Wet Age-Related Macular Degeneration
OCT Features:
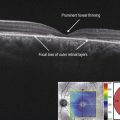
▶ Classic CNV: a classic, or type 2, CNV is present when the abnormal neovascular tissue penetrates the RPE/Bruch’s membrane complex and is present in the subretinal space (Figs 8.1.1 and 8.1.2).
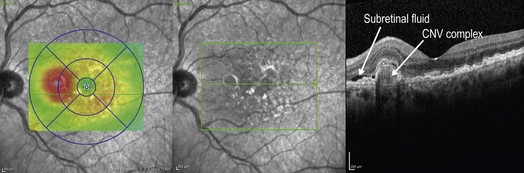
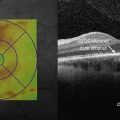
Figure 8.1.1 OCT of a classic choroidal neovascularization (far right). Corresponding thickness map (left) and infrared image (middle) are shown.
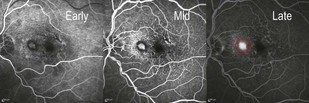
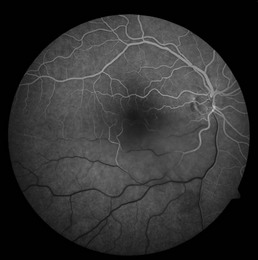
Figure 8.1.2 Fluorescein angiography (corresponding to Figure 8.1.1) shows a well-defined region of hyperfluorescence that is visible in the early frames and grows in intensity in the late frames, but does not enlarge in size, characteristic of a classic choroidal neovascularization (red circle).
▶ Occult CNV: an occult, or type 1, CNV is present when the abnormal neovascular tissue remains underneath the RPE (Figs 8.1.3 and 8.1.4).
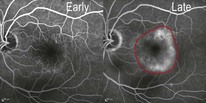
Figure 8.1.3 Fluorescein angiography shows late hyperfluorescence with ill-defined boundaries, which may represent a fibrovascular pigment epithelial detachment, and is characteristic of an occult choroidal neovascularization (within red border).
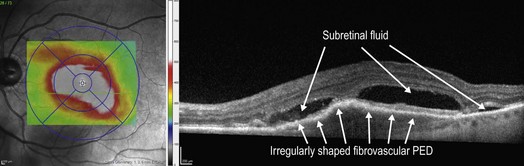
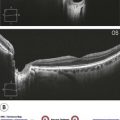
Figure 8.1.4 OCT (corresponding to Figure 8.1.3) of an occult choroidal neovascularization (right) and corresponding thickness map (left), PED.
▶ RPE tear: an RPE tear has a very characteristic OCT appearance (Figs 8.1.5 and 8.1.6), where there is a sharply demarcated region of absent RPE adjacent to an area of bunched-up RPE.
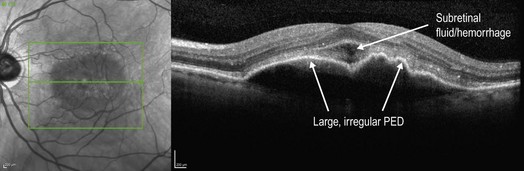
Figure 8.1.5 OCT of a choroidal neovascularization with a large, irregular pigment epithelial detachment. The corresponding infrared image is also shown (left).
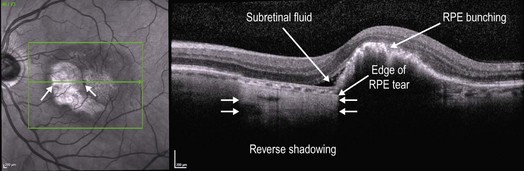
Figure 8.1.6 OCT of the same choroidal neovascularization shown in Figure 8.1.5 one month following treatment with intravitreal anti-VEGF therapy with resultant retinal pigment epithelium (RPE) tear. Due to the absence of the RPE in the region of the tear, reverse shadowing is seen in the deeper structures. The RPE is bunched up where it is still present, blocking deeper structures. There is also still a thin rim of subretinal fluid present. Corresponding infrared image is shown on the left, which helps to visualize the region of the RPE tear.
▶ Disciform scar: a disciform scar can have a varied OCT appearance but always is dominated by a hyper-reflective subretinal scar (Figs 8.1.7 and 8.1.8).
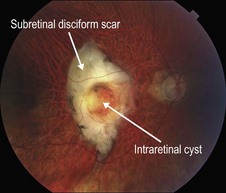
Figure 8.1.7 Color photograph of a subretinal disciform scar that is the result of end-stage wet AMD. A central intraretinal cyst is present, which is better visualized on OCT.
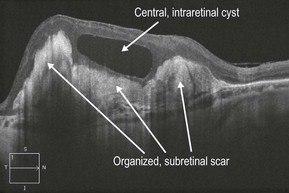
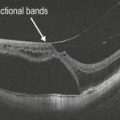
Figure 8.1.8 OCT (corresponding to Figure 8.1.7) shows highly reflective subretinal material corresponding to the organized subretinal scar. A central intraretinal cyst is also present.
▶ Treated CNV: following treatment with anti-vascular endothelial growth factor (VEGF) therapy, intra- and subretinal fluid will often improve significantly or completely resolved (Figs 8.1.9 and 8.1.10). Associated PEDs also tend to decrease in size with continued treatment.
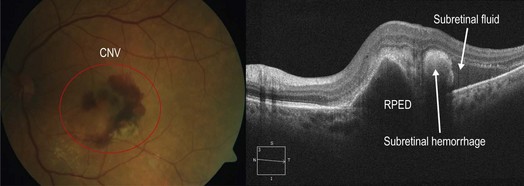
Figure 8.1.9 OCT shows all of the features of wet AMD including an irregularly shaped PED, subretinal hemorrhage, and subretinal fluid. Subretinal fluid lacks reflectivity on OCT and appears as an empty space, whereas the subretinal hemorrhage has moderate reflectivity and appears as a medium-intensity signal. The corresponding color photograph is shown on the left.
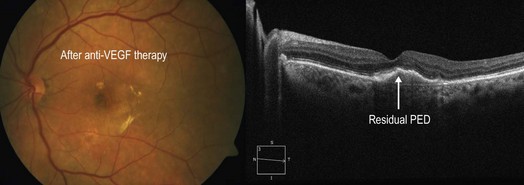
Figure 8.1.10 Following therapy with numerous intravitreal injections of an anti-vascular endothelial growth factor medication, there was significant improvement in the clinical appearance with resolution of subretinal fluid.
▶ Retinal angiomatous proliferation: type 3 CNV (or RAP), is a rare cause of exudative AMD resulting from abnormal neovascular tissue within the deep retina that typically originates within the retina and migrates towards the choriocapillaris and/or retinal surface (Figs 8.1.11 and 8.1.12).
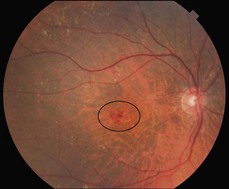
Figure 8.1.11 Color photograph of retinal angiomatous proliferation shows multiple localized intraretinal hemorrhages in an area of retinal thickening.
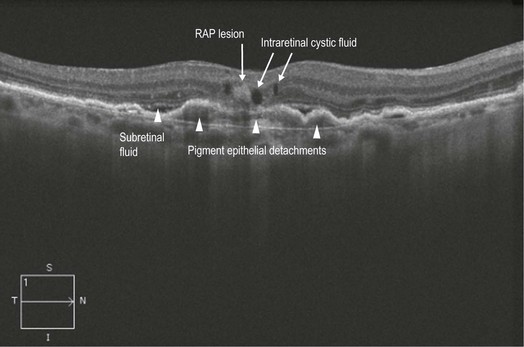
Figure 8.1.12 OCT (corresponding to Figure 8.1.10) shows a hyper-reflective area within the retina, thought to represent the retinal angiomatosis proliferation lesion. There is associated intraretinal cystic fluid and underlying subretinal fluid and pigment epithelial detachments.
▶ Polypoidal choidal vasculopathy (PCV): PCV is thought to be a variation of type 1, or occult, CNV where polyp-shaped abnormal vascular complexes are located underneath the RPE (Figs 8.1.13, 8.1.14 and Fig 8.1.15).
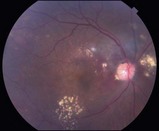
Figure 8.1.13 Color photograph shows multiple subretinal peripapillary polypoidal lesions with associated subretinal fluid and exudate.
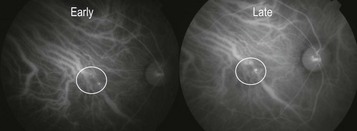
Figure 8.1.14 Indocyanine green angiogram shows small, pinpoint hyperfluorescent polyps consistent with a diagnosis of polypoidal choroidal vasculopathy in a patient who responded sub-optimally to anti-vascular endothelial growth factor therapy.
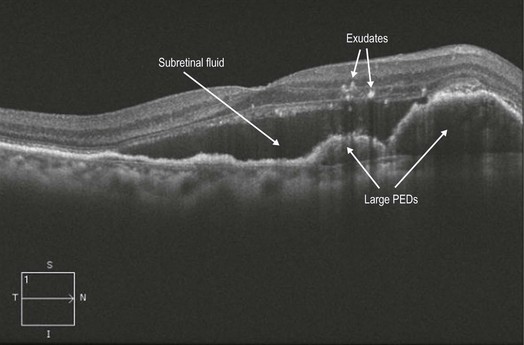
Figure 8.1.15 OCT (corresponding to Figure 8.1.12) shows multiple, large, adjacent PEDs with overlying subretinal fluid. Exudate is also visible as hyper-reflective intraretinal spots.
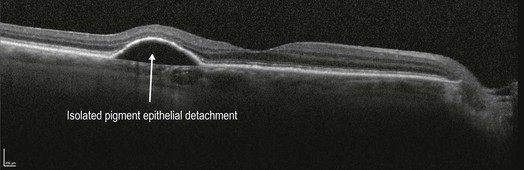
▶ Isolated PED: in the setting of a large, isolated PED, it can sometimes be difficult to determine if co-existent wet AMD is present even with the addition of an FA. OCT can help to determine if overlying intra- or subretinal fluid is present or not, which would be the case in wet AMD (Fig 8.1.16).
Ancillary Testing:
Fluorescein angiography is helpful to confirm a diagnosis of wet AMD and for subtyping the lesion (Figs 8.1.2 and 8.1.4), though this was more useful historically when treatment decisions were based on lesion type. Indocyanine green angiography (ICGA) can help to differentiate less common AMD-subtypes that do not respond well to standard anti-VEGF therapy, such as PCV (Fig 8.1.15).
Treatment:
Intravitreal anti-VEGF monotherapy is the mainstay of treatment for most eyes with wet AMD. Current treatment options include bevacizumab, ranibizumab, and aflibercept. PEDs, subretinal fluid, and subretinal hemorrhage often improve or resolve with continued anti-VEGF treatment (Fig. 8.1.9). Idiopathic choroidal vasculopathy may be best treated with a combination of focal therapy to the polyps (laser or photodynamic therapy) and anti-VEGF injections.