Authors
Proposed target proteinuria
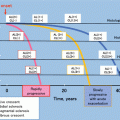
Study design and characteristics of the cohort
Partial remission or clinical remission
Definition of proteinuria remission, g/day
Timing of proteinuria measurements taken as a surrogate marker
Prospective or retrospective study design
Number of patients
Follow-up, yearsa
Type of treatment, %
RAS-I
Steroid
IS
Tonsillectomy
Reich et al. [6]
PR
<1.0
Whole follow-up
Prospective study
542
6.5 ± 4.9
53.0
12.5
15.7
ND
Le et al. [7]
PR
<0.5
Whole follow-up
Prospective study
1155
5.4 (4.1–7.2)
90.0
10.8
13.6
ND
Hotta et al. [9]
CR
<0.2b
Final observation
Retrospective study
329
6.9 ± 3.2
47.1
83.6c
28.9
76.0
Hwang et al. [11]
PR
<1.0
Within 2 years after starting anti-proteinuric treatment
Retrospective study
125
7.5 ± 3.3
100.0
20.0
14.4
ND
Hirano et al. [13]
PR
<0.4
One year after starting steroid pulse therapy
Retrospective study
141
3.8 (2.5–5.3)
44.0
100.0d
0.0
48.2
Tatematsu et al. [15]
CR
<0.2
Within 2 years of starting steroid pulse therapy
Retrospective study
109
3.3e
53.2
100.0d
0.0b
49.5
17.3 Previous Proposal of Clinical Remission Defined by the Absence of Proteinuria and Hematuria at Final Observation as Treatment Goal
Kobayashi et al. reported effectiveness of steroids for IgA nephropathy in 1988 [8]. At that time, oral steroids were the mainstay of treatment, and less than 1.0 g/day was commonly accepted as target proteinuria. With advances in treatment, target proteinuria may undergo a paradigm shift. In 2001, Hotta et al. proposed the concept of clinical remission defined by complete disappearance of proteinuria and hematuria at final observation as opposed to partial remission, as the treatment goal [9]. They reported an observational study composed by 329 cases of IgA nephropathy with a mean observation period of 6.9 years (Table 17.1). In this cohort, 197 cases received steroid pulse therapy, 250 cases had tonsillectomy, and 95 cases had immunosuppressive therapy. At the end of the observation period, among 158 cases with clinical remission, no one had 1.5 times increase in serum creatinine concentration from baseline, making it a more favorable outcome compared to the 24 cases (14 %) among 172 cases with no clinical remission. Furthermore, by multivariate analysis, steroid pulse therapy and tonsillectomy were shown to have significantly contributed to the achievement of clinical remission. Taken altogether, they recommend combination of steroid pulse therapy and tonsillectomy, and hence their suggestion of clinical remission as the goal in management of IgA nephropathy.
17.4 Limitations in Clinical Implications for the Concept of Partial and Clinical Remissions Taken from Proteinuria at Different Times During the Follow-Up
Several limitations exist when applying time-average proteinuria and final urine analysis in clinical practice. Firstly, some important information acquired during the clinical course of the disease is missed when calculating time-average proteinuria and only considering urine analysis at the end of the follow-up. Clinical course of IgA nephropathy involves periods of progression, remission, and relapse. Each period has a differing disease activity. Therefore, consideration of only average values and urine analysis at the end of the follow-up does not truly reflect the course of the disease. In the studies where the concepts of partial and clinical remissions were first described, it is uncertain as to how much attention was paid to relapses when analyzing results. Secondly, variabilities of treatment protocols were not considered in detail through analysis. Supportive treatment such as RAS inhibitors (RAS-I) and more active interventions such as steroid pulse therapy did not have different weights in the analysis, and so the results from a mixture of treatment strategies might have been interpreted as one entity. Finally, time-average proteinuria and proteinuria at the end of follow-up are measures calculated concurrently with disease progression. This means that these measures in themselves are the results of disease course and hence not strictly the indicators of prognosis.
17.5 Clinical Implications of Proteinuria Responded to Initial Treatment as a Predictor of Renal Prognosis
In a secondary study of the randomized controlled trial of omega-3 polyunsaturated fatty acids in IgA nephropathy, Donadio et al. demonstrated that proteinuria after 1 year of intervention correlated with renal prognosis in a volume-dependent manner [10]. This analysis shed new light on the link between proteinuria and renal prognosis. Nevertheless, they did not mention the threshold of proteinuria after 1 year of intervention. This was then examined by Hwang et al. in a study analyzing 125 cases of IgA nephropathy [11]. They demonstrated that those who reached proteinuria of less than 1.0 g/day 1 year after intervention had better renal prognosis (Table 17.1). However, the main problem in this study was that intervention was not standardized in that it also included RAS-I for all cases as well as a mixture of steroid and other immunosuppressive therapy for some cases.
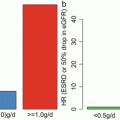
For the first time in 2004, Pozzi et al. demonstrated benefits of steroid therapy in an 8-year randomized controlled trial [12]. They reported that three courses of steroid pulse therapy given in half a year period resulted in good renal prognosis over 8 years. Although there had been studies examining at the effects of steroid therapy, the study by Pozzi was the first randomized controlled trial in this field. With their protocol, proteinuria decreased most in the first year of intervention. Leading from this, Hirano et al. standardized steroid therapy to Pozzi protocol and analyzed target proteinuria for IgA nephropathy [13], (Table 17.1). Firstly, out of proteinuria measured at three intervals, namely, before intervention, after half a year, and 1 year of intervention, proteinuria measured 1 year after intervention was most associated with renal survival. Following this, proteinuria 1 year after intervention was taken as the primary assessment point, and analysis was performed to determine the value of proteinuria above which renal function was likely to deteriorate. Regardless of baseline renal function and pathological findings, proteinuria of more than 0.4 g/day 1 year after intervention was likely to result in 1.5 times increase in creatinine concentration. Furthermore, details of relapse of IgA nephropathy with proteinuria of more than 1.0 g/day were also analyzed [14]. Risk of relapse increases rapidly when proteinuria reaches above 0.4 g/day after 1 year. For the reasons stated above, Hirano et al. proposed proteinuria of less than 0.4 g/day as the criterion for partial remission after undergoing Pozzi steroid protocol. In contrast, Tatematsu et al. who analyzed the slope of eGFR progression as the outcome suggested a more strict criterion of clinical remission within 2 years of initiating treatment as a pointer toward good renal prognosis [15], (Table 17.1). However, both studies lacked enough sample size for more solid multivariate analysis.
17.6 Future Directions of Target Proteinuria
In order to clarify target proteinuria, large prospective multicenter studies with more defined intervention protocols and specified timings of proteinuria measurements are warranted. Limitations exist in taking proteinuria as a sole indicator of prognosis in clinical practice. Therefore, in the future other markers may be used alongside proteinuria to better predict the future course of the disease. Furthermore, advances in analysis of specific subclasses of urinary protein associated with disease relapse or fibrosis may offer a new direction. An ongoing study by Suzuki et al. investigating specific serum markers for activity of IgA nephropathy may shed new light on the use of serum markers alongside proteinuria [16].
Conflict of Interest
The authors declare that they have no conflict of interest.
References
1.
KDIGO board members. KDIGO clinical practice guideline-minimal change nephrotic syndrome. Kidney Int Suppl. 2012;2:177–180.
3.
4.
5.
De Nicola L, Provenzano M, Chiodini P, Borrelli S, Garofalo C, Pacilio M, et al. Independent role of underlying kidney disease on renal prognosis of patients with chronic kidney disease under nephrology care. PLoS One. 2015;10:e0127071.CrossRefPubMedPubMedCentral
6.
7.
8.
9.
10.
11.
Hwang HS, Kim BS, Shin YS, Yoon HE, Song JC, Choi BS, et al. Predictors for progression in immunoglobulin A nephropathy with significant proteinuria. Nephrology (Carlton). 2010;15:236–41.CrossRef
12.
13.
Hirano K, Kawamura T, Tsuboi N, Okonogi H, Miyazaki Y, Ikeda M, et al. The predictive value of attenuated proteinuria at 1 year after steroid therapy for renal survival in patients with IgA nephropathy. Clin Exp Nephrol. 2013;17:555–62.CrossRefPubMedPubMedCentral
14.
Hirano K, Kawamura T, Tsuboi N, Okonogi H, Hanaoka K, Ogura M, et al. Clinical and histological parameters associated with the recurrence of proteinuria after steroid therapy in IgA nephropathy patients. J Am Soc Nephrol. 2013;24:817A.
15.