4 Western medical acupuncture in neurological conditions
Application of acupuncture in neurological conditions
Presenting symptoms may be broadly categorized as: (1) sensory symptoms; (2) motor symptoms; (3) visceral symptoms; and (4) generalized symptoms such as mood disturbance. The development of clearly reasoned treatments for patients based on Western science requires some understanding of these presentations and an awareness of current literature. This chapter will explore these presentations and consider possible treatment options, including acupuncture.
Basic mechanisms of action of acupuncture
White et al. [1] have clearly summarized the main mechanisms underlying the effects of acupuncture. These include: (1) local effects; (2) segmental effects; (3) extrasegmental and central regulatory effects; and (4) effects relating to myofascial trigger points.
Part 1 Sensory function
Absent or impaired sensation
Damage to sensory pathways within the nervous system may lead to impairments in sensation. The degree of impairment will depend on the site and extent of the lesion. Sensation and proprioceptive deficits may lead to considerable difficulties with balance and mobility as well as functioning of the upper limbs [2]. The loss of sensory information due to lesions within the pathways has a substantial effect on cortical responsiveness and topographical organization of the primary sensory cortex [3].
Prevalence of impaired sensation in neurological conditions
Sensory deficits are common in neurological conditions, with an estimated prevalence of up to 65% in stroke [4], 80–90% of those with multiple sclerosis [5] and most individuals with spinal cord injury. Sensory features are also prominent in peripheral neuropathies involving the sensory nerves, such as alcoholic peripheral neuropathy and the acute motor and sensory axonal neuropathy subtype of Guillain–Barré syndrome [6, 7].
Acupuncture for impaired sensation
We are unaware of any studies specifically using acupuncture to improve sensation, although studies by Napadow et al. [8] indicate that modifications of the primary sensory cortex may be induced by acupuncture. Many studies in neurorehabilitation have used various types of sensory stimulation, but these usually measure the outcome in terms of improved motor function rather than commenting on sensory function [9]. Perhaps this is an area that merits further exploration.
Abnormal sensations, including paraesthesia and pain
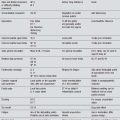
Pain is common and problematic in neurological conditions (Table 4.1). Effective management is needed and acupuncture may be of value in this situation. It is important to understand the nature of the pain presentation to guide acupuncture intervention and expected outcomes.
Table 4.1 Prevalence of pain in common neurological conditions
| Condition | Nociceptive pain | Neuropathic pain |
|---|---|---|
| Stroke | No data |
References: MacGowan et al. (147); Moulin et al. (148); Weimar et al. (149); Siddall et al. (150); Jahnsen et al. (151); Kogos et al. (152); Nampiaparampil (153); Ruts et al. (154); Beiske et al. (155); Osterberg & Boivie (5).
Is the pain nociceptive or neuropathic?
‘Nociceptive pain’ refers to pain which results from activation of nociceptors in the tissues, for example by injury, inflammation, ischaemia or degeneration [10]. It is commonly described as dull or aching and may affect the musculoskeletal system or the viscera. This type of pain is very common and often responds well to simple interventions such as analgesics, exercise, transcutaneous electrical nerve stimulation (TENS) or acupuncture (Tables 4.2 and 4.3).
| Type of Pain | IASP* Definition, 2008 | Subdivisions of pain |
|---|---|---|
| Nociceptive pain | Pain arising from stimulation of nociceptors |
* IASP, International Association for the Study of Pain.10
Table 4.3 Clinical examples of nociceptive and neuropathic pain
‘Neuropathic pain’ refers to pain which arises as a direct consequence of a lesion or disease affecting the somatosensory system [10]. As such it is common in neurological conditions. This type of pain may develop spontaneously or may be evoked by various stimuli, which are normally innocuous. It is commonly described as burning, shooting, pricking or throbbing. Neuropathic pain may affect the peripheral or central neural pathways (Tables 4.2 and 4.3). It is challenging to treat and may require the use of medications such as amitriptyline or gabapentin, psychological therapies such as cognitive-behavioural therapy and physiotherapy [11]. Refractory cases may require consideration for spinal cord stimulation or deep brain stimulation.
Mechanisms of neuropathic pain in neurological conditions
Peripheral neuropathy
Peripheral focal or generalized neuropathies involve loss of myelin and/or axonal damage of peripheral nerves. Neuropathic pain may arise from molecular changes in the area of injured or degenerating axons [12]. Hypersensitivity in the peripheral nervous system will then drive the development of hypersensitivity in the spinal cord and central pain transmission pathways.
Stroke, traumatic brain injury, spinal cord injury, multiple sclerosis
In stroke, traumatic brain injury, spinal cord injury and multiple sclerosis functional impairment of the spinothalamocortical tract conveying pain and temperature sensation from the periphery to the cortex seems crucially important in the development of neuropathic pain [5, 13–15]. In spinal cord injury evidence suggests that those individuals with more extensive reorganization of the primary somatosensory cortex experience more severe ongoing pain [16].
Parkinson’s disease
The disturbance of central processing of nociceptive information by the basal ganglia is likely to have a role in the development of central neuropathic pain in Parkinson’s disease [17, 18]. In addition dysfunction of nociceptive processing at spinal cord level is also involved [19].
Evidence for use of acupuncture for pain in neurological conditions
Peripheral neuropathy
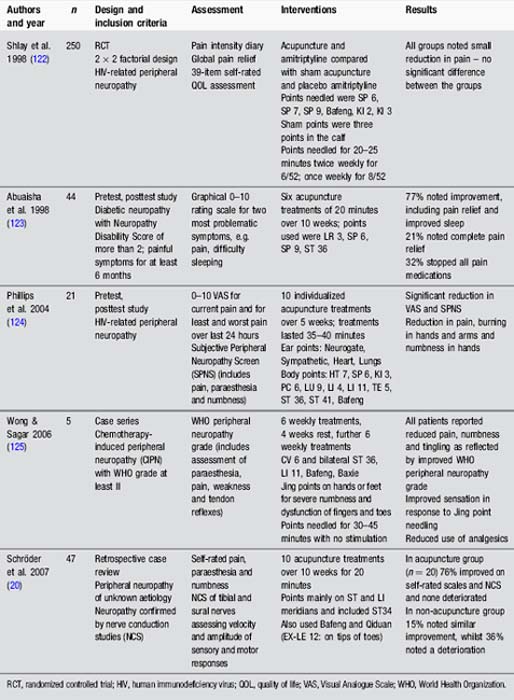
Studies have evaluated the use of acupuncture for peripheral neuropathy of various aetiologies (Table 4.4). These reveal some positive results, particularly the finding that acupuncture improved nerve conduction in tibial and sural nerves [20]. Further prospective research is needed.
Spinal cord injury
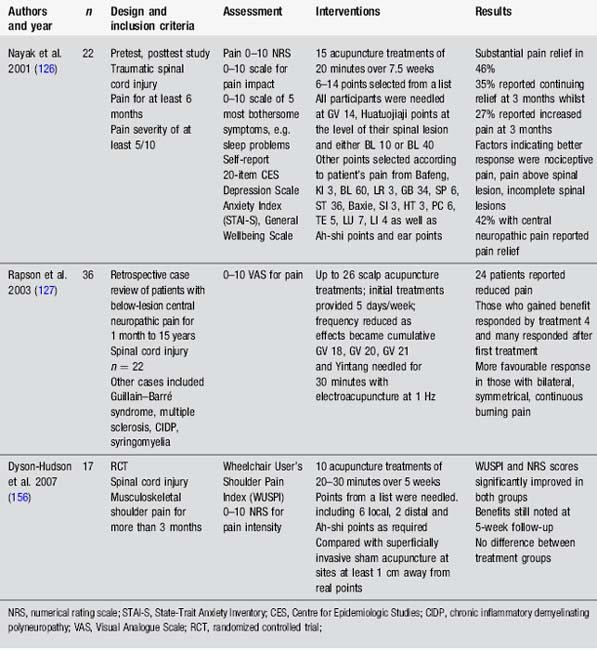
Studies in spinal cord injury are small. They provide hints that acupuncture may be useful for nociceptive and neuropathic pain (Table 4.5).
Multiple sclerosis
No large-scale trials have been conducted. Descriptive case reports suggest benefit from electroacupuncture at 50 Hz for painful trigeminal neuralgia [21], as well as body and ear acupuncture for leg pain and dysaesthesia affecting the hands [22, 23].
Stroke, traumatic brain injury, Parkinson’s disease and adult cerebral palsy
Descriptive case reports indicate benefits from acupuncture for central neuropathic pain in stroke [24], for nociceptive shoulder pain in stroke [25] and for central neuropathic pain in traumatic brain injury [26]. Implanted percutaneous electrical nerve stimulation (PENS) reduced chronic hemiplegic shoulder pain [27]. No large-scale studies have been conducted.
Paraesthesia and dysaesthesia
At this point it is worth mentioning abnormal sensations which are commonly reported in neurological conditions. ‘Paraesthesia’ refers to abnormal sensations which are not described as unpleasant [28]. This may include sensations such as tingling, prickling, pins and needles, burning, aching and tightness. ‘Dysaesthesia’ is defined as an unpleasant abnormal sensation and includes the more specific categories of allodynia and hyperalgesia. It is therefore included in the category of neuropathic pain [28]. Paraesthesia and dysaesthesia may represent a pure sensory phenomenon such as in cortical strokes affecting the postcentral gyrus, or may be accompanied by motor signs such as tonic spasms in multiple sclerosis [29, 30]. Paraesthesia and dysaesthesia may be spontaneous or evoked. They arise from abnormal activity in the nervous system such as ectopic impulse activity in the central or peripheral nerves, or ephaptic transmission between physically adjacent neurones in areas of demyelination [31]. These sensations cause substantial distress to some individuals. Management of these sensations follows the recommendations for neuropathic pain.
Acupuncture for paraesthesia and dysaesthesia
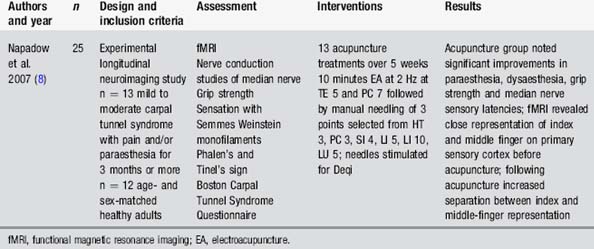
Carpal tunnel syndrome is an entrapment neuropathy of the median nerve causing paraesthesia and pain. Napadow [8, 32] reported improvements in these symptoms following a course of acupuncture (Table 4.6). Functional magnetic resonance imaging indicated changes in cortical representation as well as reduced activation of the limbic system. These changes accompanied reports of improvement from the patients. It may also be worth attempting needling with patients with dysaesthesia from central sensory lesions. No research has been conducted on this population.
Part 2 Motor function
Weakness and paralysis
Acupuncture for weakness and paralysis
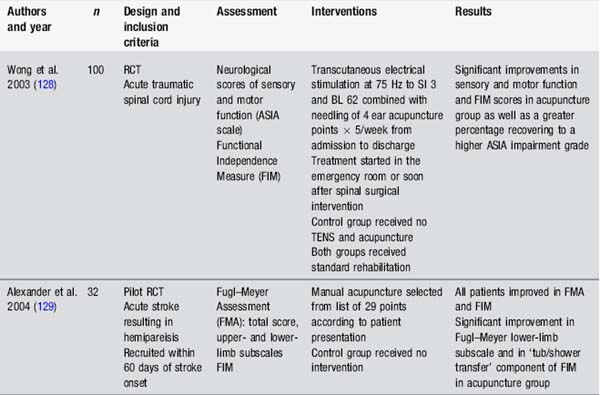
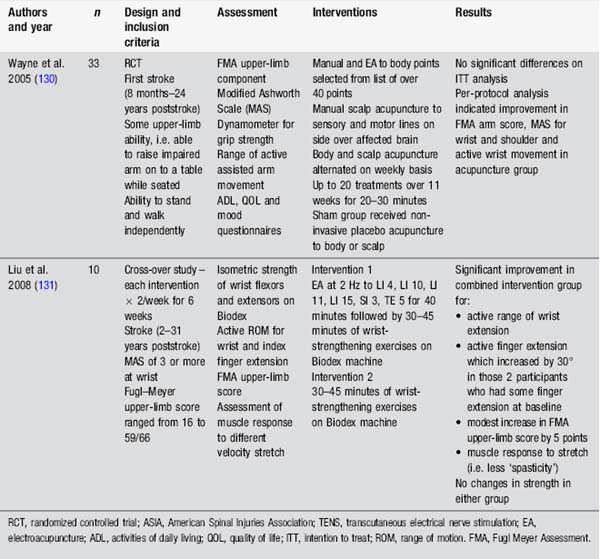
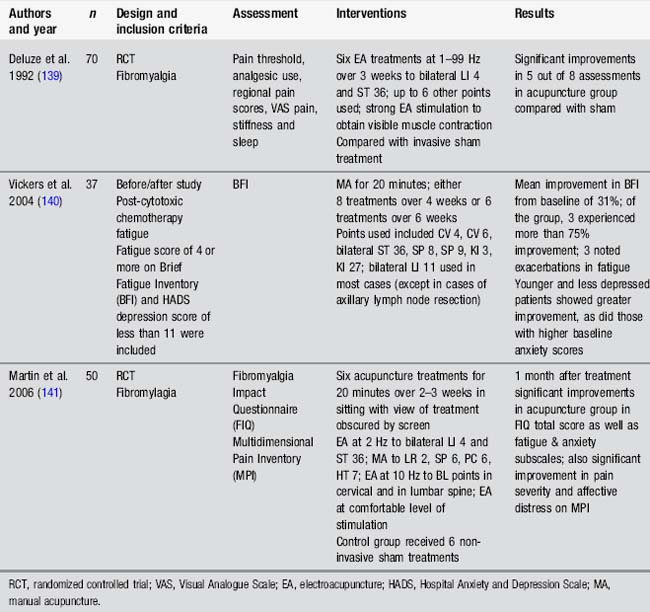
Many studies have assessed the impact of acupuncture on motor recovery in stroke. Fewer studies have assessed weakness in other populations (Table 4.7).
These studies suggest some benefits from a range of interventions, including body, ear and scalp acupuncture, as well as in combination with a strengthening programme. However these studies also raise questions about acupuncture study design, particularly regarding the upper limb. None of the acupuncture studies assessing arm function in stroke had exclusion criteria according to motor ability in the fingers or wrist. Research indicates that the ability to extend the fingers is a powerful prognostic factor for eventual arm function in stroke [33]. Other studies stipulate this ability as minimal entry level to studies, for example in constraint-induced movement therapy [34]. Interestingly, in Liu’s study [35], those individuals with some active finger extension before the acupuncture intervention gained an additional 30° with combined acupuncture and strength training. Design of studies with more defined entry criteria might provide useful information about the effects of acupuncture for motor recovery in specific subpopulations.
Spasticity
Spasticity is a movement disorder observed in individuals with lesions of the upper motor neurone pathways, for example, in stroke, multiple sclerosis or spinal cord injury. Spasticity is classically defined as a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes [36]. It is accompanied by a range of other features such as weakness and loss of dexterity [37]. The neurophysiology of spasticity is complex but involves abnormalities in proprioceptive, cutaneous and nociceptive reflexes. Persistent spasticity may lead to adaptive changes such as soft-tissue contracture. The impact of spasticity on functional ability is unclear but for many individuals spasticity is problematic [38].
Prevalence of spasticity
The prevalence of spasticity varies according to condition, with over 90% of those with cerebral palsy [39], 84% of people with multiple sclerosis [40], 65–78% of people with spinal cord injury [41] but only around 17% of people with stroke presenting with spasticity [42]. Some people with traumatic brain injury develop severe and chronic spasticity but prevalence figures are not reported [43].
Management of spasticity
Effective management of spasticity is dependent on detailed assessment. Provoking factors such as ill-fitting splints, constipation or pain need to be addressed since these have the potential to increase spasticity. Oral medications such as baclofen may be used for generalized spasticity, whilst focal treatments such as botulinum toxin injections or phenol nerve blocks would be considered when spasticity was more localized. Insertion of an intrathecal baclofen pump might be considered for cases of severe regional spasticity, for example affecting both lower limbs. Physiotherapy to improve movement control, balance and strength, as well as stretches for specific muscles, is helpful [44]. Occupational therapists can advise on many useful aids or adaptations, such as an appropriate wheelchair with suitable cushion [38].
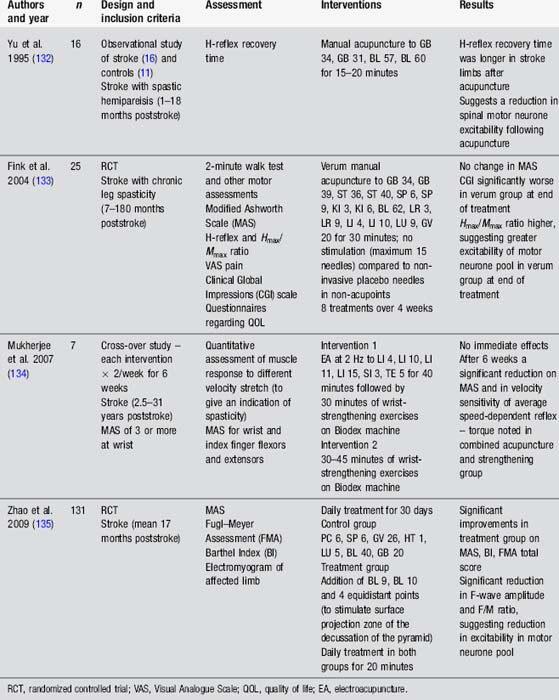
Acupuncture for spasticity
Spasticity is notoriously difficult to quantify. Electrophysiological tests such as the H-reflex, Hmax/Mmax ratio and F-wave may provide information about the excitability of the spinal motorneurone pool. These tests are moderately reliable. However, they do not always correlate with clinical assessments of spasticity. In addition commonly used assessments, such as the Modified Ashworth Scale (MAS), provide information about contracture as well as spasticity [45]. Therefore interventions directed at the dynamic component of spasticity may not result in a change in the MAS score. These methods of assessment have been used in acupuncture studies (Table 4.8).
Parkinsonian dyskinesia
Dyskinesias are abnormal involuntary movements which may appear as jerking, twisting or writhing of parts of the body. This type of movement abnormality is very common in Parkinson’s disease and may result from the disease process itself or as a side-effect of levodopa medication used to treat symptoms. Dyskinesias may be present when drugs have worn off, such as first thing in the morning (‘off’-medication dyskinesia) or during the day when the medication is working (‘on’-medication dyskinesia) [46].
The most common types of dyskinesia in Parkinson’s disease are chorea, ballism, dystonia and myoclonus. Although these movement disorders are problematic, many individuals would prefer being ‘on’ with dyskinesia rather than ‘off’ without dyskinesia [47]. Studies have indicated that dyskinesia and pain commonly coexist and some authors suggest that they may share common pathophysiological mechanisms [48].
Prevalence of parkinsonian dyskinesia
Between 22% and 40% of people with Parkinson’s disease present with ‘on’-medication dyskinesias after 5 years on levodopa medication. This rises to around 62% after 9 years [47]. ‘Off’-medication dyskinesia occurs in about 16% of people with Parkinson’s disease [46].
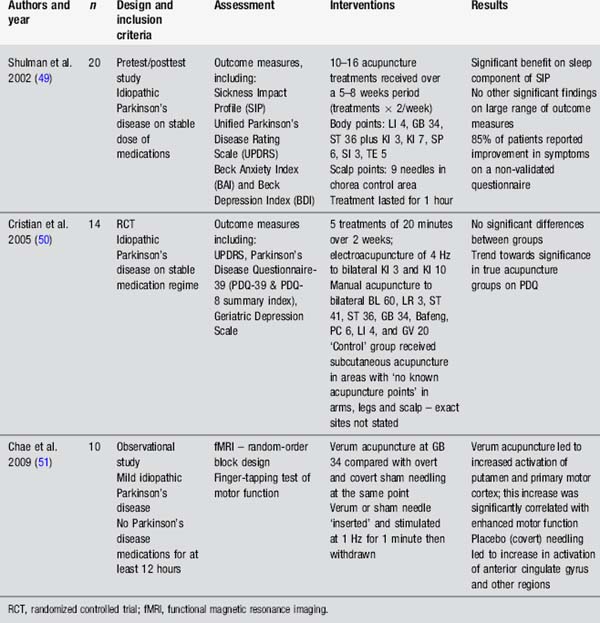
Acupuncture for Parkinson’s disease
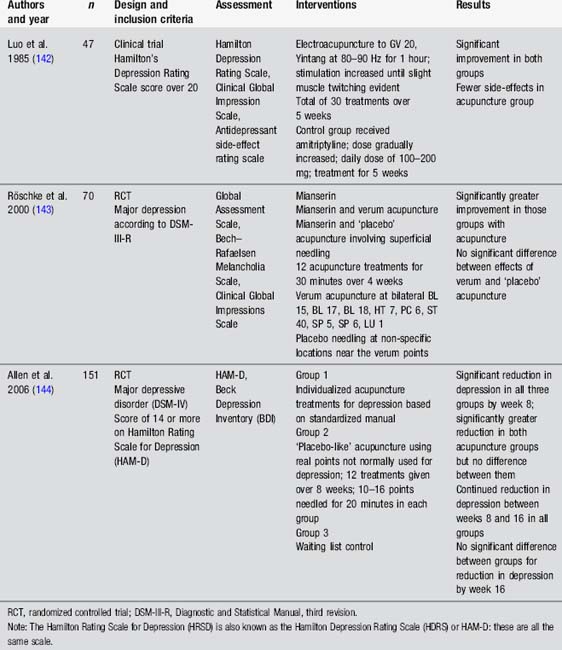
Studies evaluating the use of acupuncture in Parkinson’s disease have generally used measures assessing the global effect of acupuncture on individuals with Parkinson’s disease rather than considering specific impairments such as dyskinesia or pain (Table 4.9). The studies by Shulman et al. [49] and Cristian et al. [50] demonstrate a trend towards improvement in the acupuncture group, including improved sleep, but the evidence is not clear. It is worth noting that both studies are small and the Cristian study used an active control procedure. Chae’s team [51] have provided an interesting insight into the effects of needling GB 34 on motor function and brain activation in experimental conditions. There is a clear need for well-designed, clinically relevant studies to examine this question further.
Part 3 Visceral function
Bladder dysfunction
The bladder has two main functions: storage of urine and voiding of urine. Problems can arise from a disruption of either of these functions, leading to a range of problems such as stress incontinence, urge incontinence or a mixture of these presentations [52]. Urinary incontinence is defined as ‘any involuntary leakage of urine’. The combination of urgency, urge incontinence, daytime frequency and nocturia is termed Overactive Bladder Syndrome (OAB) and reflects hyperactivity of the detrusor [52].
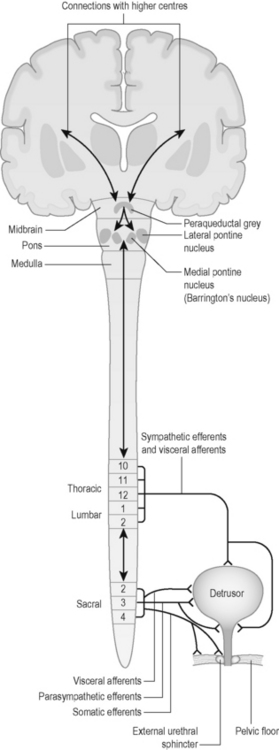
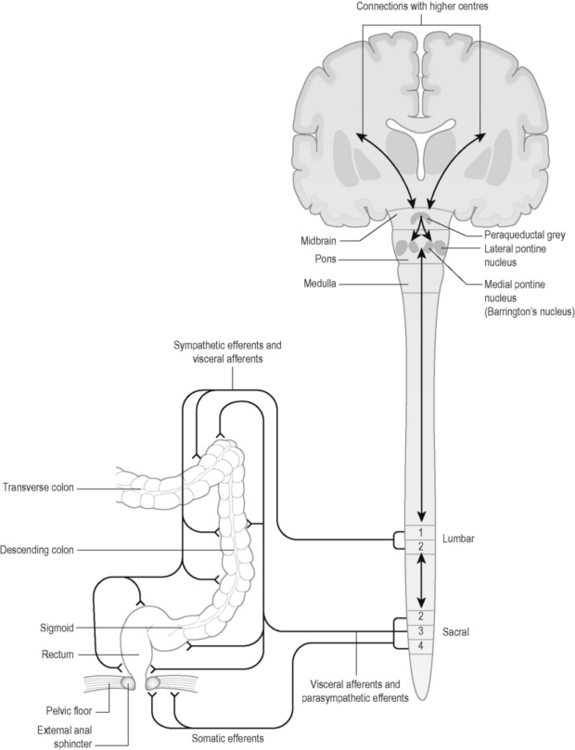
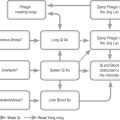
Neural control of the bladder is complex, involving autonomic and somatic pathways linking the spinal cord, brainstem and higher brain centres (Figure 4.1). Control of bladder function in the spinal cord is localized in two main regions (Table 4.10).
| Spinal cord level | Pathways | Effect |
|---|---|---|
| Thoracic 10–lumbar 2 | Visceral afferents | Conveys information regarding bladder distension and contraction |
| Sympathetic efferents |
Prevalence of urinary problems in neurological disease
Bladder dysfunction is common in neurological conditions. Estimated prevalence is more than 80% in those with multiple sclerosis [53], most people with spinal cord injury, 54% of patients with acute stroke and 32% of those at 1 year poststroke [54], up to 39% of those with Parkinson’s disease [55], and around 27% of those with Guillain–Barré syndrome [56].
Key patterns of urinary dysfunction in neurological conditions
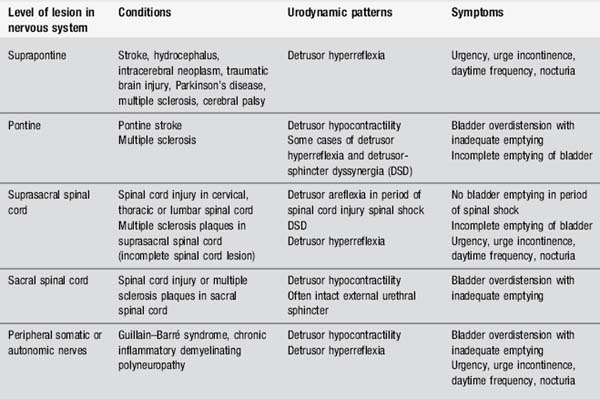
Urinary symptoms vary according to the site of the lesion within the neural pathways (Table 4.11). The primary problems seen are overactivity of the bladder (detrusor hyperreflexia), underactivity of the bladder (detrusor areflexia or hypocontractility) or incoordination of activity between the bladder and the urethral sphincters (detrusor-sphincter dyssynergia).
Management of urinary dysfunction
Management of urinary dysfunction is based on effective assessment and may include the use of bladder diaries, urine testing, postmicturition residual volumes and, when necessary, urodynamic studies [52, 57]. Different types of incontinence will require different management strategies but treatment options may include a combination of advice, education, pelvic floor exercises, bladder retraining, lifestyle modification such as adequate fluid intake and reduction of caffeine intake, as well as use of medications such as antimuscarinics, for example, oxybutynin or tolterodine. Some people may need intermittent catheterization or use of an indwelling catheter. Surgical options may be appropriate for some patients [52].
Electrical stimulation for urinary dysfunction
Electrical stimulation of pelvic floor muscles may be considered for those with stress incontinence who are unable actively to contract their pelvic floor muscles [52]. Various types of sacral nerve and posterior tibial nerve stimulation have been suggested for OAB and for urinary retention. PENS provided benefits for OAB in multiple sclerosis and Parkinson’s disease [58, 59]. Stimulation was applied to the posterior tibial nerve at the area known in acupuncture as Spleen 6. TENS over sacral dermatomes in multiple sclerosis was not effective [60]. However benefits were noted from TENS applied over the posterior tibial nerve in OAB due to multiple sclerosis, Parkinson’s disease, spinal cord injury and stroke [61]. An implanted sacral nerve root stimulator may be useful for individuals with refractory OAB [52]. However in neurological conditions botulinum toxin injections of detrusor would probably be considered first [57].
Acupuncture for bladder dysfunction
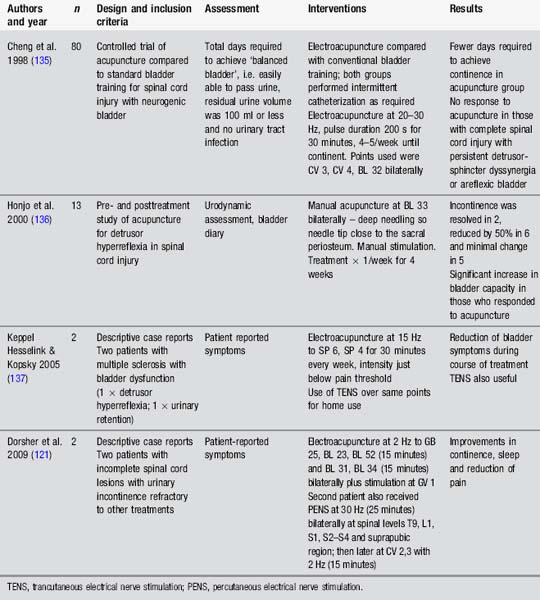
Acupuncture for bladder dysfunction has been considered in a variety of studies (Table 4.12). Early studies on spinal cats by Sato et al. [62] highlighted the influence of somatic afferent stimulation on bladder activity. More recently, Tanaka et al. [63] examined electroacupuncture to the sacral segment in rats and noted suppression of detrusor activity which was often also accompanied by changes in electroencephalogram. This suggests that acupuncture may affect the bladder as well as the sleep arousal system. These would be useful findings for those presenting with nocturia.
These studies (Table 4.12) indicate that stimulation, whether via TENS, manual acupuncture or electroacupuncture, may provide a valuable treatment option to consider in people with neurological conditions who present with urinary dysfunction, particularly OAB. Parasympathetic and sympathetic outflow to the bladder and sphincter may be influenced according to the location of afferent stimulation. Further high-quality studies with larger numbers and appropriate control groups are required.
Bowel dysfunction
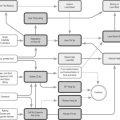
The main functions of the distal part of the large intestine or colon are to absorb water and electrolytes, move residual contents through the colon and to store faeces prior to elimination [64]. The colon and rectum are controlled by the enteric nervous system which includes sympathetic and parasympathetic neurones interconnected with local nerve networks. The maintenance of faecal continence requires the coordinated action of these different neural pathways (Figure 4.2). Spinal control of bowel function is outlined in Table 4.13.
| Spinal cord level | Pathways | Effect |
|---|---|---|
| Lumbar 1–2 | Visceral afferents | Convey information on distension and discomfort from distal colon and rectum |
| Sympathetic efferents |
Note: distal colon includes distal one-third of transverse colon, descending and sigmoid colon.
Prevalence of bowel problems in neurological conditions
Bowel problems are more common in neurological conditions than in the general population. There is an estimated prevalence of bowel dysfunction, including incontinence and constipation, in up to 73% of those with multiple sclerosis [65], 42–95% of those with spinal cord injury [64], 29–67% in Parkinson’s disease [66], up to 56% of those with cerebral palsy [67] and around 30% in patients with stroke [68].
Key patterns of bowel dysfunction in neurological conditions
Reduced activity of the colon with increased colonic transit time is common in many neurological conditions contributing to constipation [64]. Incoordination between the anal sphincter and rectal activity (defecation dyssynergia) may cause obstructed defecation and is found in Parkinson’s disease and multiple sclerosis [65, 66]. Chronic constipation in stroke appears to be related to damage of the dominant cortical area controlling defecation [68]. Faecal incontinence may be noted where there is a lack of anorectal sensation or lack of voluntary control of external anal sphincter and pelvic floor, for example, in spinal cord injury affecting the sacral cord or cauda equina [64].
Management of bowel dysfunction
Full assessment by health care professionals with relevant skills is required to identify the nature of bowel dysfunction and to recommend an appropriate plan [69]. Management options may include advice on diet, fluid intake, exercise and bowel habits, as well as the prescription of various medications. Interventions such as pelvic floor exercises, bowel retraining or biofeedback will be appropriate for some individuals. Discussion of practical issues including access to toilet, mobility, clothing and use of continence products will be relevant in some situations. Sacral nerve stimulation or surgery may be considered for some individuals [69].
Electrical stimulation for bowel dysfunction
Electrical stimulation of pelvic floor muscles has been used for those with faecal incontinence but there is currently insufficient evidence to indicate whether this is useful [70]. Sacral nerve stimulation has been used successfully to manage faecal incontinence and constipation in some patients [71]. Few studies have included people with neurological diseases but a prospective non-randomized study by Jarrett et al. [72] demonstrated a reduction in incontinent episodes in a group of 13 individuals with faecal incontinence due to partial spinal cord injury, with stimulation parameters of a frequency of 15 Hz and pulse duration 200 µs [72].
Acupuncture for bowel dysfunction
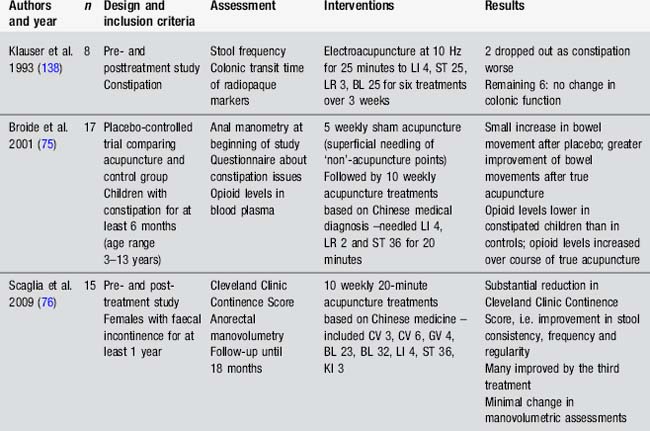
Acupuncture for bowel dysfunction has received very little attention. No placebo-controlled trials have been conducted. A few small uncontrolled trials have been conducted (Table 4.14). Animal studies demonstrate a modulatory effect of electroacupuncture at 10 Hz to Stomach 36 on the motility of the distal colon. This effect is usually to increase motility in the distal colon, although it may be the reverse in some conditions. The effect seems to be mediated through parasympathetic outflow from the sacral spinal cord as well as involving activity in brainstem nuclei [73, 74].
The small studies by Broide et al. [75] and Scaglia et al. [76] suggest benefits for those with constipation and faecal incontinence. No studies have looked at the impact of acupuncture on bowel dysfunction for those with a specific neurological condition. Anecdotal reports suggest benefit for this population but well-designed research is required to guide practice more effectively.
Part 4 Generalized symptoms
Insomnia
Insomnia relates to the difficulty in falling asleep, staying asleep, waking up too early or sleep that is of poor quality [77]. Symptoms occurring in the absence of any other medical condition are termed primary insomnia whilst secondary insomnia refers to symptoms occurring in relation to another medical or psychiatric disorder. Insomnia is typically a complex condition with many contributing factors [78]. It is thought to be a hyperarousal disorder with hyperactivity of the hypothalamic–pituitary axis [77]. Difficulty with sleeping may contribute to daytime sleepiness, fatigue, mood disturbance and poor concentration. In addition, good-quality sleep is important for consolidation of new memories such as new motor skills or declarative knowledge [79, 80].
Prevalence of insomnia in neurological conditions
Prevalence of insomnia is over 50% in stroke [81], up to 51% in people with multiple sclerosis [82], between 46% and 70% of those with traumatic brain injury [83] and between 60% and 98% in Parkinson’s disease [84]. These figures are clearly higher than those for the general population, of around 30% [77].
Insomnia in neurological conditions
Normal sleep patterns are generated and modulated by complex neural systems located mainly in the hypothalamus, brainstem and thalamus [85]. People with neurological conditions may develop sleep dysfunction due to primary involvement of these areas [84, 86, 87]. In addition insomnia may be secondary to factors which may predispose the individual to wake, such as pain, anxiety, depression, involuntary limb movements or nocturia [85].
Management of insomnia
Management of insomnia includes the treatment of any precipitating factors, education regarding good sleep habits and the use of medications as necessary. Pharmacological treatments have been widely used for the treatment of insomnia and have included benzodiazepines or other drugs such as zoplicone. These show clear benefits for short-term reduction of sleep disturbance but effects reduce with time [78]. Non-pharmacological options such as cognitive-behavioural therapy provide lasting effects for up to 6 months or longer [78, 83]. However this therapy is not always available and some patients are unwilling or unable to engage in the process.
Acupuncture for insomnia
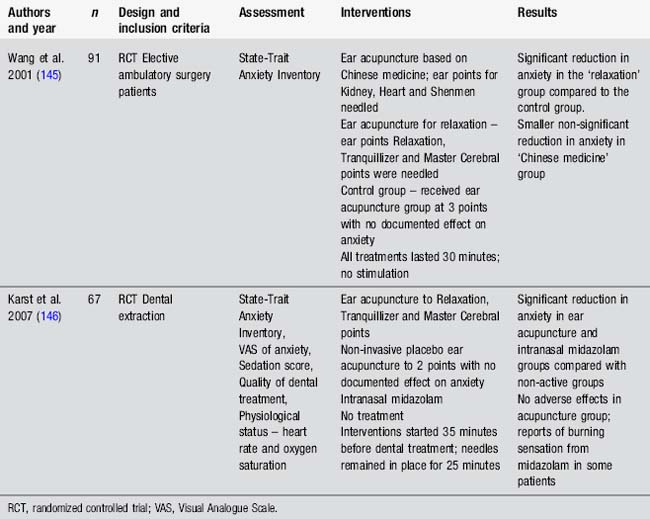
Anecdotal reports by people with neurological conditions indicate benefits from acupuncture for insomnia [88]. An interesting pretest, posttest study by Spence et al. [89] used polysomnography, nighttime melatonin levels and questionnaires to measure the effect of 10 acupuncture treatments provided over 5 weeks to 18 anxious adults [89]. Results indicated improvements in sleep as recorded by polysomnography, increases in nighttime melatonin secretion and improvements in self-rated fatigue and sleepiness. Points used were not specified. Other studies have considered the use of acupuncture in insomnia. However many of these have been small or poorly designed. Consequently a number of recent systematic reviews have concluded that whilst results are promising there is insufficient evidence to support the use of acupuncture for insomnia [90–92]. Studies evaluating acupuncture in neurological conditions have found a reduction of insomnia in patients with stroke and Parkinson’s disease [49, 93]. There is a clear need for large well-designed trials to evaluate this question further.
Fatigue
Fatigue may be defined as feelings of tiredness, exhaustion and lack of physical or mental energy which are abnormal, excessive, chronic, persistent or problematic [94]. In general the type of fatigue reported by people with neurological conditions is disproportionate to the amount of activity carried out and is generally not relieved by resting or sleeping. Fatigue has peripheral and central components. Peripheral fatigue relates to the peripheral neural and musculoskeletal system and results in difficulty performing motor activities. Central fatigue relates to dysfunction of the central nervous system and may cause difficulties in initiating and executing motor or cognitive tasks [95, 96].
Prevalence of fatigue in neurological conditions
Fatigue is reported in many neurological conditions, with prevalence higher than those for the general population. The prevalence of fatigue is reported to be between 39% and 72% of people with stroke [97], 57% of those with spinal cord injury [98], up to 58% of those with Parkinson’s disease [99], 60% of people with Guillain–Barré syndrome [100], around 70% of people with multiple sclerosis [101] and up to 73% of those with traumatic brain injury [102].
Fatigue in neurological conditions
Fatigue is complex. An element of peripheral fatigue will be evident in those individuals with muscle dysfunction such as weakness. However a substantial contribution to fatigue in neurological conditions is made by central mechanisms. The role of impaired central muscle activation and altered activation patterns of motor neurones has been reported [103]. Dopamine deficiency is likely to play a role in fatigue noted in Parkinson’s disease [96]. A role for proinflammatory cytokines in multiple sclerosis fatigue has been suggested [104]. Dysfunction of pathways involved in cognitive-attentional processes has been highlighted as important in fatigue reported in multiple sclerosis and traumatic brain injury [105, 106]. In addition a range of other factors contribute to the presence of fatigue. These include depression, anxiety, sleep disturbance, chronic pain and medication side-effects [94].
Management of fatigue in neurological conditions
Fatigue management will be tailored according to individualized assessment. Options might include medications such as modafinil or amantadine. Non-pharmacological options will broadly aim to maximize the person’s physical ability and minimize fatigue impact. Therapeutic strategies include strengthening and conditioning exercise, education regarding energy conservation and pacing of activities, as well as cognitive-behavioural therapy [94, 107]. Contributory factors such as mood or sleep disturbance also need to be addressed.
Mood disturbance
Mood disorders such as depression and anxiety are highly prevalent in people with neurological conditions (Table 4.16).
Table 4.16 Prevalence of mood disturbance in neurological conditions
| Condition | Depression | Anxiety |
|---|---|---|
| Stroke | 24–30% | 22–25% |
| Traumatic brain injury | 42% | 18–60% |
| Parkinson’s disease | 4–76% | 43% |
| Multiple sclerosis | 31–56% | 19% |
| Spinal cord injury | 37% | 30% |
| Guillain–Barré syndrome (acute phase) | 67% | 82% |
References: Hibbard et al. 1998 (157); Kreutzer et al. 2001 (158); Weiss et al. 2002 (116); De Wit et al. 2008 (159); Brown et al. 2009 (160); Migliorini et al. 2009 (161); Simuni & Sethi 2009 (84); Pontone et al. 2009 (161).
Depression and anxiety
Depression is characterized by a loss of interest in and enjoyment of ordinary things and experiences, as well as low mood, which is persistent and problematic. It is associated with behavioural and physical symptoms such as tearfulness, irritability and social withdrawal as well as less obvious symptoms of poor sleep, general pain or fatigue. Feelings of worthlessness, low self-confidence and helplessness are common. Cognitive symptoms include poor concentration, reduced attention and mental slowing. In some situations agitation and anxiety are also present [109].
Generalized anxiety disorder is characterized by excessive anxiety and worry about various events or activities. Symptoms such as restlessness, becoming fatigued easily, difficulty concentrating, irritability, muscle tension or disturbed sleep will also be evident [110].
Mood disorders in neurological conditions
Mood is regulated by intricate networks of neurones connecting areas such as the anterior cingulate cortex, prefrontal cortex and amygdala. The amygdala coordinates behavioural and autonomic activity in response to sensory information perceived as threatening. Overactivity of this structure has been found in major depression and anxiety [111]. The amygdala is regulated by activity in areas such as the prefrontal cortex and anterior cingulate cortex [112]. Problems with these modulatory circuits may predispose individuals to the development of mood disorders. A number of neurological conditions cause direct damage to these pathways, such as traumatic brain injury and multiple sclerosis [113, 114]. Additionally individuals may develop anxiety and depression as a response to real concerns and fears about how they will cope in their life with the range of difficulties they have developed as a result of their neurological condition.
Management of mood disorders in neurological disorders
Screening for mood disorders should begin early after new diagnosis of a neurological condition and be reviewed on regular basis. This is particularly relevant for those people with communication difficulties who commonly experience anxiety and depression but may be less able to express these concerns [115, 116]. Mild symptoms may respond to the provision of advice and information or the resolution of associated factors such as pain or sleep dysfunction. Symptoms which do not respond to simple measures may require referral to appropriate experts such as clinical psychologists or psychiatrists for specialized assessment. Specific interventions may include medications or psychological therapies such as cognitive-behavioural therapy [117–120].
Conclusion
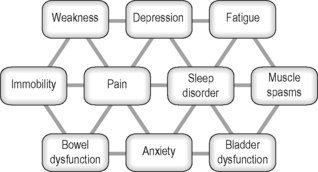
People with neurological conditions present with a wide range of symptoms which commonly interact in complex ways (Figure 4.3). Medications for these symptoms may have unexpected side-effects on other symptoms. Research indicates that acupuncture may be able to contribute to the management of some of these symptoms in some individuals. In general side-effects from acupuncture are low. It therefore represents a useful therapeutic option to consider within the overall management of people with neurological conditions. Findings from the scientific literature provide ideas regarding the application of acupuncture, although more research is required into the specific uses in this population.
[1] White A., Cummings M., Filshie J. An introduction to western medical acupuncture. Edinburgh: Churchill Livingstone, 2008.
[2] Thoumie P., Mevellec E. Relation between walking speed and muscle strength is affected by somatosensory loss in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;73(3):313-315.
[3] Corbetta M., Burton H., Sinclair R.J., et al. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A. 2002;99(26):17066-17071.
[4] Winward C.E., Halligan P.W., Wade D.T. Somatosensory recovery: a longitudinal study of the first 6 months after unilateral stroke. Disabil Rehabil. 2007;29(4):293-299.
[5] Osterberg A., Boivie J. Central pain in multiple sclerosis – sensory abnormalities. Eur J Pain. 2009. doi:10.1016/j.ejpain.2009.03.003
[6] Hughes R.A., Cornblath D.R. Guillain–Barré syndrome. Lancet. 2005;366(9497):1653-1666.
[7] Koike H., Sobue G. Alcoholic neuropathy. Curr Opin Neurol. 2006;19(5):481-486.
[8] Napadow V., Liu J., Li M., et al. Somatosensory cortical plasticity in carpal tunnel syndrome treated by acupuncture. Hum Brain Mapp. 2007;28(3):159-171.
[9] Conforto A.B., Cohen L.G., dos Santos R.L., et al. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254(3):333-339.
[10] Loeser J.D., Treede R.D. The Kyoto protocol of IASP Basic Pain Terminology. Pain. 2008;137(3):473-477.
[11] Hansson P.T., Attal N., Baron R., et al. Toward a definition of pharmacoresistant neuropathic pain. Eur J Pain. 2009;13(5):439-440.
[12] Baron R. Mechanisms of disease: neuropathic pain – a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95-106.
[13] Hari A.R., Wydenkeller S., Dokladal P., et al. Enhanced recovery of human spinothalamic function is associated with central neuropathic pain after SCI. Exp Neurol. 2009;216(2):428-430.
[14] Kumar B., Kalita J., Kumar G. Central poststroke pain: a review of pathophysiology and treatment. Anesth Analg. 2009;108(5):1645-1657.
[15] Ofek H., Defrin R. The characteristics of chronic central pain after traumatic brain injury. Pain. 2007;131(3):330-340.
[16] Wrigley P.J., Press S.R., Gustin S.M., et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141(1–2):52-59.
[17] Boivie J. Pain in Parkinson’s disease (PD). Pain. 2009;141(1–2):2-3.
[18] Chudler E.H., Dong W.K. The role of the basal ganglia in nociception and pain. Pain. 1995;60(1):3-38.
[19] Mylius V., Engau I., Teepker M., et al. Pain sensitivity and descending inhibition of pain in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80(1):24-28.
[20] Schröder S., Liepert J., Remppis A., et al. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14(3):276-281.
[21] Rampes H. Treatment of trigeminal neuralgia with electro-acupuncture in a case of multiple sclerosis. Acupunct Med. 1994;12(1):45.
[22] Blackwell R. What are the options available for the treatment of multiple sclerosis by acupuncture? University of Wales, 2001.
[23] Smith M.O., Rabinowitz N. Acupuncture treatment of multiple sclerosis: two detailed clinical presentations. Am J Acupunct. 1986;14(2):143-146.
[24] Yen H.L., Chan W. An East–West approach to the management of central post-stroke pain. Cerebrovasc Dis. 2003;16(1):27-30.
[25] Lindfield H. Chronic shoulder pain in stroke. Are we missing the acupoint? Physiother Res Int. 2002;7(1):44.
[26] Donnellan C.P. Acupuncture for central pain affecting the ribcage following traumatic brain injury and rib fractures – a case report. Acupunct Med. 2006;24(3):129-133.
[27] Chae J., Yu D.T., Walker M.E., et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12-month follow-up of a multiple-center, randomized clinical trial. Am J Phys Med Rehabil. 2005;84(11):832-842.
[28] IASP Task Force on Taxonomy. Classification of chronic pain. Part III: pain terms, a current list with definitions and notes on usage. Seattle: IASP Press, 1994.
[29] Kim J. Patterns of sensory abnormality in cortical stroke; evidence for a dichotomized sensory system. Neurology. 2007;68(3):174-180.
[30] Toru S., Yokota T., Tomimitsu H., et al. Somatosensory-evoked cortical potential during attacks of paroxysmal dysesthesia in multiple sclerosis. Eur J Neurol. 2005;12(3):233-234.
[31] Smith K., McDonald W. The pathophysiology of multiple sclerosis: the mechanisms underlying the production of symptoms and the natural history of the disease. Philos Trans R Soc Lond B Biol Sci. 1999;354(1390):1649-1673.
[32] Napadow V., Kettner N., Liu J., et al. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. 2007;130(3):254-266.
[33] Smania N., Gambarin M., Tinazzi M., et al. Are indexes of arm recovery related to daily life autonomy in patients with stroke? Eur J Phys Rehabil Med. 2009;45(3):349-354.
[34] Taub E., Uswatte G., King D.K., et al. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37(4):1045-1049.
[35] Liu W., Mukherjee M., Sun C., et al. Electroacupuncture may help motor recovery in chronic stroke survivors: a pilot study. J Rehabil Res Dev. 2008;45(4):587-595.
[36] Lance J.W. Symposium synopsis. In: Feldman R.G., Young R.R., Koella W.P., editors. Spasticity: disordered motor control. Chicago: Year Book Medical Publishers; 1980:485-494.
[37] Gracies J.M. Pathophysiology of spastic paresis. I: Paresis and soft tissue changes. Muscle Nerve. 2005;31(5):535-551.
[38] Barnes M.P., Johnson G.R. Upper motor neurone syndrome and spasticity. Cambridge: Cambridge University Press, 2008.
[39] Wichers M.J., Odding E., Stam H.J., et al. Clinical presentation, associated disorders and aetiological moments in cerebral palsy: a Dutch population-based study. Disabil Rehabil. 2005;27(10):583-589.
[40] Rizzo M.A., Hadjimichael O.C., Preiningerova J., et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10(5):589-595.
[41] Adams M.M., Hicks A.L. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577-586.
[42] Lundstrom E., Terent A., Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15(6):533-539.
[43] Francisco G.E., Latorre J.M., Ivanhoe C.B. Intrathecal baclofen therapy for spastic hypertonia in chronic traumatic brain injury. Brain Inj. 2007;21(3):335-338.
[44] Boyd R.N., Ada L. Physiotherapy management of spasticity. In: Barnes M.P., Johnson G.R., editors. Upper motor neurone syndrome and spasticity. Cambridge: Cambridge University Press; 2008:79-98.
[45] Patrick E., Ada L. The Tardieu scale differentiates contracture from spasticity whereas the Ashworth scale is confounded by it. Clin Rehabil. 2006;20(2):173-182.
[46] Cubo E., Gracies J.M., Benabou R., et al. Early morning off-medication dyskinesias, dystonia, and choreic subtypes. Arch Neurol. 2001;58(9):1379-1382.
[47] Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord. 2005;20(Suppl. 11):S11-S16.
[48] Lim S.Y., Farrell M.J., Gibson S.J., et al. Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson’s disease? Mov Disord. 2008;23(12):1689-1695.
[49] Shulman L.M., Wen X., Weiner W.J., et al. Acupuncture therapy for the symptoms of Parkinson’s disease. Mov Disord. 2002;17(4):799-802.
[50] Cristian A., Katz M., Cutrone E., et al. Evaluation of acupuncture in the treatment of Parkinson’s disease: a double-blind pilot study. Mov Disord. 2005;20(9):1185-1188.
[51] Chae Y., Lee H., Kim H., et al. Parsing brain activity associated with acupuncture treatment in Parkinson’s diseases. Mov Disord. 2009;24(12):1794-1802.
[52] NICE. Urinary incontinence: the management of urinary incontinence in women. London: National Institute for Health & Clinical Excellence/National Collaborating Centre for Women’s and Children’s Health, 2006.
[53] Ciancio S.J., Mutchnik S.E., Rivera V.M., et al. Urodynamic pattern changes in multiple sclerosis. Urology. 2001;57(2):239-245.
[54] Kolominsky-Rabas P.L., Hilz M.J., Neundoerfer B., et al. Impact of urinary incontinence after stroke: results from a prospective population-based stroke register. Neurourol Urodyn. 2003;22(4):322-327.
[55] Winge K., Fowler C.J. Bladder dysfunction in parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21(6):737-745.
[56] Sakakibara R., Uchiyama T., Kuwabara S., et al. Prevalence and mechanism of bladder dysfunction in Guillain–Barré syndrome. Neurourol Urodyn. 2009;28(5):432-437.
[57] Fowler C.J., Panicker J.N., Drake M., et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):470-477.
[58] Kabay S.C., Kabay S., Yucel M., et al. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourol Urodyn. 2009;28(1):62-67.
[59] Kabay S.C., Yucel M., Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urology. 2008;71(4):641-645.
[60] Fjorback M.V., Van Rey F.S., Rijkhoff N.J., et al. Electrical stimulation of sacral dermatomes in multiple sclerosis patients with neurogenic detrusor overactivity. Neurourol Urodyn. 2007;26(4):525-530.
[61] Amarenco G., Ismael S.S., Even-Schneider A., et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169(6):2210-2215.
[62] Sato A., Sato Y., Schmidt R.F., et al. Somato-vesical reflexes in chronic spinal cats. J Auton Nerv Syst. 1983;7(3–4):351-362.
[63] Tanaka Y., Koyama Y., Jodo E., et al. Effects of acupuncture to the sacral segment on the bladder activity and electroencephalogram. Psychiatry Clin Neurosci. 2002;56(3):249-250.
[64] Krogh K., Christensen P., Laurberg S. Colorectal symptoms in patients with neurological diseases. Acta Neurol Scand. 2001;103(6):335-343.
[65] Wiesel P.H., Norton C., Glickman S., et al. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol. 2001;13(4):441-448.
[66] Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003;2(2):107-116.
[67] Turk M.A., Scandale J., Rosenbaum P.F., et al. The health of women with cerebral palsy. Phys Med Rehabil Clin N Am.. 2001;12(1):153-168.
[68] Bracci F., Badiali D., Pezzotti P., et al. Chronic constipation in hemiplegic patients. World J Gastroenterol. 2007;13(29):3967-3972.
[69] NICE. Faecal incontinence: the management of faecal incontinence in adults. London: National Institute for Health & Clinical Excellence, 2007.
[70] Hosker G., Cody J.D., Norton C.C. Electrical stimulation for faecal incontinence in adults. Cochrane Database Syst Rev. 3, 2007. CD001310
[71] Mowatt G., Glazener C., Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 3, 2007. CD004464
[72] Jarrett M.E., Matzel K.E., Christiansen J., et al. Sacral nerve stimulation for faecal incontinence in patients with previous partial spinal injury including disc prolapsed. Br J Surg. 2005;92(6):734-739.
[73] Iwa M., Matsushima M., Nakade Y., et al. Electroacupuncture at ST-36 accelerates colonic motility and transit in freely moving conscious rats. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G285-G292.
[74] Luo D., Liu S., Xie X., et al. Electroacupuncture at acupoint ST-36 promotes contractility of distal colon via a cholinergic pathway in conscious rats. Dig Dis Sci. 2008;53(3):689-693.
[75] Broide E., Pintov S., Portnoy S., et al. Effectiveness of acupuncture for treatment of childhood constipation. Dig Dis Sci. 2001;46(6):1270-1275.
[76] Scaglia M., Delaini G., Destefano I., et al. Fecal incontinence treated with acupuncture – a pilot study. Auton Neurosci. 2009;145(1–2):89-92.
[77] Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl.):S7-S10.
[78] Sateia M.J., Nowell P.D. Insomnia. Lancet. 2004;364(9449):1959-1973.
[79] Gais S., Albouy G., Boly M., et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104(47):18778-18783.
[80] Fischer S., Nitschke M.F., Melchert U.H., et al. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005;25(49):11248-11255.
[81] Bassetti C.L. Sleep and stroke. Semin Neurol. 2005;25(1):19-32.
[82] Bamer A.M., Johnson K.L., Amtmann D., et al. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008;14(8):1127-1130.
[83] Ouellet M.C., Morin C.M. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: a single-case experimental design. Arch Phys Med Rehabil. 2007;88(12):1581-1592.
[84] Simuni T., Sethi K. Nonmotor manifestations of Parkinson’s disease. Ann Neurol. 2009;64(S2):S65-S80.
[85] Jennum P., Santamaria J. Report of an EFNS task force on management of sleep disorders in neurologic disease (degenerative neurologic disorders and stroke). Eur J Neurol. 2007;14(11):1189-1200.
[86] Parcell D.L., Ponsford J.L., Redman J.R., et al. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch Phys Med Rehabil. 2008;89(5):843-850.
[87] Zeitzer J.M., Ayas N.T., Shea S.A., et al. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. Journal of Clinical Endocrinology Metabolism. 2000;85(6):2189-2196.
[88] Vaghela S.A., Donnellan C.P. Acupuncture for back pain, knee pain and insomnia in transverse myelitis – a case report. Acupunct Med. 2008;26(3):188-192.
[89] Spence D.W., Kayumov L., Chen A., et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatry Clin Neurosci. 2004;16(1):19-28.
[90] Cheuk D.K., Yeung W.F., Chung K.F., et al. Acupuncture for insomnia. Cochrane Database Syst Rev. 3, 2007. CD005472
[91] Huang W., Kutner N., Bliwise D.L. A systematic review of the effects of acupuncture in treating insomnia. Sleep Med Rev. 2009;13(1):73-104.
[92] Yeung W.F., Chung K.F., Leung Y.K., et al. Traditional needle acupuncture treatment for insomnia: A systematic review of randomized controlled trials. Sleep Med. 2009;10(7):694-704.
[93] Kim Y.S., Lee S.H., Jung W.S., et al. Intradermal acupuncture on shen-men and nei-kuan acupoints in patients with insomnia after stroke. Am J Chin Med. 2004;32(5):771-778.
[94] De Groot M.H., Phillips S.J., Eskes G.A. Fatigue associated with stroke and other neurologic conditions: Implications for stroke rehabilitation. Arch Phys Med Rehabil. 2003;84(11):1714-1720.
[95] Levine J., Greenwald B.D. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Phys Med Rehabil Clin N Am. 2009;20(2):347-361.
[96] Lou J.S., Kearns G., Benice T., et al. Levodopa improves physical fatigue in Parkinson’s disease: a double-blind, placebo-controlled, crossover study. Mov Disord. 2003;18(10):1108-1114.
[97] Colle F., Bonan I., Gellez Leman M.C., et al. Fatigue after stroke. Ann Readapt Med Phys. 2006;49(6):272-274.
[98] Fawkes-Kirby T.M., Wheeler M.A., Anton H.A., et al. Clinical correlates of fatigue in spinal cord injury. Spinal Cord. 2008;46(1):21-25.
[99] Barone P., Antonini A., Colosimo C., et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641-1649.
[100] Garssen M.P., van K.R., van Doorn P.A. Residual fatigue is independent of antecedent events and disease severity in Guillain–Barré syndrome. J Neurol. 2006;253(9):1143-1146.
[101] Zwarts M.J., Bleijenberg G., van Engelen B.G.M. Clinical neurophysiology of fatigue. Clin Neurophysiol. 2008;119(1):2-10.
[102] Belmont A., Agar N., Hugeron C., et al. Fatigue and traumatic brain injury. Ann Readapt Med Phys. 2006;49(6):283-284.
[103] Dobkin B.H. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Repair. 2008;22(2):105-110.
[104] Heesen C., Nawrath L., Reich C., et al. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77(1):34-39.
[105] Sepulcre J., Masdeu J.C., Goni J. Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways. Mult Scler. 2009;15(3):337-344.
[106] Ziino C., Ponsford J. Selective attention deficits and subjective fatigue following traumatic brain injury. Neuropsychology. 2006;20(3):383-390.
[107] van Kessel K., Moss-Morris R., Willoughby E., et al. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70(2):205-213.
[108] Assefi N.P., Sherman K.J., Jacobsen C., et al. A randomized clinical trial of acupuncture compared with sham acupuncture in fibromyalgia. Ann Intern Med. 2005;143(1):10-19.
[109] National Collaborating Centre for Mental Health & Royal College of Psychiatrists. Depression: management of depression in primary and secondary care. National clinical practice guideline number 23. London: The British Psychological Society/The Royal College of Psychiatrists, 2004.
[110] McIntosh A., Cohen A., Turnbull N., et al. Clinical guidelines and evidence review for panic disorder and generalised anxiety disorder. Sheffield: University of Sheffield/London: National Collaborating Centre for Primary Care, 2004.
[111] Carter C.S., Krug M.K. The functional neuroanatomy of dread: Functional magnetic resonance imaging insights into generalized anxiety disorder and its treatment. Am J Psychiatry. 2009;166(3):263-265.
[112] Johnstone T., van Reekum C.M., Urry H.L., et al. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877-8884.
[113] Jorge R.E., Robinson R.G., Moser D., et al. Major depression following traumatic brain injury. Arch Gen Psychiatry. 2004;61(1):42-50.
[114] Passamonti L., Cerasa A., Liguori M., et al. Neurobiological mechanisms underlying emotional processing in relapsing-remitting multiple sclerosis. Brain. 2009. (in press)
[115] Kauhanen M.L., Korpelainen J.T., Hiltunen P., et al. Aphasia, depression, and non-verbal cognitive impairment in ischaemic stroke. Cerebrovasc Dis. 2000;10(6):455-461.
[116] Weiss H., Rastan V., Mullges W., et al. Psychotic symptoms and emotional distress in patients with Guillain–Barré syndrome. Eur Neurol. 2002;47(2):74-78.
[117] Royal College of Physicians. National clinical guideline for stroke. London: Royal College of Physicians, 2008.
[118] Royal College of Physicians and British Society of Rehabilitation Medicine. Rehabilitation following acquired brain injury: national clinical guidelines. London: Royal College of Physicians; British Society of Rehabilitation Medicine, 2003.
[119] National Collaborating Centre for Chronic Conditions. Parkinson’s disease; national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians, 2006.
[120] NICE. Multiple sclerosis: National clinical guidelines for diagnosis and management in primary and secondary care. London: National Institute for Clinical Excellence/National Collaborating Centre for Chronic Conditions, 2003.
[121] Dorsher P.T., Reimer R., Nottmeier E. Treatment of urinary incontinence due to incomplete spinal cord injury with acupuncture and percutaneous electrical nerve stimulation: two cases and literature review. Medical Acupuncture. 2009;21(1):21-26.
[122] Shlay J.C., Chaloner K., Max M.B., et al. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: a randomized controlled trial. JAMA. 1998;280(18):1590-1595.
[123] Abuaisha B.B., Costanzi J.B., Boulton A.J.M. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res Clin Pract. 1998;39(2):115-121.
[124] Phillips K.D., Skelton W.D., Hand G.A. Effect of acupuncture administered in a group setting on pain and subjective peripheral neuropathy in persons with human immunodeficiency virus disease. J Altern Complement Med. 2004;10(3):449-455.
[125] Wong R., Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy – a case series. Acupunct Med. 2006;24(2):87-91.
[126] Nayak S., Shiflett S.C., Schoenberger N.E., et al. Is acupuncture effective in treating chronic pain after spinal cord injury? Arch Phys Med Rehabil. 2001;82(11):1578-1586.
[127] Rapson L.M., Wells N., Pepper J., et al. Acupuncture as a promising treatment for below-level central neuropathic pain: a retrospective study. J Spinal Cord Med. 2003;26(1):21-26.
[128] Wong A.M., Leong C.P., Su T.Y., et al. Clinical trial of acupuncture for patients with spinal cord injuries. Am J Phys Med rehabil. 2003;82(1):21-27.
[129] Alexander D.N., Cen S., Sullivan K.J., et al. Effects of acupuncture treatment on poststroke motor recovery and physical function: a pilot study. Neurorehabil Neural Repair. 2004;18(4):259-267.
[130] Wayne P.M., Krebs D.E., Macklin E.A., et al. Acupuncture for upper-extremity rehabilitation in chronic stroke: a randomized sham-controlled study. Arch Phys Med Rehabil. 2005;86(12):2248-2255.
[131] Yu Y.H., Wang H.C., Wang Z.J. The effect of acupuncture on spinal motor neuron excitability in stroke patients. Zhonghua Yi Xue Za Zhi (Taipei). 1995;56(4):258-263.
[132] Fink M., Rollnik J.D., Bijak M., et al. Needle acupuncture in chronic poststroke leg spasticity. Arch Phys Med Rehabil. 2004;85(4):667-672.
[133] Mukherjee M., McPeak L.K., Redford J.B., et al. The effect of electro-acupuncture on spasticity of the wrist joint in chronic stroke survivors. Arch Phys Med Rehabil. 2007;88(2):159-166.
[134] Zhao J.G., Cao C.H., Liu C.Z., et al. Effect of acupuncture treatment on spastic states of stroke patients. J Neurol Sci. 2009;276(1–2):143-147.
[135] Cheng P.T., Wong M.K., Chang P.L. A therapeutic trial of acupuncture in neurogenic bladder of spinal cord injured patients – a preliminary report. Spinal Cord. 1998;36(7):476-480.
[136] Honjo H., Naya Y., Ukimura O., et al. Acupuncture on clinical symptoms and urodynamic measurements in spinal-cord-injured patients with detrusor hyperreflexia. Urol Int. 2000;65(4):190-195.
[137] Keppel Hesselink J.M., Kopsky D.J. Acupuncture for bladder dysfunctions in multiple sclerosis. Medical Acupuncture. 2005;17(1):38-39.
[138] Klauser A.G., Rubach A., Bertsche O., et al. Body acupuncture: effect on colonic function in chronic constipation. Z Gastroenterol. 1993;31(10):605-608.
[139] Deluze C., Bosia L., Zirbs A., et al. Electroacupuncture in fibromyalgia: results of a controlled trial. BMJ. 1992;305(6864):1249-1252.
[140] Vickers A.J., Straus D.J., Fearon B., et al. Acupuncture for postchemotherapy fatigue: a phase II study. J Clin Oncol. 2004;22(9):1731-1735.
[141] Martin D.P., Sletten C.D., Williams B.A., et al. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clin Proc. 2006;81(6):749-757.
[142] Luo H.C., Jia Y.K., Li Z. Electro-acupuncture vs. amitriptyline in the treatment of depressive states. J Tradit Chin Med. 1985;5(1):3-8.
[143] Röschke J., Wolf C., Muller M.J., et al. The benefit from whole body acupuncture in major depression. J Affect Disord. 2000;57(1–3):73-81.
[144] Allen J.J., Schnyer R.N., Chambers A.S., et al. Acupuncture for depression: a randomized controlled trial. J Clin Psychiatry. 2006;67(11):1665-1673.
[145] Wang S.M., Peloquin C., Kain Z.N. The use of auricular acupuncture to reduce preoperative anxiety. Anesth Analg. 2001;93(5):1178-1180. table
[146] Karst M., Winterhalter M., Munte S., et al. Auricular acupuncture for dental anxiety: a randomized controlled trial. Anesth Analg. 2007;104(2):295-300.
[147] MacGowan D.J., Janal M.N., Clark W.C., et al. Central poststroke pain and Wallenberg’s lateral medullary infarction: frequency, character, and determinants in 63 patients. Neurology. 1997;49(1):120-125.
[148] Moulin D.E., Hagen N., Feasby T.E., et al. Pain in Guillain–Barré syndrome. Neurology. 1997;48(2):328-331.
[149] Weimar C., Kloke M., Schlott M., et al. Central poststroke pain in a consecutive cohort of stroke patients. Cerebrovasc Dis. 2002;14(3–4):261-263.
[150] Siddall P.J., McClelland J.M., Rutkowski S.B., et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249-257.
[151] Jahnsen R., Villien L., Aamodt G., et al. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J Rehabil Med. 2004;36(2):78-84.
[152] Kogos S.C.Jr, Richards J.S., Banos J.H., et al. Visceral pain and life quality in persons with spinal cord Injury: a brief report. J Spinal Cord Med. 2005;28(4):333-337.
[153] Nampiaparampil D.E. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300(6):711-719.
[154] Ruts L., Rico R., van K.R., et al. Pain accompanies pure motor Guillain–Barré syndrome. J Peripher Nerv Syst. 2008;13(4):305-306.
[155] Beiske A.G., Loge J.H., Ronningen A., et al. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141(1–2):173-177.
[156] Dyson-Hudson T.A., Kadar P., LaFountaine M., et al. Acupuncture for chronic shoulder pain in persons with spinal cord injury: a small-scale clinical trial. Arch Phys Med Rehabil. 2007;88(10):1276-1283.
[157] Hibbard M.R., Uysal S., Kepler K., et al. Axis I psychopathology in individuals with traumatic brain injury. J Head Trauma Rehabil. 1998;13(4):24-39.
[158] Kreutzer J.S., Seel R.T., Gourley E. The prevalence and symptom rates of depression after traumatic brain injury: a comprehensive examination. Brain Inj. 2001;15(7):563-576.
[159] De Wit L., Putman K., Baert I., et al. Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disabil Rehabil. 2008;30(24):1858-1866.
[160] Brown R.F., Valpiani E.M., Tennant C.C. Longitudinal assessment of anxiety, depression, and fatigue in people with multiple sclerosis. Psychol Psychother. 2009;82:41-56.
[161] Migliorini C.E., New P.W., Tonge B.J. Comparison of depression, anxiety and stress in persons with traumatic and non-traumatic post-acute spinal cord injury. Spinal Cord. 2009;47(11):783-788.
[162] Pontone G.M., Williams J.R., Anderson K.E., et al. Prevalence of anxiety disorders and anxiety subtypes in patients with Parkinson’s disease. Mov Disord. 2009;24(9):1333-1338.