Chapter 74 What Is the Best Treatment for a Charcot Foot and Ankle?
Charcot, or neuropathic arthropathy, of the lower extremity is a destructive disease in which patients present with swelling, erythema, and progressive deformity of their feet. Despite diabetes becoming the most common causative agent, the incidence of Charcot neuropathy is quite low in the diabetic population. In a radiographic study,1 the incidence was noted to be less than 1%, with similar incidence in retrospective clinical studies.2 Other studies have reported the presence of Charcot radiographic bony changes in almost 16% of patients with diabetes.3 This large variability may be attributed to difference in definition, alternate diagnosis, and lack of clinical signs despite radiographic changes.
The cause of Charcot disease is related to presence of peripheral neuropathy. Two theories currently exist regarding the pathophysiology of the disease. One theory considers neurotraumatic destruction of the joint, in which multiple trauma to the insensate joint results in microfractures, which over time result in macrofractures and progressive deformity. This concept also includes major fractures that progress to clinical deformity and is similar to the pathway by which arthritic joints in neuropathic patients become Charcot joints with advanced bony changes. The second theory relies on neurosympathetic destruction in which vascular and absorptive changes occur secondary to abnormal neural function, leading to autonomic sympathectomy and vascular alterations, which eventually progresses to gross deformity.4 It is thought that a combination of both pathologies contributes to the disease process. A common finding that is not as frequently evaluated is that peripheral perfusion is maintained in this group of patients.
Biologically, the progressive bone resorption seen in Charcot joints is associated with a large number of osteoclasts lining resorptive bone lacunae. The osteoclasts outnumber osteoblasts and demonstrate immunoreactivity to cytokines: interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α). One study concludes that alteration in the synthesis, secretion, or activity of these regulatory molecules may alter bone remodeling and lead to healing without collapse or malalignment.5
CLASSIFICATION
The disease process has been divided into stages as described by Eichenholtz.6 Stage I is characterized by significant inflammatory response with hyperemia, erythema, swelling, and warmth. Radiographically, this is associated with bony fragmentation. Stage II, known as coalescence, involves decreasing inflammation and edema, with bony coalescence noted radiographically. In Stage III, the bones are consolidated with an absence of inflammatory signs leading to the presence of fixed deformity.4 A prodromal stage 0 (“pre-stage I”) category has been described in patients with neuropathy with an acute sprain or fracture that places them at risk for the development of full-blown Charcot arthropathy.7 Other classification systems have been developed since then that are based on anatomic location and type of deformity.8,9 The only classification system tested to be reliable and reproducible involved the midtarsus.9,10 In this radiographic classification, Charcot anatomic variants of midtarsus deformities are identified by Roman numerals (I-IV) beginning from the Lisfranc joints (I) and extending proximally to the transverse tarsal joints (IV). A severity stage (α [low risk] or β [high risk]) is assigned depending on radiographic features. If one of the following criteria is met, the foot is considered at high risk (β):
An α stage can be assigned when all four features are absent. By testing the system on 75 orthopedic surgeons, reliability and reproducibility were found to be high. It was believed to be useful as a tool for diagnosis, planning treatment, and assessing the prognosis.10
MANAGEMENT
Goals of treatment include the creation and maintenance of a stable plantigrade foot, wound and bone healing, elimination of infection, and prevention of deformity. Current treatment recommendations are based on the stage of disease presentation. Pinzur11 had reported that reviewing current orthopaedic textbooks reveals almost universal agreement that acute Charcot foot arthropathy, Stage I Eichenholtz, should be treated conservatively, and surgery has only been advised when accommodative treatment has failed.12,13 Despite this general consensus, significant lack of evidence-based studies exist to support this recommendation, with a majority of current data from Level III and IV studies.11 Therefore, a grade C recommendation exists regarding the use of total contact cast (TCC) for the initial management of Charcot arthropathy.
The American Orthopaedic Foot and Ankle Society (AOFAS) has taken an initiative to develop objective data for basing diagnostic and therapeutic guidelines for this disease. A two-part survey was done. Whereas the first part surveyed treatment patterns for 94 patients, the second part consisted of a questionnaire of the clinical preferences completed by 37 AOFAS members with special interest in the disease process.14 The AOFAS notes from their results that management of the Charcot foot in the community is inconsistent, and significant variability occurs from the current guidelines by both physicians in the community and the specialists.14
Adjuvant Medical Treatment
In addition to better diabetic control, a number of studies have looked at adjuvant medications during the acute phase to relieve the symptoms.15 A Level I, randomized, controlled, therapeutic trial of 39 patients with active Charcot neuroarthropathy randomly assigned patients to treatment with 90 mg pamidronate or placebo.16 The authors report the treatment group had statistically significant reduction of symptoms and warmth; there was also significant reduction in bone turnover markers, bone-specific alkaline phosphate and dehydroxypyridinoline, from 4 to 24 weeks. The authors conclude that pamidronate had a substantial effect on reducing symptoms and bone turnover markers in patients with active Charcot arthropathy.16 They also suggest that further studies may be necessary to evaluate dosing regimen and frequency of pamidronate administration. Therefore, a grade B recommendation exists for the use of pamidronate in reducing symptoms of active Charcot arthropathy.
Nonsurgical Management
Traditionally, acute Charcot arthropathy, or Eichenholtz Stage I, has been treated with non–weight bearing and TCC. In healthy subjects, plantar peak pressure was shown to be significantly less, and contact duration greater, under the metatarsal heads and heel during gait in a TCC than in a standard shoe.17 A general belief exists that the healing time for diabetic Charcot fractures may be twice as long as nondiabetic fractures15,18; nevertheless, Boddenberg’s19 literature review found no conclusive evidence to support that assumption.19 His experience with 28 patients with diabetes compared with 17 patients in a control group found a healing rate of 3.5 versus 3 months in the control group.19 Another study noted differences in healing based on the location of pathology at an average of 86 ± 45 days, with hindfoot and midfoot Charcot taking longer than forefoot disease to complete healing.20 Thus, grade B level of recommendation exists that the length of treatment of fractures in patients with Charcot arthropathy is similar or only slightly larger in nondiabetic fractures.
In a study of 55 patients with acute Charcot arthropathy who were treated with serial TCC for an average of 18.5 ± 10.6 weeks, all patients returned to permanent footwear over a period of 28.3 ± 14.5 weeks after casting.21 The authors note that the casting time to return to footwear was independent of anatomic site of involvement.21 However, patients with bilateral involvement required a longer duration of TCC treatment (28 ± 15 weeks) and a longer subsequent period for return to permanent footwear (48 ± 18 weeks; P < 0.02).21 A retrospective review of 237 patients showed successful nonoperative management with non–weight-bearing TTC in 115 (48.5%) patients. The cast was changed weekly and discontinued after dissipation of swelling and warmth.13 Another study involving 147 patients noted that 87 patients (59.2%) were successfully managed conservatively.11 An additional retrospective study of 221 neuropathic fractures or Charcot joints identified 136 that were managed without surgical reconstruction.22
However, compliance with non–weight-bearing status is variable and can be limited by physical or medical conditions, or both.15 Pinzur and colleagues15,23 managed 9 patients treated with TTC who were allowed to bear full weight on their casts. At 5 months, all patients progressed to consolidation without any complications or wound problems. In another case series, the authors used a well-padded bivalved cast (moldable, removable to check skin and padding) in 25 (51%) acute Charcot feet with good success.24 Another study by Conti and coauthors25,26 compared plantar pressure measurements between short leg casts (SLCs) and TTC in 10 healthy subjects. They identified no significant difference between SLC and TTC, although there were marginal decreased hindfoot pressures in TTC.25
In subacute (stage II) midfoot Charcot arthropathy, Myerson and coauthors27 note that TTC was successful in 70% of patients. As the stages evolved, weight bearing was advanced to full weight bearing, and patients were then placed in protective bracing to decrease the risk for flare-up or displacement.15,27
Complications of Casting
Casting is not risk free, and contraindications to total contact casting include deep infection, osteomyelitis, extensive drainage, poor skin quality, severe arterial insufficiency, and poor patient compliance.28 Guyton’s29 retrospective review of 71 patients was performed to evaluate iatrogenic complications of TCCs. The average cast was changed every 7.69 days. Complications occurred in 22 casts (5.52% per cast). These included six new pretibial ulcers, six new midfoot ulcers, four forefoot or toe ulcers, five hindfoot ulcers, and one malleolar ulcer. He notes that all the iatrogenic ulcers, except for one, healed within 3 weeks, and there was no worsening of the original wound or ulcer.29
No studies exist that have compared alternative forms of protective immobilization. It appears that a significant number of methods have been used including well-padded bivalved casts, Unna boots, compression hoses, molded splints, braces, bivalved ankle-foot orthosis (AFO), and SLCs.12,14,15,30–32 Therefore, a grade I recommendation exists for these alternative forms of protective immobilization of Charcot arthropathy.
Surgical Treatment
Indications for surgery in the Charcot foot include acute fracture or dislocation, severe or progressive deformity, and recurrent ulceration, secondary to either instability or bony prominence.22,31, 33 Surgical procedures include debridement, exostectomy of bony prominences, osteotomies with realignment, and fusion. In a Level IV series by Myerson and coauthors,27 a cohort of 41 feet with chronic Charcot, stage III, underwent total contact casting initially. Subsequent surgery in this group included arthrodesis in nine (22%) feet, exostectomy in six (14%) feet, drainage of an abscess in two (5%) feet, and amputation in two (5%) feet.27 In stage II midfoot neuroarthropathy, 3 (10%) of 30 feet were treated surgically (two arthrodeses and one exostectomy) after reaching the chronic stage, stage III.27 In another level IV study, 29 (17%) of 168 patients with Charcot arthropathy were treated surgically in either stages II or III.34 Saltzman and colleagues35 (Level IV) performed 53 procedures on 115 patients (49%), excluding amputations. Another group of 122 of 237 patients (51.5%) required surgical intervention that included bony stabilization, debridement, and partial limb amputations.13 Another group had a surgical rate of 40.8%.11
Exostectomies.
Exostectomies are indicated for stable Charcot deformities and are supported by Level IV studies, in which there are bony prominences that are promoting recurrent ulceration or hindering healing of a chronic ulcer.4,11, 36, 37 A retrospective review (Level IV) of 20 patients who underwent 27 ostectomy procedures evaluated medial and lateral column ulcerations.38 There were 18 medial and 9 lateral ulcers. After surgery, 20 wounds had resolved, resulting in a 74% healing rate. The majority of failed procedures involved lateral column wounds (six of seven). Revision surgery was required in five of the nine lateral column wounds for limb salvage. The authors warn that ostectomy for ulcers that involve the lateral column was less predictable and may require further surgery.38 Therefore, a grade C recommendation exists for exostectomy for stable Charcot arthropathy in which there are bony prominences that are promoting recurrent ulceration or hindering healing of a chronic ulcer.
Internal Fixation.
Traditionally, elective operative fusion of Charcot joints had been performed in Eichenholtz stage III or after. This protocol is followed to avoid the poorer bone quality and increased technical difficulties associated with stages I and II.15,33 Currently, no studies in the literature support that theory, however, and surgery may be indicated in stage I disease if there is gross instability, dislocation, or deformity.7 Simon and coworkers39 published the results of 14 of 43 patients with stage I disease who underwent acute extensive debridement, open reduction, and internal fixation of the tarsal-metatarsal region with autologous bone graft. They note successful union in all patients. The mean time to assisted weight bearing was 10 ± 3.3 weeks, the mean time to unassisted weight bearing was 15 ± 8.8 weeks, and the mean time to return to the use of regular shoes was 27 ± 14.4 weeks.39
Associated procedures may include Achilles tendon lengthening21,22, 33, 40 if there is associated equines contracture, bone grafting with internal or external fixation40 for defects, or increased risk for nonunion.22
Methods of Fixation.
Despite many case series recommending open reduction and internal fixation.11,12, 33, 34 No studies have looked at comparing different modalities of fixations. A variety of midfoot arthrodesis techniques has been described. Despite Charcot arthropathy being a major cause of multiple midfoot joint arthroses, most of these studies did not focus solely on the disease. Internal fixation techniques included staples, Steinmann pins, tension band wires, screws, dowels, and medial or plantar plates.41–46 A biomechanical study comparing multiscrew fixation with a plate applied to the plantar (tension) side of the medial midfoot demonstrated that the latter was stronger.47
Traditionally, fixation plates have been placed medial and plantar, areas thought to be of greatest stress.29,34, 35, 39 However, a retrospective review of nine patients undergoing fusion through a dorsally placed plate (four were secondary to Charcot arthropathy) had a 95% success rate.45 Another retrospective review of 10 patients treated with open reduction and internal fixation with iliac crest bone grafting resulted in 90% union rate.48 This included five osseous and four fibrous unions.48
For hindfoot fixation, plantar or medial plates, and screws have been utilized. A biomechanical study assessing the calcaneocuboid (CC) screw placement found that a screw placed axially from posterior to anterior starting at the calcaneal tuberosity and crossing the CC joint perpendicularly was superior to an obliquely placed screw.49
Caravaggi and researchers50 evaluated ankle arthrodesis with a compressive intramedullary nail in 14 patients. Patients had severe ankle instability with previous recommendation of undergoing below-knee amputation (BKA). After a mean follow-up of 18 months, 10 patients (71.4%) achieved a solid arthrodesis, 3 patients (21.4%) experienced development of fibrous union, after hardware failure, and 1 patient went on to a BKA.50 Another study by Pinzur and colleagues51 evaluated 21 attempted ankle fusion with a retrograde nail, of which 11 limbs had the talus absent or required complete talectomy for realignment. A significantly increased complication rate was present in this subgroup of patients compared with the group with preserved talus.51
External fixation may be used as an alternative form of fixation and deformity correction. This may be especially useful in patients with extensive open wounds and recurrent or persistent ulcerations.34 A retrospective review reported on 11 patients with significant ulcerations who underwent wound debridement, ostectomy, corrective osteotomies, and external fixation. Average time of fixator placement was 57 days, after which all patients were placed in TCC for an average of 131 days. All patients progressed to full healing, with four osseous and five fibrous unions.52 Another report by Pinzur53 advocates the use of ring external fixators for patients with high risks for wound complications for internal fixation. Based on the wide variety of surgical techniques and method of fixation, the absence of studies comparing different modality of fixation leads to a grade I recommendation.
Complications of Arthrodesis with Internal Fixation
In a series of 29 operative patients, 10 (34%) patients experienced development of pseudarthroses.34 Other complications in 19 of the 29 patients included malunion, superficial wound infection, early or late fatigue failure of screw fixation, wound slough, skin irritation and ulceration, distal tibia fracture, osteomyelitis, and BKA. However, 7 (70%) of the pseudarthroses were stable, and limb salvage was achieved in 27 (93%) patients.34 Papa and coauthors34 report a complication rate of 65% in their study of 29 patients. These included nine pseudarthroses (six tibiocalcaneal, one tibiotalar, and two talonavicular). In another series, the complication rate was 53%.54
COMPLICATIONS OF CHARCOT ARTHROPATHY
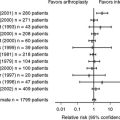
Saltzman and colleagues’35 retrospective review of 115 patients demonstrated an approximately 2.7% annual rate of amputation, a 23% risk for requiring bracing for more than 18 months, and a 49% risk for recurrent ulceration. Limbs with open ulcers at initial presentation or chronically recurrent ulcers had increased risk for amputation.35 Pinzur13 reports an overall lower extremity amputation rate of 9% (of 237 patients) during a 10-year period.
In a meta-analysis of 15 studies, the death rate was 4% (11 deaths in 301 patients) within 2.5 years after treatment for Charcot arthropathy.55 Partial or complete foot amputation had been done in 20 (7%) of 301 patients in the follow-up period. A total of 83 (28%) of 301 patients required an AFO, permanent bracing, or assistive devices.55
PREVENTION
Charcot arthropathy may occur after acute trauma in the patient with neuropathy.37 Therefore, it is prudent to treat this population group with extra caution including immobilization and protective bracing after injury. In patients with diabetes with decreased sensation, preventive patient education may include frequent inspection, extra protection against traumatic events, and low tolerance in seeking medical evaluation.37 A single-blinded, randomized, clinical trial study was conducted to evaluate the effectiveness of at-home infrared temperature monitoring for preventing the development of ulceration and wound complications in patients with diabetes at high risk.56 The authors divided 85 patients into 2 groups, with the first group undergoing standard foot care and evaluation, and the second group adding home infrared thermometer to monitor skin temperature, and reducing their activity with any detectable increase in temperature. The authors used a temperature differential of 4 degrees between corresponding sites of opposite feet to trigger the subsequent decreased activity level and to contact a healthcare worker. They note that patients in the standard management group were 10.3 times more likely to experience development of foot ulcerations than the group monitoring skin temperature changes and adjusting their activity level accordingly. Two cases of Charcot arthropathy were observed in the standard treatment group, and none was observed in the second group. Their recommendation was to use home infrared thermometry as an adjunct tool for diabetic foot care and monitoring.56
Although this one Level I study possibly supports a grade A recommendation, this is only one study, and this diagnostic modality needs to be substantiated in further studies. Table 74-1 provides a summary of recommendations for the treatment of Charcot foot and ankle.
| RECOMMENDATIONS | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
1 Smith DG, Barnes BC, Sands AK, et al. Prevalence of radiographic foot abnormalities in patients with diabetes. Foot Ankle Int. 1997;18:342-346.
2 Fabrin J, Larsen K, Holstein PE. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000;23:796-800.
3 Cavanagh PR, Young MJ, Adams JE, et al. Radiographic abnormalities in the feet of patients with diabetic neuropathy. Diabetes Care. 1994;17:201-209.
4 Brodsky JW. Charcot’s joints. In: Mann RA, Coughlin MJ, editors. Surgery of the Foot and Ankle. 8th ed. St. Louis: Mosby; 2006:1281-1368.
5 Baumhauer JF, O’Keefe RJ, Schon LC, et al. Cytokine-induced osteoclastic bone resorption in Charcot arthropathy: An immunohistochemical study. Foot Ankle Int. 2006;27:797-800.
6 Eichenholtz SN. Charcot Joints. Springfield, IL: Charles C Thomas, 1966.
7 Schon LC, Marks RM. The management of neuroarthropathic fracture-dislocations in the diabetic patient. Orthop Clin North Am. 1995;26:375-392.
8 Brodsky JW. The diabetic foot. In: Mann RA, Coughlin MJ, editors. Surgery of the Foot and Ankle. St. Louis: Mosby-Year Book; 1993:877-958.
9 Schon LC, Weinfeld SB, Horton GA, et al. Radiographic and clinical classification of acquired midtarsus deformities. Foot Ankle Int. 1998;19:394-404.
10 Schon LC, Easley ME, Cohen I, et al. The acquired midtarsus deformity classification system—interobserver reliability and intraobserver reproducibility. Foot Ankle Int. 2002;23:30-36.
11 Pinzur M. Surgical versus accommodative treatment for Charcot arthropathy of the midfoot. Foot Ankle Int. 2004;25:545-549.
12 Pinzur MS, Sage R, Stuck R, et al. Treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle Int. 1993;14:189-197.
13 Pinzur MS. Benchmark analysis of diabetic patients with neuropathic (Charcot) foot deformity. Foot Ankle Int. 1999;20:564-567.
14 Pinzur MS, Shields N, Trepman E, et al. Current practice patterns in the treatment of Charcot foot. Foot Ankle Int. 2000;21:916-920.
15 Trepman E, Nihal A, Pinzur MS. Current topics review: Charcot neuroarthropathy of the foot and ankle. Foot Ankle Int. 2005;26:46-63.
16 Jude EB, Selby PL, Burgess J, et al. Bisphosphonates in the treatment of Charcot neuroarthropathy: A double-blind randomised controlled trial. Diabetologia. 2001;44:2032-2037.
17 Baumhauer JF, Wervey R, McWilliams J, et al. A comparison study of plantar foot pressure in a standardized shoe, total contact cast, and prefabricated pneumatic walking brace. Foot Ankle Int. 1997;18:26-33.
18 Schwarz RJ, Macdonald MRC, Van der Pas M. Results of arthrodesis in neuropathic feet. J Bone Joint Surg Br. 2006;88:747-750.
19 Boddenberg U. Healing time of foot and ankle fractures in patients with diabetes mellitus: Literature review and report on own cases. Zentralbl Chir. 2004;129:453-459.
20 Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: Healing times by foot location. J Diabetes Complications. 1998;12:287-293.
21 Armstrong DG, Todd WF, Lavery LA, et al. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. Diabet Med. 1997;14:357-363.
22 Schon LC, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop. 1998;349:116-131.
23 Pinzur MS, Lio T, Posner M. Treatment of Eichenholtz stage I Charcot foot arthropathy with a weightbearing total contact cast. Foot Ankle Int. 2006;27:324-329.
24 Pinzur MS, Sage R, Stuck R, et al. A treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle. 1993;14:189-197.
25 Conti SF, Martin RL, Chaytor ER, et al. Plantar pressure measurements during ambulation in weightbearing conventional short leg casts and total contact casts. Foot Ankle Int. 1996;17:464-469.
26 Martin RL, Conti SF. Plantar pressure analysis of diabetic rockerbottom deformity in total contact casts. Foot Ankle Int. 1996;17:470-472.
27 Myerson MS, Henderson MR, Saxby T, et al. Management of midfoot diabetic neuroarthropathy. Foot Ankle Int. 1994;15:233-241.
28 Conti SF. Total contact casting. AAOS Instr Course Lect. 1999;48:305-315.
29 Guyton GP. An analysis of iatrogenic complications from the total contact cast. Foot Ankle Int. 2005;26:903-907.
30 Harrelson JM. The diabetic foot: Charcot arthropathy. Instr Course Lect. 1993;42:141-146.
31 Lesko P, Maurer RC. Talonavicular dislocations and midfoot arthropathy in neuropathic diabetic feet. Clin Orthop. 1989;240:226-231.
32 Boninger ML, Leonard JA. Use of bivalved ankle-foot orthosis in neuropathic foot and ankle lesions. J Rehabil Res Dev. 1996;33:16-22.
33 Johnson JE. Surgical treatment for neuropathic arthropathy of the foot and ankle. Instr Course Lect. 1999;48:269-277.
34 Papa J, Myerson M, Girard P. Salvage, with arthrodesis, in intractable diabetic neuropathic arthropathy of the foot and ankle. J Bone Joint Surg Am. 1993;75-A:1056-1066.
35 Saltzman CL, Hagy ML, Zimmerman B, et al. How effective is intensive nonoperative initial treatment of patients with diabetes and Charcot arthropathy of the feet? Clin Orthop Relat Res. 2005;435:185-190.
36 Bono JV, Roger DJ, Jacobs RL. Surgical arthrodesis of the neuropathic foot. A salvage procedure. Clin Orthop Relat Res. 1993:14-20.
37 Johnson JE. Operative treatment of neuropathic arthropathy of the foot and ankle. J Bone Joint Surg. 1998;80-A:1700-1709.
38 Catanzariti AR, Mendicino R, Haverstock B. Ostectomy for diabetic neuroarthropathy involving the midfoot. J Foot Ankle Surg. 2000;39:291-300.
39 Simon SR, Tejwani SG, Wilson DL, et al. Arthrodesis as an early alternative to nonoperative management of Charcot arthropathy of the diabetic foot. J Bone Joint Surg Am. 2000;82-A:939-950.
40 Deresh GM, Cohen M. Reconstruction of the diabetic Charcot foot incorporating bone grafts. J Foot Ankle Surg. 1996;35:474-488.
41 Horton GA, Olney BW. Deformity correction and arthrodesis of the midfoot with a medial plate. Foot Ankle Int. 1993;14:493-499.
42 Johnson JE, Johnson KA. Dowel arthrodesis for degenerative arthritis of the tarsometatarsal (Lisfranc) joints. Foot Ankle Int. 1986;6:243-253.
43 Kupcha PC, Fitzpatrick MJ. Application of the tension band technique for arthrodesis of the forefoot and midfoot. Foot Ankle Int. 1996;12:784.
44 Treadwell JR, Kahn MD. Lisfranc arthrodesis for chronic pain: A cannulated screw technique. J Foot Ankle Surg. 1998;37:28-36.
45 Suh J-S, Amendola A, Lee K-B, et al. Dorsal modified calcaneal plate for extensive midfoot arthrodesis. Foot Ankle Int. 2005;26:503-509.
46 Sticha RS, Frascone ST, Wertheimer SJ. Major arthrodeses in patients with neuropathic arthropathy. J Foot Ankle Surg. 1996;35:560-566.
47 Marks RM, Parks B, Schon LC. Midfoot fusion technique for neuropathic feet: Biomechanical analysis and rationale. Foot Ankle Int. 1998;19:507-510.
48 Craig Stone N, Daniels TR. Midfoot and hindfoot arthrodeses in diabetic Charcot arthropathy. Can J Surg. 2000;43:449-455.
49 Kann JN, Parks BG, Schon LC. Biomechanical evaluation of two different screw positions for fusion of the calcaneocuboid joint. Foot Ankle Int. 1999;20:33-36.
50 Caravaggi C, Cimmino M, Caruso S, et al. Intramedullary compressive nail fixation for the treatment of severe Charcot deformity of the ankle and rear foot. J Foot Ankle Surg. 2006;45:20-24.
51 Pinzur MS, Kelikian A. Charcot ankle fusion with a retrograde locked intramedullary nail. Foot Ankle Int. 1997;18:699-704.
52 Farber DC, Juliano PJ, Cavanagh PR, et al. Single stage correction with external fixation of the ulcerated foot in individuals with Charcot neuroarthropathy. Foot Ankle Int. 2002;23:130-134.
53 Pinzur MS. The role of ring external fixation in Charcot foot arthropathy. Foot Ankle Clin. 2006;11:837-847.
54 Early JS, Hansen ST. Surgical reconstruction of the diabetic foot: A salvage approach for midfoot collapse. Foot Ankle Int. 1996;17:325-330.
55 Sinacore DR, Withrington NC. Recognition and management of acute neuropathic (Charcot) arthropathies of the foot and ankle. J Orthop Sports Phys Ther. 1999;29:736-746.
56 Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to prevent ulceration. Diabetes Care. 2004;27:2642-2647.