Voltage-Regulated Potassium Channels
Myocardial Voltage-Gated K+ Channels: Transient Outward Voltage-Gated K+ Channels
Myocardial Voltage-Gated K+ Channels: Delayed Rectifier Voltage-Gated K+ Channels
Inwardly Rectifying Myocardial K+ Channels Also Contribute to Repolarization
Pore-Forming (α) Subunits of Myocardial Voltage-Gated K+ Channels
Accessory/Auxiliary Subunits of Myocardial Voltage-Gated K+ Channels
Molecular Determinants of Native Myocardial Transient Outward Voltage-Gated K+ Channels
Molecular Determinants of Native Myocardial Delayed Rectifier Voltage-Gated K+ Channels
Summary
In atrial and ventricular myocytes, the action potential upstroke, attributed to inward currents through voltage-gated Na+ (Nav) channels, is rapid (Figure 3-1). In nodal tissues, which lack functional Nav channels, the upstroke of the action potential is substantially slower and is dominated by Ca2+ influx through voltage-gated Ca2+ (Cav) channels. There are also marked regional differences in action potential heights and durations, as well as in the time courses of action potential repolarization (see Figure 3-1). These differences, which affect the normal spread of excitation in the myocardium and influence the dispersion of repolarization in the ventricles, primarily reflect regional differences in the functional expression and the properties of the outward K+, as well as the inward (Nav and Cav) currents.1
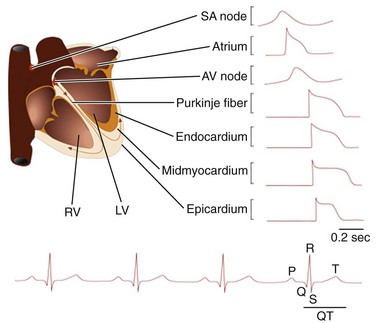
Figure 3-1 Electrical activity in the mammalian myocardium. Schematic of the human heart with the different anatomic regions labeled and representative action potential waveforms recorded in these different regions illustrated. In the lower panel, a schematic of a surface electrocardiogram is presented with four sequential beats displayed and the P, Q, R, S, and T waves marked on the last beat.
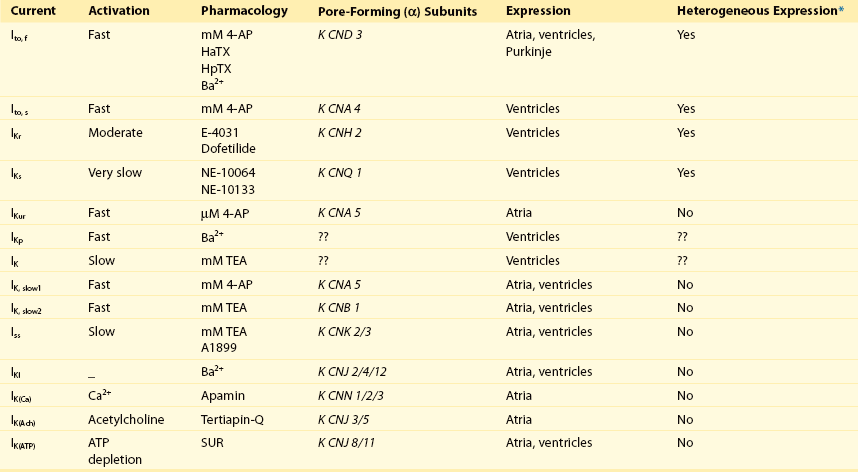
Cellular electrophysiologic studies have detailed the properties of the major inward (Nav and Cav) and outward (K+) currents that shape the waveforms of atrial and ventricular action potentials (Figure 3-2). In contrast to the cardiac Nav and Cav currents, there are multiple types of myocardial voltage-gated K+ (Kv) and non-voltage, inwardly rectifying K+ (Kir) channels (Table 3-1), many of which are differentially expressed, contributing to regional variations in myocardial action potential waveforms (see Figure 3-1) and refractoriness.1–3 In addition, changes in the densities, distributions, and properties of Kv and Kir channels are evident in a variety of myocardial diseases, and these changes affect repolarization, influence propagation and decrease rhythmicity, effects that can produce substrates for the generation of life-threatening arrhythmias.1 There is, therefore, considerable interest in defining the molecular mechanisms controlling the biophysical properties and the functional cell surface expression of these channels. A large number of Kv and Kir pore-forming α and accessory β (Table 3-2) subunits have been identified,4,5 and considerable progress has been made in defining the relationships between these subunits and functional myocardial Kv and Kir channels.1,6,7 Importantly, the studies completed to date have revealed that the molecular correlates of the various types of Kv and Kir channels distinguished electrophysiologically (see Table 3-1) are indeed distinct.1 In the long term, defining the molecular compositions of myocardial Kv and Kir channels will also facilitate studies aimed at determining the molecular mechanisms controlling the marked regional differences in the expression of these channels in the normal myocardium, as well as the derangements in the expression or functioning of these channels that occur with myocardial disease. In this chapter, the electrophysiologic and molecular diversity of repolarizing myocardial Kv and Kir channels and the molecular determinants of native myocardial K+ channels will be reviewed.
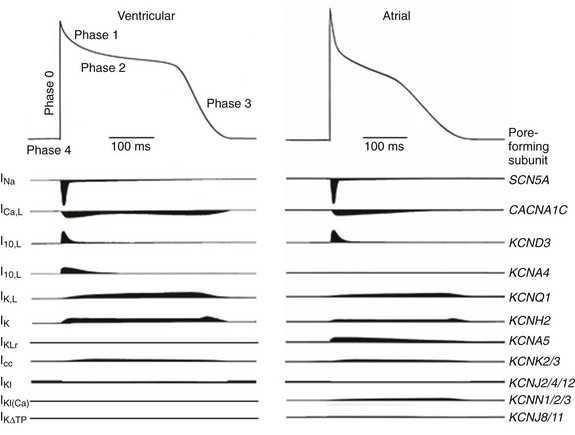
Figure 3-2 Schematics of action potential waveforms and underlying ionic currents in adult human ventricular and atrial myocytes. The major ionic currents shaping action potentials in human atrial and ventricular myocytes are schematized, and the main pore-forming α-subunits underlying these currents are listed. As discussed in the text, there are regional differences in the relative expression levels of some of the repolarizing K+ currents and the contributions of the various K+ currents to shaping action potential waveforms and controlling repolarization.
Myocardial Voltage-Gated K+ Channels: Transient Outward Voltage-Gated K+ Channels
Kv currents, activated on membrane depolarization, influence myocardial action potential amplitudes and durations and, in most cells, two broad classes of Kv currents have been distinguished: transient outward K+ currents, Ito; and delayed, outwardly rectifying K+ currents, IK (see Table 3-1). The transient currents (Ito) activate rapidly and underlie early (phase 1) repolarization, whereas the delayed rectifiers (IK) determine the latter phase (phase 3) of action potential repolarization (see Figure 3-2) back to the resting membrane potential. These classifications are broad, however, and there are actually multiple types of transient (Ito) and delayed rectifier (IK) Kv currents (see Table 3-1) expressed in cardiac cells.1,3 Electrophysiologic and pharmacologic studies, for example, have clearly demonstrated that there are two types of transient outward K+ currents, now referred to as Ito,fast (Ito,f) and Ito,slow (Ito,s), and that these currents are differentially distributed.6,7 The rapidly activating and inactivating transient outward K+ current, Ito,f, is also characterized by rapid recovery from steady-state inactivation, whereas Ito,s recovers slowly from inactivation.6,7 In addition, Ito,f is readily distinguished from other Kv currents (including Ito,s) using Heteropoda toxin-2 or -3 (see Table 3-1).
Although originally identified in Purkinje fibers, Ito,f is a prominent repolarizing Kv current in atrial and ventricular myocytes, as well as in nodal cells, in most species.1 There are, however, marked regional differences in Ito,f densities, with the highest densities typically in atrial myocytes. In addition, in mammalian ventricles, Ito,f and Ito,s are differentially distributed. In canine left ventricles (LVs), for example, Ito,f density is fivefold to sixfold higher in epicardial and midmyocardial, than in endocardial, cells.2 There are also marked regional differences in Ito,f densities in adult mouse ventricles.8–10 Specifically, Ito,f density is higher in right than in left ventricular myocytes, and within the LV, Ito,f densities are higher in apex than in base myocytes.8–10 In the mouse, even greater Kv current heterogeneity is seen in cells isolated from the septum: all ventricular septum cells express Ito,s, and most (approximately 80%) also express Ito,f.8 When present, however, Ito,f density is significantly (p < 0.001) lower in septum than in RV or LV cells.8 Ito,f and Ito,s are also differentially expressed in ferret LV, and Ito,s is detected only in endocardial LV cells.11 Despite heterogeneities in functional expression, the properties of Ito,f and Ito,s in different cardiac cell types (and species) are remarkably similar, leading to suggestions that the molecular compositions of the underlying (Ito,f and Ito,s) channels are also similar.3
Myocardial Voltage-Gated K+ Channels: Delayed Rectifier Voltage-Gated K+ Channels
Electrophysiologic and pharmacologic studies have also distinguished multiple types of cardiac delayed rectifier K+ currents, IK (see Table 3-1). In atrial myocytes, for example, the dominant repolarizing K+ current is a rapidly activating, non-inactivating K+ current, IKur (IK,ultrarapid), which is not detected in ventricular or nodal cells.1 In most ventricular myocytes, there are two prominent components of delayed rectification, IKr (IK,rapid) and IKs (IK,slow), that are different from IKur in terms of time- and voltage-dependent properties.1 The biophysical properties of IKr and IKs are distinct: IKr activates and inactivates rapidly, displays marked inward rectification, and is selectively blocked by class III anti-arrhythmics, including dofetilide and sotalol.12 In contrast, IKs activates slowly and does not display inward rectification.12
Similar to the transient outward K+ currents, there are also marked regional differences in the functional expression of IKs and IKr in mammalian ventricular myocytes.1,2 The density of IKs in canine LV, for example, is higher in epicardial and endocardial than in M cells.2 There are also regional differences in the functional expression of IKr and IKs channels in guinea pig LV.13 In cells isolated from the LV free wall, for example, the density of IKr is higher in subepicardial than in midmyocardial or subendocardial myocytes.13 At the base of the LV, in contrast, IKr and IKs densities are significantly lower in endocardial, than in either midmyocardial or epicardial, LV cells.13 Differences in Kv current densities contribute to the variations in action potential waveforms recorded in different regions (i.e., atria and ventricles, right and left ventricles, apex and base of the ventricles) of the heart, as well as in different layers (epicardial, midmyocardial, and endocardial) of the LV and RV walls.1–3,8–13
In rodent ventricles, there are additional components of IK with properties distinct from IKs and IKr (see Table 3-1). In mouse and rat ventricular myocytes, for example, there are novel delayed rectifier Kv currents that have been referred to as IK, IKslow and Iss (see Table 3-1).1,3 Mouse ventricular IK,slow was first identified as a rapidly activating and slowly inactivating K+ current with properties distinct from Ito,f, Ito,s and Iss expressed in the same cells.14 In addition, IK,slow was shown to be blocked selectively by micromolar concentrations of 4-aminopyridine (4-AP), which does not affect Ito,f or Ito,s.14 Subsequent work, however, revealed the presence of two components of mouse ventricular IK,slow: IK,slow1, which is blocked by µM 4-AP; and, IK,slow2, which is blocked selectively by TEA.15–18 In addition, it has been demonstrated that IK,slow1 and IK,slow2 reflect the expression of distinct molecular entities.15–18 In contrast to the differential distribution of Ito,f and Ito,s, however, IK,slow1, IK,slow2, and Iss appear to be uniformly expressed in mouse atrial and ventricular myocytes.8–10,15–19
Inwardly Rectifying Myocardial K+ Channels Also Contribute to Repolarization
In addition to the Kv currents, Kir currents, specifically IK1 and the ATP-dependent K+ current, IKATP (Table 3-1), contribute to shaping myocardial action potential waveforms.20–22 Similar to the Kv currents, the densities of the Kir currents vary in different regions of the heart (e.g., atria, ventricles, conducting tissue).1,3 In contrast to the Kv currents, however, myocardial Kir current densities are similar in myocytes in different regions of the ventricles.1 In mammalian atrial and ventricular myocytes, IK1 plays a role in establishing resting membrane potentials and plateau potentials, and contributes to phase 3 repolarization (see Figure 3-2). The fact that the conductances of IK1 channels are high at negative membrane potentials underlies the contribution of IK1 to atrial and ventricular resting membrane potentials.20 Although the voltage-dependent properties of IK1 channels are such that conductance is low at potentials positive to approximately −40 mV, these channels nevertheless contribute outward K+ currents during the plateau phase of the action potential in ventricular cells,20 as well as during phase 3 repolarization (see Figure 3-2), because the driving force on K+ is high at depolarized membrane potentials.
Myocardial ATP-dependent K+ channels are weak, inwardly rectifying channels that are inhibited by (elevated) intracellular ATP and activated by nucleotide diphosphates.21 In ventricular myocytes, activation of IKATP channels during periods of hypoxia and ischemia results in action potential shortening, suggesting that these channels provide a link between cellular metabolism and membrane potential.21,22 The opening of IKATP channels appears to contribute to the cardioprotection resulting from ischemic preconditioning.22 In contrast with some ventricular Kv channels, IKATP channels appear to be distributed uniformly in the right and left ventricles and through the thickness of the right or left ventricular walls. Interestingly, IKATP channels are expressed at much higher densities than other sarcolemmal myocardial K+ channels,21 suggesting that action potentials will be shortened markedly when only a few IKATP channels are activated.
Pore-Forming (α) Subunits of Myocardial Voltage-Gated K+ Channels
Kv channel pore-forming (α) subunits are six transmembrane-spanning domain proteins (Figure 3-3) with a region between the fifth and sixth transmembrane domains that contributes to the K+-selective pore.4 The positively charged fourth transmembrane domain in the Kv α-subunits (see Figure 3-3) is homologous to the corresponding regions in Nav and Cav channel α-subunits, placing them in the S4 superfamily of voltage-gated channels.3,4 In contrast to Nav and Cav channels, in which only a single α-subunit is required to form a channel, functional Kv channels comprise four α-subunits (see Figure 3-3). Similar to the diversity of functional myocardial Kv channels (see Table 3-1), however, multiple Kv α-subunits have been identified. These subunits comprise several homologous Kv α-subunit subfamilies, Kv1.x, Kv2.x, Kv3.x, Kv4.x, Kv10.x, Kv11.x, and many members of these Kv α-subunit subfamilies are expressed in the mammalian heart.1 In addition, further functional Kv channel diversity could arise through alternative splicing of transcripts and through the formation of heteromultimeric channels between two or more Kv α-subunit proteins in the same Kv α-subunit subfamily.1,3,4
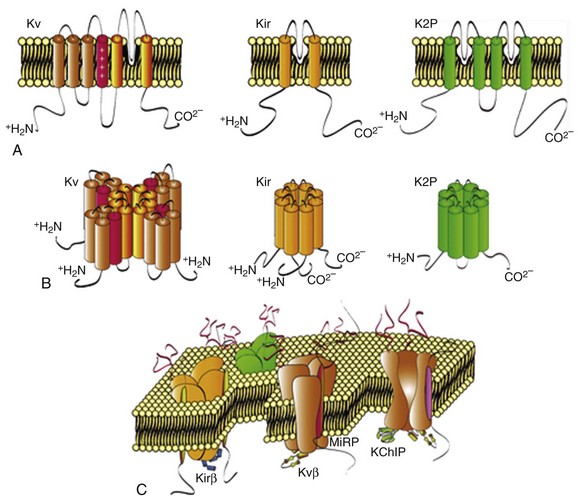
Figure 3-3 Pore-forming α-subunits and assembly of functional K+ channels. A, Linear sequences and transmembrane topologies of the pore-forming α-subunits encoding voltage-gated (Kv), inwardly rectifying (Kir), and two-pore domain (K2P) K+ channels are illustrated. B, The tetrameric assembly of Kv and Kir α-subunits and the dimeric assembly of K2P α-subunits are illustrated below the primary sequences of the α-subunits. C, Assembled, functional K+ channels composed of pore-forming α-subunits and one or more cytosolic and transmembrane accessory subunits.
Additional subfamilies of Kv α-subunits were revealed with the cloning of the human eag-related (HERG) gene, KCNH2, subsequently identified as the locus of mutations underlying one form of familial long QT-syndrome, LQT2, and KCNQ1 (KvLQT1), the locus of mutations in another inherited long QT syndrome, LQT1.23 Heterologous expression of KCNH2 (ERG1) reveals inwardly rectifying Kv currents23 with properties similar to cardiac IKr (see Table 3-1). Although there are several ERG (KCNH) subfamily members, only KCNH2 (which encodes ERG1) appears to be expressed in the myocardium.1 Heterologous expression of KCNQ1 (KvLQT1) alone reveals rapidly activating and non-inactivating Kv currents, whereas co-expression with the Kv accessory subunit, minK (see Table 3-2), produces slowly activating Kv currents that resemble the slow component of cardiac-delayed rectification, IKs.1,3,23
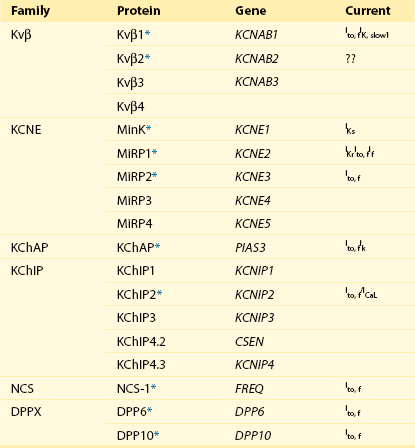
Accessory/Auxiliary Subunits of Myocardial Voltage-Gated K+ Channels
In addition to the Kv α-subunits, a number of Kv channel accessory (Kv β) subunits have also been identified (see Table 3-2). The first of these subunits was KCNE1, which encodes a small (130-aa) protein (minK) with a single transmembrane spanning domain.24 It appears that minK coassembles with KvLQT1 to form functional cardiac IKs channels.23,24 Additional minK homologs, MiRP1 (KCNE2), MiRP2 (KCNE3), and MiRP3 (KCNE4) have also been identified (see Table 3-2), and it has been suggested that MiRP1 (KCNE2) functions as an accessory subunit coassembling with ERG1 to generate cardiac IKr.25 It has also been reported that the MiRP subunits interact with multiple Kv α-subunit subfamilies and modify channel properties.24 MiRP2, for example, coassembles with Kv3.4 in mammalian skeletal muscle,26 and MiRP1 coassembles with Kv4.x α-subunits when coexpressed in heterologous cells.27 These observations suggest that the MiRP (KCNE) accessory subunits can assemble with a variety of Kv α-subunits and contribute to the formation of multiple types of myocardial Kv channels. Direct experimental support for this hypothesis, however, has not been provided, and the roles of the various KCNE subunits in the generation of functional cardiac Kv26,27 and other non-Kv28 channels remain to be defined.
Another type of Kv channel accessory subunit was revealed with the isolation of low molecular weight (approximately 45 kD) cytosolic (Kvβ) subunits from brain.5 Three homologous Kv β-subunits, Kvβ1, Kvβ2, and Kvβ3 (see Table 3-2), as well as alternatively spliced transcripts, have been identified, and both Kvβ1 and Kvβ2 are expressed in heart.1 Previous studies have shown that Kvβ subunits interact with the intracellular domains of Kv1 α-subunits, and functional studies demonstrated that Kvβ subunits affect the biophysical properties and increase the cell surface expression of heterologously expressed Kv1 α-subunit–encoded Kv currents.5 Because Kv α- and β-subunits appear to coassemble in the endoplasmic reticulum,29 the increase in functional channel expression suggests that the Kv β-subunits affect channel assembly, processing or stability or, alternatively, function as chaperone proteins. Although Kvβ1 and Kvβ2 have been shown to associate with Kv4 subunits in the mouse myocardium and the targeted deletion of Kvβ1 results in the attenuation of Kv4-encoded Ito,f in mouse ventricular myocytes,30 the roles of Kvβ subunits in the generation of myocardial Kv channels in other species have not been explored.
A yeast two-hybrid screen, using the N terminus of Kv4.2 as the bait, led to the identification of accessory Kv channel interacting proteins, (KChIPs) (see Table 3-2) in brain.31 Of the four KChIPs identified,31,32 only KChIP2 is expressed in heart,31,33 although there are several KChIP2 splice variants.34–36 Interestingly, the KChIPs belong to the recovering family of neuronal Ca2+-sensing (NCS) proteins,37 and contain four EF-hand domains.31 When coexpressed with Kv4 α-subunits, each of the KChIPs increases K+ current densities, slows inactivation, speeds recovery from inactivation, and shifts the voltage dependence of current activation.31 In contrast, KChIP coexpression does not affect the properties or the densities of Kv1.4- or Kv2.1-encoded K+ currents, consistent with the suggestion that the modulatory effects of the KChIPs are specific for Kv4 α-subunit–encoded Kv channels.31 More recent studies, however, suggest that the KChIPs modulate the functional cell surface expression of Kv1.5-encoded Kv channels,38 as well as myocardial Cav channels.39 In addition, although KChIP binding to Kv4 α-subunits is not Ca2+ dependent, mutations in EF hand domains 2, 3, and 4 eliminate the modulatory effects of KChIP1 on Kv4- and Kv1.5-encoded Kv currents,31,38 suggesting a role for voltage-dependent Ca2+ entry and intracellular Ca2+ levels in the regulation of functional cardiac (Kv4-encoded) Ito,f channels, as has been demonstrated for neuronal Kv4-encoded channels.40
The transmembrane diaminopeptidyl transferase-like protein 6 (DPP6 or DPPX) has also been suggested as an accessory subunit of cardiac41 and neuronal42 Kv4-encoded channels (see Table 3-2). Lacking enzymatic activity, DPP6 increases the cell surface expression of Kv4 α-subunits, shifts the voltage dependences of activation and inactivation of heterologously expressed Kv4 currents to more negative potentials, and accelerates the rates of current activation, inactivation, and recovery.41,42 Interestingly, heterologous coexpression of DPP6 with Kv4.3 and KChIP2 produces Kv currents that closely resemble native cardiac Ito,f.41 Another member of the DPP-like subfamily of proteins, DPP10, has been demonstrated to associate with Kv4.2 and KChIP3 in the rat brain and to have regulatory effects similar to DPP6 on heterologously expressed Kv4 (with and without KChIP) channels.43,44 In addition, DPP10 has been shown to be expressed in normal and failing human ventricles.45
Molecular Determinants of Native Myocardial Transient Outward Voltage-Gated K+ Channels
Considerable experimental evidence has accumulated demonstrating a critical role for Kv α-subunits of the Kv4 subfamily in the generation of cardiac Ito,f channels. In rat and mouse ventricular myocytes exposed to antisense oligodeoxynucleotides targeted against Kv4.2 or Kv4.3, Ito,f density is reduced by approximately 50%.46,47 Rat ventricular Ito,f density is also reduced in cells exposed to an adenoviral construct encoding a truncated Kv4.2 subunit (Kv4.2ST) that functions as a dominant negative.48 In addition, Ito,f is eliminated in ventricular and in atrial myocytes isolated from transgenic mice expressing a pore mutant of Kv4.2, Kv4.2W362F (Kv4.2DN), which also functions as a dominant negative.49,50 Although biochemical and electrophysiologic studies suggested that Kv4.2 and Kv4.3 are associated in adult mouse ventricles and that functional mouse ventricular Ito,f channels are heteromeric,47 targeted deletion of Kv4.2 eliminates mouse ventricular Ito,f,51 whereas elimination of Kv4.3 has no effect.52 In mouse ventricles, therefore, Kv4.2 is the critical α-subunit required for the generation of functional Ito,f channels.51 Given the similarities in the properties of Ito,f (see Table 3-1), it seems reasonable to suggest that Kv4 α-subunits also underlie Ito,f in other species. In canine and in human myocardium, however, the candidate subunit is Kv4.3 because Kv4.2 is barely detectable.53 Although two splice variants of Kv4.3 have been identified,54 the expression levels of the two predicted Kv4.3 proteins and the functional roles of these variants in the generation of functional cardiac Ito,f channels have been determined.
It has also been demonstrated that the Kv channel accessory subunit KChIP2 coimmunoprecipitates with Kv4 α-subunits from adult mouse ventricles, which is consistent with a role for this subunit in the generation of Kv4-encoded mouse ventricular Ito,f channels.47 In ferret and canine hearts, a gradient in KChIP2 message expression is observed through the thickness of the ventricular wall,35,36 leading to suggestions that the differential expression of KChIP2 underlies the epicardial–endocardial differences in Ito,f densities. Subsequent studies revealed that the patterns of expression of the KChIP2 message, the KChIP2 protein, and Ito,f densities in canine ventricles are indeed similar,55 which is consistent with an important role for KChIP2 in determining functional canine and human ventricular Ito,f densities. In contrast, in rat and mouse ventricles, there is little or no gradient in KChIP2 message or protein (51,56) expression, and it appears that regional differences in Kv4.2 expression underlie the heterogeneities in Ito,f densities in rodent ventricles.56,57 Molecular insights into the regulation of the observed regional variations in the expression of the Kv4.2 transcripts were provided with the demonstrations that the expression levels of the transcription factors, Irx5 and NFAT, are positively and negatively correlated, respectively, with the differences in Kv4.2 expression and Ito,f densities.58,59 Interestingly, approximately 25 transcription factors were subsequently shown to be differentially expressed in the ventricles,60 although the functional import of these findings remains to be determined.
A role for the KChIP2 splice variant, KChIP2b, in determining regional differences in ferret ventricular Ito,f densities has also been proposed.35 The KChIP-related (NCS family) protein, NCS-1, appears to be uniformly expressed in adult mouse ventricles and, in addition, coimmunoprecipitates with Kv4 α-subunits.61 The protein expression pattern of this putative Kv4 channel accessory subunit has not been examined in canine or human hearts, and a role for NCS-1 in controlling functional myocardial Ito,f densities has not been determined directly. Although accumulating evidence suggests that cardiac Ito,f channels function in macromolecular complexes, comprising Kv4 α-subunits and multiple cytosolic and transmembrane accessory subunits (Figure 3-4), the molecular compositions of native myocardial Ito,f channels have not been determined. In addition, the functional roles of additional Kv accessory subunits, including members of the KCNE2762 and DPPX4145 subfamilies, in the generation of native myocardial Ito,f channels and in determining regional differences in myocardial Ito,f densities remain to be defined.
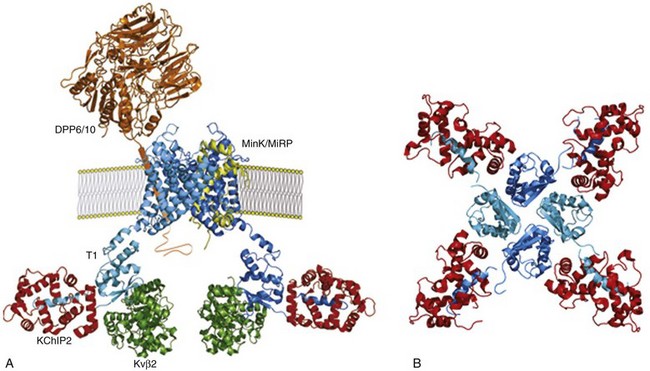
Figure 3-4 Schematic of putative Kv4.3-encoded, myocardial Ito,f channel macromolecular complex. A, Cross section of a Kv4.3 channel in a membrane showing two Kv4.3 α-subunits (blue), generated based on the structure of Kv1.2,91 each interacting with a cytosolic KChIP2 (red) and a cytosolic Kvβ (green) accessory subunit (1 : 1 : 1 : stoichiometry) through distinct, non-overlapping N-terminal domains. The transmembrane accessory subunits, DPP6/10 (brown)92 and MinK/MiRPs (yellow), which have also been proposed to interact with Kv4.3 α-subunits (1 : 2 stoichiometry) and to contribute to the formation of functional cardiac Ito,f channels. B, Structural analyses of Kv4.3N-KChIP1 complexes93 revealed a 1 : 1 stoichiometry with each KChIP (red) bridging two adjacent Kv4.3 N termini (blue), anchoring hydrophobic Kv4.3 N terminal residues in a hydrophobic binding pocket in KChIP1. Protein structures illustrated were generated based on published structural data91–93 using PyMOL.
The kinetic and pharmacologic properties of the slow transient outward K+ currents, Ito,s, in ventricular myocytes are different from Ito,f (see Table 3-1), which is interpreted as suggesting that the molecular correlates of ventricular Ito,s and Ito,f channels are also distinct. Direct experimental support for this hypothesis was provided in electrophysiologic experiments on myocytes isolated from mice with a targeted deletion of the Kv1.4 gene, Kv1.4–/–,63 demonstrating that Ito,s is undetectable in septum cells.64 The properties and the densities of Ito,f, IK,slow1, IK,slow2, and Iss in Kv1.4–/– left and right ventricular (and in atrial) myocytes, however, are indistinguishable from those measured in wild type cells.64,65 Interestingly, upregulation of Ito,s is evident in the ventricles of Kv4.2DN-expressing mice in which Ito,f is eliminated.65 Given the similarities in the time- and voltage-dependent properties of the slow transient outward K+ currents in other species with mouse Ito,s (1), it seems reasonable to suggest that Kv1.4 likely also encodes Ito,s in ferret, rabbit, human, and canine ventricular myocytes.
Molecular Determinants of Native Myocardial Delayed Rectifier Voltage-Gated K+ Channels
As noted earlier, ERG1 is the locus of mutations in LQT2, and heterologous expression of ERG1 reveals voltage-gated, inwardly rectifying K+-selective channels that are similar to cardiac IKr.23 Alternatively processed forms of ERG1, with unique N- and C-termini, have also been identified in mouse and human hearts and suggested to have roles in the generation of native cardiac IKr channels.66–68 It has also been suggested that functional cardiac IKr channels are multimeric, comprising ERG1 and minK, and biochemical studies have demonstrated coimmunoprecipitation of ERG1 and minK from equine ventricles.69 It is not clear whether ERG1 and minK or other members of the KCNE family are also found in association in other species.
Although heterologous expression of KCNQ1, the locus of mutations in LQT1, reveals rapidly activating, non-inactivating Kv currents, coexpression with minK produces slowly activating Kv currents similar to cardiac IKs.23 These observations, together with biochemical data demonstrating that heterologously expressed KvLQT1 and minK associate, were interpreted as suggesting that minK coassembles with KvLQT1 to form functional cardiac IKs channels.1,23 Direct biochemical evidence for the in situ coassembly of KvLQT1 and minK was recently provided in studies of equine ventricles.69 Similar data for human ventricular IKs are yet to be provided. Interestingly, it was reported that KvLQT1 modulates the distribution and properties of ERG1-encoded channels, an observation interpreted as suggesting that cardiac IKs and IKr channels are regulated directly through Kv α-subunit–Kv α-subunit interactions.70 The molecular mechanisms controlling the cell surface expression of functional IKs channels, the regional differences in functional ventricular IKs densities, and the interactions between cardiac IKs and IKr (and possibly other cardiac channels), however, remain to be determined.
Similar to the transient outward Kv currents, molecular genetic methods, in combination with biochemistry and electrophysiology primarily in mice, have provided molecular insights into the basis of functional delayed rectifier Kv channel diversity in the murine myocardium. A role for Kv1 α-subunits in the generation of mouse ventricular IK,slow, for example, was revealed with the demonstration that IK,slow is selectively attenuated in ventricular myocytes isolated from transgenic mice expressing a truncated, dominant negative Kv1 α-subunit, Kv1.1DN.14 It was subsequently shown, however, that IK,slow is also reduced in ventricular myocytes expressing a dominant negative Kv 2.1 mutant, Kv2.1DN,15 revealing that there are two molecularly distinct components of mouse ventricular IK,slow : IK,slow1 that is sensitive to micromolar concentrations of 4-AP and encoded by Kv1 α-subunits, and IK,slow2 that is sensitive to TEA and encoded by Kv2 α-subunits.15 Subsequent studies revealed that IK,slow1 is eliminated in ventricular myocytes isolated from mice harboring the targeted disruption of the KCNA5 (Kv1.5) locus, revealing that Kv1.5 encodes IK,slow1.16 These findings, together with the previous studies completed on cells from Kv1.4–/– animals63 in which Ito,s is eliminated,64 reveal that, in contrast to the Kv4 α-subunits, Kv4.2, and Kv4.3,47 the Kv1 α-subunits, Kv1.4 and Kv1.5, do not associate in adult mouse ventricles in situ. Rather, functional Kv1 α-subunit–encoded Kv channels in mouse ventricular myocytes are homomeric, composed of Kv1.4 α-subunits (Ito,s)63 or Kv1.5 α-subunits (IK,slow1).16 The roles of Kv accessory subunits in the generation of functional Ito,s, IK,slow1 and IK,slow2 channels and the molecular mechanisms controlling the differential expression of these channels remain to be determined.
Molecular Determinants of Myocardial Kir and K2P Channels
In cardiac and other cells, inwardly rectifying K+ (IK1) channels are encoded by a large and diverse subfamily of inward rectifier K+ (Kir) channel pore-forming α-subunit genes,4 each of which encodes a protein with two transmembrane domains that assemble as tetramers to form K+ selective pores (see Figure 3-3). Based on the properties of the currents produced in heterologous expression systems, Kir2 α-subunits were long thought to encode the strongly inwardly rectifying cardiac IK1 channels,20 and several members of the Kir2 subfamily are expressed in the myocardium.71 Direct insights into the roles of Kir2 α-subunits in the generation of cardiac IK1 channels were provided in studies completed on myocytes isolated from mice lacking KCNJ2 (Kir2.1–/–) or KCNJ12 (Kir2.2–/–).72 Although Kir2.1–/– mice have cleft palate and die shortly after birth, precluding electrophysiologic studies on myocytes from adult animals, voltage-clamp recordings from isolated newborn Kir2.1–/– ventricular myocytes revealed that IK1 is absent, whereas a quantitative reduction in IK1 was observed in adult Kir2.2–/– ventricular myocytes,72 suggesting that both Kir2.1 and Kir2.2 contribute to mouse ventricular IK1 channels and that functional cardiac IK1 channels are heteromeric. The quantitative differences between the effects of the deletion of KCNJ2 and KCNJ12 further suggest that Kv2.1 (KCNJ2) is the critical subunit underlying (mouse) IK1 channels.72
Mutations in KCNJ2 have been linked to congenital long QT (Andersen-Tawil syndrome or LQT7) and short QT syndromes,73,74 and increasing expression of Kir2.1 in the mouse heart, which results in the upregulation of IK1, is proarrhythmic.75,76 Previous studies have identified regional differences in myocardial IK1 expression and properties in adult mouse heart,77,78 and it has been suggested that these differences reflect the variable subunit compositions of the channels, as well as differences in polyamine concentrations.79 Studies focused on testing this hypothesis directly and on defining the molecular mechanisms controlling regional differences in the expression and functioning of native IK1 channels in species other than mice are also clearly warranted.
In the mammalian heart, IKATP channels appear not to have a prominent role in action potential repolarization, but are thought to be important in myocardial ischemia and preconditioning.21,22 In heterologous systems, IKATP channels can be reconstituted by coexpression of Kir6.x subunits with one or more ATP-binding cassette proteins that encode sulfonylurea receptors, SURx.80 Pharmacologic and mRNA expression studies suggested that cardiac sarcolemmal IKATP channels are encoded by Kir6.2 and SUR2A, and the essential role of Kir6.2 was demonstrated directly in experiments showing that IKATP channels are undetectable in ventricular myocytes isolated from Kir6.2–/– animals.81 In addition, cardiac IKATP channel activity is reduced in SUR2–/– myocytes82 and unaffected in SUR1-/- myocytes,83 suggesting an important role for SUR2. Interestingly, the properties of the residual IKATP channels in SUR2–/– myocytes are similar to those produced in heterologous cells on coexpression of Kir6.2 and SUR1,82 suggesting that SUR1 also contributes to the generation of functional cardiac IKATP channels by coassembling with Kir6.2 α-subunits alone or with SUR2A. Biochemical and molecular genetic strategies will need to be combined to define the molecular components of native cardiac IKATP channels directly.
Although action potential waveforms in Kir6.2–/– and wild type ventricular myocytes are indistinguishable, the action potential shortening observed in wild type cells during ischemia or metabolic blockade is abolished in the Kir6.2–/– cells,81 consistent with the hypothesis that cardiac IKATP channels have a role under pathophysiologic conditions, particularly those involving metabolic stress. Interestingly, action potential durations are largely unaffected in transgenic animals expressing mutant IKATP channels with markedly (fortyfold) reduced ATP sensitivity, suggesting that there are additional inhibitory mechanisms that regulate cardiac IKATP channel activity in vivo.84 Similar to the strong inwardly rectifying myocardial Kir currents, IK1, further study is needed to provide molecular insights into the mechanisms controlling regional differences in the functional expression of myocardial IKATP channels.
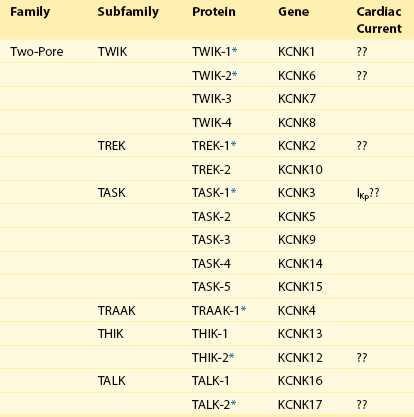
In addition to Kv and Kir channel α-subunits that assemble as tetramers, a novel type of K+ α-subunit with four transmembrane spanning regions and two pore domains (see Figure 3-3) was identified with the cloning of TWIK-1.85 Both pore domains contribute to the formation of the K+ selective pore, and TWIK-1 subunits assemble as dimers.86 A large number of two-pore domain K+ (K2P) channel α-subunit genes have been identified, and several are expressed in the mammalian myocardium (Table 3-3). Heterologous expression of K2P subunits reveals currents with distinct biophysical properties and sensitivities to several potential intracellular and extracellular modulators, including anesthetics, change in pH, and fatty acids.85
The multiplicity of K2P α-subunits (see Table 3-3), the widespread distribution of expressed subunits and the findings that the properties of the channels encoded by K2P subunits are regulated by a variety of physiologically (and pathophysiologically) relevant stimuli suggest that K2P channels likely subserve a variety of important functions. As with other cell types, the physiologic roles of these subunits and channels in the myocardium are just beginning to be explored. Both TREK-1 and TASK-1 are detected in heart, and heterologous expression of these subunits gives rise to instantaneous, non-inactivating K+ currents that display little or no voltage dependence.85 These properties suggest that K2P subunits might contribute to background or “leak” K+ channels (i.e., channels with properties similar to the steady-state, non-inactivating K+ current [Iss] that is expressed or characterized in rodent myocytes).8,9,85–87 In recent studies completed on rat atrial and ventricular myocytes, roles for TASK188–90 and TREK186,87 subunits in the generation of Iss have been suggested. Clearly, experiments focused on testing these hypotheses are needed to provide clear insights into the molecular basis of Iss and to allow further study focused on defining the mechanisms controlling the physiologic and the pathophysiologic regulation of these (Iss) channels.
Conclusions
Cellular electrophysiologic studies have distinguished multiple types of voltage-gated inward and outward currents that contribute to action potential repolarization in mammalian cardiac cells (see Table 3-1). The outward (K+) currents are more numerous and more diverse than the inward (Na+, Ca2+) currents, and most cardiac cells express a repertoire of voltage-gated and inwardly rectifying K+ channels (see Table 3-1). In addition, several of these K+ channels are expressed differentially in different myocardial cell types and through the thickness of the ventricular walls. Molecular cloning led to the identification of multiple voltage-gated (Kv), non–voltage-gated, inwardly rectifying (Kir), and weakly rectifying, non-inactivating (K2P) K+ channel pore-forming α-subunits and a number of channel accessory (β) subunits (see Table 3-2) thought to contribute to the formation of the various cardiac K+ currents that have been distinguished electrophysiologically (see Table 3-1). In recent years, considerable progress has been made in identifying the pore-forming Kv and Kir α-subunits contributing to the formation of most of the K+ channels expressed in mammalian cardiac myocytes (see Figure 3-2). In addition, biochemical studies have provided some insights into the molecular mechanisms underlying the observed heterogeneities in the expression of myocardial Kv and Kir currents. For cardiac Ito,f, regional differences in current densities are correlated with differences in Kv4.2 protein expression in adult mouse ventricles,57 whereas variable expression of the Ito,f channel accessory protein, KChIP2, has been suggested as underlying the transmural gradient in Ito,f densities in canine and human ventricles.55 For cardiac IK1 channels, in contrast, recent studies suggest that differences in Kir channel α-subunit composition or differences in the concentrations of intracellular polyamines appear to have roles in regulating the functional diversity of these channels.78
Accumulating evidence suggests that native myocardial Kv channels likely function in macromolecular protein complexes (see Figure 3-4), comprising pore-forming α-subunits and multiple cytosolic and transmembrane accessory subunits (see Table 3-2). The molecular compositions of native myocardial Kv channels, however, have not been determined. In contrast to the progress made in defining the Kv (and the Kir) α-subunits encoding native myocardial Kv (and Kir) channels or currents, however, much less is known about the functional roles of the putative accessory subunits (see Table 3-2) of these channels, as well as about the functioning of the various K2P subunits (see Table 3-3). An important focus of future work will likely be on defining the roles of the various Kvβ and the K2P α-subunits in the generation of functional myocardial K+ channels and on defining the molecular mechanisms controlling the properties and the cell surface expression of myocardial K+ channels encoded by the various Kv α and β, Kir α and β, and K2P α-subunits. In addition, as numerous studies have documented changes in functional K+ channel expression in a variety of myocardial disease states, changes that could reflect modifications in channel properties, as well as alterations in the molecular compositions of the channels or the processing of the underlying channel subunits, it seems clear that a major focus of future research will be on defining these mechanisms in detail. There are numerous possible mechanisms, including transcriptional, translational, and posttranslational, that likely are important in regulating the functional expression and the properties of myocardial K+ channels in the normal, as well as in the damaged or diseased, myocardium. Defining these mechanisms will have an important effect on the field, leading to new insights into the mechanisms that regulate myocardial K+ channel expression and functioning and to the development of novel therapeutic strategies to prevent or reverse the remodeling of these channels associated with systemic or myocardial disease.
References
1. Nerbonne, JM, Kass, RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005; 85:1205–1253.
2. Antzelevitch, C, Dumaine, R, Electrical heterogeneity in the heart: physiological, pharmacological and clinical implications. Handbook of Physiology, Section 2: The Cardiovascular System. Page, E, Fozzard, HA, Solaro, RJ, eds. Handbook of Physiology, Section 2: The Cardiovascular System; Vol. 1. Oxford, New York, 2002:654–692.
3. Nerbonne, JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol (Lond). 2000; 525:285–298.
4. Coetzee, WA, Amarillo, Y, Chiu, J, et al. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999; 868:233–285.
5. Pongs, O, Leicher, T, Berger, M, et al. Functional and molecular aspects of voltage-gated K+ channel β subunits. Ann NY Acad Sci. 1999; 868:344–355.
6. Oudit, GY, Kassiri, Z, Sah, R, et al. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol. 2001; 33:851–872.
7. Niwa, N, Nerbonne, JM. Molecular determinants of cardiac transient outward K+ current (Ito) expression and regulation. J Mol Cell Cardiol. 2010; 48:12–25.
8. Xu, H, Guo, W, Nerbonne, JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol. 1999; 113:661–678.
9. Brunet, S, Aimond, F, Li, H, et al. Heterogeneous expression of repolarizing voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004; 559:103–120.
10. Liu, J, Kim, K-H, London, B, et al. Dissection of the voltage-activated potassium outward currents in adult mouse ventricular myocytes: Ito,f, Ito,s, IKslow1, IKslow2 and Iss. Basic Res Cardiol. 2011; 106:189–204.
11. Brahmajothi, MV, Campbell, DL, Rasmussen, RL, et al. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol. 1999; 113:581–600.
12. Jurkiewicz, NK, Sanguinetti, MC. Rate-dependent prolongation of cardiac action potentials by a methanesulfonanilide class III antiarrhythmic agent. Specific block of rapidly activating delayed rectifier K+ current by dofetilide. Circ Res. 1993; 72:75–83.
13. Bryant, SM, Wan, X, Shipsey, SJ, et al. Regional differences in the delayed rectifier current (IKr and IKs) contribute to the differences in action potential duration in basal left ventricular myocytes in guinea-pig. Cardiovasc Res. 1998; 40:322–331.
14. London, B, Jeron, A, Zhou, J, et al. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998; 95:2926–2931.
15. Xu, H, Barry, DM, Li, H, et al. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999; 85:623–633.
16. London, B, Guo, W, Pan, XH, et al. Targeted replacement of Kv1. 5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of I(K,slow) and resistance to drug-induced QT prolongation. Circ Res. 2001; 88:940–946.
17. Zhou, J, Kodirov, S, Murata, M, et al. Regional upregulation of Kv2. 1-encoded current, IK,slow2, in Kv1DN mice is abolished by crossbreeding with Kv2DN mice. Am J Physiol Heart Circ Physiol. 2003; 284:H491–H500.
18. Li, H, Guo, W, Yamada, KA, et al. Selective elimination of I(K,slow1) in mouse ventricular myocytes expressing a dominant negative Kv1. 5alpha subunit. Am J Physiol Heart Circ Physiol. 2004; 286:H319–H328.
19. Bou-Abboud, E, Li, H, Nerbonne, JM. Molecular diversity of the repolarizing voltage-gated K+ currents in mouse atrial cells. J Physiol. 2000; 529:345–358.
20. Lopatin, AN, Nichols, CG. Inward rectifiers in the heart: an update on I(K1). J Mol Cell Cardiol. 2001; 33:625–638.
21. Flagg, TP, Nichols, CG. Sarcolemmal K(ATP) channels: what do we really know? J Mol Cell Cardiol. 2005; 39:61–70.
22. Grover, GJ, Garlid, KD. ATP-Sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000; 32:677–695.
23. Keating, MT, Sanguinetti, MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001; 104:569–580.
24. Abbott, GW, Goldstein, SA. A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs). Q Rev Biophys. 1998; 31:357–398.
25. Abbott, GW, Sesti, F, Splawski, I, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999; 97:175–187.
26. Abbott, GW, Butler, MH, Bendahhou, S, et al. MiRP2 forms potassium channels in skeletal muscle with Kv3. 4 and is associated with periodic paralysis. Cell. 2001; 104:217–231.
27. Zhang, M, Jiang, M, Tseng, GN. minK-related peptide 1 associates with Kv4. 2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001; 88:1012–1019.
28. Yu, H, Wu, J, Potapova, I, et al. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001; 88:E84–E87.
29. Nagaya, N, Papazian, DM. Potassium channel alpha and beta subunits assemble in the endoplasmic reticulum. J Biol Chem. 1997; 272:3022–3027.
30. Aimond, F, Kwak, SP, Rhodes, KJ, et al. Accessory Kvbeta1 subunits differentially modulate the functional expression of voltage-gated K+ channels in mouse ventricular myoctyes. Circ Res. 2005; 96:451–458.
31. An, WF, Bowlby, MR, Betty, M, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000; 403:553–556.
32. Morohashi, Y, Hatano, N, Ohya, S, et al. Molecular cloning and characterization of CALP/KChIP4, a novel EF-hand protein interacting with presenilin 2 and voltage-gated potassium channel subunit Kv4. J Biol Chem. 2002; 277:14965–14975.
33. Rosati, B, Pan, Z, Lypen, S, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001; 533:119–125.
34. Deschenes, I, DiSilvestre, D, Juang, GJ, et al. Regulation of Kv4. 3 current by KChIP2 splice variants: a component of native cardiac I(to)? Circulation. 2002; 106:423–429.
35. Patel, S, Campbell, DL, Morales, MJ, et al. Heterogeneous expression of KChIP2 isoforms in the ferret heart. J Physiol. 2002; 539:649–656.
36. Decher, N, Barth, AS, Gonzalez, T, et al. Novel KChIP2 isoforms increase functional diversity of transient outward potassium currents. J Physiol. 2004; 557:761–772.
37. Burgoyne, RD, Weiss, JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001; 353:1–12.
38. Li, H, Guo, W, Mellor, RL, et al. KChIP2 modulates the cell surface expression of Kv1. 5-encoded K(+) channels. J Mol Cell Cardiol. 2005; 39:121–132.
39. Thomsen, MB, Wang, C, Ozgen, N, et al. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ Res. 2009; 104:1382–1389.
40. Anderson, D, Mehaffey, WH, Iftinca, M, et al. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010; 13:333–337.
41. Radicke, S, Cotella, D, Graf, EM, et al. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative b-subunit of human cardiac transient outward current encoded by Kv4. 3. J Physiol. 2005; 565:751–756.
42. Nadal, MS, Ozaita, A, Amarillo, Y, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003; 37:449–461.
43. Jerng, HH, Qian, Y, Pfaffinger, PJ. Modulation of Kv4. 2 channel expression and gating by dipeptidyl peptidase 10 (DPP10). Biophys J. 2004; 87:2380–2396.
44. Ren, X, Hayashi, Y, Yoshimura, N, et al. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci. 2005; 29:320–332.
45. Radicke S, Cotella D, Bortoluzzi A, et al: Dpp10—A new putative regulatory β subunit of Ito in failing and non-failing human heart. Circulat.
46. Fiset, C, Clark, RB, Shimoni, Y, et al. Shal-type channels contribute to the Ca2+-independent transient outward K+ current in rat ventricle. J Physiol. 1997; 500:51–64.
47. Guo, W, Li, H, Aimond, F, et al. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002; 90:586–593.
48. Johns, DC, Nuss, HB, Marban, E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4. 2 constructs. J Biol Chem. 1997; 272:31598–31603.
49. Barry, DM, Xu, H, Schuessler, RB, et al. Functional knockout of the transient outward current, long-QT syndrome, and cardiac modeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998; 83:560–567.
50. Xu, H, Li, H, Nerbonne, JM. Elimination of the transient outward current and action potential prolongation in mouse atrial myocytes expressing a dominant negative Kv4 alpha subunit. J Physiol. 1999; 519:11–21.
51. Guo, W, Jung, WE, Marionneau, C, et al. Targeted deletion of Kv4. 2 eliminates I(to,f) and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005; 97:1342–1350.
52. Niwa, N, Wang, W, Sha, Q, et al. Kv4. 3 is not required for the generation of functional I(to,f) channels in adult mouse ventricles. J Mol Cell Cardiol. 2008; 44:95–104.
53. Dixon, JE, Shi, W, Wang, HS, et al. Role of the Kv4. 3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996; 79:659–668.
54. Kong, W, Po, S, Yamagishi, T, et al. Isolation and characterization of the human gene encoding Ito: further diversity by alternative mRNA splicing. Am J Physiol. 1998; 275:H1963–H1970.
55. Rosati, B, Grau, F, Rodriguez, S, et al. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol. 2003; 548:815–822.
56. Teutsch, C, Kondo, RP, Dederko, DA, et al. Spatial distributions of Kv4 channels and KChIP2 isoforms in the murine heart based on laser capture microdissection. Cardiovasc Res. 2007; 73:739–749.
57. Dixon, JE, McKinnon, D. Quantitative analysis of potassium channel mRNA expression in atrial and ventricular muscle of rats. Circ Res. 1994; 75:252–260.
58. Costantini, DL, Arruda, EP, Agarwal, P, et al. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005; 123:347–358.
59. Rossow, CF, Dilly, KW, Santana, LF. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ Res. 2006; 98:1306–1313.
60. Rosati, B, Grau, F, McKinnon, D. Regional variation in mRNA transcript abundance within the ventricular wall. J Mol Cell Cardiol. 2006; 40:295–302.
61. Guo, W, Malin, SA, Johns, DC, et al. Modulation of Kv4-encoded K+ currents in the mammalian myocardium by neuronal calcium sensor-1. J Biol Chem. 2002; 277:26436–26443.
62. Radicke, S, Cotella, D, Graf, EM, et al. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res. 2006; 71:695–703.
63. London, B, Wang, DW, Hill, JA, et al. The transient outward current in mice lacking the potassium channel gene Kv1. 4. J Physiol. 1998; 509:171–182.
64. Guo, W, Xu, H, London, B, et al. Molecular basis of transient outward K+ current diversity in mouse ventricular myocytes. J Physiol. 1999; 521:587–599.
65. Guo, W, Li, H, London, B, et al. Functional consequences of elimination of I(to,f) and I(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1. 4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000; 87:73–79.
66. Pond, AL, Scheve, BK, Benedict, AT, et al. Expression of distinct ERG proteins in rat, mouse, and human heart. Relation to functional I(Kr) channels. J Biol Chem. 2000; 275:5997–6006.
67. Jones, EM, Roti Roti, EC, Wang, J, et al. Cardiac IKr channels minimally comprise hERG 1a and 1b subunits. J Biol Chem. 2004; 279:44690–44694.
68. Phartiyal, P, Jones, EM, Robertson, GA. Heteromeric assembly of human ether-a-go-go-related gene (hERG) 1a/1b channels occurs cotranslationally via N-terminal interactions. J Biol Chem. 2007; 282:9874–9882.
69. Finley, MR, Li, Y, Hua, F, et al. Expression and coassociation of ERG1, KCNQ1, and KCNE1 potassium channel proteins in horse heart. Am J Physiol. 2002; 283:H126–H138.
70. Ehrlich, JR, Pourrier, M, Weerapura, M, et al. KvLQT1 modulates the distribution and biophysical properties of HERG. A novel alpha-subunit interaction between delayed rectifier currents. J Biol Chem. 2004; 279:1233–1241.
71. Liu, GX, Derst, C, Schlichthorl, G, et al. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001; 532:115–126.
72. Zaritsky, JJ, Redell, JB, Tempel, BL, et al. The consequences of disrupting cardiac inwardly rectifying K(+) current (IK1) as revealed by the targeted deletion of the murine Kir2. 1 and Kir2. 2 genes. J Physiol. 2001; 533:697–710.
73. Tristani-Firouzi, M, Jensen, JL, Donaldson, MR, et al. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen Syndrome). J Clin Invest. 2002; 110:381–388.
74. Priori, SG, Pandit, SV, Rivolta, I, et al. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005; 96:800–807.
75. Noujaim, SF, Pandit, SV, Berenfeld, O, et al. Up-regulation of the inward rectifier K+ current (IK1) in the mouse heart accelerates and stabilizes rotors. J Physiol. 2007; 578:315–326.
76. Piao, L, Li, J, McLerie, M, et al. Transgenic upregulation of IK1 in the mouse heart is proarrhythmic. Basic Res Cardiol. 2007; 102:416–428.
77. Dhamoon, AS, Pandit, SV, Sarmast, F, et al. Unique Kir2. x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004; 94:1332–1339.
78. Panama, BK, McLerie, M, Lopatin, AN. Heterogeneity of IK1 in the mouse heart. Am J Physiol Heart Circ Physiol. 2007; 293:H3558–H3567.
79. Panama, BK, Lopatin, AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006; 571:287–302.
80. Babenko, AP, Aguilar-Bryan, L, Bryan, J. A view of sur/KIR6. X, KATP channels. Ann Rev Physiol. 1998; 60:667–687.
81. Suzuki, M, Li, RA, Miki, T, et al. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6. 2-knockout mice. Circ Res. 2001; 88:570–577.
82. Pu, J, Wada, T, Valdivia, C, et al. Evidence of KATP channels in native cardiac cells without SUR. Biophys J. 2001; 80:625–626.
83. Seghers, V, Nakazaki, M, DeMayo, F, et al. SUR1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000; 275:9270–9277.
84. Koster, JC, Knopp, A, Flagg, TP, et al. Tolerance for ATP-insensitive K(ATP) channels in transgenic mice. Circ Res. 2001; 89:1022–1029.
85. Lesage, F, Lazdunski, M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000; 279:F793–F801.
86. Goldstein, SA, Bockenhauer, D, O’Kelly, I, et al. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci. 2001; 2:175–184.
87. Terrenoire, C, Lauritzen, I, Lesage, F, et al. A TREK-1-like potassium channel in atrial cells inhibited by beta-adrenergic stimulation and activated by volatile anesthetics. Circ Res. 2001; 89:336–342.
88. Barbuti, A, Ishii, S, Shimizu, T, et al. Block of the background K+ channel TASK-1 contributes to arrhythmogenic effects of platelet-activating factor. Am J Physiol Heart Circ Physiol. 2002; 282:H2024–H2030.
89. Besana, A, Barbuti, A, Tateyama, MA, et al. Activation of protein kinase C epsilon inhibits the two-pore domain K+ channel, TASK-1, inducing repolarization abnormalities in cardiac ventricular myocytes. J Biol Chem. 2004; 279:33154–33160.
90. Putzke, C, Wemhoner, K, Sachse, FB, et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc Res. 2007; 75:59–68.
91. Long, SB, Tao, X, Campbell, EB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2009; 450:376–382.
92. Strop, P, Bankovich, AJ, Hansen, KC, et al. Structure of a human A-type potassium channel interacting protein DPPX, a member of the dipeptidyl aminopeptidase family. J Mol Biol. 2004; 343:1055–1065.
93. Pioletti, M, Findeisen, F, Hura, GL, et al. Three-dimensional structure of the KChIP1-Kv4. 3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006; 13:987–995.