Chapter 5 What Is the Optimal Treatment for Degenerative Lumbar Spinal Stenosis?
Although it is unclear which factors account for patients who become significantly symptomatic from lumbar spinal stenosis, treatment for this condition is a common component of any spinal clinical practice. In deciding on the optimal treatment for degenerative lumbar spinal stenosis, one must first define the entity that requires treatment. Strictly speaking, spinal stenosis relates to the anatomic structural narrowing of the neural elements of the lumbar spinal canal. Some individuals are born with a morphologically narrowed canal in relation to the general population, and the term congenital lumbar spinal stenosis is used. Acquired lumbar spinal stenosis most commonly occurs because of degenerative changes with aging in the presence or absence of a congenitally small canal. It may be associated with other structural degenerative features that include spondylolisthesis. The term neurogenic claudication (or pseudoclaudication) relates to the constellation of symptoms of activity-related leg pain that is relieved with rest and is spondylogenic in origin because of structural spinal stenosis. Although the exact cause and pathophysiology of the symptoms remain evasive and poorly delineated, multiple factors have been implicated in its pathogenesis. In the era of modern generation imaging techniques, structural degenerative changes including spinal stenosis is prevalent in the general population and in individuals with minimal low back or low-related symptomatology.1 One must first carefully delineate the patients with symptoms before deciding on what may be the optimal treatment for their conditions. The degenerative process of the lumbar spine (i.e., spondylosis) with or without anatomic structural evidence for spinal stenosis can in itself be a pain generator of back pain in certain individuals, and not all individuals with spinal stenosis experience development of neurogenic claudication. The correlation between structural stenosis and the presence and severity of claudicant symptoms is poor; therefore, the clinical evaluation of a patient is paramount. Many studies reporting results relating to spinal stenosis often use the term interchangeably with neurogenic claudication. Unfortunately, the clinical presentation of symptomatic lumbar spinal stenosis is variable, and many studies group these patients with other patients with low-back–associated symptoms, for example, patients with chronic mechanical low back pain. In addition, leg symptomatology that is spondylogenic has variable presentations, and certain individuals may have primarily radiculopathic or sciatica-like symptomatology relating to structural spinal stenosis without the more classic description of a claudicant pattern. Symptoms can be unilateral or bilateral. Therefore, the comparison of a heterogeneous population of patients is a confounder to the review of literature.
OPTIONS
Nonsurgical Treatment (Grade I)
Education (Grade I).
Many professionals in the practice of medicine consider patient education to be paramount in the success of any recommended therapy. Recent spinal literature has focused on a variety of both nonsurgical and surgical therapies. Although some randomized trials evaluate the effect of educational programs as a therapeutic adjunct in the surgical treatment of patients undergoing lumbar disc decompression surgery, other trials applying educational strategies have grouped heterogeneous populations of patients.2,3 A relative paucity in recent Level I and II literature evaluating the effects of educational therapy or programs specifically in the treatment of lumbar spinal stenosis exists. Deyo and investigators4 evaluated the effect of an interactive video program in the decision-making process for patients considering surgical treatment for their lumbar spinal conditions. This prospective, randomized clinical trial at two centers enrolled a heterogeneous population of patients (171 patients with herniated discs, 110 patients with lumbar spinal stenosis, 112 with other diseases). The authors observed a greater rate of surgery in the video group (39% video and booklet vs. 29% booklet alone). However, this was not statistically significant (P = 0.34), and the authors indicated that the study was underpowered for their subgroup proportional comparisons in the patients with lumbar spinal stenosis (power analysis post hoc = 12%). The study did not observe a significant effect of the video program on symptomatic and functional results at 3 months and 1 year. In addition, there did not appear to be a significant effect on patient satisfaction with care or their satisfaction with the decision-making process comparing the two randomized groups. Overall study follow-up rate was 88% at 1 year. Compliance in the video program and booklet group was 97% for the video portion and 84% for the booklet portion, respectively, and 97% in the booklet alone group. In a follow-up study evaluating the knowledge gain as assessed by a pretreatment and post-treatment knowledge test, the combination of the interactive video with booklet produced greater knowledge gains than the book alone group in the subgroup of patients with the least knowledge at baseline.5
Medications (Grade I).
A wide gamut of oral medications is available for the potential treatment of symptomatic lumbar spinal stenosis. These include, among others, nonsteroidal agents, analgesics (narcotic and non-narcotic), and antineuritics (tricyclic antidepressants, anticonvulsants). Although there are many randomized studies of various medications for lower back disorders and low back pain, few have specifically focused on patients with stenosis. Of the few studies that have focused specifically on patients with lumbar stenosis, a couple of randomized studies on the evaluation of calcitonin treatment have been reported.6–8 Eskola and colleagues6 performed a randomized, placebo-controlled, double-blind, crossover study in 40 patients with lumbar spinal stenosis with 1-year follow-up demonstrating that calcitonin had beneficial effects on patients’ symptoms without producing significant adverse effects.6 The investigators observed primarily an analgesic effect with some positive effect on walking distance, although the authors note that the treatment effect was poor in those patients with marked limitation in walking distance caused by neurogenic claudication. Podichetty and coauthors7 randomized 55 patients with clinical lumbar canal stenosis and pseudoclaudication and pain visual analog scale (VAS) index of greater than or equal to 6 to either placebo or intranasal calcitonin for 6 weeks followed by an open-label 6-week extension during which all patients received active drug. Calcitonin was administered by nasal spray (400 IU daily) at twice the clinical dose typically used for postmenopausal women with osteoporosis. The overall study dropout rate was 22% for reasons relating to study protocol deviations, adverse event reporting, or withdrawal because of lack of perceived efficacy. Rash, erythema, and burning of the face and neck regions severe enough to cause withdrawal from treatment occurred in two patients in the experimental group. At 6 weeks, there was no difference between the two groups in change in pain VAS when compared with baseline. No difference existed between groups in time from the onset of walking to the onset of pain. Patients in both treatment groups reported improvements to their overall walking distance. However, no difference was present between the study groups. There also did not appear to be a significant effect on patient-reported functional outcome measures. The authors conclude that nasal calcitonin is not superior to placebo, and they suggest that the drug does not appear to have a role in the nonoperative treatment of lumbar canal stenosis. The study authors evaluated efficacy primarily at 6 weeks. The open-label phase during the subsequent 6 weeks suggested a trend toward improvement in patients treated with salmon calcitonin during the second phase of the trial, particularly in pain scores and 36-Item Short Form Health Survey (SF-36) results. The authors note that it is possible that the beneficial effects of nasal calcitonin could require a longer preload of drug, and that efficacy may be achieved using a different treatment schedule. In addition, the authors also indicate that the mean walking distance of patients in their study was in the more limited range where efficacy was also not demonstrated in the similar subpopulation of the study that Eskola and colleagues6 reported.
One of the more recent randomized studies specifically focusing on lumbar spinal stenosis patients evaluated the use of gabapentin, which has been used in the treatment of chronic neuropathic pain. Yaksi and coworkers9 randomized 55 patients with lumbar spinal stenosis and intermittent neurogenic claudication into 2 groups. Both randomized groups received physical therapy exercises, lumbosacral corset using a steel reinforced bracing design, and pharmacologic treatment with nonsteroidal anti-inflammatory drugs. The treatment group received in addition oral gaba-pentin administered at a dosage of 900 mg/day and increased weekly in increments of 300 mg up to a total maximal dosage of 2400 mg/day. Patients who experienced side effects (drowsiness and dizziness) were prescribed bed rest and increased oral fluid intake. Study end points to 4 months included objective assessments of walking distance, VAS scores, and proportional methods analysis of motor and sensory deficits within each group and at the end of treatment. At follow-up, both groups demonstrated improvement, with the gabapentin treatment group showing significantly better walking distance and improvements in pain scores and recovery of sensory deficit. Limitations of the study include the length of follow-up and the potential confounder of the placebo effect (Level II).
Therapeutic Exercises (Grade I).
Many randomized, controlled trials evaluating therapeutic or rehabilitative exercise programs in lumbar spinal disorders have often used a heterogenous population of patients with chronic low back pain. A small number of patients evaluated represent patients with spinal stenosis for which the severity and extent of neurologic leg symptomatology relative to back pain is poorly characterized. In addition, studies comparing therapeutic exercise with surgery have primarily evaluated fusion surgery as compared with nonsurgical treatment in the management of mechanical low back pain in lumbar spondylosis.10–12 In lumbar spinal stenosis, some authors have proposed programs that use lumbar flexion exercises with the avoidance of extension exercises because of the spinal canal and neuroforaminal narrowing produced by lumbar extension. General aerobic conditioning and aqua therapy have also been advised in the treatment of these patients. However, limited evidence is available that actually guides the recommendation of one program over another or evaluates the benefit of such programs over natural history alone. In one study by Whitman and colleagues,13 the authors performed a multicenter, randomized, controlled trial on 58 patients with lumbar spinal stenosis. Patients were randomized to one of two 6-week physical therapy programs. One program consisted of manual therapy, lumbar exercises, and body weight supported treadmill walking, whereas the other program consisted of ultrasound, lumbar flexion exercises, and treadmill walking. Patient-perceived recovery was the primary outcome with secondary measures including Oswestry Disability Index, a numeric pain rating, satisfaction, and the results of the treadmill test. Patients in both randomized groups demonstrated improvements to measured outcome parameters. Perceived recovery was greater for the program consisting of manual therapy, treadmill walking, and exercise (perceived recovery 2.6; confidence interval, 1.8–7.8). Considerations to the study results was follow-up to 1 year and that a subset of patients in each group received additional treatment during the study period consisting of epidural steroid injection, surgery, medications, and/or additional specialty physician consultations (Level II).
Therapeutic Injections (Grade I)
A variety of anesthetics, corticosteroids, or opioids can be injected into various anatomic locations in the lumbar spine. Conflicting results have been reported in the literature on their use in spinal stenosis to allow for recommendation for or against intervention. In Fukusaki and coauthors’ study,14 53 patients with neurogenic claudication of less than 20 m were randomized to either epidural injection with 8 mL saline (n = 16), epidural block with 8 mL of 1% mepivacaine (n = 18), or epidural block with 8 mL of 1% mepivacaine and 40 mg methylprednisolone (n = 19). There did not appear to be a significant advantage of epidural steroid injection as compared with epidural block with a local anesthetic alone. The study had a relative short follow-up to 3 months. Primary study outcome was walking distance in meters to intractable leg pain as quantified by an independent reviewer. By 1 week, patients in the epidural block with or without steroid groups demonstrated greater walking distances when compared with patients in the saline group. At 1- and 3-month follow-up, patients in the epidural block with or without steroids group had a greater improvement in walking distance compared with before injection. With the sample size, a statistically significant effect comparing the three randomized groups in walking distances after 1 or 3 months of follow-up did not exist. Cuckler and colleagues’15 randomized study on 73 patients with lumbar radicular pain syndromes caused by either disc herniation or lumbar stenosis did not demonstrate a significant effect of 7 mL methylprednisolone acetate and procaine over 7 mL physiologic saline solution and procaine in the treatment of patients observed for an average of 20 months. Wilson-MacDonald and coworkers’16 study randomized and compared epidural steroid injection with intramuscular injection with local anesthetic with steroid and observed better improvement in short-term pain relief in the epidural group; however, the long-term benefits or need for subsequent surgery was no different over the long term between groups. The study evaluated 93 randomized patients for a minimum of 2 years. All patients evaluated in the study were considered potential candidates for surgical treatment. Ng and colleagues17 evaluated 86 randomized patients with unilateral radicular symptoms who received either bupivacaine with methylprednisolone injection (n = 43) or bupivacaine alone (n = 43). At 3-month follow-up, both groups demonstrated improvement. However, there did not appear to be an added benefit to the use of corticosteroids in pain se-verity, claudicant walking distance, or patient-derived functional outcome.
Surgical Treatment (B)
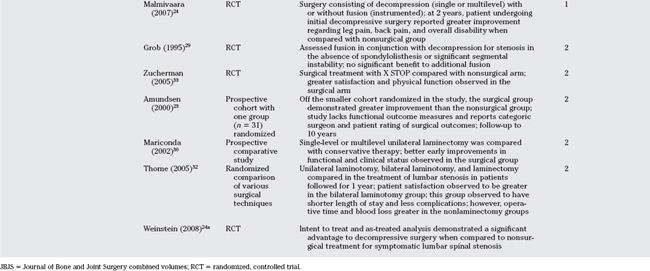
The abundance of Level I and II evidence relating to surgery in lumbar spinal stenosis is more focused on variations in surgical techniques than on evaluating the specific efficacy of surgical versus nonsurgical strategies. With the realization that there really is a lack of grade A or consistent grade B evidence for many nonsurgical therapies that are commonly practiced, the mainstay of lumbar spinal stenosis surgery can be more broadly categorized into surgical decompressive techniques with or without adjuvant spondylodesis or spinal fusion. The surgical principles involve decompression of compressive elements in lumbar canal stenosis (overgrown bony facets, ligamentous hypertrophy, disc herniations/extrusions) with or without adjuvant fusion of grossly degenerate levels or those levels with instability. Instability in the context of lumbar spinal stenosis in association with degenerative spondylolisthesis is discussed elsewhere in this textbook and is not included in this chapter (Chapter 4). The results of surgical intervention are generally positive in the relief of neurogenic claudicant symptomatology and patient-related quality of life, although its effect on objective physical parameters of function in the literature has been more variable. The notion that surgery for lumbar stenosis is typically more successful in the relief of claudicant symptomatology versus the relief of mechanical low back pain is generally accepted (Levels IV/V). However, there is a lack of large randomized trials comparing surgery with nonsurgical therapy in a homogeneous population with symptomatic lumbar spinal stenosis. The most recent Cochrane review of published randomized clinical trials for the surgical treatment of degenerative lumbar stenosis has included a review of heterogeneous studies, with seven relating to spondylolisthesis, spinal stenosis, and nerve compression.18 In reviewing those Class I and II studies that specifically compare surgical with nonsurgical treatment in lumbar stenosis, several reports relate to the long-term results from the Maine Lumbar Spine Study. These studies have compared a prospective cohort of patients treated a priori with either surgery or nonsurgical therapy19–21 (Level II). In the most recent report of 148 eligible consenting patients who were initially enrolled, 105 were alive after 10 years.21 Among surviving patients, long-term follow-up of between 8 and 10 years was available for 97 of 123 (79%) patients. As anticipated, patients undergoing surgery had worse baseline symptoms and functional status than those initially treated without surgery. Outcomes at 1 and 4 years favored those patients who underwent initial surgery. After 8 to10 years, there was no difference comparing treatment groups in the percentage of patients who reported that their back pain was improved (53% vs. 50%, surgical vs. nonsurgical; P = 0.8), improvements in predominant symptom of either back or leg pain (54% vs. 42%; P = 0.3), and satisfaction with their current status (55% vs. 49%; P = 0.5). Leg pain relief and greater back-related functional status continued to favor those initially treated surgically. By 10 years, 23% of surgical patients had undergone at least a second lumbar spine operation, and those patients who required additional surgeries faired worse when compared with those patients who continued with their initial treatment. No difference was reported in outcomes accordant to actual treatment received at 10 years. The study limitations include its observational and nonrandomized design, although baseline differences among treatment groups were considered and adjusted for in the analysis. Surgery in their Maine Lumbar Spine study consisted predominantly but not exclusively of decompressive nonfusion surgery, and the authors were not able to provide substantive clinical details for why subsequent surgeries were required in certain patients and what type of procedure was subsequently required.
Herno and investigators22 performed a matched-pair study of surgically and nonsurgically treated patients with lumbar spinal stenosis (Level III). A total of 496 patients who underwent surgery between 1974 and 1987, and 57 patients treated conservatively between 1980 and 1987 were evaluated at an average of 4 years after recommended treatment. Sex, age, myelographic findings, major symptom, and duration of symptoms were matched. At follow-up, subjective disability was assessed by Oswestry questionnaire, and functional status was evaluated by clinical examination. No statistical difference was found in outcome between the matched-pair groups, although male patients who underwent surgery fared better when compared with male patients who underwent conservative treatment. Functional status was good in both treatment groups and for both sex groups.
In Amundsen and colleagues’ study,23 a prospective cohort of 100 patients with symptomatic lumbar spinal stenosis was provided surgical or conservative treatment and followed for 10 years (Level II). Nineteen patients with what was considered to be severe symptoms were treated with surgery, 50 patients with moderate symptoms were treated nonoperatively, and 31 patients were randomized to either conservative (n = 18) or surgical (n = 13) treatment. Patients with an unsatisfactory result from conservative treatment were offered delayed surgery at a median of 3.5 months. The results of patients randomized to surgery were better than for patients randomized to conservative treatment. The treatment results of delayed surgery were similar to that of the initial group. Clinically significant deterioration of symptoms during the final 6 years of the study period was not observed. Patients with significant multilevel pathology did not respond as well as those with primarily single-level pathology. Limitations of the study included a relatively small number of patients randomized and the lack of patient-derived functional outcome measures.
In a more recent study that randomized 94 patients into surgical and nonsurgical groups, Malmivaara and investigators24 performed a multicenter prospective study evaluating outcome based primarily on assessment of functional disability using the Oswestry Disability Index. Inclusion criteria included back pain with radiation to buttocks or lower limbs, fatigue or loss of sensation in the lower limbs aggravated by walking, persistent pain without progressive neurologic dysfunction, imaging consistent with lumbar stenosis (midsagittal diameter <10 mm2 or cross-sectional dural area <75 mm2), and symptoms and signs for longer than 6 months. In the 50 patients randomized to surgery, surgery consisted of decompressive laminectomy of the stenotic segment(s), and in 10 patients, adjuvant transpedicular fusion was performed. The nonsurgical group was followed by a physiatrist who assessed the need for individualized treatment that included medications such as nonsteroidal anti-inflammatories or active/passive physiotherapy programs. Baseline low back or lower limb pain scores, Oswestry Disability Index scores, or walking ability was not significantly different comparing the two randomized groups, although there was a greater proportion of female patients and patients with good perceived health randomized to the surgical group. At 2-year follow-up, patients in both randomized groups reported improvements to their condition. In the 44 patients randomized to the nonoperative group, 4 patients required surgery by 2 years because of persistent symptoms. The authors observed at 2-year follow-up that those patients who underwent initial decompressive surgery reported greater improvement regarding leg pain, back pain, and overall disability when compared with the nonsurgical group. Limitations to the study include the length of follow-up and varying surgery type being performed in the surgical arm. The most recent study by Weinstein et al.24a described a randomized study of surgical versus nonsurgical therapy for lumbar spinal stenosis. Surgical candidates with at least 12 weeks of symptoms and spinal stenosis without spondylolisthesis were randomized to decompressive surgery or nonsurgical care. The primary outcomes were bodily pain and physical function on the Medical Outcomes Study 36-item SF36 and modified Oswestry Disability Index at 6 weeks through 2 years. Of the 289 patients enrolled in the randomized cohort and 365 patients enrolled in the observational cohort, there was significant cross-over with 67% of patients randomized to surgery receiving surgery and 43% of patients randomized to nonsurgical care also undergoing surgery by the study 2 year follow-up. The intention-to-treat analysis of the randomized cohort favored surgery on the SF-36 scale for bodily pain with a mean change from baseline of 7.8 points (95% confidence interval, 1.5 to 14.1). The as-treated analysis, adjusted for potential confounders, demonstrated a significant advantage for surgery by 3 months for all primary outcomes that remained significant at 2 years.
In a study on the radiographic severity in lumbar spinal stenosis, Hurri and colleagues25 reviewed 12-year data on 75 patients with myelographic changes diagnostic for stenosis. The authors observed that the severity of stenosis radiographically predicted disability after adjusting for the effects of age, sex, therapy regimen, and body mass index. Surgical and nonsurgical therapy was not a significant correlate with later disability as quantified by Oswestry Disability Index. Using more recent radiographic imaging, Weiner and investigators26 prospectively evaluated 27 consecutive patients undergoing isolated surgical decompression at L4-5 for lumbar canal stenosis. Using magnetic resonance imaging (MRI) evaluation of stenosis, the authors observed that a greater than 50% reduction in cross-sectional area or preoperative MRI was more likely to have a successful surgical outcome as quantified by Weiner and Fraser’s neurogenic claudication outcome score when compared with those individuals with less than 50% reduction in cross-sectional area.26 Less consistent evidence has been reported in larger prospective cohort, noncontrolled, surgical series regarding radiographic severity and surgical outcome; although with larger series, one may anticipate a more heterogeneous population of study patients as it pertains to the number of involved lumbar motion segments, and variation in type and extent of lumbar surgery performed.27,28
For surgery beyond lumbar spinal decompression, there does not appear to be significant evidence to support or refute the use of adjuvant lumbar fusion in patients undergoing surgery for stenosis in the absence of spondylolisthesis or significant segmental instability. In Grob and coauthors’29 study, 45 patients were randomized to 1 of 3 treatment groups accordant to the day that patients were admitted to the hospital. The average study follow-up was 28 months, and surgery was performed by a single surgeon. Fifteen patients received lumbar decompressive laminotomy and medial facetectomy, 15 patients received decompression followed by arthrodesis of the most stenotic segment, and 15 patients received decompression followed by arthrodesis of all decompressed spinal levels. Patients in all groups reported improvements in pain and walking distance after surgery. The authors did not observe significant differences in the results among the three groups with regard to the relief of pain, although the study was limited by sample size and also lacked validated patient-derived functional outcome measures. In the prospective multicenter observational study by Katz and colleagues,28 the authors reviewed 272 patients who underwent lumbar surgery (Level II). The authors acknowledge limitations of this nonrandomized study in terms of the number of participating surgeons and a modest sample size. With this caveat, the authors indicated that the individual surgeon was a more accurate correlate of the decision to perform arthrodesis versus clinical parameters such as spondylolisthesis, that noninstrumented lumbar fusion resulted in greater relief of back pain, and that the costs relating to adjunctive instrumentation in lumbar fusion were not an insignificant consideration.
More recent surgical strategies have included the application of less invasive surgical decompressive techniques, dynamic stabilization techniques as an alternative to lumbar instrumented fusion, and minimally invasive techniques utilizing the concept of affording indirect lumbar spinal decompression. Many of these studies are limited in sample size, heterogeneity of the study population of interest, and lack long-term data. Newer, less invasive surgical strategies have involved modifications to conventional laminectomy and partial facetectomy to balance the degree and extent of bony and soft-tissue dissection/resection necessary to achieve adequate restoration of spinal canal space.30–32 Several equivalency randomized trials have corroborated in the short term that, in the correct surgical hands, patient outcomes appear to be favorable when compared with conventional laminectomy.31,32 In Cho and colleagues’31 study, split-spinous process laminotomy and discectomy were compared with conventional laminectomy (30 patients), with or without discectomy in 70 patients randomized and followed prospectively. The follow-up ranged from 10 to 18 months, with a mean of 15.1 months for the split-spinous process group and 14.8 months for the conventional laminectomy group. There was a shorter mean postoperative duration until ambulation without assistance, a reduction in mean duration of hospital stay, a lower mean creatine phosphokinase-muscular–type isoenzyme level, and a lower VAS score for back pain at 1-year follow-up for the split-spinous process group. Operative time and surgical blood loss, however, were greater for this group. The authors conclude that although the split-spinous process method required more operative time than laminectomy, earlier mobilization and shortened length of stay with reduction in pain and satisfactory neurologic and functional outcomes with the method was an attractive consideration in the context of surgical procedures aimed to address structural lumbar stenosis. In Thome and colleagues’32 study, 120 consecutive patients with 207 levels of lumbar stenosis (without instability or disc herniation) underwent randomization to bilateral laminotomy, unilateral laminotomy, or laminectomy. Patients were managed for 1 year with visual analogue pain and functional outcome measures (Roland-Morris Scale and SF-36). Complications were lowest in the bilateral laminotomy group, and the authors observed the bilateral laminotomy group to have favorable outcomes when compared with either laminectomy or unilateral laminotomy groups.32 In addition, there have been short- to intermediate-term randomized studies comparing minimally invasive strategies using indirect lumbar decompressive techniques/devices for patients with stenosis when compared with nonsurgical treatment. In their study, Zucherman and coauthors33 reviewed the 2-year results of patients randomized to either surgical treatment using the X STOP device or to nonsurgical treatment. The rationale of the device is to increase the interspinous process distance to indirectly decompress the spinal canal positioning the lumbar spine into a relatively more flexed position (minimizing the extent of lumbar extension over the motion segment), which is analogous to the physical therapeutic posturing techniques (e.g., William’s flexion program) that may transiently improve patient symptoms relating to lumbar stenosis. One hundred patients were evaluated in the surgical arm and 91 in the control group. The primary measure was the Zurich Claudication questionnaire (ZCQ). At 2 years, experimental patients improved by 45.4% over the mean baseline symptom severity score when compared with 7.4% in the control group. There was greater satisfaction in the treatment group (73.1%) when compared with the control group (35.9%). Observed differences in the ZCQ physical function component favored the treatment group. The control group consisted of an epidural steroid injection after enrollment. Fifty-nine percent of control patients received more than one injection over the study period. Control patients were also prescribed medications and physiotherapy as necessary. Limitations of this study included the lack of comparison with conventional surgical treatment, lack of consistency in the nonsurgical treatment arm, and the study length of follow-up. In their study, Kondrashov and colleagues34 reviewed a subset of patients who participated in the FDA clinical trial on X STOP and identified 18 patients whose subsequent analysis at 4 years suggested that surgical outcomes were stable as measured by the Oswestry Disability Index. Clearly, longer term follow-up with a larger sample size and validation by independent investigators is warranted before consideration of its use over currently reported strategies. Finally, newer strategies have also included surgical dynamic stabilization utilizing newer implants/devices as a potential alternative to lumbar fusion.35 Literature on its use has focused primarily on comparisons with conventional techniques of instrumented surgical fusion. The theoretical consideration of dynamic stabilization may have merit as an alternative to lumbar fusion. However, larger randomized series evaluating such technology have coupled the application of these implants/devices to lumbar fusion. Given the lack of high-level evidence with consistent funding to support the use of fusion strategies in general as an adjunct to decompression in lumbar spinal stenosis, future studies evaluating dynamic stabilization require appropriate comparison with surgical decompression without fusion and nonsurgical control subjects. Insufficient evidence exists to support or refute the potential application of many newer surgical strategies in the treatment of lumbar spinal stenosis, and additional studies are required before providing an evidence-based opinion in recommendation(s).
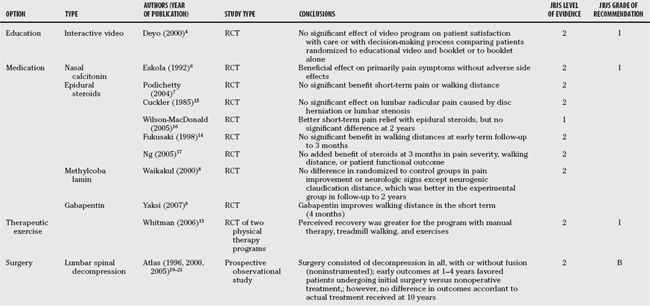
In summary, the strength of evidence according to the JBJS grades of recommendation is insufficient (I) or fair (B) at best for many options that are available to treat patients with symptomatic lumbar spinal stenosis. Many studies have evaluated these patients in the broader context of patients with chronic low back pain. In general, a lack of good evidence (JBJS grade A) exists as it pertains to Level I studies with consistent findings that would guide evidenced-based recommendations for intervention (Table 5-1). Despite anticipated awareness and improvements to evidence-based practice and study design with an increase in Level I studies being reported, much of the current treatment of symptomatic lumbar spinal stenosis is based on expert opinion and medicalconsensus. Appropriate control arms with consistent and comparable inclusion criteria are required to further strengthen existing literature in this area. Understandably, some of the difficulty in characterizing this condition and ensuring consistency in a homogenous population of study has been described in the introduction. It highlights what many of us encounter in the management of patients with symptomatic lumbar spinal stenosis—a chronic condition with a heterogeneous presentation that changes over time. With this consideration in mind, several themes are available on review of current literature. Symptomatic patients can often be managed through nonsurgical approaches, although insufficient evidence is available to support one specific type of approach over another. It would make inherent sense that patient education is paramount and additional Level I/II studies may further guide strategies that will optimize informing patients of the appropriate knowledge necessary to understand their conditions and treatment options. Many commonly used oral medications have not been convincingly proved effective specifically in the treatment of lumbar stenosis, although some renewed interest in antineuritic medications such as gabapentin warrant further validation and longer term study in patients with symptomatic claudication from spinal stenosis. There is insufficient evidence to substantiate therapeutic exercises over other alternatives in the management of patient-related symptoms apart from the possibly related general health benefits of aerobic conditioning on the cardiovascular system. Epidural steroids are mixed in their results in the literature. The natural history of the condition would appear to be favorable, and nonsurgical therapy in many patients is not necessarily associated with significant clinical deterioration over time. There is a lack of sufficient good evidence and Level I studies to make a strong recommendation of surgery over nonsurgical therapies. It would appear, however, that surgery can be of significant benefit in certain patient subpopulations that require ongoing characterization. As such, fair evidence (JBJS grade B) in the role of surgery in the treatment of persistently symptomatic lumbar stenosis exists. Patients should be appropriately informed that the results of surgery if required at a later stage are not convincingly lessened if a nonsurgical approach is initially chosen. Of the surgical treatment options, insufficient evidence exists to recommend many of the available options beyond a decompressive posterior lumbar procedure. The historical standard of care has been a lumbar laminectomy with or without partial facetectomy. This consideration also needs to be weighed in the context of patients who elect to choose the surgical route because there is an appreciable risk for requiring an additional lumbar procedure over time for their condition, and the results of subsequent lumbar surgical procedures are not as successful as index procedures. Although the structural severity of the stenosis may relate to success of surgery, it is also cautioned that the severity of stenosis radiographically is not a good correlate to patient symptom severity or perceived function. Rapid, progressive neurologic deterioration appears uncommon with any of the available therapies. The optimal timing for surgical intervention in the context among patient symptom severity, structural stenosis severity, and self-perceived quality of life and physical function warrants additional study. In conclusion, until stronger evidence is available for recommending therapeutic intervention in symptomatic lumbar stenosis, treatment for this condition needs to be individualized. Currently, insufficient evidence exists to recommend an optimal treatment regimen for a patient with symptomatic lumbar spinal stenosis.
| Summary of Recommendations | ||
|---|---|---|
| STATEMENT | EE O E IDENCE ADE O ECOMMENDATION | E EENCES |
1 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408.
2 Selkowitz DM, Kulig K, Poppert EM, et al. The immediate and long-term effects of exercise and patient education on physical, functional, and quality-of-life outcome measures after single-level lumbar microdiscectomy: a randomized controlled trial protocol. BMC Musculoskelet Disord. 2006;7:70.
3 Burton AK, Waddell G, Tillotson KM, et al. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine. 1999;24:2484-2491.
4 Deyo RA, Cherkin DC, Weinstein J, et al. Involving patients in clinical decisions: Impact of an interactive video program on use of back surgery. Med Care. 2000;38:959-969.
5 Phelan EA, Deyo RA, Cherkin DC, et al. Helping patients decide about back surgery: A randomized trial of an interactive video program. Spine. 2001;26:206-212.
6 Eskola A, Pohjolainen T, Alaranta H, et al. Calcitonin treatment in lumbar spinal stenosis: A randomized, placebo-controlled, double-blind, cross-over study with one-year follow-up. Calcif Tissue Int. 1992;50:400-403.
7 Podichetty VK, Segal AM, Lieber M, et al. Effectiveness of salmon calcitonin nasal spray in the treatment of lumbar canal stenosis: A double-blind, randomized, placebo-controlled, parallel group trial. Spine. 2004;29:2343-2349.
8 Waikakul W, Waikakul S. Methylcobalamin as an adjuvant medication in conservative treatment of lumbar spinal stenosis. J Med Assoc Thai. 2000;83:825-831.
9 Yaksi A, Ozgonenel L, Ozgonenel B. The efficiency of gaba-pentin therapy in patients with lumbar spinal stenosis. Spine. 2007;32:939-942.
10 Sculco AD, Paup DC, Fernhall B, et al. Effects of aerobic exercise on low back pain patients in treatment. Spine J. 2001;1:95-101.
11 Fritzell P, Hagg O, Wessberg P, et al. 2001 Volvo Award Winner in Clinical Studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine. 2001;26:2521-2534.
12 Brox JI, Sorensen R, Friis A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine. 2003;28:1913-1921.
13 Whitman JM, Flynn TW, Childs JD, et al. A comparison between two physical therapy treatment programs for patients with lumbar spinal stenosis: A randomized clinical trial. Spine. 2006;31:2541-2549.
14 Fukusaki M, Kobayashi I, Hara T, et al. Symptoms of spinal stenosis do not improve after epidural steroid injection. Clin J Pain. 1998;14:148-151.
15 Cuckler JM, Bernini PA, Wiesel SW, et al. The use of epidural steroids in the treatment of lumbar radicular pain. A prospective, randomized, double-blind study. J Bone Joint Surg Am. 1985;67:63-66.
16 Wilson-MacDonald J, Burt G, Griffin D, et al. Epidural steroid injection for nerve root compression. A randomised, controlled trial. J Bone Joint Surg Br. 2005;87:352-355.
17 Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: A randomized, double-blind, controlled trial. Spine. 2005;30:857-862.
18 Gibson JN, Waddell G. Surgery for degenerative lumbar spondylosis: Updated Cochrane Review. Spine. 2005;30:2312-2320.
19 Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, Part III. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996;21:1787-1795.
20 Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of lumbar spinal stenosis: Four-year outcomes from the Maine lumbar spine study. Spine. 2000;25:556-562.
21 Atlas SJ, Keller RB, Wu YA, et al. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8 to 10 year results from the Maine lumbar spine study. Spine. 2005;30:936-943.
22 Herno A, Airaksinen O, Saari T, et al. Lumbar spinal stenosis: A matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996;10:461-465.
23 Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: Conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424-1436.
24 Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or nonoperative treatment for lumbar spinal stenosis? A randomized controlled trial. Spine. 2007;32:1-8.
24a Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:818-824.
25 Hurri H, Slatis P, Soini J, et al. Lumbar spinal stenosis: Assessment of long-term outcome 12 years after operative and conservative treatment. J Spinal Disord. 1998;11:110-115.
26 Weiner BK, Patel NM, Walker MA. Outcomes of decompression for lumbar spinal canal stenosis based upon preoperative radiographic severity. J Orthop Surg. 2007;2:3.
27 Jonsson B, Annertz M, Sjoberg C, et al. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: Five-year follow-up by an independent observer. Spine. 1997;22:2938-2944.
28 Katz JN, Stucki G, Lipson SJ, et al. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;24:2229-2233.
29 Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am. 1995;77:1036-1041.
30 Mariconda M, Fava R, Gatto A, et al. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: A prospective comparative study with conservatively treated patients. J Spinal Disord Tech. 2002;15:39-46.
31 Cho DY, Lin HL, Lee WY, et al. Split-spinous process laminotomy and discectomy for degenerative lumbar spinal stenosis: A preliminary report. J Neurosurg Spine. 2007;6:229-239.
32 Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: A randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005;3:129-141.
33 Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: Two-year follow-up results. Spine. 2005;30:1351-1358.
34 Kondrashov DG, Hannibal M, Hsu KY, et al. Interspinous process decompression with the X-STOP device for lumbar spinal stenosis: A 4-year follow-up study. J Spinal Disord Tech. 2006;19:323-327.
35 Korovessis P, Papazisis Z, Koureas G, et al. Rigid, semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis: A correlative radiological and clinical analysis of short-term results. Spine. 2004;29:735-742.