Chapter 6 What Is the Optimal Treatment for Thoracolumbar Burst Fractures?
Despite injury prevention initiatives and safer automobile designs, the incidence of thoracolumbar high-energy trauma remains significant.1 Burst fractures of the thoracolumbar spine account for approximately 45% of all thoracolumbar trauma cases, and half of these patients remain neurologically intact after injury.2 The 1990s and 2000s have brought significant technologic advancements, specifically the widespread use of pedicle screw fixation in the thoracic spine,3–7 the design of stiffer and more rigid instrumentation,3,8, 9 the ability to reconstruct the anterior spinal column with expandable cages10 and biologics,11 and less invasive spinal surgical approaches.12 The treatment of thoracolumbar trauma, however, and specifically that of burst fractures, continues to be one of the most controversial areas in spine trauma care despite the high incidence of these injuries and extensive published research.
OPTIONS
It is important to define the population discussed here. Patients with burst fractures between T10-L2 inclusive, with or without neurologic deficit, who are a minimum age of 16 years are included. The term burst fracture refers to a fracture of the vertebral body with fracture lines that extend into the posterior vertebral body wall and result in a separation or widening of the pedicles.13 Burst fractures may be associated with varying degrees of disruption to the posterior vertebral elements, specifically the facet and laminar complex and the posterior ligamentous complex.14
Essentially, two major decisions need to be made by the treating physician: First, and more fundamentally, should the patient be treated with or without surgery? Second, if surgery is to be selected, what approach and technique should be used? Nonoperative care may involve the use of a thoracolumbar sacral orthosis (TLSO), body cast, hyperextension brace, or no orthosis at all, whereas operative treatment may involve anterior surgery alone, posterior surgery alone, or a combination of both. The posterior surgical fixation options include hook or wire constructs,7,15, 16 short-segment pedicle screw fixation at one level above and one below the fractured vertebra,3,4, 6, 7, 17 and long-segment fixation, characteristically two or three segments of fixation above and below the fracture.7,18, 19 When the anterior column of the spine is surgically reconstructed, the vertebral body and discs may be approached indirectly through transpedicular bone3,7, 20 or cement augmentation,21,22 or by an indirect posterolateral approach. A direct anterior approach facilitates vertebral body resection, decompression of the anterior spinal canal, anterior reconstruction of the vertebra, and also anterior fixation with either plates or screw-rod constructs.23,24 Prosthetic devices (fixed and expanding cages), as well as autograft and allograft, are the most commonly used anterior vertebral reconstruction options.10,23–28
EVIDENCE
Operative versus Nonoperative Treatment
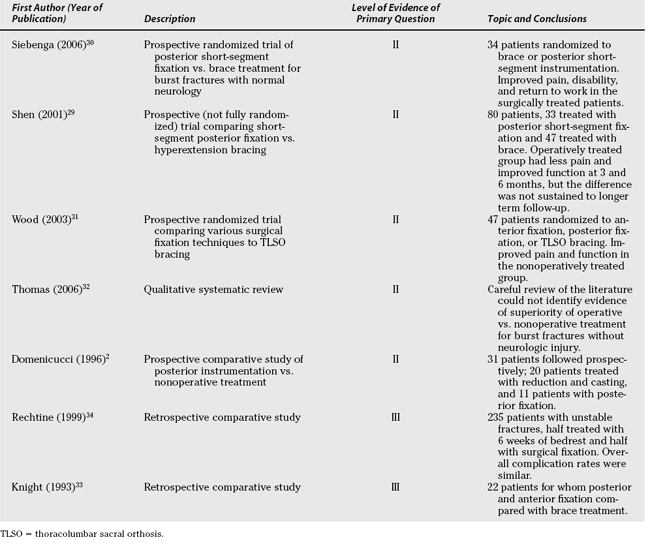
Five Level II studies directly compare operative with nonoperative care for thoracolumbar burst fractures (Table 6-1).2,29–32 All of these studies include thoracolumbar burst fractures with normal neurology. Some of the fractures included would be described as unstable with significant kyphosis and some degree of posterior ligamentous disruption, though most would be described as stable.
Wood and colleagues31 recruited 53 patients, 27 of whom were randomized to the nonoperative treatment arm, which contained two forms of nonoperative treatment, either a postural reduction and cast or a hyperextension custom-molded jacket TLSO worn for 12 to 16 weeks. Twenty-six patients were treated by a variety of surgical techniques, either a posterior screw/hook construct and fusion spanning between two to five levels or an anterior vertebrectomy, rib strut graft, and instrumentation. Patients’ outcome evaluation included the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36), modified Roland Morris Disability Scale (RMDS) score, Oswestry Questionnaire, visual analogue pain scale (VAS), and a radiographic evaluation. The study does have significant limitations, including alack of standardization, multiple treatment options, outcome measures reported at varying intervals, lack of a priori determination of a primary outcome, no power calculations, and multiple comparisons without statistical adjustment.
A statistically significant difference between operative and nonoperative treatment was observed favoring nonoperative treatment, for physical function (P = 0.002) and role physical (P = 0.003). Wood and colleagues31 report an average Roland Disability score of 8.2 for the operative group and 3.9 for the nonoperative group (P = 0.02). These authors also used the Oswestry questionnaire, reporting an average score of 20.75 and 10.66 for the surgical and nonsurgical groups, respectively. For both the Roland and Oswestry instruments, a lower score signifies better function.
In contradistinction with Wood and colleagues’31 study, Siebenga and investigators30 included a homogeneously defined cohort and carefully standardized treatment of both the operative and nonoperative groups. By randomizing 34 patients to brace treatment and posterior short-segment fixation, Siebenga and investigators30 showed a significant difference in pain (72 vs. 87 mm; P = 0.033), Rolland Morris Disability scores (8.9 vs. 3.1; P = 0.030), and return to work (38% vs. 85%; P = 0.018), each in favor of operative treatment. The methodology and uniformity of treatment applied to each group make this a strong study, whereas the lack of an a priori power calculation and an a priori description of a primary outcome prevent it from attaining Level I evidence status.
Shen and coauthors29 attempted to randomize patients to receive short-segment posterior instrumentation and fusion or nonoperative care using a hyperextension brace. Because of recruitment difficulties, some patients were not randomized, and as such, this study should be considered a prospective cohort study. Outcomes were measured by an independent assessor at 1, 3, 6, 12, 18, and 24 months. Also using a VAS, at 2-year follow-up, Shen and coauthors29 note the VAS to be 1.5 and 1.8 for the nonoperative and operative cohorts, respectively. For the 3- and 6-month follow-up, Shen and coauthors29 show improved pain in the surgically compared with the brace-treated patients. The authors note a better Greenough low back outcome score in the surgically treated group for up to 6 months, but this effect was not observed with longer follow-up.
The systematic review by Thomas and investigators32 was performed before the study by Siebenga and colleagues,30 and concludes that there was no evidence of superiority of operative over nonoperative treatment for neurologically intact thoracolum-bar burst fractures. The final of the five Level II studies is a prospective comparative study by Domenicucci and coworkers,2 which has multiple methodologic flaws, and although it favors surgery for patients with increased radiographic deformity (kyphosis over 20 degrees), issues of power and bias are significant.
Two Level III studies compare operative and nonoperative care (see Table 6-1).33,34 Rechtine and colleagues’34 article is thought provoking in that it brings to mind the issues of costs of care, as well as patient preference, and shows that even in severely injured individuals, satisfactory outcomes may be obtained with or without surgery, however, with different treatment approaches, resource implications, costs, and risk.
In addition, a number of Level IV studies show satisfactory outcomes with nonoperative treatment of a variety of these thoracolumbar fractures.33,35–40 An example of one of these is an article by Mumford and coauthors,40 who reviewed 41 of 47 patients treated with a variable period of bed rest (range, 7–68 days) followed by a custom-made TLSO for an average of 12 weeks. Inclusion criteria included burst fractures between T11-L5, and the data for each individual patient were reported. For patients treated without surgery, Mumford and coauthors40 found that 50% of patients had little to no pain at final follow-up, as measured on a Likert Scale.
In summary, strong evidence has been reported to support satisfactory outcomes with both operative and nonoperative treatment. Two Level II studies29,30 suggest improved outcomes with operative treatment, one of which shows improved outcomes only at 3 and 6 months, and not sustained out to longer follow-up.29 Wood and colleagues’31 study has significant enough methodologic defects to make its conclusions nebulous.
Choice of Operative Approach
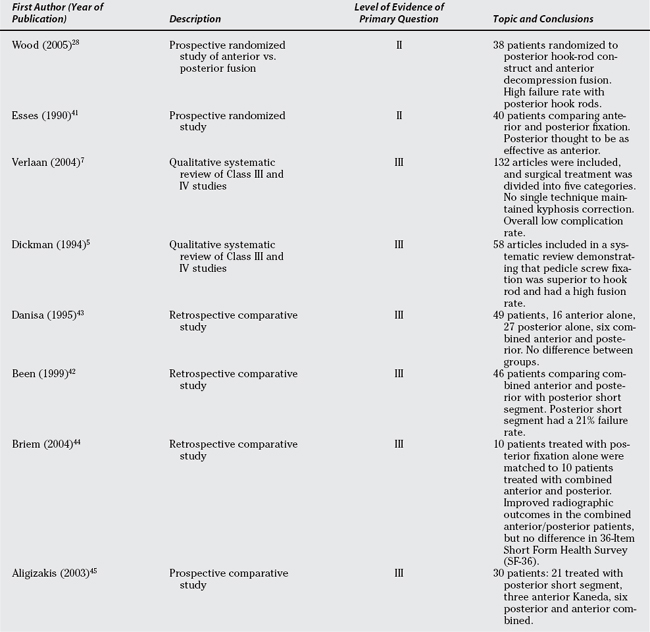
Specifically looking at the decision to operate from an anterior approach alone, posterior alone, or combined anterior and posterior approaches, two Level II studies28,41 and six Level III studies5,7,35,42–44 address this as their primary question (Table 6-2).
Wood and colleagues,28 in an article that appears to be a subset of a previously reported randomized trial,31 randomly assigned 43 patients to either anterior partial vertebrectomy and Kaneda or Isola instrumentation or posterior Isola rod-hook stabilization. Unfortunately, the use of posterior rod-hook constructs is likely inferior to the more commonly used pedicle screw-rod systems currently used; thus, the high rate (11/18) of implant-related complications in the posterior surgery group is not that surprising. The generally good clinical outcomes in the anterior surgery group and the low complication rate make this a potentially reasonable option in the neurologically intact thoracolumbar burst fracture that requires surgical treatment.
Esses and coauthors’41 study strongly favors short-segment posterior fixation over an anterior fixation system (Kostuik–Harrington device) that tended to fail as frequently as the posterior short-segment fixator and required a greater degree of complexity and risk for its insertion.
Two Level III systematic reviews5,7 compare various surgical approaches and techniques. Though suffering from a lack of high-quality studies in his systematic review, Dickman and coworkers5 confidently state that segmental pedicle screw fixation of thoracolumbar fractures has a higher fusion rate than do rod-screw constructs or anterior fixation devices. Verlaan and investigators7 in a meta-analysis of 132 articles divided treatment into 5 categories, including posterior long- and short-segment instrumentation, a mixture of short- and long-segment instrumentation, anterior alone, and combined anterior and posterior fixation. Verlaan and investigators7 conclude that none of the five techniques reliably maintains alignment, and there is no compelling evidence of the superiority of one of these techniques over another.
Been and Bouma,42 in a retrospective, comparative study of short-segment posterior fixation, with and without concomitant anterior strut grafting, reported a 21% instrumentation failure rate with the posterior short-segment instrumentation, reduced to 4% when an anterior strut graft is added. In another retrospective comparative study, Danisa and coauthors43 compared anterior alone (16 patients), posterior alone (27 patients), and combined anterior and posterior(6 patients); even with significant selection bias, there was no significant difference in kyphosis, pain, return to work, or neurologic recovery among the three groups. Danisa and coauthors43 recommend posterior fixation as the least complex procedure. Briem and colleagues44 performed a Level III matched-pairs analysis comparing posterior instrumentation alone with combined posterior and anterior fixation. Although the combined anterior and posterior procedure maintained sagittal alignment more effectively than posterior alone, there was no difference in SF-36 between the two groups. The SF-36 likely lacks the sensitivity to detect change that a disease-specific outcome measure would have. Finally, Aligizakis and investigators,45 in a parallel study of several cohorts, attempted to use the load sharing classification toguide the choice of surgical approach and technique. This study45 reports good outcomes of several selected cohorts where the addition of an anterior approach is based on the numeric score of the load sharing classification. Aligizakis and investigators45 treated simple burst fractures with posterior alone short-segment instrumentation (21 patients), whereas “complete” burst fractures (three patients with significant vertebral body comminution) were treated with anterior alone Kaneda devices and burst fractures with posterior element distraction (six patients with flexion/distraction injuries) were treated with posterior instrumentation and an anterior load-bearing strut graft.45
Choice of Operative Technique
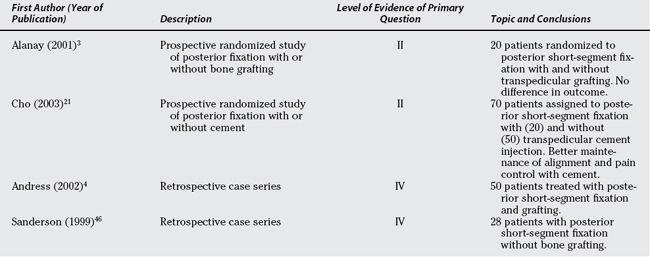
Andress and colleagues4 report on 50 patients treated with short-segment pedicle screw instrumentation that was routinely removed 9 to 15 months after surgery (Table 6-3). The mean 46-month follow-up included a radiographic evaluation, preinjury (assigned retrospectively after fracture) and postinjury Hanover score, and a seven-point clinical assessment scale. Sanderson and coworkers46 evaluated a cohort of 28 patients who were treated between 1990 and 1993, using pedicle screw instrumentation two levels above and below without fusion, followed by routine hardware removal 6 to 12 months after surgery. Surgical indications were listed as kyphosis greater than 20 degrees and/or greater than 50% loss of anterior vertebral height. In these and other reports of short-segment posterior fixation, the failure rate of the posterior instrumentation has been variously reported as 14%,46 17%,8 21%,42 22%,21 50%,47 and 53%.17 Despite the popularity and relative surgical simplicity of this technique, the predictable high mechanical failure rate clearly makes it difficult to accept this procedure as the optimal treatment option.
Several attempts have been made to minimize the collapse of the vertebral body and resultant instrument failure, one of these being the addition of transpedicular intracorporeal grafting,48 and this is the focus of one Level II study20 and is prominent in a Level III sys-tematic review.7 Alanay and coworkers20 randomized 20 consecutive patients to short-segment pedicle instrumentation with or without transpedicular intracorporeal grafting. The technique of transpedicular intracorporeal grafting did not influence the collapse of the fractured vertebra, and this finding was confirmed by Verlaan and investigators7 in a systematic review of a number of articles. The technique of transpedicular intracorporeal bone grafting can be described confidently as ineffective.
In a novel modification of the short-segment posterior fixation technique, several authors have proposed transpedicular intracorporeal injection of cements.21,22 In a particularly interesting, prospective, comparative Level II study, Cho and colleagues21 compare posterior short-segment fixation with and without intracorporeal cement injection. The cement injection was shown to maintain vertebral height and kyphosis correction, whereas a 22% instrumentation failure rate occurred in the group without cement injection. The long-term significance of cement in a vertebral body of a young patient is unknown; thus, this technique must remain experimental.
AREAS OF UNCERTAINTY
Based on the literature reviewed earlier, it is remarkable how our efforts to stratify patients with thoracolumbar burst fractures into subgroups and tailor their treatment remain unclear and ineffective. Most studies treat the 20-year-old laborer in the same way that they treat the 70-year-old adult who falls in the bath. Similarly, classification systems are only now becoming available that guide treatment to some degree.14 The principal areas where we believe attention should be directed are as follows:
From individual studies and from several meta-analyses, it appears that the rates of serious complication for even the most extensive surgical treatments are relatively low. Documented neurologic deterioration after baseline assessment, regardless of treatment, is extremely rare,8,23,42,43,49–54 although one study does report this problem.13 Further careful consideration of complication rates and patterns is required.
GUIDELINES
Fair evidence exists to recommend nonoperative treatment as an option for the majority of thoracolumbar burst fractures as long as they do not appear to have neurologic impairment, significant posterior element disruption, or a significant deformity (kyphosis over 25–30 degrees, although this is debatable). Level II and III studies have consistently shown satisfactory clinical outcomes with nonoperative treatment in this patient population, and no one has been able to conclusively link the degree of eventual deformity to the quality of clinical outcome. It appears as if it is the achievement of “stability” or healing and not necessarily the alignment of the spine that determines a good clinical outcome.
Superimposed on the earlier fairly consistent and weighty body of literature, there are several recent studies that provide fair evidence that, although nonoperative outcomes appear to be satisfactory, the surgical treatment of thoracolumbar burst fractures may improve pain relief, function, and return to work. The small sample sizes in studies such as Cho and colleagues,21 Siebenga and investigators,30 and Shen and coauthors29 require further study and verification before operative treatment can be strongly considered. Furthermore, the fairly consistent reports of instrumentation-related failure with the most common surgical technique, namely, short-segment posterior fixation, makes the authors uneasy in recommending the abandonment of nonoperative treatment in pursuit of the improved outcomes promised by an operative treatment that has such a high mechanical failure rate.
Satisfactory clinical results have been consistently achieved with acceptable complication rates using several operative approaches and techniques. The criteria on which one selects anterior vertebrectomy graft and plate (or rod) stabilization, posterior short- or long-segment fixation, or a combination of anterior and posterior fixation are not clearly defined. Several statements can be made based on what are fairly consist findings in Level III and IV clinical studies. Posterior instrumentation with pedicle screws provides better fixation than hook constructs.5 Longer segment instrumentation (two motion segments above and two below the fractured vertebra) will reduce the risk for instrument failure at the bone-screw interface or within the instrumentation itself.15,47 Short-segment posterior fixation and, to some degree, most operative treatment techniques will fail to maintain the operatively achieved alignment and will drift into kyphosis approximately 10 degrees greater than that achieved at surgery.7 Kyphosis does not appear to be related to clinical outcome, at least not when it is less than 25 to 30 degrees.7,55
Finally, some techniques have been shown not to be associated with satisfactory results, and these include the transpedicular injection of bone20 or OP-1,11 anterior fixation utilizing the Slot–Zielke divice,23 and anterior grafting using bovine bone.27
When the segmental kyphosis is on the order of 25 or 30 degrees, either because of the degree of anterior vertebral body comminution or posterior ligamentous complex incompetence, then surgical treatment is the preferred treatment option, although nonoperative treatment remains an option. The studies by Siebenga and investigators30 and Shen and coauthors29 are both well done and suggest improved outcomes with surgery. Therefore, there is likely a place for surgery and experience. Widespread practice would support that surgery would be reserved for higher degrees of kyphosis and posterior ligament injuries (Level V).
In the presence of neurologic injury, it is widespread practice in North America to treat these injuries surgically, either simply stabilizing the fracture with posterior instrumentation or adding a concomitant decompression. Because of the lack of convincing data to favor one surgical approach over another, and the reported high failure rate of short-segment posterior instrumentation, the authors use posterior instrumentation utilizing pedicle screws at two segments above and two below as the most reliable and lowest risk surgical procedure. Posterior stabilization may be followed by reimaging (computed tomography or MRI) to assess the degree of spinal canal occlusion (in the case of neurologic deficit) and vertebral comminution, and the surgeon can use clinical judgment, as well as guidance from the load sharing classification,45 in deciding when to perform an additional anterior corpectomy and strut grafting.
| Summary of Recommendations | ||
|---|---|---|
| STATEMENT | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION | REFERENCES |
1 Fisher CG, Noonan VK, Dvorak MF. Changing face of spine trauma care in North America. Spine. 2006;31:S2-S8.
2 Domenicucci M, Preite R, Ramieri A, et al. Thoracolumbar fractures without neurosurgical involvement: Surgical or conservative treatment? J Neurosurg Sci. 1996;40:1-10.
3 Alanay A, Acaroglu E, Yazici M, et al. Short-segment pedicle instrumentation of thoracolumbar burst fractures: Does transpedicular intracorporeal grafting prevent early failure? Spine. 2001;26:213-217.
4 Andress HJ, Braun H, Helmberger T, et al. Long-term results after posterior fixation of thoraco-lumbar burst fractures. Injury. 2002;33:357-365.
5 Dickman CA, Yahiro MA, Lu HT, Melkerson MN. Surgical treatment alternatives for fixation of unstable fractures of the thoracic and lumbar spine. A meta-analysis. Spine. 1994;19:2266S-2273S.
6 Kramer DL, Rodgers WB, Mansfield FL. Transpedicular instrumentation and short-segment fusion of thoracolumbar fractures: A prospective study using a single instrumentation system. J Orthop Trauma. 1995;9:499-506.
7 Verlaan JJ, Diekerhof CH, Buskens E, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: A systematic review of the literature on techniques, complications, and outcome. Spine. 2004;29:803-814.
8 Liu CL, Wang ST, Lin HJ, et al. AO fixateur interne in treating burst fractures of the thoracolumbar spine. Chung Hua I Hsueh Tsa Chih (Taipei). 1999;62:619-625.
9 Wang ST, Ma HL, Liu CL, et al. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine? A prospective, randomized study. Spine. 2006;31:2646-2652.
10 Lange U, Knop C, Bastian L, Blauth M. Prospective multicenter study with a new implant for thoracolumbar vertebral body replacement. Arch Orthop Trauma Surg. 2003;123:203-208.
11 Laursen M, Hoy K, Hansen ES, et al. Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: Preliminary results. Eur Spine J. 1999;8:485-490.
12 Oner FC, Dhert WJ, Verlaan JJ. Less invasive anterior column reconstruction in thoracolumbar fractures. Injury. 2005;36(suppl 2):B82-B89.
13 Denis F, Armstrong GWD, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. A comparison between operative and nonoperative treatment. Clin Orthop. 1984;189:142-149.
14 Vaccaro AR, Lehman RAJr, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005;30:2325-2333.
15 Serin E, Karakurt L, Yilmaz E, et al. Effects of two-levels, four-levels, and four-levels plus offset-hook posterior fixation techniques on protecting the surgical correction of unstable thoracolumbar vertebral fractures: A clinical study. Eur J Orthop Traumatol. 2004;14:1-6.
16 Vornanen MJ, Bostman OM, Myllynen PJ. Reduction of bone retropulsed into the spinal canal in thoracolumbar vertebral body compression burst fractures. A prospective randomized comparative study between Harrington rods and two transpedicular devices. Spine. 1995;20:1699-1703.
17 McLain RF, Sparling E, Benson DR. Early failure of short-segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am. 1993;75:162-167.
18 Akbarnia BA, Crandall DG, Burkus K, Matthews T. Use of long rods and a short arthrodesis for burst fractures of the thoracolumbar spine. A long-term follow-up study. J Bone Joint Surg Am. 1994;76:1629-1635.
19 Korovessis P, Baikousis A, Koureas G, Zacharatos S. Correlative analysis of the results of surgical treatment of thoracolumbar injuries with long Texas Scottish rite hospital construct: Is the use of pedicle screws versus hooks advantageous in the lumbar spine? J Spinal Disord Tech. 2004;17:195-205.
20 Alanay A, Acarolu E, Yazici M, et al. The effect of transpedicular intracorporeal grafting in the treatment of thoracolumbar burst fractures on canal remodeling. Eur Spine J. 2001;10:512-516.
21 Cho DY, Lee WY, Sheu PC, et al. Treatment of thoracolumbar burst fractures with polymethyl methacrylate vertebroplasty and short-segment pedicle screw fixation. Neurosurgery. 2003;53:1354-1361.
22 Verlaan JJ, Oner FC, Dhert WJ. Anterior spinal column augmentation with injectable bone cements. Biomaterials. 2006;27:290-301.
23 Been HD. Anterior decompression and stabilization of thoracolumbar burst fractures by the use of the Slot-Zielke device. Spine. 1991;16:70-77.
24 McDonough PW, Davis R, Tribus C, Zdeblick TA. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine. 2004;29:1901-1908.
25 Dvorak MF, Kwon BK, Fisher CG, et al. Effectiveness of titanium mesh cylindrical cages in anterior column reconstruction after thoracic and lumbar vertebral body resection. Spine. 2003;28:902-908.
26 Schnee CL, Ansell LV. Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg. 1997;86:48-55.
27 Schultheiss M, Sarkar M, Arand M, et al. Solvent-preserved, bovine cancellous bone blocks used for reconstruction of thoracolumbar fractures in minimally invasive spinal surgery—First clinical results. Eur Spine J. 2005;14:192-196.
28 Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: A prospective, randomized study. J Spinal Disord Tech. 2005;18(suppl):S15-S23.
29 Shen WJ, Liu TJ, Shen YS. Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine. 2001;26:1038-1045.
30 Siebenga J, Leferink VJM, Segers MJM, et al. Treatment of traumatic thoracolumbar spine fractures: A multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881-2890.
31 Wood K, Buttermann G, Mehbod A, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study [erratum appears in J Bone Joint Surg Am 2004 Jun;86-A(6):1283]. J Bone Joint Surg Am. 2003;85:773-781.
32 Thomas KC, Bailey CS, Dvorak MF, et al. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: A systematic review. J Neurosurg Spine. 2006;4:351-358.
33 Knight RQ, Stornelli DP, Chan DP, et al. Comparison of operative versus nonoperative treatment of lumbar burst fractures. Clin Orthop Relat Res.; 293; 1993; 112-121.
34 Rechtine IG, Cahill D, Chrin AM. Treatment of thoracolumbar trauma: Comparison of complications of operative versus nonoperative treatment. J Spinal Disord. 1999;12:406-409.
35 Aligizakis A, Katonis P, Stergiopoulos K, et al. Functional outcome of burst fractures of the thoracolumbar spine managed non-operatively, with early ambulation, evaluated using the load sharing classification. Acta Orthop Belg. 2002;68:279-287.
36 Cantor JB, Lebwohl NH, Garvey T, Eismont FJ. Nonoperative management of stable thoracolumbar burst fractures with early ambulation and bracing. Spine. 1993;18:971-976.
37 Chow GH, Nelson BJ, Gebhard JS, et al. Functional outcome of thoracolumbar burst fractures managed with hyperextension casting or bracing and early mobilization. Spine. 1996;21:2170-2175.
38 Hitchon PW, Torner JC, Haddad SF, et al. Management options in thoracolumbar burst fractures. Surg Neurol. 1998;49:619-627.
39 Kraemer WJ, Schemitsch EH, Lever J, et al. Functional outcome of thoracolumbar burst fractures without neurological deficit. J Orthop Trauma. 1996;10:541-544.
40 Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine. 1993;18:955-970.
41 Esses SI, Botsford DJ, Kostuik JP. Evaluation of surgical treatment for burst fractures. Spine. 1990;15:667-673.
42 Been HD, Bouma GJ. Comparison of two types of surgery for thoraco-lumbar burst fractures: Combined anterior and posterior stabilisation vs. posterior instrumentation only. Acta Neurochir (Wien). 1999;141:349-357.
43 Danisa OA, Shaffrey CI, Jane JA, et al. Surgical approaches for the correction of unstable thoracolumbar burst fractures: A retrospective analysis of treatment outcomes. J Neurosurg. 1995;83:977-983.
44 Briem D, Lehmann W, Ruecker AH, et al. Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch Orthop Trauma Surg. 2004;124:461-468.
45 Aligizakis AC, Katonis PG, Sapkas G, et al. Gertzbein and load sharing classifications for unstable thoracolumbar fractures. Clin Orthop Relat Res.; 411; 2003; 77-85.
46 Sanderson PL, Fraser RD, Hall DJ, et al. Short segment fixation of thoracolumbar burst fractures without fusion. Eur Spine J. 1999;8:495-500.
47 Moon MS, Choi WT, Moon YW, et al. Stabilisation of fractured thoracic and lumbar spine with Cotrel-Dubousset instrument. J Orthop Surg (Hong Kong). 2003;11:59-66.
48 Knop C, Fabian HF, Bastian L, et al. Fate of the transpedicular intervertebral bone graft after posterior stabilisation of thoracolumbar fractures. Eur Spine J. 2002;11:251-257.
49 Kirkpatrick JS, Wilber RG, Likavec M, et al. Anterior stabilization of thoracolumbar burst fractures using the Kaneda device: A preliminary report. Orthopedics. 1995;18:673-678.
50 Knop C, Bastian L, Lange U, et al. Complications in surgical treatment of thoracolumbar injuries. Eur Spine J. 2002;11:214-226.
51 Ruan D-K, Shen G-B, Chui H-X. Shen instrumentation for the management of unstable thoracolumbar fractures. Spine. 1998;23:1324-1332.
52 Sasso RC, Cotler HB. Posterior instrumentation and fusion for unstable fractures and fracture-dislocations of the thoracic and lumbar spine: A comparative study of three fixation devices in 70 patients. Spine. 1993;18:450-460.
53 Shen W-J, Shen Y-S. Nonsurgical treatment of three-column thoracolumbar junction burst fractures without neurologic deficit. Spine. 1999;24:412-415.
54 Shiba K, Katsuki M, Ueta T, et al. Transpedicular fixation with Zielke instrumentation in the treatment of thoracolumbar and lumbar injuries. Spine. 1994;19:1940-1949.
55 Knop C, Blauth M, Bühren V, et al. [Surgical treatment of injuries of the thoracolumbar transition—3: Follow-up examination. Results of a prospective multi-center study by the “Spinal” Study Group of the German Society of Trauma Surgery]. Unfallchirurg. 2001;104:583-600.