Weapons of Mass Destruction
Perspective
The results of an attack with WMD, although admittedly of low probability, are potentially catastrophic. According to a World Health Organization estimate, 50 kg of anthrax spores aerosolized above a city of 5 million people would result in 100,000 deaths, with an additional 150,000 people seriously infected. The cost of managing 100,000 cases of anthrax exposure is estimated at between $6.4 and $26.2 billion.1 Given these considerations, most authorities believe that preparedness for such threats is a priority.
The use of WMD has been predominantly by the military during times of conflict. In recent history, however, the use of these agents has taken an ominous turn. Nonaffiliated groups have begun using WMD directed at civilians to achieve political ends. The Bhagwan cult sprayed salad bars in Oregon with Salmonella in an attempt to influence an election in 1984.2 The Aum Shinrikyo used the nerve agent sarin in an unsuccessful 1994 assassination attempt on three judges in Matsumoto, Japan. This same group used sarin again in the 1995 Tokyo subway attack that killed 11 people.3 The United States experienced multiple anthrax hoaxes during 1997 and 1998, motivated by personal or political agendas. Terrorists initiated an actual anthrax attack using the U.S. mail in 2001 that resulted in 11 deaths. No one has yet used radiologic or nuclear devices in a successful mass terrorist attack, but at least one attempt has occurred. In addition, several highly radioactive sources have been stolen from U.S. medical facilities, and a Russian dissident, Alexander Litvinenko, was assassinated with a radiologic agent (polonium-210) in 2006.
Many agents are potential candidates for weaponization, and some represent a substantial risk (Box 194-1). Management strategies for patients exposed to WMD are frequently similar to strategies for hazardous materials exposure. However, several features associated with WMD make these events unique (Box 194-2). Additional knowledge and skills are required in the evaluation and treatment of WMD victims. These plans represent only one small part of an overall comprehensive emergency management strategy for all hazards (see Chapter 193). Names of departments, bureaus, and agencies that can assist with planning and response to WMD events are listed in Table 194-1.
Table 194-1
Resources and Contacts for Planning and Response to Events Involving Weapons of Mass Destruction
| ORGANIZATION | WEBSITE | TELEPHONE |
| Radiation Emergency Assistance Center/Training Site (REAC/TS) | http://orise.orau.gov/reacts | Daytime: 865-576-3131 Emergency: 865-576-1005 |
| State and local health departments | www.statepublichealth.org | |
| www.cdc.gov/other.htm#states | ||
| Centers for Disease Control and Prevention (CDC) | www.bt.cdc.gov | 800-CDC-INFO |
| Federal Bureau of Investigation (FBI) | www.fbi.gov | |
| Federal Emergency Management Agency (FEMA) | www.fema.gov | 800-621-FEMA |
| U.S. Army Medical Research Institute of Chemical Defense | http://chemdef.apgea.army.mil |
Nuclear and Radiologic Devices
Instead, simple radiologic devices, such as those used by hospitals for radiation therapy, are thought to be the source of choice. These sources are plentiful. They do not detonate on their own and give no warning of their presence unless they are dispersed by a conventional explosive (radiologic dispersal device). Thefts of radiotherapy sources have occurred in the United States. Accidental dispersion from a stolen hospital therapy source in Brazil resulted in the screening of 112,000 people for contamination. A total of 249 people were found to be exposed, 4 of whom ultimately died.2 Placement of such a device at an information kiosk in a crowded mall during a busy holiday shopping season would silently expose countless persons to significant radiation.
Ionizing radiation, regardless of its type, causes injury at the cellular level, usually by damaging DNA. Rapidly dividing cells are the most sensitive. Patients have symptoms within hours to days, depending on the dose. Common syndromes associated with radiation exposure include dermal burns, bone marrow failure, and gastrointestinal dysfunction (e.g., vomiting and gastrointestinal bleeding) (see Chapter 146). A U.S. Department of Homeland Security task force developed an expert consensus document on medical treatment of radiologic casualties, and the results were subsequently published in the peer-reviewed literature.4
Contaminated patients are more challenging, and early involvement of the radiation safety officer is critical. This individual evaluates the degree of the victim’s contamination and monitors radioactivity levels throughout the decontamination process. Internally contaminated patients present a therapeutic challenge because they have radioactive material inside their bodies (e.g., lungs and gastrointestinal tract) or incorporated into their cells. They should be placed in an isolation room, where all secretions and body fluids can be collected. Various medications are available for administration to internally contaminated patients and can limit uptake or facilitate removal of certain radioactive elements. These medications include Radiogardase (Prussian blue) for cesium and thallium ingestions and diethylenetriaminepentaacetic acid (DTPA) for plutonium exposure. Health care providers can receive assistance by calling the Radiation Emergency Assistance Center/Training Site (REAC/TS; http://orise.orau.gov/reacts) at 865-576-3131 (emergency number: 865-576-1005).
Externally contaminated victims have radioactive material on their skin or clothing and are decontaminated by removal of clothing and washing with soap and water. Washing by protected personnel should continue until monitoring by the radiation safety officer demonstrates the absence of radioactivity. If wounds are present, they are decontaminated first. After the wounds are covered with a sterile, waterproof dressing, the remaining skin is washed. Hospitals must be prepared to decontaminate patients because historical data suggest that up to 80% of patients do not receive this intervention before arrival.2 Decontamination before hospital entry is crucial because these individuals can expose caregivers to radiation and contaminate the entire hospital through the ventilation system. Removal of clothing and covering of the head with a surgical cap can reduce contamination by 80% to permit stabilization in the decontamination unit, but complete decontamination should occur before exposure of unprotected staff if the patient’s medical condition permits.
Initial triage of radiation casualties is based on their overall pathologic condition, not on exposure.5 Even patients who have received a lethal dose of radiation do not die immediately as a consequence of the ionizing exposure. Therefore, a patient in acute distress from a myocardial infarction or urosepsis would be triaged ahead of a radiation victim with stable vital signs, regardless of the dose received. If a radiation casualty also suffers a severe injury or illness, immediate intervention is required. Most of the immediate morbidity and mortality associated with a radiologic dispersion device is related to traumatic injuries from the explosion and not to radiation exposure.4
Although many radioactive elements are candidates for use in a terrorist attack, 131I and related isotopes deserve additional discussion because of heightened interest. 131I is found only after a nuclear detonation or in reactor fuel rods. Although it is not impossible, the probability that terrorists could tap either of these sources is very small. The use of 131I in a radiologic dispersal device is unlikely because of its short half-life (8 days). Even if such a device could be made, it is extremely unlikely that the radiologic dispersal device could disperse sufficient radioactive material to pose an immediate health hazard.6 Given these facts, the probability that any significant exposure of the population (especially children) to 131I will occur is equally small. The large number of childhood thyroid cancers that occurred after the accident at the Chernobyl nuclear power plant resulted, to a significant degree, from situations that will not occur in the United States. These include delayed reporting of a breach in the reactor containment vessel preventing timely evacuation of all exposed populations, failure to effectively quarantine contaminated milk and vegetables, and significant iodine deficiency in the exposed population.7 The risk to children in communities surrounding the Fukushima nuclear power plant is also an issue that will require long-term monitoring. Nonetheless, concern about treatment to prevent thyroid cancer after potential exposure to 131I remains. Current recommendations for treatment with potassium iodide, which blocks uptake of 131I by the thyroid, are listed in Table 194-2. Caveats for use of this table include increasing the amount of potassium iodide for adolescents approaching 70 kg to the adult dose (130 mg) and monitoring thyroid-stimulating hormone and free T4 levels in neonates when possible. Nonpregnant adults older than 40 years are unlikely to benefit from this intervention.
Biologic Weapons
Patients exposed to biologic agents usually present with vague symptoms associated with an influenza-like illnesses. Unless a biologic attack is announced or suspected, the emergency department staff may not realize that they are treating victims. Indeed, it is not always possible to distinguish natural occurrences from engineered outbreaks of diseases. Examples of non-terrorist outbreaks of anthrax include cutaneous disease in intravenous heroin users in Europe and an outbreak of cutaneous anthrax in Bangladesh in 2010 with more than 400 cases. Because of the challenges in identifying the true etiology of acute events, personnel should be vigilant and at least consider the possibility, especially when warning signs are present (Box 194-3). For example, large numbers of patients suddenly presenting with “the flu” not during influenza season should cause concern. For these reasons, health surveillance will be paramount in identifying agents and potential sources. The emergency department should have a working relationship with local and state health departments as well as with local law enforcement and stay apprised of Centers for Disease Control and Prevention (CDC) and Department of Homeland Security guidelines.
Several infectious agents with potential for use as biologic weapons can spread in a hospital environment. Examples include Ebola and smallpox.2,8 Hospitals need protocols for PPE and patient isolation to ensure a safe environment.9–12 Fortunately, such protocols are similar to those applied to other infectious diseases (Box 194-4). Implementation of such precautions is credited with halting of the in-hospital spread of the Ebola virus in the 1995 Zaire outbreak. Decontamination is not a priority unless the exposure is immediate. Standard (universal) precautions are generally sufficient, and special suits (e.g., levels A, B, and C) are unnecessary.13
Whereas the CDC lists six Category A (high threat) agents (www.bt.cdc.gov/agent/agentlist-category.asp), this chapter focuses on three biologic agents—anthrax, plague, and smallpox—that represent the greatest interest.14
Anthrax
Russia and the United States have developed anthrax into a biologic weapon. The effectiveness of this agent was clearly demonstrated by two events: an accidental release of spores from a biologic weapons facility in the former Soviet Union town of Sverdlovsk in 1979 and the intentional distribution of anthrax spores through the mail along the eastern seaboard of the United States in 2001. After the Sverdlovsk release, at least 66 people died downwind from the compound during the next several weeks, and animal cases of anthrax were reported 30 miles away.15,16 The ability of non–state-sponsored terrorist groups to develop anthrax as a weapon is uncertain. The Japanese organization Aum Shinrikyo made several attempts to disperse anthrax throughout Tokyo without success.1 The individual believed responsible for the U.S. anthrax attack was not a foreign national. This is consistent with the fact that the strain of anthrax used in the attack (Ames strain) was developed by the U.S. government.
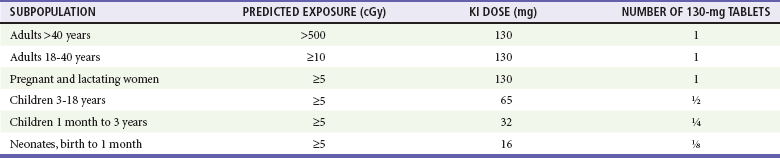
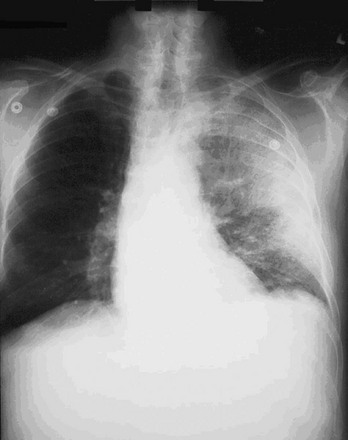
Inhalational anthrax is the most lethal form of the disease and is caused by inhalation of spores into the lungs. The mortality rate was thought to exceed 90%. However, data from the 2001 anthrax exposure call this figure into question (5 deaths in 11 cases). Although the actual mortality rate is unknown, it is probably in the 50% range.17,18 The minimum number of spores required to produce disease is unknown. The original number quoted in the literature, 104 spores, appears high given recent experience.19 After phagocytosis by macrophages, the spores germinate and are transported to the tracheobronchial lymph nodes, where the bacteria multiply. During 2 to 10 days, patients have an influenza-like illness, with malaise, fever, and nonproductive cough. This initial phase can be delayed for more than 1 month in some patients. Within 24 to 48 hours, abrupt deterioration occurs, with overwhelming sepsis, shock, hemorrhagic mediastinitis, dyspnea, and stridor. A chest radiograph obtained at this time may show a widened mediastinum and hilar adenopathy, but typical radiographic findings are not dramatic and could be missed (Fig. 194-1). Computed tomography scanning of the chest is more sensitive and should be performed if the disease is suspected. Bloody pleural effusions can also occur, and examination of the lung fields frequently reveals consolidation. This can easily be confused with pneumonia (Fig. 194-2). Death usually results within 3 days, and 50% of patients have hemorrhagic meningitis. Human-to-human transmission has not been reported with inhalational anthrax.
Initial diagnosis is generally made clinically on the basis of an influenza-like or septic illness; a suspicious chest radiograph or computed tomography scan demonstrating hilar adenopathy, infiltrates, or pleural effusions; and a reason to consider anthrax in the first place (e.g., current outbreak or warning from authorities). Several clinical algorithms exist that attempt to separate patients with influenza from those with anthrax.20,21 Unfortunately, they are based on a handful of anthrax cases, and their usefulness remains in doubt.18 Sputum culture, Gram’s stain, and blood cultures are not helpful until late in the course of the disease. Tests to confirm the diagnosis of inhalational anthrax include the polymerase chain reaction for identification of anthrax markers in pleural fluid, serologic detection of immunoglobulin to protective antigen, and immunohistochemical testing of biopsy specimens.
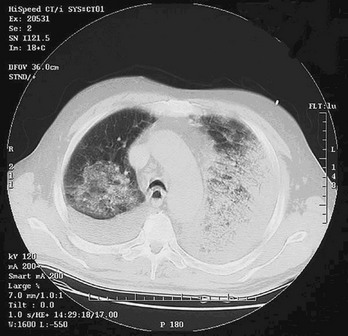
In addition to inhalational anthrax, cutaneous anthrax can occur in any area where large numbers of spores are released, as was the case in the United States in 2001. This form of the disease occurs when spores are introduced into the skin, usually through open wounds or abrasions. The mortality rate is approximately 20% without treatment and 1% with treatment. Antibiotics do not affect the course of local disease but are used to prevent dissemination and death. After an incubation period of 1 to 5 days, a papule develops, progressing to form a large vesicle during the next several days. Severe edema occurs around the lesion and is associated with regional lymphadenitis. The lesions are not tender, and the patient may or may not be febrile (Fig. 194-3). After approximately 1 week, the lesion ruptures, forming a black eschar (thus the name anthrax, from the Greek word for “coal”). In the next 2 or 3 weeks, either the eschar sloughs off and the illness is over or the organism disseminates and the patient dies. As with inhalational anthrax, the diagnosis is predominantly clinical. Confirmation is established by culture of the lesion, punch biopsy, or serologic testing. A total of 11 cutaneous anthrax cases occurred in the United States after the 2001 attack.22
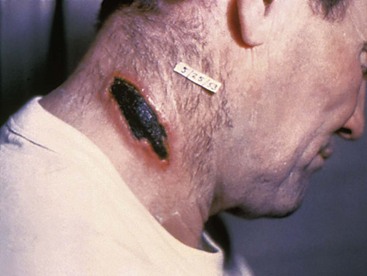
Figure 194-3 Adult with cutaneous anthrax. (Courtesy the U.S. Centers for Disease Control and Prevention.)
Treatment
Traditional treatment of anthrax infection has been penicillin. However, weapons-grade anthrax is probably resistant to penicillin (although this was not the case with the U.S. attack). Current treatment recommendations reflect this fact (Box 194-5).1,2 These consensus recommendations include fluoroquinolones and tetracycline for all children, regardless of age. Balancing the potential risks of such drugs against the consequences of infection by drug-resistant anthrax strains, the benefits justify the recommendations. Nontoxic victims with cutaneous anthrax can be treated as outpatients with oral ciprofloxacin or doxycycline for 7 to 10 days. Victims with inhalational, cutaneous, or gastrointestinal disease and toxicity require intravenous therapy with ciprofloxacin or doxycycline plus at least two other antibiotics (e.g., rifampin, clindamycin, imipenem, or an aminoglycoside). Patients can be switched to oral antibiotics when toxicity resolves. Other modalities that may be helpful include chest tube drainage of pleural effusions and possibly tracheal intubation and mechanical ventilation.22 Surprisingly, the latter intervention did not improve mortality. All patients intubated after the U.S. anthrax attack died.17,19
Treatment must continue for 60 days or until the patient has received three doses of the anthrax vaccine, given on days 0, 14, and 28. The complete vaccine course requires 18 months. This treatment regimen is also recommended for children and pregnant women. If the anthrax strain proves susceptible, patients can be switched to intravenous penicillin or oral amoxicillin. In vitro studies suggest that ofloxacin or levofloxacin can be substituted for ciprofloxacin.1
For postexposure prophylaxis, oral ciprofloxacin (500 mg) or doxycycline (100 mg) twice a day is recommended. Amoxicillin can be substituted if sensitive strains are identified. Antibiotic prophylaxis must continue for 60 days or until patients have received at least three doses of the vaccine. The vaccine is approved by the U.S. Food and Drug Administration (FDA) for adults but not for children. A review by the Institute of Medicine found the vaccine both safe and effective for prophylaxis against inhalational anthrax.23
Plague
Bubonic plague occurs when organisms are inoculated into the skin, usually from a flea bite. During the 2- or 3-day incubation period, the bacilli migrate to regional lymph nodes, where they multiply and cause inflammation and necrosis of lymphatic tissue, resulting in large, tender nodes, or buboes. Typically, buboes develop in the groin, axilla, or cervical region and are so painful that the patient will refrain from moving the affected area (Fig. 194-4). Individuals experience fever, chills, and weakness. In approximately 25% of patients, vesicles or ulcerations occur at the site of the flea bite. The buboes are usually nonfluctuant but rarely can suppurate. Organisms can be aspirated from the nodes for diagnosis, but incision and drainage are not recommended because the lymphadenitis resolves with antibiotic treatment, and practitioners could become infected if they are exposed during the procedure.
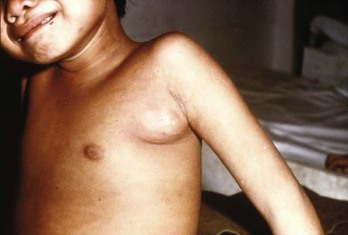
Figure 194-4 Child with bubonic plague demonstrating axillary bubo. (Courtesy Frederick M. Burkle, Jr., MD, MPH.)
Treatment
Antibiotic treatment is essentially identical for all three types of plague (Box 194-6).24 The same caveats for the use of fluoroquinolones and tetracycline in children with anthrax apply to plague. In the case of pneumonic and septicemic plague, treatment should be started within 24 hours of symptoms for outcome to be improved. Antibiotics are given for a minimum of 10 days. As patients improve, oral antibiotics are substituted for intravenous therapy to complete the course. Respiratory isolation of patients with pneumonic plague is necessary for 4 days after beginning of antibiotics to guarantee sterilization of sputum. Patients with septicemic and bubonic plague require isolation for 48 hours. If they do not have pneumonia or draining lesions during this time, isolation can be discontinued. Nonseptic patients with mild bubonic plague can be treated at home with oral doxycycline or tetracycline for 10 days.
Smallpox
Smallpox was eradicated in 1980. The only known repositories of the variola virus, the etiologic agent of smallpox, are in the United States and Russia. However, Russia was successful in weaponizing the virus, which may have been sold or smuggled out of the country.8 The effectiveness of Russia’s weaponized strain was demonstrated in 1971 when an individual became infected while traveling on a ship 15 km downwind from a Soviet bioweapons test site (Vozrozhdeniye Island in the Aral Sea).25 In addition, most of the world’s population is no longer fully immune to infection because vaccination against smallpox ceased approximately 40 years ago in nonmilitary personnel. Given its high infectivity and lethality, this makes smallpox an excellent biologic weapon.
The infection begins when the virus is inhaled. After migrating to regional lymph nodes, the virus replicates for 3 or 4 days and then asymptomatically spreads to the spleen, bone marrow, other lymphoid tissue, and liver. A second viremia occurs 8 to 12 days later and is associated with fever, prostration, and headache. Mental status changes can occur. During this phase, which lasts 2 or 3 days, the virus localizes to the skin and pharyngeal mucosa. A maculopapular rash soon appears, which becomes vesicular and finally pustular. In contrast to chickenpox, the rash first appears on the face and forearms, later spreading to the legs and trunk. All the lesions in any one area of the body are the same age (Fig. 194-5). During the next 8 to 14 days, the pustules crust over and separate from the skin, leaving pitted scars.
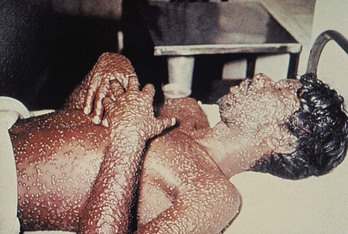
Figure 194-5 Man with smallpox. (From the U.S. Centers for Disease Control and Prevention Public Health Image Library.)
A clinical algorithm developed by the CDC can assist in assessing the probability that an individual has smallpox. It relies on three major and five minor criteria. The major criteria are a febrile prodrome, classic smallpox lesions, and lesions in the same stage of development. The minor criteria are centrifugal distribution of pustules; first lesions on the oral mucosa, face, or forearms; toxic appearance; slow evolution of lesions; and pustules on the palms and soles. A patient with all three major criteria is at high risk and should be isolated and reported to public health authorities and law enforcement agencies immediately. Patients with the febrile prodrome and either four minor criteria or one other major criterion are at moderate risk. Consultation with infectious disease and dermatology specialists should be sought and tests ordered to confirm varicella. If smallpox cannot be ruled out after these interventions, the patient should be treated as high risk. If a patient does not have the febrile prodrome or has the prodrome but no other major criteria and has fewer than four minor criteria, the individual is at low risk for smallpox. These patients can be managed as clinically indicated.26
Differential Diagnosis
As with anthrax and plague, the initial diagnosis of smallpox is clinical. Other illnesses resembling smallpox include chickenpox, herpes simplex, and monkeypox. Unlike with variola, the rash associated with chickenpox (varicella) is seen first on the trunk and then spreads to the extremities and face. In addition, the pustules are in different stages of evolution in any one area of the body. If the first case seen is hemorrhagic or malignant smallpox, the diagnosis will probably be missed until more typical cases present. For confirmation of the diagnosis, vesicular fluid or scabs were traditionally sent for electron microscopic examination. Polymerase chain reaction techniques appear promising for rapid viral identification, with sensitivities and specificities in the 98% range.27
Treatment
No effective therapy exists for victims infected with smallpox who become symptomatic. However, potential antiviral agents, such as cidofovir, show promise. In mice exposed to a lethal cowpox challenge, administration of an oral lipid prodrug of cidofovir in modest doses once a day for 5 days produced 100% survival.28 Vaccinia immune globulin (VIG) has no role in the treatment of active disease. Some practitioners suggest that most smallpox patients should be isolated at home or other nonhospital facilities because the virus spreads easily in a hospital environment and the disease is currently untreatable.8
The smallpox vaccine supply situation has improved dramatically in the United States. The CDC has 100 million doses of the traditional smallpox vaccine (Dryvax). These vials can be diluted 5 : 1 to produce a total of 500 million doses without any decrement in the vaccine’s efficacy.29 In 2004, a British company delivered an additional 209 million doses of a second-generation vaccine derived from the same vaccinia virus used in Dryvax but grown in cell cultures. In addition, work is commencing on production of an improved modified vaccinia Ankara vaccine. The virus used in the modified vaccinia Ankara vaccine is so attenuated that it does not replicate in a human host. This may make it safe for administration to immunocompromised individuals.
Chemical Weapons
Unlike victims of biologic weapons, casualties exposed to chemical agents manifest symptoms quickly, from immediately to a few hours after chemical contact.30 Therefore, surveillance and recognition are less problematic. The challenge is decontamination and treatment.
Terrorism with chemical weapons produces casualties similar to those seen in hazardous materials incidents, and medical management is comparable. However, the unique features of such events, including the volume of patients and the risk of hospital contamination, necessitate additional preparation. For example, the Tokyo subway attack with use of sarin in 1995 resulted in 11 deaths and more than 5000 patients converging on local emergency departments. Although the majority of these patients had subclinical exposure or psychological symptoms alone, the health care system was severely stressed. Secondary contamination by direct contact or vaporization occurred in ambulances and at the hospitals.3
Health care facilities should have protocols in place to deal with the eventuality of chemically contaminated patients (Box 194-7).31,32 Current recommendations for levels of PPE and types of decontamination facilities necessary in a hospital setting have been advanced, but evidence-based recommendations remain inconclusive.33,34 Collection of waste water from the decontamination process in a containment vessel is ideal but not required if this would impede necessary and appropriate actions to protect human life (www.epa.gov/oem/docs/chem/runoff.pdf).
Nerve Agents (Sarin, Tabun, Soman, and VX)
Nerve agents are organophosphates. They inhibit the enzyme acetylcholinesterase, blocking the degradation of acetylcholine at the postsynaptic membrane. Acetylcholine accumulates, resulting in overstimulation of muscarinic and nicotinic receptors. Symptoms are receptor dependent. Stimulation of muscarinic receptors produces miosis, salivation, rhinorrhea, lacrimation, bronchorrhea, bronchospasm, vomiting, and defecation. The major life threat associated with this syndrome is ventilatory compromise from profound bronchorrhea and bronchoconstriction. Stimulation of nicotinic receptors produces muscle fasciculations, flaccid paralysis, tachycardia, and hypertension. Unlike with typical organophosphates, exposure to nerve agents has not been associated with urination. In addition, bradycardia is rare, and miosis does not respond to systemic therapy.2
Treatment
The treatment of nerve agent victims depends on the form (liquid or vapor) and level of exposure (mild, moderate, or severe) (Box 194-8). Three drugs are the mainstay of treatment: atropine for the muscarinic effects (improves ventilation), pralidoxime chloride (2-PAM) for the nicotinic effects (reverses paralysis), and diazepam for the prevention and treatment of seizures (Box 194-9). The dose of atropine is titrated to the drying of respiratory secretions and not to heart rate or pupil size. If diazepam is unavailable, other benzodiazepines can be used. 2-PAM is most effective if it is administered within 4 to 6 hours of sarin exposure. After this period, the drug’s impact wanes because of “aging,” defined as the permanent attachment of sarin to the acetylcholinesterase enzyme. Hypertension can occur during 2-PAM administration and is controlled by intravenous phentolamine, 5 mg for adults and 1 mg in repeated doses for children. Recently, investigators have published data that question the efficacy of oximes in the treatment of commercial insecticide exposure. In one study, the use of oximes did not improve clinically important outcomes.35 Whether these results apply to nerve agents remains uncertain.
An autoinjector kit (Mark I) approved by the FDA consists of two cartridges, one containing atropine (2 mg) and the other 2-PAM (600 mg). Mark I kits are available as part of civilian pharmaceutical caches strategically located throughout the United States. A newer version with both drugs combined in a single autoinjector (Antidote Treatment Nerve Agent Auto-Injector referred to as DuoDote) will gradually replace the Mark I kits. An autoinjector containing 10 mg of diazepam is an additional option if intravenous access is not possible. Doses should be adjusted for pediatric and geriatric patients. Atropine autoinjectors are manufactured in three doses (2 mg, 1 mg, and 0.5 mg) for these groups. Use of the Mark I kits to treat children and the elderly can be problematic because of difficulty in adjusting the dose. An alternative solution is to inject the medication into a sterile vial. The drug can then be reaspirated in an appropriate amount for the patient’s weight or age and administered.36
Vesicants (Mustard)
Vesicants (blistering agents) are chemical warfare agents that induce blister formation on contact with skin. Terrorists could use several of these compounds, but mustard is considered the chemical of choice. Mustard is a liquid at room temperature but has both liquid and vapor toxicity. Injury from mustard exposure occurs in 1 or 2 minutes, but symptoms do not develop for 4 to 8 hours. The exact mechanism is unknown, but the agent damages DNA, causing eventual cell death. Mustard has both local and systemic toxicity. Local effects occur from direct exposure to the skin, eyes, and airway. Systemic effects result from the impact of absorbed mustard on the bone marrow. Treatment is supportive and includes decontamination (to prevent secondary contamination) and airway maintenance. Although no specific antidote for mustard is currently available, a topical iodine preparation shows promise. When it is applied within 1 hour after mustard exposure to the skin of guinea pigs, an iodine-tetraglycol solution reduced vesicle formation and signs of inflammation.37 Application of the solution more than 1 hour after exposure was not effective in preventing symptoms.
Blood Agents (Cyanide)
Victims should be removed from the area, have their clothing discarded, and receive oxygen. If no improvement occurs, the cyanide antidote is given. This has traditionally been the sequential administration of amyl nitrate, sodium nitrite, and sodium thiosulfate. However, the FDA has approved intravenous hydroxocobalamin for treatment of cyanide exposure. The initial dose is 5 g and can be repeated if necessary.38
References
1. DeAngelo, G. Cost minimization and medical examinations: The case of anthrax. J Homeland Security Emergency Management. 2008;5:Article 30.
2. Chemical and Biological Defense Command. Domestic Preparedness Training Program: Hospital Provider Manual. Edgewood Arsenal, Md: Chemical and Biological Defense Command; 1999.
3. Okumura, T, et al. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28:129.
4. Koenig, KL, et al. Medical treatment of radiologic casualties: Current concepts. Ann Emerg Med. 2005;45:643.
5. Hatchett, RJ, Kaminiski, JM, Goans, RE. Nuclear and radiologic events. In: Koenig KL, Schultz CH, eds. Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices. New York: Cambridge University Press, 2010.
6. Health Physics Society, Guidance for Protective Actions Following a Radiological Terrorist Event. Position Statement of the Health Physics Society. McLean, Va:Health Physics Society; 2004. http://hps.org/documents/rddpags_ps019-s010.pdf.
7. Bennett B, Repacholi M, Carr Z, eds. Health Effects of the Chernobyl Accident and Special Health Care Programmes: Report of the UN Chernobyl Forum Health Expert Group. World Health Organization: Geneva, 2006. www.who.int/ionizing_radiation/chernobyl/WHO%20Report%20on%20Chernobyl%20Health%20Effects%20July%2006.pdf.
8. Henderson, DA, et al. Smallpox as a biological weapon: Medical and public health management. JAMA. 1999;281:2127.
9. Koenig, KL, et al. Health care facilities’ “war on terrorism”: A deliberate process for recommending personal protective equipment. Am J Emerg Med. 2007;25:185.
10. Koenig, KL, et al. Health care facility–based decontamination of victims exposed to chemical, biological, and radiological material. Am J Emerg Med. 2008;26:71.
11. Koenig, KL, Kahn, CA, Schultz, CH. Medical strategies to handle mass casualties from the use of biological weapons. Clin Lab Med. 2006;26:313.
12. Shih, F, Koenig, KL. Improving surge capacity for biothreats: Experience from Taiwan. Acad Emerg Med. 2006;13:1114.
13. Schultz, CH, Mothershead, JL, Field, M. Bioterrorism preparedness I: The emergency department and hospital. Emerg Med Clin North Am. 2002;20:437.
14. Darling, R, Woods, JB, Cieslak, TJ. Biological events. In: Koenig KL, Schultz CH, eds. Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices. New York: Cambridge University Press, 2010.
15. Purcell, BK, Worsham, PL, Friedlander, AM. Anthrax. In: Lenhart MK, Lounesbury DE, Martin JM, eds. Medical Aspects of Biological Warfare, Textbook of Military Medicine. Washington, DC: Office of the Surgeon General, U.S. Army; 2007:69–90.
16. Dixon, TC, et al. Anthrax. N Engl J Med. 1999;341:815.
17. Jernigan, JA, et al. Bioterrorism-related inhalational anthrax: The first 10 cases reported in the United States. Emerg Infect Dis. 2001;7:933.
18. Schultz, CH. Chinese curses, anthrax, and the risk of bioterrorism. Ann Emerg Med. 2004;43:329.
19. Centers for Disease Control and Prevention. Update: Investigation of bioterrorism-related inhalational anthrax—Connecticut, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:1029.
20. Hupert, N, Bearman, GML, Mushlin, AI, Callahan, MA. Accuracy of screening for inhalational anthrax after a bioterrorist attack. Ann Intern Med. 2003;139:337.
21. Kuehnert, MJ, et al. Clinical features that discriminate inhalational anthrax from other acute respiratory illnesses. Clin Infect Dis. 2003;36:328.
22. Bell, DM, et al. Conference Summary: Clinical issues in the prophylaxis, diagnosis, and treatment of anthrax. Emerg Infect Dis. 2002;8:222.
23. Joellenbeck, LM, et al. The Anthrax Vaccine—Is it safe? Does it work?. Washington, DC: Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Institute of Medicine, National Academy Press; 2002.
24. Inglesby, TV, et al. Plague as a biological weapon: Medical and public health management. JAMA. 2000;283:2281.
25. Tucker, JB, Zilinskas, RA. The 1971 Smallpox Epidemic in Aralsk, Kazakhstan, and the Soviet Biological Warfare Program, Occasional Paper No. 9. Monterey, Calif: Chemical and Biological Weapons Nonproliferation Project, Center for Nonproliferation Studies, Monterey Institute of International Studies; 2002.
26. Centers for Disease Control and Prevention. Evaluating Patients for Smallpox: Acute, Generalized Vesicular or Pustular Rash Illness Protocol. http://emergency.cdc.gov/agent/smallpox/diagnosis/pdf/poxalgorithm11-14-07.pdf.
27. Aitichou, M, Javorschi, S, Ibrahim, MS. Two-color multiplex assay for the identification of orthopox viruses with real-time LUX-PCR. Mol Cell Probes. 2005;19:323.
28. De Clercq, E. Cidofovir in the therapy and short-term prophylaxis of poxvirus infections. Trends Pharmacol Sci. 2002;23:456.
29. Frey, SE, et al. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346:1265.
30. Urbanetti, J. Clinical aspects of large-scale chemical events. In: Koenig KL, Schultz CH, eds. Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices. New York: Cambridge University Press, 2010.
31. Macintyre, AG, et al. Weapons of mass destruction events with contaminated casualties: Effective planning for health care facilities. JAMA. 2000;283:242.
32. Burgess, JL, et al. Emergency department hazardous material protocol for contaminated patients. Ann Emerg Med. 1999;34:205.
33. Koenig, KL, et al. Health care facilities’ “war on terrorism”: A deliberate process for recommending personal protective equipment. Am J Emerg Med. 2007;25:185–195.
34. Koenig, KL, et al. Healthcare facility–based decontamination of victims exposed to chemical, biological, and radiological material. Am J Emerg Med. 2008;26:71–80.
35. Eddleston, M, et al. Pralidoxime in acute organophosphorus insecticide poisoning—a randomised controlled trial. PLoS Med. 2009;6:e1000104.
36. Henretig, FM, Mechem, C, Jew, R. Potential use of autoinjector-packaged antidotes for treatment of pediatric nerve agent toxicity. Ann Emerg Med. 2002;40:405.
37. Wormser, U, et al. Topical iodine preparation as therapy against sulfur mustard–induced skin lesions. Toxicol Appl Pharmacol. 2000;169:33.
38. Borron, SW, et al. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49:794.