Chapter 82 What Is the Role of Antibiotic Cement in Total Joint Replacement?
Deep wound infection after total joint arthroplasty can be a devastating complication for the patient. Data from the Swedish Knee Registry demonstrates a deep infection rate after total knee arthroplasty (TKA) of 1.7% in patients with osteoarthrosis and 4.4% in patients with rheumatoid arthritis.1 Data from other large series of patients have shown infection rates to be between 0.2% to 1%.2–4 Antibiotic-loaded bone cement (ALBC) is a well-accepted adjunct in the treatment of an established infection. However, its role in the prevention of infection remains controversial because of issues regarding drug resistance, sensitivity, efficacy, and cost.
BACKGROUND
The course and amount of antibiotic that is released from the cement depends on the porosity and the overall surface area of the bone cement exposed. Antibiotic is released from the surface of the cement, and also from cracks and voids in the cement.5,6
Palacos (Zimmer, Warsaw, IN, USA) bone cement has been shown to have greater elution levels than other types of bone cement.7,8 This difference is attributed to the increased porosity of Palacos cement. Although the majority of the antibiotic release occurs in the first 9 weeks, late fracture of the cement mantle can liberate substantial levels of antibiotic many years after implantation.9,10
Commercially prepared antibiotic cement may be superior to cement in which antibiotics are mixed intraoperatively. Elution of gentamicin and tobramycin from surgeon-mixed Simplex (Stryker, Mahwah, NJ, USA) or Palacos beads compared with elution from commercially prepared gentamicin-PMMA (Septopal, Biomet, Warsaw, IN, USA) beads showed that more total antibiotic was released from the latter and was maintained at greater concentrations than it was in the cement to which antibiotics were mixed by hand.11
Some antibiotics elute better from bone cement than others.12 A study of antibiotic elution from Simplex bone cement examined cefazolin (4.5 g per 40 g cement powder), ciprofloxacin (6 g per 40 g powder), clindamycin (6 g per 40 g powder), ticarcillin (12 g per 40 g powder), tobramycin (9.8 g per 40 g powder), and vancomycin (4 g per 40 g powder). The authors conclude that clindamycin, vancomycin, and tobramycin displayed the best elution characteristics into bone and granulation tissue.13
The use of local antibiotic delivery from ALBC in the treatment of musculoskeletal infection is well established.14–17 It has been shown that at least 3.6 g antibiotic per 40 g cement is optimal for the best elution kinetics and sustained therapeutic levels.18 Doses as high as 6 to 8 g antibiotic per 40 g bone cement have been shown to be safe clinically.17 The use of these high doses is important for the sustained release of antibiotics at levels that are bacteriocidal for the organisms being treated.
PROPHYLAXIS
Total Knee Arthroplasty
The use of ALBC for treating active infections in joint arthroplasty has been well established. The basis for the use of ALBC as a prophylactic measure is to reduce the prevalence of deep joint infection. Gentamicin, cefuroxime, and tobramycin are the antibiotics most commonly used in bone cement in clinical studies worldwide.19–22 Of the three antibiotics, gentamicin has been used most frequently and studied most extensively.23 We are not aware of any clinical studies comparing the efficacy of one antibiotic over another as a prophylaxis in cement.
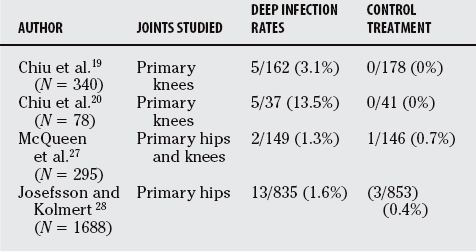
In a randomized clinical trial of 340 primary TKAs, Chiu and colleagues19 evaluated the deep infection rates for patients with cefuroxime bone cement as compared with standard bone cement. No deep infections occurred in the study group, whereas a deep infection developed in 5 of the 162 knees (3.1%) in the control group (P = 0.02). Even with the small sample size, a significant reduction in deep infections was noted with the use of antibiotic bone cement.
The same authors then performed a second prospective randomized trial on just 78 patients with diabetes undergoing primary TKA.20 Once again, cefuroxime bone cement was used in one group and standard bone cement in the other. The authors note a significant reduction in deep infection rate in this high-risk patient population with the use of antibiotic bone cement (P < 0.02). The reported a deep infection rate of 13.5% (5/37) in the control group and no infections in the ALBC group. This overall infection rate for patients with diabetes is greater than that reported in the literature of 3.1% to 7%.24–26
In another randomized trial, McQueen and coworkers27 found that, in 295 patients undergoing primary hip or knee replacement, there was no difference in infection rates between the use of cefuroximeimpregnated bone cement or administration of the cefuroxime intravenously. They report an overall infection rate of 6.8%.
Prophylaxis: Total Hip Arthroplasty
Josefsson and Kolmert28 performed a large randomized trial of 1688 patients undergoing hip replacement and found that at 2 years, the group treated with intravenous antibiotics had a deep infection rate of 1.6% (13/835) versus 0.4% (3/853) in the group treated with gentamycin-impregnated bone cement (P < 0.05).
Espehaug and coauthors21 report on 10,905 primary cemented total hip replacements from the Norwegian Arthroplasty Registry. There were four treatment groups: (1) systemic antibiotics and antibiotic-impregnated bone cement, (2) systemic antibiotics only, (3) antibiotic-impregnated bone cement only, and (4) no antibiotic prophylaxis. The overall infection rate was 0.4%. The use of antibiotic bone cement and systemic antibiotics was found to be significantly more effective in preventing deep infection than using either systemic antibiotics or antibiotic bone cement alone (P < 0.001). The adjusted failure rate ratios in the remaining groups were 4.3 (systemic antibiotics only), 6.3 (antibiotic-impregnated cement only), and 11.5 (no antibiotic prophylaxis). The authors conclude that the best prophylaxis against infection was a combination of systemic antibiotics and antibiotic-impregnated cement.
The Swedish Hip Registry data consisted of 92,675 primary and revision total hip replacements performed between 1978 and 1990. Malchau and researchers22 found that the quality of the operating room ventilation and the use of gentamycin-impregnated antibiotic bone cement were the only significant factors in reducing deep infection (P < 0.001). Also, the benefit was greater for revision surgery than for primary hip replacements. Interestingly, the data showed a decrease in deep infection rates from 1979 to 1991 in all patients, regardless of the use of ALBC, because of other measures of infection control introduced over this period.
A large retrospective study of 22,170 primary total hip replacements from the Norwegian Arthroplasty Register during the period of 1987 to 2001 was reported by Engesaeter and investigators.29 Patients who received only systemic antibiotic prophylaxis (5960 patients) had a 1.8 times greater rate of infection than patients who received systemic antibiotic prophylaxis combined with gentamicin-loaded bone cement (15,676 patients) (P = 0.01).
In another retrospective review of 1542 total hip replacements, no difference was found in infection rate between primary total hip replacements performed with (1.65%) compared with those performed without gentamicin-loaded bone cement (1.72%).30 However, with revision surgery, gentamicin-loaded bone cement provided significantly better results, with a 0.81% infection rate, as compared with a rate of 3.46% after those done with plain cement.30
Tunney and colleagues’31 in vitro study examined the efficacy of gentamicin-impregnated bone cement and preoperative and postoperative administration of cefuroxime in the prevention of biofilm formation. The authors conclude that with a low bacterial inoculum, the combination of gentamicin and cefuroxime was effective in preventing biofilm formation but had no effect in an environment of high bacterial concentrations (Table 82-1).
STUDY LIMITATIONS
In the literature, the event rate for deep infection has been reported at about 1% in many large series.1–4 Taking a 50% reduction in infection rate as significant and assuming a power of 0.8, a well-powered, randomized, controlled study would need approximately 5000 patients to adequately document a difference between groups.
The randomization process in the two studies by Chiu and colleagues19,20 used a system based on even and odd medical record numbers as opposed to a formal, computer-generated randomization schedule.
Potential Disadvantages of Routine Use of Antibiotic-Loaded Bone Cement
The unanswered question remains whether the benefits of prophylaxis with ALBC in the current era of joint arthroplasty with an extremely low rate of infection are outweighed by the potential disadvantages associated with its routine use. The issues of toxicity and detrimental effects to the mechanical properties of bone cement are inconsequential when using low-dose ALBC (0.5–1.0 g antibiotic per 40 g cement). Biomechanical testing has shown that, in contrast with the use of high-dose antibiotics, which can weaken bone cement, the low-dose, antibiotic-impregnated bone cements that are used in practice have negligible reductions in fatigue strength, and fixation is not compromised.32–34
Two reports showed that the addition of gentamicin powder into Palacos R bone cement (Zimmer) or either erythromycin plus colistin or tobramycin powder into Simplex P (Stryker) bone cement did not decrease the fatigue strength compared with that of the respective plain-cement controls.35,36 In contrast, DeLuise and Scott6 showed that hand-mixing tobramycin into Simplex P bone cement results in a 36% decrease in the strength of the cement compared with the strength of commercially prepared, tobramycin-loaded bone cement (Simplex T) and that of plain Simplex P cement. Radiostereometric analysis studies have revealed comparable fixation of cemented implants with and without commercially prepared antibiotic-impregnated bone cement.34
Other concerns are the potential for an allergic reaction to the antibiotic being used and the potential development of drug-resistant organisms. No reports have been made of allergic reactions in more than 100,000 cases of ALBC thus far in the literature.37 Some authors have described gentamycin-resistant organisms and have suggested the use of other antibiotics such as the cephalosporins ciprofloxacin, erythromycin, or vancomycin as alternative choices.31,38, 39
The cost of commercially available ALBC products is considerable. Compared with the cost of plain bone cement, the cost of equivalent ALBC is increased from $284 to $349 (U.S currency) per 40-g package.40 This increased cost needs to be balanced against the potential cost savings from avoiding a deep prosthetic joint infection.
Potentially High-Risk Populations
Patients with the following characteristics are at potentially high risk for infection:
The use of ALBC in prophylaxis in primary total joint arthroplasty remains controversial. ALBC should be considered as a defense against direct contamination at the time of surgery or during the early postoperative period as the wound seals. In a survey of 1015 American adult arthroplasty surgeons, only 56% routinely use ALBC in their practice.43
Kurtz and colleagues44 have projected that between the years of 2005 and 2030, the revision burden in the United States for hip and knee surgery will increase by 137% and 601%, respectively. The healthcare costs for treating joint sepsis after arthroplasty has been estimated at $40 to $80 million annually in the United States.45 Sculco46 estimates the direct costs of revision surgery for deep infection at more than $55,000.
With concerns of cost and antibiotic-resistant organisms, the use of ALBC in all routine primary hip and knee replacements cannot be recommended based on the current evidence. Antibiotics in bone cement has a more definitive role in those populations at high risk described earlier and in revision joint arthroplasty. Table 82-2 provides a summary of recommendations.
| RECOMMENDATIONS | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION | REFERENCES |
|---|---|---|
ALBC, antibiotic-loaded bone cement; THA, total hip arthroplasty; TKA, total knee arthroplasty.
1 Robertsson O, Knutson K, Lewold S, et al. The Swedish knee arthroplasty register 1975–1997: An update with special emphasis of 41,223 knees operated on in 1988–1997. Acta Orthop Scand. 2001;72:603.
2 Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691.
3 Mahomed NN, Barrett J, Katz JN, et al. Epidemiology of total knee replacement in the United States Medicare population. J Bone Joint Surg Am. 2005;87:1222-1228.
4 Phillips CB, Barrett JA, Losina E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85:20-26.
5 Neut D, van de Belt H, van Horn JR, et al. The effect of mixing on gentamicin release from polymethylmethacrylate bone cements. Acta Orthop Scand. 2003;74:670-676.
6 DeLuise M, Scott CP. Addition of hand-blended generic tobramycin in bone cement: Effect on mechanical strength. Orthopedics. 2004;27:1289-1291.
7 Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty. 1999;14:209-214.
8 Baker AS, Greenham LW. Release of gentamicin from acrylic bone cement. Elution and diffusion studies. J Bone Joint Surg Am. 1988;70:1551-1557.
9 Powles JW, Spencer RF, Lovering AM. Gentamicin release from old cement during revision hip arthroplasty. J Bone Joint Surg Br. 1998;80:607-610.
10 Fletcher MD, Spencer RF, Langkamer VG, Lovering AM. Gentamicin concentrations in diagnostic aspirates from 25 patients with hip and knee arthroplasties. Acta Orthop Scand. 2004;75:173-176.
11 Nelson CL, Griffin FM, Harrison BH, Cooper RE. In vitro elution characteristics of commercially and noncommercially prepared antibiotic PMMA beads. Clin Orthop. 1992;284:303-309.
12 Lawson KJ, Marks KE, Brems J, Rehm S. Vancomycin vs tobramycin elution from polymethylmethacrylate: An in vitro study. Orthopedics. 1990;13:521-524.
13 Adams K, Couch L, Cierny G, et al. In vitro and in vivo evaluation of antibiotic diffusion from antibiotic-impregnated polymethylmethacrylate beads. Clin Orthop Relat Res.; 278; 1992; 244-252.
14 Buchholz HW, Elson RA, Engelbrecht E, et al. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63:342-353.
15 Duncan CP, Masri BA. The role of antibiotic-loaded cement in the treatment of an infection after a hip replacement. Instr Course Lect. 1995;44:305-313.
16 Hanssen AD, Rand JA, Osmon DR. Treatment of the infected total knee arthroplasty with insertion of another prosthesis. The effect of antibiotic-impregnated bone cement. Clin Orthop Relat Res.; 309; 1994; 44-55.
17 Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res.; 427; 2004; 47-51.
18 Penner MJ, Duncan CP, Masri BA. The in vitro elution characteristics of antibiotic-loaded CMW and Palacos-R bone cements. J Arthroplasty. 1999;14:209.
19 Chiu FY, Chen CM, Lin CFJ, et al. Cefuroxime impregnated cement in primary total knee arthroplasty. J Bone Joint Surg Am. 2002;84:759.
20 Chiu FY, Lin CF, Chen CM, et al. Cefuroxime-impregnated cement at primary total knee arthroplasty in diabetes mellitus. A prospective, randomized study. J Bone Joint Surg Br. 2001;83:691.
21 Espehaug B, Engesaeter LB, Vollset SE, et al. Antibiotic prophylaxis in total hip arthroplasty: Review of 10,905 primary cemented total hip replacements reported to the Norwegian arthroplasty register, 1987 to 1995. J Bone Joint Surg Br. 1997;79:590.
22 Malchau H, Herberts P, Ahngelt L. Prognosis of total hip replacement in Sweden. Follow-up of 92,675 operations performed 1978-1990. Acta Orthop Scand. 1993;64:497-506.
23 Hanssen AD. Prophylactic use of antibiotic bone cement: An emerging standard—in opposition. J Arthroplasty. 2004;19(4 suppl 1):73-77.
24 Yang K, Yeo SJ, Lee BP, Lo NN. Total knee arthroplasty in diabetic patients: A study of 109 consecutive cases. J Arthroplasty. 2001;16:102-106.
25 England SP, Stern SH, Insall JN, Windsor RE. Total knee arthroplasty in diabetes mellitus. Clin Orthop Relat Res.; 260; 1990; 130-134.
26 Meding JB, Reddleman K, Keating ME, et al. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res.; 416; 2003; 208-216.
27 McQueen M, Littlejohn A, Hughes SP. A comparison of systemic cefuroxime and cefuroxime loaded bone cement in the prevention of early infection after total joint replacement. Int Orthop. 1987;11:241-243.
28 Josefsson G, Kolmert L. Prophylaxis with systematic antibiotics versus gentamicin bone cement in total hip arthroplasty. A ten-year survey of 1,688 hips. Clin Orthop Relat Res.; 292; 1993; 210-214.
29 Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: Effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0-14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand. 2003;74:644-651.
30 Lynch M, Esser MP, Shelley P, Wroblewski BM. Deep infection in Charnley low friction arthroplasty. Comparison of plain and gentamicin-loaded cement. J Bone Joint Surg Br. 1987;69:355-360.
31 Tunney MM, Ramage G, Patrick S, et al. Antimicrobial susceptibility of bacteria isolated from orthopedic implants following revision hip surgery. Antimicrob Agents Chemother. 1998;42:3002.
32 Davies JP, Harris WH. Effect of hand mixing tobramycin on the fatigue strength of Simplex P. J Biomed Mater Res. 1991;25:1409.
33 Masari BA, Duncan CP, Beauchamp CP. Long-term elution of antibiotics from bone cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system. J Arthroplasty. 1998;13:331.
34 Adelberth G, Nilsson KG, Karrholm J, et al. Fixation of the tibial component using CMW-1 or Palacos bone cement with gentamicin: Similar outcome in a randomized radiostereometric study of 51 total knee arthroplasties. Acta Orthop Scand. 2002;73:531.
35 Davies JP, Harris WH. Effect of hand mixing tobramycin on the fatigue strength of Simplex P. J Biomed Mater Res. 1991;25:1409-1414.
36 Davies JP, O’Connor DO, Burke DW, Harris WH. Influence of antibiotic impregnation on the fatigue life of Simplex P and Palacos R acrylic bone cements, with and without centrifugation. J Biomed Mater Res. 1989;23:379-397.
37 Bourne R. Prophylactic use of antibiotic bone cement an emerging standard—in the affirmative. J Arthroplasty. 2004;19:69-72.
38 Sanzen L, Walder M. Antibiotic resistance of coagulase-negative staphylococci in an orthopaedic department. J Hosp Infect. 1988;12:103.
39 Taggart T, Kerry RM, Norman P, et al. The use of vancomycin-impregnated cement beads in the management of infection of prosthetic joints. J Bone Joint Surg Br. 2002;84:70.
40 Jiranek W, Hanssen AB, Greenwalk AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487-2500.
41 Lentino JR. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157-1161.
42 Blom AW, Brown J, Taylor AH, et al. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688-691.
43 Heck D, Rosenberg A, Schink-Ascani M, et al. Use of antibiotic impregnated cement during hip and knee arthroplasty in the United States. J Arthroplasty. 1995;10:470-475.
44 Smabrekke A, Espehaug B, Havelin LI, Fumes O. Operating time and survival of primary total hip replacements: an analysis of 31,745 primary cemented and uncemented total hip replacements from local hospitals reported to the Norwegian Arthroplasty Register 1987-2001. Acta Orthop Scand. 2004;75:524-532.
45 Brause BD. Infections with prostheses in bones and joints. In: Mandell GL, Bennett Je, Dolin R, editors. Principles and Practice of Infectious Diseases. 4th ed. New York: Churchill Livingstone Inc.; 1995:1051-1055.
46 Sculco TP. The economic impact of infected total joint arthroplasty. Instr Course Lect. 1993;42:349-351.