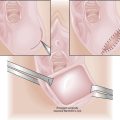
Chapter 40 Vulvar and Vaginal Cancer
 Intraepithelial Neoplasia
Intraepithelial Neoplasia
SQUAMOUS CELL CARCINOMA IN SITU: VULVAR INTRAEPITHELIAL NEOPLASIA TYPE III
Clinical Features
Itching is the most common symptom, although some patients present with palpable or visible abnormalities of the vulva. About half of patients are asymptomatic. There is no absolutely diagnostic appearance. Most lesions are elevated, but the color may be white, red, pink, gray, or brown (Figure 40-1). About 20% of the lesions have a “warty” appearance, and the lesions are multicentric in about two thirds of cases.
 Invasive Vulvar Cancer
Invasive Vulvar Cancer
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma accounts for about 90% of vulvar cancers.
Clinical Features
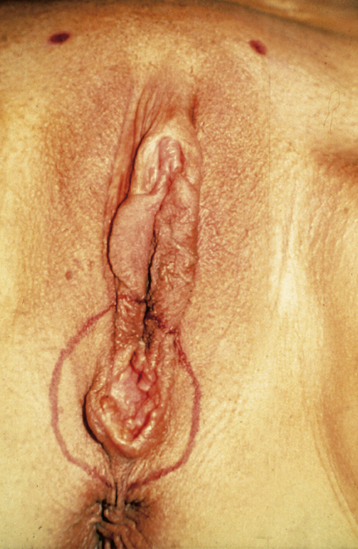
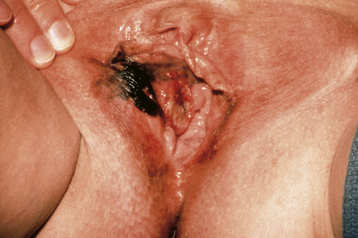
Patients generally present with a vulvar lump, although long-standing pruritus is common. The lesions may be raised, ulcerated, pigmented, or warty in appearance, and definitive diagnosis requires biopsy under local anesthesia. Most lesions occur on the labia majora; the labia minora are the next most common sites. Less commonly, the clitoris or the perineum is involved (Figure 40-2). About 5% of cases are multifocal.
Methods of Spread
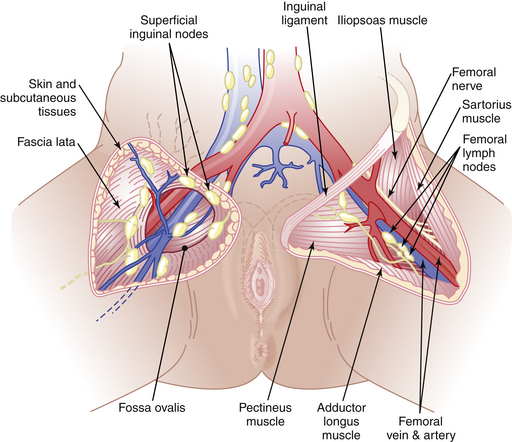
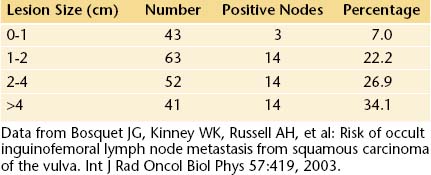
Vulvar cancer spreads by direct extension to adjacent structures, such as the vagina, urethra, and anus; by lymphatic embolization to regional lymph nodes; and by hematogenous spread to distant sites, including the lungs, liver, and bone. In most cases, the initial lymphatic metastases are to the inguinal lymph nodes, located between Camper’s fascia and the fascia lata. From these superficial nodes, spread occurs to the femoral nodes located medial to the femoral vein. Cloquet’s node, which is situated beneath the inguinal ligament, is the most cephalad of the femoral node group. From the inguinofemoral nodes, spread occurs to the pelvic nodes, particularly the external iliac group (Figure 40-3).
The incidence of lymph node metastases in vulvar cancer is about 30%. It is related to the size of the lesion (Table 40-1). About 5% of patients have metastases to pelvic lymph nodes. Such patients usually have three or more positive unilateral inguinofemoral lymph nodes. Hematogenous spread usually occurs late in the disease and rarely occurs in the absence of lymphatic metastases.
Staging
In 1989, the International Federation of Gynecology and Obstetrics (FIGO) Cancer Committee introduced a surgical staging system for vulvar cancer. This system was revised in 1994, and the present FIGO staging system is shown in Table 40-2.
TABLE 40-2 INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS (FIGO) STAGING OF VULVAR CARCINOMA (1994)
| Stage 0 | Carcinoma in situ, intraepithelial carcinoma |
| Stage I | Tumor confined to the vulva or perineum, or both, and 2 cm or less in greatest dimension; no nodal metastasis |
| Stage Ia | As above with stromal invasion ≤1 mm |
| Stage Ib | As above with stromal invasion >1 mm |
| Stage II | Tumor confined to the vulva or perineum, or both, and more than 2 cm in greatest dimension; no nodal metastasis |
| Stage III | Tumor of any size with: |
| 1. Adjacent spread to the urethra and/or vagina and/or anus | |
| 2. Unilateral regional lymph node metastasis, or a combination | |
| Stage IV | |
| Stage IVa | Tumor invades any of the following: upper urethra, bladder mucosa, rectal mucosa, pelvic bone or bilateral regional node metastasis, or a combination |
| Stage IVb | Any distant metastasis including pelvic lymph nodes |
MANAGEMENT OF PATIENTS WITH POSITIVE NODES
Prognosis
The overall survival rate for vulvar carcinoma is about 70%. Survival by FIGO stage is shown in Table 40-3. Survival also correlates significantly with lymph node status because patients with positive nodes have a 5-year survival rate of about 50%, whereas those with negative nodes have a 5-year survival rate of about 90%. Patients with one involved node have a good prognosis, regardless of stage, whereas those with three or more involved nodes do poorly, regardless of stage.
TABLE 40-3 SURVIVAL FOR VULVAR CANCER BY FIGO STAGE (EPIDERMOID INVASIVE CANCER ONLY)
| Stage | No. of Patients | Five-Year Survival (%) |
|---|---|---|
| I | 160 | 76.9 |
| II | 202 | 54.8 |
| III | 125 | 30.8 |
| IV | 44 | 8.3 |
Data from the Annual Report on the Results of Treatment in Gynecological Cancer. Patients treated 1996-1998. J Epidemiol Biostat 83:7-26, 2003.
MALIGNANT MELANOMA
Malignant melanoma is the second most common type of vulvar cancer. Melanomas may arise de novo or from a preexisting junctional or compound nevus. They occur predominantly in postmenopausal white women and most commonly involve the labia minora or clitoris (Figure 40-4).
 Vaginal Neoplasms
Vaginal Neoplasms
SQUAMOUS CELL CARCINOMA OF THE VAGINA
Staging
The FIGO staging for vaginal cancer is clinical, as shown in Table 40-4. In practice, all patients should have at least a chest radiograph and pelvic and abdominal computed tomography or magnetic resonance imaging to detect evidence of metastatic spread, including bulky pelvic or para-aortic lymph nodes. Positron emission tomography is increasingly used to look for metastatic disease.
TABLE 40-4 INTERNATIONAL FEDERATION OF GYNECOLOGY AND OBSTETRICS (FIGO) STAGING OF VAGINAL CANCER
| Stage I | Carcinoma limited to the vaginal wall |
| Stage II | Carcinoma has involved the subvaginal tissue but has not extended onto the pelvic side wall |
| Stage III | Carcinoma has extended to the pelvic side wall |
| Stage IV | Carcinoma has extended beyond the true pelvis or has involved the mucosa of the bladder or rectum |
| Stage IVa | Spread to bladder or rectum |
| Stage IVb | Spread to distant organs |
Chan J.K., Sugiyama V., Pham H., et al. Margin distance and other clinico-pathologic factors in vulvar carcinoma: A multivariate analysis. Gynecol Oncol. 2007;104:636-641.
Hacker N.F. Vulvar cancer. In Berek J.S., Hacker N.F., editors: Practical Gynecologic Oncology, 5th ed, Philadelphia: Lippincott Williams & Wilkins, 2009.
Hakim A.A., Terada K.Y., et al. Sentinel node dissection in vulvar cancer: Current treatment options in oncology. 2006;7:85-91.
Jones R.W., Baranyai J., Stables S. Trends in squamous cell carcinoma of the vulva: The influence of vulvar intraepithelial neoplasia. Obstet Gynecol. 1997;90:448-452.
Landrum L.M., Lanneau G.S., Skaggs V.J., et al. Gynecologic Oncology Group risk groups for vulvar carcinoma: Improvement in survival in the modern era. Gynecol Oncol. 2007;106:521-525.
Tran P.T., Su Z., Lee P., Lavori P., et al. Prognostic factors for outcome and complications for primary squamous cell carcinoma of the vagina treated with radiation. Gynecol Oncol. 2007;105:641-649.

 Vulvar Neoplasms
Vulvar Neoplasms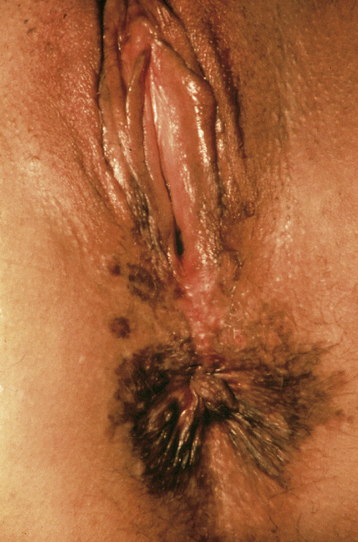
 Diethylstilbestrol Exposure in Utero
Diethylstilbestrol Exposure in Utero