Chapter 19 What Is the Best Surgical Treatment for Cuff Tear Arthropathy?
HISTORICAL OVERVIEW
Cuff tear arthropathy (CTA) is the term Neer1,2 originally proposed to describe a form of shoulder arthritis in association with long-standing rotator cuff deficiency. It is distinct clinically and radiographically from other forms of shoulder arthropathy in which joint destruction coexists with cuff deficiency such as rheumatoid arthritis or osteoarthritis with traumatically acquired cuff tear. The causative factor was first attributed to a crystal-mediated synovitis (the Milwaukee shoulder),3,4 and it is still recognized that there is a correlation between large rotator cuff tears and high levels of calcium phosphate crystals in synovial fluid.5 It is now generally agreed that this unique pattern of joint destruction occurs because of, not simply in association with, rotator cuff deficiency. Mechanical and nutritional factors associated with progressive upward humeral head migration from loss of cuff integrity are its causes. This combination of pain, glenohumeral joint destruction, and a nonfunctioning rotator cuff present treatment challenges that stand apart from other forms of shoulder arthropathy.
PATHOMECHANICS
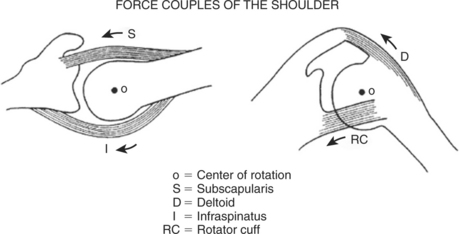
An understanding of shoulder biomechanics is necessary to appreciate surgical treatment options. The fact that shoulder muscles function as force couples in the coronal plane was first appreciated by Inman and colleagues6 and expanded subsequently by Burkhart,7 who described a transverse force couple as well. Applying this theory to explain why full shoulder elevation is still possible in some shoulders with massive cuff tears and not in others, Burkhart8 identified three groups of fulcrum kinematics. Despite major cuff tearing, transverse and coronal force couples may remain intact and the fulcrum is stable; the humeral head is contained within the glenoid socket, and full elevation is possible. When force couples are destroyed by cuff tearing, the humeral head rises out of the socket on attempted arm elevation; that is, the fulcrum is unstable, and so-called pseudoparalysis of elevation results. In some cases, the humeral head may restabilize in a superiorly subluxated position under an intact coracoacromial arch, the so-called captured fulcrum, again allowing arm elevation (Fig. 19-1).
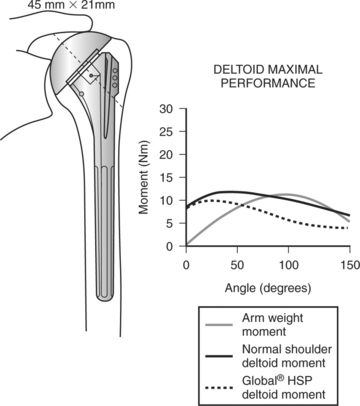
The dynamic action of muscle forces including those of the rotator cuff, together with other capsuloligamentous restraints, stabilize the humeral head through the concavity compression mechanism. As explained by Matsen and coauthors9 and in the biomechanical analysis of De Wilde and coworkers,10 a loss of any of the normal osseous, capsuloligamentous, or muscular constraints leads to glenohumeral instability. Superior instability results from loss of the compression of the rotator cuff and spacer effect of the normal supraspinatus. Upward displacement of the humerus slackens the deltoid, making it less effective in humeral elevation and weakness, or even pseudoparalysis is the result. The coracoacromial arch is then the only remaining barrier to further upward migration. When this barrier has been compromised by frictional wear or aggressive surgical acromioplasty, anterosuperior escape of the humeral head occurs, further compounding pseudoparalysis.
CLINICAL PRESENTATION
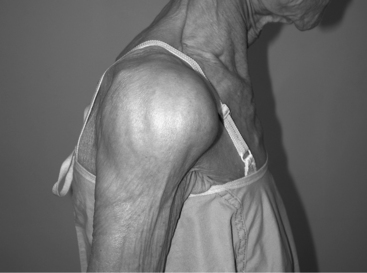
CTA is the end stage of a continuum which begins with a simple cuff tear. The incidence of CTA is estimated to be anywhere from 4% to 20% of cuff tears.11,12 Patients with CTA typically present with pain and weakness of the shoulder and glenohumeral joint destruction on radiographs. Jensen and investigators13 collected clinical data on 104 cases from 10 different published reports and found the majority were elderly women (average age, 77.5 years), and almost 50% had bilateral involvement. Neer and researchers2 describe the findings in 26 cases averaging 69 years of which 75% were women. No history of trauma was reported in 75%. The shoulders typically were swollen (Fig. 19-2), muscles were atrophic, and the long head of biceps ruptured. Passive motion was limited to an average of 90-degree elevation and 20-degree external rotation. Only 2 of 26 were able to actively elevate greater than 90 degrees. Positive lag signs for cuff deficiency14 are frequently seen. These include the lift-off test for subscapularis,15,16 the external rotation lag sign for supraspinatus and infraspinatus, and the drop sign for infraspinatus. In the presence of a normal deltoid, the drop-arm sign is indicative of a massive posterosuperior cuff tear with loss of the ability to create a stable fulcrum. The term pseudoparalysis17 is also used when active shoulder elevation is less than 90 degrees in the presence of free anterior elevation with an intact deltoid.
RADIOGRAPHIC FEATURES
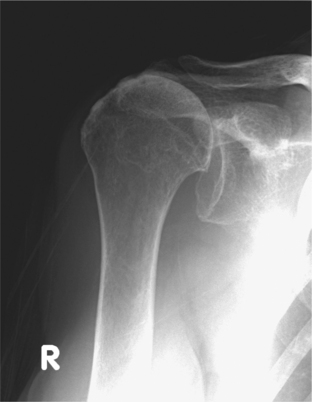
Radiographic indicators of major cuff deficiency such as acromiohumeral narrowing and superior migration of the humeral head represent the earliest phases in a continuum that eventually leads to the joint destruction characteristic of true CTA.18 Chronic wear of the superiorly displaced humeral head sculpts the undersurface of the coracoacromial arch and glenoid, termed acetabularization. Rounding off of the tuberosities leads to femoralization of the humerus (Fig. 19-3). In advanced cases, there may be collapse of the humeral head.2
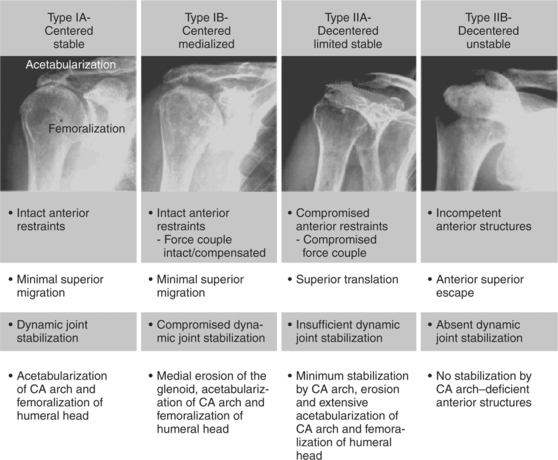
Seebauer19 has proposed a classification of CTA (Fig. 19-4) that combines radiographic features with an appreciation of the altered fulcrum mechanics that Burkhart8 described. The four distinct groups are distinguished by the degree of superior migration from the center of rotation and the amount of instability at the center of rotation. In the most advanced category, IIB, the humeral head has escaped superiorly, removing any remaining fulcrum stability.
TREATMENT
Quantifying Treatment Results in Cuff Tear Arthropathy
Many grading systems have become popular for clinical assessment of shoulder outcomes. Neer and researchers20 recognized the need for a “limited goals” category in the case of patients with poorly functioning cuff musculature. This acknowledges that a result can still be considered successful if there is good pain relief and “useful” shoulder function even if the rigid criteria of success in those with intact cuff muscles are not met. Here, a result is considered successful if no significant pain is felt, the patient is pleased with the procedure, the patient remains independent for activities of daily living, and the patient has at least 90 degrees of arm elevation and 20-degree external rotation.
Surgical Management
Arthroscopic Debridement.
Arthroscopic irrigation to remove activated enzymes and crystals offers only limited short-term relief21 (Level IV). Any surgical violation of the coracoacromial arch, if still intact, is to be vigorously discouraged for fear of further destabilizing the humeral head.13
Arthrodesis.
Glenohumeral arthrodesis has the potential to stabilize the glenohumeral joint and relieve pain but at the cost of reduced motion and function. However, most patients with CTA are unsuitable for arthrodesis because they are older, intolerant of prolonged immobilization, and frequently have bilateral disease.2 Furthermore, osteoporosis in these typically elderly women presents problems with internal fixation. Arthrodesis is best reserved for younger high-demand patients disabled by an irreparable cuff tear, often with a nonfunctioning deltoid, and who require a strong stable shoulder girdle22 (Level V). It may also have a place in other instances of massive irreparable cuff tear with deltoid dysfunction.
Unconstrained Shoulder Replacement.
Shoulder prostheses with no built-in constraint rely on an intact and functioning rotator cuff to stabilize the humeral head within the socket. Although it is agreed that small cuff tears, regardless of whether they are repaired, have no effect on the outcome of total shoulder arthroplasty23 (Level III); the same is not true for cases of major cuff deficiency. Franklin and coauthors24 report a direct association between superior migration of the humeral component and a deficient rotator cuff (Level IV). They called the eccentric superior loading of the glenoid component the “rocking horse” phenomenon that leads to loosening and superior tilt. Therefore, unconstrained prostheses are not recommended for cuff tear arthroplasty.
Semiconstrained Shoulder Replacement.
Semiconstrained glenoid components have also been used to give resistance to ascent of the humeral head in cuff deficient shoulders. Neer and researchers2 experimented with 200% and 600% glenoid components for CTA in 11 cases followed for an average of 30 months, but they soon abandoned such designs because of the greater risk for failure and the difficulty of closing residual cuff defects25 (Level IV). Amstutz and colleagues26 and Gristina and investigators27 have described the use of hooded glenoid components for use with nonconstrained systems but reported only short-term results (Level IV). It has been shown by finite element analysis28 that the superior constraints of the component intending to prevent humeral head ascent increase stresses and may cause earlier loosening than with unconstrained designs.
Bipolar Shoulder Replacement.
Bipolar hemiarthroplasty was introduced by Swanson and coworkers29 for the treatment of advanced glenohumeral arthritis associated with superior migration of the humeral head and loss of rotator cuff function. It uses a larger diameter mobile shell around the head in an attempt to gain several advantages: increased mobility because of the lateralization of the center of rotation and the increase in the muscle lever arm; increased stability of the prosthetic shoulder; and reduced glenoid and acromial wear in contact with the prosthetic head.30 Few published31–33 and unpublished studies34 exist that detail results but in general these indicate that even if pain relief is achieved, function remains low after the procedure (Level IV). Noting the better results reported with simple hemiarthroplasty particularly about mobility, Duranthon and investigators33 conclude that the deltoid lever arm was being increased at the expense of overstretching capsular and residual cuff tissues, and that it was better to use a head size closer to normal anatomy. In addition to this overstuffing effect, concerns have been raised regarding rupture of the subscapularis tendon because of the vertical orientation of the component and the potential for excessive polyethylene wear.35
Hemiarthroplasty.
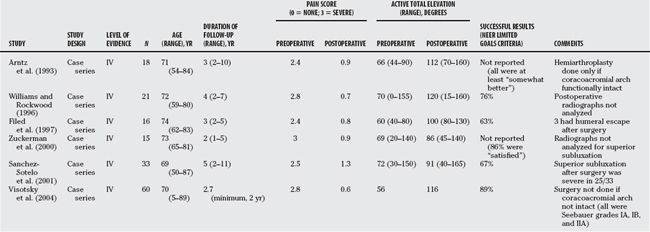
The difficulties encountered with prosthetic designs that attempt to control the altered biomechanics of major cuff deficiency have led many to return to simple nonconstrained hemireplacement as the preferred procedure. Table 19-1 summarizes the published results. In the studies by Arntz and colleagues and Visotsky and coworkers,19 close attention was paid to the issue of superior humeral escape (grade IIB in Seebauer’s classification), and hemiarthroplasty was avoided in those cases. In Sanchez-Sotelo and coauthors’51 study, postoperative anterosuperior instability occurred in seven of the eight cases that had had prior acromioplasty and resection of the coracoacromial arch. Although these studies show that symptoms can be improved by hemiarthroplasty, it is clear that these results fall far short of what can be achieved by replacement arthroplasty in other diagnoses, for example, total shoulder replacement for osteoarthritis, for both pain relief and arm elevation. The biomechanical computer analysis of De Wilde and coworkers10 shows why simple hemiarthroplasty cannot be expected to solve the problem of weak arm elevation if the rotator cuff is not working and the humeral head is superiorly subluxated (Seebauer grade IIA), even if humeral escape had not yet occurred (Fig. 19-5). Simple hemiarthroplasty in these cases does not restore normal biomechanics, though it may relieve some pain.
Constrained Shoulder Replacement.
Prosthetic arthroplasty with a constrained prosthesis has been used to treat all forms of shoulder arthropathy including cuff-deficient shoulders with arthritis. The fixed fulcrum mechanics of the Stanmore and Michael Reese prostheses used for many types of glenohumeral arthritis would appear to compensate for absence of functioning cuff but have resulted in high rates of mechanical failure and loosening of the glenoid component.36–39 Table 19-2 summarizes the published results. Neer40 abandoned his work in fixed fulcrum designs as early as 1974, concluding that the poor quality of scapular bone precludes adequate fixation of the glenoid component in a constrained prosthesis (Level IV).
Reverse Ball and Socket Replacement.
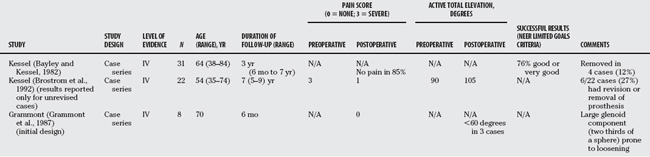
A number of prostheses were introduced based on a reverse ball and socket concept to address the disappointing results reported earlier. Most remained essentially experimental. The Kessel (Fig. 19-6) and the early Grammont prosthesis featured a center of rotation well lateral to the glenoid surface, resulting in excessive torque or shear forces on the glenoid component and early loosening (Table 19-3). Recognizing this weakness, Grammont modified his original design with two important innovations (Fig. 19-7): The glenoid component diameter was enlarged, but the size of the sphere was reduced from two-thirds to half, thereby placing the center of rotation at the glenoid surface, and thus decreasing shear forces; the large diameter allows for a greater arc of motion before impingement occurs. The humeral component has a small cup almost vertically oriented that covers less than half of the glenosphere, effectively lengthening or tensioning the deltoid (Fig. 19-8). This modified design offers several important biomechanical advantages41 (Table 19-4). Although restoration of active elevation more than 90 degrees is achieved, active external rotation is often limited, particularly when the teres minor is not functioning. Internal rotation is also rarely restored because of the design limitations of the prosthesis.
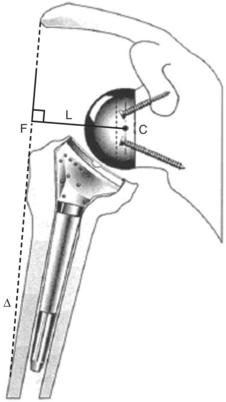
FIGURE 19-8 Diagram of a Grammont reverse prosthesis. The center of rotation (C) lies on the surface of the glenoid, protecting it from excessive shearing forces, and the humerus is lowered. L, lever arc of the force vector (F) and the deltoid (Δ).23
TABLE 19-4 Biomechanical Advantage of Reverse Ball and Socket Prosthesis
A feature unique to this prosthesis is bone loss believed to be caused by abutment of the adducted humeral prosthesis against the scapular neck. Termed scapular notching, it is seen in more than 50% of cases.41 This contact is a direct consequence of the absence of a prosthetic neck to the glenosphere and the horizontal orientation of the humeral cup, which are the biomechanical solutions to avoid excessive forces on the glenoid component and improve the power of the deltoid. The degree of notching can be classified42 as grade 1 if confined to the scapular pillar, grade 2 when it is in contact with the lower screw, grade 3 when it is over the lower screw, and grade 4 when it extends into the baseplate (Fig. 19-9). In a study using cadaveric shoulders, Nyffeler and investigators43 showed that placing the glenoid component 2 to 4 mm lower than originally recommended significantly improves adduction and may reduce the risk for notching. Simovitch and colleagues44 were able predict scapular notching by radiographic measurement of glenosphere placement and scapular neck angle. They correlated notching with significantly poorer outcomes in terms of Constant score, strength, and active flexion. Other causes for concern are polyethylene wear, prosthetic instability related to improper tensioning, and component disassociation.23
Clinical experience with the reverse ball and socket in the treatment of CTA has been impressive, with most patients achieving excellent pain relief and regaining elevation above horizontal. Some of the more recent reports are presented in Table 19-5. Sirveaux and researchers’42 multicenter study does not specify inclusion criteria, so presumably the procedure was offered to all comers with painful CTA and none was offered hemiarthroplasty because of being younger, having a stabilized humeral head, or still being capable of active elevation of more than 90 degrees. The reoperation rate was 5.0% (glenoid loosening, infection, and component disassociation). Survivorship in this series (based on reoperation for component failure or significant pain) was 88% at 5 years and 29% at 7 years, which is a high rate of failure. Those with an intact teres minor had better Constant scores. Scapular notching was noted in 64% of cases. In cases with more advanced notching, the Constant score was also adversely affected.
TABLE 19-5 Published Series for Reverse Ball and Socket Prostheses: Latest Design (Cuff Tear Arthropathy)
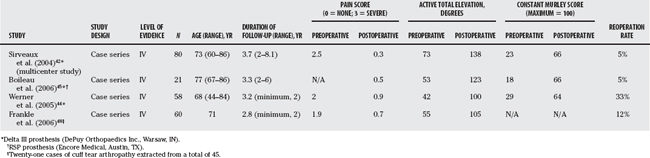
Boileau45 presented the results of 45 Grammont prostheses, 21 of which were for CTA. In the whole group, the reoperation rate was 22% and the incidence rate of major complications was 31%. Results for CTA were better and were met with fewer complications. Again, the importance of an intact teres minor was stressed. Scapular notching was seen in 68% of cases.
Werner and coauthors46 reviewed the results of 58 patients using the Grammont prosthesis. All had severe pain and pseudoparalysis (active elevation >,90 degrees). In all cases, the rotator cuff was considered irreparable (chronic pseudoparalysis, acromiohumeral interval >,7 mm, and fatty infiltration of the surpaspinatus and infraspinatus at least Goutallier47 grade 2). Substantial pain relief and improved function were achieved in all cases. Improvement was similar in all groups. Complications (including haematomas) occurred in 50% of cases but affected the outcome in only 10%. Component loosening or disassociation occurred in 9% of cases. Reoperation occurred in 33% overall but was 38% when there was a prior prosthesis and 40% when there was a prior nonprosthetic operation.
Frankle and colleagues48 used a reverse ball and socket prosthesis of their own design (Reverse Shoulder Prosthesis; Encore Medical, Austin, TX) to treat 60 cases of CTA with good results. In their brief report, they note a 17% complication rate and a reoperation rate of 12%. They did not mention scapular notching possibly because the center of rotation is more lateralized.
Guery and researchers49 studied survivorship of the Grammont prosthesis (Delta III) in 80 cases mostly for CTA. Using the end points of prosthetic revision and loosening, the survival rates were 91% and 84%, respectively, at 120 months for CTA, which they found significantly better than for other diagnoses. However, the survival rate was only 58% at 120 months using the end point of a Constant score less than 30.
In summary, several authors9,49, 50 have expressed caution regarding the use of a reverse ball and socket prosthesis in regard to complications and long-term survivorship. Currently, it should be reserved for the treatment of disabling CTA and exclusively in patients older than 70 years with low functional demands.
To begin with, it must be recognized that most of the available evidence on which to base treatment decisions is only Level IV. With this in mind, however, rational and reasonable choices can still be made. The selection of the best surgical treatment for CTA begins with a thorough clinical assessment including history, physical, and imaging (plain radiographs and magnetic resonance imaging [MRI] in most cases). Not all cases of advanced cuff disease will have arthritis because the disease represents a continuum. Thorough assessment will establish the diagnosis and rule out other causes of joint destruction, particularly infection. The patient’s fitness, age, and functional expectations (high or low demand) must be determined. Details of previous surgery are important. Physical examination should categorize the status of shoulder muscle force couples (active total elevation above or below 90 degrees). Up-to-date radiographs should be examined for the presence of acromiohumeral narrowing, superior humeral subluxation, and superior humeral head escape (deficient coracoacromial arch). An attempt to categorize the status of superior stability (stable or unstable fulcrum) should be made by assessing for pseudoparalysis and from inspection of plain radiographs (see Fig. 19-4). An MRI scan will reveal residual intact cuff, especially inferior subscapularis (which should be carefully preserved in any surgery) and teres minor (which will determine external rotation after surgery). The appropriate care can be selected with this information. The stage of the cuff disease may then be determined (Table 19-6).
| History |
ADL, activities of daily living; CA, coracoacromial.
In all cases of prosthetic replacement, patients must be followed at regular intervals afterward. With humeral head replacement, continued superior migration of the prosthesis has been observed.51 With reverse shoulder replacement, there are serious concerns regarding late complications such as loosening and component disassociation. Table 19-7 provides a summary of recommendations for the surgical treatment of rotator cuff disease.
TABLE 19-7 Summary of Recommendations
| STAGE | RECOMMENDATION | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|---|
| Reparable rotator cuff tear without arthropathy | Repair torn cuff | C |
| Irreparable cuff without arthropathy | Tendon transfer | C |
| Cuff tear arthrop-athy with stable fulcrum | Humeral head replacement | C |
| Cuff tear arthrop-athy with unstable fulcrum elderly, low demand | Reverse shoulder prosthesis | C |
| Cuff tear arthrop-athy with unstable fulcrum young, high demand | Glenohumeral arthrodesis | C |
1 Neer CSI, Cruess RL, Sledge CB, Wilde AH. Total glenohumeral replacement. A preliminary report. Orthop Trans. 1977;1:244-245.
2 Neer CS, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232-1244.
3 Garancis JC, Cheung HS, Halverson PB, McCarty DJ. “Milwaukee shoulder”—association of microspheroids containing hydroxyapatite crystals, active collagenase, ad neutral protease with rotator cuff defects. III. Morphologic and biochemical studies of an excised synovium showing chondromatosis. Arthritis Rheum. 1981;24:484-491.
4 McCarty DJ. Milwaukee shoulder syndrome. X. Trans Am Clin Climatol Assoc. 1990;102:271-283.
5 Antoniou J, Tsai A, Baker D, et al. Milwaukee shoulder: Correlating possible etiologic variables. Clin Orthop Relat Res.; 407; 2003; 79-85.
6 Inman VT, Saunders CM, Abbott LC. Observations on the function of the shoulder joint. J Bone Joint Surg. 1944;26:1-30.
7 Burkhart SS. Arthroscopic treatment of massive rotator cuff tears. Clinical results and biomechanical rationale. Clin Orthop. 1991;267:45-56.
8 Burkhart SS. Fluoroscopic comparison of kinematic patterns in massive rotator cuff tears. A suspension bridge model. Clin Orthop. 1992;284:144-152.
9 Matsen FAIII, Boileau P, Walch G, et al. The reverse total shoulder arthroplasty. J Bone Joint Surg Am. 2007;89:660-667.
10 De Wilde LF, Audenaert EA, Berghs BM. Shoulder prostheses treating cuff tear arthropathy: A comparative biomechanical study. J Orthop Res. 2004;22:1222-1230.
11 Neer CS, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319-337.
12 Worland RL, Jessup DE, Arredondo J, Warburton KJ. Bipolar shoulder arthroplasty for rotator cuff arthropathy. J Shoulder Elbow Surg. 1997;6:512-515.
13 Jensen KL, Williams GR, Russell IJ, Rockwood CA. Current concepts review—rotator cuff tear arthropathy. J Bone Joint Surg Am. 1999;81:1312-1324.
14 Hertel R, Ballmer FT, Lombert SM, Gerber C. Lag signs in the diagnosis of rotator cuff rupture. J Shoulder Elbow Surg. 1996;5:307-313.
15 Gerber C, Krushell R. Isolated ruptured of the tendon if the subscapularis muscle clinical features in 16 cases. J Bone Joint Surg Br. 1991;73:389-394.
16 Gerber C, Hersche O, Farron A. Isolated rupture of the subscapularis tendon. J Bone Joint Surg Am. 1996;78:1015-1023.
17 Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476-1486.
18 Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin Orthop Relat Res.; 254; 1990; 92-96.
19 Visotsky JL, Basamania C, Seebauer L, et al. Cuff tear arthropathy: Pathogenesis, classification, and algorithm for treatment. J Bone Joint Surg Am. 2004;86(suppl 2):35-40.
20 Neer CS2, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319-337.
21 Caporali R, Rossi S, Montecucco C. Tidal irrigation in Milwaukee shoulder syndrome. J Rheumatol. 1994;21:1781-1782.
22 Dines DM, Moynihan DP, Dines JS, McCann P. Irreparable rotator cuff tears: What to do and when to do it; the surgeon’s dilemma. J Bone Joint Surg Am. 2006;88:2294-2302.
23 Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88:562-575.
24 Franklin JL, Barrett WP, Jackins SE, Matsen FA. Glenoid loosening in total shoulder arthroplasty. Association with rotator cuff deficiency. J Arthroplasty. 1988;3:39-46.
25 Neer CSI. Glenohumeral arthroplasty. Shoulder reconstruction. Philadelphia: W.B. Saunders Company, 1990;153.
26 Amstutz HC, Thomas BJ, Kabo JM, et al. The Dana total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70:1174-1182.
27 Gristina AG, Romano RL, Kammire GC, Webb LX. Total shoulder replacement. Orthop Clin North Am. 1987;18:445-453.
28 Orr TE, Carter DR, Schurman DJ. Stress analyses of glenoid component designs. Clin Orthop Relat Res.; 232; 1988; 217-224.
29 Swanson AB, de Groot SG, Sattel AB, et al. Bipolar implant shoulder arthroplasty. Long-term results. Clin Orthop Relat Res.; 249; 1989; 227-247.
30 Gregory T, Hansen U, Emery RJ, et al. Developments in shoulder arthroplasty. Proc Inst Mech Eng [H]. 2007;221:87-96.
31 Sarris IK, Papadimitriou NG, Sotereanos DG. Bipolar hemiarthroplasty for chronic rotator cuff tear arthropathy. J Arthroplasty. 2003;18:169-173.
32 Petroff E, Mestdagh H, Maynou C, Delobelle JM. [Arthroplasty with a mobile cup for shoulder arthrosis with irreparable rotator cuff rupture: Preliminary results and cineradiographic study]. [French]. Revue de Chirurgie Orthopedique et Reparatrice de l Appareil Moteur. 1999;85:245-256.
33 Duranthon LD, Augereau B, Thomazeau H, et al. [Bipolar arthroplasty in rotator cuff arthropathy: 13 cases]. [French]. Revue de Chirurgie Orthopedique et Reparatrice de l Appareil Moteur. 2002;88:28-34.
34 Worland RL, Jessup DE, Arredondo J, Warburton KJ. Bipolar shoulder arthroplasty for rotator cuff arthropathy. J Shoulder Elbow Surg. 1997;6:512-515.
35 Calton TF, Fehring TK, Griffin WL, McCoy TH. Failure of the polyethylene after bipolar hemiarthroplasty of the hip. A report of five cases. J Bone Joint Surg Am. 1998;80:420-423.
36 Coughlin MJ, Morris JM, West WF. The semiconstrained total shoulder arthroplasty. J Bone Joint Surg Am. 1979;61:574-581.
37 Lettin AW, Copeland SA, Scales JT. The Stanmore total shoulder replacement. J Bone Joint Surg Br. 1982;64:47-51.
38 Post M, Jablon M. Constrained total shoulder arthroplasty. Long-term follow-up observations. Clin Orthop Relat Res.; 173; 1983; 109-116.
39 Post M, Haskell SS, Jablon M. Total shoulder replacement with a constrained prosthesis. J Bone Joint Surg Am. 1980;62:327-335.
40 Neer CSI. Glenohumeral arthroplasty. Shoulder reconstruction. Philadelphia: W.B. Saunders Company, 1990;148-150.
41 Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: Design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
42 Sirveaux F, Favard L, Oudet D, et al. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388-395.
43 Nyffeler RW, Sheikh R, Atkinson TS, et al. Effects of glenoid component version on humeral head displacement and joint reaction forces: An experimental study. J Shoulder Elbow Surg. 2006;15:625-629.
44 Simovitch RW, Zumstein MA, Lohri E, et al. Predictors of scapular notching in patients managed with the delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588-600.
45 Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005. The Grammont reverse shoulder prosthesis: Results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15:527-540.
46 Werner CML, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476-1486.
47 Goutallier D, Postel JM, Gleyze P, et al. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550-554.
48 Frankle M, Levy JC, Pupello D, et al. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients surgical technique. J Bone Joint Surg Am. 2006;88(1 suppl 2):178-190.
49 Guery J, Favard L, Sirveaux F, et al. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742-1747.
50 Wirth MA, Rockwood CAJr. Complications of shoulder arthroplasty. Clin Orthop.; 307; 1994; 47-69.
51 Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am. 2001;83:1814-1822.