of the suture on both sides of the wound. Misplaced sutures will result in uneven wound edges and poor skin edge eversion. An equal distribution of wound closure tension is achieved by inserting the suture needle below and parallel to the surface of the skin, following the curve of the needle and exiting below and parallel to skin on the contralateral side of the wound. Poor eversion of skin edges results when the needle enters too far from the skin edge or at an improper angle.
Once the subcutaneous tissue layers of a wound have been approximated, the method of cutaneous closure must be determined. Suture remains the “gold standard” material for repair of skin incisions. Sutures are highly adaptable to complex wounds and may be tailored to each individual wound. The limiting aspect for all cutaneous suture techniques is the time required to perform the procedure. When suture closure is performed, with or without deep sutures, the surgeon must use precise techniques to promote skin eversion (Fig. 4-6). At the completion of an ideal wound closure, the skin edges will be slightly everted. In time, with wound contraction, the everted skin edge will retract to leave an aesthetic cutaneous scar. In contrast, when skin edges are approximated without eversion, contractile forces may result in a depressed or widened scar. Sutures that are placed too far from the wound edge may result in telltale suture marks (i.e., train tracks) from pressure necrosis (Fig. 4-7).
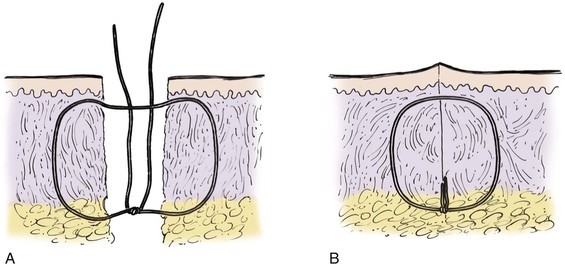
FIGURE 4-6 Schematic diagram of deep suture placement. Placement of deep sutures should eliminate dead space and accomplish wound edge approximation and eversion.
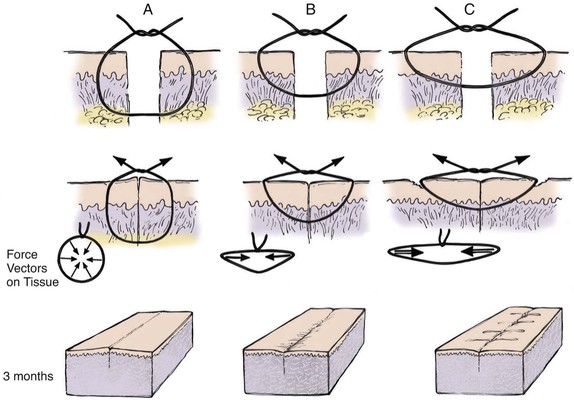
FIGURE 4-7 A, Proper placement of cutaneous suture showing equal distribution of tension along entire wound edge. Everted surgical wound edges provide aesthetic result. B, Improper placement of cutaneous suture with surgical bite too far from skin edge and too superficial. This creates vector forces that are greatest in a horizontal direction and produces inverted wound. C, Improper placement of cutaneous suture farther from skin edge with more superficial surgical bite. Force vectors on wound are greater in a horizontal direction and create more wound inversion. In addition, permanent suture marks result in areas of pressure necrosis where wound closure tension is greatest.
The most basic cutaneous suture is the simple interrupted suture (Fig. 4-8). This technique enables the surgeon to adjust to variations in the level of the wound edges with each individual suture. Whereas an excellent wound closure can be achieved with this technique, it is time-consuming. If the level of the wound edges does not require many adjustments, they may be approximated with a simple continuous (running) suture (see Fig. 4-8). This method provides a rapid wound repair and an even distribution of wound tension along the length of the incision. Wounds near an area of increased motion, such as the lips or medial cheeks, may benefit from an interrupted suture repair. More stress is placed on cutaneous sutures in these areas compared with other regions of the face, and as a result, there is a greater risk for sutures to break. Should this occur to a continuous suture, wound dehiscence is likely.
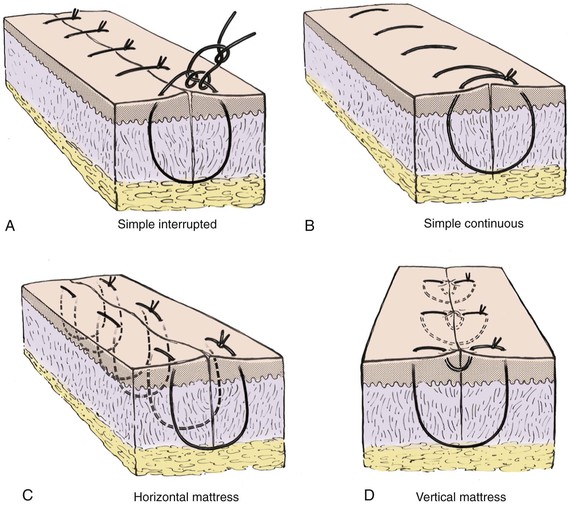
FIGURE 4-8 A, Simple interrupted suture. B, Simple continuous suture. C, Horizontal mattress suture. D, Vertical mattress suture.
The cutaneous suture that creates maximum wound edge eversion is the vertical mattress suture. This suture passes in arcs from two opposing points distal to the wound margin to two opposing points immediately adjacent to the wound margins (see Fig. 4-8). When it is tied, this suture provides vectors of force that maximize wound edge eversion. It also has the benefit of decreasing dead space compared with a simple interrupted or simple continuous cutaneous suture. The horizontal mattress suture does little to decrease dead space, but like the vertical mattress suture, it provides maximum wound edge eversion (see Fig. 4-8). A horizontal mattress suture is placed by taking two equidistant bites of tissue on either side of the wound in a purse-string fashion.
Depending on the location of the wound and the type of suture used, intradermal sutures may be beneficial. The intracuticular technique is a continuous suture that is placed in alternating loops completely within the dermis (Fig. 4-9). Because the suture never penetrates the epidermis, there are no external suture marks. The technique is technically difficult because the suture must be placed at exactly the same depth within the dermis with each insertion of the needle. When suture depth varies, the wound edges will tend to invert and create unfavorable scarring.
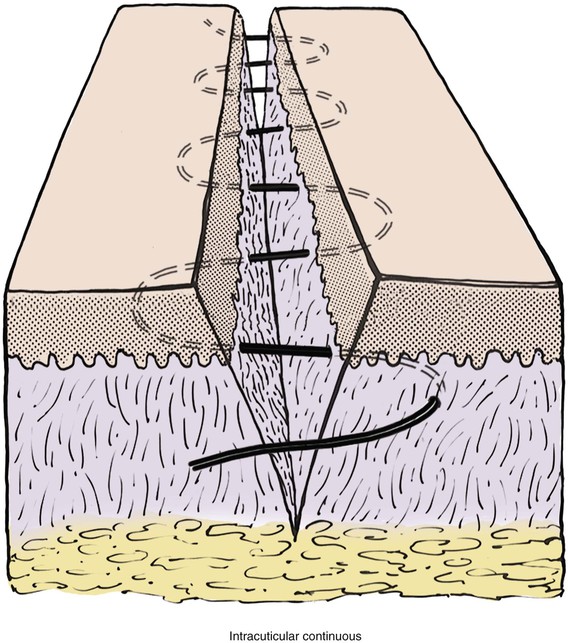
FIGURE 4-9 Intracuticular continuous suture. Cross section of skin shows continuous suture at level of dermis to achieve maximal wound edge eversion.
The use of cutaneous sutures allows selective adjustment of wound margin apposition when there is a discrepancy in the thickness of the opposing skin edges (Fig. 4-10). To alleviate disparities in skin thickness on either side of the wound, deep sutures must first be placed to align the dermal-subdermal tissue planes along the border of tissue apposition (Fig. 4-10B, C). Alignment of the skin edges is accomplished by taking a deeper dermal bite of the thinner skin and a more superficial dermal bite of the thicker skin (Fig. 4-10D, E).
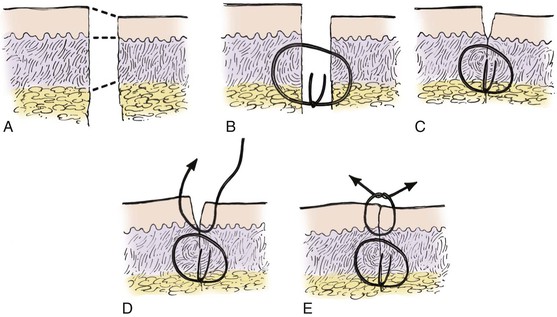
FIGURE 4-10 A, Schematic diagram illustrating two skin edges of unequal thickness. B, C, Placement of deep sutures in mismatched tissue to achieve maximal cutaneous alignment. Note that suture is placed in dermis at deeper plane of thinner skin edge. D, E, Placement of cutaneous sutures in mismatched tissue to achieve maximal tissue eversion.
Wound repair with tape is effective in properly selected patients. The ideal wound is one that is located on a flat body surface. The repair must have no wound closure tension at the cutaneous level. Rather than achieving cutaneous approximation, paper tape essentially maintains an already approximated wound in the form of strips that traverse the wound. Tape closure offers the advantage of ease of use and improved patient comfort.
The technique for tape closure begins once the wound is completely approximated. A skin adhesive is applied immediately adjacent to the wound edges, and strips of tape are placed perpendicular to the line of closure without tension. Tissue forceps should be used to align skin edges during the process. Once it is complete, the tape bandage acts to distribute wound closure tension evenly over the skin-tape interface (Fig. 4-11). When surgical tape is applied to an improperly approximated wound, shearing forces create tension at the cutaneous interface (see Fig. 4-11). The uneven distribution of tension by the tape closure can result in inversion of skin edges and poor wound healing.
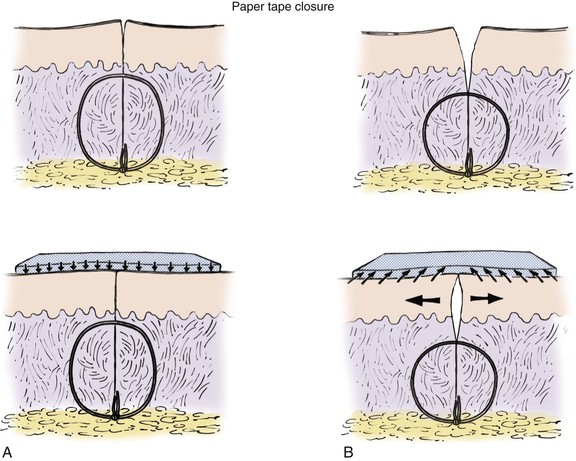
FIGURE 4-11 A, Appropriately selected wound for surgical taping. Adequate deep tissue approximation allows minimum and well-distributed tension between surgical tape and skin surface. B, Poorly selected wound for surgical taping. Inadequate deep tissue closure creates gaping of wound and increased wound closure tension that is poorly distributed between skin surface and surgical tape interface. This creates shearing on skin surface, which results in wound edge inversion and epidermolysis.
Staples are an excellent option for repair of certain skin incisions. The location and characteristic of the wound must be carefully determined before this option is selected. The wound surface should be relatively flat and not located over major bone convexities or concavities. Staples should not be used with delicate tissue (e.g., eyelid) or at the border of certain aesthetic boundaries (e.g., vermilion border). Furthermore, because of the relative lack of precise skin edge approximation when staples are used, there should be minimal disparity in thickness of the skin at the wound edges. With a properly selected wound, staples achieve an aesthetic result similar to that of suture closure.12
As with all cutaneous closures, the staple technique begins with an appropriate subcutaneous tissue approximation (Fig. 4-12). The skin edges are then grasped with a tissue forceps to position them in an everted orientation. Staples are then applied and are placed at intervals necessary to maintain eversion of the wound edge. Properly applied staples produce a transverse vector force that maintains wound approximation and eversion.
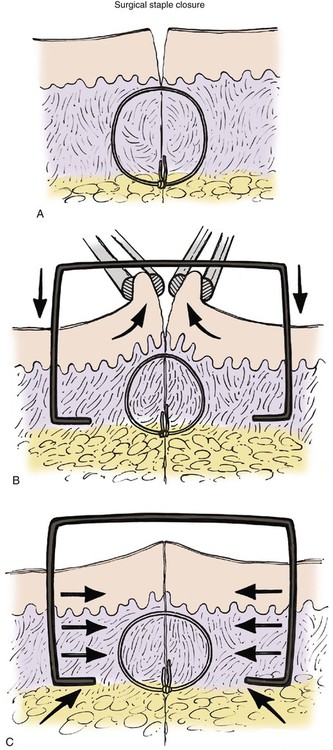
FIGURE 4-12 A, Deep tissue approximation required before placement of surgical staple. B, Tissue forceps approximating and everting skin edge during application of surgical staple. C, Appearance of surgical wound after appropriate placement of surgical staple. Note equal and well-distributed horizontal vector forces on tissue edge after appropriate placement.
Postoperative Dressing
When a skin defect is left open to heal by secondary intention, several strategies may be employed to hasten the healing process. Foremost is providing a moist healing environment. If an open skin wound is allowed to dry, a significant loss of fluids and electrolytes may occur. Dry wounds develop an eschar. Whereas the eschar provides an element of protection to the wound, its presence delays wound healing and usually results in greater scar tissue formation than when wounds heal in a moist environment.13 Thus wounds healing by secondary intention are kept moist by the periodic application of a petroleum-based ointment. Although this is an effective strategy, it requires frequent reapplication by the patient. Hydrogel products are a new class of semiocclusive dressings that provide a moist environment for optimal re-epithelialization of the wound. The semipermeable nature of the dressing prevents maceration of the surrounding tissues and decreases the incidence of infection. There are multiple commercial hydrogel dressings, such as Vigilon, CarraDres, and NuGel. Although they are ideal for wounds that are healing by secondary intention, the hydrogel dressings are also useful for skin care after dermabrasion. Application of a hydrogel dressing minimizes postprocedure crusting and speeds re-epithelialization, resulting in reduced wound healing time.
Suture Material
Suture Characteristics
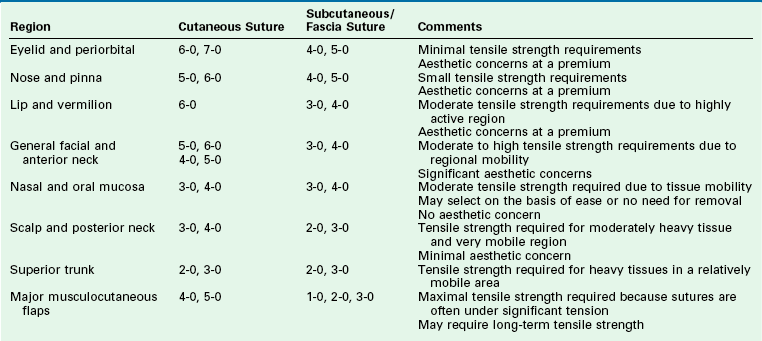
The ideal suture material should be easy to handle, have high tensile strength, and have no tissue reaction (Table 4-2). A variety of suture material is available for soft tissue surgery. Selection of a particular type is dependent on the physical characteristics of the material. A set of common terms and definitions are presented in Table 4-3. Given the multitude of requirements of cutaneous and reconstructive procedures, there is no single suture suited for all wound closures. In part, the outcome of wound repair depends on the type of suture material and needle architecture selected. Inappropriate needle or suture material can create tissue damage at the wound interface and affect the ultimate aesthetic results. One important characteristic of suture material is its coefficient of friction. Different types of suture filaments have varied levels of friction (Fig. 4-13). A higher level of friction results in increased local tissue damage. In general, monofilament sutures have the lowest coefficient of friction. Examples of monofilament sutures are nylon and polypropylene. Braided sutures with higher coefficients of friction can be made more “slippery” with coating agents. Common coating agents include silicone, organic waxes, and polymers of suture material (such as polyglactin 370 and polycaprolate). Another important suture characteristic is elasticity. During wound healing, there is local tissue edema, and the suture material used for repair of the wound should have sufficient elasticity to accommodate the change in tissue volume without compromising wound approximation. Poliglecaprone 25 (Monocryl) and polybutester (Novofil) are two materials that have excellent elasticity profiles.
TABLE 4-2
Ideal Suture Material Characteristics
Minimal tissue injury
Minimal tissue reaction
Ease of handling
High tensile strength
Favorable absorption profile
Resistance to infection
TABLE 4-3
Suture Material: Definition of Terms
| Absorbable Suture | Memory |
| Undergoes progressive loss of mass or volume (does not correlate with loss of tensile strength) | A suture’s inherent capability to return to or maintain its original gross morphologic shape; it is a function of elasticity, plasticity, and diameter |
| Breaking Strength | Nonabsorbable Suture |
| Limit of tensile strength at which material (or tissue) failure occurs | Undergoes no significant loss of mass or volume (does not correlate with retention of tensile strength) |
| Capillarity | Plasticity |
| Extent to which absorbed fluid is transferred along the strand | A measure of a suture’s ability to deform without breaking and to maintain that new form after relief of the deforming force |
| Coefficient of Friction | Straight-pull Tensile Strength |
| A numeric value that quantifies a material’s surface characteristics that act to resist motion against another surface | Linear breaking strength of suture material |
| Elasticity | Suture Pullout Value |
| A measure of a suture’s ability to regain its original form and length after deformation | Limit of strength of a particular tissue as measured by the force applied to a loop of suture at which tissue failure occurs |
| Fluid Absorption | Tensile Strength |
| Ability to take up fluid after immersion | A material’s or tissue’s ability to resist deformation and breakage |
| Knot-pull Tensile Strength | Wound Breaking Strength |
| Breaking strength of a suture material, which can be 10% to 40% weaker after deformation by knot placement | Limit of tensile strength of a healing wound at which wound separation occurs |
| Knot Strength | |
| Amount of force necessary to cause a knot to slip, which is directly proportional to the coefficient of static friction and plasticity of a given material |

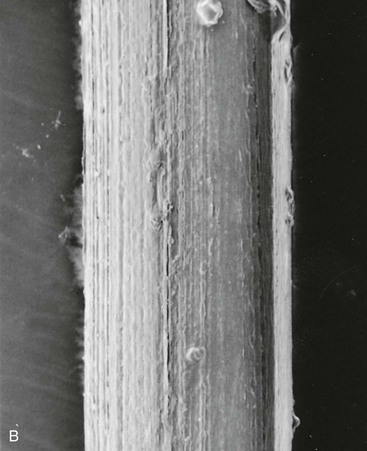
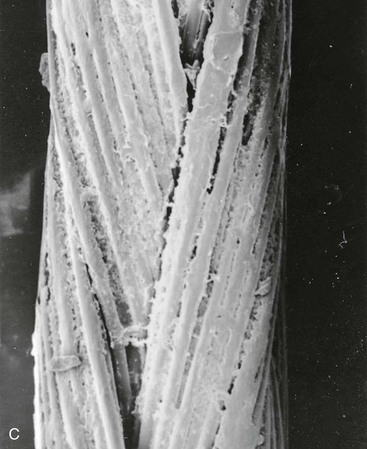
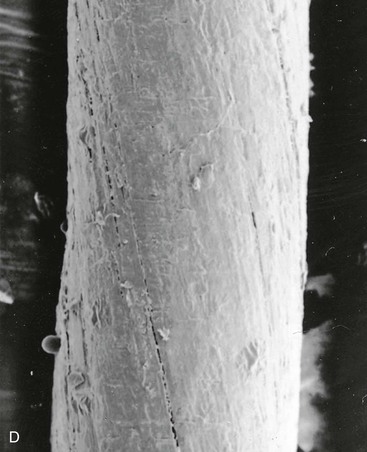
FIGURE 4-13 Scanning electron micrograph (40×) of various suture materials: A, silk suture; B, nylon suture; C, coated synthetic braided suture (Vicryl); D, chromic gut suture. In general, monofilament sutures (like nylon) have lowest coefficient of friction.
All suture material elicits an inflammatory response in the tissue where the suture is placed. The response may be related to the physical nature of the suture material (coating materials) or a patient’s immune reaction to the material. Chromic and plain gut sutures are associated with significant tissue reactions.14 These collagen-based sutures are absorbed by neutrophil-mediated proteolysis. A nonspecific inflammatory response accompanies the arrival of neutrophils, resulting in local tissue reaction. In contrast, a variety of synthetic absorbable suture fibers are absorbed by simple hydrolysis, which is associated with limited inflammation. Nonabsorbable fibers do not undergo local degradation and elicit the least inflammatory response.
The tensile strength of suture material is important to maintain wound approximation. For a given wound closure, a smaller gauge suture may be used if it possesses sufficient tensile strength. Conversely, mechanically weaker suture material must have a larger caliber to close similar wounds. In repairs in which there is significant wound closure tension, smaller gauge sutures with high tensile strength can cut through the wound edges as it transmits wound closure tension to the local tissue. For best results, the suture used should be no stronger than the tissue it is approximating. The strength of individual tissue types is detailed in Table 4-4.
The United States Pharmacopoeia (USP) classification system was established in 1937 for standardization and comparison of suture materials. The graded system has a direct correlation to metric measures. Suture size describes the diameter of the suture strand and is graded by a series of zeros. Somewhat paradoxically, the more zeros a suture has, the smaller it is (e.g., 5-0 is smaller than 4-0). Suture materials are also divided into three classes: collagen, synthetic absorbable, and nonabsorbable. USP specifications for each class are detailed in Tables 4-5 to 4-7. Review of these specifications reveals several inconsistencies of the USP system. The true metric size and USP size have some variation between the three classes. In addition, metric size does not perfectly correlate with suture knot tensile strength. Last, whereas the USP specifications set the minimum tensile strength for a given suture size, they do not set an upper limit. Therefore, sutures in the same USP class may have different tensile strengths.
TABLE 4-5
USP Specifications for Collagen Suture
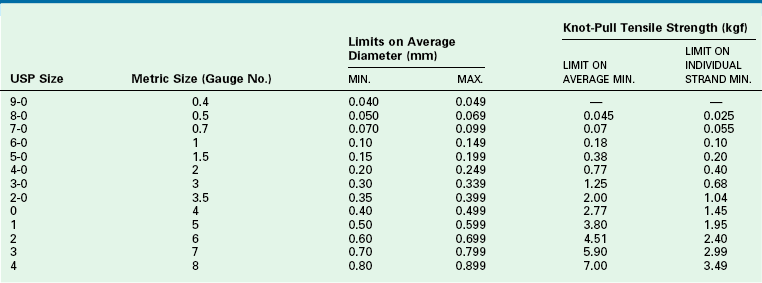
USP36-NF31 ©2013 United States Pharmacopeial Convention. Used by Permission.
TABLE 4-6
USP Specifications for Synthetic Absorbable Suture
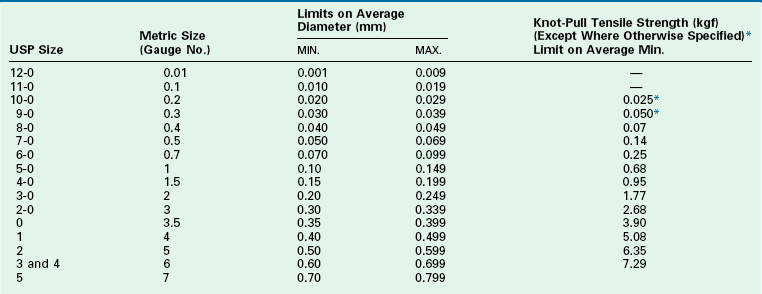
*The tensile strength of the specific USP size is measured by straight pull.
USP36-NF31 ©2013 United States Pharmacopeial Convention. Used by Permission.
TABLE 4-7
USP Specifications for Nonabsorbable Surgical Suture
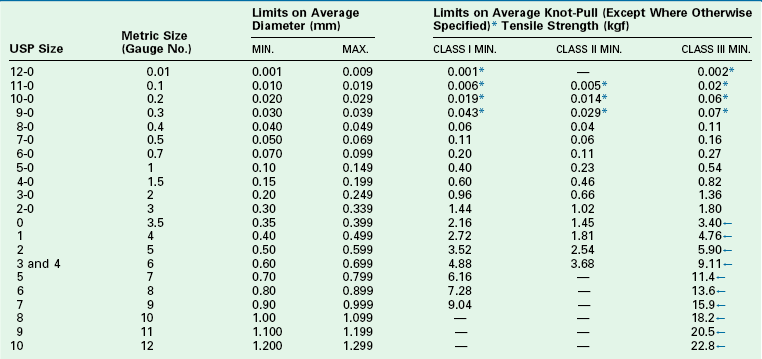
Class III: composed of monofilament or multifilament metal wire.
*Tensile strength of sizes smaller than USP size 8-0 (metric size 0.4) is measured by straight pull. Tensile strength of sizes larger than USP size 2-0 (metric size 3) of monofilament class III (metallic) nonabsorbable surgical suture is measured by straight pull.
←Silver wire meets the tensile strength values of class I sutures but is tested in the same manner as class III sutures.
USP36-NF31 ©2013 United States Pharmacopeial Convention. Used by Permission.
Natural Fiber Suture
Multiple natural fiber sutures are available. The characteristics of natural fiber sutures are listed in Table 4-8. Natural fiber materials may be absorbable or nonabsorbable. Absorbable sutures consist of collagen and varieties include gut, fast gut, and chromic gut. Collagen suture is derived from either sheep or beef intestine. Plain gut suture maintains tensile strength for 7 to 10 days. Absorption is complete by 70 days. Fast-absorbing gut maintains tensile strength for 5 days with complete absorption by 60 days. Chromic gut has a longer duration of action and its tensile strength is maintained for 10 to 14 days. The treatment of the collagen material with chromium salts retards absorption, slowing the process to 90 days. Whereas plain and chromic gut may be used internally, fast-absorbing gut is intended for external use.
TABLE 4-8
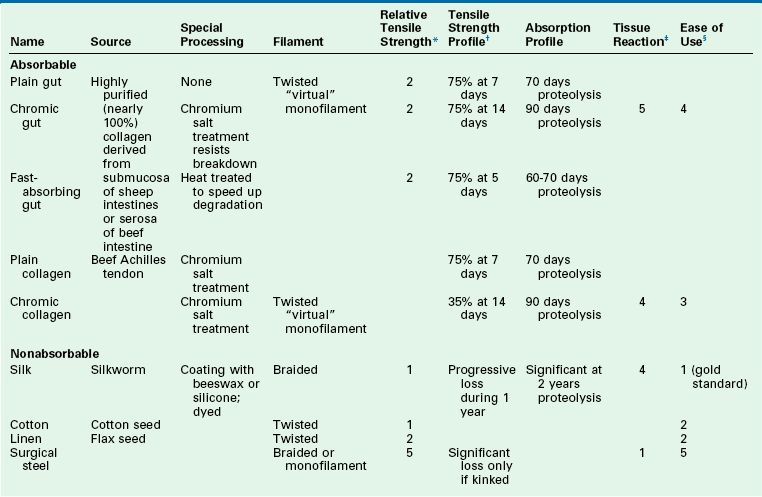
*Relative tensile strength scale: 1 = least, 5 = greatest strength. When accompanied by ( ), indicates relative rank within a relative strength class: (1) = greatest, (5) = least.
†Represents percentage of original dry, out-of-package tensile strength.
‡Represents relative tissue reaction: 1 = least, 5 = greatest reaction.
§Represents relative overall ease of use: 1 = easiest, 5 = most difficult.
Natural fiber suture that is nonabsorbable is less commonly used in facial plastic surgery. Available materials include silk and cotton. Surgical silk is made from the natural raw product of silkworms. For many years, silk suture was considered the ideal material, given its ease of handling. Silk is classified as a nonabsorbable material, but in many cases it is absorbed by 2 years. Tensile strength of silk suture decreases over time and is lost by 1 year. Surgical cotton is rarely used. Because it is nonabsorbable, in time it becomes an encapsulated foreign body. The tensile strength decreases over time and is reduced to one-third of its original strength by 2 years.
Synthetic Fibers
Absorbable synthetic sutures are polymers that are degraded by hydrolysis, which causes less inflammation than with natural fiber sutures. Popular synthetic absorbable sutures include Vicryl, Monocryl, and polydioxanone (PDS). These products are detailed in Table 4-9. Vicryl is a braided multifilament suture coated with lactide and glycolide. These copolymers are hydrophobic, which prolongs the life of the suture. Nearly two-thirds of the tensile strength remains at 14 days. Absorption is not complete until 60 to 70 days after implantation. Vicryl is an ideal suture for approximating the depths of wounds, including fascia and muscle. Monocryl is a synthetic monofilament that is composed of glycolide and ε-caprolactone. Initial tensile strength is maintained during the first week, with subsequent rapid degradation by 3 weeks. The pliable nature of Monocryl makes it ideal for delicate subcuticular closures. PDS is another monofilament and is a polyester derivative. This suture has a pliability similar to that of Monocryl but with a longer duration of tensile strength. Tensile strength is maintained for up to 6 weeks. PDS is used for repairs that may have increased wound closure tension.
TABLE 4-9
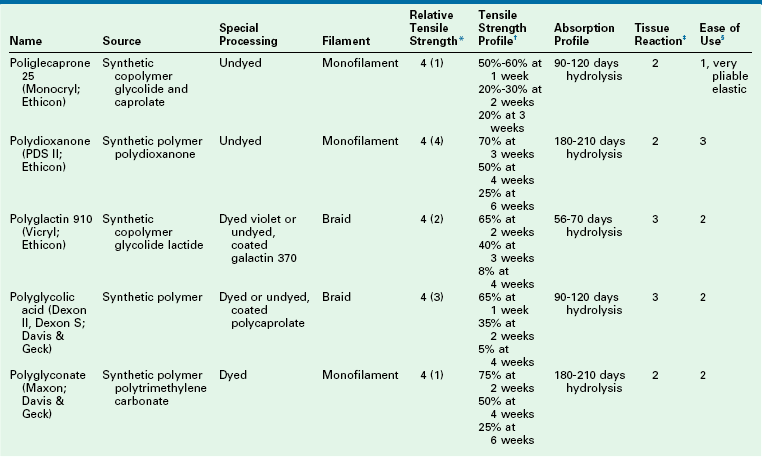
*Relative tensile strength scale: 1 = least, 5 = greatest strength. When accompanied by ( ), indicates relative rank within a relative strength class: (1) = greatest, (5) = least.
†Represents percentage of original dry, out-of-package tensile strength.
‡Represents relative tissue reaction: 1 = least, 5 = greatest reaction.
§Represents relative overall ease of use: 1 = easiest, 5 = most difficult.
Synthetic nonabsorbable sutures, such as nylon and Prolene, have long been the standard for cutaneous approximation. Other synthetics, such as Mersilene, are used for deep permanent sutures. The characteristics of these sutures are noted in Table 4-10. Nylon is a polyamide polymer and is available in a monofilament and braided formulation. The suture has a high level of elasticity and is useful for cutaneous closures. When it is used as an internal permanent suture, nylon has an impressive duration of tensile strength. As much as two-thirds of the original tensile strength is present at 10 years after implantation. Although nylon undergoes eventual hydrolysis, this is minimized by the foreign body encapsulation that occurs. Polybutester (Novofil) is a true permanent suture that does not lose tensile strength or become absorbed. Another polyester fiber (polyethylene terephthalate) is a multifilament braided fiber that is available in coated (Ethibond) and uncoated (Mersilene) versions. This suture has an indefinite permanence. With its ease of use and long life, the suture is ideal for use with implants and in procedures requiring cartilage sculpting, such as otoplasty. Prolene is a propylene polymer monofilament suture. As a monofilament, Prolene has excellent pliability and minimal friction. Of all the synthetic monofilaments, Prolene is the best for holding a knot.
TABLE 4-10
Nonabsorbable Synthetic Suture
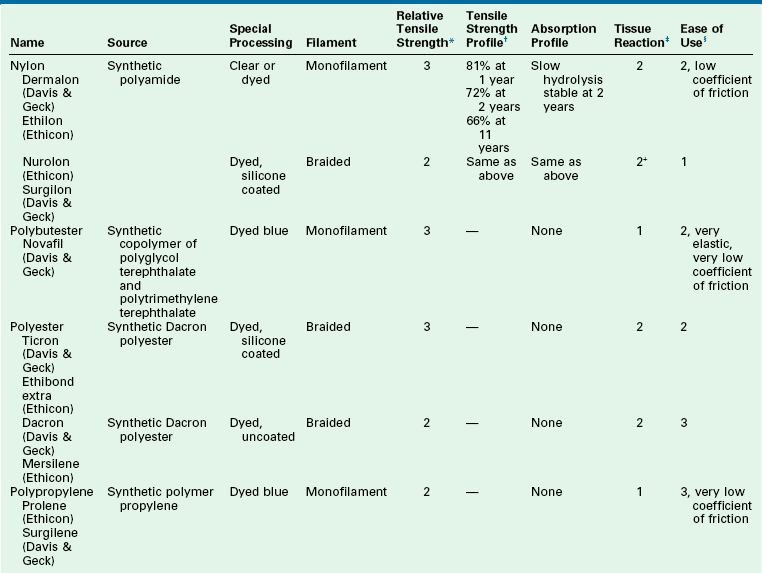
*Relative tensile strength scale: 1 = least, 5 = greatest strength. When accompanied by ( ), indicates relative rank within a relative strength class: (1) = greatest, (5) = least.
†Represents percentage of original dry, out-of-package tensile strength.
‡Represents relative tissue reaction: 1 = least, 5 = greatest reaction.
§Represents relative overall ease of use: 1 = easiest, 5 = most difficult.
Suture Needles
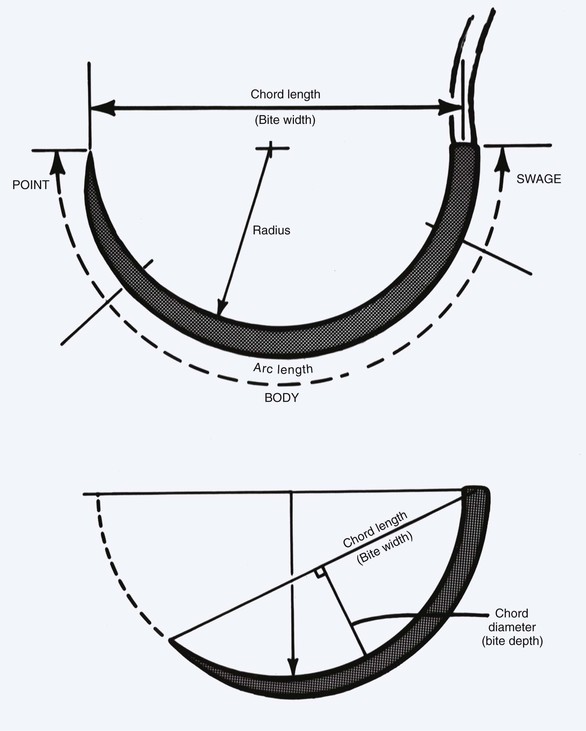
Similar to the large selection of sutures, a number of suture needles are also available in a variety of styles. Commonly used nomenclature for needles is listed in Table 4-11. The basic architecture of the needle is noted in Figure 4-14. All needles have the same three basic elements: point, body, and swage. The point is the portion of the needle that begins at the tip and ends when the needle reaches its full diameter. The body forms the majority of the needle and ends at the contour change that is the start of the swage. The swage continues to the end of the needle where the suture is attached.
TABLE 4-11
Commonly Used Nomenclature of Suture Needles
| BV | Blood vessel |
| TE | Three-eighths |
| P | Plastic surgery |
| PS | Plastic surgery |
| CPS | Conventional plastic surgery |
| PC | Precision cosmetic |
| OPS | Oculoplastic surgery |
| M | Muscle |
| J | Conjunctiva |
| FS | For skin |
| FSL | For skin large |
| X | Exodontial |
| KS | Keith straight |
| SC | Straight cutting |
| FN | For tonsil |
| SH | Small half |
| MH | Medium half |
| CT | Circle taper |
| ST | Straight taper |
| CP | Cutting point |
| T | Taper |
| E | Three-eighths |
| O | One-fourth |
| SC | Standard cutting |
| S | Straight |
| CTE | Cutting taper three-eighths |
| PR | Plastic reconstructive |
| PRE | Plastic reconstructive three-eighths |
The point of the needle represents the initial interface of the structure with the tissue through which it passes. A silicone coating on the stainless steel of the needle determines the level of friction that occurs with each pass through tissue. An improved quality of steel and silicone coating maximizes the durability of the needle point. There are multiple styles of needle points. The three most common types are taper, cutting, and reverse cutting (Fig. 4-15; Table 4-12).
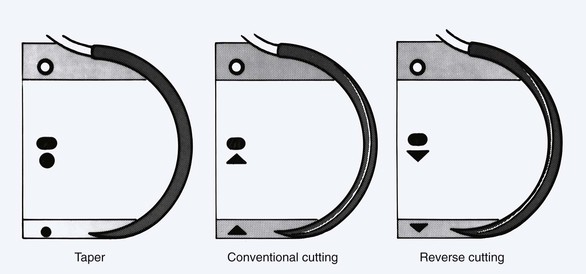
FIGURE 4-15 Schematic diagram of commonly used suture needles. Cross-sectional geometry of needle point, needle body, and needle swage is shown in inset drawings.
The tapered needle is round and works by spreading the tissue as it passes through it. Its sharpness is directly related to the rapidity of its taper. This is known as the taper ratio. Most tapered needles have a taper ratio of 8 : 1 to 12 : 1.The higher the ratio, the sharper is the needle tip. The function of the taper needle makes it suitable for use in soft elastic tissues, such as muscle and subcutaneous fat.
The body of the needle plays a role in the function of the needle. Its greatest importance is in the interaction with the needle holder (Fig. 4-16). The geometry of the needle body determines the amount of force transmitted to the needle point. Round-bodied needles have limited surface contact area with a needle holder and thus transmit the least force to the needle tip. Ovoid bodies have greater surface contact area with the needle holder and transmit greater force to the needle point. For this reason, most needles have an ovoid contour. A needle holder of appropriate size is important when a specific needle is selected. Needle holder jaw width should be no wider than 30% to 50% of the needle radius (Fig. 4-17). A needle holder that is too large straightens the needle, and a holder that is too small allows the needle to rotate around the needle’s long axis.
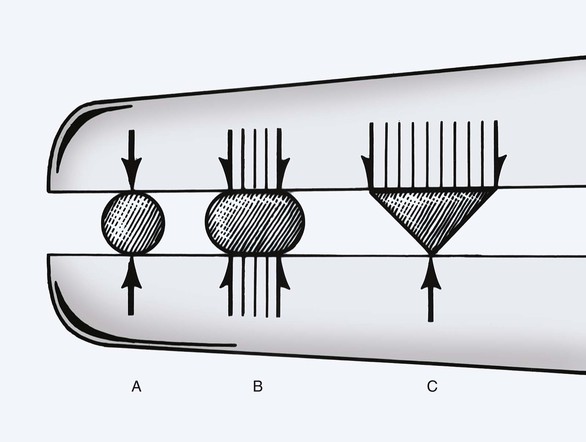
FIGURE 4-16 Profile of typical needle holders grasping three different geometric-shaped needle bodies. B, Oval needle maximizes surface contact with holder, transmitting greatest force to needle tip.
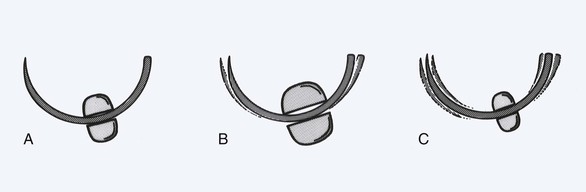
FIGURE 4-17 Schematic diagram of interaction between needle holder and suture needle. A, Needle holder of appropriate size. B, Needle holder too large for needle shown, which creates inadvertent straightening of needle. C, Needle holder too small for needle shown, which allows needle rotation around long axis of needle holder.
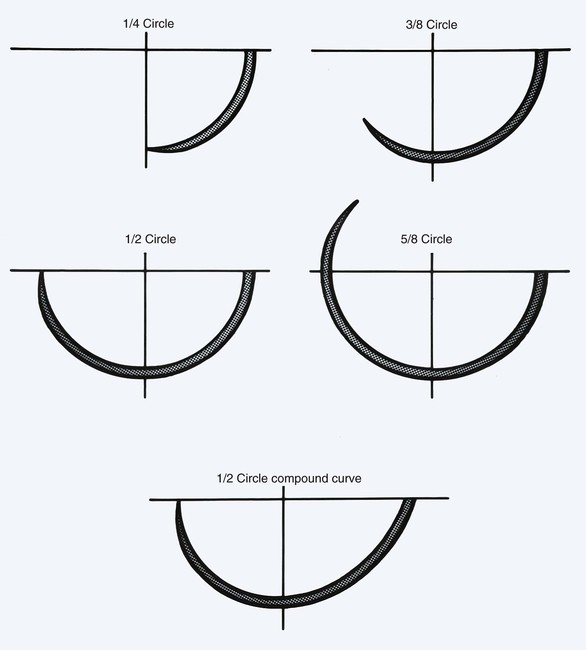
In addition to the cross-sectional design of the needle body, the arc of the needle body is also highly variable. The arc or shape of the needle body influences its applications in surgery (Table 4-13). The most common design used in facial surgery is semicircular. This allows optimum distribution of tension on the suture and a favorable skin edge eversion. The arc of a needle body is described by the fraction of a complete circle that it approximates (Fig. 4-18). A standard suture bite that enters and exits equidistant from the wound edge requires a 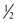 circle needle. As the depth of the bite increases, the fraction of a circle required increases. For example, a
circle needle. As the depth of the bite increases, the fraction of a circle required increases. For example, a 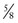 circle needle will place a suture at the same equidistant points as a
circle needle will place a suture at the same equidistant points as a 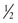 circle needle but with deeper tissue penetration. Table 4-14 lists the definition of terms that play a role in the characteristics of a suture needle in relationship to the needle holder and the interaction of a needle with tissue it encounters.
circle needle but with deeper tissue penetration. Table 4-14 lists the definition of terms that play a role in the characteristics of a suture needle in relationship to the needle holder and the interaction of a needle with tissue it encounters.
TABLE 4-14
Suture Needle and Needle Holder: Definition of Terms
Angle of Needle Failure
Angle of deformation at which a needle rapidly loses resistance to plastic deformation
Bending Moment
Force applied to needle creating angular deformation
Needle Ductility
Resistance to needle breakage
Needle Holder Clamping Moment
Force applied to a suture needle by a needle holder; this applied force may cause deformation of a curved needle
Taper Ratio
Length-to-width ratio of the needle point; a measurement of the sharpness of a needle point
Tip Angle
Angle described by two straight lines drawn from the tip of the needle toward the body that best match the slightly curved contour of the taper; a measurement of the sharpness of a needle point
Ultimate Moment
Greatest bending moment at which the limits of nonreversible (plastic) deformation are reached
Yield Moment
Greatest bending moment at which reversible (elastic) deformation occurs
Tissue Sealants
Fibrin Sealants
The use of fibrin-based tissue sealants has a long history. Their initial use was as early as the First World War. Pooled human plasma was used as the source for fibrin and associated factors. Commercial production was achieved soon after, but further development was limited by concern for safety.15 Improvement in viral screening and safety in the 1970s has prompted re-examination of these useful surgical adjuncts. Production of fibrin sealants has occurred in Europe and Canada. A more protracted approval process culminated with the approval of the Food and Drug Administration (FDA) for fibrin-based sealants in 1997.
The basic model for fibrin-based sealants involves the combination of two distinct components, which results in rapid coagulation. The primary components are purified fibrinogen, factor XIII, and various plasma-derived proteins. The secondary component, which acts as the catalyst, is concentrated thrombin and calcium chloride. The mixing of these components induces activation of the coagulation cascade. The result is conversion of individual fibrin molecules to a cross-linked polymer of fibrin.16
The physiology of this basic reaction can be altered by producing fibrin and thrombin products with variable properties. In general, these products are classified as either tissue sealants or glues. Sealants provide moderate tissue adhesion and excellent hemostasis. Fibrin glues have greater tensile strength for improved tissue adhesion.17 A third class of products uses a collagen base with activated thrombin to produce a significantly hemostatic agent. This combination is commercially available as Floseal (Baxter) and has a much stronger hemostatic profile with the ability to inhibit active arterial hemorrhage.
There are many indications for fibrin-based sealant products in facial plastic and reconstructive surgery. For soft tissue procedures that involve the use of full- or split-thickness skin grafts, studies have demonstrated a significant improvement in outcome when a fibrin sealant is used.18 Fibrin glue has been shown to improve the survival rate of skin grafts. The improved survival rate is particularly notable at grafting sites that involve an element of unavoidable motion. Bacterial wound infections have also been reduced by the hemostatic properties of fibrin products. Experimental studies demonstrate that fibrin sealants are an ideal delivery vehicle for exogenous growth factors.19 This strategy may be used in the near future to accelerate wound healing.
Fibrin sealants have also proved useful in local flap techniques. Improved flap adhesion and accelerated hemostasis contribute to the long-term success of these procedures. Early flap adhesion reduces soft tissue shearing forces that can delay neovascularization. Fibrin sealants help reduce surgical dead space. Thus, the use of fibrin sealants may be particularly useful when creating large local flaps, such as cervicofacial rotation flaps. A significant decrease in postoperative drainage as well as in hematoma formation has been demonstrated when fibrin sealants are used during rhytidectomy.20
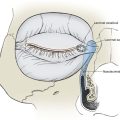
After reagent preparation is complete, the surgical technique for the use of a fibrin sealant depends on the location and nature of the procedure. In all cases, the basic delivery device is the same: a syringe with dual chambers and a single ejection port. The reagents combine and begin the coagulation cascade as they are ejected. For delivery in small-volume areas (e.g., small full-thickness skin grafts, small local flaps), an angiocatheter style tip is adequate for coating the graft or flap recipient site. For larger procedures (e.g., cervicofacial flaps), the delivery system can be combined with a forced-air system to produce an aerosol delivery. This is ideal for coating of a large area with a thin volume of fibrin sealant (Fig. 4-19). After application is complete, the flap or graft should be stabilized in the desired location with gentle pressure. The time required for complete adhesion varies from 60 seconds to several minutes, depending on the concentration of the reagents. The surgeon can modify the rapidity of the adhesion on the basis of the surgical procedure. The sealant is adjusted for rapid onset for simple grafts and flaps. For larger or more complex local flaps, the sealant should be at a slower mixture setting to allow ideal tissue positioning.
A new type of wound healing reagent introduced in recent years is a starch compound that is purified from potatoes and has been FDA approved for enhancement of intraoperative hemostasis. Branded as Arista, the compound has a number of advantages: it is significantly less expensive than fibrin-based products, it has minimal tissue reactivity because of its chemical nature, and it is effective in promoting hemostasis in an open wound.21
Tissue Adhesives
The development of tissue adhesives parallels the history of fibrin sealants.22 The chemical compounds (cyanoacrylates) were discovered in the 1940s, but complete commercialization was delayed until FDA approval in 1998. These compounds demonstrate excellent adhesion and tensile strength. The initial formulation of these substances was not suited for medical use because of the brisk inflammatory reaction that they induced. Industrial use in the 1950s became popular. A modification to the formula in the early 1970s created a more useful compound that, although off-label, was used for wound closure during the Vietnam War. During the 1980s and early 1990s, the cyanoacrylates were used widely in veterinary medicine. Initial successes in studies with human wounds eventually led to commercialization and FDA approval for human use.23
The cyanoacrylate tissue adhesives combine cyanoacetate and formaldehyde and a base to form a liquid monomer. Moisture on the skin’s surface then chemically induces a polymerization. Once it is fully polymerized, the cyanoacrylate is bound to the top epithelial layer. The polymerized cyanoacrylate bridges the wound edges together, and normal healing occurs at the subepithelial level. It is essential that wound edges be well apposed to avoid contamination of the subepithelial portions of the wound, which can lead to foreign body reaction and wound failure.24
References
1. Dubay, DA, Franz, MG. Acute wound healing: the biology of acute wound failure. Surg Clin North Am. 2003; 83:463.
2. Carlson, MA, Longaker, MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004; 12:134.
3. Li, J, Zhang, YP, Kirsner, RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003; 60:107.
4. Falcone, PA, Caldwell, MD. Wound metabolism. Clin Plast Surg. 1990; 17:443.
5. Kim, SE, Lee, JH, Kwon, HB, et al. Greater collagen deposition with the microneedle therapy system than with intense pulsed light. Dermatol Surg. 2011; 37:336–341.
6. Choe, JH, Park, YL, Kim, BJ, et al. Prevention of thyroidectomy scar using a new 1,550-nm fractional erbium-glass laser. Dermatol Surg. 2009; 35:1199–1205.
7. Achar, S, Kundu, S. Principles of office anesthesia: part I. Infiltrative anesthesia. Am Fam Physician. 2002; 66:91.
8. Mather, LE, Cousins, MJ. Local anesthetics and their current clinical use. Drugs. 1979; 18:185.
9. Bartfield, JM, Ford, DT, Homer, PJ. Buffered versus plain lidocaine for digital nerve blocks. Ann Emerg Med. 1993; 22:216.
10. Brissett, AE, Sherris, DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001; 17:263.
11. Topaz, M, Carmel, NN, Silberman, A, et al. The TopClosure 3S System, for skin stretching and a secure wound closure. Eur J Plast Surg. 2012; 35:533–543.
12. Edlich, RF, Becker, DG, Thacker, JG. Scientific basis for selecting staple and tape skin closures. Clin Plast Surg. 1990; 17:571.
13. Svensjo, T, Pomahac, B, Yao, F, et al. Accelerated healing of full-thickness skin wounds in a wet environment. Plast Reconstr Surg. 2000; 106:602.
14. Swanson, NA, Tromovitch, TA. Suture materials, 1980s: properties, uses and abuses. Int J Dermatol. 1982; 21:373.
15. Reece, TB, Maxey, TS, Kron, IL. A prospectus on tissue adhesives. Am J Surg. 2001; 182(Suppl):40S.
16. Spotnitz, WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001; 182(Suppl):8S.
17. Clark, RA. Fibrin glue for wound repair: facts and fancy. Thromb Haemost. 2003; 90:1003.
18. Currie, LJ, Sharpe, JR, Martin, R. The use of fibrin glue in skin grafts and tissue-engineered skin replacements: a review. Plast Reconstr Surg. 2001; 108:1713.
19. Greiling, D, Clark, RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997; 110:861.
20. Oliver, DW, Hamilton, S, Figle, AA, et al. A prospective, randomized, double-blind trial of the use of fibrin sealant for face lifts. Plast Reconstr Surg. 2001; 108:2101.
21. Sindwani, R. Use of novel hemostatic powder MPH for endoscopic sinus surgery: initial impressions. Otolaryngol Head Neck Surg. 2009; 140:614.
22. Mobley, SR, Hilinski, J, Toriumi, DM. Surgical tissue adhesives. Facial Plast Surg Clin North Am. 2002; 10:147.
23. Toriumi, DM, O’Grady, K, Desai, D, et al. Use of octyl-2-cyanoacrylate for skin closure in facial plastic surgery. Plast Reconstr Surg. 1998; 102:2209.
24. Edmonson, MB. Foreign body reactions to dermabond. Am J Emerg Med. 2001; 19:240.