Chapter 19
Viscerosensory Pathways
Ascending Pathway for Sympathetic Afferents
Projections to Reticular Formation
Pathways for Parasympathetic Afferents
Sacral Parasympathetic Afferents
The somatosensory system conveys information from sensory receptors in the skin, joints, and skeletal muscles that allows one to perceive and respond to stimuli arising either from the external environment or from the position of or the movement of the body. Functioning in parallel with somatosensory pathways are fibers that convey information about the status of visceral organs. This input allows the body to maintain homeostasis by making appropriate responses to changes in its internal environment.
Viscerosensory Receptors
Viscerosensory receptors may be categorized as either nociceptors or physiologic receptors (Table 19-1). Nociceptors in the viscera are the free nerve endings of Aδ and C fibers located in the heart, respiratory structures, gastrointestinal tract, and urogenital tract (Table 19-1). These receptors respond to stimuli that have the potential to damage tissue or to stimuli resulting from the presence of damaged tissue. For example, intense mechanical stimuli (such as overdistention or traction), ischemia, and endogenous compounds (including bradykinin, prostaglandins, and hydrogen and potassium ions) can activate visceral nociceptors and produce pain. These receptors signal changes in visceral structures that result from pathologic processes, such as myocardial ischemia or appendicitis, or from benign conditions, such as gastrointestinal cramping or bloating. Visceral pain is often described as being diffuse and difficult to localize and is frequently referred to an overlying somatic body location (discussed later in this chapter).
Table 19-1 Classification of Nociceptive Receptors and Physiologic Receptors in the Viscera and Their Adequate Stimuli
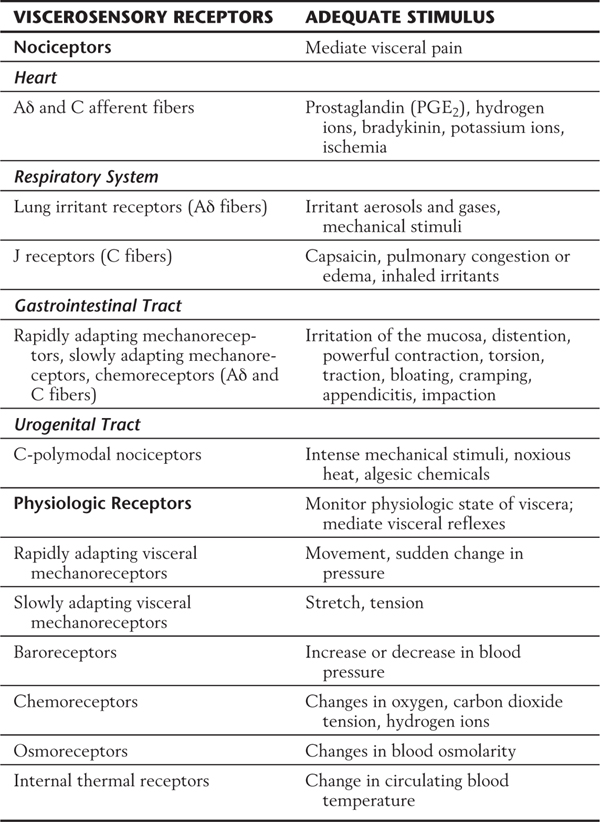
Physiologic receptors are responsive to innocuous stimuli, and they monitor the functions of visceral structures on a continuing basis. These receptors also mediate normal visceral reflexes, such as the baroreceptor reflex. Examples of physiologic receptors are (1) rapidly adapting mechanoreceptors, (2) slowly adapting mechanoreceptors, and (3) various types of specialized receptors.
Rapidly adapting mechanoreceptors (Table 19-1) signal the occurrence of dynamic events, such as movement or sudden changes in pressure. This class of receptor is present in organs of the thoracic, abdominal, and pelvic cavities. In the thoracic cavity, it is represented by free nerve endings that exist in the epithelia of pulmonary airways. Because these nerve endings are sensitive to the presence of inhaled particles, they have been referred to as cough receptors. Rapidly adapting mechanoreceptors in the abdominal and pelvic cavities vary greatly in size and location and may be either unencapsulated or encapsulated. The largest example of a rapidly adapting mechanoreceptor is the Pacinian corpuscle.
Slowly adapting mechanoreceptors (Table 19-1) signal the presence of stretch or tension within a visceral structure. These typically unencapsulated receptors are located in the smooth muscle layer of the pulmonary airways and in the smooth muscle layers of hollow abdominal and pelvic viscera. They provide the afferent limbs of some visceral reflexes, for example, the emptying reflexes of the rectum or bladder. Slowly adapting mechanoreceptors are also essential for the perception of a sense of fullness in certain viscera, such as the stomach or bladder.
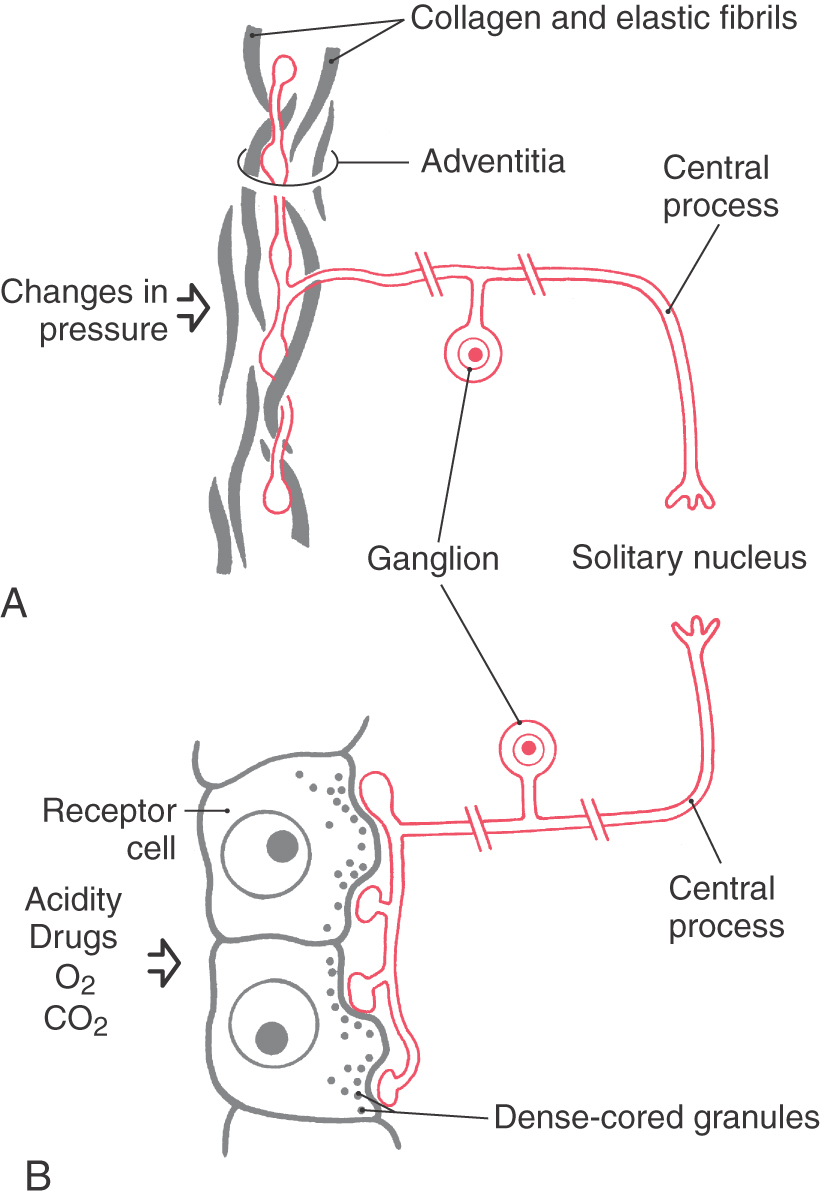
Certain specialized receptors (Table 19-1) are unique to the viscerosensory system. These include baroreceptors, chemoreceptors, osmoreceptors, and internal thermal receptors. Baroreceptors (Fig. 19-1A) are found in the walls of the aortic arch and carotid sinus and respond to rapid increases or decreases in blood pressure. For baroreceptors to effectively perform this task, blood pressure must be in the range of 30 to 150 mm Hg. Chemoreceptors (Fig. 19-1B) are found in structures called carotid bodies (located at the bifurcation of the common carotid artery) and aortic bodies (located in the aortic arch) and are activated by changes in the composition of arterial blood. These changes include alterations in oxygen and carbon dioxide tension and in acidity.
Additional specialized visceroreceptors also reside in the hypothalamus as chemoreceptors, osmoreceptors, and internal thermal receptors. These viscerosensory receptors are activated by changes in blood chemistry or osmolarity or by changes in the temperature of blood circulating through the hypothalamus. Hypothalamic neurons that respond to these changes by altering their firing rates are considered to be the “receptor” cells.
VISCEROSENSORY FIBERS
Sympathetic and parasympathetic divisions of the autonomic (visceral motor) nervous system (see Chapter 29) have traditionally been considered to consist of only visceromotor (visceral efferent [VE]) fibers. These fibers travel through sympathetic nerves (such as splanchnic and cardiac nerves) or through parasympathetic nerves (such as vagus and pelvic nerves). However, these sympathetic and parasympathetic nerves also contain viscerosensory (visceral afferent [VA]) fibers that serve many important functions. In this chapter, the terms “sympathetic afferent” and “parasympathetic afferent” are used to describe viscerosensory fibers contained in sympathetic and parasympathetic nerves, respectively. In addition to its conciseness, this usage complies with the terminology introduced by Langley, a pioneer in studies on the autonomic nervous system.
Visceral afferents tend to predominate in parasympathetic nerves but are comparatively sparse in sympathetic nerves. For example, more than 80% of the fibers in the vagus nerve (a parasympathetic nerve) are viscerosensory, whereas less than 20% of the fibers in the greater splanchnic nerve (a sympathetic nerve) are visceral afferents. Most visceral afferents (90%; both sympathetic and parasympathetic) are either unmyelinated or thinly myelinated and therefore are slowly conducting fibers.
There is a division of responsibility between parasympathetic and sympathetic nerves in terms of viscerosensory input. Information originating from physiologic receptors (innocuous input) is conveyed primarily by fibers contained in parasympathetic nerves. In contrast, input from nociceptors is conducted almost exclusively by sympathetic nerves. This feature can be exploited in treatment of intractable pain arising from disease of the abdominal viscera. For example, injection of an agent that blocks action potentials on nerve fibers passing through the celiac plexus can, in some cases, relieve the pain associated with terminal cancer of the pancreas.
ASCENDING PATHWAY FOR SYMPATHETIC AFFERENTS
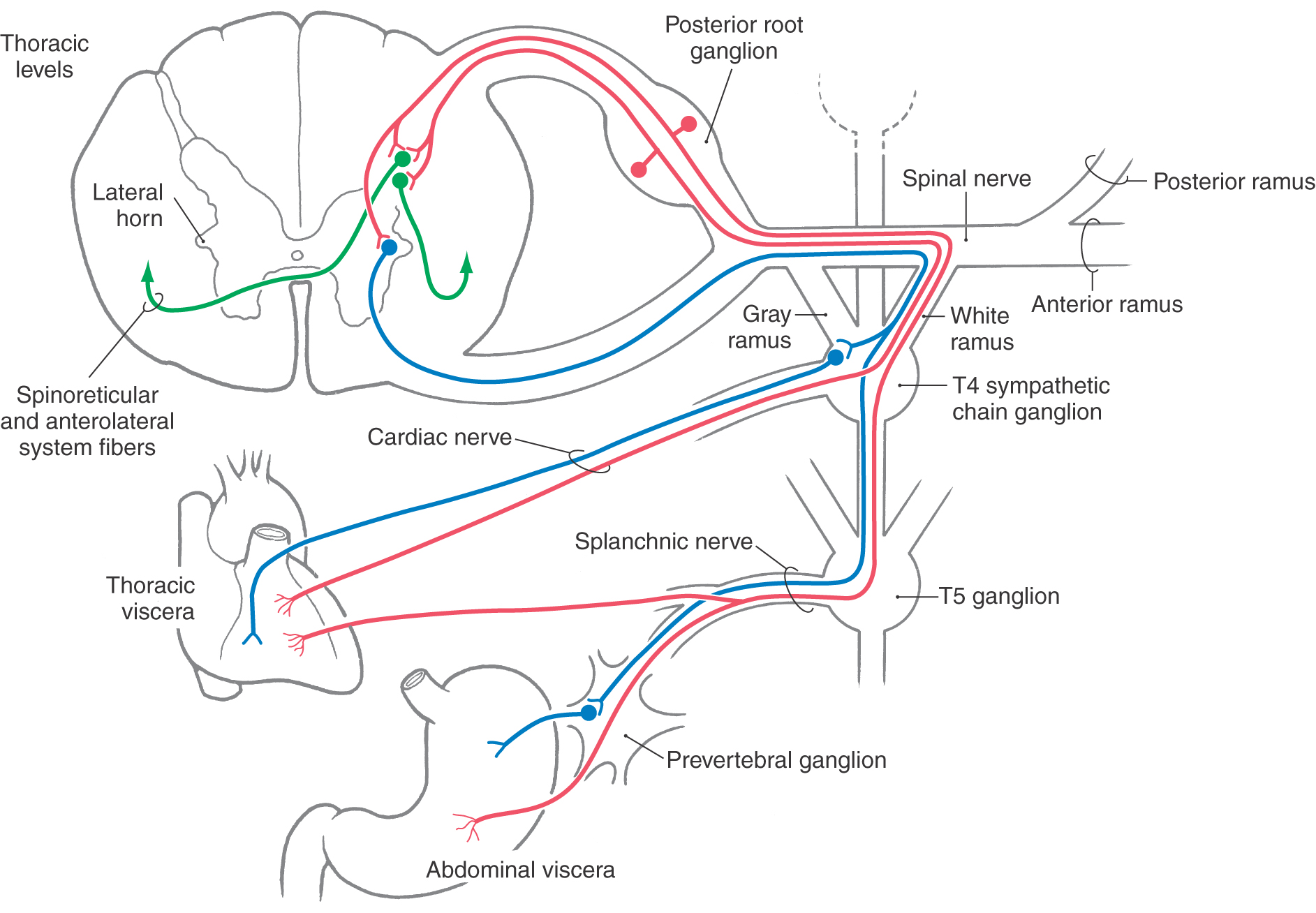
Afferent fibers conveying nociceptive information from thoracic and abdominal viscera travel via the cardiac and splanchnic nerves (Fig. 19-2). For example, primary sensory fibers that originate from the stomach join the greater splanchnic nerve, enter the sympathetic trunk, and pass through a white ramus to join the spinal nerve. Nociceptive input from pelvic viscera such as the prostate and sigmoid colon is conveyed by viscerosensory fibers traveling through the hypogastric plexus and lumbar splanchnic nerves.
The cell bodies of origin of sympathetic afferent fibers are located in posterior root ganglia at about levels T1 to L2 (Fig. 19-2). The central processes of these fibers enter the spinal cord via the lateral division of the posterior root. They may ascend or descend one or two spinal levels in the posterolateral fasciculus before terminating in laminae I and V or laminae VII and VIII. Cells in laminae I and V project mainly to the contralateral side as part of the anterolateral system (ALS), whereas the neurons in laminae VII and VIII project bilaterally as spinoreticular fibers. In addition, some primary viscerosensory fibers terminate on preganglionic sympathetic cell bodies located in the intermediolateral cell column at spinal levels T1 to L2 (Fig. 19-2). The axons of these cells in turn exit through the anterior root as VE preganglionic sympathetic fibers.
In general, viscerosensory fibers that enter the spinal cord at a particular level originate from structures that receive VE input from the same spinal level (Fig. 19-2). For example, visceral afferent fibers from the stomach enter the spinal cord over the posterior roots of T5 to T9 and terminate in the same spinal segments that convey visceral efferent outflow to the stomach.
Projections to Thalamus
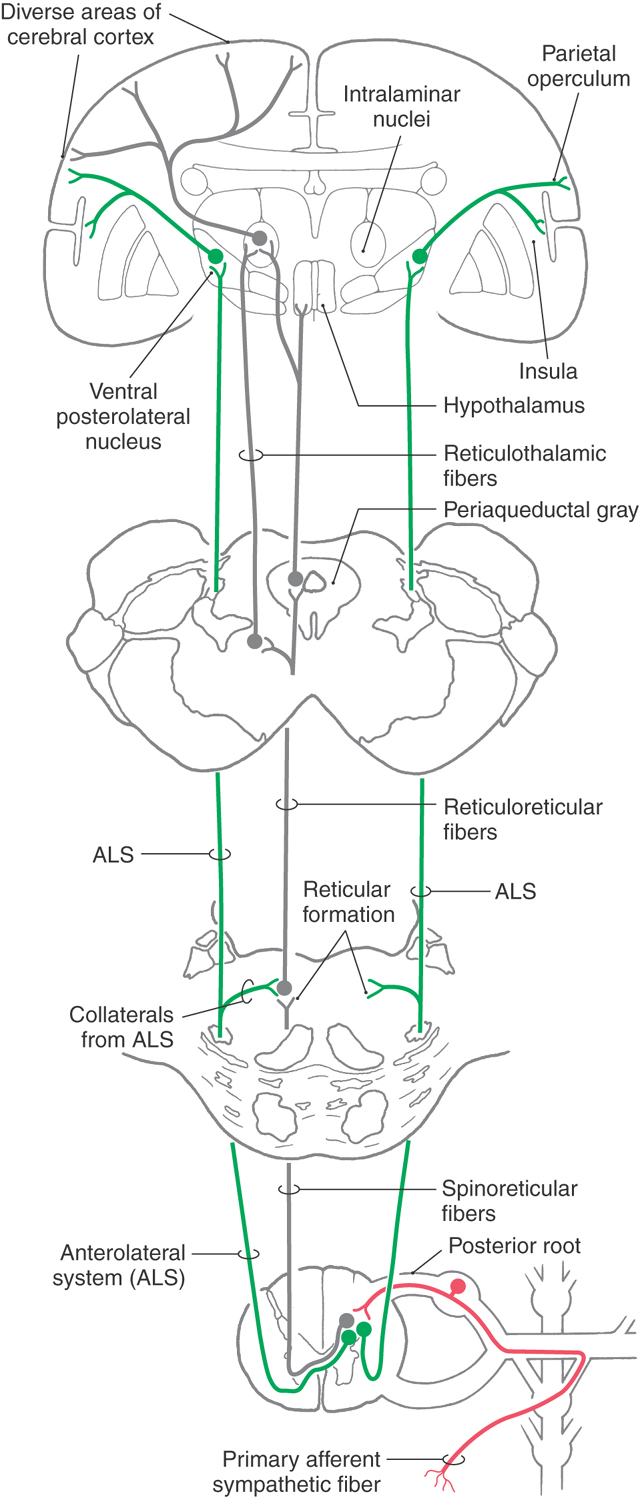
Some neurons located in laminae I and V receive nociceptive input from sympathetic afferent fibers and send their axons rostrally via two routes in the ALS (Fig. 19-3). Some fibers cross in the anterior white commissure and ascend in the ALS, whereas others ascend in this bundle on the ipsilateral side. These ALS fibers terminate in the ventral posterolateral nucleus (VPL) of the thalamus, which in turn projects to the inferolateral part of the postcentral gyrus (the parietal operculum) and to the insular cortex (Fig. 19-3). The location from which this visceral nociceptive information originated is encoded in these particular regions of the cerebral cortex. However, visceral pain is poorly localized (lacks detailed point-to-point representation) because receptor density is low and receptive fields are correspondingly large and because this input converges in the pathway. Consequently, it is not possible to distinguish, for example, whether perceived pain is coming from the stomach or the duodenum; rather, it can be determined only that the pain is coming from the general area of the upper abdomen (epigastric pain).
Projections to Reticular Formation
In addition to the direct path to the thalamus and sensory cortex via the ALS and VPL, there are indirect routes via the reticular formation through which visceral nociceptive information can reach the cortex. The reticular formation receives spinoreticular inputs (mainly from laminae VII and VIII) and collaterals from the ALS (Fig. 19-3). In turn, cells of the reticular formation project to progressively higher levels of the neuraxis, thus relaying viscerosensory information in a multisynaptic fashion to progressively higher levels of the brain. Neurons located in the reticular formation and in the periaqueductal gray ultimately project to the hypothalamus and to the intralaminar nuclei of the thalamus (Fig. 19-3). These latter cell groups project to the cortex.
Reticulohypothalamic fibers travel via the dorsal longitudinal fasciculus, the mammillary peduncle, and the medial forebrain bundle. The first originates mainly from the periaqueductal gray, and the last two originate mainly from the mesencephalic reticular formation. These midbrain centers receive both viscerosensory and somatosensory input, and through their projections, hypothalamic centers may be influenced by either system. For example, viscerosensory input resulting from distention of the bowel may result in increased heart rate or cutaneous flushing. On the other hand, somatosensory stimuli such as those associated with coitus or suckling may increase the release of the hypothalamic hormone oxytocin.
Referred Pain
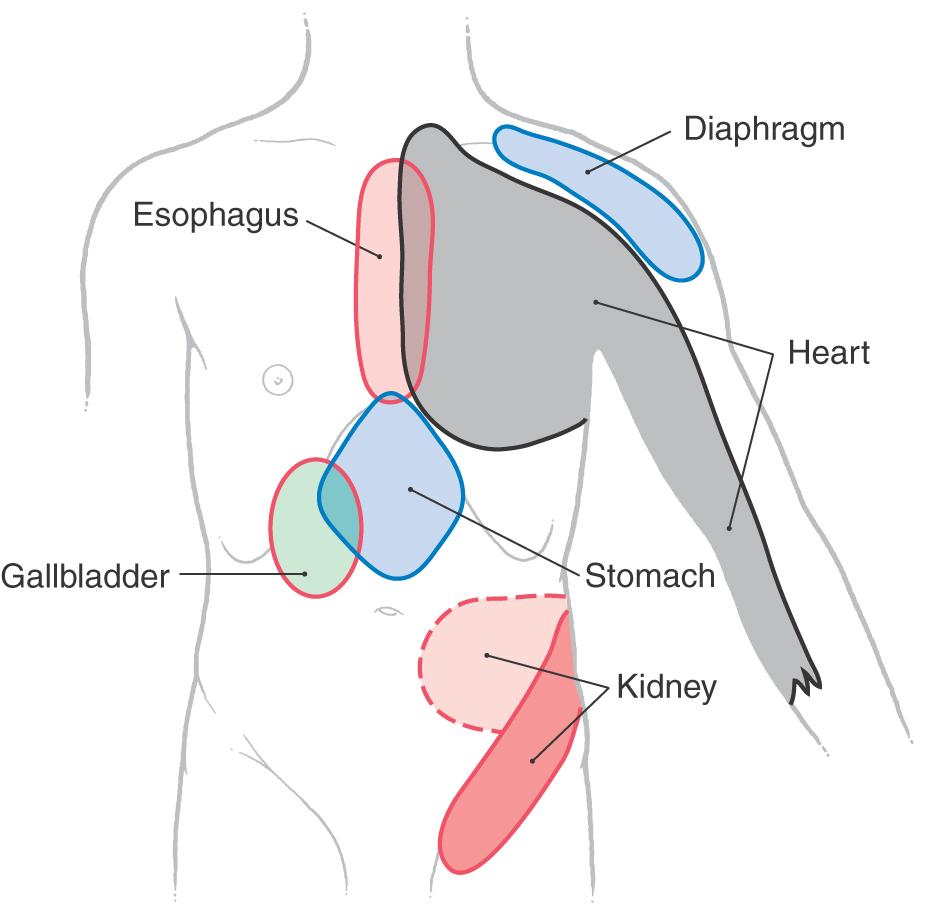
Referred pain is the phenomenon whereby noxious stimuli that originate in a visceral structure, such as the heart or the stomach, are perceived by the patient as pain arising from a somatic portion of the body wall, such as the skin, bones, or skeletal muscles (Fig. 19-4). Although such referral of pain may mask the true origin of the information, certain patterns of referred pain are useful in the clinical setting because they are clearly diagnostic of diseases in particular visceral locations. For example, pain in the chest (sometimes perceived as intense pressure) that radiates down the left arm may be indicative of a serious heart problem. A stomach condition may be perceived as pain in the epigastric region. Visceral pain is transmitted by sympathetic sensory fibers and is typically referred to those somatic structures whose afferents enter the cord via the same posterior roots.
Figure 19-4. Superficial areas to which pain is commonly referred from the corresponding deep structures.
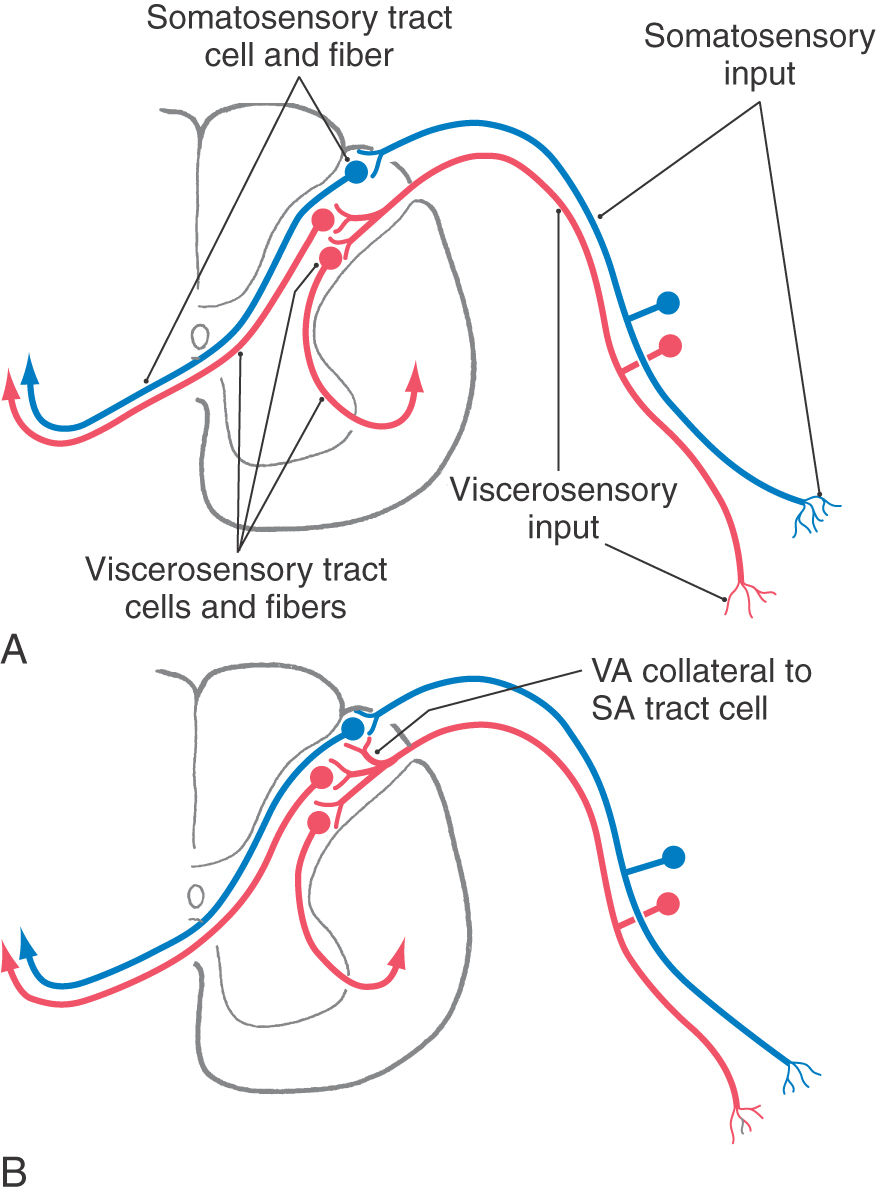
The mechanism underlying referred pain is thought to involve a convergence of somatic and visceral afferent information onto pools of posterior horn neurons, the axons of which ascend to higher levels of the neuraxis (Fig. 19-5). Normally, a visceral nociceptive fiber (e.g., from the heart) synapses on spinothalamic tract cells whose axons project to the VPL, and from there the information is relayed to visceral parts of the sensory cortex. Consequently, this sensory input is perceived as arising from deep within one of the body’s cavities (e.g., the thoracic cavity) (Fig. 19-5A). However, collaterals of visceral afferent fibers may also synapse on and excite posterior horn tract cells that usually transmit only somatosensory information (Fig. 19-5B). In this case, the tract cells are activated by visceral afferent collaterals but send information via the VPL to a part of the somatosensory cortex that represents the body wall (Fig. 19-5B). Consequently, the pain is referred to (interpreted as coming from) the surface of the body (Fig. 19-4) even though the stimulus actually originated from a visceral structure.
Angina
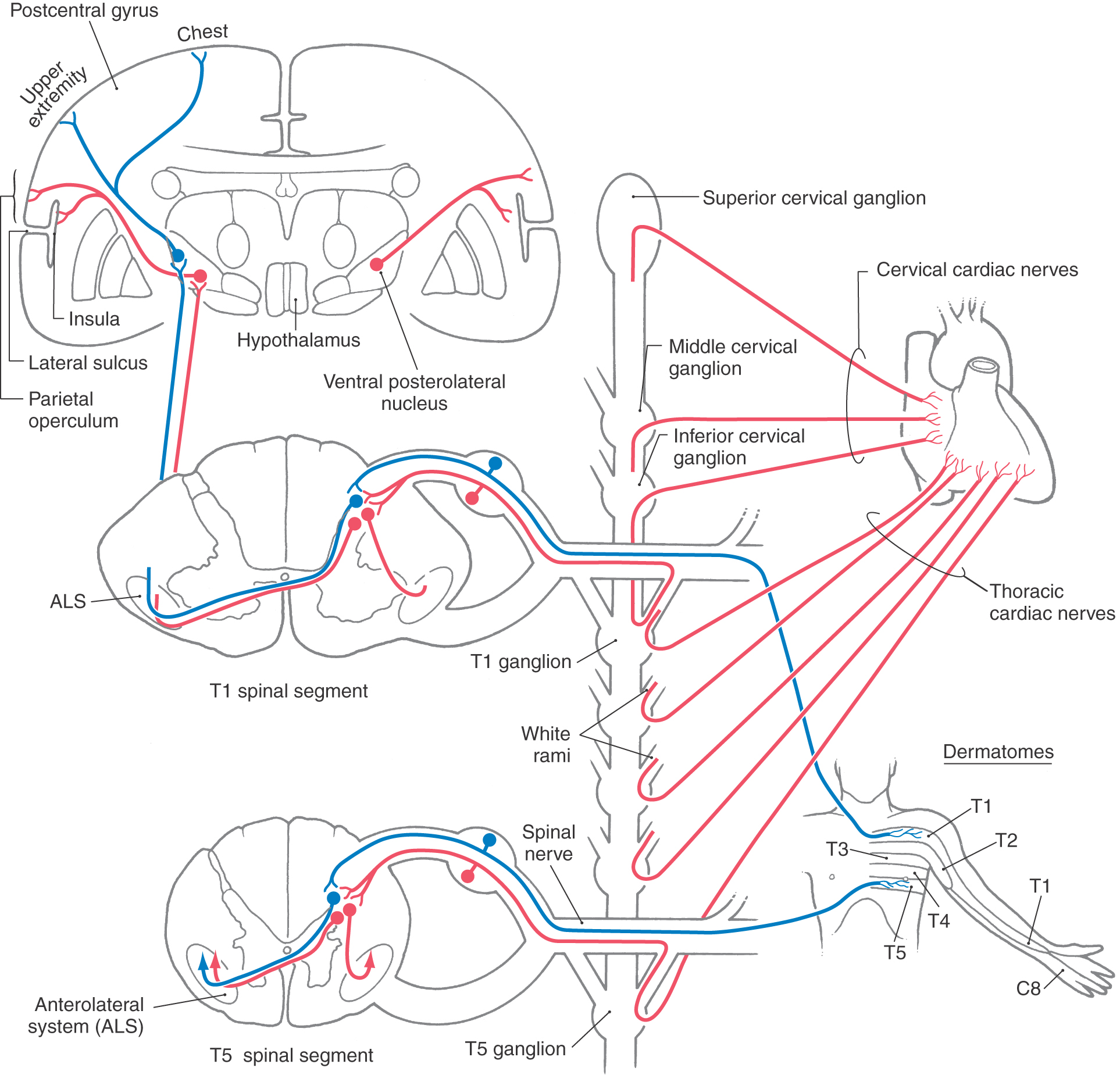
Referred pain may occur in conjunction with disease affecting any internal organ; however, it is most frequently associated with diseases of the heart (Fig. 19-4). The pain resulting from heart disease is termed angina. In about 80% of patients, angina is initially perceived as an unpleasant squeezing sensation originating from behind the sternum. This discomfort may also be perceived as pain radiating down the left arm or, more rarely, down both arms. On rare occasions, the pain has been reported to radiate bilaterally into the neck, jaw, and temporomandibular joints. The predilection of the pain for the left side of the chest or extending down the left arm reflects the predominance of myocardial disease in the left side of the heart. Consequently, nociception from the left side of the heart is referred to the left side of the body. Because angina is typically perceived as a pain of the chest, including the sternum and pectoral muscles, it is frequently called angina pectoris.
The pathways involved in angina are shown in Figure 19-6. Afferent fibers from the heart enter the sympathetic trunk through either the cervical cardiac or thoracic cardiac nerves. The cervical cardiac nerves join the sympathetic chain at superior, middle, and inferior cervical ganglia, whereas the thoracic cardiac nerves join the sympathetic ganglia associated with spinal nerves T1 to T5 (Fig. 19-6). These primary viscerosensory fibers enter the spinal cord and terminate in laminae I and V of the posterior horn. These same spinal segments also receive cutaneous somatosensory input from dermatomes of the chest wall and arm (Fig. 19-6). Tract cells in the posterior horn that receive primarily somatosensory input may also be activated, as noted previously, by collaterals of visceral afferent fibers from the heart. Consequently, the cerebral cortex interprets the pain as originating from the surface of the body (over the upper chest or arm) when actually the stimulus that has produced the painful input is located in a visceral structure (the heart).
PATHWAYS FOR PARASYMPATHETIC AFFERENTS
Sacral Parasympathetic Afferents
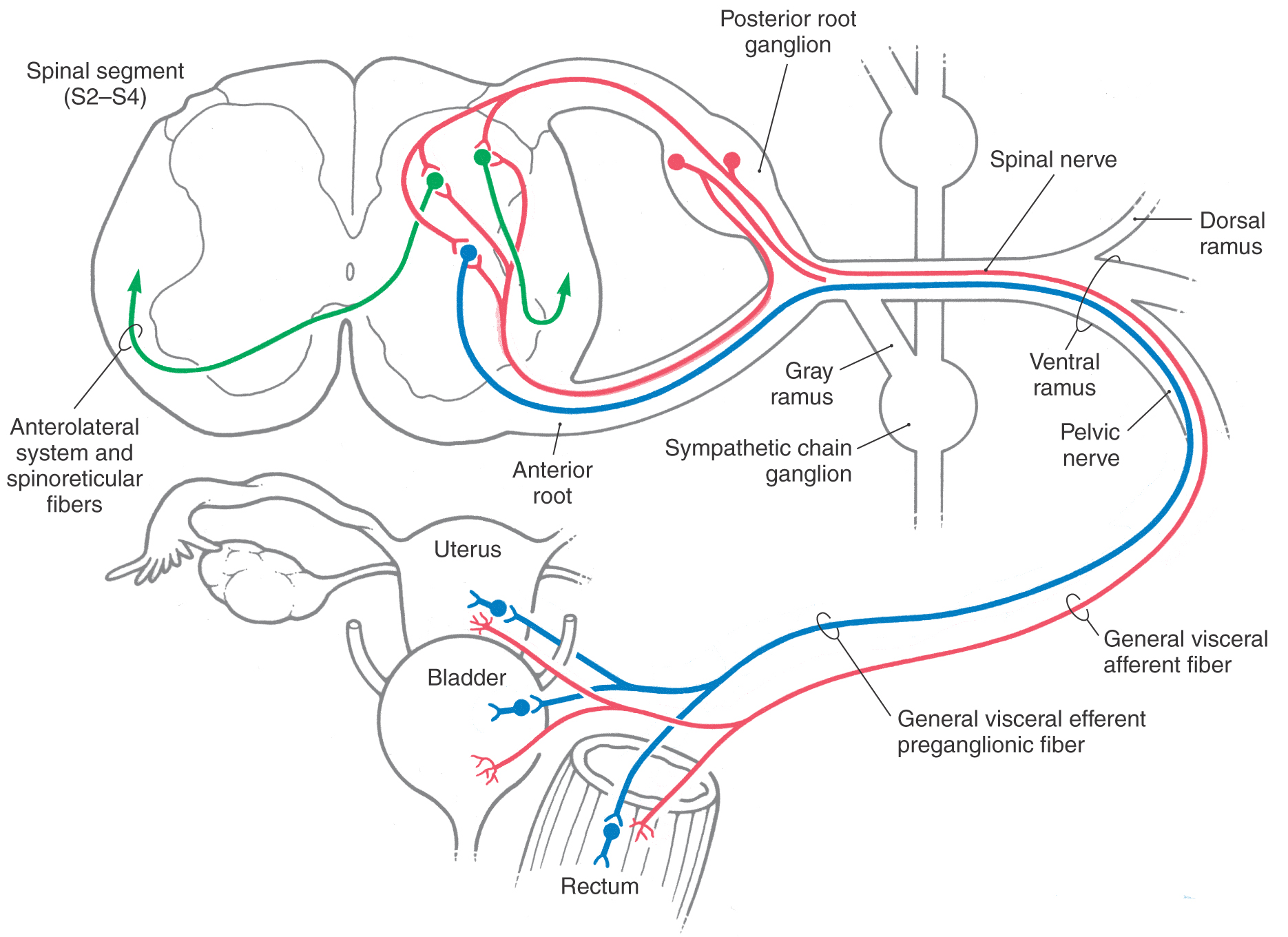
The pelvic nerves are parasympathetic and contain viscerosensory fibers passing to cord levels S2 to S4 and VE preganglionic fibers originating from these levels. These primary sensory parasympathetic fibers pass through the pelvic nerves, enter the spinal nerves, and have their cell bodies in posterior root ganglia of S2 to S4 (Fig. 19-7). Many of the central processes then pass through the posterior root to enter the spinal cord. However, a large number of other central processes, known as recurrent fibers, enter the spinal cord by traversing the anterior root (Fig. 19-7).
Once in the cord, these viscerosensory fibers terminate in the posterior horn and in the immediate vicinity of the visceral efferent preganglionic motor neurons. The posterior horn cells relay information on bladder or bowel distention (a sense of “fullness”) to the VPL via the ALS and spinoreticular pathways described earlier and then, through this thalamic nucleus, to the insular and parietal opercular cortices (Figs. 19-3 and 19-6). In this way, a full bladder is perceived and interpreted as such. In addition, ascending input from the pelvic viscera is shunted into the hypothalamus for the initiation of supraspinal autonomic reflexes. Those VE cell groups in S2 to S4 that receive viscerosensory afferents give rise to parasympathetic preganglionic axons that synapse on postganglionic cells located in pelvic viscera (Fig. 19-7). This relationship between viscerosensory fibers and the VE cell groups to which they project forms the basis for spinal autonomic reflexes.
Cranial Parasympathetic Afferents
Cranial nerves III, VII, IX, and X contain fibers of VE preganglionic parasympathetic motor neurons. However, only cranial nerves VII, IX, and X have sensory ganglia. Of these, only cranial nerves IX (the glossopharyngeal) and X (the vagus) have significant numbers of parasympathetic afferent fibers.
Visceral afferent fibers traveling in the glossopharyngeal nerve originate primarily from chemoreceptors of the carotid body and baroreceptors of the carotid sinus wall (Fig. 19-8). In addition, nociceptive and tactile input from the oropharynx (the general area of the palatine tonsil) is also conveyed on the ninth cranial nerve. These sensory fibers form the afferent limb of the gag reflex.
The carotid body is composed of specialized neural elements, chemoreceptors, and is innervated by viscerosensory branches of the glossopharyngeal nerve. Within the carotid body, these chemoreceptors are located close to a fenestrated capillary network. As a result, they are responsive to changes in arterial oxygen and carbon dioxide tension, to the acidity of the blood, and to drugs. Carotid baroreceptors are located in the carotid sinus wall and respond to rapid changes in arterial blood pressure (Fig. 19-8). The aortic arch also contains chemoreceptors and baroreceptors that are similar in structure and function to those found in the carotid body and sinus. These specialized receptors, however, are innervated by aortic or cardiac branches of the vagus nerve.
Fibers of the vagus nerve transmit a wide variety of physiologic information from thoracic viscera and from all viscera of the abdominal cavity above the level of the splenic flexure of the large colon. These vagal fibers convey information about the functional status of these structures but are not responsible for conveying information on pain.
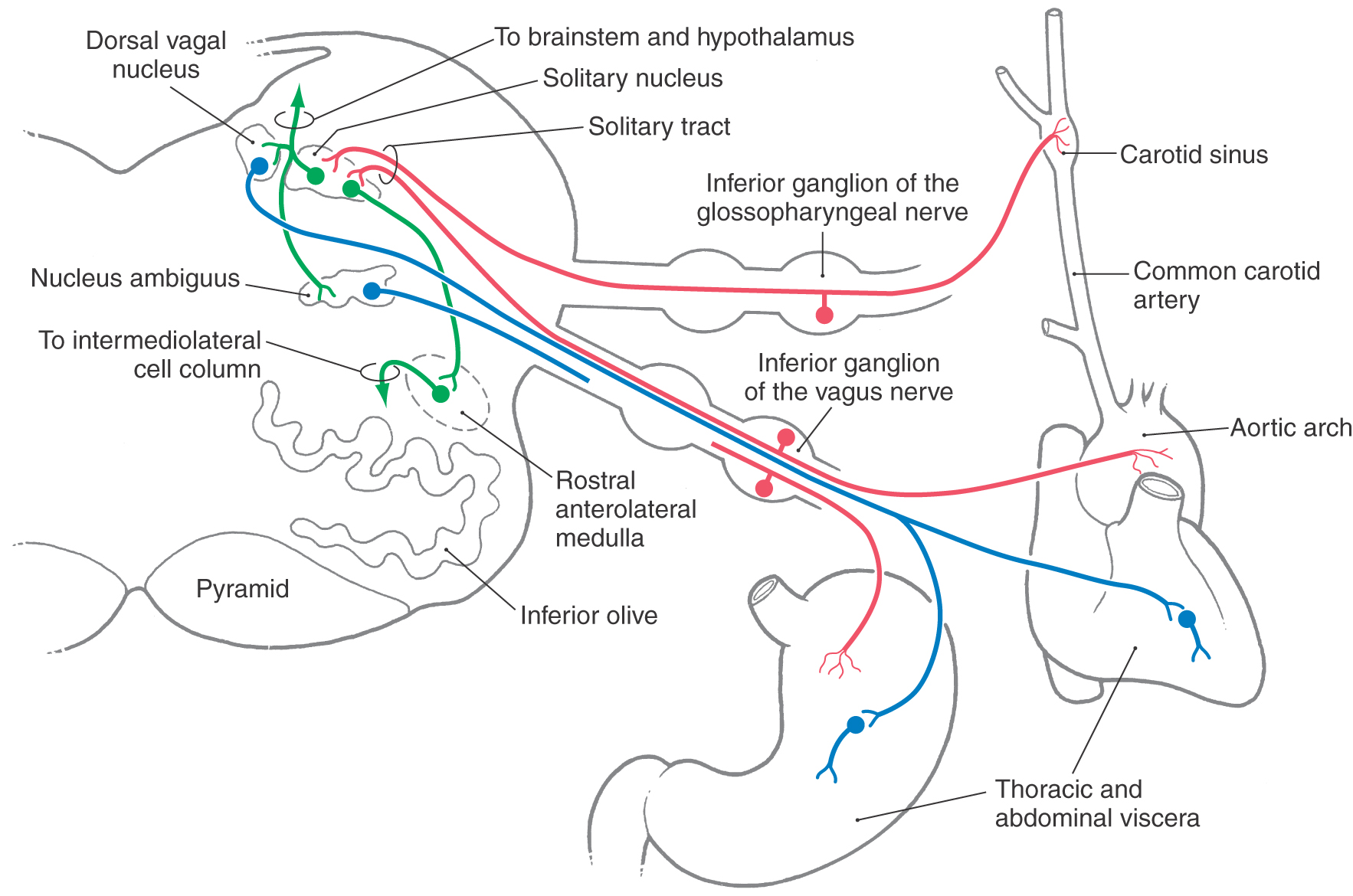
The peripheral viscerosensory fibers, traveling in the glossopharyngeal and vagus nerves, enter the skull through the jugular foramen. Within this foramen, there is a superior and an inferior ganglion on each nerve (Fig. 19-8). Cell bodies of primary viscerosensory neurons (VA) are found within the inferior ganglion, whereas the superior ganglion contains the cell bodies of primary somatosensory neurons (general somatic afferent [SA]).
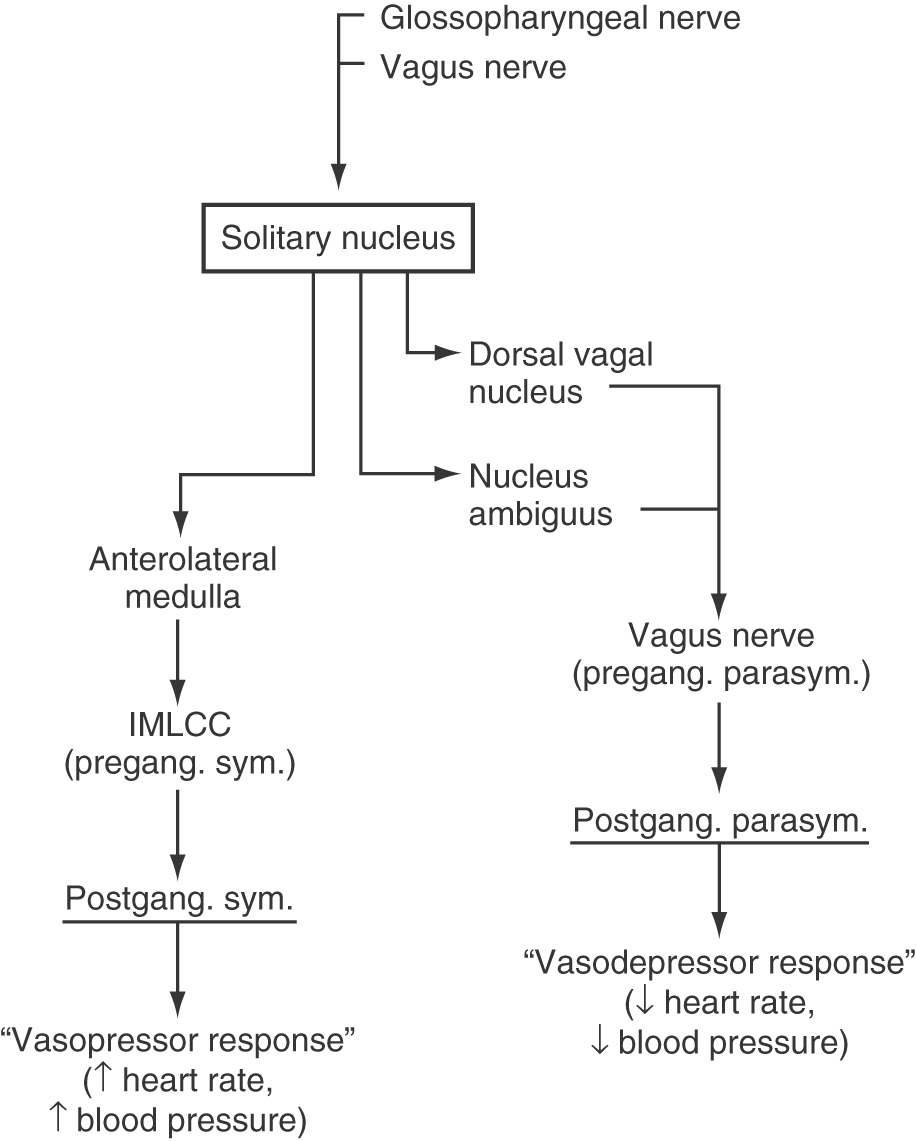
The central processes of primary visceral fibers in cranial nerves IX and X enter the medulla, form the solitary tract, and synapse with neurons of the adjacent solitary nucleus (Fig. 19-8). Some of these fibers use substance P and cholecystokinin as their transmitters. Second-order neurons in the solitary nucleus project to and influence a variety of neurons in the brainstem and hypothalamus. These targets include the dorsal vagal nucleus, the nucleus ambiguus, rostral areas of the anterolateral medulla, and the parabrachial nuclei. The dorsal vagal nucleus is the primary source of preganglionic parasympathetic neurons that project to thoracic and abdominal viscera. In addition, the nucleus ambiguus may contain some parasympathetic visceromotor cells (VE, autonomic) that innervate cardiac ganglia. However, the targets of most motor neurons in the nucleus ambiguus cells are muscles of the larynx, pharynx, and esophagus.
The few visceral efferent motor neurons of the nucleus ambiguus and the major population in the dorsal vagal nucleus receive input from the solitary nucleus and project, via the vagus nerve, to parasympathetic ganglia of the heart. Activation of this pathway causes a decrease in heart rate and a corresponding decrease in blood pressure, a “vasodepressor” response (Fig. 19-9). Conversely, neurons located in rostral parts of the anterolateral medulla receive solitary input and project to the spinal cord, where they influence the activity of preganglionic sympathetic motor neurons in the intermediolateral cell column. In doing so, this medullary center causes an increase in blood pressure and thereby serves a “vasopressor” function (Fig. 19-9).
Figure 19-9. A diagrammatic representation of the pathways that mediate the vasodepressor and vasopressor responses. The initial parts of this pathway are illustrated in Figure 19-8. IMLCC, intermediolateral cell column.
Baroreceptor Reflex
Projections from the solitary nucleus to the dorsal vagal nucleus, to cells associated with the nucleus ambiguus, and to rostral parts of the anterolateral medulla are essential to the normal operation of the baroreceptor reflex (Fig. 19-8). In this reflex, afferent input from carotid and aortic baroreceptors enters the medulla on cranial nerves IX and X and terminates in the solitary nucleus. Increases in blood pressure cause the baroreceptors to increase their discharge frequency, whereas decreases in blood pressure result in a lower rate of baroreceptor discharge. In this manner, blood pressure is continuously monitored and the resulting information is forwarded to the solitary nucleus. Within this nucleus, neurons projecting to the dorsal vagal nucleus and the nucleus ambiguus respond in a manner opposite to that of neurons projecting to the rostral parts of the anterolateral medulla. For example, during a period of acute hypertension, solitary neurons excite vasodepressor cells of the dorsal vagal nucleus and nucleus ambiguus and inhibit vasopressor neurons of the rostral anterolateral medulla (Figs. 19-8 and 19-9). The inhibitory solitary neurons may be GABAergic, whereas the excitatory neurons presumably use one of the excitatory amino acids. As a result of this dual influence from solitary neurons, blood pressure is lowered, and the hypertension is diminished. Conversely, during a period of acute hypotension, projections from the solitary nucleus inhibit vasodepressor cells and excite vasopressor neurons, leading to an elevation of blood pressure. Consequently, blood pressure is elevated and the hypotension relieved.
In addition to projections from the solitary nucleus to ambiguus, dorsal vagal, and medullary nuclei, solitary neurons also project into the reticular formation. Consequently, visceral afferent information entering the medulla on the vagal and glossopharyngeal nerves may also influence the hypothalamus and diverse areas of the cerebral cortex. The solitary nucleus does not relay any appreciable amount of general visceral sensation to the dorsal thalamus. Consequently, most of the visceral afferent information conveyed by the glossopharyngeal and vagal nerves does not reach a level of consciousness.
VISCERAL INPUT TO THE RETICULAR ACTIVATING SYSTEM
The reticular formation of the brainstem receives a wide range of inputs and projects to, among other targets, the intralaminar nuclei of the thalamus. These cell groups, in turn, send their axons to broad areas of the cerebral cortex, with the largest number terminating in the frontal lobe. These reticulothalamic and thalamocortical pathways “alert” or “activate” the cerebral cortex as a whole and constitute one important part of the ascending reticular activating system (ARAS) (see also Chapters 18 and 32). The function of the ARAS is essential to activities of daily living; damage to this ascending pathway may have profound consequences. For example, vascular compromise of the anterior portions of the thalamus (as in occlusion or damage to the thalamoperforating arteries, about 8% arising from a common trunk on one side) results in an infarct of the vascular territory served, and the patient is in a persistent coma. Such lesions interrupt the ascending message en route to the frontal cortex.
As discussed previously, the reticular formation receives viscerosensory input via spinoreticular fibers and collaterals from the ALS. Some of these ascending fibers to the reticular formation convey nociceptive visceral afferent information originating from the gut on sympathetic afferent fibers. Other ascending fibers convey a sense of bladder (or bowel) fullness originating from pelvic viscera on parasympathetic afferents. Both types of viscerosensory input feed into the reticular formation and participate in the “arousal” of the cerebral cortex through the ARAS. For example, either sudden pain from the stomach or small intestine or the stimulus of a full bladder will excite the reticulothalamocortical circuit and wake a person from a deep sleep. The initial sensation is not one of specific information (full bladder, stomach pain) but rather the sense of just being awakened. However, once the cortex has been “alerted,” the conscious/perceptive part of the brain takes over (recognizes the source of the arousal) and addresses the problem.
Sources and Additional Reading
Ammons WS. Cardiopulmonary sympathetic afferent excitation of lower thoracic spinoreticular and spinothalamic neurons. J Neurophysiol. 1990;64:1907–1916.
Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80.