2 What cancer is
How cells should work
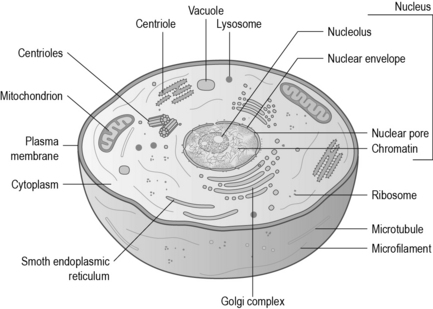
A cell (Fig. 2.1) is the basic building unit of life. All animal cells are similar in their components, although they may have different functions. Similar cells are grouped together to create tissues which carry out specific functions. For example, there are four main types of tissue that make up the human body: muscle, epithelial, nervous and connective tissues.
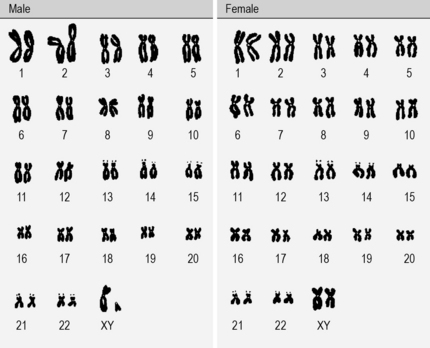
Each cell contains a complete copy of our genome in the form of 23 separate pairs of chromosomes (one set from each of our parents) (Fig. 2.2). For each chromosome and each gene, we have two slightly different copies.
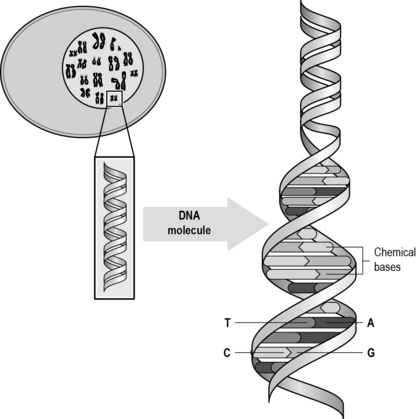
DNA is made up of individual molecules called nucleotides, which are in turn made up of a sugar (deoxyribose), a phosphate and a nitrogenous base. The DNA molecule is comprised of two chains of nucleotide bases, arranged in a double helix (Fig. 2.3). There are four bases which are grouped into two types: purines (adenine (A) and guanine (G)) and pyrimidines (thymine (T) and cytosine (C)). Each base is paired up with another base: A pairs with T and C pairs with G. Each base is a slightly different length which gives the double helix its twisted shape. The bases can occur in any sequence. It is the sequence of the bases that makes up the instruction, a bit like the words of an instruction manual. Depending on the sequence of the bases, a particular protein will be produced. The proteins in turn will enable the cell to function in a particular way, including cell replication.
Cell cycle
From the time of conception, all of our cells continue to multiply in order for us to grow into an adult. Once we reach adulthood our cells only divide when there is need to repair and replace old damaged cells and to reproduce. To do this, cells go through a process called the cell cycle (Fig. 2.4). The phases of the cell cycle are:
• G0: resting phase, not in cell division.
• G1 and G2: the cell builds up energy and prepares for the next stage.
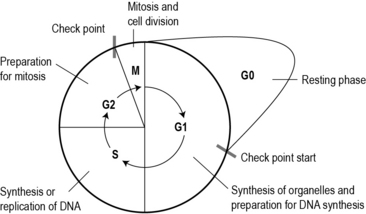
Fig 2.4 The cell cycle.
(Reproduced with permission from Kearney N, Richardson A (2005) Nursing patients with cancer: principles and practice. Churchill Livingstone (Fig. 5.4, page 78))
Normal cells only divide when they are needed, such as during growth (from conception to adulthood) and to replace old and damaged cells. This replication is carefully controlled by a number of checkpoints (see Fig. 2.4), which ensure that cell division only occurs when really necessary and occurs accurately. Certain genes control cell division, known as proto-oncogenes. These genes code for proteins and growth factors which signal to the nucleus of the cell to turn the cell cycle on, a bit like an accelerator of a car. There are four main groups of proteins produced by the proto-oncogenes: growth factors, growth factor receptors, signal transducers and nuclear proto-oncogenes and transcription factors.
What goes wrong to allow a cancer cell to develop?
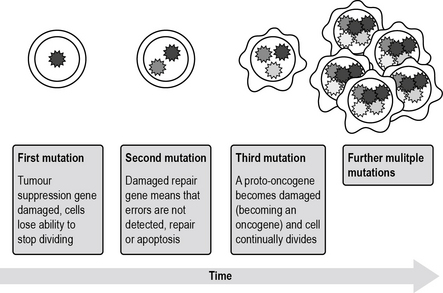
Usually there is more than one mutation in the DNA. For instance, many individuals who develop cancer will have mutations in a proto-oncogene (when a proto-oncogene becomes damaged it is known as an oncogene), a tumour suppressor gene and a repair gene. These errors will code for different proteins and subsequently the cell will behave differently and cell cycle regulation may be overridden, resulting in uncontrolled growth (Fig. 2.5).
There are several other key changes in a cancer cell:
• Growth: instead of the telomeres shortening each time the cell divides, cancer cells produce an enzyme called telomerase which prevents the shortening and extends the life of the cell.
• Differentiation: this is the process where cells mature and become specialised in their function and form. This is a bit like student nurses who, once they become qualified, specialise in cardiac or theatre or cancer nursing. Cells develop a unique function such as a heart or muscle or skin or nerve cell and each is specialised. Usually, the more specialised the cell, the more likely it spends more time in the G0 phase of the cell cycle. Cancer cells lose their differentiation as they become more and more mutated each time they divide. In doing so, they lose their intended function and behave in an inappropriate manner.
• Tumour heterogeneity: as a cancer grows and becomes advanced, the cells become more and more mutated, becoming different from one another. This is why some patients do not respond to treatment – some cells are killed, but others survive.
• Metastatic spread: a common feature of cancer is its ability to move from one part of the body to another site (often to a distant site). This is known as metastatic spread, sometimes known as secondary disease. Cancer cells are determined to survive and, once they have outgrown their blood, oxygen and nutrient supply, they will seek a new home that will provide all that they need.
How cancer cells spread to other parts of the body?
What causes the DNA to become damaged?
Biological factors
• Human papillomavirus (HPV) accounts for 80–90% of all cervical cancer cases as well as increasing numbers of oral cancers.
• Hepatitis B and C increase the risk of developing liver cancer by 80%.
• Epstein–Barr virus has been linked with Birkett’s and Hodgkin’s lymphoma.
• Human immunodeficiency virus (HIV) has been linked with Kaposi’s sarcoma and non-Hodgkin’s lymphoma.
Chemical factors
Industrial processes and chemicals such as dioxins, benzene, vinyl chlorides, nickel and arsenic have all been connected with certain cancers and their use is subsequently restricted by environmental and health and safety regulations, such as the Control of Substances Hazardous to Health Regulations (COSHH) (Health and Safety Executive 2002) in the UK.
Sex hormones
• The age of menarche and menopause is significant – the younger a woman is when she starts her periods and the older she is when she finishes ovulating completely.
• Age at first full-term pregnancy.
• Length of time a woman breastfeeds.
• Exogenous hormones taken such as hormone replacement therapy (HRT).
Inherited damage
Other examples of inherited cancers are the following:
• Hereditary non-polyposis: this accounts for 5–10% of all colorectal cancers. Again, it is worth remembering that even if someone carries this defective gene they will not necessarily develop cancer, but they have a 25% chance of developing it by the age of 65 years old.
• Familial adenomatous polyposis (FAP): 1% of patients with colorectal cancer test positive for FAP. This, on the other hand, carries a 100% risk of developing cancer by the age of 50 years old. Both these conditions require regular surveillance by undergoing a sigmoidoscopy or colonoscopy.
• There are some hereditary syndromes associated with cancer, such as Li Frameni, Cowden and Von Hippel Lindau, however these are very rare.
Health and Safety Executive. The Control of Substances Hazardous to Health regulations. Approved code of practice and guidance. Online. Available at: 5th ed, London:HSE ;2002.http://www.hse.gov.uk/coshh/ (accessed May 2011)
Waugh A., Grant A. Ross and Wilson anatomy and physiology in health and illness. Edinburgh: Churchill Livingstone; 2010.
Kearney N., Richardson A. Nursing patients with cancer: principles and practice. Edinburgh: Churchill Livingstone; 2006.
King R.J.B., Robins M.W. Cancer biology, 3rd ed. Harlow: Prentice Hall; 2006.
Pecorino L. Molecular biology of cancer: mechanisms, targets and therapeutics, 2nd ed. Oxford: Oxford University Press; 2008.
Scotting P. Cancer: a beginners guide. Oxford: Oneworld; 2010.