Chapter 14 What Is the Best Method of Rehabilitation after Flexor Tendon Repair in Zone II: Passive Mobilization or Early Active Motion? What Is the Best Suture Configuration for Repair of Flexor Tendon Lacerations?
Restoration of satisfactory digital function after flexor tendon laceration and repair continues to be one of the most difficult problems in hand surgery. Early efforts to improve the performance of flexor tendon repairs are largely based on individual anecdotal experience, historical precedence, and clinical experimentation with little or no scientific support. Methods to repair flexor tendons have undergone a notable evolution since the 1950s. Early primary repair of flexor tendons in zone II, once called “no man’s land,” has replaced tendon grafting as the standard of care. Rehabilitation after repair of flexor tendon injuries has also evolved from complete immobilization to early passive motion and now early active motion. Nonetheless, the optimal treatment of a flexor tendon laceration in zone II remains an unresolved challenge for hand surgeons to define.1 The basic tenet of current and historical investigative efforts has been to improve the strength of tendon repair to allow for earlier motion, thereby preventing adhesion formation. Recent studies have contributed to a better understanding of the biology of flexor tendon injuries, improved methods of tendon repair, a greater emphasis on flexor sheath and pulley management, and the development of early controlled motion rehabilitation protocols leading to better clinical results.2 The purpose of this chapter is to provide a concise review of Level I and II studies on flexor tendon injury repair techniques and postoperative rehabilitation protocols.
TENDON REPAIR CONSIDERATIONS
During early phases of tendon healing, repair site strength is primarily dependent on the strength of the suture repair method. Strickland2 describes six principles of an ideal repair: (1) easy placement of sutures in the tendon, (2) secure suture knots, (3) smooth juncture of tendon ends, (4) minimal gapping at the repair site, (5) minimal interference with tendon vascularity, and (6) sufficient strength throughout healing to permit the application of early motion stress to the tendon.2 To satisfy these characteristics and therefore permit earlier active tendon mobilization, various suture techniques have been described that reportedly provide increased strength. Initial repair-site strength is roughly proportional to the intrinsic properties of the type of suture used, the number of suture strands traversing the repair site, and the number of grasping loops incorporated into the repair. Currently, hand surgeons agree that flexor tendon repairs should include a grasping or locking suture within the tendon, the “core” suture, and a continuous circumferential or “epitendinous” suture around the laceration site (Level IV). Addition of an epitendinous finishing suture has been shown to be of benefit in providing added tensile strength and gap resistance, as well as preventing triggering from uneven suture lines (Level IV).
CORE SUTURE CONFIGURATION
Early reports of active motion of tendons repaired with conventional two-strand repair documented rupture rates of up to 10%. Traditional two-strand suture methods are not sufficiently strong to consistently allow for early active digital motion. For this reason, several multistrand tendon suture techniques have been described. These techniques include the Kessler, Tajima, Savage, Lee, Tsuge, Tang, Sandow, and cruciate repair of Wolfe.3–8 Recent in vitro studies have evaluated the biomechanical properties of various suture methods for flexor tendon repair in the canine model. Increased ultimate strength of repair was reported for the multistrand and multiple-grasping methods of Lee (38 N), Savage (53 N), and an eight-strand technique.9 In vivo analysis demonstrated significant increases in strength for multistrand repair methods compared with traditional two-strand repairs at both 3 and 6 weeks in canine models. Reported ultimate strength values for an eight-strand repair was 52.6 and 70.9 N at 3 and 6 weeks, respectively.10 Ex vivo and in vivo studies in human and canine models suggest that core suture configurations with the greatest tensile strength are those in which there are multiple sites of tendon suture interaction. The addition of a circumferential suture may increase the strength of core repairs by 10% to 50%, reduce gapping between tendon ends, and smooth the repair site.11 Other variables shown to have a positive effect on the repair strength include a dorsovolar location of the core suture, adding locking or grasping stitches, and increasing the cross-sectional area of tendon that is grasped or locked by the redirecting loop of suture.12 Ex vivo human model studies have demonstrated that greater strength is achieved with more dorsal rather than volar placement of the core suture within the tendon.13–15 Positioning the redirecting loop of the core suture to “lock” rather than “grasp” the tendon stumps and increasing the number of suture locks or grasps further provides greater tensile strength of the repair site.16,17
Schuind and colleagues18 report forces across flexor tendons of 0.9 kilogram force (kgf; 8.9 N) and 3.5 kgf (34.4 N) for passive and active digital motion, respectively, in an in vivo study of patients undergoing carpal tunnel surgery (Table 14-1). These values increased significantly to 12 kgf (117.5 N) with fingertip pinch. Urbaniak and coauthors19 report an average tension in a human profundus tendon to be 14.7 N and found that tensile strength of tendon repairs decreases to approximately one fifth of its initial strength at 1 week. Taking into account increased resistance from edema after surgery and a decrease in suture strength during the initial weeks after repair, Urbaniak and coauthors19 and Savage20 therefore suggest that initial repair strength equal or exceed five times the average tension, 73.5 N, to withstand gentle or moderate active finger motion. To this end, numerous published investigations base treatment recommendations on Urbaniak19 and Schuind’s18 reported in vivo force values.
| TYPE | KILOGRAMS FORCE | NEWTONS |
|---|---|---|
| Passive mobilization of wrist | 0.6 | 5.9 |
| Passive mobilization of fingers | 0.9 | 8.9 |
| Unresisted active mobilization of wrist | 0.4 | 3.9 |
| Unresisted active mobilization of fingers | 3.5 | 34.4 |
| Grasp (flexor digitorum profundus) | 6.4 | 62.9 |
| Tip pinch | 12.0 | 117.5 |
Data from Schuind F, Garcia-Elias M, Cooney WP 3rd, An KN: Flexor tendon forces: In vivo measurements. J Hand Surg [Am] 17:291–298, 1992.
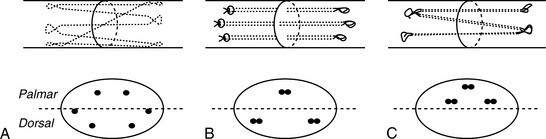
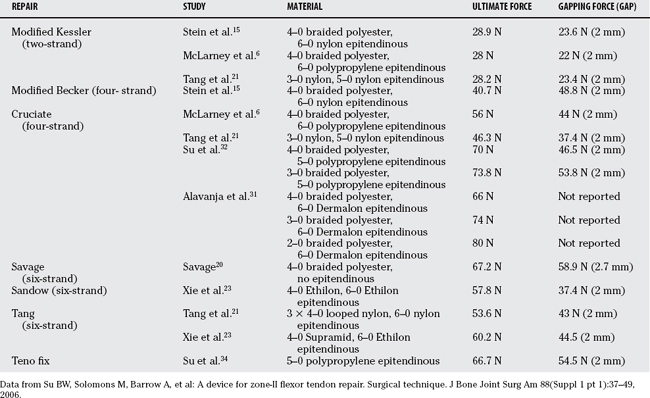
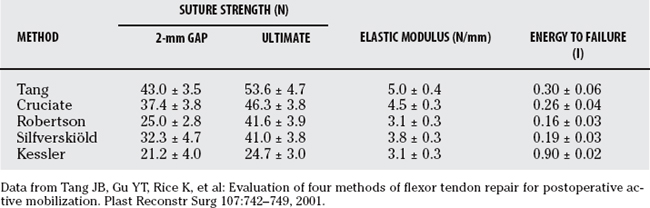
Tang and coworkers21 demonstrate in a human cadaver study that four newly developed suture methods (Fig. 14-1), the Tang, Silfverskiöld, Robertson, and cruciate, were biomechanically superior to the modified Kessler suture method when subjected to mechanical loads using the Instron tensile machine.The Tang method possessed the greatest ultimate strength (53.6 N), 2-mm gap formation force (43.0 N), energy to failure, and capacity to resist tendon deformation among the five tested techniques. The cruciate method showed statistically higher tensile strength and energy to failure compared with the Robertson, Silfverskiöld, and modified Kessler methods. The gap formation force, ultimate strength, elastic modulus, and energy to failure were lowest for the modified Kessler method (Table 14-2).
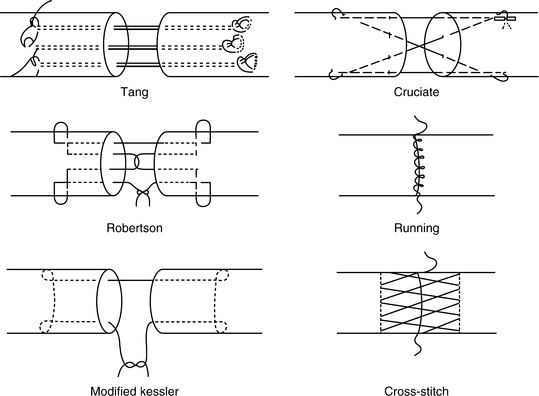
FIGURE 14-1 Schematic illustration of tendon suture techniques.
(Adapted from Tang JB, Gu YT, Rice K, et al: Evaluation of four methods of flexor tendon repair for postoperative active mobilization. Plast Reconstr Surg 107:742–749, 2001, by permission.)
TABLE 14-2 Gap Formation Force, Ultimate Strength, and Energy to Failure of Repaired Tendons (Mean ± Standard Deviation)
Labana and investigators22 similarly evaluated the biomechanical properties of three types of repairs—the standard Kessler–Tajima, double-loop (four-strand) modified Tsuge, and triple-loop (six-strand) modified Tsuge—in a human cadaver study. After subjecting the various repairs to mechanical testing using the Instron machine, the authors showed that the six-strand Tsuge suture was significantly stronger than both repairs, and that the four-strand Tsuge was significantly stronger than the Kessler–Tajima suture in terms of force to failure and initial gapping. Supramid 4–0 was used for core stitches in the modified Tsuge repairs and braided 4–0 nylon in the Kessler–Tajima repairs. 6–0 Prolene was used for epitenon repair in all cases. Labana and investigators22 conclude that an ultimate tensile strength of 64 N for the six-strand modified Tsuge repair and 48 N for the four-strand modified Tsuge repair exceeds the forces measured by Schuind and colleagues18 and, therefore, should withstand early range-ofmotion rehabilitation protocols (Table 14-3).
TABLE 14-3 Mean Force at Failure and Force to Initial Gapping for Three Repairs
| REPAIR TYPE | MEAN ULTIMATE FORCE TO FAILURE (SD), N | MEAN INITIAL GAPPING (SD), N |
|---|---|---|
| Kessler-Tajima | 31.8 (8.8) | 29.6 (9.2) |
| Four-strand Tsuge | 48.4 (10.7) | 40.7 (12.3) |
| Six-strand Tsuge | 64.2 (11.0) | 56.1 (9.7) |
SD, standard deviation.
Data from Labana N, Messer T, Lautenschlager E, et al: A biomechanical analysis of the modified Tsuge suture technique for repair of flexor tendon lacerations. J Hand Surg [Br] 26:297–300, 2001.
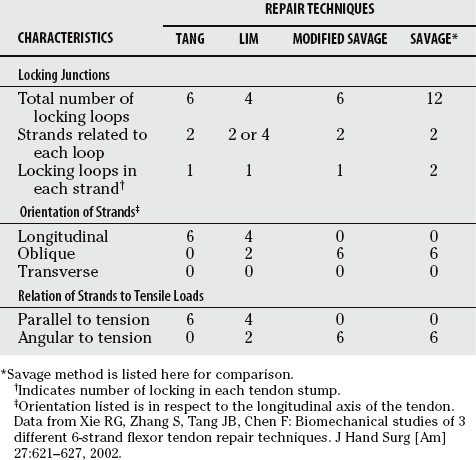
Xie and researchers23 compared the biomechanical properties of three six-strand tendon repair techniques with different configurations of core sutures: the modified Savage (Sandow’s method), Tang, and Lim (Fig. 14-2; Table 14-4). 4–0 Ethilon was used in the modified Savage repairs and 4–0 Supramid in the Tang and Lim repairs in addition to a 6–0 Ethilon running peripheral suture in all cases. Statistically, ultimate strength of the modified Savage (57.8 N) and Tang (60.2 N) methods were similar and significantly higher than that of the Lim method (51.3 N). Gap formation force was also greater in the Tang method compared with the modified Savage and Lim methods. In addition, results indicate a significant difference between mode of failure between the modified Savage and the Tang or Lim methods. Tendons repaired with the modified Savage method failed predominantly by suture breakage, which suggests that this repair may be strengthened by a larger caliber suture, whereas tendons repaired with the Tang and Lim methods failed mostly by suture pullout. The results of this study demonstrate that repair strength significantly varies with different configurations of six-strand repairs, and that location, number of locking junctions, and orientation of core sutures play an important role in repair strength despite an equal number of strands crossing the repair site.
TABLE 14-4 Characteristics in Locking Junctions with the Tendon and Orientations of Core Sutures in Three Six-Strand Repair Techniques
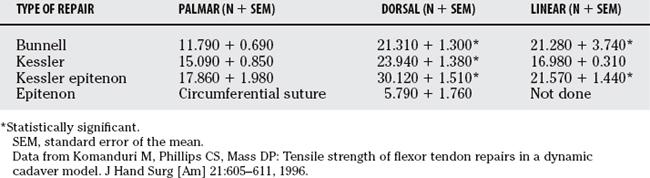
Although in vitro studies can provide basic biomechanical data concerning specific repair techniques, it is impossible to reproduce in vitro the in vivo physiologic environment in which repaired human tendons heal. The weakness of in vitro studies is that actual healing does not take place, and mechanical testing in tendons without prior trauma may not accurately account for increases in work of flexion from postsurgical edema, stiffness, and adhesion formation. Until recently, most in vitro studies used a simple linear testing model to evaluate tensile strength in terms of extra-anatomic longitudinally applied loads. Komanduri and colleagues14 propose using a dynamic “curvilinear” human cadaver model to test the strength of tendon repairs to more accurately simulate repair strength in vivo and account for biomechanical factors such as angulation at the repair site, differential loading, and frictional interference.
Komanduri and colleagues14 show that dorsal tendon repairs using Kessler or Bunnell core suture techniques were stronger than the standard volar repair.14 Suture material was limited to 4–0 nylon core sutures and 6–0 nylon circumferential sutures. In all cases with and without epitenon repair, dorsally placed sutures provided significantly more tensile strength than palmarly placed sutures (Table 14-5).
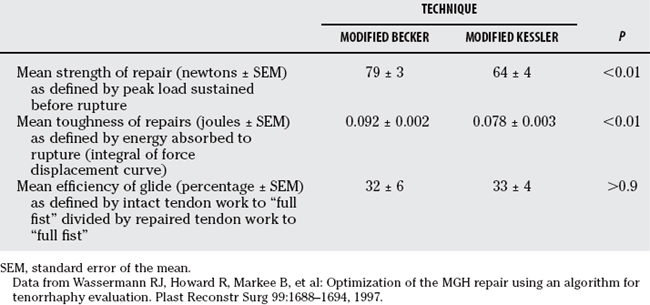
Historically, concern has existed that crossing or cruciate sutures may not be as desirable as linear suture techniques because they may interfere with the blood supply of the tendon. In addition, the blood supply to the tendon is located in the dorsal portion of the tendon, which happens to be the stronger part of the tendon to anchor sutures. Because tendon nutrition is predominantly synovial, the effect compromising the blood supply by suture is theoretical and has not been evaluated experimentally or clinically. Expanding on Komanduri’s work and using the same curvilinear model, Stein and colleagues15 tested newer suture techniques: the Strickland, Robertson, and modified Becker. All core sutures were performed with 4–0 Ethibond and 6–0 nylon for epitenon repair. Stein and colleagues15 demonstrate statistically significant increases in dorsal versus volar grasping strength with Kessler and Robertson repairs. No differences were found with the locking Strickland and modified Becker repairs. The four-strand techniques (Robertson and modified Becker) were also significantly stronger than the two-strand techniques (Kessler and Strickland). Wasserman and coworkers24 further demonstrated the modified Becker repair to be significantly stronger and tougher than the modified Kessler repair whereas allowing equally efficient glide in a dynamic human cadaveric model (Table 14-6).
SUTURE MATERIAL AND SIZE
Choice of suture material is an important factor in flexor tendon repair, which is reflected in efforts to determine the best tendon suture material. Few studies have investigated the range of suture materials available to the surgeon.25 Monofilament stainless steel sutures have the greatest tensile strength but are difficult to use, tend to pull through the tendon, and may weaken by kinking.26 The rate of material absorption and strength reduction of absorbable sutures in tendon repair often preclude their use in clinical settings.27,28 Most surgeons use nonresorbable 3–0 or 4–0 braided polyester sutures because they have been found to be nearly as strong as stainless steel in comparable sizes and have much easier handling properties.19
Hatanaka and Manske29 demonstrated a strength advantage of larger core sutures in a linear distraction model. Taras and investigators30 similarly showed that larger caliber sutures significantly increase repair strength. In a cadaveric study using ananatomic curvilinear model, Alavanja and coauthors31 compared the mechanical properties of a four-strand locked cruciate repair technique using 2–0, 3–0, and 4–0 braided polyester sutures. This study demonstrates that increasing suture caliber from 4–0 to 2–0 significantly increases maximum tensile strength but also results in an increased work of flexion and gliding resistance that may preclude its use in the clinical setting (Table 14-7). Gapping was not affected by suture caliber, and no significant difference between 3–0 or 4–0 braided polyester sutures was shown in terms of strength or gliding function. Repairs using 3–0 and 4–0 sutures in this study provided sufficient mechanical strength to withstand unrestricted finger flexion according to Schuind18 and Urbaniak.19
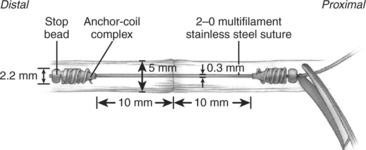
Stainless-steel sutures have been shown to have increased tensile strength but are not widely used in flexor tendon surgery because of difficulty with handling and knot-tying. Su and coworkers32 compared the biomechanical characteristics of the single-strand multifilament stainless-steel Teno Fix device (Ortheon Medical, Columbus, OH) designed for flexor tendon repair with a four-strand locked cruciate (3–0 or 4–0 braided polyester) suture repair using a linear model (Table 14-8). The Teno Fix is a knotless anchoring device composed of two intratendinous stainless steel anchors that adjoins transected tendon ends with a single multifilament 2–0 stainless-steel suture (Fig. 14-3).33 The device was developed to take advantage of stainless-steel suture strength whereas avoiding difficulties with handling and knot-tying. The 2-mm gapping force was significantly greater for the Teno Fix and 3–0 repairs compared with 4–0 repairs. No statistically significant difference in peak force or energy absorbed at peak force was found among the three different repair techniques. This study also confirmed that addition of a circumferential epitendinous suture using 5–0 monofilament polypropylene to the Teno Fix repair increased the 2-mm gapping force by 40% and peak force by 48%. To further evaluate the clinical effectiveness of the Teno Fix, Su and coworkers34 conducted a blinded, randomized clinical trial comparing its use with four-strand cruciate repairs using a single 4–0 or 3–0 polypropylene suture (depending on tendon size) with 6–0 nylon epitendinous repair.34 None of the Teno Fix repairs ruptured compared with 18% (9/51) of tendons repaired with the cruciate technique. Five of the nine ruptures were attributed to resistive motion against medical advice in noncompliant patients. No differences were reported between the two groups for range of motion, DASH (disability of the arm, shoulder, and hand) score, pinch and grip strength, pain, or swelling. The authors conclude that the Teno Fix is safe and effective for flexor tendon repair if tendon size and exposure are sufficient.
NEW DEVELOPMENTS
Mentzel and researchers35 compared the functional results 12 weeks after flexor tendon repair surgery with and without the use of Adcon-T/N. No significant difference was found with regard to total active motion and total extension lag between treated and untreated patients. Golash and investigators36 also showed in a prospective, double-blinded, randomized, controlled, clinical trial that Adcon-T/N had no statistically significant effect on total active motion at 3, 6, and 12 months. However, the time taken to achieve final range of motion was significantly shorter in treated patients (10 vs. 14 weeks; P = 0.02). The authors suggest that Adcon-T/N may therefore limit adhesion formation in the early stages of healing. Greater rates of late rupture were observed in patients treated with Adcon-T/N (33% compared with 20% at a mean of 4 weeks compared with 2 weeks; P = 0.016), resulting in early conclusion of the study because of potential inhibitory effects of Adcon-T/N on tendon healing. In a similar double-blind, randomized, clinical study, Liew and coauthors37 report a rupture rate of 25% in patients with tendon repairs treated with Adcon-T/N at an average of 33 days compared with a control group rupture rate of 26% at an average of 23 days. At 6-month follow-up examination, patients in the group treated with Adcon-T/N regained significantly more motion at the proximal interphalangeal joint (87% vs. 68%; P = 0.005), but not distal interphalangeal joint motion, hand grip, or pinch strength. Based on these results from early clinical studies, the benefit of using Adcon, as well as other mechanical barriers, in flexor tendon repairs remains unproven and, therefore, has not gained acceptance in clinical practice (grade B).
POSTOPERATIVE REHABILITATION
Postoperative rehabilitation protocols have evolved alongside advancement in flexor tendon repair techniques. Rehabilitation after flexor tendon repair must achieve a balance between protection of the repair from disrupting forces and prevention of adhesions. Studies have shown that early controlled forces applied to flexor tendon repairs are beneficial in providing more rapid recovery of tensile strength, fewer adhesions, improved tendon excursion, and minimal repair-site deformation in canine models.38 Gelberman and coworkers38 demonstrate an increase in tendon strength with passive motion at 12 weeks to within 50% of control, whereas a tendon completely immobilized had a strength equivalent to 20% of control. Tendon motion studies also show that immobilization results in 20% of normal excursion, whereas immediate mobilization produces up to 95% of normal tendon gliding. Motion clearly provides a stimulus for tendon healing.
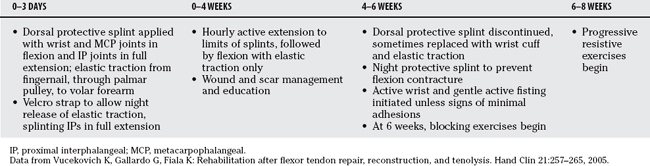
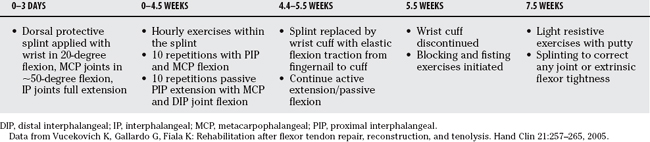
Lister and colleagues39 were among the first to report remarkable clinical results using active extension-passive flexion mobilization with the aid of a dynamic traction splint (Level IV). Two basic passive motion programs serve as the basis for other passive motion protocols: the Kleinert method and the Duran method40 (Level IV). An effective postoperative program requires full range of passive flexion of both the distal and proximal interphalangeal joints. Both the Kleinert and Duran methods accomplish this goal when performed properly. Tables 14-9 and 14-10 outline the basic Kleinert and Duran protocols, respectively, that have been adapted and modified by hand surgeons.41
The timing, duration, and progression of rehabilitation, as well as optimal frequency of finger motion after flexor tendon repair, also have not been clearly established. Rehabilitation can begin anytime within 1 week after repair. Many surgeons report initiation of rehabilitation as immediate or starting the day after surgery. In a randomized clinical study, Adolfsson and researchers42 investigated the effect of a shortened postoperative mobilization program after flexor tendon repair. All tendons were repaired with a modified 4–0 Maxon Kessler suture and running circumferential 6–0 Prolene suture. Patients were immobilized in a dorsal plaster splint with the wrist in 30 degrees and metacarpophalangeal (MCP) joints in 70 degrees of flexion. During the first 4 weeks, a passive flexion-active extension protocol described by Karlander42a was instituted followed by active flexion and extension without load for an additional 2 weeks. After the 6th week, patients were randomized to receive gradually increasing load and unrestricted activity at either 8 or 10 weeks. No significant differences were observed between the two groups in terms of functional results (Louisville, Tsuge, and Buck–Gramko), rupture rates, grip strength, or subjective assessments at 6 months. The authors conclude that a postoperative mobilization program after flexor tendon repair in zone II of the hand can be reduced to 8 weeks using the described regimen without significantly increasing risk for rupture or poorer functional results.
In a prospective, randomized, clinical study, Gelberman and coworkers43 compared a traditional early passive motion protocol with a continuous passive motion protocol. All tendons were repaired with a Kessler 4–0 braided suture and 6–0 nylon epitenon suture. After surgery, dorsal plaster splints were applied with the wrist in 30-degree flexion, and MCP joints in 70-degree flexion. Patients were randomized into two treatments groups: (1) passive-motion rehabilitation using a continuous motion device for the first 4 weeks followed by combination active-motion rehabilitation alternating with continuous motion for a mean interval of 75 hours/week, and (2) controlled intermittent passive motion for the first 4 weeks followed by alternating active-motion and controlled-passive motion for a mean interval of controlled passive motion of 4 hours/week. Range of motion, both total active and mean active motion, as measured by the Strickland and Glogovac criteria showed a statistically significant difference between groups in favor of continuous motion.
More recently, early active motion protocols have been developed in response to experimental and clinical studies that demonstrate beneficial effects of early active motion. Early controlled active mobilization after flexor tendon repair may actually improve differential gliding between the flexor digitorum profundus (FDP) and flexor digitorum superficialis FDS tendons, and therefore reduce finger flexion contractures.44 Strickland introduced an early active motion protocol for a four-strand repair with an epitendinous suture (Table 14-11).45 Evans, Silfverskiöld, May, and others have also introduced protocols that incorporate early active motion exercises (performed only under therapy supervision for the first few weeks) whereas using a Kleinert-type dorsal blocking splint.46
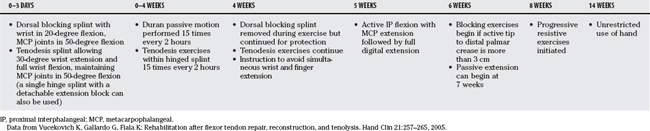
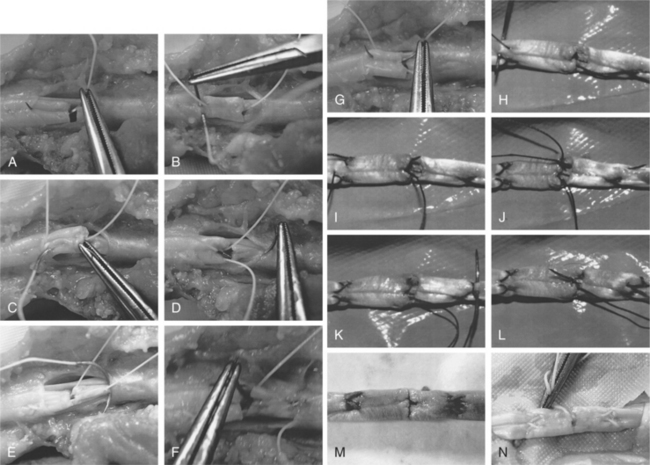
Based on the summary recommendations of evidence (Table 14-12), we recommend a four-strand (or higher) core repair supplemented by a running epitendinous suture. We prefer a 3–0 (or 4–0 in smaller tendons) braided polyester core suture with Teflon coating and a 6–0 running Nylon epitendinous suture. We use a modified Savage technique as described by Sandow and McMahon49 for repair of flexor tendons. The latest modification uses a four-strand, single cross core suture reinforced with a cross-stitch epitendinous suture (Fig. 14-4).50,51 This repair method is technically easier and possesses gapping and strength characteristics as the original Savage technique strong enough to allow protected early active motion. After surgery, we recommend a modified Duran passive motion and place and hold rehabilitation protocol (Table 14-13.
| STATEMENT | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION | REFERENCES |
|---|---|---|
Data from Hunter J, Macklin E, Callahan A: Rehabilitation of the Hand. St Louis, CV Mosby, 1995. Diagnosis & Treatment Manual for Physicians and Therapies, 4th ed. The Hand Rehabilitation Center of Indiana, Indianapolis, Indiana, 2001.
TABLE 14-13 University of California Davis Hand Flexor Tendon Zone II Protocol52,53
DIP, distal interphalangeal; FDP, flexor digitorum profundus; IP, interphalangeal; MCP, metacarpophalangeal; PIP, proximal interphalangeal.
Despite the various active and passive motion protocols described in the literature, the optimal rehabilitation program after flexor tendon repair is yet to be determined. Kneafsey et al47 reports no significant differences between controlled passive flexion with active extension (modified Kleinert) and controlled passive mobilization (modified Duran) in terms of active range of motion, power grip, pinch grip, and maximum finger pressure. In a Cochrane review, Thien and coworkers48 conclude that there is insufficient evidence from randomized, controlled clinical trials to define the best mobilization strategy after flexor tendon repair.48 The authors suggest that rehabilitation protocols using some early active mobilization should be used with multiple strand suture methods in compliant patients.
1 Strickland JW. Development of flexor tendon surgery: Twenty-five years of progress. J Hand Surg [Am]. 2000;25:214-235.
2 Strickland JW. Flexor tendon injuries: I. Foundations of treatment. J Am Acad Orthop Surg. 1995;3:44-54.
3 Lee H. Double loop locking suture: A technique of tendon repair for early active mobilization. Part I: Evolution of technique and experimental study. J Hand Surg [Am]. 1990;15:945-952.
4 Lee H. Double loop locking suture: A technique of tendon repair for early active mobilization. Part II: Clinical experience. J Hand Surg [Am]. 1990;15:953-958.
5 Tang JB, Shi D, Gu YQ, et al. Double and multiple looped suture tendon repair. J Hand Surg [Br]. 1994;19:699-703.
6 McLarney E, Hoffman H, Wolfe SW. Biomechanical analysis of the cruciate four-strand flexor tendon repair. J Hand Surg [Am]. 1999;24:295-301.
7 Tsuge K, Ikuta Y, Matsuishi Y. Intra-tendinous tendon suture in the hand—a new technique. Hand. 1975;7:250-255.
8 Kessler I, Nissim F. Primary repair without immobilization of flexor tendon division within the digital sheath. An experimental and clinical study. Acta Orthop Scand. 1969;40:587-601.
9 Noguchi M, Seiler JG3rd, Gelberman RH, et al. In vitro biomechanical analysis of suture methods for flexor tendon repair. J Orthop Res. 1993;11:603-611.
10 Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: A biomechanical study in dogs. J Hand Surg [Am]. 1998;23:97-104.
11 Wade PJ, Wetherell RG, Amis AA. Flexor tendon repair: Significant gain in strength from the Halsted peripheral suture technique. J Hand Surg [Br]. 1989;14:232-235.
12 Boyer MI, Strickland JW, Engles D, et al. Flexor tendon repair and rehabilitation: State of the art in 2002. Instr Course Lect. 2003;52:137-161.
13 Soejima O, Diao E, Lotz JC, Hariharan JS. Comparative mechanical analysis of dorsal versus palmar placement of core suture for flexor tendon repairs. J Hand Surg [Am]. 1995;20:801-807.
14 Komanduri M, Phillips CS, Mass DP. Tensile strength of flexor tendon repairs in a dynamic cadaver model. J Hand Surg [Am]. 1996;21:605-611.
15 Stein T, Ali A, Hamman J, Mass DP. A randomized biomechanical study of zone II human flexor tendon repairs analyzed in an in vitro model. J Hand Surg [Am]. 1998;23:1046-1051.
16 Hatanaka H, Zhang J, Manske PR. An in vivo study of locking and grasping techniques using a passive mobilization protocol in experimental animals. J Hand Surg [Am]. 2000;25:260-269.
17 Hatanaka H, Manske PR. Effect of the cross-sectional area of locking loops in flexor tendon repair. J Hand Surg [Am]. 1999;24:751-760.
18 Schuind F, Garcia-Elias M, Cooney WP3rd, An KN. Flexor tendon forces: In vivo measurements. J Hand Surg [Am]. 1992;17:291-298.
19 Urbaniak JR, Cahill JD, Mortenson RA. Tendon suture methods: Analysis of tensile strength, in AAOS Symposium on Tendon Surgery in the Hand. St. Louis: Mosby, 1975;70-80.
20 Savage R. In vitro studies of a new method of flexor tendon repair. J Hand Surg [Br]. 1985;10:135-141.
21 Tang JB, Gu YT, Rice K, et al. Evaluation of four methods of flexor tendon repair for postoperative active mobilization. Plast Reconstr Surg. 2001;107:742-749.
22 Labana N, Messer T, Lautenschlager E, et al. A biomechanical analysis of the modified Tsuge suture technique for repair of flexor tendon lacerations. J Hand Surg [Br]. 2001;26:297-300.
23 Xie RG, Zhang S, Tang JB, Chen F. Biomechanical studies of 3 different 6-strand flexor tendon repair techniques. J Hand Surg [Am]. 2002;27:621-627.
24 Wassermann RJ, Howard R, Markee B, et al. Optimization of the MGH repair using an algorithm for tenorrhaphy evaluation. Plast Reconstr Surg. 1997;99:1688-1694.
25 Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg [Am]. 2005;30:836-841.
26 Nystrom B, Holmlund D. Separation of sutured tendon ends when different suture techniques and different suture materials are used. An experimental study in rabbits. Scand J Plast Reconstr Surg. 1983;17:19-23.
27 Mashadi ZB, Amis AA. Variation of holding strength of synthetic absorbable flexor tendon sutures with time. J Hand Surg [Br]. 1992;17:278-281.
28 O’Broin ES, Early MJ, Smyth H, Hooper AC. Absorbable sutures in tendon repair. A comparison of PDS with prolene in rabbit tendon repair. J Hand Surg [Br]. 1995;20:505-508.
29 Hatanaka H, Manske PR. Effect of suture size on locking and grasping flexor tendon repair techniques. Clin Orthop Relat Res.; 375; 2000; 267-274.
30 Taras JS, Raphael JS, Marczyk SC, Bauerle WB. Evaluation of suture caliber in flexor tendon repair. J Hand Surg [Am]. 2001;26:1100-1104.
31 Alavanja G, Dailey E, Mass DP. Repair of zone II flexor digitorum profundus lacerations using varying suture sizes: A comparative biomechanical study. J Hand Surg [Am]. 2005;30:448-454.
32 Su BW, Protopsaltis TS, Koff MF, et al. The biomechanical analysis of a tendon fixation device for flexor tendon repair. J Hand Surg [Am]. 2005;30:237-245.
33 Su BW, Solomons M, Barrow A, et al. A device for zone-II flexor tendon repair. Surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 pt 1):37-49.
34 Su BW, Solomons M, Barrow A, et al. Device for zone-II flexor tendon repair. A multicenter, randomized, blinded, clinical trial. J Bone Joint Surg Am. 2005;87:923-935.
35 Mentzel M, Hoss H, Keppler P, et al. The effectiveness of ADCON-T/N, a new anti-adhesion barrier gel, in fresh divisions of the flexor tendons in Zone II. J Hand Surg [Br]. 2000;25:590-592.
36 Golash A, Kay A, Warner JG, et al. Efficacy of ADCON-T/N after primary flexor tendon repair in Zone II: A controlled clinical trial. J Hand Surg [Br]. 2003;28:113-115.
37 Liew SH, Potokar T, Bantick GL, et al. The use of ADCON-T/N after repair of zone II flexor tendons. Chir Main. 2001;20:384-387.
38 Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am]. 1982;7:170-175.
39 Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg [Am]. 1977;2:441-451.
40 Vucekovich K, Gallardo G, Fiala K. Rehabilitation after flexor tendon repair, reconstruction, and tenolysis. Hand Clin. 2005;21:257-265.
41 Duran RJ, House RG. Controlled passive motion following flexor tendon repairs in zones 2 and 3,. AAOS Symposium on Tendon Surgery in the Hand. St. Louis: Mosby, 1975.
42 Adolfsson L, Söderberg G, Larsson M, Karlander LE. The effects of a shortened postoperative mobilization programme after flexor tendon repair in zone 2. J Hand Surg [Br]. 1996;21:67-71.
42a Karlander LE, Berggren M, Larsson M, Soderberg G, Nylander G. Improved results in zone 2 flexor tendon injuries with a modified technique of immediate controlled mobilization. J Hand Surg [Br]. 1993;18(1):26-30.
43 Gelberman RH, Nunley JA2nd, Osterman AL, et al. Influences of the protected passive mobilization interval on flexor tendon healing. A prospective randomized clinical study. Clin Orthop Relat Res.; 264; 1991; 189-196.
44 Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg [Br]. 2007;32:118-129.
45 Strickland JW, Cannon NM. Flexor tendon repair—Indiana method. Indiana Hand Center Newsletter. 1993:1-19.
46 Silfverskiold KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg [Am]. 1994;19:53-60.
47 Kneafsey B, O’Shaughnessy M, Vidal P, et al. Controlled mobilization after flexor tendon repair: A prospective comparison of two methods [abstract]. J Hand Surgery [Br]. 1994;19(suppl 1):37-38.
48 Thien TB, Becker JH, Theis JC. Rehabilitation after surgery for flexor tendon injuries in the hand. Cochrane Database Syst Rev.; 4; 2004; CD003979.
49 Sandow MJ, McMahon MM. Single cross-grasp six-strand repair for flexor tenorrhaphy modified Savage technique. Atlas Hand Clinics. 1996;1:41-64.
50 Bernstein MA, Taras JS. Flexor tendon suture: A description of two core suture techniques and the Silfverskiold epitendinous suture. Tech Hand Up Extrem Surg. 2003;7:119-129.
51 Wakefield Orthopedic Clinic. Upper limb research: Single-cross-grasp four strangd (Adelaide) tenorrhaphy. Available at: http://woc.com.au/content/14. Accessed December 2007.
52 Hunter J, Macklin E, Callahan A. Rehabilitation of the Hand. St. Louis: CV Mosby, 1995.
53 Diagnosis & Treatment Manual for Physicians and Therapists, 4th ed. The Hand Rehabilitation Center of Indiana, Indianapolis, Indiana, 2001.