Chapter 2 Vital Signs
Sir William Osler, JAMA 26:999, 1896.
“A quartan fever kills old men and heals young.” — Italian proverb
Generalities
C. Temperature
5 Are there any other conditions that can alter oral temperature?
Beside tachypnea (which decreases oral temperature by 0.5°C for every 10 breaths/minute increase in respiratory rate), recent ingestion of cold or hot substances (including smoking a cigarette) may also change it—something well known to anyone who ever tried to play hooky from school (see factitious fever in question 17).
8 How long does it take for a thermometer to equilibrate when placed under the tongue?
Three minutes with the old mercury thermometers, and around one with the newest models.
10 What are the most commonly encountered fever patterns?
 Sustained or continued (little variability from day to day): This used to be the pattern of lobar pneumonia, steady until abruptly resolving by either crisis or death. Nowadays, a sustained pattern is mostly seen in gram-negative sepsis, but also in central nervous system (CNS) diseases.
Sustained or continued (little variability from day to day): This used to be the pattern of lobar pneumonia, steady until abruptly resolving by either crisis or death. Nowadays, a sustained pattern is mostly seen in gram-negative sepsis, but also in central nervous system (CNS) diseases.
 Intermittent: With complete resolution between episodes (see later)
Intermittent: With complete resolution between episodes (see later)
 Remittent: Abating every day, but still not completely resolving. This used to be the pattern of typhoid fever.
Remittent: Abating every day, but still not completely resolving. This used to be the pattern of typhoid fever.
 Relapsing: With a series of febrile attacks, each lasting several days, and all separated by afebrile intervals of about the same length. A relapsing fever is usually infectious (brucellosis, borreliosis, or relapsing typhoid, but also tuberculosis [TB]), but can occur in Hodgkin’s, too, or familial Mediterranean fever.
Relapsing: With a series of febrile attacks, each lasting several days, and all separated by afebrile intervals of about the same length. A relapsing fever is usually infectious (brucellosis, borreliosis, or relapsing typhoid, but also tuberculosis [TB]), but can occur in Hodgkin’s, too, or familial Mediterranean fever.
11 What are the most common fever types?
It might also suggest a noninfectious etiology, such as an occult neoplasm or autoimmune disease.
12 What are the main types of “intermittent” fever?
 Quotidian fever: From the Latin quotidianus, daily. This is a fever whose paroxysm (and resolution) occurs every day. It is usually caused by a double tertian malaria, due to infection by two distinct groups of Plasmodium vivax, alternately sporulating every 48 hours. It may also be caused by the most pernicious malarial parasite (P. falciparum), combined with vivax, or by two distinct falciparum generations that mature on different days, thus resulting in a fever that occurs twice a day. Note that a double quotidian fever is a daily two-spikes fever that is not malarial, but gonococcal. It used to be present in 50% of endocarditis cases, but today is mostly extinct.
Quotidian fever: From the Latin quotidianus, daily. This is a fever whose paroxysm (and resolution) occurs every day. It is usually caused by a double tertian malaria, due to infection by two distinct groups of Plasmodium vivax, alternately sporulating every 48 hours. It may also be caused by the most pernicious malarial parasite (P. falciparum), combined with vivax, or by two distinct falciparum generations that mature on different days, thus resulting in a fever that occurs twice a day. Note that a double quotidian fever is a daily two-spikes fever that is not malarial, but gonococcal. It used to be present in 50% of endocarditis cases, but today is mostly extinct.
 Tertian fever: From the Latin tertianus, third. This is a P. vivax fever that recurs every third day, counting the day of an episode as the first. Hence, it occurs every 48 hours (every other day).
Tertian fever: From the Latin tertianus, third. This is a P. vivax fever that recurs every third day, counting the day of an episode as the first. Hence, it occurs every 48 hours (every other day).
 Quartan fever: From the Latin quartanus, fourth. This is a P. malariae fever that recurs every fourth day, counting the day of an episode as the first. Hence, it occurs every 72 hours. Note that a double quartan is instead an infection with two independent groups of quartan parasites, so that the febrile paroxysms occur on two successive days, followed by one without fever.
Quartan fever: From the Latin quartanus, fourth. This is a P. malariae fever that recurs every fourth day, counting the day of an episode as the first. Hence, it occurs every 72 hours. Note that a double quartan is instead an infection with two independent groups of quartan parasites, so that the febrile paroxysms occur on two successive days, followed by one without fever.
 Malignant tertian fever: This is the fever of P. falciparum (falciparum fever, or aestivo-autumnal fever, or Roman fever because it was a common ailment in the countryside of Rome up to World War II). It is characterized by 48-hour paroxysms of a severe form of malaria, occurring with acute cerebral, renal, or gastrointestinal manifestations. These are usually due to clumping of the infected red blood cells, causing secondary capillary obstruction and ischemia.
Malignant tertian fever: This is the fever of P. falciparum (falciparum fever, or aestivo-autumnal fever, or Roman fever because it was a common ailment in the countryside of Rome up to World War II). It is characterized by 48-hour paroxysms of a severe form of malaria, occurring with acute cerebral, renal, or gastrointestinal manifestations. These are usually due to clumping of the infected red blood cells, causing secondary capillary obstruction and ischemia.
18 Are there any other terms in this alphabet soup of “fevers”?
Quite a few, once again primarily of historic value. For a smorgasbord:
 Ephemeral fever: A febrile episode lasting only a day or two
Ephemeral fever: A febrile episode lasting only a day or two
 Epimastical fever: From the Greek epakmastikos, coming to a height. A fever that increases steadily until reaching an acme, and then declines by crisis or lysis (crisis indicates a sudden drop, whereas lysis indicates a more gradual defervescence).
Epimastical fever: From the Greek epakmastikos, coming to a height. A fever that increases steadily until reaching an acme, and then declines by crisis or lysis (crisis indicates a sudden drop, whereas lysis indicates a more gradual defervescence).
 Exanthematous fever: A fever associated with an exanthem, i.e., a skin rash
Exanthematous fever: A fever associated with an exanthem, i.e., a skin rash
 Fatigue (exhaustion) fever: An elevation of body temperature that follows excessive and continued muscular exertion. It may last sometimes for up to several days.
Fatigue (exhaustion) fever: An elevation of body temperature that follows excessive and continued muscular exertion. It may last sometimes for up to several days.
 Miliary fever: An infectious fever characterized by profuse sweating and the production of sudamina (i.e., minute vesicles of fluid retention in sweat follicles, a.k.a. “milia”). Typical of past epidemics.
Miliary fever: An infectious fever characterized by profuse sweating and the production of sudamina (i.e., minute vesicles of fluid retention in sweat follicles, a.k.a. “milia”). Typical of past epidemics.
 Monoleptic fever: A continued fever that has only one paroxysm
Monoleptic fever: A continued fever that has only one paroxysm
 Polyleptic fever: From the Greek poly (multiple) and lepsis (paroxysm). A fever that occurs in two or more paroxysms, as typically seen in malaria.
Polyleptic fever: From the Greek poly (multiple) and lepsis (paroxysm). A fever that occurs in two or more paroxysms, as typically seen in malaria.
 Undulant fever: The long and wavy temperature curve of brucellosis
Undulant fever: The long and wavy temperature curve of brucellosis
24 What other physical findings may help identify the cause of a fever?
 Anhidrosis argues in favor of either heat stroke or drugs interfering with diaphoresis.
Anhidrosis argues in favor of either heat stroke or drugs interfering with diaphoresis.
 Muscle rigidity suggests neuroleptic malignant syndrome or malignant hyperthermia.
Muscle rigidity suggests neuroleptic malignant syndrome or malignant hyperthermia.
 Jaundice may be seen in bacterial infections, independent of their direct involvement of the hepatobiliary system (such as cholangitis or hepatitis, see question 14).
Jaundice may be seen in bacterial infections, independent of their direct involvement of the hepatobiliary system (such as cholangitis or hepatitis, see question 14).
 Shaking chills argue only modestly in favor of bacteremia. Conversely:
Shaking chills argue only modestly in favor of bacteremia. Conversely:
26 What are the causes of hypothermia?
Various. Based on its mechanism, hypothermia is usually classified as:
27 What are the signs and symptoms of hypothermia?
They vary, depending on the degree of hypothermia and the type of underlying disorder (a stroke, for example, may obscure the signs of hypothermia). Moreover, symptoms and signs are often a continuum, and there is major variability among patients (Table 2-1).
| Mild Hypothermia | Moderate Hypothermia | Severe Hypothermia |
|---|---|---|
| Confusion | Level of consciousness diminishes | Unresponsiveness or coma |
| Tachypnea | Delirium | May appear dead* |
| Tachycardia | Bradycardia | Loss of reflexes |
| Vasoconstriction | Bradypnea | Very cold skin |
| Lethargy | Shivering stops | Hypotension |
| Shivering | Reflexes slowed | Pulmonary edema |
| Ataxia | Cold diuresis | Respiratory failure |
| Dysarthria | Profound acidemia and ventricular fibrillation | |
| Loss of fine motor coordination |
* Hence, you are never dead until you are warm and dead (see Chapter 20, Coma).
D. Heart Rate and Rhythm
29 How should the pulse be examined?
It depends. If you are simply assessing rate and rhythm, the best (and most accurate) technique is to count the pulse at the wrist for 30 seconds, and then double the figure. Alternatively, you could count the apical rate, which is more accurate in situations of pronounced tachycardia, especially atrial fibrillation (where pulse deficit commonly occurs). In this case, counting 60 seconds may further improve accuracy. Finally, if you want to assess the characteristics of the waveform, then you should assess the pulse of a central artery (see Chapter 10, questions 3–56).
34 What bedside information can help to evaluate an arrhythmia?
In times of electrocardiograms (ECGs), it seems almost anachronistic to discuss the physical examination of arrhythmias. Yet a thorough and astute exam, comprising an assessment of arterial pulse (especially when coordinated with apical impulse—see pulse deficit in question 30), venous waveform, and characteristics of heart sounds, can often deliver a diagnosis.
35 What are the features of the pulse one should consider when evaluating arrhythmias?
Its regularity (or lack thereof) and its response to vagal maneuvers. In this regard:
 A regularly irregular tachycardia is a sign of bigeminy or trigeminy. But it can also indicate atrial flutter with variable atrioventricular block (in this case, look for flutter waves in the neck veins) or a second-degree heart block in which skipped beats occur at regular intervals.
A regularly irregular tachycardia is a sign of bigeminy or trigeminy. But it can also indicate atrial flutter with variable atrioventricular block (in this case, look for flutter waves in the neck veins) or a second-degree heart block in which skipped beats occur at regular intervals.
 An irregularly irregular tachycardia is most commonly seen in atrial fibrillation. This is differentiated from frequent premature contractions because the latter may present with occasional cannon “A” waves (see Chapter 10, questions 78–88).
An irregularly irregular tachycardia is most commonly seen in atrial fibrillation. This is differentiated from frequent premature contractions because the latter may present with occasional cannon “A” waves (see Chapter 10, questions 78–88).
 A regularly regular tachycardia can be due to atrial flutter (with constant A-V block), paroxysmal atrial tachycardia, ventricular tachycardia, and, of course, sinus tachycardia. Response to vagal maneuvers might be helpful in separating these entities.
A regularly regular tachycardia can be due to atrial flutter (with constant A-V block), paroxysmal atrial tachycardia, ventricular tachycardia, and, of course, sinus tachycardia. Response to vagal maneuvers might be helpful in separating these entities.
 A tachycardia that resolves abruptly after either Valsalva’s maneuver or carotid artery massage is a paroxysmal atrial tachycardia (typically associated with a unique feeling of “pounding in the neck” due to the simultaneous occurrence of carotid pulsations and cannon “A” waves).
A tachycardia that resolves abruptly after either Valsalva’s maneuver or carotid artery massage is a paroxysmal atrial tachycardia (typically associated with a unique feeling of “pounding in the neck” due to the simultaneous occurrence of carotid pulsations and cannon “A” waves).
 One that only slows down is usually sinus tachycardia.
One that only slows down is usually sinus tachycardia.
 One that halves in rate is typically atrial flutter.
One that halves in rate is typically atrial flutter.
 Ventricular tachycardia is usually unchanged by vagal maneuvers. Ventricular tachycardia, however, typically presents with findings of atrioventricular dissociation, such as cannon “A” waves, and variable intensity of S1.
Ventricular tachycardia is usually unchanged by vagal maneuvers. Ventricular tachycardia, however, typically presents with findings of atrioventricular dissociation, such as cannon “A” waves, and variable intensity of S1.
36 What other findings might help to recognize an arrhythmia?
 The presence and characteristics of a pause between heartbeats. A pause preceded by a premature beat, for example, usually indicates an atrial (or a ventricular) premature contraction, whereas one not preceded by a premature beat usually indicates a heart block.
The presence and characteristics of a pause between heartbeats. A pause preceded by a premature beat, for example, usually indicates an atrial (or a ventricular) premature contraction, whereas one not preceded by a premature beat usually indicates a heart block.
 A premature beat associated with a cannon “A” wave on venous exam (see Chapter 10, questions 86 and 87) usually indicates an atrial contraction against a closed tricuspid valve and, therefore, a ventricular rather than an atrial premature contraction. Cannon “A” waves might also consistently occur in paroxysmal atrial tachycardia due to the almost simultaneous contraction of atria and ventricles in this condition. Conversely, they might occur randomly in complete heart block.
A premature beat associated with a cannon “A” wave on venous exam (see Chapter 10, questions 86 and 87) usually indicates an atrial contraction against a closed tricuspid valve and, therefore, a ventricular rather than an atrial premature contraction. Cannon “A” waves might also consistently occur in paroxysmal atrial tachycardia due to the almost simultaneous contraction of atria and ventricles in this condition. Conversely, they might occur randomly in complete heart block.
 A very loud, almost “cannon-like,” S1 occurring at times in patients with regular rhythm usually suggests the coincidental contraction of atria just before ventricles. This argues in favor of an escape ventricular rhythm from complete heart block.
A very loud, almost “cannon-like,” S1 occurring at times in patients with regular rhythm usually suggests the coincidental contraction of atria just before ventricles. This argues in favor of an escape ventricular rhythm from complete heart block.
 A “regular” pulse deficit (for example, a rate at the wrist that is exactly half of that at the apex) argues in favor of bigeminy, with the premature beats being always unable to achieve ejection. This needs to be differentiated from pulsus alternans (see Chapter 10, question 23).
A “regular” pulse deficit (for example, a rate at the wrist that is exactly half of that at the apex) argues in favor of bigeminy, with the premature beats being always unable to achieve ejection. This needs to be differentiated from pulsus alternans (see Chapter 10, question 23).
E. Blood Pressure
39 What does sphygmomanometer mean?
It is Greek for “measure of a weak pulse” (sphygmos, pulse; manos, scanty; metron, measure).
43 Who first thought of the mercury sphygmomanometer?
 He used the brachial rather than the radial artery, making measurements easier and more accurate.
He used the brachial rather than the radial artery, making measurements easier and more accurate.
 He used a wraparound inflatable rubber cuff that greatly reduced the frequency of Potain’s over-readings (later Von Recklinghausen increased the cuff width from 5 to 13 cm).
He used a wraparound inflatable rubber cuff that greatly reduced the frequency of Potain’s over-readings (later Von Recklinghausen increased the cuff width from 5 to 13 cm).
 He suggested guidelines for the correct use of the instrument, aimed at minimizing errors.
He suggested guidelines for the correct use of the instrument, aimed at minimizing errors.
 He proposed an instrument so simple and easy to carry that it made blood pressure measurement feasible even at the bedside. Indeed, with only minor modifications, his original sphygmomanometer is still very much in use 100 years later.
He proposed an instrument so simple and easy to carry that it made blood pressure measurement feasible even at the bedside. Indeed, with only minor modifications, his original sphygmomanometer is still very much in use 100 years later.
 Finally, Riva-Rocci was also the first to describe the “white-coat” effect of blood pressure measurement (see question 61).
Finally, Riva-Rocci was also the first to describe the “white-coat” effect of blood pressure measurement (see question 61).
46 What is the systolic pressure?
It is the highest intra-arterial pressure that can be produced during ventricular systole.
47 What is the diastolic pressure?
It is the lowest intra-arterial pressure prior to the next systolic event.
48 What are the various Korotkoff phases?
50 What is the proper technique for indirect measurement of blood pressure?
The American Heart Association has published guidelines for indirect (auscultatory) measurement (Table 2-2). Italicized parts ought to be paid special attention. Note that you should use the stethoscope’s bell and not the diaphragm (because Korotkoff sounds are low in frequency).
| The intent and purpose of the measurement should be explained to the patient in a reassuring manner, and every effort should be made to put the patient at ease. (Include a 5-minute rest before the first measurement.) The sequential steps for measuring the blood pressure in the upper extremity, as for routine screening and monitoring purposes, should include the following: |
| 1. Have paper and pen at hand for immediate recording of the pressure. |
| 2. Seat the patient in a quiet, calm environment [with feet flat on the floor, and back supported against the chair] with his or her bared arm resting on a standard table or other support so that the midpoint of the upper arm is at the level of the heart. |
| 3. Estimate by inspection, or measure with a tape, the circumference of the bare upper arm at midpoint between acromium and olecranon, and select an appropriately sized cuff. The bladder inside the cuff should encircle 80% of the arm in adults and 100% in children less than 13 years old. If in doubt, use a larger cuff. If the available cuff is too small, this should be noted. |
| 4. Palpate the brachial artery and place the cuff so that the midline of the bladder is over the arterial pulsation; then wrap and secure the cuff snugly around the patient’s bare arm. Avoid rolling up the sleeve in such a manner that it forms a tight tourniquet around the upper arm. Loose application of the cuff results in overestimation of the pressure. The lower edge of the cuff should be 1 in (2 cm) above the antecubital fossa where the head of the stethoscope is to be placed. |
| 5. Place the manometer so that the center of the mercury column (or aneroid dial) is at eye level (except for tilted-column floor models) and easily visible, and tubing from cuff is unobstructed. |
| 6. Inflate the cuff rapidly to 70 mmHg, and increase by 10 mmHg increments while palpating the radial pulse. Note the level of pressure at which the pulse disappears and subsequently reappears during deflation. This palpatory method provides a necessary preliminary approximation of the systolic BP, and ensures an adequate level of inflation for the actual, auscultatory measurement. The palpatory method is particularly useful to avoid underinflation of the cuff in patients with an auscultatory gap and overinflation in those with very low BP. |
| 7. Place the earpieces of the stethoscope into the ear canals, angled forward to fit snugly. Switch the stethoscope head to the low-frequency position (bell). The setting can be confirmed by listening as the stethoscope head (i.e., the bell orifice) is tapped gently. |
| 8. Place the head of the stethoscope over the brachial artery pulsation, just above and medial to the antecubital fossa but below the edge of the cuff, and hold it firmly (but not too tightly) in place, making sure that the head makes contact with the skin around its entire circumference. Wedging the head of the stethoscope under the edge of the cuff may free one hand but results in considerable extraneous noise (and is nearly impossible with the bell in any event). |
| 9. Inflate the bladder rapidly and steadily to a pressure 20–30 mmHg above the level previously determined by palpation; then partially unscrew (open) the valve and deflate the bladder at 2 mm[Hg]/sec while listening for the appearance of the Korotkoff sounds. |
| 10. As pressure in the bladder falls, note the level of pressure on the manometer at the first appearance of repetitive sounds (Phase I), at the muffling of these sounds (Phase IV), and when they disappear (Phase V). While Korotkoff sounds are audible, rate of deflation should be no more than 2 mm per pulse beat, thus compensating for both rapid and slow heart rates. |
| 11. After the Korotkoff sound is heard, the cuff should be deflated slowly for at least another 10 mmHg to ensure that no further sounds are audible and then rapidly and completely deflated. The patient should be allowed to rest for at least 30 seconds. |
| 12. The systolic (Phase I) and diastolic (Phase V) pressures should be recorded immediately, rounded off (upward) to the nearest 2 mmHg. In children, and when sounds are heard nearly to a level of 0 mmHg, Phase IV pressure also should be recorded (example: 108/65/56 mmHg). All values should be recorded together with the name of the patient, the date and time of the measurement, the arm on which the measurement was made, the patient’s position, and the cuff size (when a nonstandard size is used). |
| 13. Measurement should be repeated after at least 30 seconds, and the two readings averaged. Additional measurements can be made in the same or opposite arm, same or alternative position. |
Copyright 1993 American Heart Association. Reproduced with permission.
(Adapted from Reeves RA: Does this patient have hypertension? How to measure blood pressure. JAMA 273:1211–17, 1995.)
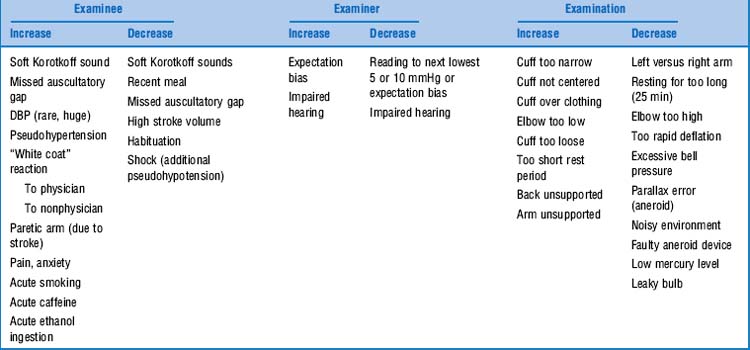
54 Which factors can affect the accuracy of blood pressure measurement?
Several, and these can be related to patient, equipment, or examiner (Table 2-3). Conversely, some factors have no effect on blood pressure: menstrual phase, chronic caffeine ingestion, phenylephrine nasal spray, cuff self-inflation, discordance in gender or race of examinee and examiner, thin shirt sleeve under cuff, bell versus diaphragm, cuff inflation per se, hour of day (during work hours), and room temperature.
58 How do you calibrate an aneroid sphygmomanometer?
gainst a mercury unit, and by using a Y connector to link the cuff to both devices.
61 How accurate is blood pressure measurement by sphygmomanometer?
Accurate, but with some limitations:
 Values recorded indirectly (auscultatory method) correlate quite well with simultaneous direct intra-arterial recordings (r = 0.94 to 0.98). Still, Korotkoff phase I sounds do not appear until 4–15 mmHg below direct systolic blood pressure, whereas Korotkoff phase V sounds disappear above the direct diastolic value (by 3–6 mmHg). Hence, there is some minor underestimation and overestimation.
Values recorded indirectly (auscultatory method) correlate quite well with simultaneous direct intra-arterial recordings (r = 0.94 to 0.98). Still, Korotkoff phase I sounds do not appear until 4–15 mmHg below direct systolic blood pressure, whereas Korotkoff phase V sounds disappear above the direct diastolic value (by 3–6 mmHg). Hence, there is some minor underestimation and overestimation.
 Physicians may also cause inaccuracies. For example, despite previously agreeing to use three readings for diagnosis, a group of British general practitioners diagnosed hypertension after only one measurement in half of the cases. Similarly, 37% of German ambulatory physicians determined diastolic pressure using Korotkoff phase IV (muffling), rather than the more accurate phase V. Still, the most common physician’s error is failure to use sufficiently large cuffs. In one survey, only 25% of primary care offices had them available. Of interest, auscultatory automatic monitors have fewer discrepancies than experienced clinicians.
Physicians may also cause inaccuracies. For example, despite previously agreeing to use three readings for diagnosis, a group of British general practitioners diagnosed hypertension after only one measurement in half of the cases. Similarly, 37% of German ambulatory physicians determined diastolic pressure using Korotkoff phase IV (muffling), rather than the more accurate phase V. Still, the most common physician’s error is failure to use sufficiently large cuffs. In one survey, only 25% of primary care offices had them available. Of interest, auscultatory automatic monitors have fewer discrepancies than experienced clinicians.
 Finally, in some patients the blood pressure measured in the physician’s office is considerably and consistently higher than the daytime ambulatory value. This phenomenon is called the “white coat” effect and is seen in as many as 10–40% of untreated and borderline hypertensive patients. Even treated patients often show blood pressure differences that are >20/10 mmHg. The phenomenon is more pronounced in female than male patients and results more often in responses to the white coat of doctors than to that of nurses.
Finally, in some patients the blood pressure measured in the physician’s office is considerably and consistently higher than the daytime ambulatory value. This phenomenon is called the “white coat” effect and is seen in as many as 10–40% of untreated and borderline hypertensive patients. Even treated patients often show blood pressure differences that are >20/10 mmHg. The phenomenon is more pronounced in female than male patients and results more often in responses to the white coat of doctors than to that of nurses.
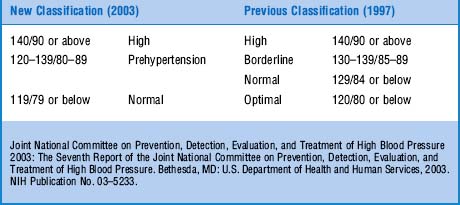
67 What are the key aspects of the latest guidelines?
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (released in 2003) is an update of the 1997 guidelines (Table 2-4). It combines stages 2 and 3 hypertension, and it also contains a new category of prehypertension (120/80–139/89 mmHg). Prehypertensive patients are at increased risk of progressing to hypertension (those in the 130/80–139/89 mm Hg range have twice the risk of developing hypertension as those with lower values) and thus should initiate lifestyle modifications. These include weight reduction, a DASH diet (Dietary Approaches to Stop Hypertension), lowering of sodium intake, increased physical activity, moderate consumption of alcohol, and no tobacco.
79 What is the significance of a wide pulse pressure in aortic regurgitation (AR)?
If >80 mmHg, it reflects moderate to severe AR—a finding moderately sensitive but highly specific.
80 What is the significance of a wide pulse pressure in only one extremity?
It indicates the presence of an A-V fistula in that extremity. A Branham’s sign would confirm it.
88 Describe the pathophysiology of pulsus paradoxus
 Inspiration lowers intrathoracic pressure, which then increases venous return to the “right” ventricle. Conversely, it reduces venous return to the left ventricle (because of blood pooling in the inflated lungs and a leftward shift in the ventricular septum). In turn, the smaller end-diastolic left ventricular volume results in lower stroke volume and, thus, lower systolic pressure.
Inspiration lowers intrathoracic pressure, which then increases venous return to the “right” ventricle. Conversely, it reduces venous return to the left ventricle (because of blood pooling in the inflated lungs and a leftward shift in the ventricular septum). In turn, the smaller end-diastolic left ventricular volume results in lower stroke volume and, thus, lower systolic pressure.
 Exhalation, on the other hand, increases left ventricular filling (because of blood being “squeezed” by lung deflation, and also because of rightward shift in the ventricular septum). This increased ventricular filling results in higher left-ventricular stroke volume and systolic pressure.
Exhalation, on the other hand, increases left ventricular filling (because of blood being “squeezed” by lung deflation, and also because of rightward shift in the ventricular septum). This increased ventricular filling results in higher left-ventricular stroke volume and systolic pressure.
89 How does one measure pulsus paradoxus?
 Position yourself at the bedside in such a way to simultaneously monitor the respiratory movements of the patient and the column of the sphygmomanometer.
Position yourself at the bedside in such a way to simultaneously monitor the respiratory movements of the patient and the column of the sphygmomanometer.
 Don’t ask the patient to breathe too vigorously, since this might generate an abnormal pulsus paradoxus even in the normal subject.
Don’t ask the patient to breathe too vigorously, since this might generate an abnormal pulsus paradoxus even in the normal subject.
 Fully inflate the blood pressure cuff until you achieve auscultatory silence.
Fully inflate the blood pressure cuff until you achieve auscultatory silence.
 Start deflating the cuff very slowly, while at the same time paying attention to both chest and abdominal wall expansions.
Start deflating the cuff very slowly, while at the same time paying attention to both chest and abdominal wall expansions.
 As soon as you hear the first Korotkoff sounds, stop deflating the cuff, and record the systolic pressure reading. You will notice that sounds are only heard in exhalation.
As soon as you hear the first Korotkoff sounds, stop deflating the cuff, and record the systolic pressure reading. You will notice that sounds are only heard in exhalation.
 Restart deflating the cuff, very slowly, until you hear Korotkoff sounds both in inspiration and expiration. Record the second systolic blood pressure reading.
Restart deflating the cuff, very slowly, until you hear Korotkoff sounds both in inspiration and expiration. Record the second systolic blood pressure reading.
 The difference between the two systolic recordings, in mmHg, is the pulsus paradoxus.
The difference between the two systolic recordings, in mmHg, is the pulsus paradoxus.
90 Can pulsus paradoxus be identified on arterial tracing?
Yes, since it causes visible inspiratory and expiratory swings in systolic pressure.
95 In addition to pulsus paradoxus, what are the other clinical features of tamponade?
They consist of a tetrad, present in close to 100% of patients:
97 Can pulsus paradoxus be falsely negative in tamponade?
 Isolated right heart tamponade: This has been described in patients with chronic renal failure who are on hemodialysis. Regional tamponade can also occur in loculated pericardial effusions. In both cases, the pericardial “water bag” is too asymmetric to cause a “real estate” competition between ventricles.
Isolated right heart tamponade: This has been described in patients with chronic renal failure who are on hemodialysis. Regional tamponade can also occur in loculated pericardial effusions. In both cases, the pericardial “water bag” is too asymmetric to cause a “real estate” competition between ventricles.
 Aortic regurgitation (AR): In AR, the left ventricle can fill from the aorta during inspiration, which then prevents the development of a “pulsus.” Hence, patients with aortic dissection (who have both AR and tamponade) may often present without pulsus paradoxus.
Aortic regurgitation (AR): In AR, the left ventricle can fill from the aorta during inspiration, which then prevents the development of a “pulsus.” Hence, patients with aortic dissection (who have both AR and tamponade) may often present without pulsus paradoxus.
 Large atrial septal defect: The normal inspiratory increase in systemic venous return is counterbalanced by a decrease in left-to-right shunt, resulting in minimal change in right ventricular volume.
Large atrial septal defect: The normal inspiratory increase in systemic venous return is counterbalanced by a decrease in left-to-right shunt, resulting in minimal change in right ventricular volume.
 Elevated left ventricular diastolic pressures: These occur in cases of severe left ventricular dysfunction. The left ventricular pressure is too high to allow any ipsilateral septal shift in inspiration.
Elevated left ventricular diastolic pressures: These occur in cases of severe left ventricular dysfunction. The left ventricular pressure is too high to allow any ipsilateral septal shift in inspiration.
 Severe rheumatoid spondylitis or disease of the bony thorax: Wide changes in intrathoracic pressure are prevented by the relative immobility of the chest wall.
Severe rheumatoid spondylitis or disease of the bony thorax: Wide changes in intrathoracic pressure are prevented by the relative immobility of the chest wall.
 Coexistent conditions producing “reversed pulsus paradoxus” (see question 101)
Coexistent conditions producing “reversed pulsus paradoxus” (see question 101)
98 What other conditions can cause pulsus paradoxus >10 mmHg?
Table 2-5 Conditions responsible for pulsus paradoxus >10 mmHg
| Cardiac causes | Extracardiac pulmonary causes | Extracardiac nonpulmonary causes |
|---|---|---|
| Cardiac tamponade | Bronchial asthma | Anaphylactic shock (during urokinase administration) |
| Pericardial effusion | Tension pneumothorax | |
| Constrictive pericarditis* | Hypovolemic shock | |
| Restrictive cardiomyopathy | Volvulus of the stomach | |
| Pulmonary embolism | Diaphragmatic hernia | |
| Right ventricular infarction | Superior vena cava obstruction | |
| Right ventricular failure | Extreme obesity | |
| Cardiogenic shock |
* Seen in 30–45% of patients, but the condition must have an exudative component and not be completely “dry.”
Modified from Khasnis A, Lokhandwala Y: Clinical signs in medicine: pulsus paradoxus. J Postgrad Med 48:46–49, 2002.
100 How does pulsus paradoxus behave in intubated and mechanically ventilated patients?
It may present as respiratory variations in the baseline tracing of pulse oximetry (with the height of oscillation correlating with the severity of pulsus and the degree of auto-PEEP [positive end-expiratory pressure]). Hence, pulse oximetry assessment of pulsus paradoxus can provide a useful noninvasive monitor of air trapping severity. Still, beware that positive pressure ventilation can reverse the respiratory changes in intrathoracic pressure, so that the lowest systolic blood pressure will be recorded during expiration (reversed pulsus paradoxus—see question 101), rather than inspiration. Although the correlation between pulsus paradoxus and pulse oximetry recordings might not be influenced by the state of respiration (spontaneous or mechanical ventilation), reversed pulsus paradoxus is not a good indicator of disease severity in patients receiving mechanical ventilation because the magnitude of the pulsus depends (at least in part) on the applied ventilatory pressures.
103 What is the usefulness of Kussmaul’s sign in pulsus paradoxus?
Kussmaul’s sign is a paradoxical increase in venous distention (and pressure) during inspiration (see Chapter 10, The Cardiovascular Exam). This should not be confused with the exaggeration of the normal expiratory increase in venous pressure that is often seen in patients with pulmonary disease. Instead, Kussmaul’s sign reflects some sort of obstruction to right-sided venous return, like superior vena cava syndrome, tricuspid stenosis, right ventricular hypertrophy or infarction, constrictive pericarditis, pulmonary emboli, and severe pulmonary hypertension. Of interest, patients with tamponade do not demonstrate Kussmaul’s sign; yet, they do demonstrate pulsus paradoxus. Conversely, “pulsus” (albeit one that is never >21 mmHg) may occur in some patients with Kussmaul’s.
104 What is Trousseau’s sign?
There are actually two Trousseau’s signs:
 Recurrent thrombophlebitis associated with a visceral carcinoma (Trousseau’s syndrome). The phlebitis may be superficial or deep, often with a migratory pattern (thrombophlebitis migrans). This presents with successive crops of tender nodules in the affected vessels, with different veins being involved either simultaneously or randomly. It reflects a prothrombotic state from an underlying visceral malignancy, usually an adenocarcinoma (pancreas or lung, but also stomach, breast, and prostate). First described in 1861 by Trousseau, who in 1867 recognized it on himself as part of the pancreatic cancer that eventually killed him.
Recurrent thrombophlebitis associated with a visceral carcinoma (Trousseau’s syndrome). The phlebitis may be superficial or deep, often with a migratory pattern (thrombophlebitis migrans). This presents with successive crops of tender nodules in the affected vessels, with different veins being involved either simultaneously or randomly. It reflects a prothrombotic state from an underlying visceral malignancy, usually an adenocarcinoma (pancreas or lung, but also stomach, breast, and prostate). First described in 1861 by Trousseau, who in 1867 recognized it on himself as part of the pancreatic cancer that eventually killed him.
 Carpal spasm in patients with overt tetany (Trousseau’s phenomenon). This is associated with extension of the foot (carpopedal spasm), extension of the body, and opisthotonos. The spasm of the hand involves wrist flexors and finger extensors, so that the fingers are flexed at the metacarpophalangeal joints and extended at the phalangeal joints; the thumb is flexed and adducted into the palm. Thus shaped, the hand so typically resembles that of a physician making a vaginal examination to often be referred to as the “obstetrician’s hand” (main d’accoucheur, from the original Trousseau’s description).
Carpal spasm in patients with overt tetany (Trousseau’s phenomenon). This is associated with extension of the foot (carpopedal spasm), extension of the body, and opisthotonos. The spasm of the hand involves wrist flexors and finger extensors, so that the fingers are flexed at the metacarpophalangeal joints and extended at the phalangeal joints; the thumb is flexed and adducted into the palm. Thus shaped, the hand so typically resembles that of a physician making a vaginal examination to often be referred to as the “obstetrician’s hand” (main d’accoucheur, from the original Trousseau’s description).
105 What are the causes of an “obstetrician’s hand”?
Those predisposing to tetany: alkalemia, hypocalcemia, hypomagnesemia, or hypophosphatemia.
106 How can one trigger carpal spasm in patients with “latent” tetany?
 By occluding the arterial pulse for 5 minutes with a blood pressure cuff. This has sensitivity of 66% for hypocalcemia, with a false positive rate of 4%. Hence, it does not eliminate the need for blood testing.
By occluding the arterial pulse for 5 minutes with a blood pressure cuff. This has sensitivity of 66% for hypocalcemia, with a false positive rate of 4%. Hence, it does not eliminate the need for blood testing.
 By Chvostek’s sign. This test of muscular hyperexcitability is performed by tapping the bone anterior to the ear, which corresponds to the exit point of cranial nerve VII. The test is positive when it triggers facial twitching. Chvostek’s sign has low sensitivity for latent tetany (27%), and very high false positive rates (19–74% in children and 4–29% in adults).
By Chvostek’s sign. This test of muscular hyperexcitability is performed by tapping the bone anterior to the ear, which corresponds to the exit point of cranial nerve VII. The test is positive when it triggers facial twitching. Chvostek’s sign has low sensitivity for latent tetany (27%), and very high false positive rates (19–74% in children and 4–29% in adults).
114 Is Hill’s sign specific for AR?
No. It also can be encountered in other hyperdynamic states (previously discussed).
118 How is the ABPI measured?
According to the guidelines of the Standards Division of the Society of Interventional Radiology:
1. With the patient supine, measure brachial and ankle systolic pressure by handheld Doppler.
2. To obtain an ABPI, divide the highest systolic pressure of the dorsalis pedis (or tibialis posterior) for each foot by the highest systolic pressure at the arm. For example:
 To obtain the left ABPI, first measure the systolic brachial pressure in both left and right arms. Select the higher value as your brachial artery pressure measurement. There should be a difference of less than 10 mmHg between each brachial pressure measurement.
To obtain the left ABPI, first measure the systolic brachial pressure in both left and right arms. Select the higher value as your brachial artery pressure measurement. There should be a difference of less than 10 mmHg between each brachial pressure measurement.121 What is the Valsalva’s maneuver? How does it modify blood pressure?
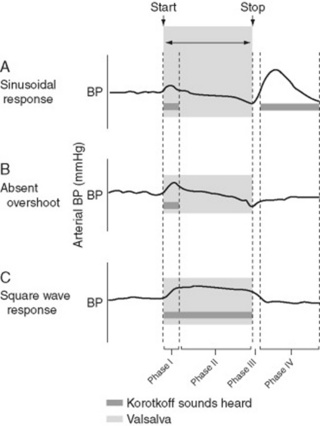
Valsalva is a great little test for assessing the reflex autonomic control of the cardiovascular system, both sympathetic and vagal. It does so by modifying blood pressure, heart rate, and venous return—all as a result of respiratory swings in intrathoracic pressures. It can be difficult to perform, though, and thus should always be well explained to the patient, especially the need to keep on straining until told to stop, and to breathe as quietly as possible after stopping straining. Valsalva consists of two major periods, comprising a total of four phases (Fig. 2-1):
Period 1: a held (or strain) period. This is carried out by asking the patient to fully inspire and then forcefully exhale against closed glottis for at least 10 seconds. It can be easily accomplished by having the patient “bear down as if having a bowel movement,” or alternatively, by placing a fist onto the midabdomen of a supine patient and then having him or her strain against it (it could even be accomplished more formally by having the patient blow for 10 seconds against an aneroid manometer at a constant pressure of 40 mmHg). Whatever the technique, the resulting strain causes an increase in intrathoracic pressure, a drop in venous return, a reduction in left ventricular diameter, and a fall in cardiac output. These can be quite dramatic (in fact, when first experimenting with Valsalva’s, Weber, in typical Teutonic fashion, managed to give himself a syncope and a seizure; then he recovered and wrote the paper). This “strain” comprises two phases:
 Phase I: After onset of straining, systolic pressure increases (due to aortic compression), and heart rate decreases (due to reflex bradycardia from baroreceptor activation).
Phase I: After onset of straining, systolic pressure increases (due to aortic compression), and heart rate decreases (due to reflex bradycardia from baroreceptor activation). Phase II: Characterized instead by a drop in venous return because of straining-induced compression of the vena cava. This eventually leads to a fall in cardiac output, a secondary fall in aortic pressure (which therefore slowly returns to the baseline level), and a secondary increase in heart rate (still mediated by the baroreceptor reflex). During the rest of the straining period, the arterial mean and pulse pressures continue to slowly fall, and the heart rate continues to slowly increase.
Phase II: Characterized instead by a drop in venous return because of straining-induced compression of the vena cava. This eventually leads to a fall in cardiac output, a secondary fall in aortic pressure (which therefore slowly returns to the baseline level), and a secondary increase in heart rate (still mediated by the baroreceptor reflex). During the rest of the straining period, the arterial mean and pulse pressures continue to slowly fall, and the heart rate continues to slowly increase.Period 2: a release period. This is carried out by asking the patient to stop bearing down, or alternatively, by releasing the fist pressure on the abdomen. It also comprises two phases:
 Phase III: As soon as straining is released (and the subject starts breathing again), there is a small but transient drop in systolic pressure due to sudden loss of aortic compression. This, in turn, causes a further reflex acceleration of the heart rate.
Phase III: As soon as straining is released (and the subject starts breathing again), there is a small but transient drop in systolic pressure due to sudden loss of aortic compression. This, in turn, causes a further reflex acceleration of the heart rate. Phase IV: When the vena cava compression completely resolves, venous return suddenly increases. This causes a rapid rise in cardiac output, which, in turn, makes the systolic pressure overshoot above baseline values (due to increased systemic resistance from phase II sympathetic activation) and heart rate to drop (because of baroreceptor reflex).
Phase IV: When the vena cava compression completely resolves, venous return suddenly increases. This causes a rapid rise in cardiac output, which, in turn, makes the systolic pressure overshoot above baseline values (due to increased systemic resistance from phase II sympathetic activation) and heart rate to drop (because of baroreceptor reflex).This normal hemodynamic response to Valsalva can be quite altered in congestive heart failure.
123 In addition to an abnormal Valsalva response, are there other findings that might diagnose congestive heart failure (CHF)?
Yes, and they involve most of the five “fingers” of the cardiovascular exam (see Chapter 20, Cardiovascular Examination). On the venous side, for example, the presence of either end-inspiratory crackles or distended neck veins has high specificity (90–100%) but low sensitivity (10–50%) for increased left-sided filling pressure due to either systolic or diastolic dysfunction. Of these two signs, only an elevated jugular venous pressure has a significant positive likelihood ratio (3.9). Positive abdominojugular reflux has equally high specificity, but better sensitivity (55–85%), and an even stronger likelihood ratio (8.0). S3 gallop, downward and lateral displacement of the apical impulse, and peripheral edema also have high specificity (>95%) but low sensitivity (1–40%) for elevated diastolic filling pressures; of them, only the S3 and the displaced apical impulse have a positive likelihood ratio (5.7 and 5.8, respectively). Given their negative likelihood ratios, only an absent abdominojugular reflux and an abnormal Valsalva response argue against the presence of high filling pressures. Finally, S4 has high sensitivity (71%), but low specificity (50%), and nonsignificant likelihood ratios.
127 What are the diagnostic and therapeutic uses of Valsalva?
Two major diagnostic applications:
 Identification of congestive heart failure (previously discussed)
Identification of congestive heart failure (previously discussed)
 Enhancement of the murmurs of hypertrophic obstructive cardiomyopathy (HOCM) and mitral valve prolapse (MVP) (see Chapter 21, Cardiac Murmurs)
Enhancement of the murmurs of hypertrophic obstructive cardiomyopathy (HOCM) and mitral valve prolapse (MVP) (see Chapter 21, Cardiac Murmurs)
Valsalva also has four therapeutic applications:
 Interrupting supraventricular tachyarrhythmias (by increasing vagal tone)
Interrupting supraventricular tachyarrhythmias (by increasing vagal tone)
 Helping patients with multiple sclerosis whose flaccid bladder cannot fully empty
Helping patients with multiple sclerosis whose flaccid bladder cannot fully empty
 On rare occasions, diminishing chest pain in patients with mild coronary disease
On rare occasions, diminishing chest pain in patients with mild coronary disease
 Last but not least, helping men avoid premature ejaculation (I am not making this up)
Last but not least, helping men avoid premature ejaculation (I am not making this up)
F. Respiration
Respiratory Rate and Rhythm
128 How useful is it to assess the patient’s rate, rhythm, and depth of respiration?
Very useful. In fact, an intelligent observation of these respiratory parameters may generate an entire alphabet soup of terminology, often conducive to specific diagnoses. For a more detailed description of these terms and disease processes, please refer to Chapter 13, Chest Inspection, Palpation, and Percussion; and Chapter 14, Lung Auscultation.
1 Bailey R, Bauer JH. A review of common errors in the indirect measurement of blood pressure (sphygmomanometry). Arch Intern Med. 1993;153:2741-2748.
2 Bor DH, Makadon HJ, Friedland G, et al. Fever in hospitalized medical patients: Characteristics and significance. J Gen Intern Med. 1988;3:119-125.
3 Cavallini MC, Roman MJ, Blank SG, et al. Association of the auscultatory gap with vascular disease in hypertensive patients. Ann Intern Med. 1996;124:883.
4 Dock W. Korotkoff’s sounds. N Engl J Med. 1980;302:1264-1267.
5 Enselberg CD. Palpatory measurement of diastolic blood pressure. N Engl J Med. 1961;265:272-274.
6 Erickson RS, Kirklin SK. Comparison of ear-based, bladder, oral, and axillary methods for core temperature measurement. Crit Care Med. 1993;21:1528-1534.
7 Hla KM, Samsa GP, Stoneking HT, Feussner JR. Observer variability of Osler’s maneuver in detection of pseudohypertension. J Clin Epidemiol. 1991;44:513-518.
8 Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 2003. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda, MD: U.S. Department of Health and Human Services, 2003. NIH Publication No. 03–5233
9 Kishan CV, Talley JD. Hill’s sign: A non-invasive clue of the severity of chronic aortic regurgitation. J Ark Med Soc. 1999;95:501-502.
10 Kuwajima I, Hoh E, Suzuki Y, et al. Pseudohypertension in the elderly. J Hypertens. 1990;8:429-432.
11 Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578-1580.
12 McGee S. Evidence-Based Physical Diagnosis. Philadelphia: W.B. Saunders, 2001.
13 Musher DM, Fainstein V, Young EJ, et al. Fever patterns: Lack of clinical significance. Arch Intern Med. 1979;139:1225-1228.
14 Neufeld PD, Johnson DL. Observer error in blood pressure measurement. Can Med Assoc J. 1986;135:633-637.
15 Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697-716.
16 Reeves RA. Does this patient have hypertension? How to measure blood pressure. JAMA. 1995;273:1211-1218.
17 Sacks D, Bakal CW, Beatty PT, et al. Position statement on the use of the ankle brachial index in the evaluation of patients with peripheral vascular disease. J Vasc Interv Radiol. 2003;14:S389.
18 Tsapatsaris NP, Napolitana GT, Rothchild J. Osler’s maneuver in an outpatient clinic setting. Arch Intern Med. 1991;151:2209-2211.