Chapter 38 What Is the Best Treatment of Malignant Bone Tumors in Children?
SYSTEMIC THERAPY
The two most common primary tumors of bone are osteosarcoma (OS) and Ewing’s sarcoma (ES) with an incidence of 4.8 cases/million and 2.9 cases/million, respectively, in people younger than 20 years.1 ES is part of a family of small, round, blue cell tumors whose exact cell of origin remains unknown. The mainstay of therapy includes multiagent cytotoxic chemotherapy and effective local control with surgery and/or radiation. The following section focuses on the medical therapy of localized OS. Improvement in the survival of patients with OS has been elusive since the therapeutic breakthroughs of the late 1970s to early 1980s, despite the efforts of large multinational cooperative groups.
CHEMOTHERAPY IN OSTEOSARCOMA
It is clear that with surgery alone, 80% of patients with malignant bone tumors will develop pulmonary metastases.2 Therefore, the treatment of OS has evolved to include systemic chemotherapy in addition to surgical resection. OS is relatively resistant to radiotherapy, so this particular modality is not used with curative intent. With current chemotherapy regimens, event-free survival remains at approximately 70% at 5 years.
Multiple fundamental issues regarding the treatment of OS remain incompletely resolved:
Which Drugs Are Most Effective?
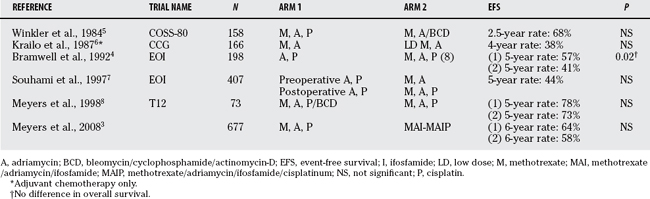
It is generally accepted that cisplatin, adriamycin, ifosfamide, and methotrexate are active against OS. Various combinations of chemotherapy have been tested in randomized, controlled trials, none of which has demonstrated robust superiority over another in improving survival. A recent large trial involving 677 patients demonstrated a 6-year event-free survival rate of 64% with the use of these agents; survival did not improve with the addition of ifosfamide.3 Although it is widely used, the role of methotrexate in OS remains uncertain4 and is currently under investigation4a (Table 38-1).
Does Tailoring Postsurgical Chemotherapy According to the Degree of Tumor Response Improve Survival?
One advantage to neoadjuvant (pre-operative) chemotherapy is the opportunity provided for assessment of the tumor’s response to the drugs being administered. Histologic assessment of the tumor after resection is most commonly described as per the Huvos system. A major distinction is drawn between tumors in which there are fewer than 10% residual viable cells and those with more than 10%.9 The degree of histologic response to chemotherapy is of prognostic relevance with “responders” (patients >90% necrosis) having significantly better outcome than “nonresponders” (patients <90% necrosis). It is less clear whether changing the therapeutic regimen in patients with poor response will improve survival. Unfortunately, all studies that have attempted to address this question have been single-arm case series. None of these studies demonstrated the ability to salvage patients who had a poor response by an alteration in postoperative therapy.10–13 Currently, an international study is under way to address this question in a randomized fashion.13a
Is Intra-Arterial or Intravenous Chemotherapy Better?
Some investigators have advocated the use of intra-arterial cisplatin or adriamycin to increase the local response rate that was found to be predictive of survival. Although many centers have attempted intra-arterial chemotherapy in OS, only a few randomized studies exist. Histologic response at the time of surgery improved from 43%–46% good response to 64%–77% with intravenous cisplatin compared with intra-arterial administration, respectively.14–16 However, there was no difference in survival.
Should Patients Be Treated with Chemotherapy before or after Surgery?
Neo-adjuvant chemotherapy, which is the current standard in the treatment of OS, initially evolved in order to allow time for the construction of endoprosthetic devices. The rationale for the administration of neo-adjuvant chemotherapy includes the early treatment of micrometastatic disease, optimization of the opportunity for limb salvage procedures, and the ability to gauge tumor response to therapy. The impact of timing of surgery was recently evaluated.17 No difference was found in survival between patients randomized to immediate surgery or neoadjuvant chemotherapy.
LOCAL CONTROL
General Issues
Biopsy.
What constitutes a safe biopsy is taught by experts, and there is no prospective study to confirm the validity of expert opinion and anecdotal evidence in this regard. Nonetheless, a comparison of outcomes in patients who underwent appropriately or poorly performed biopsy as judged retrospectively by experts revealed the latter result in about a 10% incidence rate of otherwise unnecessary functional or anatomic loss.18
Limb Salvage Surgery.
To investigate oncologic outcomes from the late 1970s through the 1980s when limb salvage surgery was supplanting amputation for local control of bone sarcoma, researchers performed retrospective comparative studies. Survival is equivalent between amputation and limb salvage cohorts of patients with OS.19,20
Surgical Margins.
The incidence of local recurrence after limb salvage procedures of the distal femur is greater than that after above-knee amputation. Local recurrence is less likely with more proximal levels of amputation.19,21, 22 These findings imply that the magnitude of a negative surgical margin is related to the ability to obtain local control but cannot specify what constitutes an adequate margin. Contradictory findings regarding rates of local recurrence after limb salvage or amputation have also been published,23 suggesting that surgical selection bias influences the outcome in these retrospective comparisons.
It takes great care to plan the distal resection margin of a malignant metaphyseal bone tumor in a skeletally immature individual. Preservation of the epiphysis, and therefore the native adjacent joint, would likely be a functionally optimal and durable option. However, it is often difficult to be certain of the distal extent of the tumor. It has been shown that the accuracy of magnetic resonance imaging (MRI) in detecting involvement of the epiphysis by tumor is 90.3% using histology of the same tumors as a reference. Importantly, there are no false-negative results by computed tomography or MRI.24 The surgical margin is therefore likely to safely correlate with preoperative MRI findings.
Surgical Complications
Two comparative studies highlight the importance of primary wound healing in the setting of limb-sparing procedures. Risk factors for developing infection of an orthopedic implant were assessed in a retrospective comparative fashion.25 The overall implant infection incidence rate was 21%, and multivariate analysis revealed that the only modifiable risk factor in those who experienced development of implant infections compared with those who did not was local wound infection. Those who experienced implant infection were more likely to undergo amputation and had poorer functional outcomes. A prospective comparison of patients who did and did not receive primary muscle flap coverage of a structural allograft reconstruction revealed that those in the former group were less likely to undergo reoperation for bone-related complications.26
Nonunion of a structural allograft that is used to reconstruct skeletal defects after resection of a sarcoma is another limb-threatening complication. In a retrospective comparison of a large number of patients, it was found that those who underwent chemotherapy were more likely to develop allograft nonunion than those who did not.27 Of course, this risk factor is not readily modifiable, but it highlights the anticipated difficult course for limb salvage reconstructions. Other retrospective comparisons have shown that locking plates improve the union rate of host-allograft junctions compared with ordinary plates,28 and that intramedullary cement can decrease the incidence of allograft fractures.29 In a comparison of reconstructions that used allograft alone versus allograft-vascularized fibular composites, it was shown that complication rates were comparable.30 The presence of a vascularized fibula, however, hastened the time to union.
Surgery versus Radiotherapy for Ewing’s Sarcoma
Radiotherapy and surgery have been used individually or combined for the local control of ES. Multiple retrospective comparisons of these 2 modalities have been performed as part of large cooperative group studies. In these studies, the treating physicians chose the modality for local control. Most studies demonstrate superior local control after surgery alone or when surgery and radiation are combined compared with radiotherapy alone,31 whereas others show equivalent results.31,32 However, some of these findings may reflect bias in the selection of radiation for larger tumors. Intralesional debulking surgery combined with radiotherapy results in local recurrence rates similar to local treatment with radiotherapy alone.31 The additional risks posed by the use of radiotherapy, such as development of a second malignant neoplasm,33 need to be considered when making decisions to undertake local control. A comparison of large, prospective studies reveals that if radiotherapy is used, survival is better when it is administered earlier in the course of chemotherapy as opposed to later.31
SPECIFIC RECONSTRUCTIVE OPTIONS
Upper Extremity
Reconstructive options after extra-articular resection of the shoulder together with the abductor mechanism include suspension arthroplasty with a spacer (metal or bone graft) and allograft intercalary fusion of the remaining native scapula to the native distal humerus. In a retrospective comparison of patients who underwent one of these two types of reconstruction, it was shown that physician-administered functional outcome measures (Musculoskeletal Tumor Society) are superior in those with a fusion.34 There are, of course, other factors to consider when choosing such a reconstruction, such as patient preference, the relative incidence of complications, and the planned strategy to manage anticipated limb-length discrepancy in young children.
Lower Extremity
When the articular surface of the distal femur or proximal tibia needs to be resected, the reconstructive options include replacement of the joint with a mega-endoprosthesis, an allograft-endoprosthetic composite, or an osteoarticular allograft; ablation of the joint by above-knee amputation or by rotationplasty; or arthrodesis of the joint. The use of an intercalary allograft to perform an arthrodesis of the knee as a limb-sparing reconstruction is associated with a greater rate of complications compared with allograft use in other settings.27,35 Osteoarticular allografts were found to be less predictably successful than endoprostheses in a mixed-age population,36 and allograft-endoprosthetic composites are less predictably successful than mega-endoprostheses in adults.37
Many pediatric cases are frequently amenable to what are likely the most reasonable two options: endoprosthetic replacement or rotationplasty. The decision to undertake one or the other is often individualized and is largely made by the patient and their family based on the anticipated relative functionality, appearance, durability, and risk for complications. Retrospective comparisons38–40 have shown that the functional outcome and quality of life after rotationplasty is comparable with that of endoprosthetic reconstruction both with regard to physician-administered (Musculoskeletal Tumor Society) and patient-derived (Toronto Extremity Salvage Score, Short Form 36, European Organization for Research and Treatment of Cancer) scores. The outcome of both of these reconstructive options score modestly greater than that of above-knee amputation. In general, outcome scores are better with limb salvage compared with amputation, except when the amputation is below the knee.41 Internal hemipelvectomy for pelvic tumors is also advantageous over hind-quarter amputation.41 No reconstruction of the pelvis after resection is associated with lower complication rates and at least comparable function compared with reconstruction of pelvic defects in children.42,43
Expandable endoprostheses bear mention because of their increasing popularity. Multiple case series involving various generations of these implants seem to document a relatively high rate of complications such as implant fracture,44 which are not common in adult series. No comparisons have been made yet to biological reconstruction or to reconstruction with a nonexpandable endoprosthesis combined with an alternative approach for the management of limb-length discrepancy from which to draw conclusions.
SUMMARY
Table 38-2 provides a summary of recommendations for treatment of malignant bone tumors in children.
| STATEMENT | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION | REFERENCES |
|---|---|---|
1 Gurney JG, Bulterys M. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. In: Ries LAG, Gurney JG, editors. Malignant bone tumors. Bethesda, MD: National Institutes of Health; 1999:99-110.
2 Link MP, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600-1606.
3 Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival–a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633-638.
4 Bramwell VH, et al. A comparison of two short intensive adjuvant chemotherapy regimens in operable osteosarcoma of limbs in children and young adults: The first study of the European Osteosarcoma Intergroup. J Clin Oncol. 1992;10:1579-1591.
4a De Camargo B, Van den Berg H, Van Dalen EC. Methotrexate for high-grade osteosarcoma in children and young adults. Cochrane Database of Systematic Reviews. 2008;2:CD 006325.
5 Winkler K, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: Results of a Cooperative German/Austrian study. J Clin Oncol. 1984;2:617-624.
6 Krailo M, et al. A randomized study comparing high-dose methotrexate with moderate-dose methotrexate as components of adjuvant chemotherapy in childhood nonmetastatic osteosarcoma: A report from the Childrens Cancer Study Group. Med Pediatr Oncol. 1987;15:69-77.
7 Souhami RL, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911-917.
8 Meyers PA, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: Results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452-2458.
9 Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: Pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14-18.
10 Rosen G, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221-1230.
11 Winkler K, et al. Neoadjuvant chemotherapy of osteosarcoma: Results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329-337.
12 Saeter G, et al. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: A Scandinavian Sarcoma Group study. J Clin Oncol. 1991;9:1766-1775.
13 Provisor AJ, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: A report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76-84.
13a National Cancer Institute. Combination chemotherapy, PEG-interferon alfa-2b, and surgery in treating patients with osteosarcoma. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=438714&version=patient&protocolsearchid=4812149. Accessed July 2008.
14 Bacci G, et al. Effect of intra-arterial versus intravenous cisplatin in addition to systemic adriamycin and high-dose methotrexate on histologic tumor response of osteosarcoma of the extremities. J Chemother. 1992;4:189-195.
15 Bacci G, et al. A comparison of methods of loco-regional chemotherapy combined with systemic chemotherapy as neo-adjuvant treatment of osteosarcoma of the extremity. Eur J Surg Oncol. 2001;27:98-104.
16 Ferrari S, et al. Nonmetastatic osteosarcoma of the extremity: Results of a neoadjuvant chemotherapy protocol (IOR/OS-3) with high-dose methotrexate, intraarterial or intravenous cisplatin, doxorubicin, and salvage chemotherapy based on histologic tumor response. Tumori. 1999;85:458-464.
17 Goorin AM, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574-1580.
18 Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656-663.
19 Rougraff BT, et al. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649-656.
20 Simon MA, et al. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331-1337.
21 Bacci G, et al. Predictive factors for local recurrence in osteosarcoma: 540 patients with extremity tumors followed for minimum 2.5 years after neoadjuvant chemotherapy. Acta Orthop Scand. 1998;69:230-236.
22 Picci P, et al. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699-2705.
23 Sluga M, et al. Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop Relat Res.; 358; 1999; 120-127.
24 San-Julian M, et al. Indications for epiphyseal preservation in metaphyseal malignant bone tumors of children: Relationship between image methods and histological findings. J Pediatr Orthop. 1999;19:543-548.
25 Gaur AH, et al. Infections in children and young adults with bone malignancies undergoing limb-sparing surgery. Cancer. 2005;104:602-610.
26 Mastorakos DP, et al. Soft-tissue flap coverage maximizes limb salvage after allograft bone extremity reconstruction. Plast Reconstr Surg. 2002;109:1567-1573.
27 Hornicek FJ, et al. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res.; 382; 2001; 87-98.
28 Buecker PJ, et al. Locking versus standard plates for allograft fixation after tumor resection in children and adolescents. J Pediatr Orthop. 2006;26:680-685.
29 Ozaki T, et al. Intramedullary, antibiotic-loaded cemented, massive allografts for skeletal reconstruction. 26 cases compared with 19 uncemented allografts. Acta Orthop Scand. 1997;68:387-391.
30 Belt PJ, Dickinson IC, Theile DR. Vascularised free fibular flap in bone resection and reconstruction. Br J Plast Surg. 2005;58:425-430.
31 Dunst J, Schuck A. Role of radiotherapy in Ewing tumors. Pediatr Blood Cancer. 2004;42:465-470.
32 Yock TI, et al. Local control in pelvic Ewing sarcoma: Analysis from INT-0091—a report from the Children’s Oncology Group. J Clin Oncol. 2006;24:3838-3843.
33 Henderson TO, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99:300-308.
34 O’Connor MI, Sim FH, Chao EY. Limb salvage for neoplasms of the shoulder girdle. Intermediate reconstructive and functional results. J Bone Joint Surg Am. 1996;78:1872-1888.
35 Donati D, et al. Allograft arthrodesis treatment of bone tumors: A two-center study. Clin Orthop Relat Res.; 400; 2002; 217-224.
36 Brien EW, et al. Allograft reconstruction after proximal tibial resection for bone tumors. An analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res.; 303; 1994; 116-127.
37 Wunder JS, et al. Comparison of two methods of reconstruction for primary malignant tumors at the knee: A sequential cohort study. J Surg Oncol. 2001;77:89-100.
38 Cammisa FPJr, et al. The Van Nes tibial rotationplasty. A functionally viable reconstructive procedure in children who have a tumor of the distal end of the femur. J Bone Joint Surg Am. 1990;72:1541-1547.
39 Hillmann A, et al. Malignant tumor of the distal part of the femur or the proximal part of the tibia: endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. J Bone Joint Surg Am. 1999;81:462-468.
40 Hopyan S, et al. Function and upright time following limb salvage, amputation, and rotationplasty for pediatric sarcoma of bone. J Pediatr Orthop. 2006;26:405-408.
41 Pardasaney PK, et al. Advantage of limb salvage over amputation for proximal lower extremity tumors. Clin Orthop Relat Res. 2006;444:201-208.
42 Hillmann A, et al. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340-344.
43 Schwameis E, et al. Reconstruction of the pelvis after tumor resection in children and adolescents. Clin Orthop Relat Res.; 402; 2002; 220-235.
44 Gitelis S, et al. The use of a closed expandable prosthesis for pediatric sarcomas. Chir Organi Mov. 2003;88:327-333.