12 Vitreous and Vitreoretinal Disorders
Anatomy and Embryology of the Vitreous
Rhegmatogenous Retinal Detachment
Differential Diagnosis of Rhegmatogenous Retinal Detachment
Special Types of Rhegmatogenous Retinal Detachment
Extraretinal Neovascularization
Macular Vitreoretinal Pathology
Macular Epiretinal Membrane (Pucker)
Principles of Vitreoretinal Surgery for Rhegmatogenous Retinal Detachment
ANATOMY AND EMBRYOLOGY OF THE VITREOUS
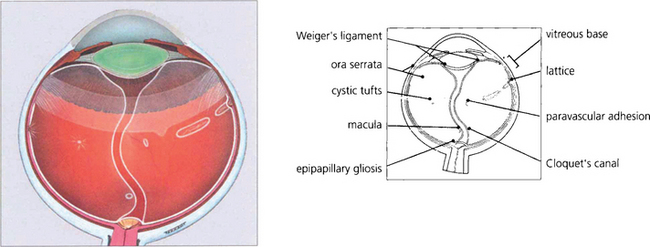
During early development the invaginated optic vesicle (optic cup) contains the primary vitreous, a vascularized tissue supplying the lens and retina, both of which are of ectodermal origin. During the third month of gestation the primary vitreous gradually loses its vascularity and is replaced by the secondary vitreous which is derived from the anterior retina and ciliary body. The principal remnants of the primary vitreous are Cloquet’s canal, a central tubular stucture that stretches sinuously between the lens and the optic disc and some epipapillary gliosis. In later life an exaggeration of the latter is seen as Bergmeister’s papilla and a Mittendorf’s dot is a primary vitreous remnant seen on the posterior lens capsule (see Ch. 17). The most common and severe developmental anomaly of the vitreous is persistent hyperplastic primary vitreous. This usually presents in infancy as a microphthalmic squinting eye with leukocoria. Pupil dilatation may demonstrate dragging of the ciliary processes towards a central plaque of fibrovascular tissue; this invades the lens posteriorly and ultimately causes complete cataract and secondary angle closure glaucoma (see Ch. 8).
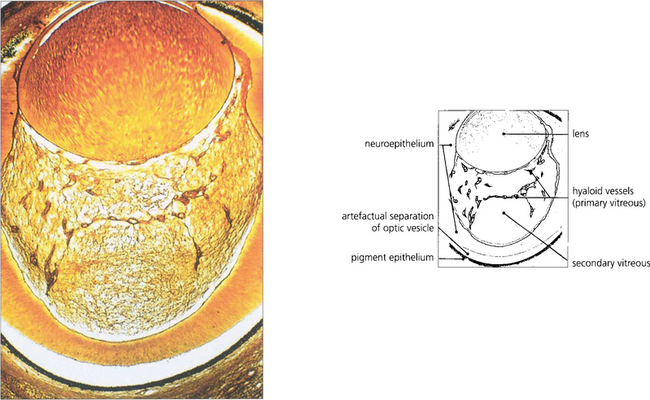
Fig. 12.1 Light micrograph of a section through the eye of a 13-mm human fetus showing primary and secondary vitreous with artefactual re-establishment of optic vesicle (silver stain).
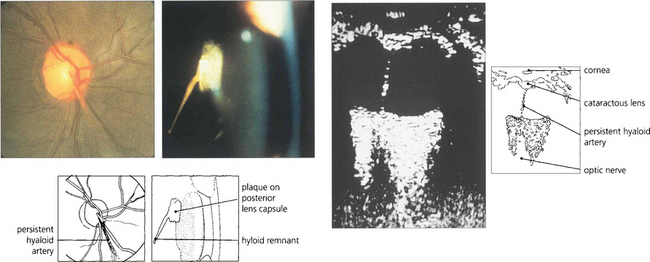
Fig. 12.2 The hyaloid artery may occasionally persist as a vascular channel between the central gel and optic disc (left), or as a glial plaque on the posterior lens capsule (centre). The B scan (right) demonstrates a persistent hyaloid artery.
VITREOUS CHANGES
POSTERIOR VITREOUS DETACHMENT
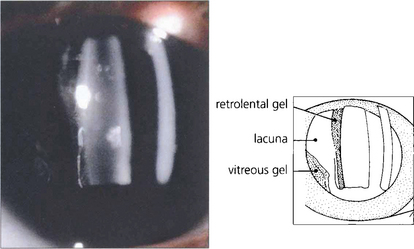
Fig. 12.4 With ageing vitreous degenerates to cause syneresis (condensation) and lacuna (cavity) formation and collapse. At slit-lamp examination the detached posterior hyaloid membrane looks like a wrinkled hanging curtain moving freely on eye movement. It separates from the retina up to the posterior border of the vitreous base (or the posterior aspect of any vitreoretinal adhesions).
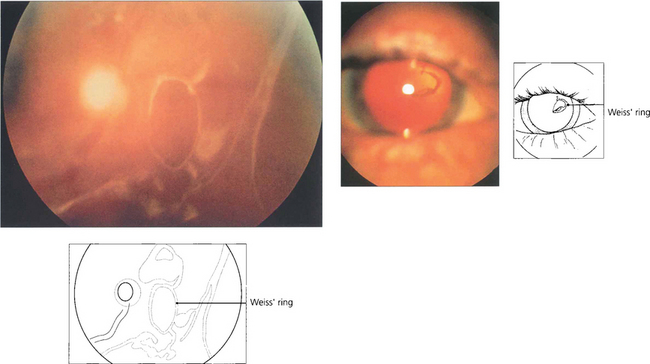
Fig. 12.5 On posterior vitreous detachment epipapillary glial tissue can become avulsed from the disc margin and is seen ophthalmoscopically as a ring of tissue on the detached posterior hyaloid interface situated in front of the optic disc (Weiss’ ring). The patient sees a circular or oval floater (depending on how it tilts) or describes a ‘cobweb’ or ‘spider’ that moves with the eye. Aggregations of collagen fibrils can also produce symptomatic floaters. ‘Lightening flashes’ may be seen in the temporal periphery, each typically lasting for a few seconds and induced by eye movement, possibly because photoreceptors depolarize when the vitreous base tugs on the retina; these flashes are often more noticeable on the transition from light to dark.
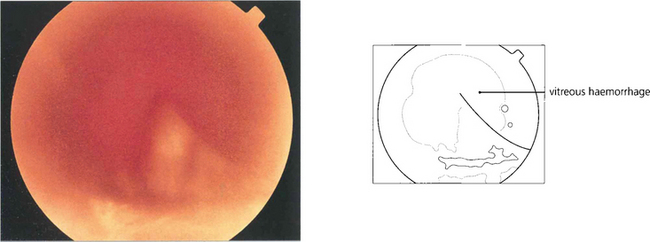
VITREOUS HAEMORRHAGE
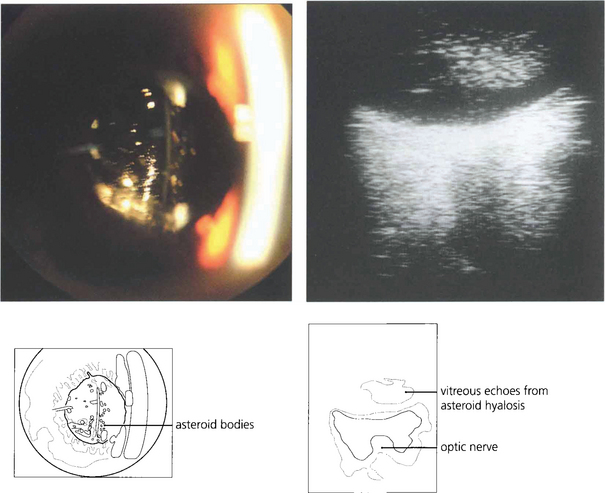
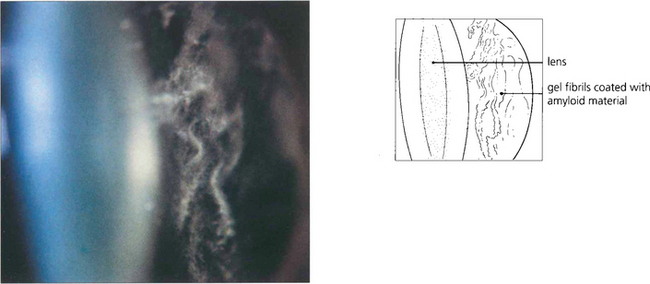
The blood in the vitreous gel initially forms a localized clot but subsequently disperses throughout the gel following fibrinolysis. During haemolysis biconcave erythrocytes loose haemoglobin to become spheroidal erythroclasts. Biodegraded haemoglobin stains the gel ochre-yellow or orange. Erythroclast clogging of the vitreous cortex produces an ‘ochre membrane’ at the posterior hyaloid face. The mechanisms by which vitreous haemorrhage is spontaneously absorbed are not clear although phagocytosis by macrophages, outflow through the trabecular meshwork and syneretic disintegration of the gel play a part. Erythroclasts in the trabecular meshwork can reduce outflow to produce raised intraocular pressure and ‘erythroclastic glaucoma’ (see Ch. 8). Rarely, vitreous haemorrhage causes ‘synchysis scintillans’, a localized form of cholesterolosis bulbi characterized by cholesterol crystal accumulation in the vitreous cavity. The crystals sediment inferiorly but shower through the vitreous cavity with eye movement.
VITRITIS
Inflammatory cell infiltration of the vitreous is a feature of posterior uveitis, bacterial, viral, yeast and fungal infections, and intraocular lymphoma (see Ch. 10). Vitreous biopsy or vitrectomy has an increasing role in the management of these patients to obtain specimens for cellular diagnosis, microbial culture, removal of the vitreous scaffold and delivery of therapeutic drugs.
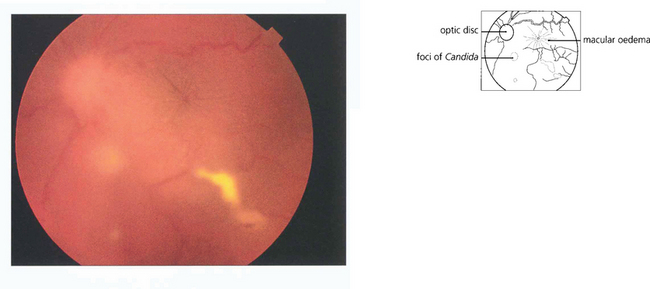
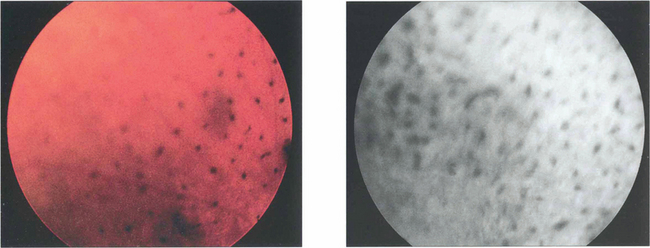
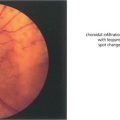
Fig. 12.8 Candidaemia in patients taking broad-spectrum antibiotics or with indwelling intravenous catheters or in intravenous drug users (Candida can grow in the lemon juice that may be used to dissolve heroin), carries a significant risk of metastatic endophthalmitis which manifests as intravitreal white puff-balls. Candida endophthalmitis, although more slowly progressive than bacterial endophthalmitis, usually requires vitrectomy to remove the infected gel, to administer intravitreal medication and to restore vision. Vitreous puff-balls are seen in this intravenous drug user with Candida endophthalmitis.
RHEGMATOGENOUS RETINAL DETACHMENT
FORMATION OF RETINAL BREAKS
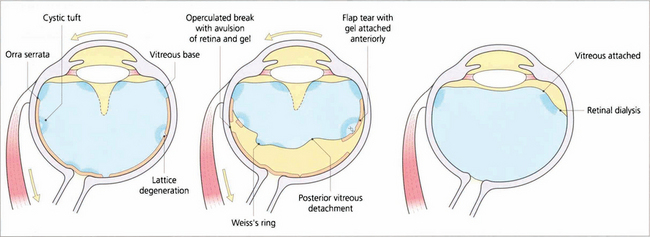
Fig. 12.11 Diagram illustrating the concept of dynamic vitreoretinal traction after posterior vitreous detachment and how this generates a flap-tear or an operculated tear. In contrast with a dialysis the vitreous remains attached and there is no posterior vitreous detachment.
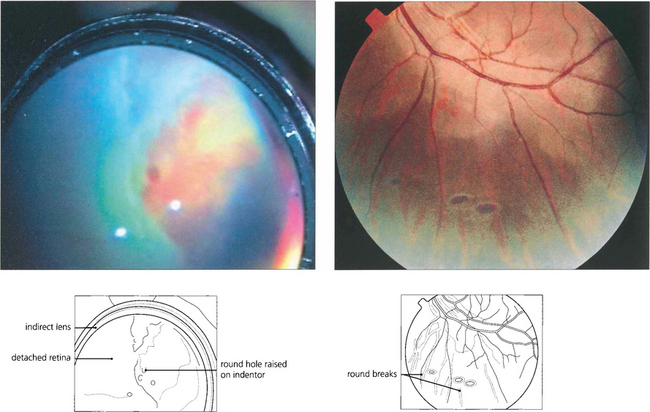
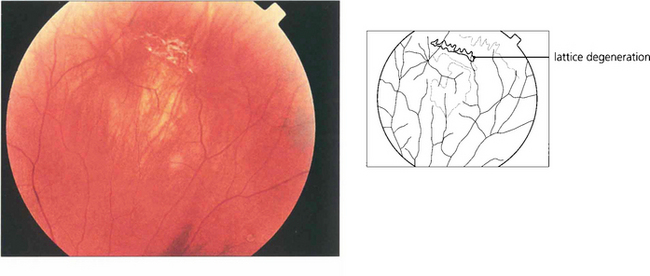
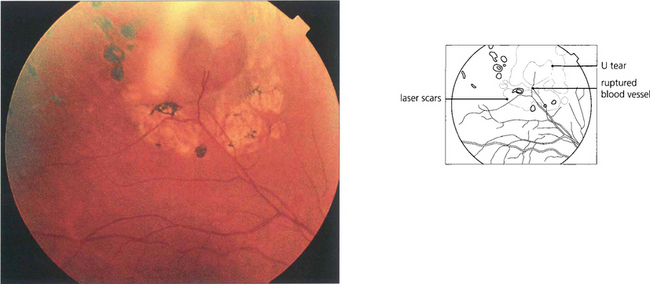
Fig. 12.15 Atrophic round retinal breaks are seen with an attached vitreous gel. They are usually equatorial and often associated with lattice degeneration. Recruitment of fluid with round hole detachment probably occurs by connection of the hole to vitreous lacunae; this may cause a stepwise detachment with multiple demarcation lines in a chronic-looking retinal detachment (see Fig. 12.24). The vast majority will not cause retinal detachment and prophylactic therapy is generally regarded as unnecessary.
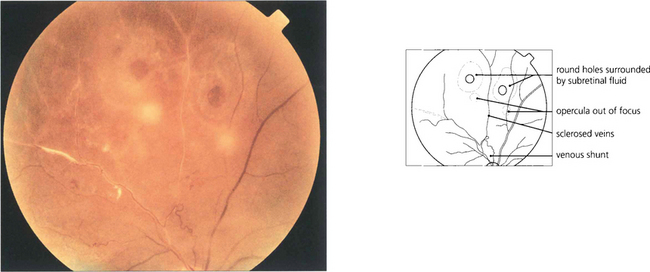
Fig. 12.16 Retinal breaks are seen in a patient with retinal vasculitis in whom the vitreous has separated avulsing small discs of retina that remain attached to the posterior vitreous surface as opercula. Round retinal tears result.
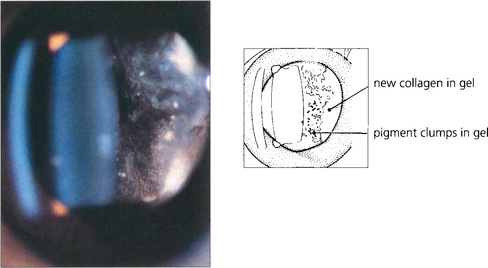
Fig. 12.17 Slit-beam photograph of the anterior gel after a retinal break showing RPE cells in the retrolental gel (the ‘tobacco dust’ or Shafer’s sign). Retinal tears are usually associated with release of RPE cells into the vitreous cavity and, when seen, suggest that a retinal break is present. This is of particular value when assessing a patient following an acute posterior vitreous detachment. These cells transform into fibroblast-like cells in proliferative vitreoretinopathy.
SUBRETINAL FLUID ACCUMULATION
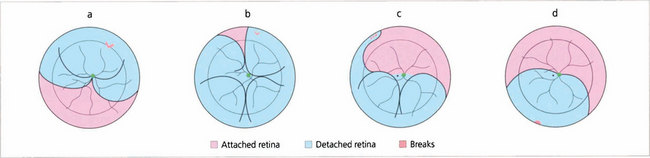
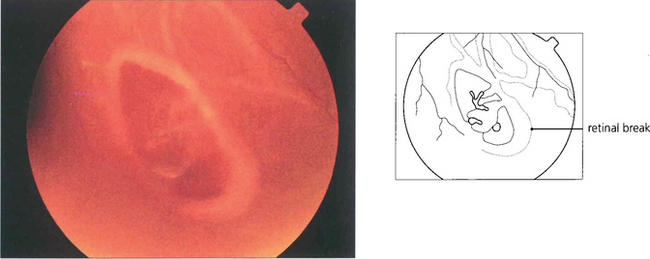
Fig. 12.19 Gravity causes subretinal fluid to spread in a pattern that can help the surgeon localize a retinal break. A tear between the 11 and 1 o’clock positions causes a retinal detachment which becomes total (a). Tears above the horizontal meridian (from 3 to 9 o’clock) produce subtotal detachments. Fluid recruitment progresses downwards on the same side as the tear, at first producing a concave edge to the detachment, and then upwards as a convex edge on the opposite side of the disc but to a level lower than that on the side of the tear (b,c). Inferior subretinal fluid from a superior tear tends to separate partially into two bullae leaving a cleft or ‘cleavage’ of less elevated retina in the 6 o’clock meridian (b,c). A break located below the horizontal meridian tends to accumulate fluid more slowly than superior breaks (d). The upper limits of the detachment form convex curved edges on each side, the higher edge indicating the side of the break. Bullae are not seen with inferior breaks. Occasionally a small anterior and superior tear leaks fluid down the post-oral retina, producing an inferior retinal detachment. Therefore, inferior retinal detachments can occur from both superior and inferior tears.
NATURAL HISTORY OF RHEGMATOGENOUS RETINAL DETACHMENT
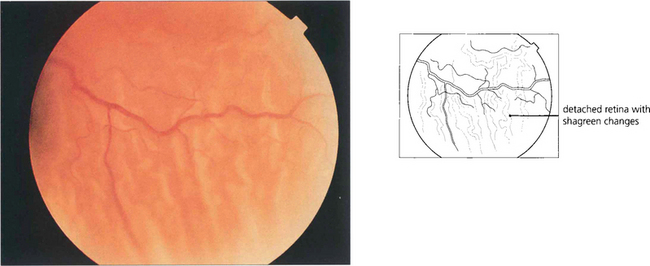
Fig. 12.20 Characteristic wrinkling of the retina from outer retinal swelling (outer retinal shagreen) is seen after detachment.
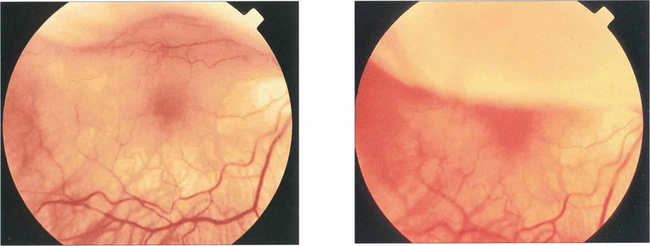
Fig. 12.21 These two photographs demonstrate a bullous superior retinal detachment with movement of subretinal fluid that spares the fovea as the eye rapidly looks up and down; the retinal detachment is ‘macula on’ and freely mobile with no retinal fibrotic changes.
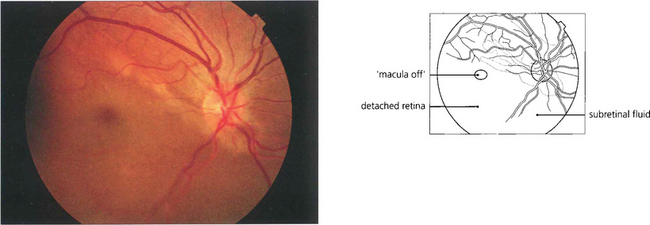
Fig. 12.22 The fovea has been elevated by subretinal fluid in this ‘macula off’ inferior retinal detachment.
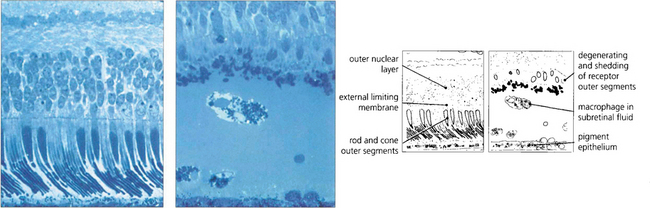
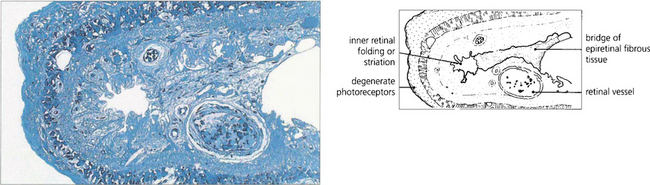
Fig. 12.23 A comparison of the histological appearance of normal and detached retina demonstrates shedding and shortening of rod outer segments. Pigment-laden macrophages are seen in the subretinal space.
By courtesy of Professor J Marshall.
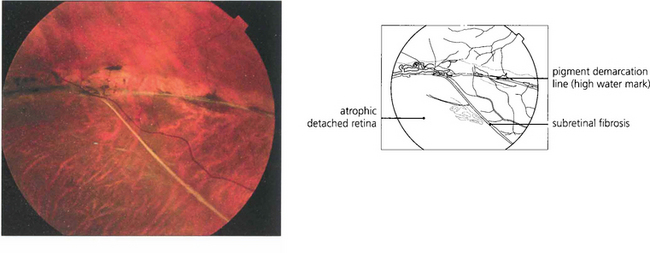
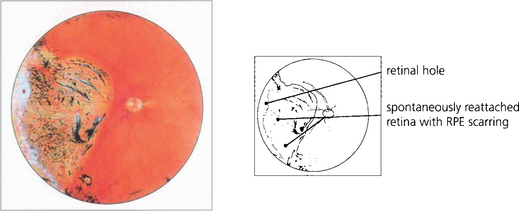
Fig. 12.24 The pigmented demarcation line along the superior edge with thin strands of subretinal fibrosis and a rather atrophic appearance suggests that this retinal detachment is chronic.
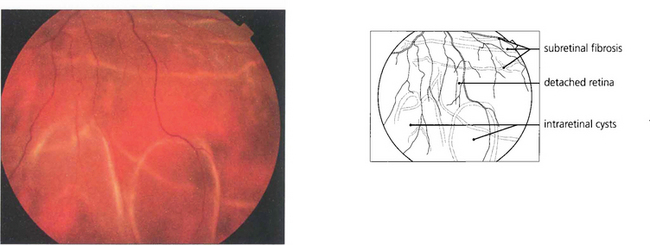
Fig. 12.25 Intraretinal cysts in this retinal detachment suggests that the retina has been elevated for at least a year.
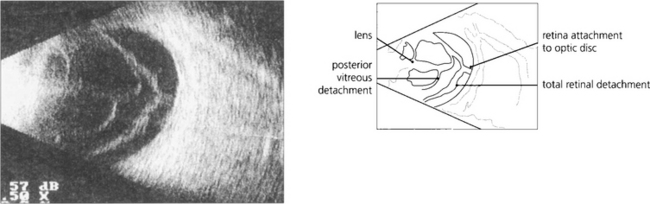
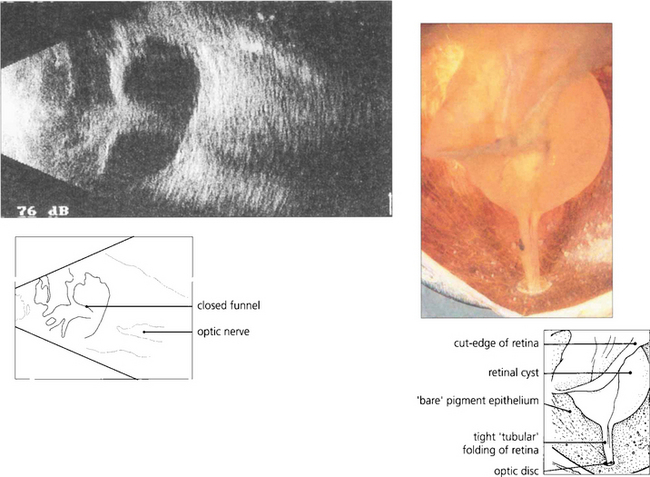
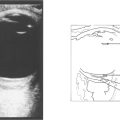
Fig. 12.26 A B scan shows a posterior vitreous detachment and total retinal detachment with the retinal attachment to the optic disc visible. The degree of retinal fibrosis can be assessed by watching the retinal mobility on dynamic B scan. The more fibrotic the retina the less retinal movement on ocular movement.
PROLIFERATIVE VITREORETINOPATHY
Grade A— clumping of RPE cells and stiffening of the vitreous
Grade B— partial-thickness folding of the inner retina
Grade C— full-thickness fixed folding of the retina, commencing as localized star folds and progressing to an open funnel detachment, then to a closed funnel in the final stages. Grade C proliferative vitreoretinopathy can be further classified according to where the folding occurs as either anterior (A) or posterior (P) to the equator, and by the number of clock-hours of retina involved (1–12).
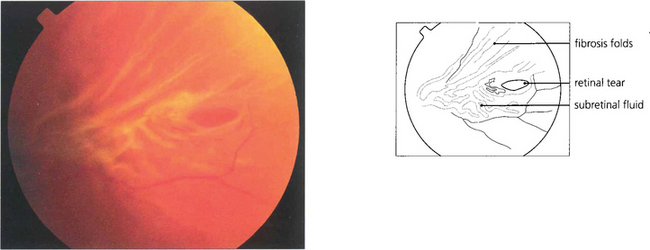
Fig. 12.28 This tear is distorted by early proliferative vitreoretinopathy. The tear will be splinted open by the fibrosis unless this is removed during surgery.
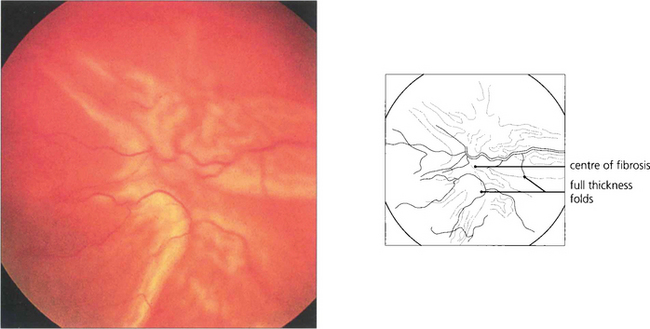
Fig. 12.29 This retina is wrinkled by preretinal fibrosis from proliferative vitreoretinopathy and graded as CP2 (full-thickness folds posterior to the equator extending for 2 clock-hours).
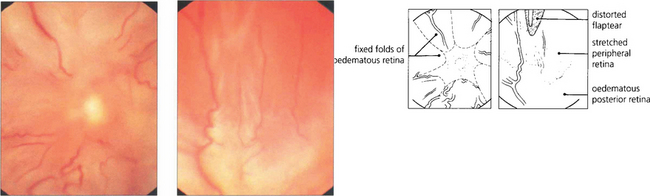
Fig. 12.30 Fundus photographs from an eye with a total retinal detachment and massive preretinal proliferation. The immobilized central retina is drawn into an open funnel-shaped configuration while stretching of the peripheral retina distorts and immobilizes the original flap-tear.
DIFFERENTIAL DIAGNOSIS OF RHEGMATOGENOUS RETINAL DETACHMENT
The majority of retinal detachments are rhegmatogenous, caused by a break in the retinal neuroepithelium. ‘Traction’ detachments reflect the effect of ‘static’ tractional forces from fibrotic tissue directed either along the posterior hyaloid membrane or on the retinal surface. A combined rhegmatogenous and traction detachment can occur in PVR when abnormal epiretinal proliferation and contraction complicate the initial rhegmatogenous retinal detachment. Alternatively, in other ‘combined’ detachments, tangential traction from epiretinal proliferation is the primary event with subsequent retinal break formation, as frequently happens in proliferative diabetic retinopathy. Two other broad groups of retinal detachment are recognized in the absence of retinal breaks: ‘solid’ detachments (for instance those due to choroidal malignant melanoma; see Ch. 9) and ‘serous’ detachments (uveal effusion syndrome, Harada’s disease, central serous retinopathy, polypoidal choroidal vasculopathy; see Chs 9, 10 and 16). Disturbances in other coats of the eye may mimic retinal detachment; these include scleral infolding (e.g. from hypotony), scleral swelling (e.g. from posterior scleritis), choroidal detachment, RPE detachment and retinoschisis.
RETINOSCHISIS
Retinoschisis is a splitting of the neuroretina into two layers. It is classically divided into ‘juvenile’ and ‘senile’ varieties. Juvenile retinoschisis is a rare X-linked recessive dystrophy affecting young males (see Ch. 16) and must be considered when a boy presents with an apparent retinal detachment. A common presentation is vitreous haemorrhage, and central vision may be impaired by associated foveal schisis. The retina is split between the nerve fibre layer and ganglion cell layer.
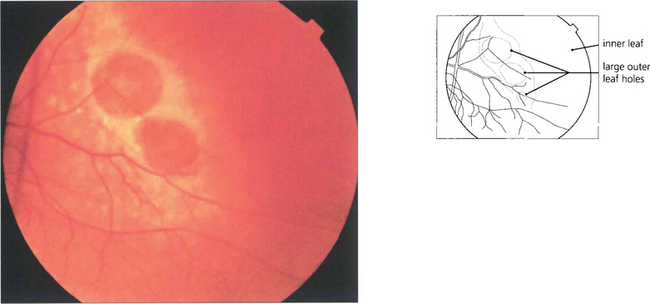
Fig. 12.33 A retinoschisis with large outer leaf breaks in the temporal peripheral retina. Outer leaf breaks tend to be large with rolled edges whereas inner leaf breaks are usually small and round and best seen in the thin diaphenous retina by an oblique view with indirect ophthalmoscopy, with a fundus lens or three-mirror lens.
UVEAL EFFUSION SYNDROME
The uveal effusion syndrome (see Ch. 9) is an unusual condition, often mistaken for either a rhegmatogenous detachment complicated by choroidal detachment or a ‘ring melanoma’ of the anterior choroid. It is characterized by choroidal detachment, mottling of the overlying RPE (leopard spots) and serous retinal detachment which exhibits marked ‘shifting fluid’ (movement of subretinal fluid with gravity). PVR is not seen as there is no retinal break. The aetiology appears to be an abnormality of trans-scleral fluid outflow from the vitreous through an abnormally thickened sclera in a normally sized eye or vortex vein compression in a nanophthalmic eye. Spontaneous resolution may occasionally occur over months; otherwise the effusion responds to scleral thinning and decompression procedures.
SPECIAL TYPES OF RHEGMATOGENOUS RETINAL DETACHMENT
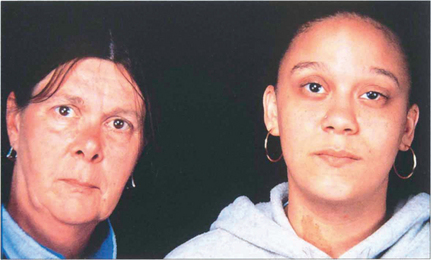
Fig. 12.35 Stickler’s syndrome is characterized by myopia, paravascular pigmentary changes, dragging of major vessels at the optic disc, ‘veils’ or condensations of cortical vitreous around large lacunae or dehiscences in the gel and multiple posterior vitreoretinal adhesions. Patients have a characteristic facies with a flattened nasal bridge, a short mandible and long philtrum; other associations are a high palate and arthralgia. The gene has been localized to COLIIA1.
RHEGMATOGENOUS MACULAR HOLE DETACHMENT
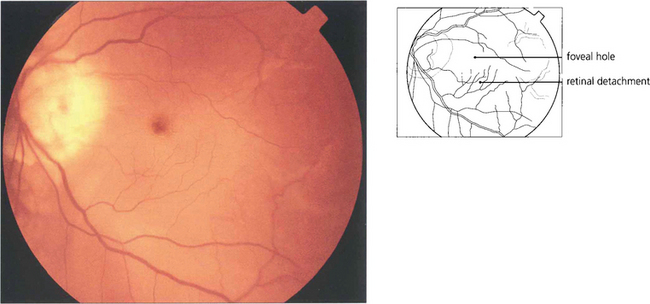
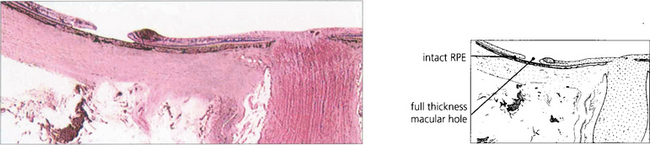
Fig. 12.36 Breaks in the macula of emmetropic eyes do not usually cause retinal detachment (e.g. age related macular holes). In highly myopic eyes posterior breaks especially at the macula or temporal to the optic disc, often associated with areas of chorioretinal atrophy and posterior staphylomas, can cause retinal detachment. A macular hole has caused the retina to detach in this myopic eye with the subretinal fluid accumulating at the posterior pole. The detachment usually remains localized at the posterior pole but occasionally extends anteriorly if peripheral breaks are also present.
GIANT TEARS
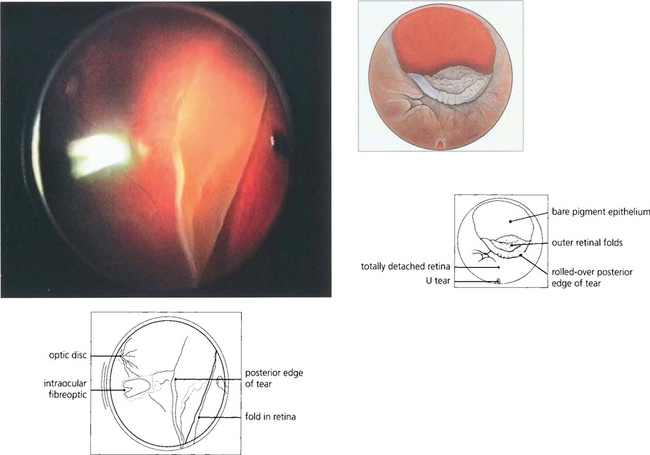
Fig. 12.37 Other breaks are noted for their size. Giant retinal breaks are defined as extending for over 90° of the retinal circumference with a freely mobile posterior flap. They are located immediately post-orally or, less frequently, equatorially. They are caused by vitreous traction (the gel being attached to the anterior margin of the break). The posterior flap moves independently of the gel, essentially by gravity, and breaks may extend radially and posteriorly, especially at their upper limit. Giant tears may extend from 90° to 360° and often have satellite U tears. Giant tears are commonly associated with congenital myopia, Stickler’s syndrome and trauma and have a high risk of developing PVR.
TRAUMATIC RETINAL DETACHMENT
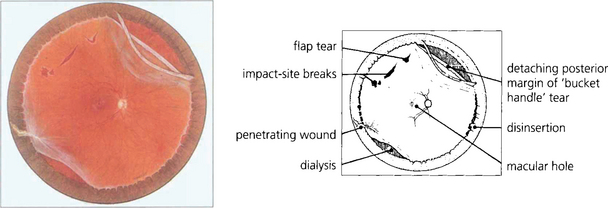
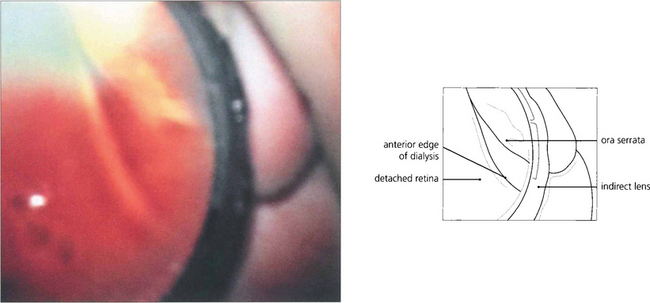
Fig. 12.38 Composite fundus painting demonstrating some of the types of retinal break seen after trauma. With blunt trauma the commonest break is an inferotemporal dialysis which, because of the slow onset of detachment, remains asymptomatic for months or years after the trauma until the macula detaches. The free posterior edge of torn retina may also become detached. Severe blunt trauma can produce commotio retinae which rarely can develop into a large ragged degenerative break. Occasionally an avulsion of the vitreous base is seen which comprises of a strip of ciliary epithelium, ora serrata and immediately post-oral retina, into which the basal vitreous gel remains inserted; this ‘bucket handle’ is most often found in the supranasal quadrant and hangs down in the vitreous cavity. Penetrating trauma can tear the retina at the site of injury or incarcerate vitreous, retina and choroid which progressively scars to shorten the vitreous and retina and produce further tears either at the incarceration site or, due to transvitreal traction, elsewhere.
INTRAOCULAR FOREIGN BODIES
Surgical removal of the foreign body is indicated to avoid severe posterior segment complications such as vitritis and endophthalmitis (infectious or toxic, e.g. acute chalcosis). Siderosis results from the ocular absorption of retained toxic ferrous material. Glaucoma and cataract appear several months after the injury (see Ch. 8). Destruction of photoreceptors produces characteristic ERG changes that are useful in assessing the remaining visual potential.
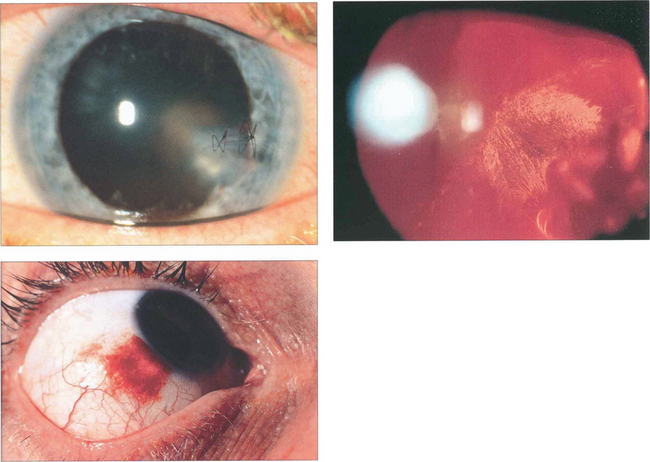
Fig. 12.39 This patient has evidence of an intraocular foreign body with a corneal laceration (repaired with suture) (top left), iris damage, cataract and a poor red reflex from vitreous haemorrhage (top right). In another patient the entry site lies over the sclera at the limbus (bottom left).
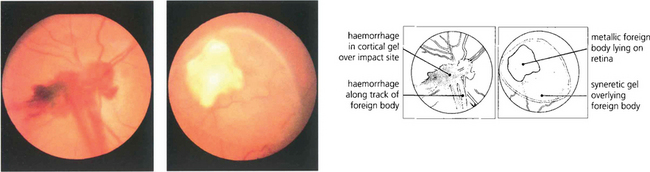
Fig. 12.40 Fundus photograph of another patient shows a foreign body impaction site with prepapillary haemorrhage in the cortical gel and along the track of the foreign body (left). A lacuna of syneretic gel is seen overlying a large reactive foreign body on the retinal surface, viewed some days after injury (right).
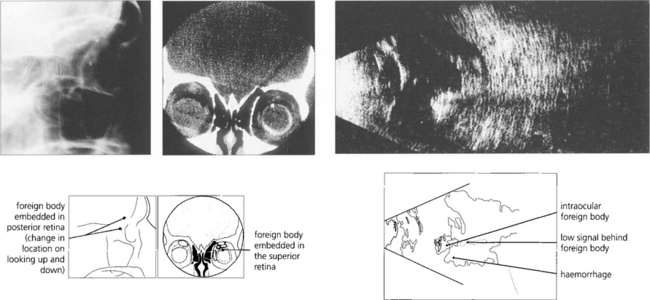
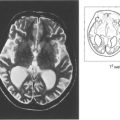
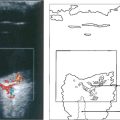
Fig. 12.41 Radiological detection and localization of a foreign body by an ‘eye mover’ lateral film (left) and by computed tomography (centre). In another eye B-mode ultrasongraphy shows a large intravitreal foreign body with surrounding haemorrhage or vitreous inflammatory reaction (right).
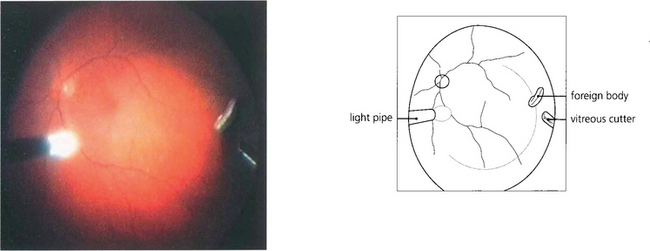
Fig. 12.42 Metallic foreign bodies in the posterior segment are best removed by vitrectomy. This shows the operative view at the time of surgical removal.
EXTRARETINAL NEOVASCULARIZATION
Vascularized epiretinal membrane formation in response to retinal hypoxia and ischaemia may be complicated by vitreous haemorrhage, vitreoretinal traction and retinal detachment. Causes of extraretinal neovascularization include retinal vein occlusion, haemoglobin SC disease and retinal vasculitis but the most common is diabetic retinopathy. Ischaemic diabetic retinopathy characteristically affects the mid-peripheral retina outside the major temporal vascular arcades and nasal to the optic disc (see Ch. 15). Neovascularization generally develops near the junction of ischaemic and normal retina (frequently at the optic disc and along the major vascular arcades). Abnormal neovascular tissue arising from intraretinal venules grows out through the inner limiting membrane and proliferates on the retinal surface or within the most cortical part of the vitreous gel as a vascularized epiretinal membrane (flat new vessels). New vessels do not grow into the central gel except occasionally within Cloquet’s canal. The membranes incarcerate the gel on which they proliferate producing vitreoretinal adhesions.
Fibroblasts within the vascularized membranes contract to cause tangential traction that is consolidated by subsequent collagen synthesis. Tangential traction is exerted along the retinal surface. It initially causes folding of the inner retinal layers (internal limiting membrane and nerve fibre layer) and may then progress to full-thickness retinal folding and to traction retinal detachment. Anteroposterior traction is exerted along the incompletely detached hyaloid face between the vitreous base and the point of vitreoretinal attachment. Contraction of membranes combined with vitreous gel shrinkage pulls the retina at these points of adhesion towards the centre of the eye. Without a retinal hole, subretinal fluid does not accumulate and the detached retina has a concave configuration. The vitreous detachment is taut and stretches from vitreous base to the neovascular membrane and between membranes. Areas of retinal detachment are often multifocal and surround neovascular membranes on the retinal arcades. Eventually the macula detaches whilst the periphery remains flat. If a hole appears in the fragile ischaemic retina subretinal fluid will accumulate; the retinal detachment then becomes convex and may extend anteriorly in a bullous fashion.
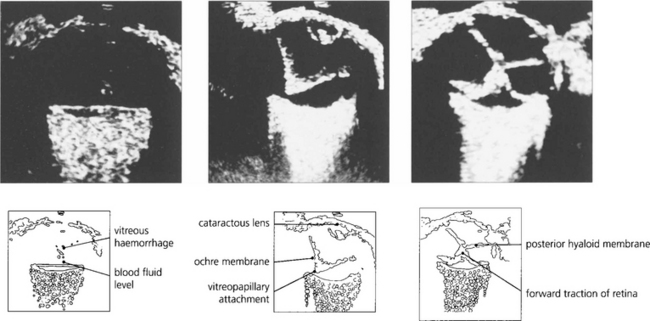
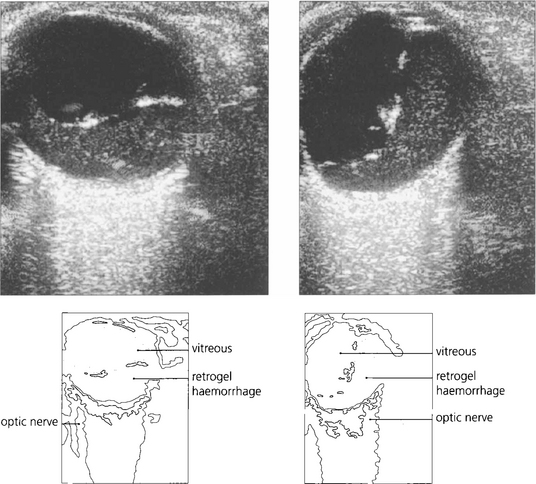
Fig. 12.44 B scans showing a fluid level from retrogel haemorrhage over the posterior pole (left) and an intragel ‘ochre membrane’ (centre). A traction retinal detachment is seen in (right).
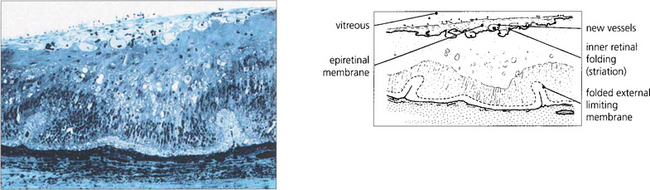
Fig. 12.45 Light microscopy of a diabetic retina shows a fibrovascular epiretinal membrane producing outer retinal folding.
By courtesy of Professor J Marshall.
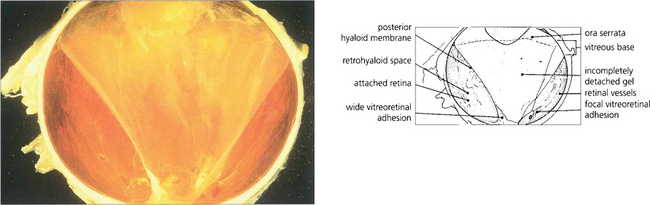
Fig. 12.46 Macrophotograph of a hemisected diabetic globe shows an incomplete posterior vitreous detachment adherent to the optic disc and temporal parapapillary retina.
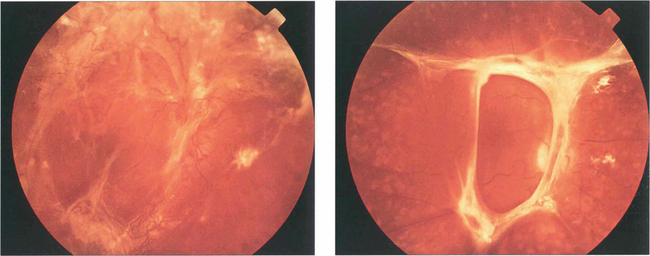
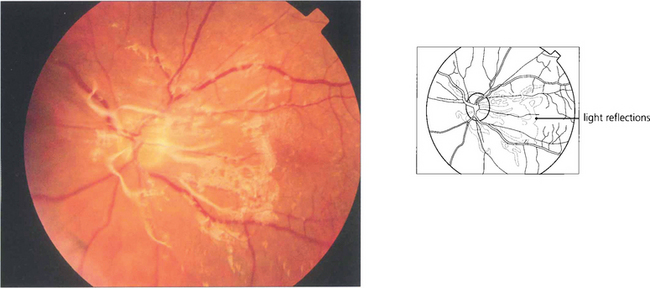
Fig. 12.47 (Left) This diabetic shows gross neovascularization, fibrosis and vitreoretinal traction along the temporal vascular arcades with tractional retinal detachment of the posterior pole. (Right) Inactive fibrotic changes are seen along the temporal vascular arcades with some tractional retinal detachment temporal to the macula.
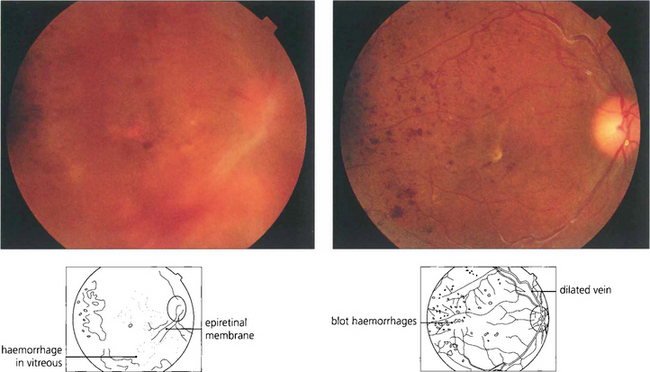
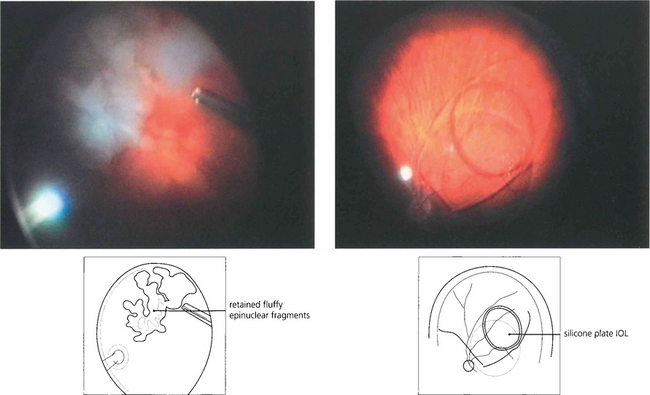
Fig. 12.48 This is the appearance of a diabetic traction detachment before and after operation following vitrectomy, membrane dissection and intravitreal injection of gas to flatten the retina. The acuity improved from HM to 20/80. A considerable amount of retinal ischaemia is evident in the posterior pole; this will require further laser photocoagulation.
MACULAR VITREORETINAL PATHOLOGY
AGE-RELATED MACULAR HOLE
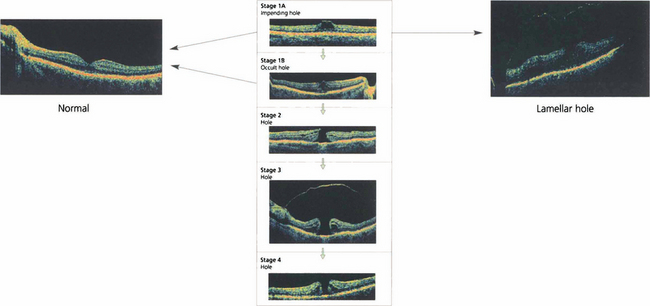
Fig. 12.49 Classification of macular holes. Stage 1A is an intrafoveal cyst. A yellow spot is seen with no PVD on biomicroscopy although there may be perifoveal detachment on OCT. Sometimes the anterior lamellae may separate at the stage 1A creating a lamellar hole. Stage 1B is an intrafoveal cyst with a posterior lamellar split. A yellow ring is seen on biomicroscopy with perifoveal detachment on OCT. The patient is usually asymptomatic or may have some metamorphopsia. Stage 1A and 1B may resolve spontaneously with posterior hyaloid separation or may progress to a full thickness hole with visual symptoms in 50% of cases, initially as a small hole of less than 300μm with posterior hyaloid attached to the lip (stage 2), enlarging to a hole of more than 300μm diameter, the vitreous separated from the fovea but with the PVD still incomplete (stage 3) and finally to a hole with complete posterior vitreous detachment (stage 4) over a few months.
By courtesy of Mr G Duguid.
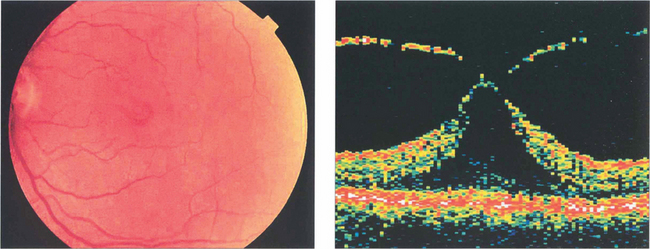
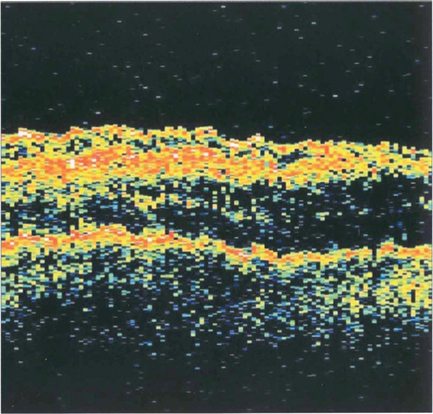
Fig. 12.50 This fundus photograph shows a stage 1B hole. OCT shows that the neuroretina has been elevated by vitreous traction. Many stage 1 holes resolve or remain static.
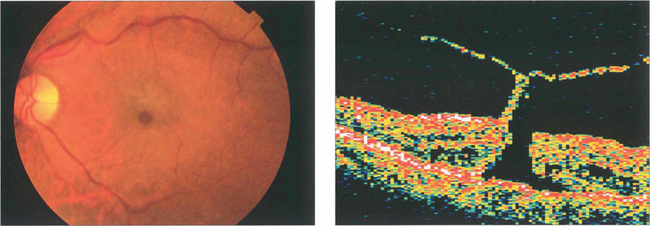
Fig. 12.51 A small grade 2 macular hole (left) and an OCT scan showing foveal dehiscence with separation of the posterior hyaloid membrane and a tiny operculum (right). Cystoid spaces are present in the edge of the hole.
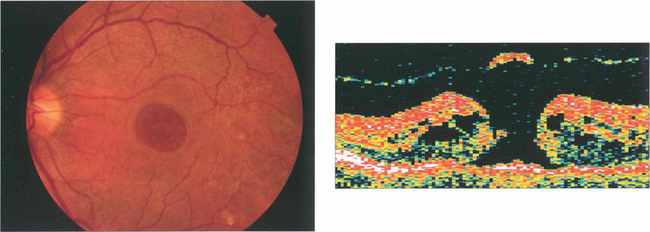
Fig. 12.52 A larger grade 3 macular hole. The visual falls to about 20/200 or CF. Typically the retina surrounding the hole is thickened and small yellowish excrescences can be seen on the exposed RPE. Fluoroscein angiography will show mild hyperfluorescence corresponding to the hole. OCT shows a partial vitreous separation from the fovea, an operculum and a large hole with rolled everted edges, adjacent cystoid intraretinal spaces and a shallow rim of subretinal fluid.
MACULAR EPIRETINAL MEMBRANE (PUCKER)
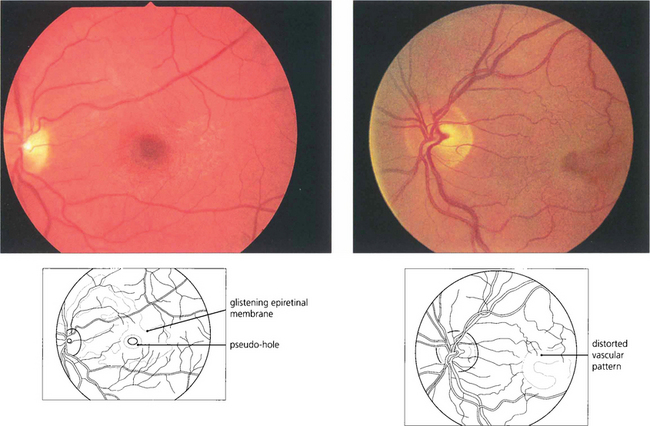
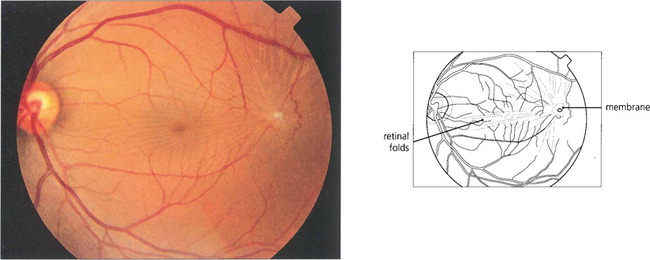
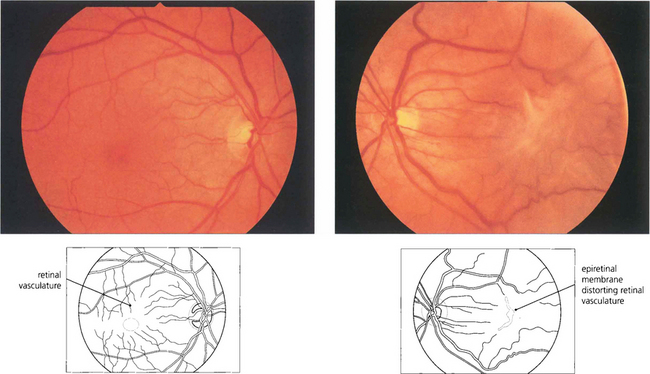
Fig. 12.55 (Left) A fine epiretinal membrane is seen over this macula with vascular distortion and an appearance of a pseudo-macular hole. Note the crinkly macular sheen from the epiretinal membrane giving the appearance of a pseudo-macular hole. Although the patient had metamorphopsia the acuity remained good. On the right an epiretinal membrane can be seen distorting the blood vessels in the posterior pole.
PRINCIPLES OF VITREORETINAL SURGERY FOR RHEGMATOGENOUS RETINAL DETACHMENT
RETINOPEXY
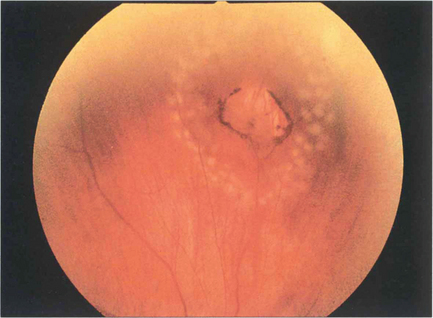
Fig. 12.58 Two rows of fresh argon laser burns seal this operculated peripheral round hole; within days they will start to become pigmented and atrophic producing a permanent chorioretinal adhesion.
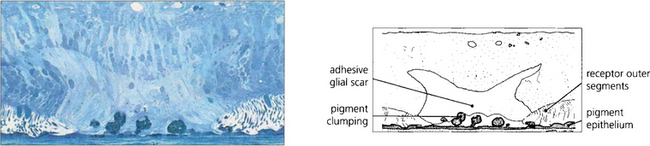
Fig. 12.59 The histological appearance of a chorioretinal scar induced by laser photocoagulation shows damage at the level of the RPE and outer retina. Reparative glial tissue from the outer retina establishes a direct connection to Bruch’s membrane. RPE cells have migrated forwards in clumps.
By courtesy of Professor J Marshall.
REATTACHING THE RETINA, RELIEVING TRACTION AND TAMPONADE
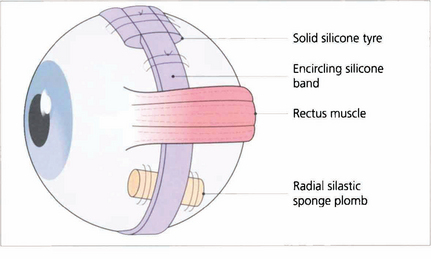
Fig. 12.60 A large variety of shapes and sizes of episcleral implants are available. This figure shows an encircling band, a circumferentially placed tyre and a radial plomb.
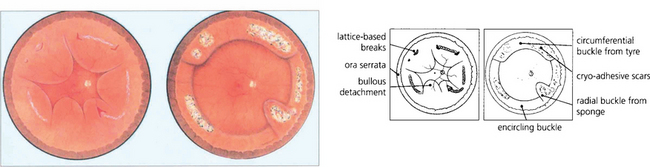
Fig. 12.61 Fundus painting of total retinal detachment with multiple breaks in lattice degeneration shows how explants can be used and the postoperative appearance after cryotherapy.