Chapter 96 What Is the Best Treatment for Chondral Defects in the Knee?
Damage to the articular cartilage comprises a spectrum of disease entities ranging from single, focal chondral defects to more advanced degenerative disease. Long implicated in the subsequent development of osteoarthritis, focal chondral defects result from various causative factors. Patients are approximately evenly split in reporting a traumatic versus an insidious onset of symptoms; athletic activities are the most common inciting event associated with the diagnosis of a chondral lesions.1 Traumatic events and developmental causative agents such as osteochondritis dissecans (OCD) predominate in younger age groups. Several large studies have found high-grade chondral lesions (Outerbridge grades III and IV) in 5% to 11% of younger patients (<40 years) and up to 60% of older patients.1–3 The most common locations for these defects are the medial femoral condyle (up to 32%) and the patella,2,3 and most are detected incidentally during meniscectomy or anterior cruciate ligament reconstruction.1,4 Notably, despite this relatively high incidence, many of these defects are incidental in nature and asymptomatic. It is agreed that articular cartilage lesions have no spontaneous repair potential and a propensity to worsen with time. Even though the natural history is still not completely understood, those involved in cartilage repair agree that one must look for background factors that predispose to the formation of these defects—malalignment and compartment overload of the tibiofemoral or patellofemoral compartments, joint laxity, contracture, meniscal insufficiency, and of course, genetic predisposition to osteoarthritis—for which to date clinical, biological, or genetic markers are lacking. Therefore, various techniques have evolved to stimulate defect repair or overtly replace these defects. The high costs and extensive rehabilitation associated with many of these procedures necessitate careful evaluation to establish their respective clinical and cost-effectiveness.
OPTIONS
Before the development of modern bioengineering techniques, orthopedists were restricted to procedures that aimed to palliate the effects of chondral lesions or attempted to stimulate a healing response initiated from the subchondral bone resulting in the formation of fibrocartilage to fill the defect. Simple arthroscopic lavage and debridement of lesions has been used since the 1940s in an effort to reduce symptoms resulting from loose bodies and cartilage flaps, and it is a common first-line treatment, especially for coincidental defects. Marrow stimulation techniques (MST), such as abrasion arthroplasty, drilling, and microfracture, attempt to induce a reparative response by perforation of the subchondral bone after radical debridement of damaged cartilage and removal of the tide mark “calcified” zone to enhance the integration of repair tissue. The resultant blood clot, and the primitive mesenchymal cells contained within, may differentiate into a fibrocartilaginous repair tissue that fills the defect. Unlike hyaline cartilage, this fibrocartilage largely consists of type I collagen and is mechanically less stable and less durable.5 The pluripotential marrow-derived cells may also form bone, another mode of MST-related failure that is increasingly becoming recognized.6 Although closely related, MSTs vary by the degree of trauma to the subchondral bone, which has been recognized as a factor in the failure of these techniques. Abrasion arthroplasty (or also abrasion chondroplasty) decorticated the superficial subchondral bone with a bur to expose the more porous bone below but also destabilized the subchondral bone with the risk for fracture. Drilling utilizes small drill bits or K-wires to perforate the subchondral bone, which can result in heat necrosis; microfracture avoids this issue by using special awls (microfracture or Steadman awls). Currently, most surgeons have abandoned the older methods in favor of microfracture, which is usually performed in an all-arthroscopic fashion.
Restorative cartilage repair techniques such as autologous chondrocyte implantation (ACI) introduce chondrogenic cells into the defect area, resulting in the formation of a repair tissue that more closely resembles the collagen type-II rich hyaline cartilage. The original technique of ACI was developed in the 1980s7 and has been used in the United States to treat more than 10,000 patients since its approval by the U.S. Food and Drug Administration (FDA) in 1997. ACI is indicated for the treatment of medium to large chondral defects with no or shallow associated osseous deficits. It originally received FDA approval for application in the femoral condyle (medial, lateral, and trochlea) but has also been used successfully to treat patellar defects. ACI in its current form is a two-stage procedure with an initial arthroscopic cartilage biopsy, followed by a staged reimplantation through an arthrotomy. The next generation ACI-c (collagen-covered) technique was developed to reduce the reoperation rate because of hypertrophy of the periosteal patch used to cover the defect. This was achieved by substitution of periosteum with a collagen membrane, frequently consisting of a porcine type-I/III collagen bilayer membrane. The latest generation of ACI, termed MACI (membrane associated), cultures the chondrocytes directly on the aforementioned collagen membrane, which is then implanted arthroscopically or through a mini-open approach with fibrin glue or limited suturing.
Cartilage replacement techniques include osteochondral autograft and allograft transfers, such as the osteochondral autograft transfer system (OATS; Arthrex, Naples, FL), mosaicplasty (Smith & Nephew, Andover, MA), and mega-OATS techniques. Osteochondral autograft transplantation is used to address small to medium defects (1–4 cm2), often with associated bone loss. Osteochondral cylinders are harvested from lesser marginal weight-bearing areas of the knee joint and press-fitted into the prepared defect. Commonly, multiple cylinders have to be transplanted to fill larger defects. Osteochondral autografting is limited by the amount of cartilage that can be harvested without violating the weight-bearing articular surface.8 The main advantage lies in its autogenicity, avoidance of disease transmission, immediate graft availability through harvesting of the patient’s own tissue, and decreased cost of this single-stage procedure.
The treatment of chondral defects with fresh osteochondral allografts has garnered significant attention because of its potential to restore and resurface even extensive areas of damaged cartilage and bone. Osteochondral allograft transplantation is used predominantly in the treatment of large and deep osteochondral lesions resulting from OCD, osteonecrosis, and traumatic osteochondral fractures, but it can also be used to treat peripherally uncontained cartilage and bone defects. Furthermore, osteochondral allografting presents a viable salvage option after failure of other cartilage resurfacing procedures. The main advantages over autograft transplantation are the ability to closely match the curvature of the articular surface by harvesting the graft from a corresponding location in the donor condyle, the ability to transplant large grafts, and the avoidance of donor-site morbidity. The main concerns with allograft transplantation are failure to incorporate with subchondral collapse and the risk for disease transmission (estimated at 1 in 1.6 million for the transmission of HIV9).
EVIDENCE
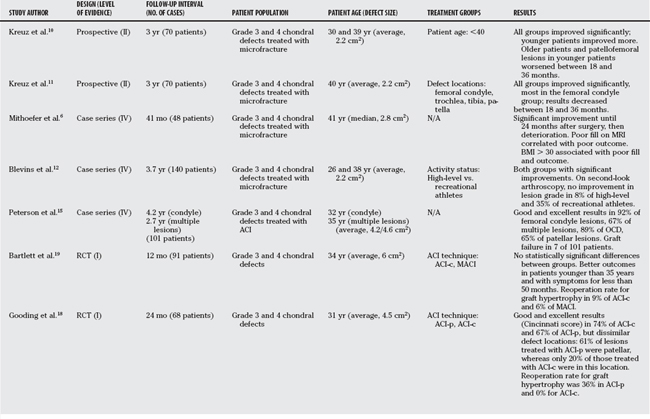
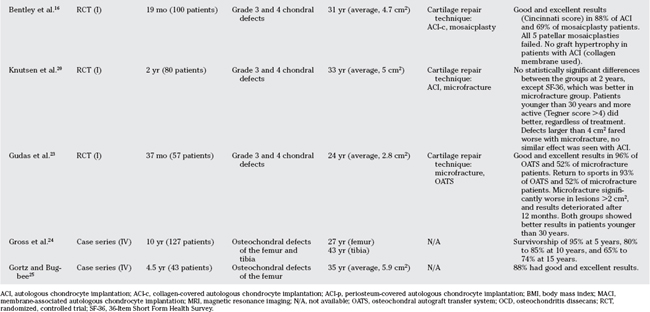
Table 96-1 provides an overview of cartilage repair studies. Table 96-2 provides a summary of treatment recommendations and respective levels of evidence for chondral defects in the knee.
TABLE 96-2 Treatment Recommendations and Respective Level of Evidence
| RECOMMENDATION | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
ACI, autologous chondrocyte implantation; ACI-c collagen-covered autologous chondrocyte implantation; BMI, body mass index; MACI, membrane-associated autologous chondrocyte implantation.
Microfracture
Most studies show good outcomes in 60% to 80% of patients. Several studies have tried to define the indications for microfracture in regards to patient and defect characteristics. Kreuz and Erggelet10 have demonstrated improved results in patients younger than 40 years, both in regard to validated scores and to magnetic resonance imaging (MRI) findings of better fill and quality. They also demonstrated a worsening between the 18- and 36-month follow-up scores for all patients in the older age group and for younger patients with patellofemoral defects (Level of Evidence II). In another study, the same authors report on the association between outcomes and defect locations, and found the best outcomes in femoral condyle lesions, whereas those in the patella group fared the worst (Level II).11 Other authors have described better results in smaller lesions, body mass index less than 30, and shorter duration between onset of symptoms and start of treatment (Level IV).6,12, 13
Autologous Chondrocyte Implantation
Several long-term case series have reported good-to-excellent results in 70% to 90% of patients after ACI in the knee, depending on the location (patella and femoral condyle) or the clinical series (Level IV).14,15 Bentley and colleagues16 compared ACI-c with mosaicplasty in a prospective randomized controlled trial (RCT), showing good and excellent results (Cincinnati score) in 88% of patients with ACI, but only 69% of mosaicplasties. All 5 patellar mosaicplasties were considered failures (Level I). In a similar study by Horas and coworkers,17 mosaicplasty performed significantly better than ACI. Biopsies were obtained from a subgroup of patients and uniformly revealed fibrous tissue in all defects treated with ACI. This study, however, was limited by the use of nonstandard cell-culturing facilities (Level I).
In a prospective RCT comparing ACI-p (periosteum-covered) with ACI-c, (Gooding and coworkers18 found no statistically significant differences in the clinical outcome, but a reoperation rate for symptomatic graft hypertrophy of 36% in the periosteal covered group versus 0% with the collagen membrane (Level I). Bartlett and coauthors19 presented a prospective RCT comparing the advanced techniques ACI-c and MACI, and reported no statistically significant differences between the groups. They also found improved outcomes in patients younger than 35 years and with pre-operative symptoms for less than 50 months (Level I).19 Knutsen compared ACI with microfracture and found no significant differences. The 36-Item Short Form Health Survey (SF-36) improved more in the microfracture group, and the authors hypothesized whether this could be because of the more invasive nature of ACI. Furthermore, the authors observed better results in younger (<30 years) and more active (Tegner scale score >4) patients. Defects larger than 4 cm2 fared worse than smaller lesions after microfracture treatment; no such effect was seen after ACI (Level I).20
Graft hypertrophy resulting in mechanical symptoms such as clicking and popping occurs in up to 25% to 30% of patients, typically 7 to 9 months after the procedure,21 and can be addressed with arthroscopic debridement of the hypertrophic tissue. Newer techniques such as ACI-c and MACI have decreased the rate of symptomatic graft hypertrophy to less than 10% (Level I).19
Osteochondral Autograft Transplantation
Patients treated with osteochondral autograft transplantation experienced good-to-excellent results in approximately 90% of condylar lesions, 80% of tibial defects, and 70% of trochlear lesions.22 The treatment of patellar defects remains controversial, with some groups reporting almost universal failure in this location.16 Gudas and investigators23 compared OATS with microfracture in a group of athletes, and demonstrated good and excellent results in 96% of patients treated with OATS versus 52% with microfracture. Rate of return to sports was 93% with OATS and 52% with microfracture. Microfracture had significantly worse results in lesions larger than 2 cm2, and results started to deteriorate after the 12-month examination. Both groups showed better results in patients younger than 30 years (Level I).23
Osteochondral Allograft Transplantation
Gross reported on a large series of fresh osteochondral allografts for the treatment of predominately traumatic distal femoral and proximal tibial defects. Survivorship analysis demonstrated intact and well-functioning grafts (Hospital for Special Surgery score >70) in 95% of patients at 5 years, which decreased to 80% to 85% at 10 years and 65% to 74% at 15 years (Level IV).24 Gortz and Bugbee25 report good and excellent results in 88% of 43 fresh osteochondral allografts of the distal femur at an average follow-up time of 4.5 years (Level IV).
AREAS OF UNCERTAINTY
BACKGROUND FACTORS
With the exception of clearly traumatic defects, most cartilage defects do not exist in isolation, but rather are associated with other pathologic entities. Careful evaluation can often identify structural abnormalities, such as malalignment, patellar instability, or insufficiency of the ligamentous and meniscal structures. The presence of these associated abnormalities introduces an additional layer of variability into studies because of the reproducibility of assessing background factors between surgeons and treating these before or concomitantly with the treatment of the chondral defect varies. To date, this correlation has not been firmly established. The disappointing early results of cartilage repair have been explained by the failure to properly diagnose and correct these associated bony and ligamentous abnormalities; for example, in early studies of patellar defects treated with ACI alone, good and excellent results were found in only one third of patients.7 Later studies, however, identified patellar maltracking as an important associated abnormality, and concurrent use of a corrective osteotomy led to 71% good or excellent results.28 These reports emphasize the importance of a thorough patient evaluation to correctly identify and treat all associated abnormalities to ensure the long-term success of chondral repair.
1 Aroen A, Loken S, Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-215.
2 Curl WW, Krome J, Gordon ES, et al. Cartilage injuries: A review of 31,516 knee arthroscopies. Arthroscopy. 1997;13:456-460.
3 Hjelle K, Solheim E, Strand T, et al. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-734.
4 Piasecki DP, Spindler KP, Warren TA, et al. Intraarticular injuries associated with anterior cruciate ligament tear: Findings at ligament reconstruction in high school and recreational athletes. An analysis of sex-based differences. Am J Sports Med. 2003;31:601-605.
5 Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res.; 365; 1999; 149-162.
6 Mithoefer K, Williams RJ3rd, Warren RF, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-1920.
7 Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-895.
8 Garretson RB3rd, Katolik LI, Verma N, et al. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004;32:967-974.
9 Gitelis S, Cole BJ. The use of allografts in orthopaedic surgery. Instr Course Lect. 2002;51:507-520.
10 Kreuz PC, Erggelet C, Steinwachs MR, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22:1180-1186.
11 Kreuz PC, Steinwachs MR, Erggelet C, et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119-1125.
12 Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: An analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761-778.
13 Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy. 2003;19:477-484.
14 Minas T. The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. Instr Course Lect. 1999;48:629-643.
15 Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res.; 374; 2000; 212-234.
16 Bentley G, Biant LC, Carrington RW, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223-230.
17 Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185-192.
18 Gooding CR, Bartlett W, Bentley G, et al. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-210.
19 Bartlett W, Skinner JA, Gooding CR, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-645.
20 Knutsen G, et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455-464.
21 Micheli LJ, Browne JE, Erggelet C, et al. Autologous chondrocyte implantation of the knee: Multicenter experience and minimum 3-year follow-up. Clin J Sport Med. 2001;11:223-228.
22 Hangody L, Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: Ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A(suppl 2):25-32.
23 Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005;21:1066-1075.
24 Gross AE, Shasha N, Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res.; 435; 2005; 79-87.
25 Gortz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect. 2007;56:469-481.
26 Jakobsen RB, Engebretsen L: An analysis of the quality of cartilage repair studies – an update. In ISAKOS Current Concepts Winter 2007. Edited, ISAKOS, 2007.
27 Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-2239.
28 Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res.; 436; 2005; 30-39.