Chapter 10 Vitreoretinal Diseases
Introduction
Vitreoretinal diseases are the most common indication for ultrasonographic imaging of the posterior segment. Although most conditions of the posterior segment can be directly viewed, in situations where there is media opacity, for example due to vitreous hemorrhage, echography allows for evaluation of the vitreous, retina, and choroid that would otherwise be impossible.1,2 Using ultrasonography it is possible to identify, evaluate, and follow up a large number of posterior segment conditions such as retinal tears,3,4 vitreous and retinal detachments,5–8 retinoschisis,9 retinal pigment epithelium (RPE) detachment,10 subretinal hemorrhage,11 and eccentric disciform lesions.12
Methods of ultrasonographic evaluation of the posterior segment are described elsewhere (Chapter 3). It is imperative to conduct a thorough examination of all the quadrants to avoid missing any pathology, and to evaluate the vitreous body, posterior hyaloid, subvitreal space, retina, choroid, sclera, optic disc, and macular region.
Vitreous
Vitreous hemorrhage
The vitreous is an avascular structure. Vitreous hemorrhage (VH) occurs by the extravasation of blood into the space limited anteriorly by the posterior lens capsule, posteriorly by the internal limiting membrane and laterally by the ciliary body and lens zonular fibers. VH can be caused by bleeding from normal, diseased or abnormal new retinal vessels, traumatic insult or extension of hemorrhage from any other source. The incidence of VH in the general population is seven cases per 100 000 per year.13 The most common causes of VH vary based on the population studied, with the two most common causes being posterior vitreous detachment (PVD) with or without retinal tear and proliferative diabetic retinopathy, followed by ocular trauma and neovascularization secondary to retinal vein occlusion.14–17
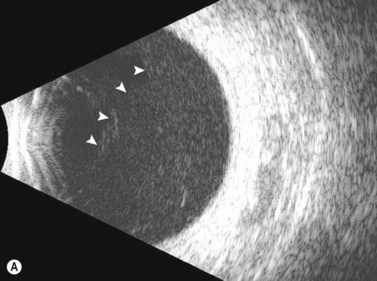
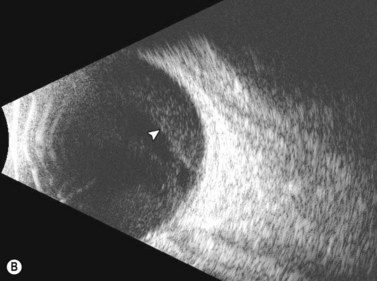
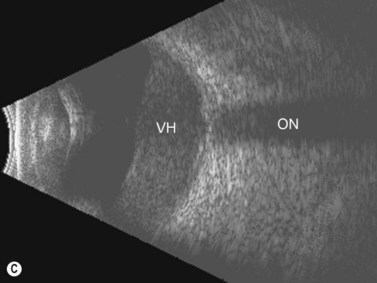
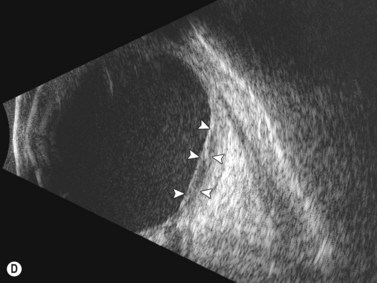
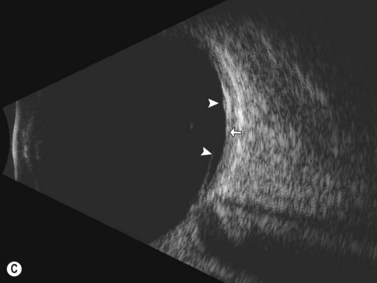
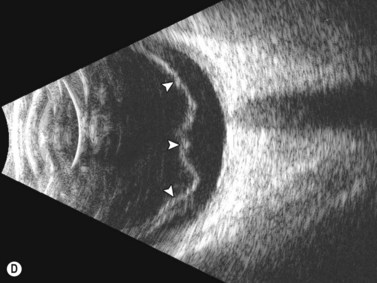
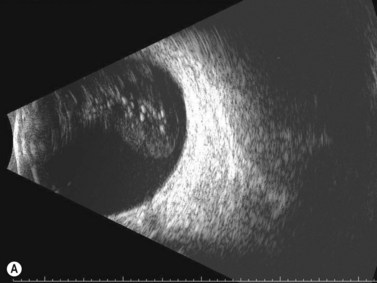
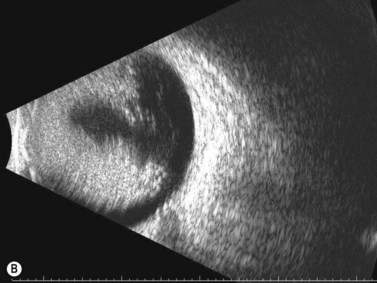
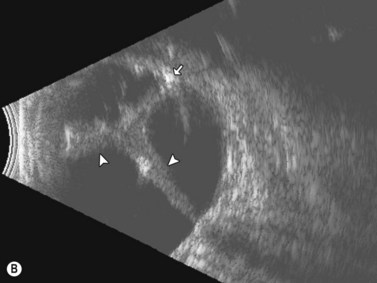
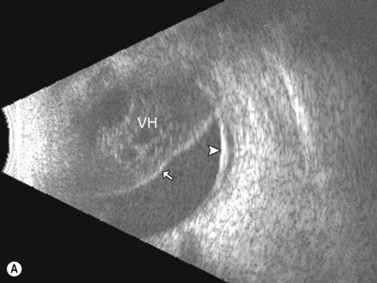
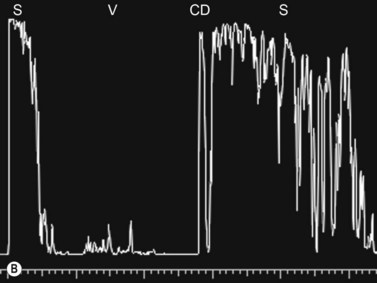
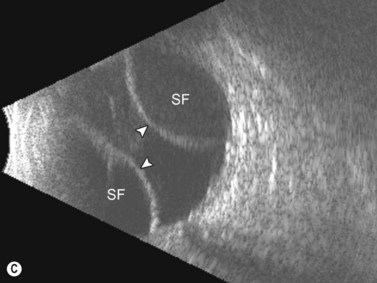
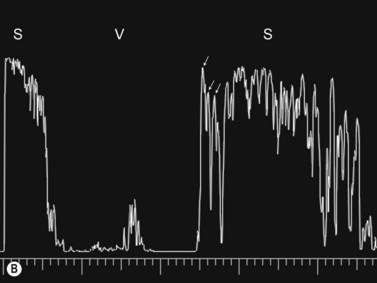
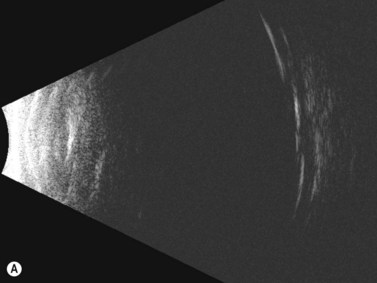
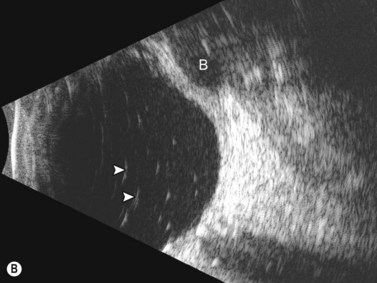
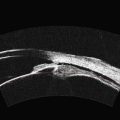
Dynamic A- and B-scan ultrasonographic examinations should be performed to rule out retinal tears, detachment, or other intraocular pathology as the source of vitreous hemorrhage.![]() See Clip 10.1 A fresh vitreous hemorrhage appears as diffuse opacities of low to medium reflectivity on B-scan, with multiple low intensity spikes on A-scan (Figure 10.1A).18 As the blood organizes, it forms pseudomembranous surfaces on B-scan, corresponding to slightly higher intensity spikes on A-scan (Figure 10.1B). Signal intensity on both A- and B-scan directly correlates with the density of the hemorrhage (Figure 10.1C). Layering of blood inferiorly results in very high reflectivity on B-scan and in a static exam may be mistaken for a retinal detachment (RD) (Figure 10.1D).
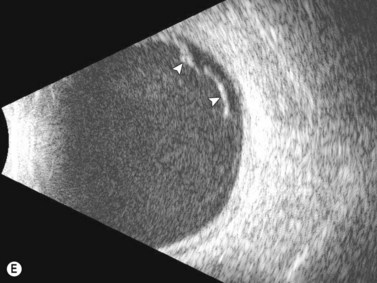
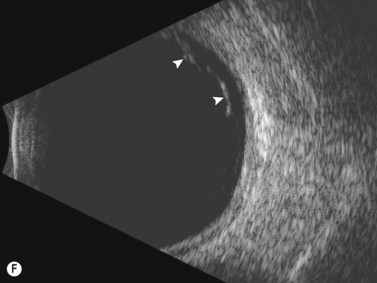
See Clip 10.1 A fresh vitreous hemorrhage appears as diffuse opacities of low to medium reflectivity on B-scan, with multiple low intensity spikes on A-scan (Figure 10.1A).18 As the blood organizes, it forms pseudomembranous surfaces on B-scan, corresponding to slightly higher intensity spikes on A-scan (Figure 10.1B). Signal intensity on both A- and B-scan directly correlates with the density of the hemorrhage (Figure 10.1C). Layering of blood inferiorly results in very high reflectivity on B-scan and in a static exam may be mistaken for a retinal detachment (RD) (Figure 10.1D).![]() See Clip 10.2 In a vitrectomized eye, blood can remain in a liquefied state and often requires the use of high gain settings to visualize the hemorrhage (Figure 10.1E and F).
See Clip 10.2 In a vitrectomized eye, blood can remain in a liquefied state and often requires the use of high gain settings to visualize the hemorrhage (Figure 10.1E and F).
If PVD is absent, a retinal tear or rhegmatogenous retinal detachment (RD) is unlikely and therefore, other causes of the VH must be explored. If a PVD is present and RD is not observed, PVD is most likely not the cause of the VH. However, a small anterior retinal detachment may not be detected by an inexperienced ultrasonographer.19 In addition, presence of a PVD does not exclude other causes of the VH since the PVD may have been present prior to the VH.
Posterior vitreous detachment
Posterior vitreous detachment is a common degenerative process of the vitreous in which the vitreous gel loses its attachment to the internal limiting membrane. The causative factor for PVD can vary, but is most commonly senile degeneration of the vitreous gel. The vitreous is very strongly attached (vitreous base) in a band extending 360° around the anterior limits of the retina (ora serrata) and only weakly adherent to the macula and optic disc; thus the site of detachment is usually located in the posterior pole.20 In nearly half of patients the PVD is incomplete and some portions of the vitreous remain attached to and can exert traction on the retina.21 Retinal tears often occur just posterior to the vitreous base, due to traction placed on the retina as the vitreous pulls away from the retina.
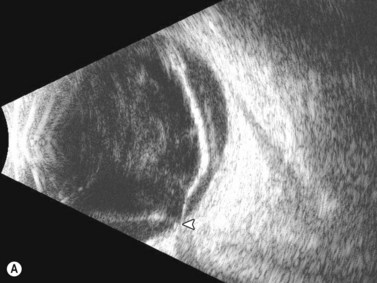
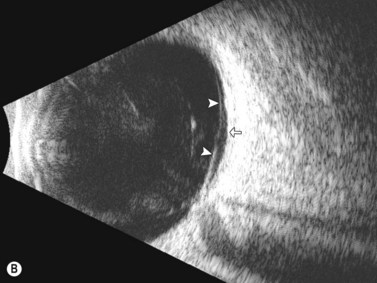
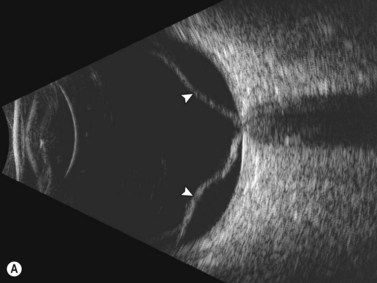
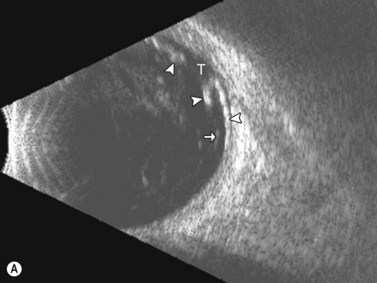
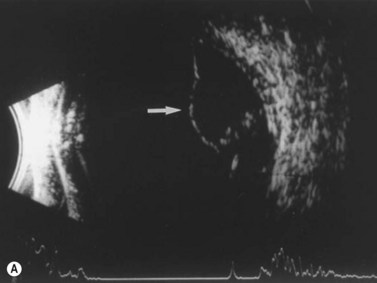
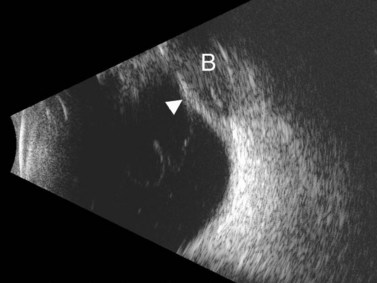
Ultrasonographically, PVD appears as a thin, smooth membrane that may retain its attachment to the retina at the site of retinal tears, areas of neovascularization, optic disc, and/or at the vitreous base. A PVD can mimic a RD on ultrasonography when the posterior hyaloid remains attached to the optic disc; however, there are specific clues that can be used to differentiate these two entities (Figure 10.2A, Table 10.1). A PVD demonstrates significant movement and after movement on dynamic B-scan.![]() See Clip 10.3 In cases of inflammation and trauma, the PVD may be much less mobile. In this situation, it is usually possible to differentiate PVD from a RD based on the reflectivity profiles of the tissues. In the absence of dense vitreous hemorrhage, a PVD appears as a low to medium reflective membrane on both A and B-scan, while a retinal detachment is always highly reflective. A PVD is visible only at high gain settings whereas the retina is visible at low and high gain settings (Figure 10.2B, C). Layering of blood along the surface of a PVD may result in a thickened appearance on B-scan and very high reflectivity on A-scan (Figure 10.2D). Therefore, to differentiate a hemorrhagic PVD from a RD, it is necessary to examine different portions of the membrane for a decrease in reflectivity suggestive of a vitreous membrane. Posteriorly, both the retina and vitreous membranes can appear as highly reflective structures. Anteriorly, however, the retina is much more highly reflective than vitreous membranes.22 In patients with vitreous hemorrhage secondary to proliferative vitreoretinopathy (PVR), localization of focal traction on the retina can be the differential diagnostic indicator.
See Clip 10.3 In cases of inflammation and trauma, the PVD may be much less mobile. In this situation, it is usually possible to differentiate PVD from a RD based on the reflectivity profiles of the tissues. In the absence of dense vitreous hemorrhage, a PVD appears as a low to medium reflective membrane on both A and B-scan, while a retinal detachment is always highly reflective. A PVD is visible only at high gain settings whereas the retina is visible at low and high gain settings (Figure 10.2B, C). Layering of blood along the surface of a PVD may result in a thickened appearance on B-scan and very high reflectivity on A-scan (Figure 10.2D). Therefore, to differentiate a hemorrhagic PVD from a RD, it is necessary to examine different portions of the membrane for a decrease in reflectivity suggestive of a vitreous membrane. Posteriorly, both the retina and vitreous membranes can appear as highly reflective structures. Anteriorly, however, the retina is much more highly reflective than vitreous membranes.22 In patients with vitreous hemorrhage secondary to proliferative vitreoretinopathy (PVR), localization of focal traction on the retina can be the differential diagnostic indicator.
Table 10.1 Ultrasonographic differentiating features between posterior vitreous detachment and retinal detachment.
| Feature | Posterior vitreous detachment | Retinal detachment |
|---|---|---|
| Echogenicity | Low–medium echogenicity | High echogenicity |
| Change with gain (dB) | Disappears with low gain | Visible with low gain |
| Mobility | High mobility | Low mobility |
| Optic disc attachment | Present or absent | Always present |
Asteroid hyalosis
Asteroid hyalosis (AH) is an uncommon, predominantly unilateral, condition that rises in prevalence with age, although a link to systemic diseases has been suggested.23–25 Clinically, it appears as multiple small spheres scattered throughout the vitreous consisting of condensations of calcium and phospholipid. Most patients with AH are asymptomatic. On A-scan, asteroid hyalosis appears as medium to highly reflective spikes that move with the vitreous. There have been a few reports of falsely shortened axial length measurements on A-scan in eyes with AH, but the majority of eyes will show no change in axial length measurement due to AH.26–28 On B-scan, the asteroid bodies appear as both diffuse and focal point-like highly reflective sources with an area of clear vitreous between the posterior border of the asteroid bodies and the retina (Figure 10.3).
Retinal detachment
Retinal detachments occur when the neurosensory retina separates from the underlying retinal pigment epithelium. Retinal detachments are divided into four main types: rhegmatogenous, tractional, exudative (serous), and combined tractional/rhegmatogenous retinal detachment.29
Rhegmatogenous retinal detachment
Rhegmatogenous retinal detachment is the most common type of detachment and is characterized by the presence of a full-thickness retinal tear. There are three prerequisites for the development of rhegmatogenous retinal detachment: liquefaction of the vitreous gel, tractional forces to produce a retinal tear, and a retinal tear that allows fluid access from the liquefied vitreous into the subretinal space.29,30 The annual incidence of rhegmatogenous retinal detachments in the general population of the United States is about 12 cases per 100 000 people (0.01% annual risk). There are about 36 000 cases annually, with anatomic surgical success rates up to 95%.31–33 The major risk factors are high myopia, trauma, cataract surgery, ocular infections, lattice degeneration, and glaucoma.
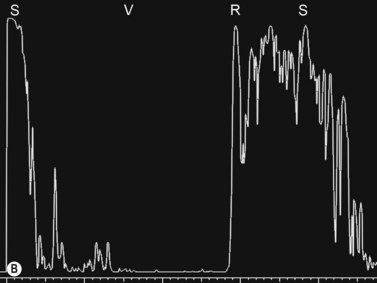
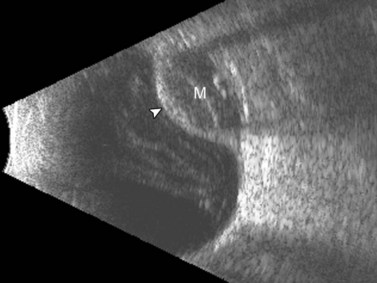
In the setting of media opacity such as a vitreous hemorrhage, differentiating a PVD from a retinal detachment can sometimes be challenging (Table 10.1). Retinal detachments can present with variable mobility, but will always be less mobile than vitreous membranes.34 Retinal detachments are highly reflective with a thickened, rope-like appearance and always have optic disc attachment, while a PVD can retain attachment to the optic disc or be completely detached (Figure 10.4A). On A-scan, the retina demonstrates close to 100% reflectivity (Figure 10.4B).
Tractional retinal detachment
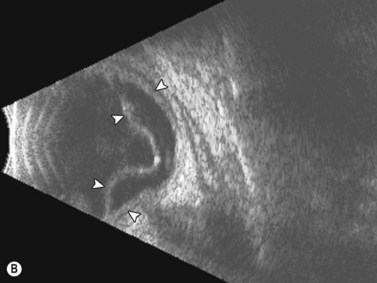
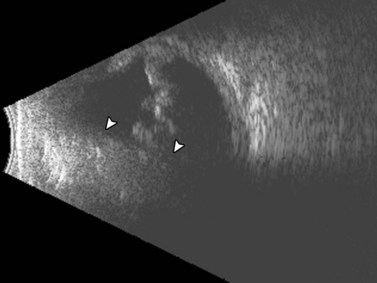
Tractional retinal detachments (TRD) are the second most common type of retinal detachment.32 TRDs can occur due to PVR, penetrating trauma, retinopathy of prematurity, and severe diabetic retinopathy. TRDs occur due to vitreoretinal adhesions that cause mechanical separation of the retina from the underlying RPE causing a retinal detachment. The detachment has a tent-like configuration that does not extend to the ora serrata. On B-scan TRDs demonstrate reduced mobility compared to rhegmatogenous retinal detachments due to the traction placed on the retina (Figure 10.5).![]() See Clip 10.435
See Clip 10.435
Exudative retinal detachment
Exudative retinal detachments are the result of processes that cause the accumulation of fluid between the retina and the RPE in the absence of a retinal break. There is a long list of conditions that may cause exudative retinal detachments and these include idiopathic exudative vascular conditions such as Coat’s disease, central serous chorioretinopathy and hypertension, inflammatory conditions such as scleritis, Vogt–Koyanagi–Harada syndrome and choroiditis (e.g., sarcoid or syphilitic choroiditis), neoplastic such as retinoblastoma or choroidal metastasis and iatrogenic such as excessive photocoagulation or scleral buckling. Exudative retinal detachments can be distinguished clinically from rhegmatogenous detachments by their smooth surface, the absence of rugae, the absence of a retinal break, and shifting of the subretinal fluid with movement to the most dependent part of the eye. B-scan ultrasonography will show the smooth, sometimes convex surface and the absence of rugae and retinal breaks. Most importantly on ultrasonography, as the patient’s head position is changed, the subretinal fluid will “shift” to the most dependent portion. Depending on the etiology of the exudation, the B-scan may also pick up choroidal masses or a thickened choroid or sclera (Chapters 11 and 12).
Total retinal detachment
Open and closed funnel detachments are total retinal detachments attached at the optic disc at one end, (like all retinal detachments), and attached anteriorly at the ora serrata. In a closed funnel detachment, the two sides of the retina forming the “funnel” are stuck together or “closed” from posterior to anterior, usually due to proliferative vitreoretinopathy. On B-scan the open funnel retinal detachment appears as a wavy, ropelike membrane of high reflectivity with mild to moderate mobility (Figure 10.6A).![]() See Clip 10.5 A closed funnel or “T-shaped” chronic retinal detachment appears as a thickened, highly reflective membrane with complete loss of mobility (Figure 10.6B).
See Clip 10.5 A closed funnel or “T-shaped” chronic retinal detachment appears as a thickened, highly reflective membrane with complete loss of mobility (Figure 10.6B).
Differential diagnosis
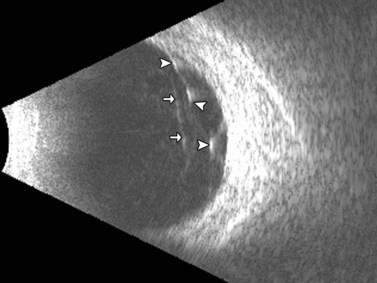
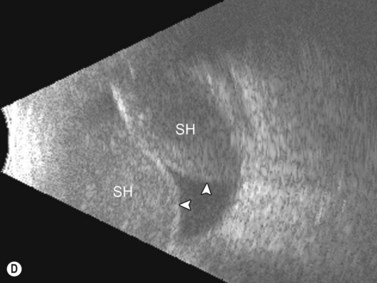
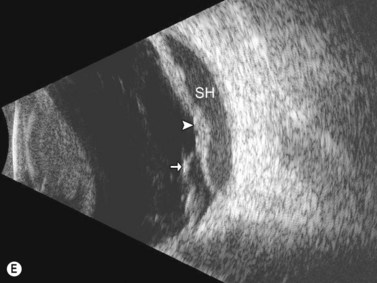
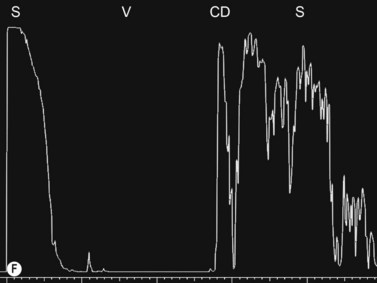
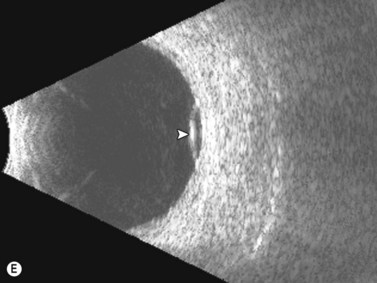
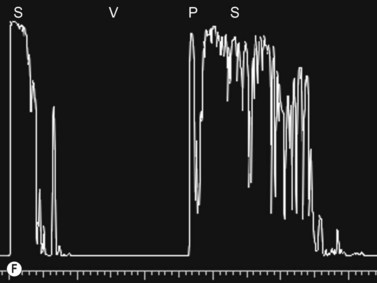
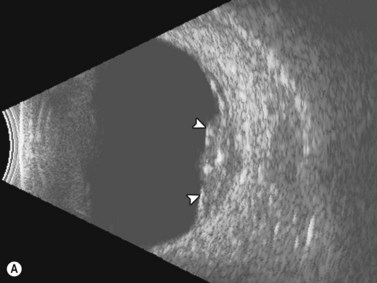
Entities that may be mistaken for RD include suprachoroidal hemorrhage, serous choroidal detachment (CD), and a hemorrhagic PVD.8 A suprachoroidal hemorrhage can be differentiated from an RD by the smooth, thick, convex shape and immobility with little after-movement on dynamic B-scan. Serous choroidal detachments can be smooth, dome shaped, or flat on B-scan, have minimal or absent after-movement, and lack of attachment at the optic disc (Figure 10.7A).22,36 On diagnostic A-scan, both suprachoroidal hemorrhage and serous choroidal detachments show a steep, thick, 100% double-peaked spike on A-scan and are differentiated from retinal detachments that show only a single peaked spike on B-scan (Figure 10.7B). Ultrasonography can be used to differentiate between serous CD and suprachoroidal hemorrhage (hemorrhagic choroidal detachment). Serous CD demonstrates echolucent areas beneath the choroid while the suprachoroidal hemorrhage shows dense suprachoridal opacities (Figure 10.7C, D).37 The presence of both choroidal detachment and retinal detachment can be differentiated from retinal detachment with subretinal hemorrhage by the double peak spike corresponding to the choroidal detachment on A-scan (Figure 10.7E, F).
Retinal tear
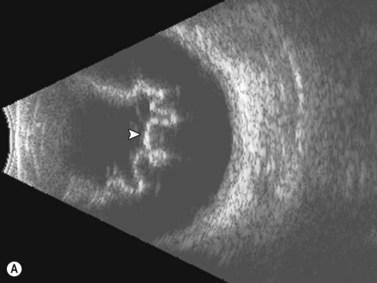
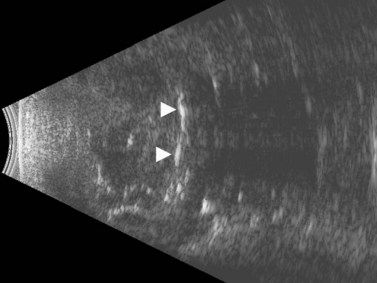
Men have a significantly higher risk of developing retinal tears after PVD.38 Retinal tears are associated with vitreous hemorrhage in 35% of cases. Ultrasonography is very accurate at detecting small retinal tears, with a sensitivity and specificity of more than 90%.2,3 On ultrasonography, retinal tears are seen as a focal elevation of the retina that usually has an adherent strand of vitreous, with high reflectivity in the retinal portion, very little movement, and lower reflectivity in the vitreous strand (Figure 10.8A). The vitreous strand will disappear with reductions in gain, while the retina will remain visible at low gain.
Associated retinal detachment
A focal, shallow RD may be found close to the retinal tear and care should be taken to avoid overlooking this finding. In presence of a concomitant RD, retinal tears are usually located within two clock hours of the area of greatest retinal elevation.7
Giant retinal tear
A giant retinal tear is defined as a tear that spans more than one quadrant (3 clock hours) of the retina. Giant retinal tears should be suspected whenever there is an area of lucency in the retina spanning more than one quadrant of the retina.4 A giant retinal tear will appear as two membranes attached to the optic disc on ultrasonography, with the echo discontinuous with the optic disc representing the inverted posterior flap of the tear and the second echo representing the detached retina (Figure 10.8B). The ultrasonographic findings in giant retinal tears can be extremely varied as they usually occur in combination with other traumatic changes to the posterior segment (Chapter 16).
Differential diagnosis
The main differential diagnosis of a retinal tear in vitreous hemorrhage is an area of neovascularization.3 Areas of neovascularization also extend from the retina to the posterior vitreous face. They are differentiated from retinal tears by their location; usually occurring in the posterior pole, lack of discontinuity of the retinal echo, and acoustic enhancement at the site of attachment to the posterior vitreous face.
Retinal pigment epithelium detachment
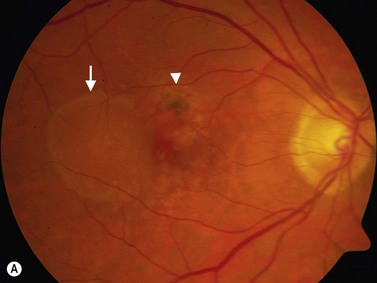
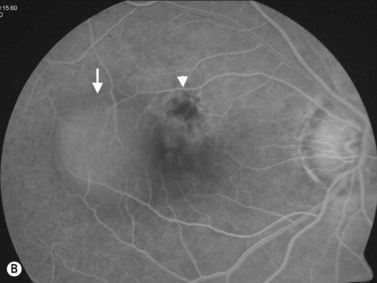
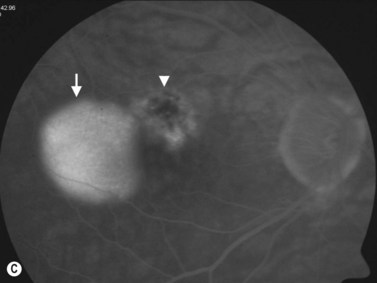
Retinal pigment epithelium detachment (PED) is observed in several chorioretinal diseases of inflammatory, degenerative, ischemic, or idiopathic origin. Pigment epithelial detachments occur most commonly as a result of age-related thickening of Bruch’s membrane secondary to lipid deposits, which can lead to photoreceptor dysfunction and loss of the original architecture.39–41 A common complication of PED is RPE tear, which can be accompanied by bleeding that may extend into the vitreous cavity causing a vitreous hemorrhage.42,43
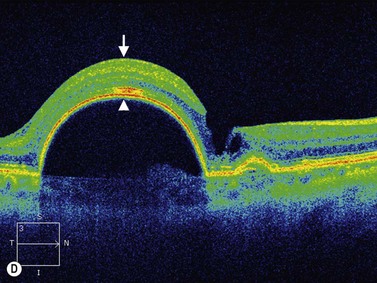
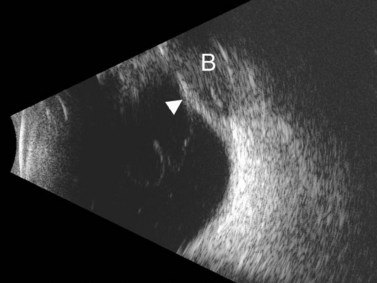
PEDs are usually asymptomatic, but if the foveal area is involved, the patient can complain of blurred vision and visual distortion. Clinically, PEDs are detected on a fundus examination and are best characterized by fluorescein angiography and optical coherence tomography (OCT) (Figure 10.9).44 The role of ultrasonography in this setting is helpful in cases where vitreous hemorrhage is obstructing the view to the posterior segment. On B-scan PEDs appear as a thick, non-mobile, dome shaped membrane and high reflectivity on A-scan.
Retinoschisis
Retinoschisis involves splitting of the sensory retina into inner and outer layers with the formation of cystic spaces in between the layers.45 Retinoschisis occurs in two forms: degenerative and juvenile. Degenerative retinoschisis is an idiopathic, age-related process with a prevalence of 0.7%, and is most frequently found in the inferotemporal quadrant.46 Juvenile retinoschisis is an X-linked retinal dystrophy that typically presents in school age children.
Both retinoschisis and retinal detachment are highly reflective on B-scan, but retinoschisis is usually of lower amplitude and is thinner than a retinal detachment.9 Retinoschisis can be differentiated from retinal detachment by its focal, smooth, dome shape (Figure 10.10A). Retinoschisis is differentiated from choroidal detachment by its thinner appearance on B-scan and a single peak on A-scan, while a choroidal detachment has a double peak (Figure 10.10B). The diagnosis of retinoschisis can be greatly aided by OCT imaging, which shows the splitting of the retina with cystic spaces between the two layers.47
Disciform lesions
Disciform lesions are typically characterized by irregular structure and mainly high reflectivity. On B-scan, disciform lesions appear as an elevation of the retina that may be calcified in long standing lesions (Figure 10.11).12 Over time the height of the lesion will usually decrease.48 Utrasonography is useful in the setting of a large, peripheral disciform lesion that may be confused with a choroidal melanoma (Chapter 11).
Retinal cyst
It is not uncommon for intraretinal macrocysts to develop in a chronic retinal detachment (Figure 10.12).49 Parasitic cysts due to intraocular cysticercosis, whilst uncommon in developed nations, are frequently observed in developing nations (Figure 10.13).50,51
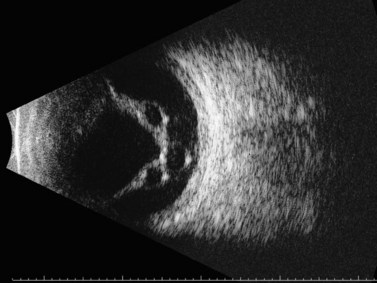
Figure 10.12 Retinal cyst. B-scan ultrasonogram showing multiple intraretinal macrocysts in a chronic retinal detachment.
Post-surgical changes
Scleral buckle
Scleral buckles produce a convex indentation of the ocular wall and strong sound attenuation due to the extremely high reflectivity of the buckling material (Figure 10.14). A clue to the presence of a scleral buckle is the encircling band, which will often produce a lower elevation peripheral to the buckle. The ultrasonographic appearance of the scleral buckle can vary significantly based upon the type of material used for the buckle.
MIRAgel implant
A hydrogel implant, MIRAgel implant was commonly used for scleral buckling in the 1980s and early 1990s. Due to its physical properties, the implant would swell extensively over time (>10 years) causing conjunctival bulging, limitation of ocular motility, diplopia, ocular pain, ocular inflammation, and protrusion of the implant.52,53 Rarely, the swollen MIRAgel implant can present as an orbital tumor.54 The epibulbar location of the implant, density consistent with a scleral buckle, and orbital shadowing allows for differentiation from an orbital mass. The extensive swelling of the implant can necessitate removal of the buckle which is often complicated by fragmentation of the implant on removal.55
On ultrasonography the MIRAgel implant causes intrusion of the retina, choroid and sclera into the vitreous cavity, similar to all scleral buckles. MIRAgel implants have lower reflectivity than a regular buckle, but still cause shadowing behind the implant (Figure 10.15). The implant may also extrude through the sclera into the vitreous cavity.
Gas/air bubbles
Sound penetration is possible through a gas bubble that completely fills the vitreous cavity. However, if the bubble is small enough it can be moved by head position to allow ultrasonographic evaluation of the posterior segment (Figure 10.16).
Silicone oil
Silicone oil tamponade is utilized in lieu of gas/air bubbles in cases of severe retinal detachment caused by proliferative diabetic retinopathy, proliferative vitreoretinopathy, giant retinal tears, in repeat operations for retinal detachment, and if the patient is unable to comply with positioning requirements of gas/air bubbles.56 Silicone oil has a lower specific gravity than water and will rise to the top of the vitreous cavity when the patient is upright; therefore it is best suited for cases where the detachment/tear is located superiorly. Once stable attachment of the retina has been achieved the silicone oil is removed, usually between 6 weeks and 3 months postoperatively.
Silicone oil has a significantly lower sound velocity than the vitreous resulting in significant reductions in penetration of the ultrasound signal and limiting observation of the posterior ocular wall (Figure 10.17A). The lower sound velocity also causes a 50% echographic elongation of the vitreous cavity.57 Secondary to these acoustic boundaries, conventional ophthalmic B-scan is unreliable in the differential diagnosis of intraocular structures in silicone filled globes. There is usually a small amount of silicone oil remaining in the eye after it is removed surgically, which on ultrasonography appear as highly reflective echoes scattered in the vitreous cavity (Figure 10.17B).
Retained perfluorocarbon liquids
Perfluorocarbon liquids are often used as a vitreous substitute during vitreoretinal surgery to aid in the repair of complicated retinal detachments due to their very high specific gravity and ability to provide counter-traction and retinal stabilization.58 Small amounts of perfluorocarbon liquid can be retained postoperatively due to poor visualization of the liquid.59 Several studies have demonstrated there is significant retinal and corneal toxicity associated with intraocular retention of perfluorocarbon liquids.60,61 Retained perfluorocarbon liquids can be visualized on ultrasonography as highly reflective echoes causing shadowing of the orbit (Figure 10.18).
1 Green RL. The echographic evaluation of spontaneous vitreous hemorrhage. In: Ossoinig K, editor. Ophthalmic Echography. Dordrecht, the Netherlands: Dr W Junk; 1984:233-238.
2 Nischal KK, James JN, McAllister J. The use of dynamic ultrasound B-scan to detect retinal tears in spontaneous vitreous haemorrhage. Eye. 1995;9(Pt 4):502-506.
3 DiBernardo C, Blodi B, Byrne SF. Echographic evaluation of retinal tears in patients with spontaneous vitreous hemorrhage. Arch Ophthalmol. 1992;110(4):511-514.
4 Jalkh AE, Jabbour N, Avila MP, et al. Ultrasonographic findings in eyes with giant retinal tears and opaque media. Retina. 1983;3(3):154-158.
5 Coleman DJ, Jack RL. B-scan ultrasonography in diagnosis and management of retinal detachments. Arch Ophthalmol. 1973;90(1):29-34.
6 Sutherland GR, Forrester JV, Railton R. Echography in the diagnosis and management of retinal detachment. Br J Radiol. 1975;48(574):796-800.
7 Blumenkranz MS, Byrne SF. Standardized echography (ultrasonography) for the detection and characterization of retinal detachment. Ophthalmology. 1982;89(7):821-831.
8 Kerman BM, Coleman DJ. B-scan ultrasonography of retinal detachments. Ann Ophthalmol. 1978;10(7):903-911.
9 Hillman JS, Ridgway AE. Retinoschisis and retinal detachment, an ultrasonic comparison. Bibl Ophthalmol. 1975;83:63-67.
10 Silva VB, Brockhurst RJ. Hemorrhagic detachment of the peripheral retinal pigment epithelium. Arch Ophthalmol. 1976;94(8):1295-1300.
11 Bloome MA, Ruiz RS. Massive spontaneous subretinal hemorrhage. Am J Ophthalmol. 1978;86(5):630-637.
12 Valencia M, Green RL, Lopez PF. Echographic findings in hemorrhagic disciform lesions. Ophthalmology. 1994;101(8):1379-1383.
13 Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol. 1997;42(1):3-39.
14 Morse PH, Aminlari A, Scheie HG. Spontaneous vitreous hemorrhage. Arch Ophthalmol. 1974;92(4):297-298.
15 Lean JS, Gregor Z. The acute vitreous haemorrhage. Br J Ophthalmol. 1980;64(7):469-471.
16 Butner RW, McPherson AR. Spontaneous vitreous hemorrhage. Ann Ophthalmol. 1982;14(3):268-270.
17 Manuchehri K, Kirkby G. Vitreous haemorrhage in elderly patients: management and prevention. Drugs Aging. 2003;20(9):655-661.
18 Green RL, Byrne SF. Diagnostic ophthalmic ultrasound. 4th ed. Ryan SJ, editor. Retina. vol 1. Philadelphia: Elsevier Mosby; 2006:265-350.
19 Kocabora MS, Gulkilik G, Yilmazli C, et al. The predictive value of echography in diabetic vitreous hemorrhage. Int Ophthalmol. 2005;26(6):215-219.
20 Sebag J. Ageing of the vitreous. Eye. 1987;1(Pt 2):254-262.
21 Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9(4):253-260.
22 Freyler H, Egerer I. Echography and histological studies in various eye conditions. Arch Ophthalmol. 1977;95(8):1387-1394.
23 Mitchell P, Wang MY, Wang JJ. Asteroid hyalosis in an older population: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10(5):331-335.
24 Moss SE, Klein R, Klein BE. Asteroid hyalosis in a population: the Beaver Dam eye study. Am J Ophthalmol. 2001;132(1):70-75.
25 Fawzi AA, Vo B, Kriwanek R, et al. Asteroid hyalosis in an autopsy population: The University of California at Los Angeles (UCLA) experience. Arch Ophthalmol. 2005;123(4):486-490.
26 Erkin EF, Tarhan S, Ozturk F. Axial length measurement and asteroid hyalosis. J Cataract Refract Surg. 1999;25(10):1400-1403.
27 Allison KL, Price J, Odin L. Asteroid hyalosis and axial length measurement using automated biometry. J Cataract Refract Surg. 1991;17(2):181-186.
28 Hartstein I, Barke RM. Axial length measurement discrepancies in asteroid hyalosis. Br J Ophthalmol. 1991;75(3):191.
29 Sodhi A, Leung LS, Do DV, et al. Recent trends in the management of rhegmatogenous retinal detachment. Surv Ophthalmol. 2008;53(1):50-67.
30 Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16(4):411-421.
31 Christensen U, Villumsen J. Prognosis of pseudophakic retinal detachment. J Cataract Refract Surg. 2005;31(2):354-358.
32 Haimann MH, Burton TC, Brown CK. Epidemiology of retinal detachment. Arch Ophthalmol. 1982;100(2):289-292.
33 Wilkes SR, Beard CM, Kurland LT, et al. The incidence of retinal detachment in Rochester, Minnesota, 1970–1978. Am J Ophthalmol. 1982;94(5):670-673.
34 Forrester JV, Sutherland GR. B-scan ultrasonography in the evaluation of retinal detachment. Br J Ophthalmol. 1974;58(8):746-751.
35 Jalkh AE, Avila MP, El-Markabi H, et al. Immersion A- and B-scan ultrasonography. Its use in preoperative evaluation of diabetic vitreous hemorrhage. Arch Ophthalmol. 1984;102(5):686-690.
36 Portney GL, Kohl JW. Ultrasonic localization of choroidal detachment associated with flat anterior chamber. Ophthalmic Surg. 1975;6(3):86-88.
37 Wing GL, Schepens CL, Trempe CL, et al. Serous choroidal detachment and the thickened-choroid sign detected by ultrasonography. Am J Ophthalmol. 1982;94(4):499-505.
38 Novak MA, Welch RB. Complications of acute symptomatic posterior vitreous detachment. Am J Ophthalmol. 1984;97(3):308-314.
39 Zayit-Soudry S, Moroz I, Loewenstein A. Retinal pigment epithelial detachment. Surv Ophthalmol. 2007;52(3):227-243.
40 Bird AC. Bruch’s membrane change with age. Br J Ophthalmol. 1992;76(3):166-168.
41 Ramrattan RS, van der Schaft TL, Mooy CM, et al. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857-2864.
42 Coscas G, Koenig F, Soubrane G. The pretear characteristics of pigment epithelial detachments. A study of 40 eyes. Arch Ophthalmol. 1990;108(12):1687-1693.
43 Chang LK, Sarraf D. Tears of the retinal pigment epithelium: an old problem in a new era. Retina. 2007;27(5):523-534.
44 Hee MR, Baumal CR, Puliafito CA, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103(8):1260-1270.
45 Straatsma BR, Foss RY. Typical and reticular degenerative retinoschisis. Am J Ophthalmol. 1973;75(4):551-575.
46 Lewis H. Peripheral retinal degenerations and the risk of retinal detachment. Am J Ophthalmol. 2003;136(1):155-160.
47 Azzolini C, Pierro L, Codenotti M, et al. OCT images and surgery of juvenile macular retinoschisis. Eur J Ophthalmol. 1997;7(2):196-200.
48 Davis GJ, Wong HC. Peripapillary disciform lesions in the elderly. Aust N Z J Ophthalmol. 1994;22(2):101-104.
49 Marcus DF, Aaberg TM. Intraretinal macrocysts in retinal detachment. Arch Ophthalmol Jl. 1979;97(7):1273-1275.
50 Chung GW, Lai WW, Thulborn KR, et al. Magnetic resonance imaging in the diagnosis of subretinal cysticercosis. Am J Ophthalmol. 2002;134(6):931-932.
51 Rathinam SR, Ashok KA. Ocular manifestations of systemic disease: ocular parasitosis. Curr Opin Ophthalmol. 2010;21(6):478-484.
52 Tolentino FI, Refojo MF, Schepens CL. A hydrophilic acrylate implant for scleral buckling: technique and clinical experience. Retina. 1981;1(4):281-286.
53 Ho PC, Chan IM, Refojo MF, et al. The MAI hydrophilic implant for scleral buckling: a review. Ophthalmic Surg. 1984;15(6):511-515.
54 Shields CL, Demirci H, Marr BP, et al. Expanding MIRAgel scleral buckle simulating an orbital tumor in four cases. Ophthal Plast Reconstr Surg. 2005;21(1):32-38.
55 Li K, Lim KS, Wong D. Miragel explant fragmentation 10 years after scleral buckling surgery. Eye. 2003;17(2):248-250.
56 Yeo JH, Glaser BM, Michels RG. Silicone oil in the treatment of complicated retinal detachments. Ophthalmology. 1987;94(9):1109-1113.
57 Clemens S, Kroll P, Rochels R. Ultrasonic findings after treatment of retinal detachment by intravitreal silicone instillation. Am J Ophthalmol. 1984;98(3):369-373.
58 Crafoord S, Larsson J, Hansson LJ, et al. The use of perfluorocarbon liquids in vitreoretinal surgery. Acta Ophthalmol Scand. 1995;73(5):442-445.
59 Scott IU, Murray TG, Flynn HWJr, et al. Outcomes and complications associated with perfluoro-n-octane and perfluoroperhydrophenanthrene in complex retinal detachment repair. Ophthalmology. 2000;107(5):860-865.
60 Stolba U, Binder S, Velikay M, et al. Use of perfluorocarbon liquids in proliferative vitreoretinopathy: results and complications. Br J Ophthalmol. 1995;79(12):1106-1110.
61 Lee GA, Finnegan SJ, Bourke RD. Subretinal perfluorodecalin toxicity. Aust N Z J Ophthalmol. 1998;26(1):57-60.