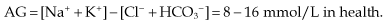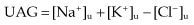11 Water, electrolyte and acid-base balance
Salt and volume distribution
Water and volume
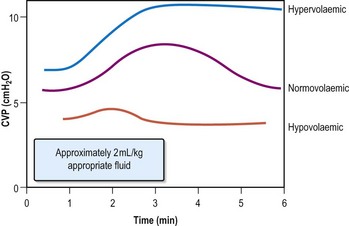
Assessing volume status
Thirst is a symptom of dehydration; oedema indicates increased volume.
Fluid therapy in hospital
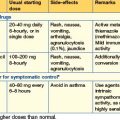
Two common circumstances necessitate fluids as therapy (Table 11.1) in hospital:
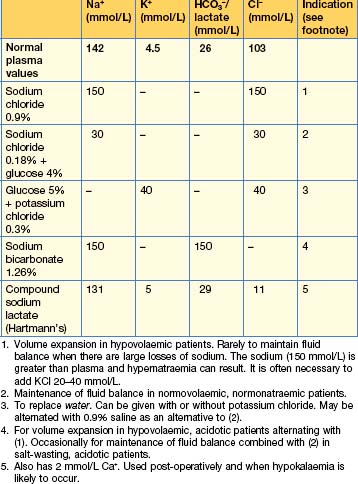
The volume administered varies according to the state of hydration; in otherwise well ‘nil by mouth’ patients with no losses, aim for 2 L/24 hours of saline (0.18%) and glucose (4%), usually with added potassium 20 mmol/L. Post-surgical fluid therapy should reflect the nature of the surgery (and the likely ongoing losses).
Sodium
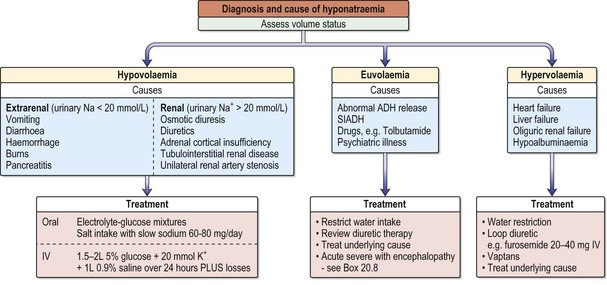
Hyponatraemia
Investigations
Hyponatraemia with a normal extracellular volume
Excess body water results from an abnormally high intake.
•Treating hyponatraemia without encephalopathy
•Treating hyponatraemia with encephalopathy, see Chapter 20, Emergencies in medicine, p. 711
Hyponatraemia with a decreased extracellular volume
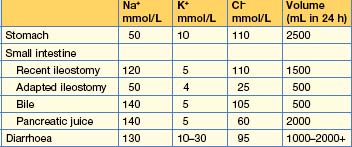
A decreased volume stimulates thirst and vasopressin release, which in turn inhibits water excretion. Losses from the kidney occur in tubulointerstitial disease, the recovery phase of acute tubular necrosis, unilateral renal artery stenosis, excessive use of diuretics, adrenocortical insufficiency and osmotic diuresis (e.g. hyperglycaemia, severe uraemia). Losses from the gastrointestinal tract are due to vomiting, diarrhoea, naso-gastric suction and haemorrhage (Table 11.2). Losses can also occur from the skin (burns), pericarditis and peritonitis.
Hyponatraemia with an increased extracellular volume
Hypernatraemia
Causes
Water deficit is due to the following:
Management
Always treat underlying cause.
So, in a 50 kg female with a serum Na+ of 155 mmol/L:
Include ongoing losses in the deficit (allow 1 L insensible losses/day).
Potassium
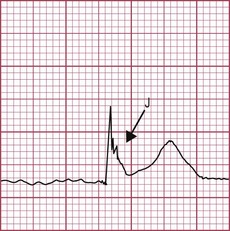
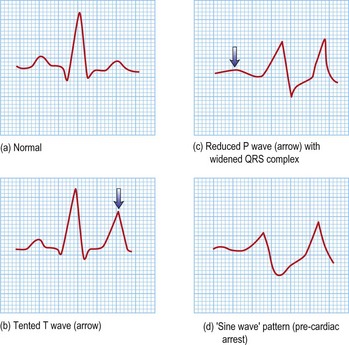
Hyperkalaemia
Management (See Emergencies in medicine p. 711)
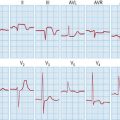
Hypokalaemia
Causes
Management of asymptomatic or mild hypokalaemia (K > 2.5 mmol/L)
Magnesium
Hypomagnesaemia
Management
Withdraw any causal agents. ECG evidence of hypomagnesaemia includes prolonged PR interval, broad flattened T waves and a widening QRS complex leading to fatal ventricular arrhythmias. Oral magnesium is poorly absorbed and tolerated. Even mild hypomagnesaemia (> 0.4 mmol/L) should be treated with IV magnesium sulphate (20–60 mmol/day in 0.9% saline). Acute or severe hypomagnesaemia is associated with torsades de pointes (p. 409). Give 2–4 mL 50% magnesium sulphate as a bolus.
Phosphate
Check Ca2+, Na+, K+, renal function and bicarbonate, glucose and parathormone levels.
Hypophosphataemia
Management
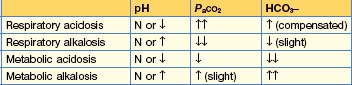
Acid–base disorders
Diagnostic tests
In diarrhoea, urinary ammonium chloride (NH4Cl) is high, giving a negative UAG. A positive (or zero) UAG is seen in renal disease and distal renal tubular acidosis (low urinary NH4 production).
Metabolic acidosis
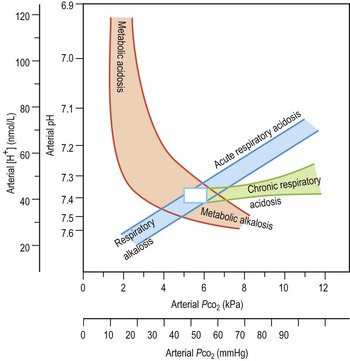
Metabolic acidosis (Fig. 11.4) is defined as a fall in systemic pH to < 7.35 with a primary decrease in plasma bicarbonate. It may be caused by excess generation or retention of acid, or by increased bicarbonate losses.
Metabolic acidosis with normal AG
Metabolic acidosis with high AG
Causes
•Management
Metabolic alkalosis
The retention of bicarbonate or loss of H+ leads to a metabolic alkalosis (Fig. 11.4), usually due to primary chloride or potassium depletion. Any significant loss of Cl− (vomiting, diuretics) will lead to HCO3− retention to maintain electrical neutrality. Any cause of secondary hyperaldosteronism (e.g. hypovolaemia) will lead to Na+ retention, and potassium and H+ depletion. Hypoventilation may buffer an evolving alkalosis to a degree, and modest elevations of PCO2 are usual.

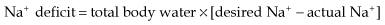
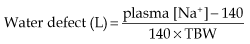
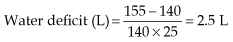
 CO2 + H2O
CO2 + H2O