Chapter 28
Visual Motor Systems
Sympathetic Supply to the Orbit
Extraocular Muscle Motor Neurons
Horizontal and Vertical Gaze Centers
All animals use their sensory organs to scan the environment in search of information. Often these organs are actively oriented toward relevant targets. This orienting behavior is exhibited by creatures from honey bees to humans. Movement of the eyes, for example, allows closer inspection of the visual environment. Moreover, human eyes have a fovea, a small portion of the central retina that has exquisite visual sensitivity. Accurate direction of the fovea to targets of interest represents a crucial orienting behavior in humans. Extraocular muscles orient our highly mobile eyes. These muscles are supplied by cranial nerves III, IV, and VI, and the oculomotor (or ocular motor) system controls these muscles. It is one of several visual motor systems that support the function of visual sensation.
OVERVIEW
The oculomotor system includes gaze systems that redirect the eyes to each new target. There are three basic types of “targeting” movements: (1) saccades, rapid movements that direct the eyes to each new target; (2) smooth pursuit, slower movements that allow the eyes to follow moving targets; and (3) vergence movements, which adjust for target distance by changing the angle between the eyes. Vergence is coupled with changes in the curvature of the lens and the size of the pupil that focus the target image on the fovea. Saccades and smooth pursuit are conjugate movements in which the eyes move in the same direction, often with accompanying movements of the head and body. Vergence movements are disconjugate (Table 28-1).
Table 28-1 Summary of Eye Movement Characteristics
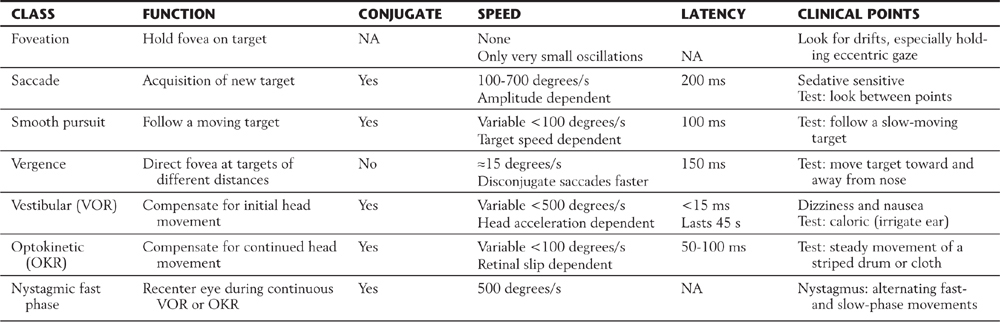
OKR, optokinetic reflex; VOR, vestibuloocular reflex; NA, not applicable.
Visual motor systems also mediate a set of reflex actions. Compensatory reflexes keep the eyes on target despite body movements. Sensory inputs from the vestibular and visual systems tell the brain that the body is in motion. During movement, the vestibuloocular reflex compensates for acceleration, which is sensed by the vestibular labyrinth, whereas the optokinetic reflex compensates for velocity, which is indicated by movement of the whole visual field (Table 28-1). Visual motor systems also compensate for the amount of light falling on the retina. The pupillary light reflex maintains the level of retinal illumination within the working range of the photopigments in the photoreceptor cells (rods or cones). Finally, rhythmic and reflex blinks of the eyelids protect the eye.
Disturbances of the visual motor systems are common and often produce the first symptoms recognized by a patient. Understanding of these ocular signs provides for timely and effective diagnosis. For instance, strabismus is a defect in which the eyes are misaligned. If this defect is left untreated, the brain reacts to the constant diplopia (double vision) by ignoring the input from one eye and failing to focus it (amblyopia) and, eventually, failing even to orient it. However, amblyopia can be avoided through early treatment of strabismus.
PERIPHERAL STRUCTURES
Extraocular Muscles
The eye is moved in the orbit by six extraocular muscles (Figs. 28-1 and 28-2). These muscles produce movements in the horizontal plane (left and right) around a vertical axis, movements in the vertical plane (up and down) around a horizontal axis, and torsional movements (clockwise and counterclockwise) around an axis running through the center of the pupil to the fovea. There are two pair of rectus muscles, with the members of each antagonistic pair arranged opposite one another on the globe, and a single pair of oblique muscles, which also act as antagonists (Figs. 28-1 and 28-2). For horizontal eye movements, the medial rectus muscle rotates the eye toward the nose (adduction) and the lateral rectus muscle rotates the eye toward the temple (abduction). For vertical eye movements, the primary action of the superior rectus is to rotate the eye upward (elevation), and the primary action of the inferior rectus is to rotate the eye downward (depression). The direction of pull of the superior oblique muscle (Figs. 28-1 and 28-2A, B) is modified because its tendon passes through a loop of connective tissue, the trochlea, on the medial wall of the bony orbit. Its insertion on the globe is caudal to that of the superior rectus. As a consequence, the actions of the superior oblique are intorsion, depression, and abduction. Conversely, the actions of the inferior oblique muscle (Figs. 28-1 and 28-2A, C) are extorsion and elevation as well as abduction.
Figure 28-2. Coronal (A, B) and sagittal (C) T2-weighted magnetic resonance images showing the relationships of the extraocular muscles within the orbit. The coronal planes are through (A) and caudal to (B), the bulb of the eye. Compare these images with the drawing in Figure 28-1.
The extraocular muscles are supplied by three cranial nerves. The superior oblique muscle is innervated by the trochlear nerve; the lateral rectus muscle, by the abducens nerve; and the medial, superior, and inferior recti and inferior oblique muscles, by the oculomotor nerve. The formula (SO4 LR6) 3 (superior oblique 4, lateral rectus 6, all others 3) is a convenient memory aid for this pattern.
Extraocular muscles are striated and contain fibers adapted to produce extremely high contraction velocities and nearly constant tension. These muscles contain some of the smallest motor units in the body, giving them great precision of movement. The muscles run through connective tissue sheaths that act as pulleys controlling their pulling directions (Fig. 28-3). Each muscle has an inner global layer and an outer orbital layer. The thick global layer inserts onto the sclera of the eye, whereas the thin orbital layer inserts into the connective tissue sheath to regulate the muscle’s pulling direction (Fig. 28-3). Some forms of strabismus may be due to misaligned pulleys. The trigeminal nerve carries sensory information from the extraocular muscles. This proprioceptive signal appears to be critical for normal development of stereoscopic vision (perception of three-dimensional space) and may have other roles. Visual feedback is the primary source of information revealing eye movement accuracy.
Figure 28-3. Frontal section through the orbit showing the extraocular muscles and the connective sheaths (pulleys) through which these muscles pass. These pulleys help define the pulling directions of the respective muscle; see also Figure 28-1. (Photo courtesy of Dr. Joseph Demer.)
Intraocular Muscles
The eyes contain three intrinsic smooth muscles (Fig. 28-4). The ciliary muscle changes the curvature of the lens to bring visual targets into focus on the retina. The sphincter (or constrictor) pupillae muscle and dilator pupillae muscle control the size of the pupils in an antagonistic fashion to regulate the amount of light entering the eyes and the depth of field.
The ciliary muscle, found in the ciliary body, is connected to the lens by the suspensory ligaments (zonule of Zinn). These fine connective tissue threads resemble the spokes of a bicycle wheel. The action of the ciliary muscle changes the shape of the lens (via the zonule) to adjust its refractive state and focus the image on the retina (Fig. 28-4E, F). These changes are termed lens accommodation. Constriction of the ciliary muscle is produced by activation of cholinergic postganglionic parasympathetic fibers from the ciliary ganglion. With age, the lens grows less elastic, so that the actions of the ciliary muscle have less effect on refraction. This loss of accommodation produces a blurring of near vision termed presbyopia. Myopia, a loss of distant acuity, generally appears at an early age and may be due to genetic factors or overwork at close focal distances (e.g., reading).
The sphincter pupillae is a ring-shaped muscle that lies along the pupillary margin (Fig. 28-4A, C). It contracts in response to activation of cholinergic parasympathetic fibers from the ciliary ganglion to constrict the pupil (miosis). The dilator muscle is radially arranged, so that its action folds the iris and draws open the pupil (mydriasis). The dilator is activated by adrenergic postganglionic sympathetic fibers from the superior cervical ganglion.
Eyelid
The eyelid is controlled by the levator palpebrae superioris, the orbicularis oculi, and the tarsal or Müller muscles (Fig. 28-5; see p. 395). The levator palpebrae is supplied by cranial nerve III. This muscle originates with and travels parallel to the superior rectus but continues forward to insert into the upper lid (Fig. 28-2A, C). The levator holds the eyelid up when the eyes are open, and it functions in concert with the superior rectus, increasing the elevation of the lids, when the eyes look up. The orbicularis oculi, supplied by cranial nerve VII, closes the eyes by depressing the upper lid and elevating the lower lid. At the same time, co-contraction of the rectus muscles retracts the eye. The tarsal muscles are small smooth muscles at the edge of the bony orbit. They are supplied by postganglionic sympathetic fibers and help keep the lids open.
Figure 28-5. The structure of the upper eyelid.
CENTRAL STRUCTURES
Oculomotor Nucleus
The oculomotor nucleus (Fig. 28-6A, B; see p. 395) lies near the midline in the inferior part of the periaqueductal gray of the rostral midbrain. Beneath it lie the fibers of the medial longitudinal fasciculus, many of which synapse within the oculomotor nucleus. Axons from oculomotor motor neurons generally pass medial to the red nucleus and exit the midbrain just medial to the crus cerebri. These structures are supplied by paramedian branches of the basilar artery and the proximal part of the posterior cerebral artery (P1 segment). Vascular lesions in this region produce oculomotor deficits in combination with other symptoms (Table 28-2).
Table 28-2 Summary of Lesions of the Exiting Oculomotor Nerve
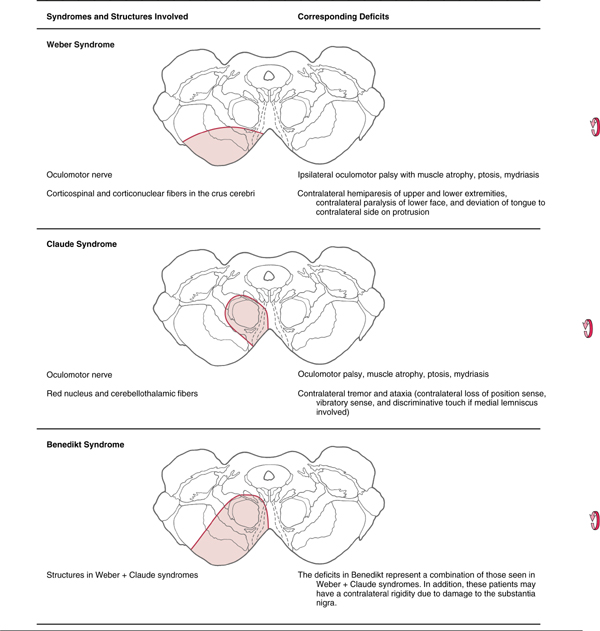
The third cranial nerve passes along the wall of the cavernous sinus and enters the ipsilateral orbit by way of the superior orbital fissure. Its three branches supply the superior rectus and levator palpebrae muscles, the inferior rectus and inferior oblique muscles, and the medial rectus muscle. The motor neurons supplying each of these individual muscles form rostrocaudally oriented columns within the nucleus (Fig. 28-7; see p. 396). The motor neurons supplying the levator palpebrae superioris muscle form a separate dorsal midline subnucleus called the caudal central subdivision.
Edinger-Westphal Complex
The Edinger-Westphal complexconsists of the Edinger-Westphal centrally projecting nucleus and the Edinger-Westphal preganglionic nucleus. In humans, the centrally projecting cell group wraps around the posterior medial corner of the oculomotor nucleus but is not related to oculomotor function. These neurons project diffusely in the brainstem and spinal cord and are peptidergic. The preganglionic nucleus lies on either side of the midline, immediately posterior to the centrally projecting group. It contains cholinergic, preganglionic motor neurons whose axons project to the ciliary ganglion to control the lens and pupil (Figs. 28-6 and 28-7). The preganglionic fibers travel with the ipsilateral oculomotor nerve and synapse in the ciliary ganglion. Cholinergic, postganglionic motor neurons send their axons to the globe via the short ciliary nerves. These motor neurons supply the ciliary muscle and pupillary constrictor, with the great majority supplying the ciliary muscle. Thus, in Weber syndrome (Table 28-2) or other lesions of the oculomotor nerve, loss of the preganglionic fibers may result in ipsilateral mydriasis (dilation of the pupil) and paralysis of accommodation.
Trochlear Nucleus
The trochlear nucleus is a small, ovoid group of neurons nestled in the medial longitudinal fasciculus of the caudal midbrain (Fig. 28-8; see p. 396). These motor neurons supply the contralateral superior oblique muscle. The axons forming the trochlear nerve arch dorsally and caudally around the periaqueductal gray, cross the midline in the anterior medullary velum, and exit the dorsal surface of the brainstem at the base of the inferior colliculus. The nerve courses laterally and then anteriorly, hugging the surface of the midbrain. It passes rostrally along the wall of the cavernous sinus before entering the orbit via the superior orbital fissure to innervate the superior oblique muscle.
Abducens Nucleus
The abducens nucleus is a spherical cell group located in the facial colliculus, adjacent to the internal genu of the facial nerve in the caudal pons (Fig. 28-9 [see p. 396]; also see Figs. 12-10 and 12-12). Abducens fibers pass slightly caudally and then anteriorly (ventrally) to exit near the midline at the pontomedullary junction. En route, they pass adjacent to the medial lemniscus and corticospinal tract. Owing to this relationship, loss of paramedian branches of the basilar artery compromises both the corticospinal tract and the exiting abducens fibers, resulting in a contralateral hemiplegia and paralysis of abduction in the ipsilateral eye (Foville syndrome). This pairing of motor symptoms is called an alternating hemiplegia or crossed deficit. Crossed deficits can also occur in association with cranial nerves III and XII. After exiting the brainstem, the abducens nerve (cranial nerve VI) enters the dura and ascends the clivus. It then passes beneath the petroclinoid ligament (the Dorello canal) to enter and pass through the cavernous sinus. Cranial nerves III, IV, and VI and branches of cranial nerve V may be damaged in this sinus, singly or in combination, by pituitary tumors or carotid aneurysms. Cranial nerve VI supplies the lateral rectus muscle, so damage to this nerve in isolation will paralyze abduction ipsilaterally (Fig. 28-10; see p. 397).
Figure 28-9. Human abducens nucleus and adjacent structures in Nissl-stained cross section showing neuron cell bodies.
Abducens Internuclear Neurons
Conjugate eye movement is achieved in the horizontal plane by a pathway from the abducens nucleus to the contralateral medial rectus subdivision of the oculomotor nucleus (Fig. 28-13, green). Inputs to the abducens nucleus supply motor neurons but also drive abducens internuclear neurons found in this nucleus. Abducens internuclear neurons transmit signals to medial rectus motor neurons on the contralateral side via the medial longitudinal fasciculus. In this way, the lateral rectus of one eye works in tandem with the medial rectus of the other eye. Lesions in the medial longitudinal fasciculus between the abducens and oculomotor nuclei damage the abducens internuclear neuron axons, producing internuclear ophthalmoplegia. In this situation, the lateral rectus contracts appropriately during horizontal eye movements, but the medial rectus for the opposite eye does not. However, because the oculomotor nerve and nucleus are intact, no deficits are present on convergence. The presence of internuclear neurons also explains why the symptoms of abducens nerve lesions differ from those of abducens nucleus lesions. In the abducens nucleus lesions, the action of the contralateral medial rectus muscle is impaired during conjugate horizontal movements in addition to the expected paralysis of the ipsilateral lateral rectus muscle. Large abducens nucleus lesions may also include the crossing fibers entering the medial longitudinal fasciculus from the opposite abducens. This produces a one-and-a-half syndrome in which the ipsilateral eye does not move in either direction for horizontal gaze and the contralateral eye can only abduct.
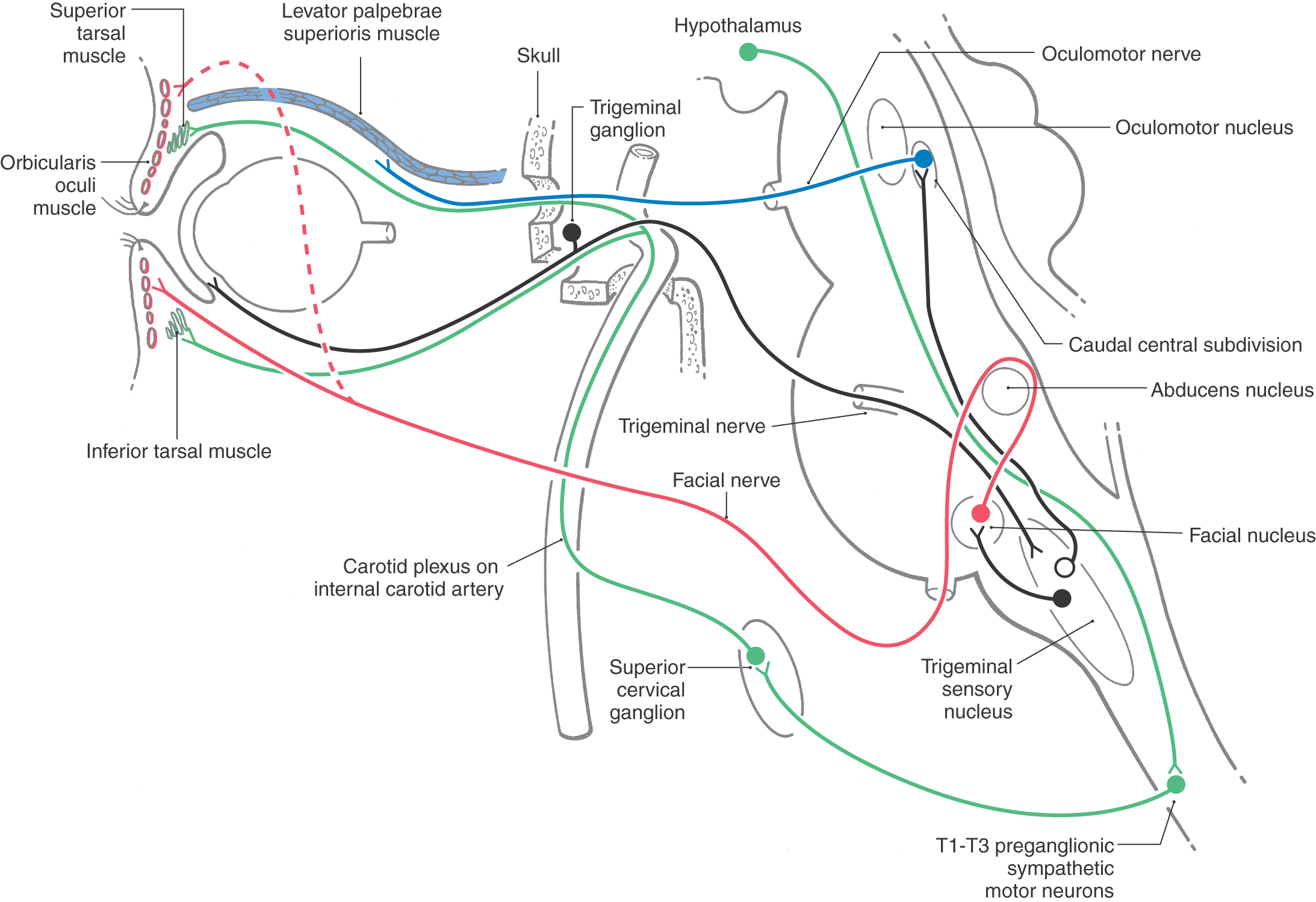
Sympathetic Supply to the Orbit
The sympathetic innervation of the orbital contents is from the ipsilateral superior cervical ganglion (Fig. 28-11; see p. 397). These adrenergic postganglionic fibers enter the cranium on the internal carotid artery. In the cavernous sinus, they run briefly with cranial nerve VI and then join cranial nerves III and V. Sympathetic fibers traveling with the levator branch of cranial nerve III supply the superior tarsal muscle. Fibers traveling with the trigeminal nasociliary nerve exit as the long ciliary nerves to supply the eye, including the dilator pupillae muscle.
The cholinergic preganglionic motor neurons that supply the superior cervical ganglion are located at spinal cord levels T1 to T3. Their axons enter and ascend in the sympathetic trunk to terminate in the ipsilateral superior cervical ganglion. Damage along this long path produces a Horner syndrome. The cardinal symptoms of this loss of sympathetic input to the head are partial ptosis (a drooping lid resulting from relaxation of the superior tarsal muscle), miosis (constriction of the pupil that occurs because the action of the sphincter is no longer opposed by the action of the dilator), and anhidrosis (loss of facial sweating). A Horner syndrome may also result from interruption of pathways linking the hypothalamus and brainstem to preganglionic motor neurons in the thoracic cord.
TARGETING MOVEMENTS
Saccades
To obtain detailed information about the visual world, the eyes make a series of very rapid movements (200 to 700 degrees/second) from one point to another, stopping briefly at each point to allow detailed foveal inspection. These rapid conjugate eye movements are saccades, and the places at which the detailed visual inspection occurs are fixation points. During the saccade, the visual system suppresses incoming visual input. Consequently, one is not aware of these movements. Other processes in visual association cortex provide visual constancy by weaving together the information obtained at each fixation into a seamless view of the visual world.
Extraocular Muscle Motor Neurons
Extraocular muscle motor neurons have a characteristic burst-tonic firing pattern for saccadic eye movements. For example, before a leftward horizontal movement (Fig. 28-12, points A to B; see p. 397), left lateral and right medial rectus motor neurons show an initial burst of action potentials, which is then reduced to a sustained tonic level of activity. At the new eye position, the firing rate is slower than during the initial burst but higher than the rate for the previous eye position (Fig. 28-12, points B to C).
These two parts of the motor neuron and target muscle response are referred to as a pulse and step of activity. The pulse, the initial burst of motor neuron activity, directs the phasic portion of the movement, producing the muscle contraction necessary to overcome the viscosity of the orbit and to send the globe toward the target. The step in the activity supports the tonic action of the muscle, which is required to maintain the eye at its new position. The activity of the antagonists is silenced for the saccade and then resumes at a lower rate. However, when the eyes look to the right (Fig. 28-12, points C to D), motor neurons for the left medial rectus and right lateral rectus muscles are activated. The brainstem distribution of activated motor neurons defines which muscles are activated and hence the direction of movement. The number of cells activated and their firing rates define the speed and distance (metrics) of the movement.
Horizontal and Vertical Gaze Centers
The brainstem circuitry that controls saccades is subdivided into systems controlling horizontal and vertical components of eye movement. The pontine reticular formation near the midline, which contains neurons that project to the extraocular motor nuclei, is sometimes called the paramedian pontine reticular formation (PPRF) or the horizontal gaze center (Figs. 28-13 and 28-14). The PPRF occupies portions of the oral and caudal pontine reticular nuclei. Cells of this premotor region show activity related to horizontal saccades, and lesions in this region produce horizontal gaze palsies. PPRF cells that project to extraocular motor neurons include excitatory burst neurons (EBNs), found rostral to the sixth nucleus, and inhibitory burst neurons (IBNs), found caudal to the sixth nucleus. Both of these cell types have phasic activity patterns; that is, they produce a burst of action potentials that slightly precedes the activity of the motor neurons (Fig. 28-13). When gaze shifts to the right, the EBNs activate the abducens nucleus neurons on the right side, as the IBNs suppress the abducens nucleus neurons on the left side. If this inhibition of antagonists does not occur, eye movements are slowed and undershoot.
 Figure 28-13. The positions of the horizontal and vertical gaze centers in the sagittal (A) and frontal (B–E) planes.
Figure 28-13. The positions of the horizontal and vertical gaze centers in the sagittal (A) and frontal (B–E) planes.
The pattern of activity in PPRF neurons is a product of the signal (coded in the cell firing pattern) sent from supranuclear structures, including the superior colliculus and the frontal eye field, and processed through interneurons within the PPRF. In addition, the burst of action potentials produced by EBNs and IBNs is gated by inhibition from cells found in the midline of the pontine tegmentum (Figs. 28-13 and 28-14). These inhibitory neurons are called omnipause cells because they fire spontaneously during fixation but are silent during a saccadic eye movement in any direction. Thus the burst of action potentials in PPRF neurons is due, in part, to release from inhibition. PPRF neurons directly contribute to the pulse of motor neuron activity that produces a saccade but not to the step change in activity that maintains eye position. The “where the eye is going” signal produced by the PPRF is transformed (integrated) into a “keep the eye in that position” signal by another brainstem structure. The likely source of this tonic position signal for horizontal saccades is the nucleus prepositus hypoglossi (Figs. 28-13 and 28-14).
Vertical gaze palsies are often encountered with lesions of the midbrain-diencephalon junction. The vertical gaze center is located in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), found at the rostral end of the medial longitudinal fasciculus (Fig. 28-12). This region receives supranuclear input from the superior colliculus and frontal eye field as well as input from omnipause cells. It also contains burst neurons. These neurons provide the phasic signal for the saccade-related pulse of activity present in vertical gaze motor neurons. The tonic signal for the step in vertical gaze motor neuron activity is provided by neurons in the interstitial nucleus of Cajal (Fig. 28-13). Because the superior and inferior rectus muscles act in pairs during vertical eye movements, projections of the riMLF and interstitial nucleus of Cajal on both sides of the brainstem must work in concert. (Unilateral activation produces torsion.) The connections between the two interstitial nuclei of Cajal and the crossed projections to the oculomotor nucleus pass through the posterior commissure. Consequently, pinealomas pressing on this commissure produce vertical gaze deficits. Oblique saccades are produced by the vertical and horizontal gaze centers working in concert.
Saccadic eye movements are often accompanied by orienting movements of the head. Cells in or near the PPRF, riMLF, and interstitial nucleus of Cajal are responsible for combined head and eye movements. These areas receive collicular and cortical projections, and they project to the extraocular motor neurons and to the cervical spinal cord as reticulospinal and interstitiospinal fibers. The superior colliculus also projects to the cervical spinal cord, but the tectospinal portion of the tectoreticulospinal system is very small.
Supranuclear Control
For each saccade, the central nervous system must determine the position of the next target of interest and transform this position, which is coded in a sensory map, into the appropriate pattern of motor neuron activity. The areas of the brain that direct saccadic eye movements include the cortical eye fields and the superior colliculus. In each area, stimulation will initiate contralaterally directed saccades, and single cell recordings reveal neuronal activity before saccades occur. Specifically, stimulation of a location in which cells are active before a 20-degree saccade to the left will produce a 20-degree leftward saccade.
In humans, the frontal eye field is located rostral to the motor cortex, mainly in caudal portions of the middle frontal gyrus in Brodmann area 6 with some extension into area 8 (Figs. 28-15 and 28-16). It is apprised of the location of targets via input from visual association cortex and thalamic relays (paralamellar dorsomedial nucleus) (Fig. 28-16). The frontal eye field influences eye movements through projections to the vertical and horizontal gaze centers and to the superior colliculus. Additional cortical regions influencing saccades include the supplemental eye field and the parietal eye field in the lateral intraparietal cortex (area 7 of Brodmann) (Fig. 28-15). They have features similar to those of the frontal eye field but are less directly connected to the brainstem saccade circuits. These three cortical eye fields are reciprocally connected, and all three project to the superior colliculus. Perhaps for this reason, loss of any one of these four structures produces few visual motor symptoms.
Figure 28-15. Locations and interconnections of the cortical eye fields on medial (upper) and lateral (lower) views of the cerebral cortex.
The superior colliculus (optic tectum) is a layered structure found in the roof of the midbrain (Fig. 28-17). This area of the midbrain tectum receives its blood supply from the quadrigeminal artery, a branch of the P1 segment of the posterior cerebral artery. The superficial layer of the superior colliculus is visual sensory. It is a target of retinal axons with M and K type physiologic characteristics and projects to the dorsal lateral geniculate and pulvinar nuclei. In contrast, the intermediate layer is visual motor. It is the source of the crossed tectoreticulospinal system that runs ventral to the medial longitudinal fasciculus and terminates in the vertical and horizontal gaze centers (Fig. 28-18).
Major inputs to the intermediate layers of the superior colliculus arise from the cortical eye fields and substantia nigra (Fig. 28-18). GABAergic nigrotectal cells in the substantia nigra pars lateralis and pars reticulata are spontaneously active but cease firing before saccadic eye movements. Tectal saccade-related activity is partly the result of release from this nigral inhibition. Similar pathways connect the nigra and frontal eye fields. Basal nuclei diseases produce eye movement disorders; for example, patients with Parkinson disease have a dearth of spontaneous eye movements because of unmodulated activity in the nigrotectal pathway.
The superior colliculus and the frontal eye field differ in the types of saccades they control. The frontal eye field is important for voluntary and memory-guided eye movements, and the superior colliculus directs reflexive orienting movements. With loss of either structure, few deficits are seen after recovery because the remaining structure compensates for the loss, but loss of both produces profound visuomotor impairment.
Smooth Pursuit
The eyes also make conjugate movements that allow the foveae to follow a moving target (Fig. 28-19). Usually, smooth pursuit eye movements are used to follow slow-moving, predictable targets (30 degrees/second or less). They are, however, capable of following targets at speeds up to 100 degrees/second. The lateral parietal and midtemporal cortices contain neurons that are sensitive to the speed and direction of a target moving across the retina (Fig. 28-20). This input determines the speed and direction of the pursuit eye movements needed to keep the foveae on target. Neurons that display pursuit-related motor activity are found in a portion of the frontal eye field. These three cortical regions project to the flocculus and paraflocculus of the cerebellum by way of a synaptic relay in the posterolateral (dorsolateral) pons. This portion of the cerebellum, in turn, provides input to the vestibular nuclei. Although located in a “sensory” nucleus, vestibular smooth pursuit cells fire with respect to the position and velocity of the eyes, not the visual sensory input. They are, in fact, premotor neurons that project to the third, fourth, and sixth cranial nerve nuclei.
Smooth pursuit premotor neurons fire in a graded manner, depending on the degree and rate of eye excursion. This firing pattern is similar to that displayed by their motor neuron targets during smooth pursuit movements (Fig. 28-19). They do not have the pulse-and-step form seen with saccades. Presumably, the cerebellum plays a role in precisely determining the rate of movement and predicting target trajectory. Floccular lesions do, in fact, produce deficits in smooth pursuit movements.
Vergence Movements and the Near Triad
Foveation involves directing the eyes toward targets in three-dimensional space. To look from a distant target to a closer one, three changes are made in the eyes. First, the eyes converge by the simultaneous activation of both medial rectus muscles to point both foveae at the closer target (Fig. 28-12, points E to F). This is a disconjugate movement because the eyes move in opposite directions. Second, the curvature of the lens is increased, producing increased refractive power to focus the closer target on the fovea (Fig. 28-4). Third, the pupil is constricted, thereby increasing the depth of field of the eye. The combination of these three actions is termed the near triad or near response. The opposite effects (divergence, flattening of the lens, and pupillary dilation) occur when the gaze is shifted from a closer target to one farther away.
As the term “near triad” suggests, the three actions are generally yoked. Although vergence and accommodation can be dissociated under special conditions (closing one eye and bringing the target straight at the open eye), under normal conditions the vergence angle is used by the brain to adjust the accommodation of the lens. Other cues used to direct the near response include focus, which is indicated by blurring of the image, and retinal disparity. For retinal disparity, cells in the visual cortex with binocular visual fields are activated when the retinal images have a specific degree of disparity (the difference in the points on each retina where an image falls). This difference is an appropriate signal for the control of vergence.
Vergence movements are generally slower than saccades, and the initial burst of motor neuron activity is less evident (Fig. 28-12, points E to F). Premotor neurons whose activity correlates with the near response are found in a midbrain near response region located in the supraoculomotor area (SOA) (Fig. 28-21). Cells in the SOA project to the medial and lateral rectus motor neurons and also to the preganglionic motor neurons in the Edinger-Westphal preganglionic nucleus that control the lens and pupil. The cerebellum influences vergence and accommodation through projections to the SOA, but other near response pathways remain undefined.
Vergence and saccade movements often occur in combination. When combined, they have a similar time course, but the saccadic and vergence systems can also act independently. For instance, after a lesion in the paramedian pontine reticular formation, horizontal saccades are disrupted, but vergence movements in the horizontal plane are unaltered.
REFLEX MOVEMENTS
The body is equipped with compensatory systems that keep the eyes directed at a target despite external perturbations of the body or head. The vestibular system specializes in sensing the acceleration that usually occurs at the beginning of a movement, and it also senses gravity. Activation of the vestibular labyrinths elicits a series of compensatory eye movements commonly termed the vestibuloocular reflexes. The pathways subserving these reflexes are discussed in Chapter 22. In contrast, the optokinetic system compensates for continuous-velocity movements. This section covers the optokinetic system as well as the pupillary and blink reflexes.
Optokinetic Eye Movements
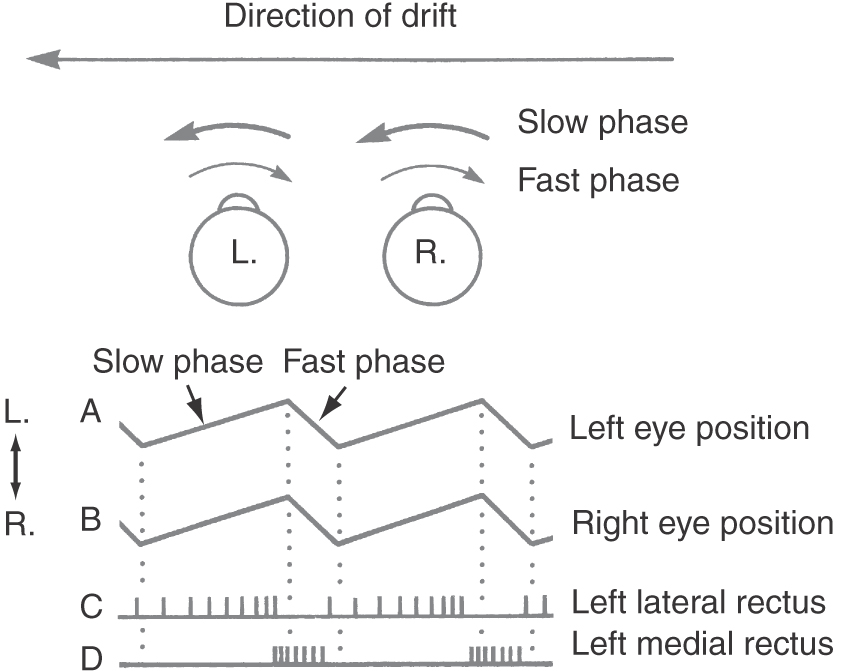
As we move in the world or move our heads, the entire visual scene moves across the retina. This whole-field movement of the visual scene is called retinal slip. Under these conditions, the eyes automatically move in a compensatory fashion to stabilize the image on the retina. For example, if the body is rotating to the left, the visual world will seem to move to the right, and rightward compensatory eye movements match the velocity and direction of this retinal slip (Fig. 28-22). As with smooth pursuit, these optokinetic eye movements are produced by graded increases and decreases in the tonic firing rate of the appropriate motor neurons (Fig. 28-22). When the eyes approach the limit of their rotation, a quick saccade brings them back to their primary position and another slow following movement begins. This set of alternating slow (compensatory) and fast (saccadic) phases of movement is called optokinetic nystagmus (Fig. 28-22). Clinically, the direction of optokinetic nystagmus is specified by the fast-phase direction. A series of stripes can be moved in front of a subject to elicit nystagmus and to test the optokinetic reflex.
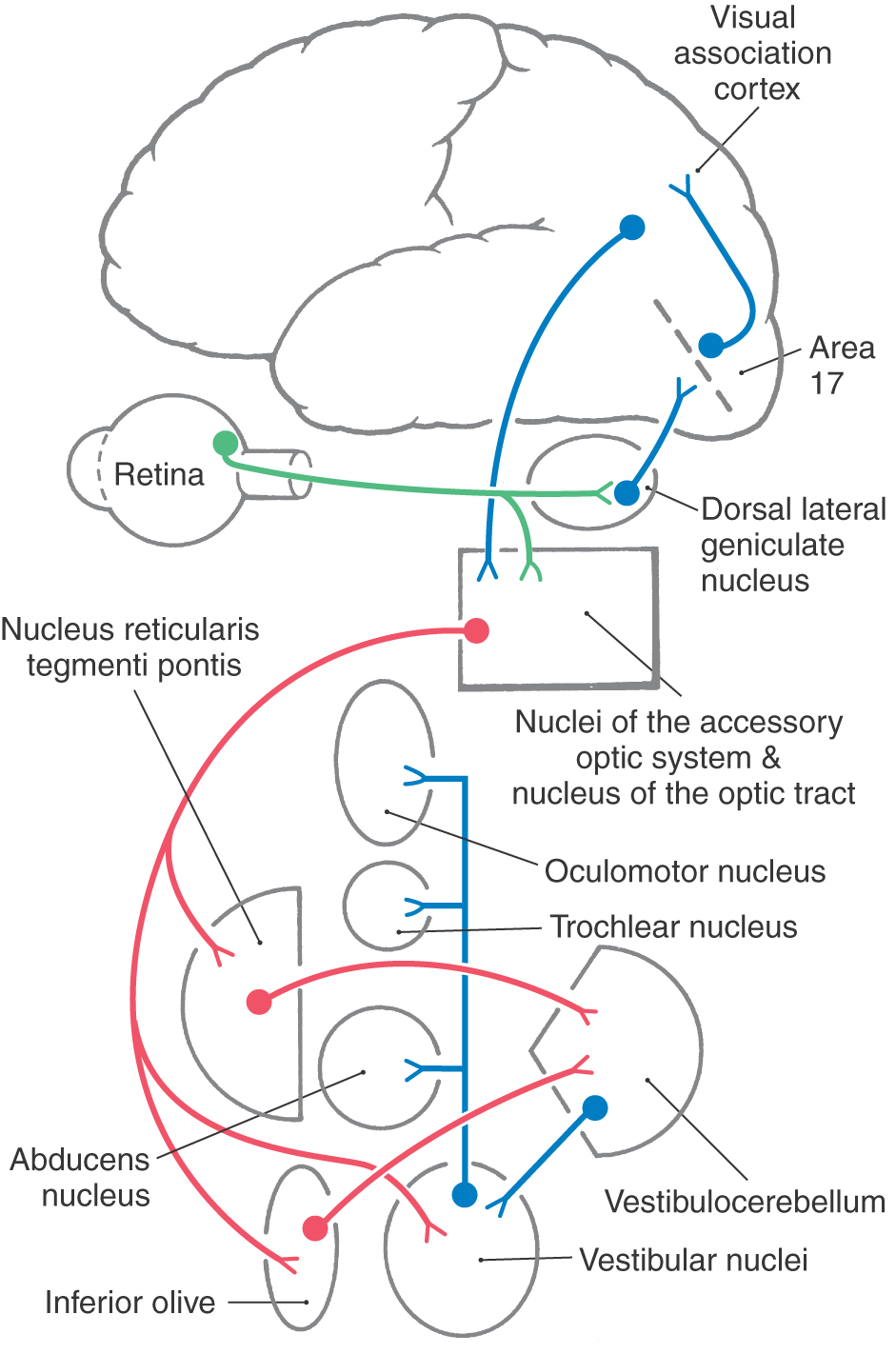
The afferent limb of this reflex begins with stimulation of wide-field retinal ganglion cells that are sensitive to slow movements of the whole receptive field (Fig. 28-23). These receptive fields are tuned to directions that are comparable to the orientations of the vestibular semicircular canals. Axons of these retinal ganglion cells terminate in a series of small nuclei along the incoming optic tract, which together compose the accessory optic system (AOS) (Fig. 28-21). Each nucleus of the AOS contains cells that are activated by retinal slip in specific directions. For example, the nucleus of the optic tract is sensitive to temporal-to-nasal retinal slip. So, activation of one nucleus of the optic tract indicates a turn toward the activated side, and bilateral activation indicates backward movement. The AOS nuclei also receive input from visual sensory association cortex, presumably from neurons of the smooth pursuit system (Fig. 28-23). In fact, the human optokinetic reflex comes into play only when the AOS indicates retinal slip and the pursuit system notes equivalent foveal target movement. Otherwise, the pursuit system overrides the optokinetic system.
Figure 28-23. Pathway for the optokinetic system.
The AOS nuclei project to the portions of the nucleus reticularis tegmenti pontis and the inferior olive that supply the vestibulocerebellum and to the vestibular nuclei (Fig. 28-23). In addition to vestibuloocular premotor neurons, the vestibular nuclei contain optokinetic neurons that influence extraocular motor neurons. In fact, lesions in the vestibular nuclei produce severe deficits in both reflexes. The pathways through the cerebellum are involved in adapting the gains of the optokinetic and vestibuloocular reflexes so that they combine to produce appropriate eye movements.
Pupillary Light Reflex
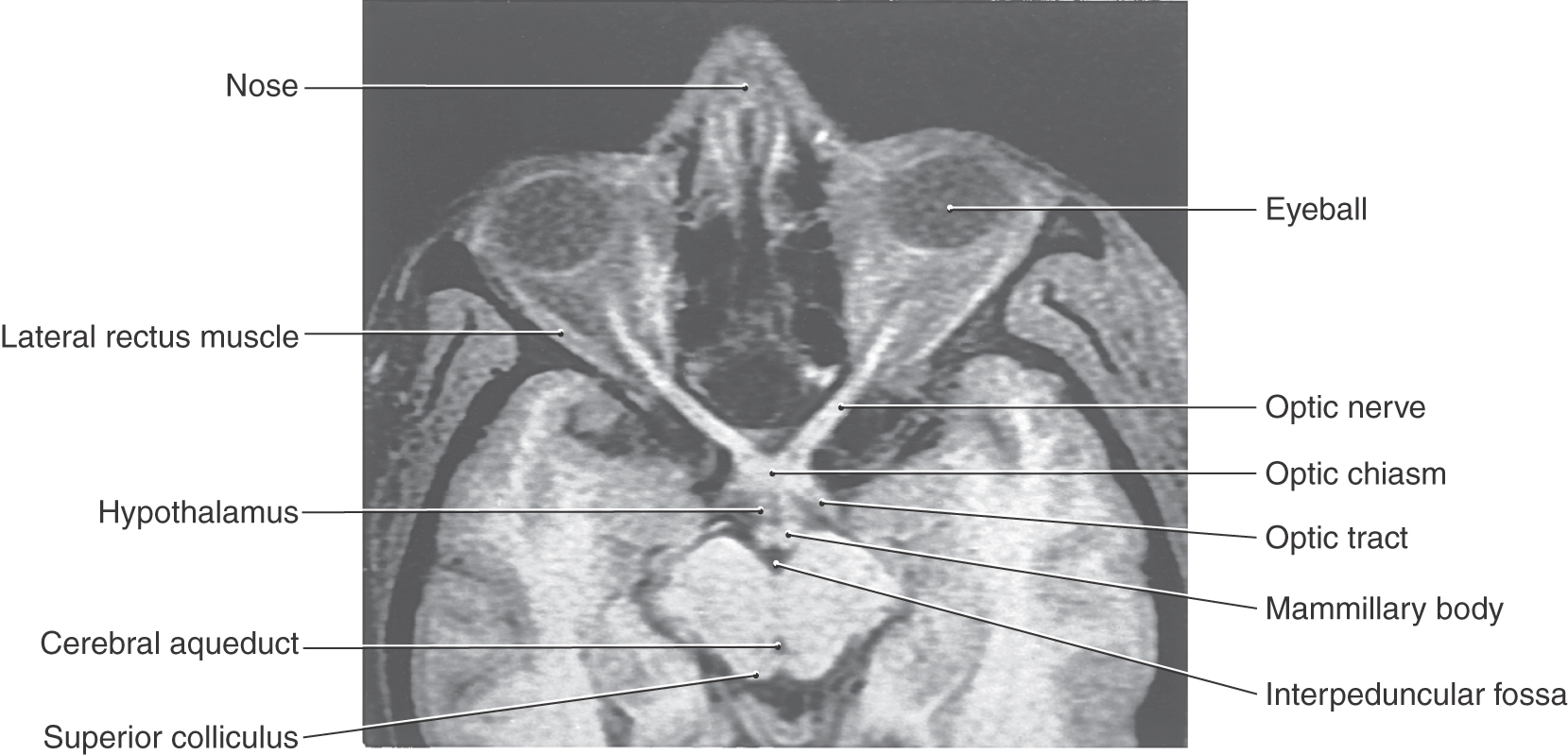
In addition to the changes in pupillary size that result from the near response, the pupil also responds to the amount of ambient light. The actions of the iris in each eye are yoked, so that light directed into one eye results in pupillary constriction in both the illuminated eye (direct response) and the opposite eye (consensual response). The pupil changes diameter to maintain luminance of the retina in the optimal range of the receptor photopigments. Many of the structures involved in the pupillary light reflex pathway are clearly visible in a magnetic resonance image oriented specifically to the long axis of the optic nerve (Fig. 28-24).
Figure 28-24. T1-weighted magnetic resonance image of optic structures (nerve, chiasm, and tract) in relation to the hypothalamus and midbrain. Compare with Figure 28-25.
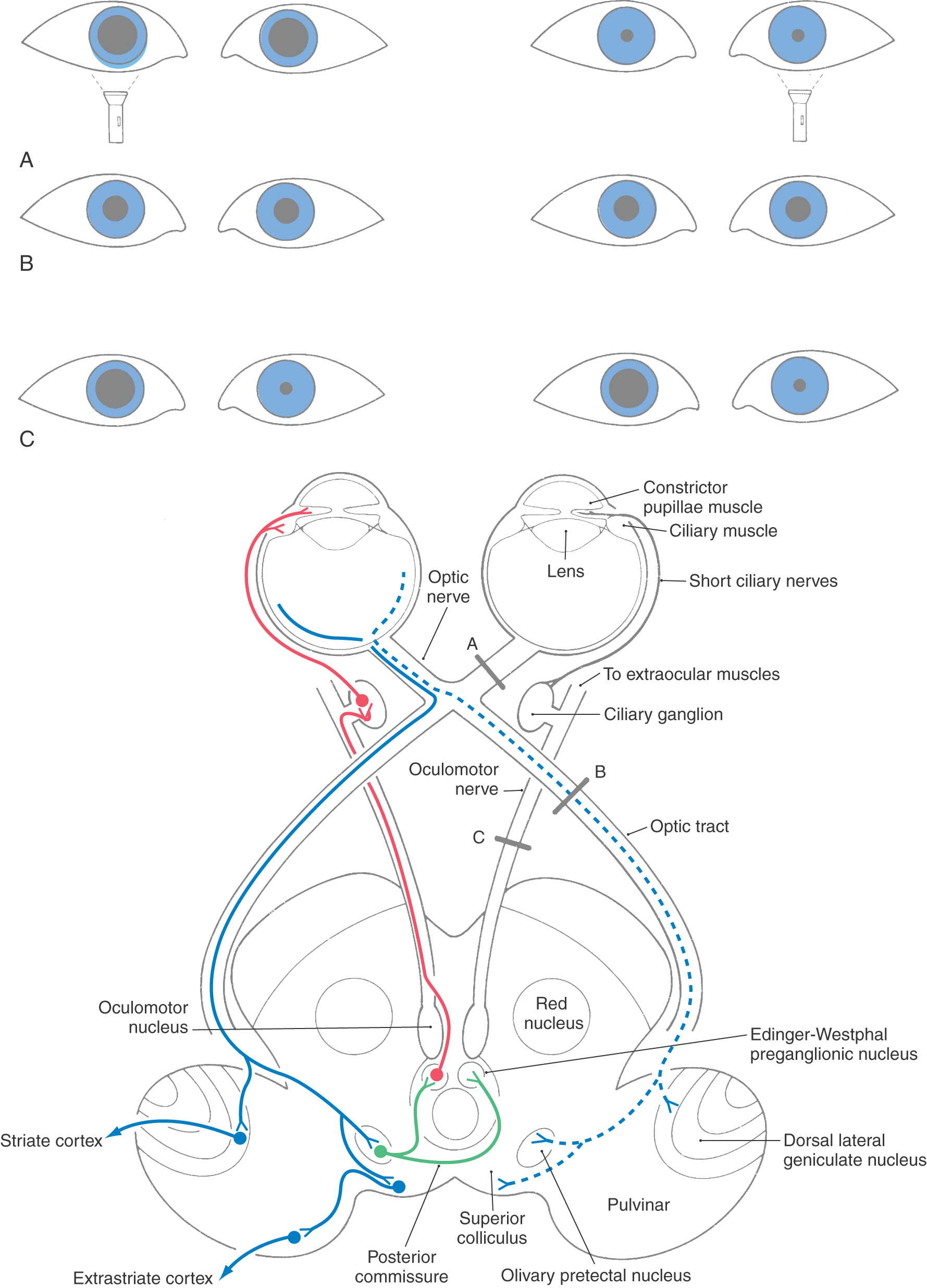
The pupillary light reflex is a four-neuron arc (Figs. 28-24 and 28-25). A set of retinal ganglion cells that respond in a linear fashion to luminance levels project via the optic nerve and tract to the midbrain. The decussation of approximately half of these fibers in the chiasm is one of the structural features responsible for the consensual response. The retinal axons terminate in the pretectum within the olivary pretectal nucleus (commonly called the pretectal nucleus), which in turn projects bilaterally to the Edinger-Westphal preganglionic nucleus, with the decussating fibers crossing in the posterior commissure. Parasympathetic, preganglionic fibers from the Edinger-Westphal preganglionic nucleus exit with the oculomotor nerve and terminate in the ciliary ganglion. The postganglionic fibers reach the iris, where they excite the pupillary constrictor muscle. Damage to these postganglionic fibers produces a tonic dilated pupil (the Adie syndrome) in which the constrictor muscle is supersensitive to cholinergic drugs.
The pupillary light pathway described before is activated when light is transduced by rods and cones, initiating a synaptic cascade that results in firing of action potentials by ganglion cells. Thus, when light is shined into the eye of a patient, not only does it produce direct and consensual pupillary responses, but the patient will also “see” the light because of an intact retinogeniculostriate pathway. However, an alternative and clinically relevant process has recently been established. The light reflex can also be initiated by a class of ganglion cells that are directly activated by light, without synaptic input from photoreceptors. These melanopsin-containing ganglion cells have large cell bodies and very large dendritic fields. They represent less than 1% of the total human retinal ganglion cell population and do not directly participate in visual sensory image formation. Their presence explains why individuals with, for example, advanced retinitis pigmentosa still have a pupillary light response when light is shined in the eye, even though they can no longer “see” the light. The pupillary light reflex is a useful diagnostic tool to test brainstem and cranial nerve function (Figs. 28-24 and 28-25). Lesions may result in loss of the direct or consensual pupillary response or in uneven pupil size (anisocoria). A dilated, unresponsive (fixed) pupil (or pupils) in the unconscious victim of head trauma is a grave sign. For example, it may indicate that a space-occupying lesion has forced the parahippocampal gyrus or uncus over the edge of the tentorium (uncal herniation), compressing the third cranial nerve. The pupillary fibers are superficially located in the oculomotor nerve and are particularly sensitive to pressure. Their loss may indicate that compression of the brainstem is imminent.
Disease of the retina, the optic nerve, or in some cases the optic chiasm may result in asymmetry of the pupillary response; this is a relative afferent pupillary defect (also called a Marcus Gunn pupil). To test the direct and consensual responses, a penlight is shined just below the visual axis of first one eye and then the other. In the diseased eye, the pupillary response is less brisk (slower) and not as complete (the pupil constricts less), and the pupil does not stay constricted for as long as it does when the light is shined in the normal eye. Because the penlight is quickly moved back and forth between the two eyes, this is commonly called the swinging-light or alternating-light test. The effects of lesions in the sympathetic pathways (Horner syndrome) have already been discussed. Another syndrome, called Argyll Robertson pupil, is found in cases of tabes dorsalis (central nervous system syphilis). Affected patients show small pupils with very weak or absent pupillary light reflexes bilaterally, but there is no loss of visual acuity, and the pupils do constrict in the near response. This pattern of sparing indicates that the afferent and efferent limbs of the pupillary light reflex must be intact. Consequently, it is assumed that bilateral degeneration in either the olivary pretectal nuclei or their projections must be the source of the pupillary dysfunction.
Pupil size also reflects visceromotor tone. Greater levels of excitement, including desire, result in dilation of the pupil via sympathetic activation. This fact was known to Elizabethan women, who used tincture of belladonna to dilate their eyes for cosmetic purposes. Today, cholinergic blockers are used to dilate the pupils for ophthalmologic examination.
Blinking and Other Lid Movements
The delicate structures of the eye are protected by the eyelids. Blinks, some of which occur in response to somatosensory stimulation, ensure protection for the eye. The corneal blink reflex is used to assess trigeminal sensory and facial motor nerve function as well as the integrity of the lid pathways through the lateral pons. Trigeminal nerve fibers with free nerve endings in the cornea and lash follicle receptors have central processes terminating in the spinal portion of the trigeminal sensory nucleus (Fig. 28-26). Second-order trigeminal neurons project directly and indirectly to the facial nucleus to excite orbicularis oculi motor neurons, which produce lid closure. In addition, an inhibitory pathway suppresses the activity of antagonist levator palpebrae motor neurons in the oculomotor nucleus.
Blinks also occur at regular intervals (averaging 12 blinks/minute) as automatically triggered movements that spread the tear film over the cornea. The constant dispersal of the tear film produced by rhythmic blinks prevents corneal lesions and scarring. Blepharospasm is a disorder in this rhythmic behavior that results in bouts of high-frequency blinking, whereas parkinsonism produces a decreased blink rate. To keep out of the line of vision, the lids also move with the eyes during vertical eye movements. These movements are produced by actions of the levator palpebrae muscle, which works in concert with the superior rectus. Levator motor neurons receive inputs from cells in and near the vertical gaze centers. The orbicularis oculi muscle is not involved. Consequently, Bell palsy, in which the facial nerve is damaged, often by a herpetic infection of the nerve, results in a loss of the blink reflex on the affected side but no ptosis or loss of vertical gaze–related lid movements. Lesions of the oculomotor nerve produce just the opposite results.
The tarsal muscles help keep the lids open, as indicated by the partial ptosis present in the Horner syndrome. Their sympathetic innervation suggests that they regulate lid position with respect to emotional state. For example, high sympathetic tone produces widely opened eyes. Relaxation of the tarsal muscles leads to the feeling of “heavy lids,” which signals the general tone of the autonomic system as the brain prepares to rest.
Sources and Additional Reading
Brandt T. Vertigo: Its Multisensory Syndromes. ed 2 London: Springer-Verlag; 1999. p 503
Evinger C. A brainstem reflex in the blink of an eye. News Physiol Sci. 1995;10:147–153.
Ong E, Ciuffreda KJ. Nearwork-induced transient myopia. Doc Ophthalmol. 1995;91:57–85.
Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200.

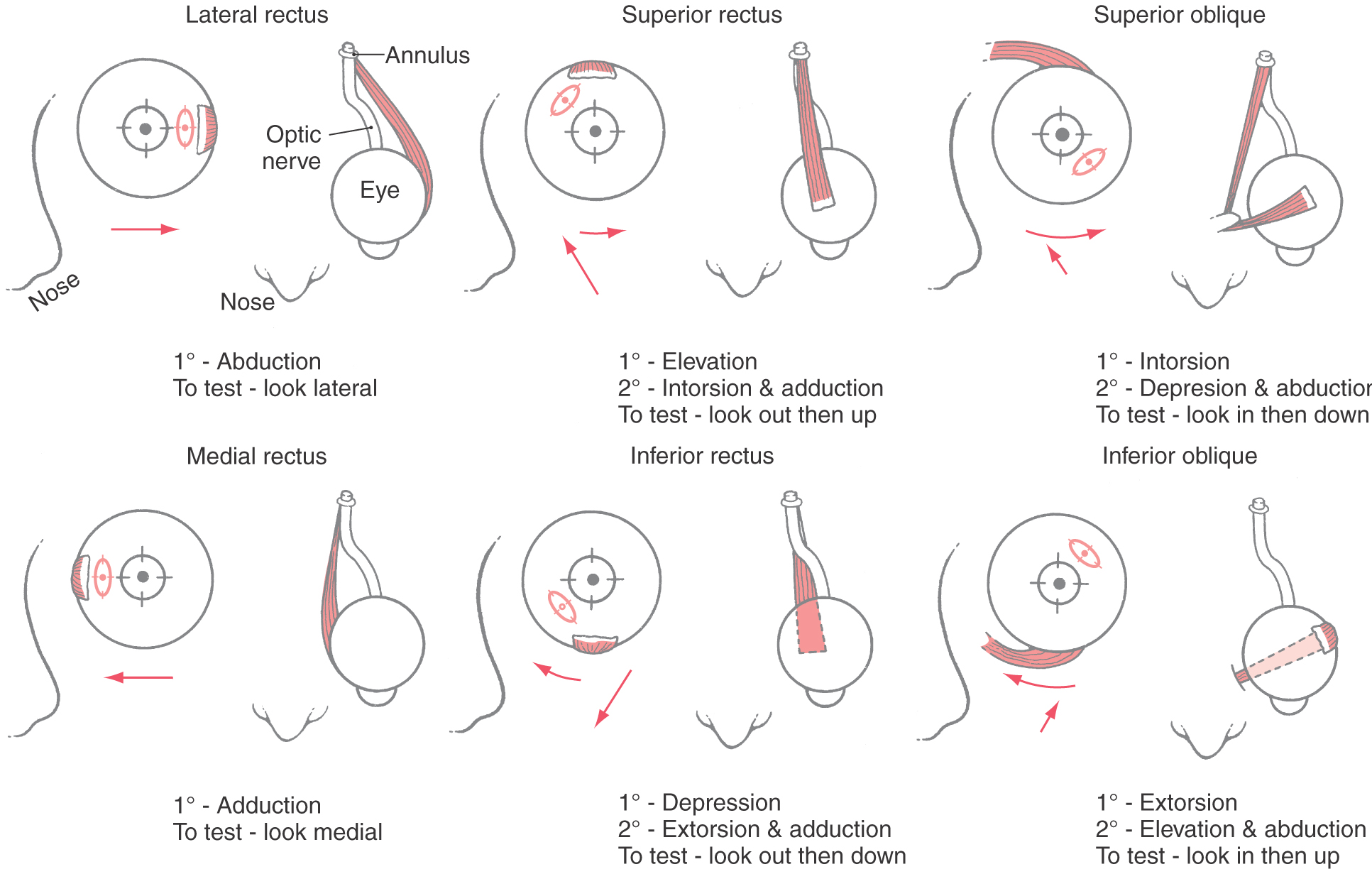
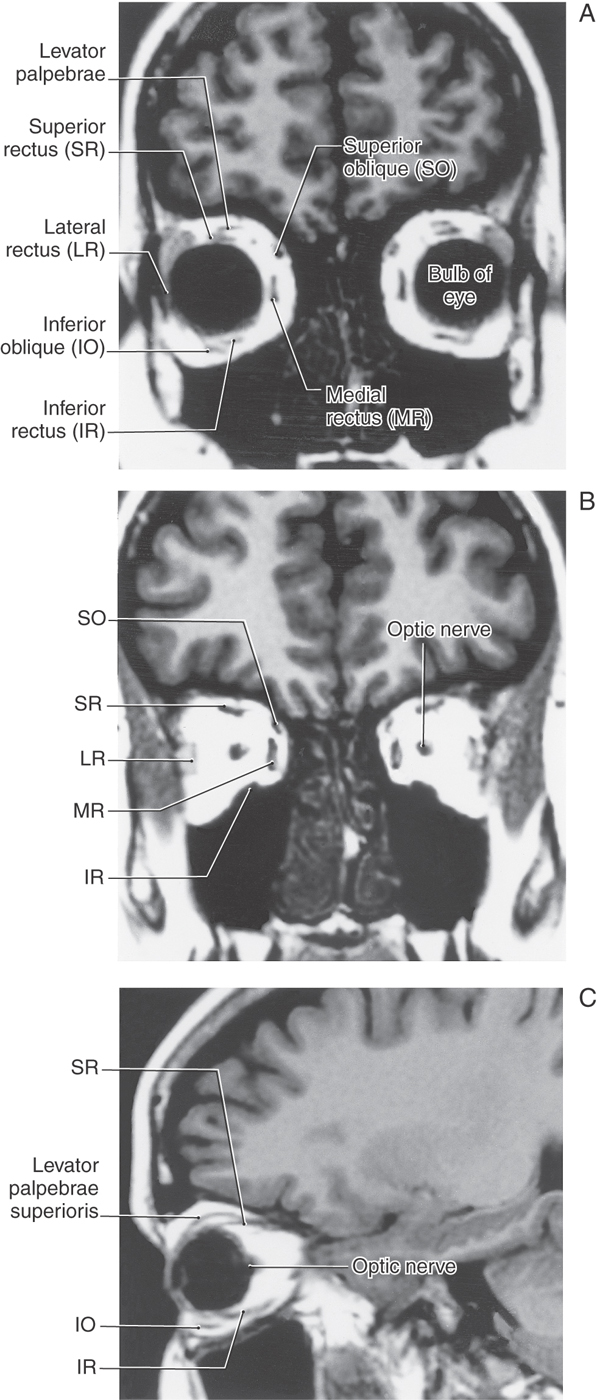
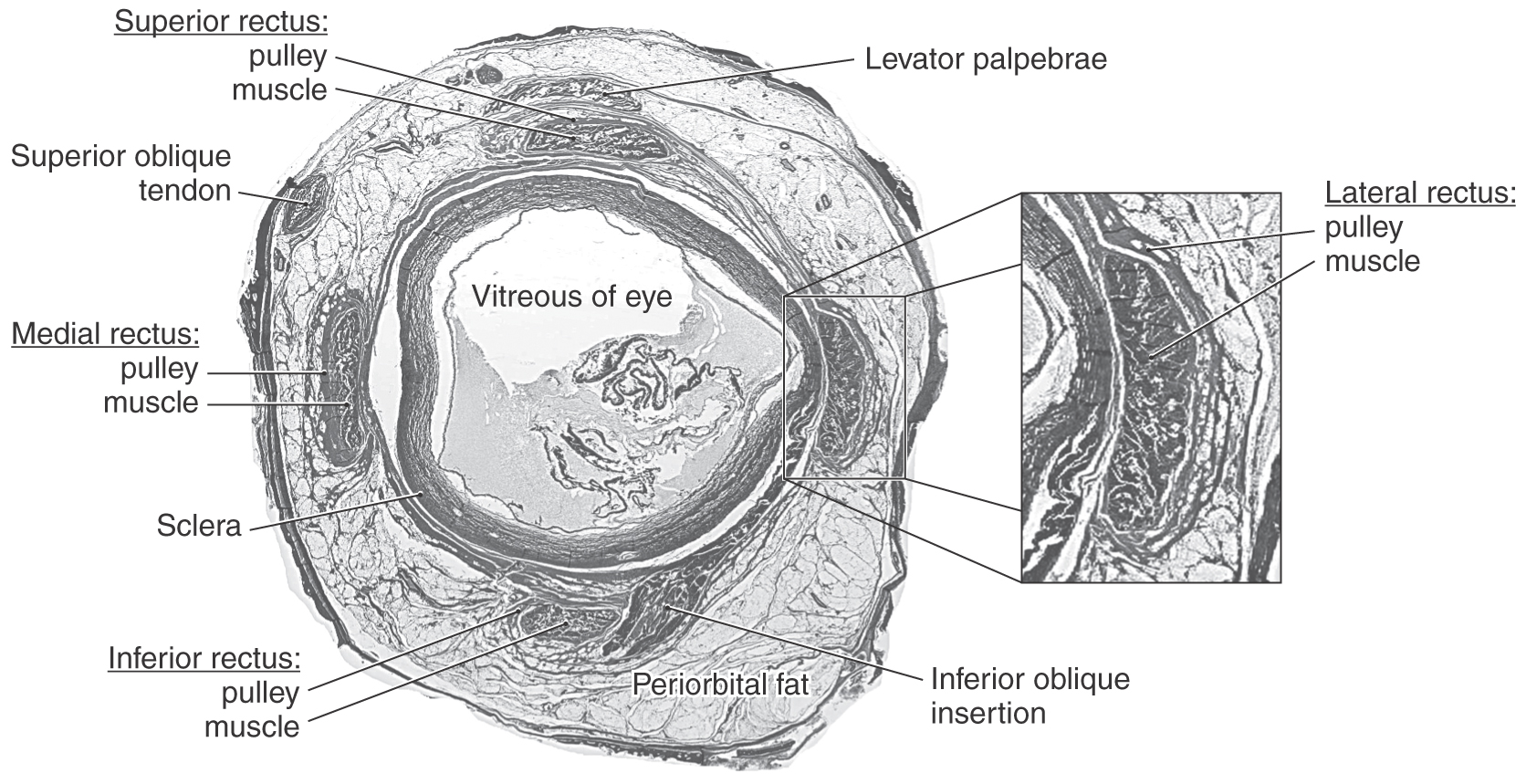
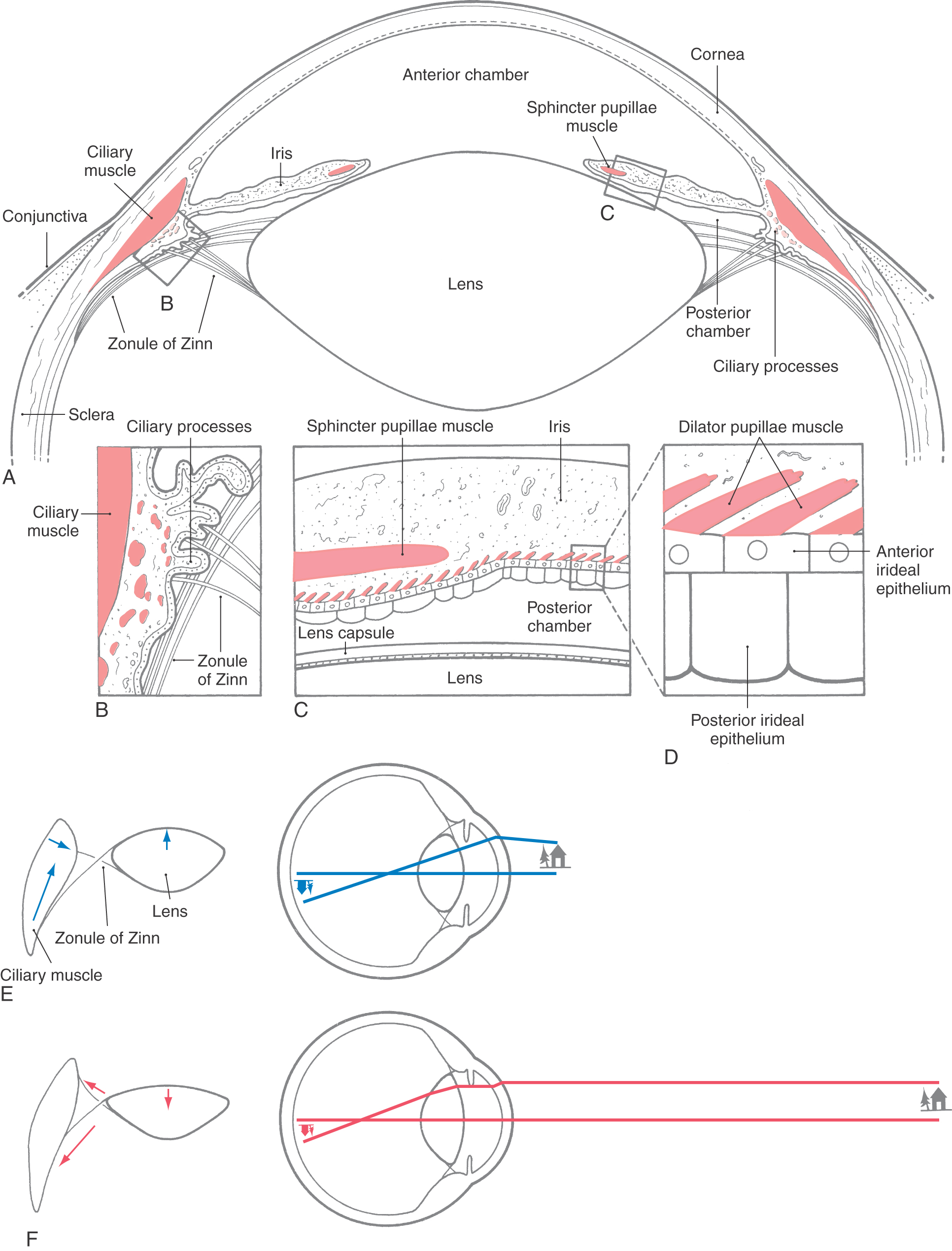
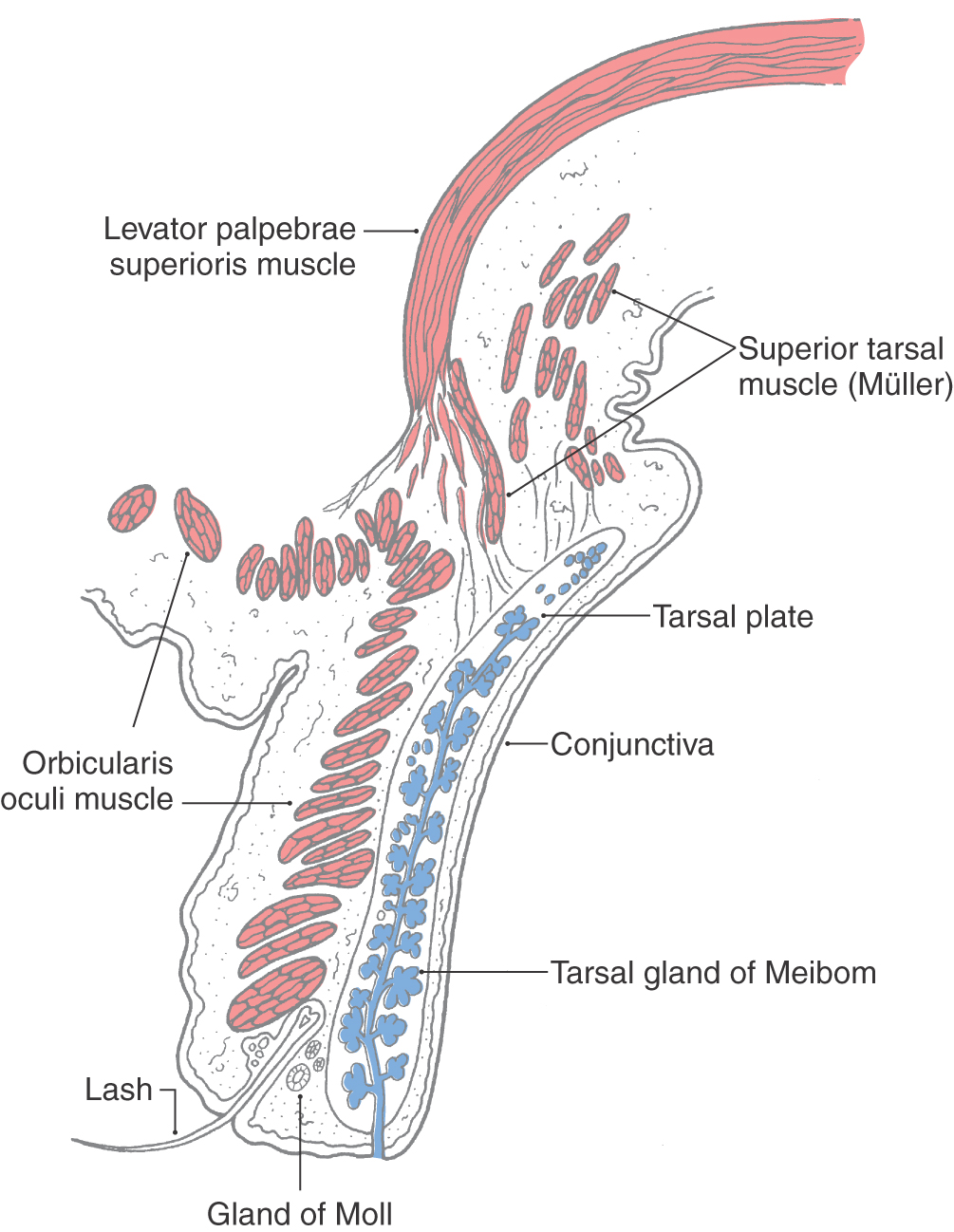
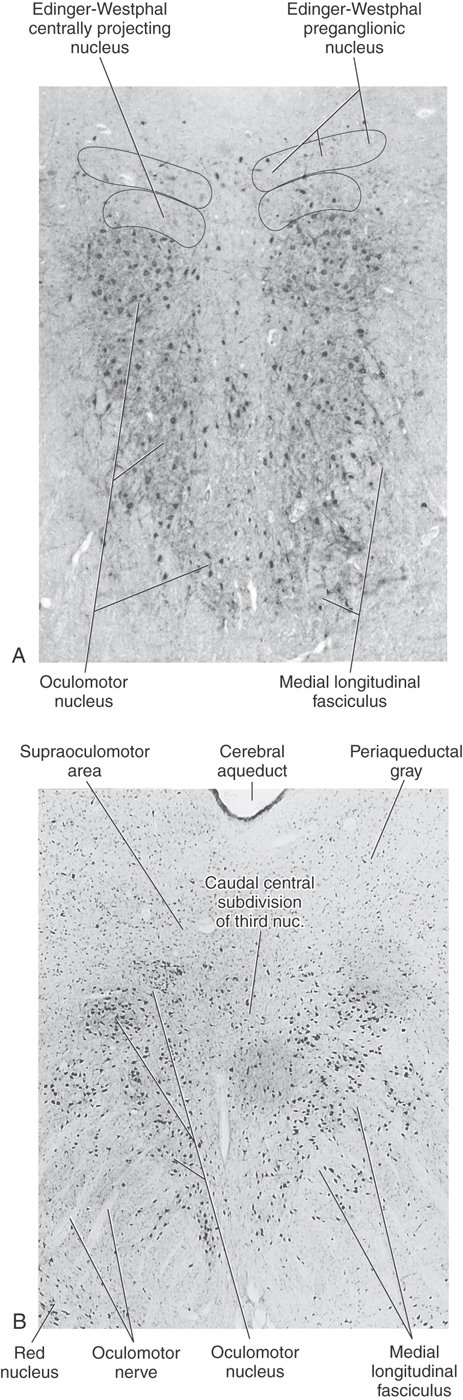
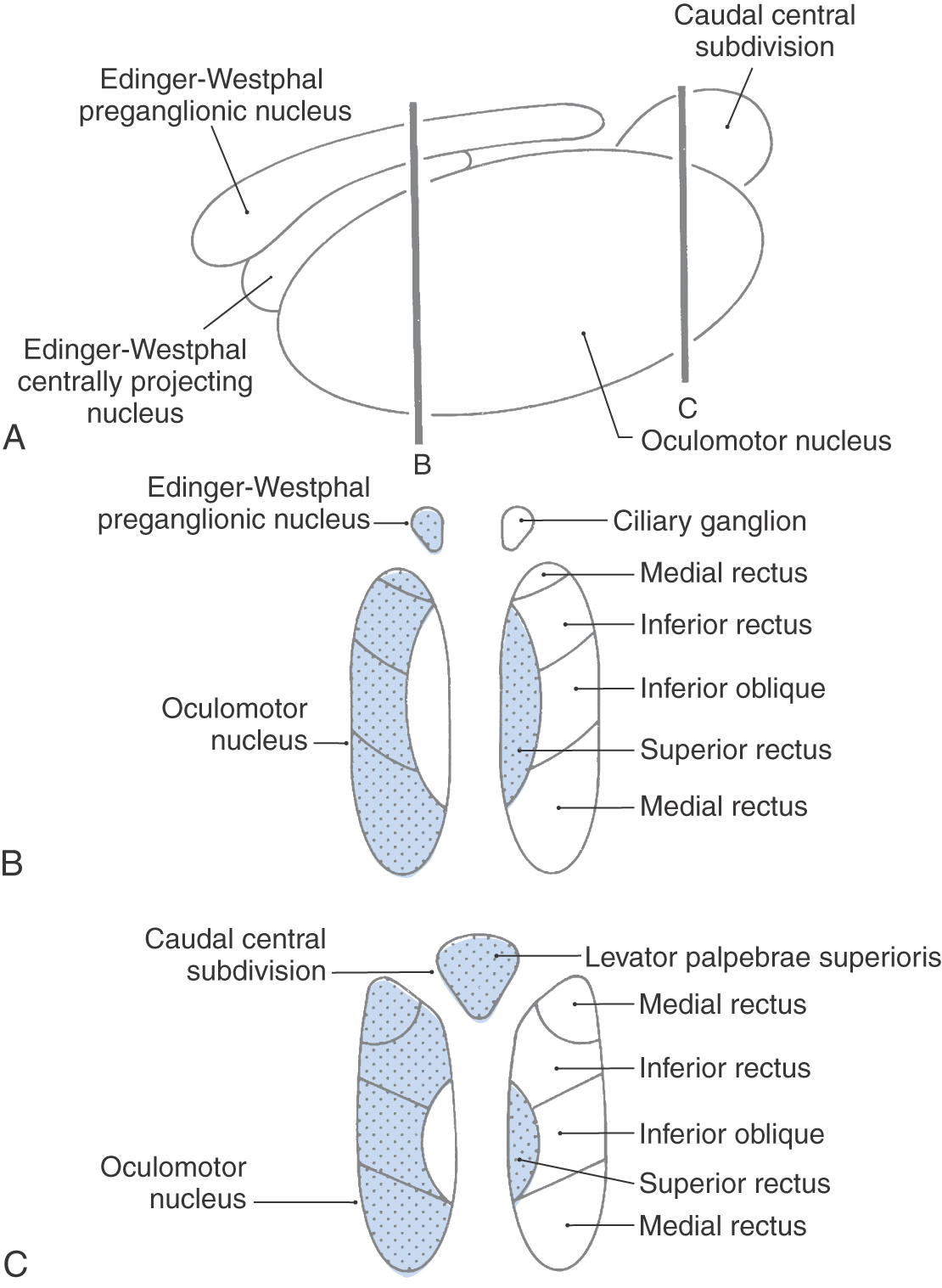
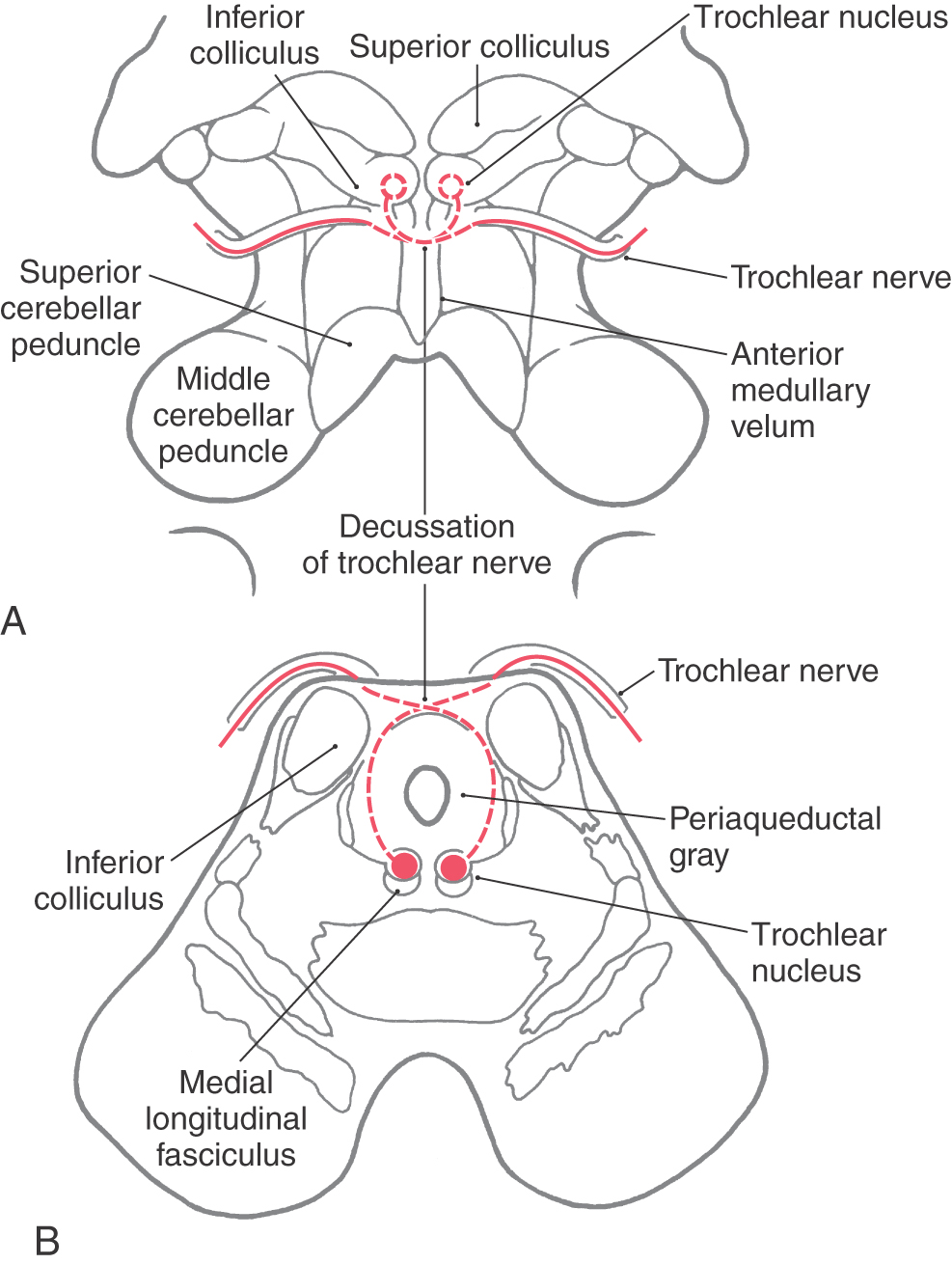
 Figure 28-8.
Figure 28-8. 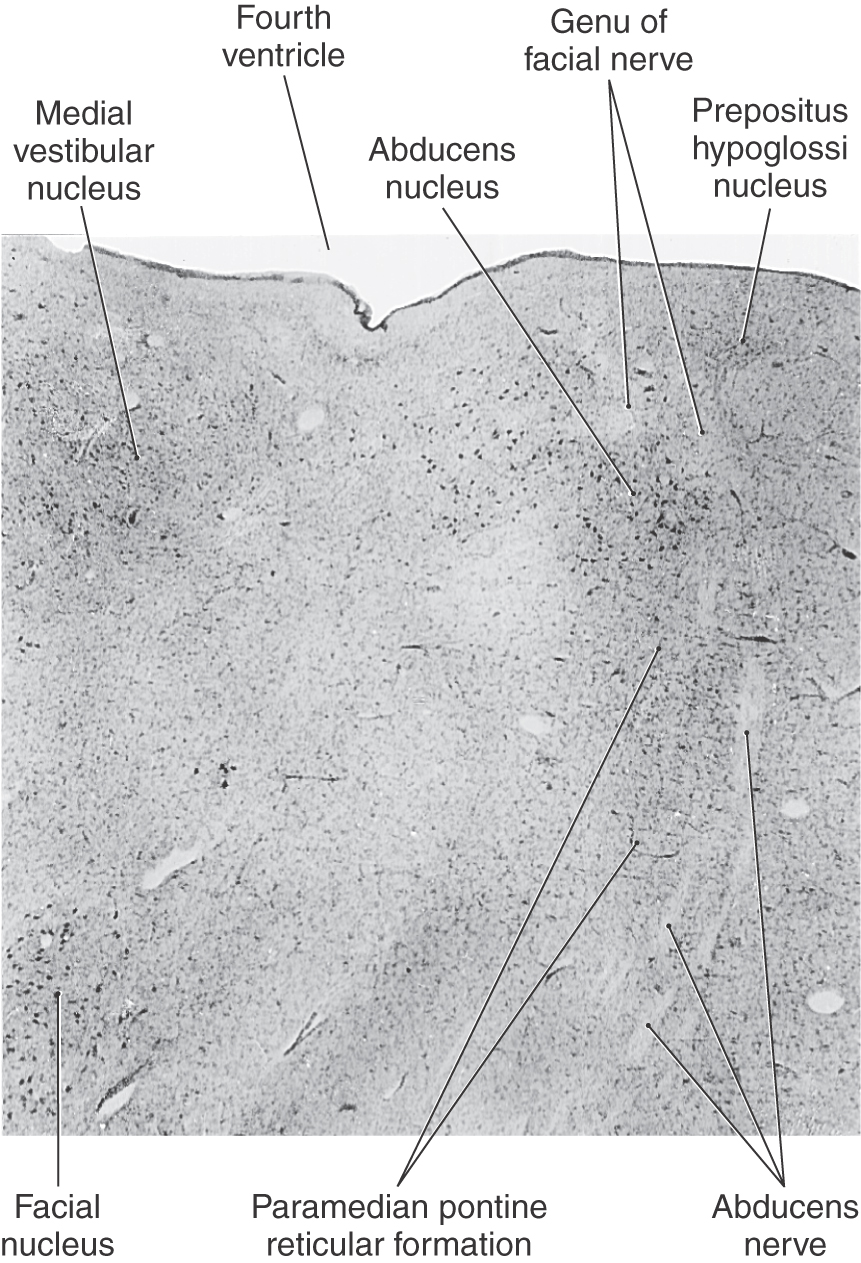
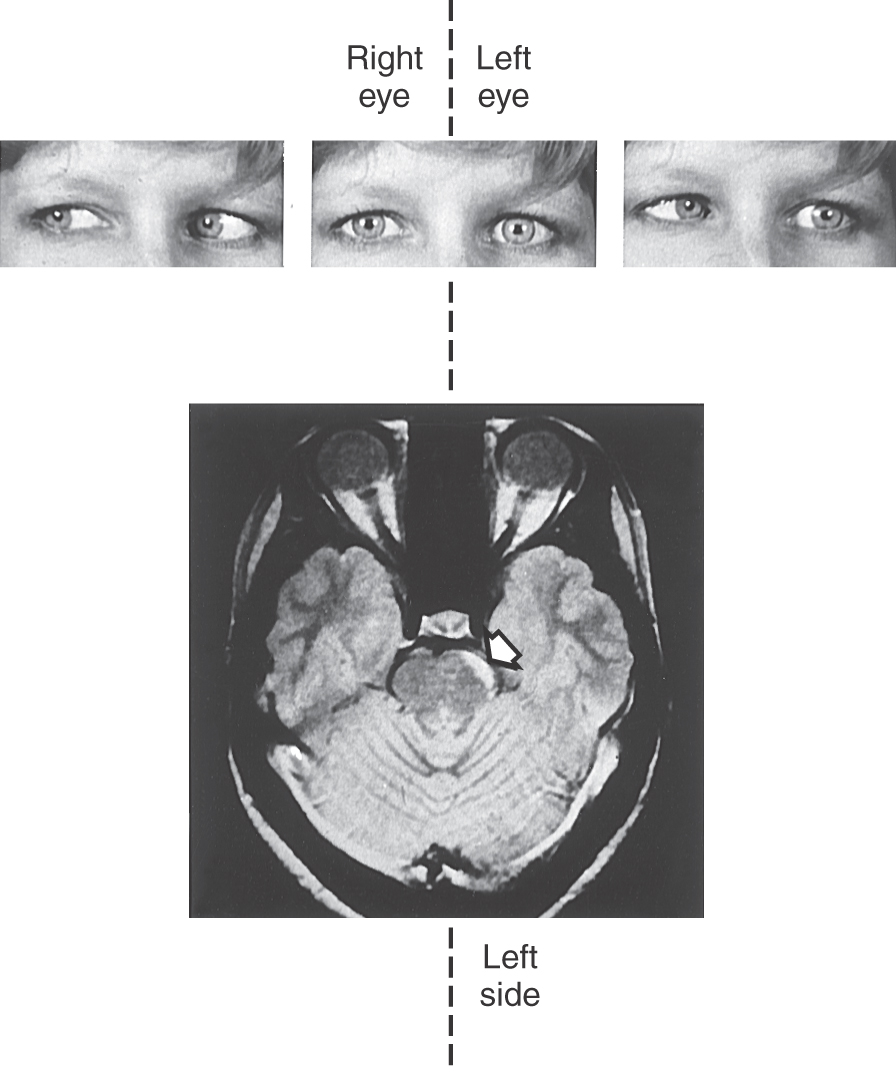
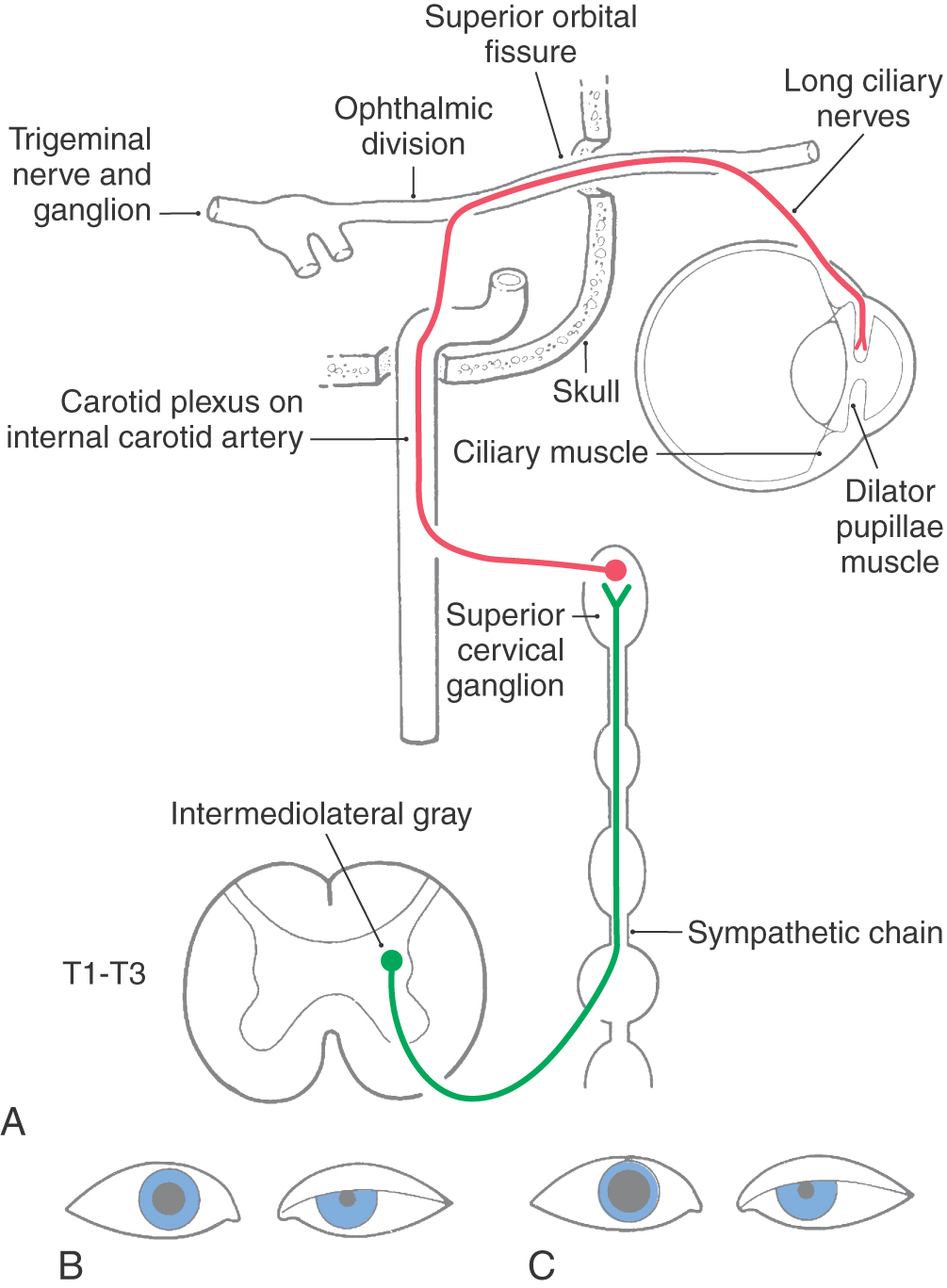
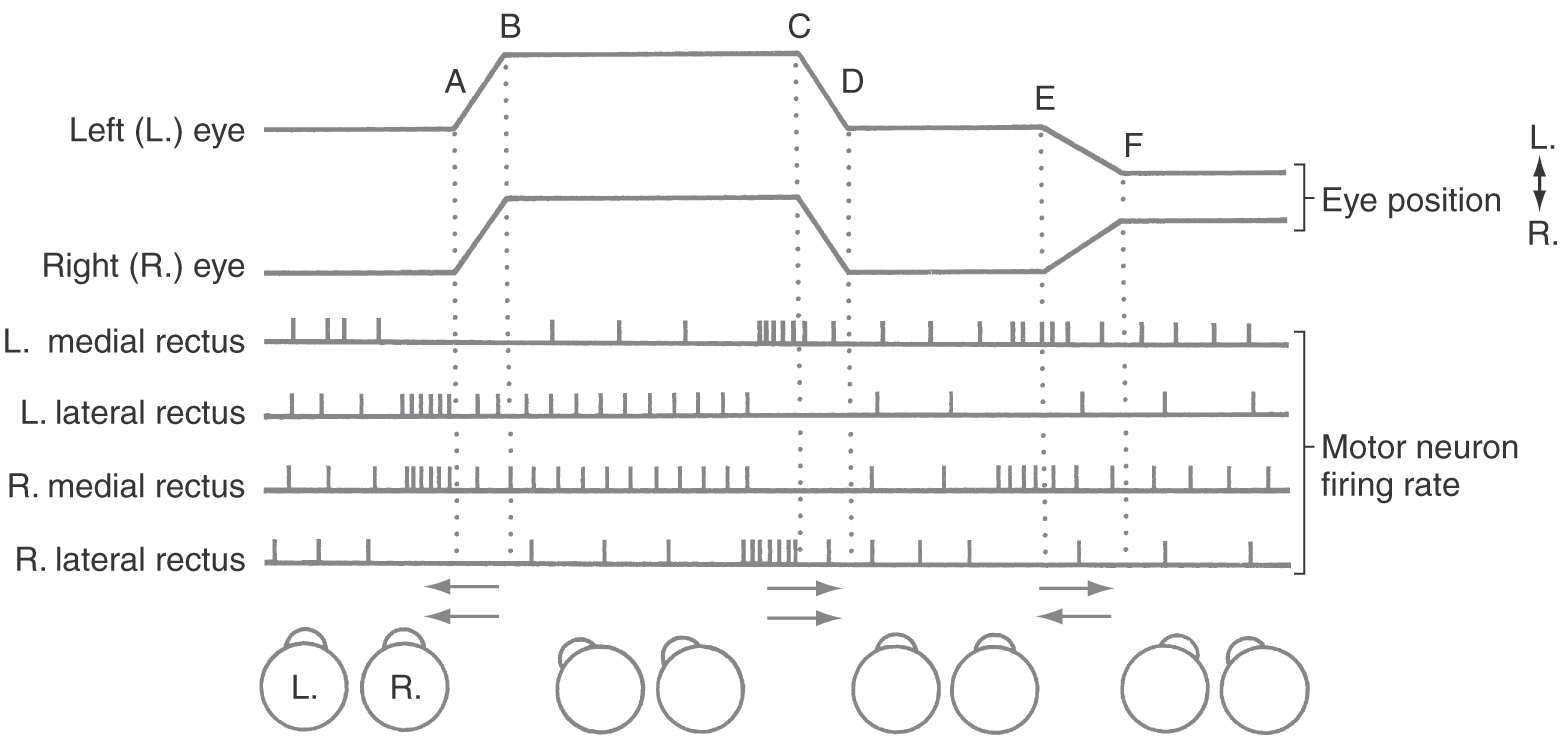
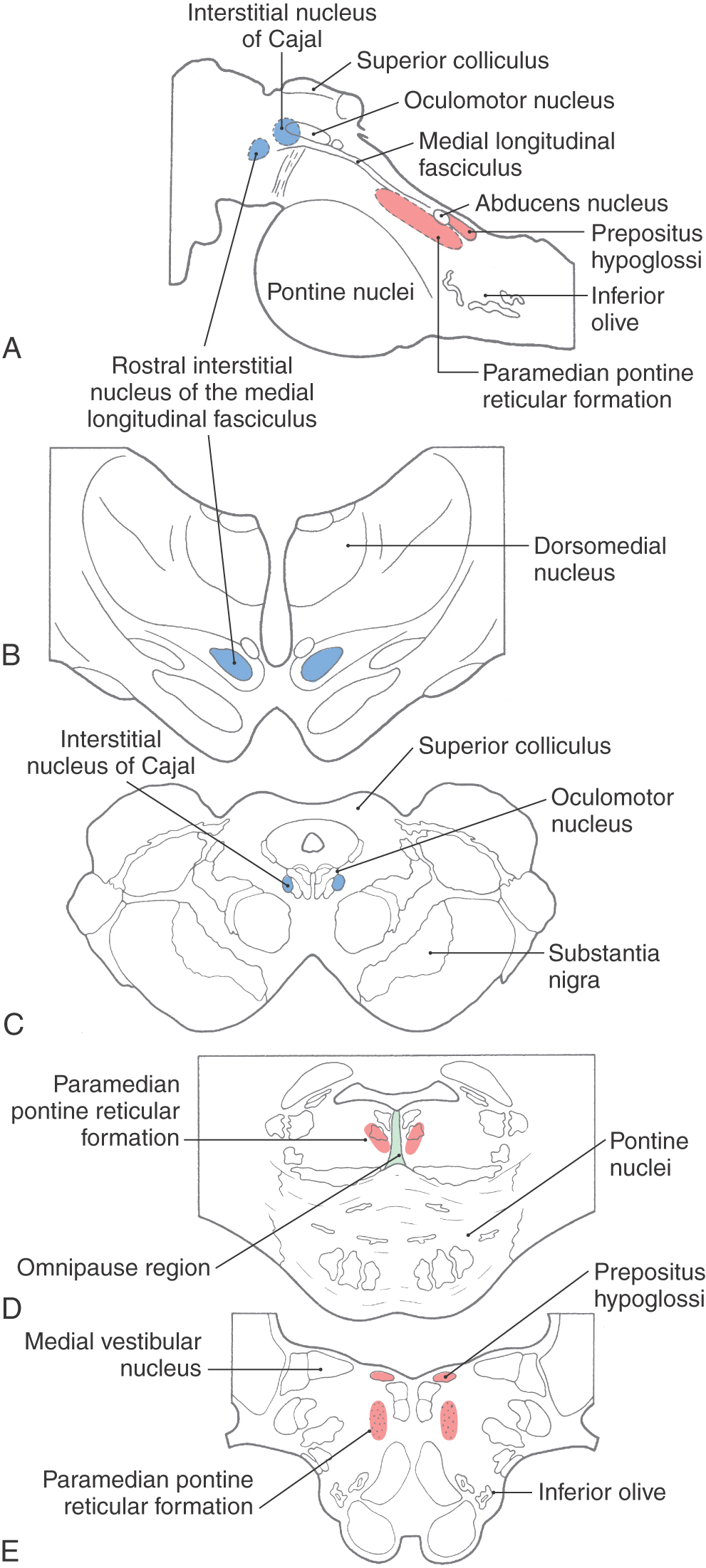
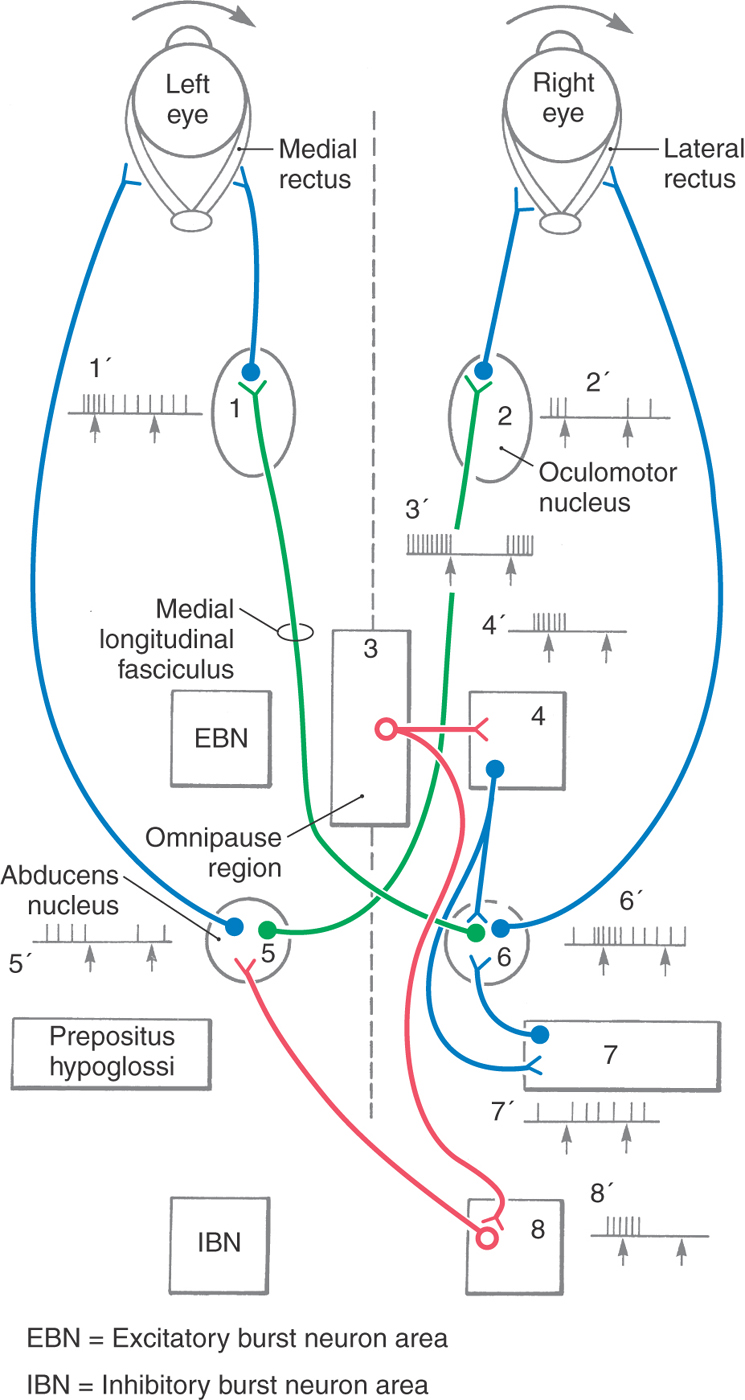
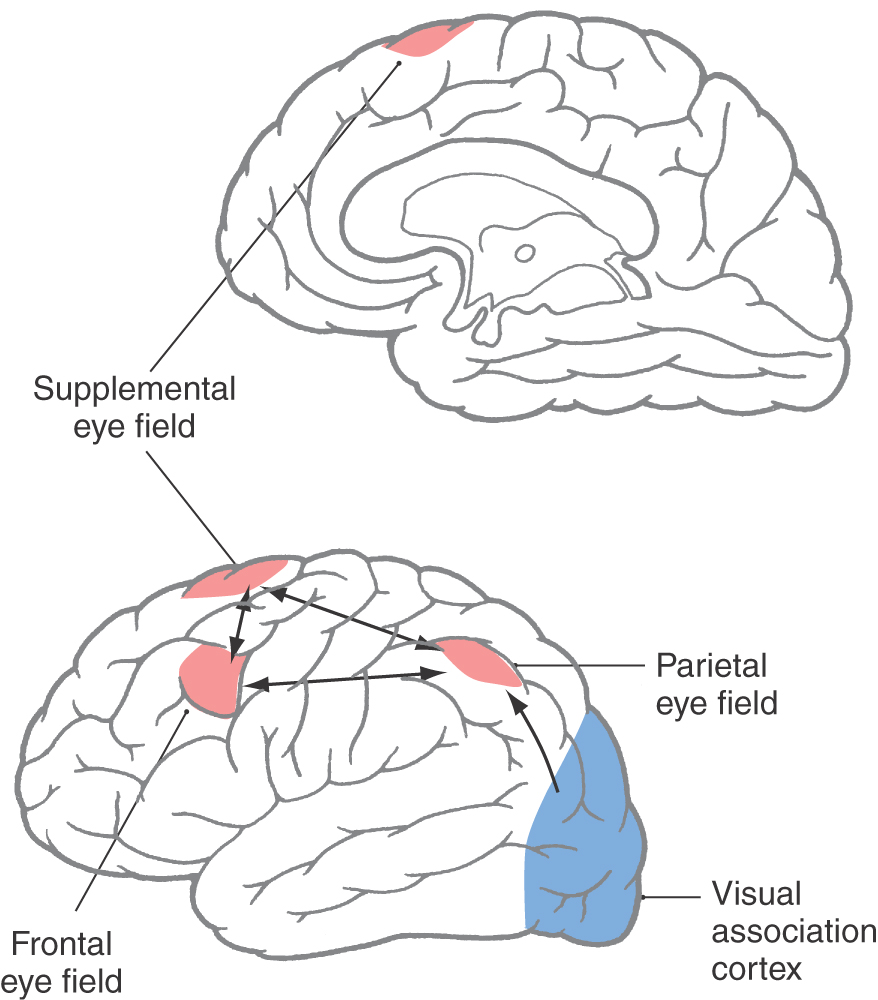
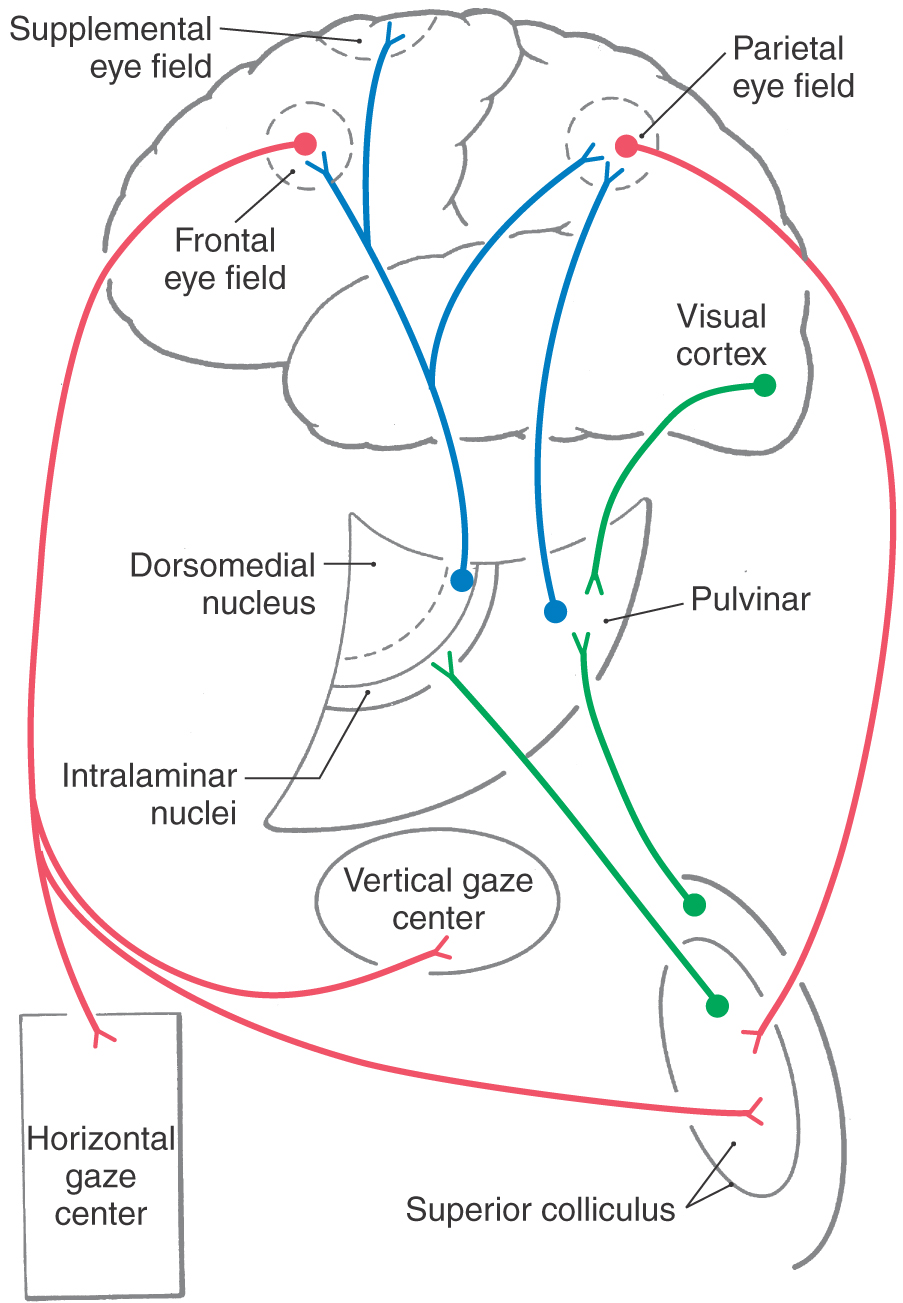
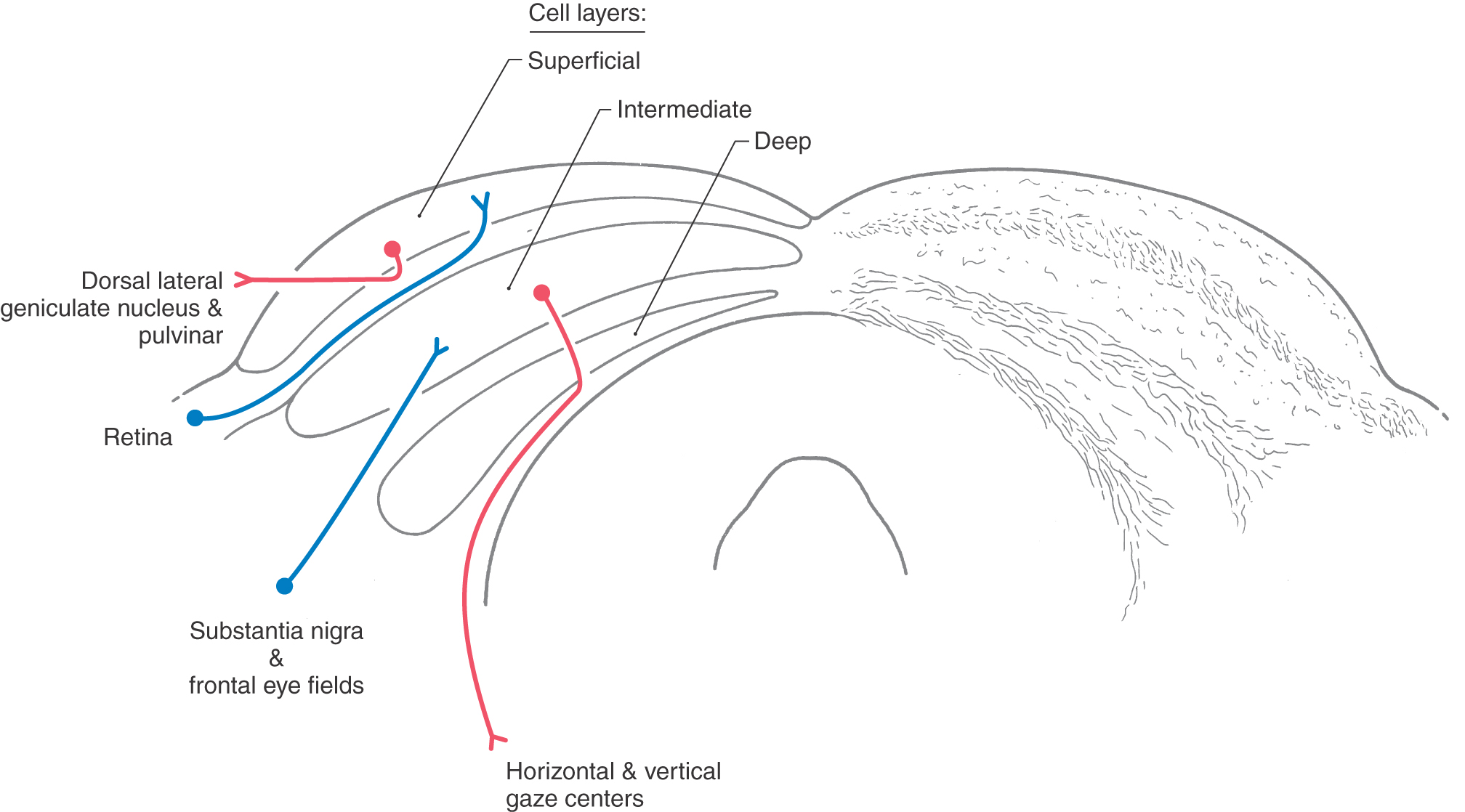
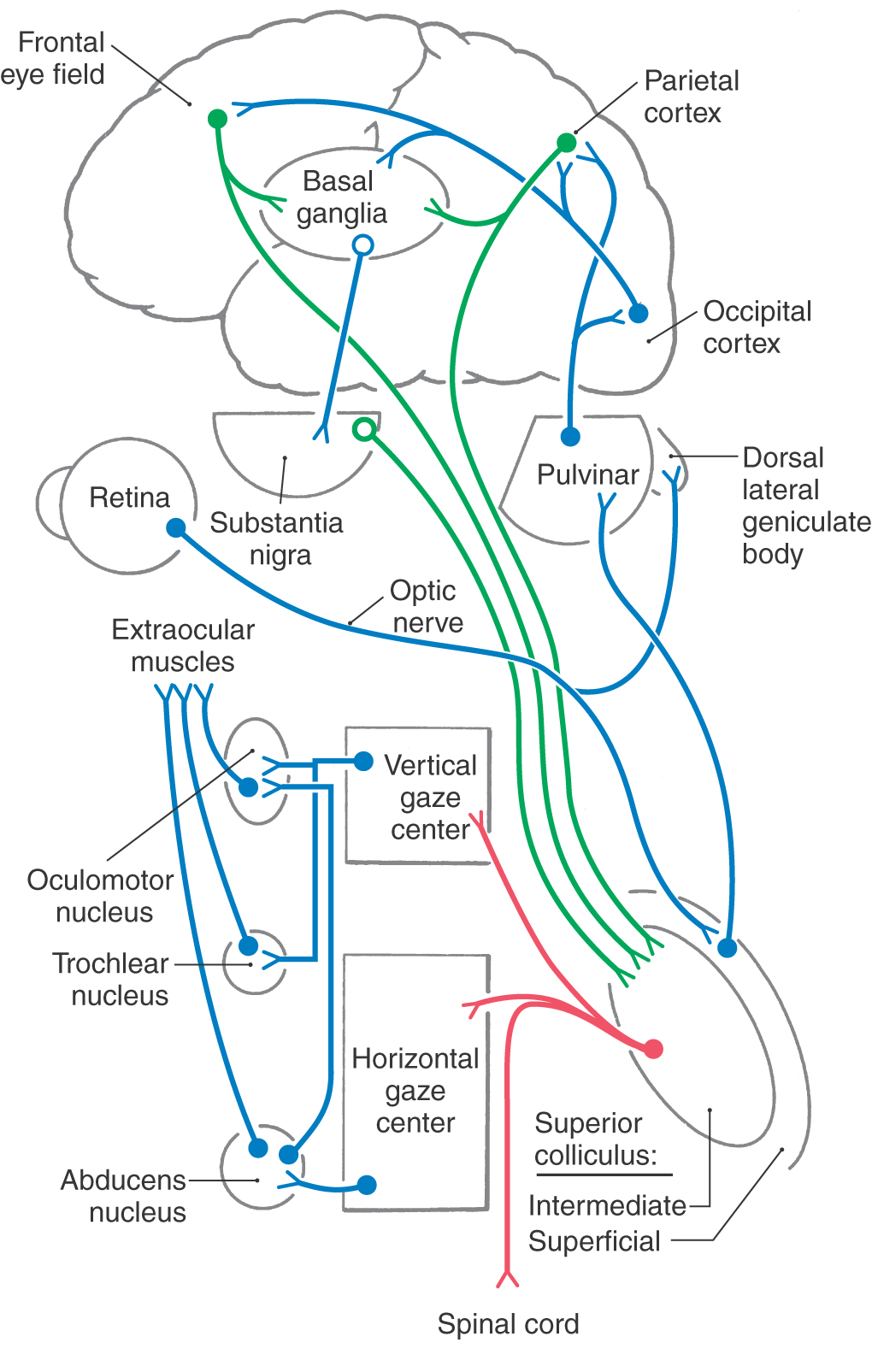
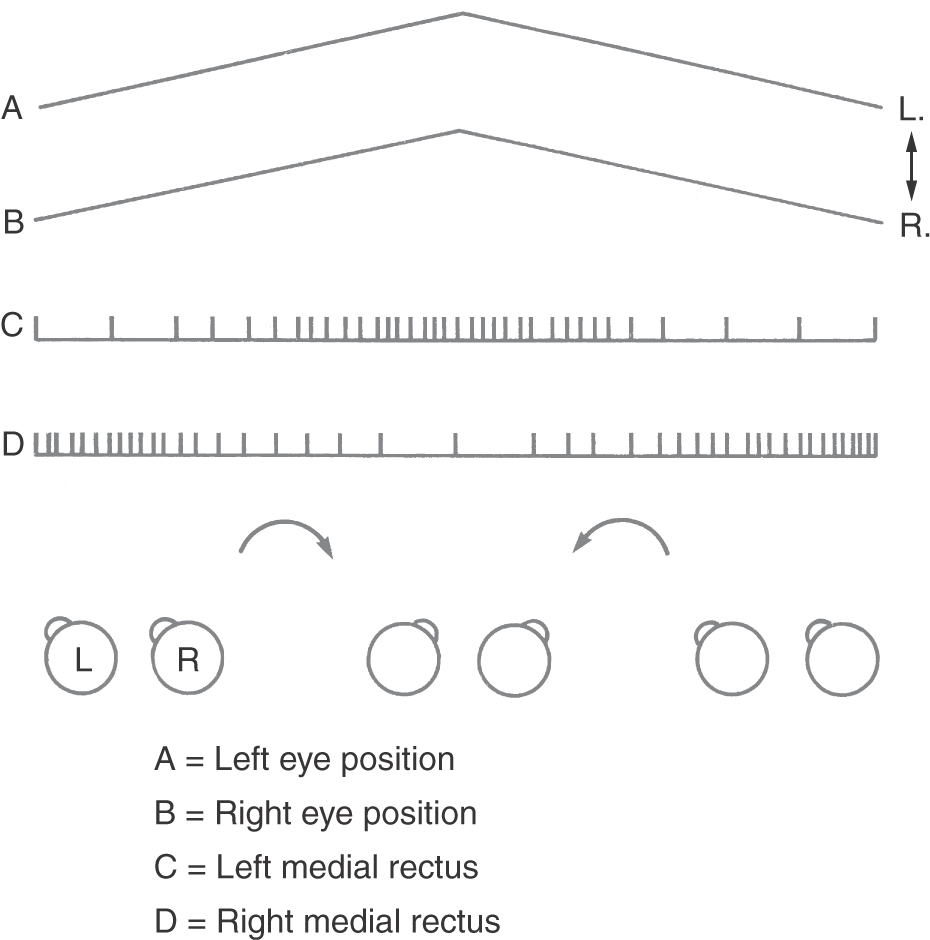
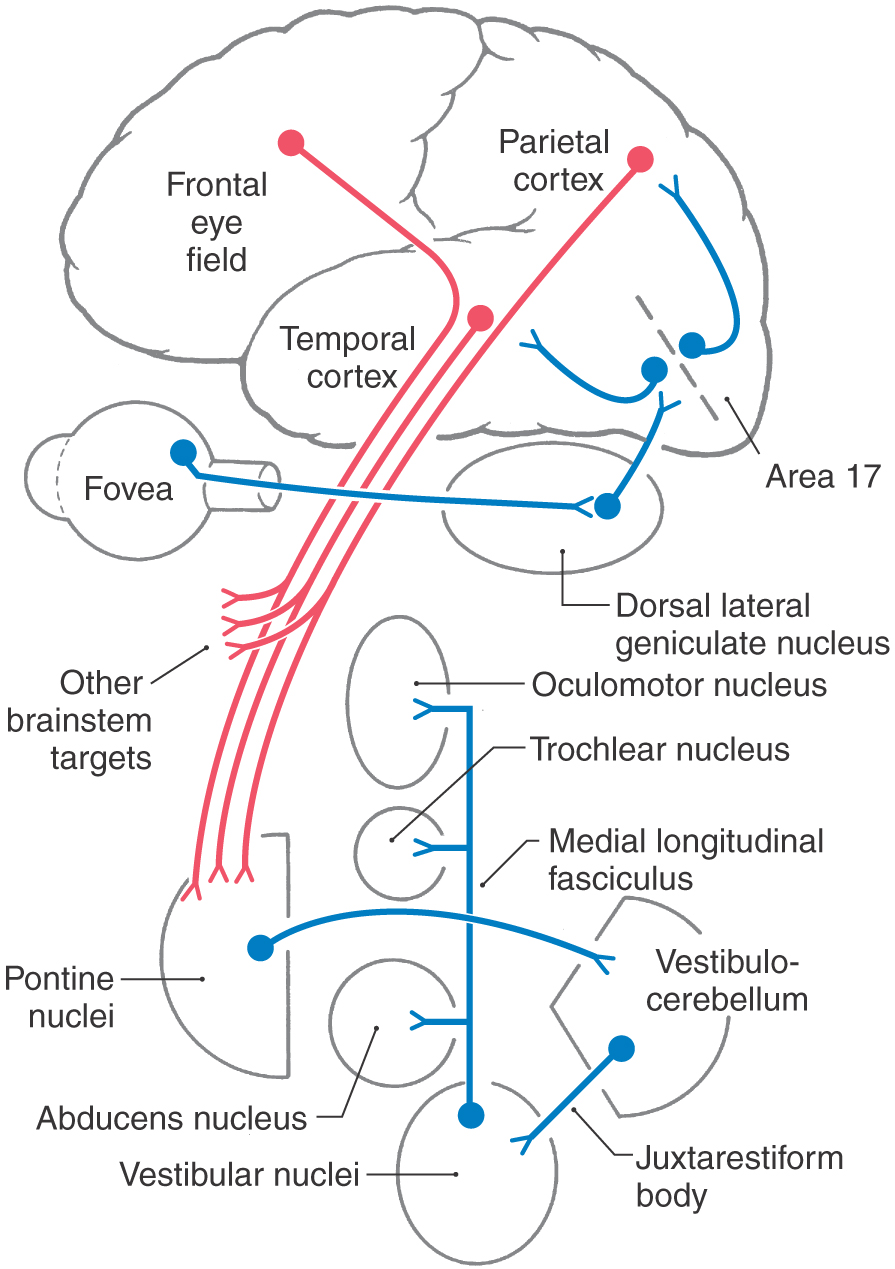
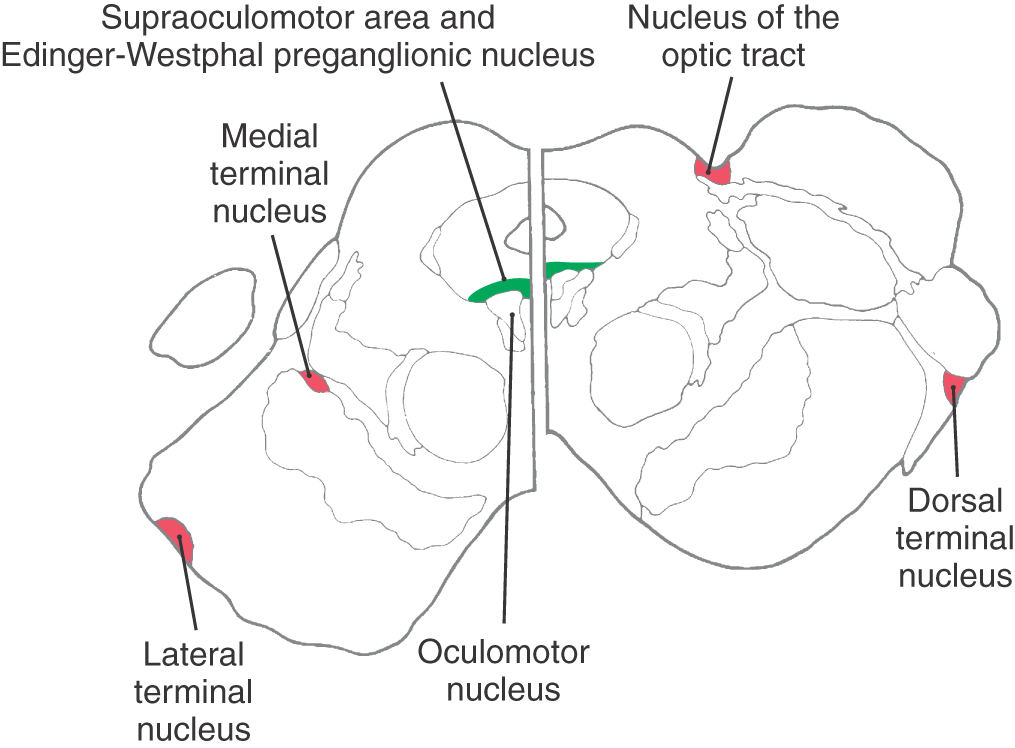
 Figure 28-21.
Figure 28-21.