Chapter 29
Visceral Motor Pathways
Organization of the Visceral Motor System
Targets of Visceral Motor Outflow
General Features of Peripheral Visceral Motor Outflow
Preganglionic Visceral Motor Neurons
Postganglionic Visceral Motor Neurons
Sympathetic Preganglionic Neurons
Internal Organization of Sympathetic Ganglia
Functional and Chemical Coding
Receptor Types in Sympathetic Targets
Preganglionic and Postganglionic Neurons
Parasympathetic Outflow Pathways
Functional and Chemical Coding
Receptor Types in Parasympathetic Targets
Regulation of Visceral Motor Outflow
The primary function of the visceral motor system is the regulation of cardiovascular, respiratory, digestive, urinary, integumentary, and reproductive organs. These organs are the main effectors of homeostasis, the maintenance of a stable internal environment against perturbing influences, both external and internal. In general, visceral motor neurons innervate smooth and cardiac muscles and glandular epithelium or structures made up of combinations of these tissues.
OVERVIEW
The visceral motor (autonomic) system ensures that tissues of the body receive appropriate nutrients, electrolytes, and oxygen and that functions such as osmolarity and temperature are properly regulated. The nervous system contributes significantly to the control and coordination of homeostatic mechanisms in response to continually changing requirements. Two overlapping control systems influence visceral effectors. One is humoral (endocrine). Hormonal responses tend to develop slowly, but the effects are prolonged. The other system is neural (autonomic). Visceral motor responses tend to be immediate, but their effects are short term.
The endocrine and autonomic systems are interdependent. They are both under the control of widely distributed central nervous system (CNS) structures, which generate commands after integrating inputs from a wide variety of sources. Thus visceral motor output is influenced by emotional status as well as by sensory signals reporting conditions inside and outside the body.
The visceral motor system has two major subdivisions, sympathetic and parasympathetic. In addition, neurons located in the wall of the alimentary canal form a somewhat autonomous component called the enteric nervous system. This is sometimes regarded as a third subdivision of the autonomic system. Within each of these components are populations of chemically coded, target-specific neurons.
ORGANIZATION OF THE VISCERAL MOTOR SYSTEM
Targets of Visceral Motor Outflow
The autonomic system provides neural control of smooth muscle, cardiac muscle, or glandular secretory cells or combinations of these tissues. For example, the gut wall is composed of smooth muscle and glandular epithelium. The sympathetic and parasympathetic divisions have overlapping and generally antagonistic influences on those viscera located in body cavities and on some structures of the head, such as the iris (Table 29-1). There are also visceral targets in the body wall and limbs. These are found in skeletal muscle (blood vessels) and in the skin (blood vessels, sweat glands, and arrector pili muscles). Visceral structures of the body wall and extremities are generally regulated by the sympathetic division alone. The sympathetic outflow thus has a global distribution in that it innervates visceral structures in all parts of the body, whereas the parasympathetic outflow is restricted to targets in the head and body cavities (Table 29-1).
Table 29-1 Comparison of Effects of Sympathetic and Parasympathetic Activity on Some Visceral Functions
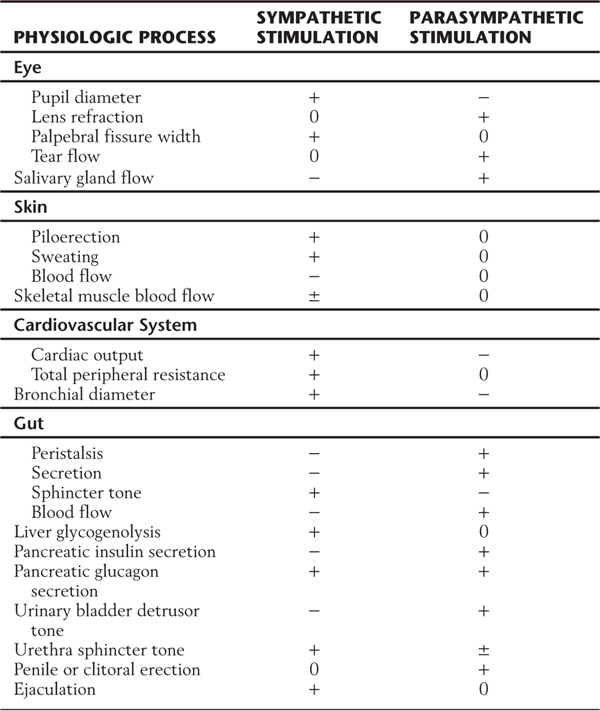
+, positive effect; −, negative effect; 0, no effect; ±, variable effect.
General Features of Peripheral Visceral Motor Outflow
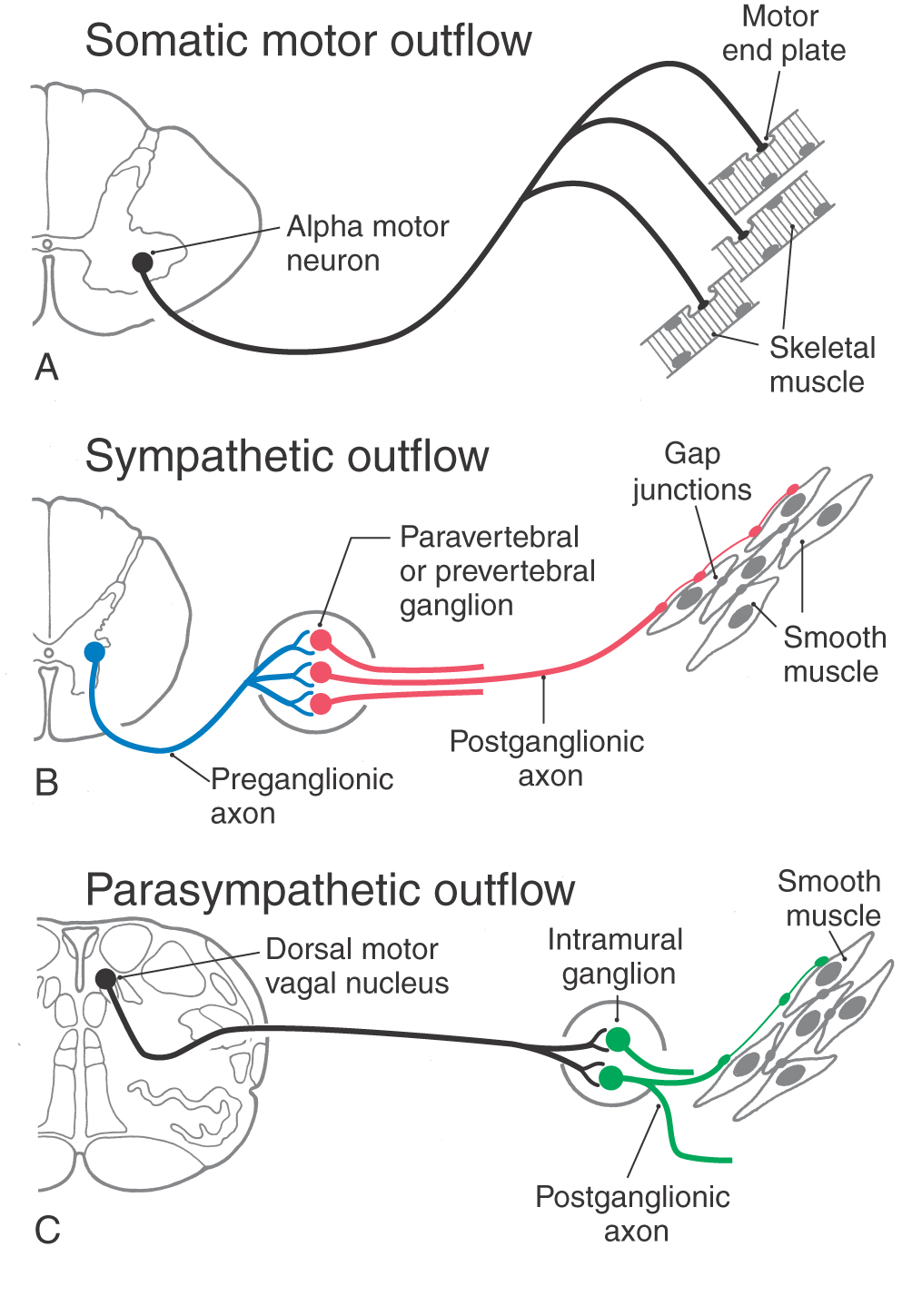
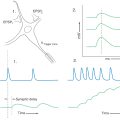
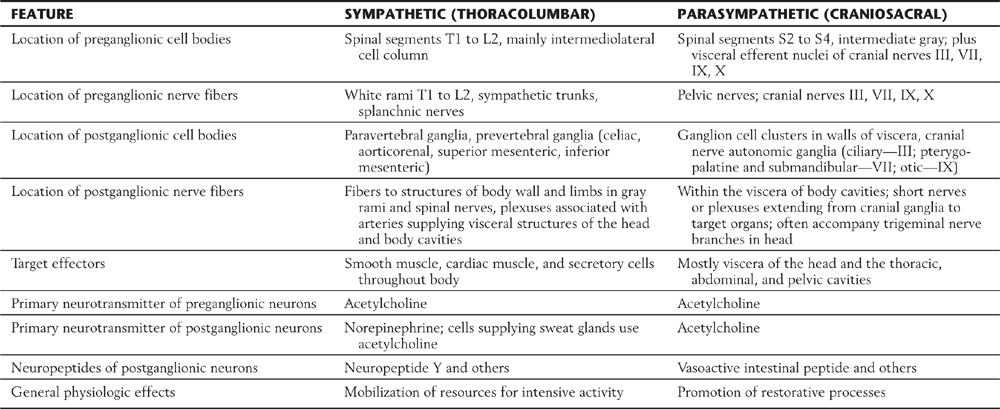
There are similarities and differences between the neural control of skeletal muscle and visceral effectors such as smooth muscle (Fig. 29-1). As described in Chapter 24, lower motor neurons (alpha motor neurons) function as the final common pathway linking the CNS to skeletal muscle fibers (Fig. 29-1A). Similarly, sympathetic and parasympathetic outflows serve as the final but often dual common neural pathway from the CNS to visceral effectors. However, unlike the somatic motor system, the peripheral visceral motor pathway consists of two neurons (Fig. 29-1B, C). The first, the preganglionic neuron, has its cell body in either the brainstem or the spinal cord. Its axon projects as a thinly myelinated preganglionic fiber to an autonomic ganglion. The second, the postganglionic neuron, has its cell body in the ganglion and sends an unmyelinated axon (postganglionic fiber) to visceral effector cells such as smooth muscle. In general, parasympathetic ganglia are close to the effector tissue and sympathetic ganglia are close to the CNS. Consequently, parasympathetic pathways typically have long preganglionic fibers and short postganglionic fibers, whereas sympathetic pathways more often have short preganglionic fibers and long postganglionic fibers.
 Figure 29-1. Comparison of somatic motor outflow (A) with sympathetic (B) and parasympathetic (C) outflow.
Figure 29-1. Comparison of somatic motor outflow (A) with sympathetic (B) and parasympathetic (C) outflow.
Visceral motor neurons and their targets are not organized into discrete motor units like those of the somatic motor system. Recall that an alpha motor neuron makes synaptic contacts with a definite group of skeletal muscle fibers over which it has exclusive control. In contrast, the terminal branches of a postganglionic visceral motor axon typically have a series of swellings containing neurotransmitter vesicles along their length, giving them a beaded (varicose) appearance (Fig. 29-1B, C). The neurotransmitters released from these terminals may act on effector cells at a distance of up to 100 µm. Moreover, unlike skeletal muscle fibers, cardiac muscle fibers and the smooth muscle cells of some organs are electrically coupled by gap junctions. Because of this, neurochemical signaling to a few cells is sufficient to regulate a large group of cells that act as a unit. The main features of sympathetic and parasympathetic divisions are summarized in Table 29-2.
Table 29-2 Comparison of the Sympathetic and Parasympathetic Divisions of Autonomic Outflow
DEVELOPMENT
Preganglionic Visceral Motor Neurons
Cell bodies of these neurons are located in nuclei or cell columns embryologically derived from the visceral efferent cell column. This column arises from neuroblasts in the basal (motor) plate of the brainstem and spinal cord portions of the neural tube.
Postganglionic Visceral Motor Neurons
Cell bodies of these multipolar neurons are located in autonomic ganglia, which may be either well-defined, encapsulated structures, such as the superior cervical ganglion, or clusters of somata found in nerve plexuses or in the walls and capsules of visceral organs. Like most primary sensory neurons, autonomic ganglion cells are derived from neural crest cells that migrate to appropriate locations during development.
One result of this cell migration is the advent, in the adult, of the myenteric (Auerbach) and the submucosal (Meissner) plexuses and the normal muscular and secretory functions of the intestinal wall.
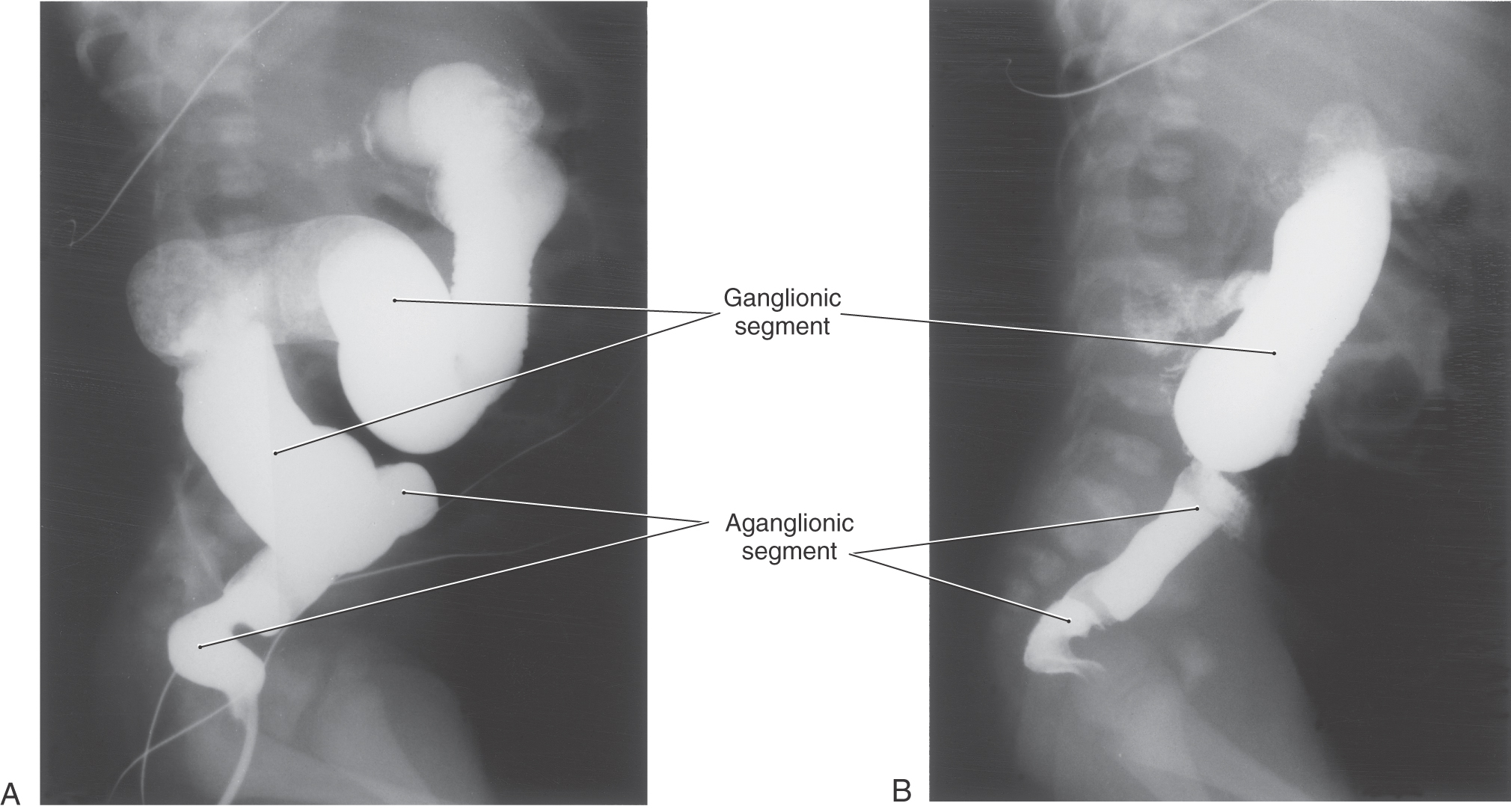
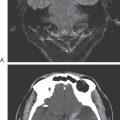
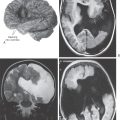
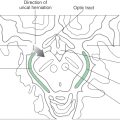
Congenital megacolon, or Hirschsprung disease, results from a failure of these enteric neuronal precursor cells to migrate into the wall of the developing lower gut. As a result, the affected segment of the gut (the portion lacking enteric ganglion cells, the aganglionic segment), usually the colon, is paralyzed in a constricted state, with consequent distention of the proximal and normally innervated portion of the intestine (the portion containing enteric ganglion cells, the ganglionic segment) (Fig. 29-2). This disease is most commonly seen in the very young (newborn to 6 years) but may be seen in adults. Although the presentation of the disease is strikingly characteristic on a radiograph or with magnetic resonance imaging (Fig. 29-2), definitive diagnosis relies on a biopsy and histologic confirmation of a lack of enteric ganglion neurons in the affected segment. The treatment of choice is to resect the aganglionic segment and join the remaining normal portions of the gut.
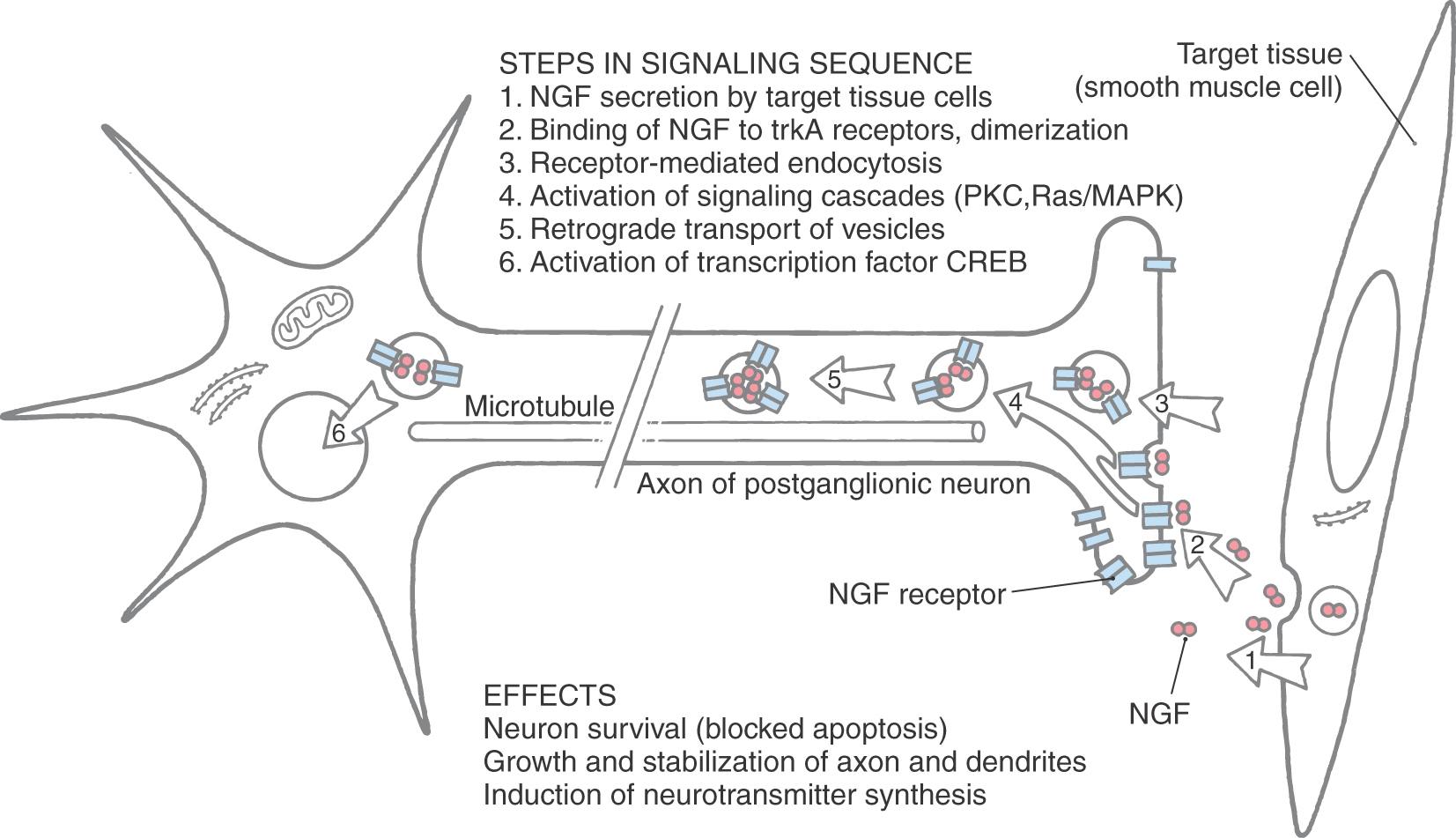
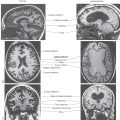
Development of the autonomic nervous system requires an elaborate sequence of intercellular signaling that involves two major families of neurotrophic factors. One is the glial cell line–derived neurotrophic factor (GDNF) family, which consists of several distinct signaling molecules and their receptors. Mutations of one of these receptors, designated RET, is the underlying cause of some cases of congenital megacolon. The neurotrophins are the other large family of neurotrophic factors. As with the GDNF family, each neurotrophin regulates development and function of specific populations of peripheral nervous system and CNS neurons via binding to specific receptors. The existence of these neuronal growth factors was first demonstrated when the neurotrophin nerve growth factor (NGF) was discovered as a target-derived messenger molecule that is absolutely essential for survival and development of sympathetic postganglionic neurons (Fig. 29-3) as well as those primary sensory neurons that are involved in pain (Fig. 29-3). The pathologic changes in animals deprived of NGF or its high-affinity receptor are similar to those seen in patients with congenital insensitivity to pain with anhidrosis (hereditary sensory and autonomic neuropathy type IV), an autosomal recessive disease. Indeed, mutations that impair the function of the NGF receptor trkA have been identified in patients with this disease.
SYMPATHETIC DIVISION
Sympathetic Preganglionic Neurons
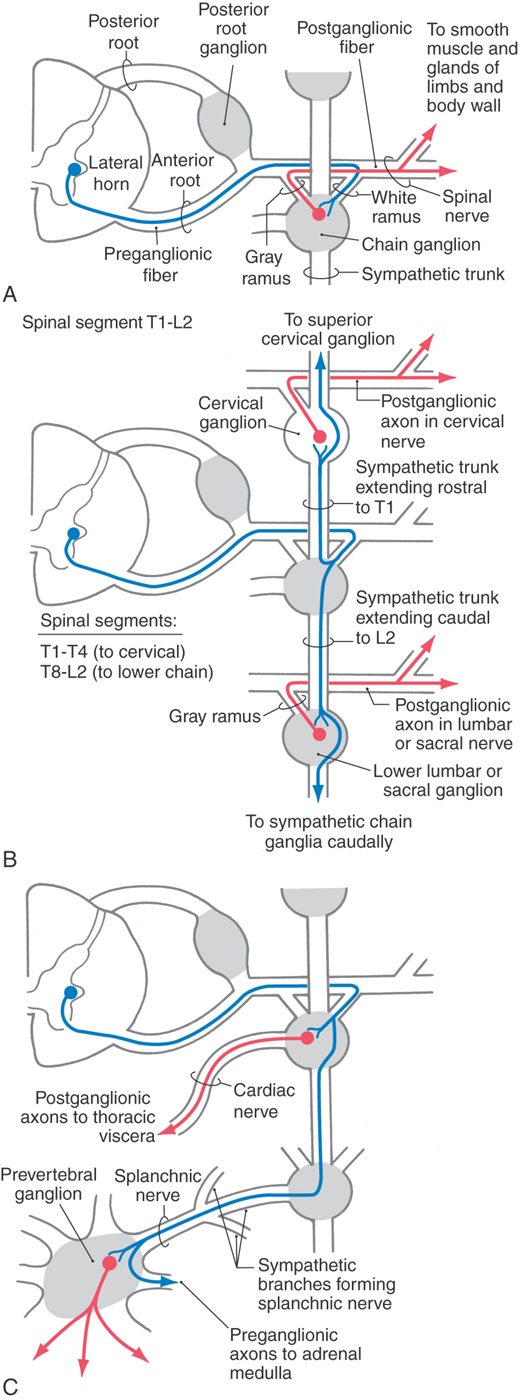
Although the sympathetic outflow influences visceral targets throughout the body, sympathetic preganglionic neurons are found only in spinal cord segments T1 through L2 (sometimes in C8 and L3). These cell bodies are located in Rexed lamina VII, primarily in the intermediolateral nucleus (cell column) of the lateral horn. The axons of these preganglionic cells exit the spinal cord in the ventral root and enter the sympathetic trunk via the white communicating ramus. A preganglionic fiber takes one of three courses after entering the sympathetic chain: (1) it may synapse at that level with postganglionic neurons whose unmyelinated axons join that spinal nerve via a gray communicating ramus; (2) it may ascend or descend in the sympathetic chain to synapse on postganglionic neurons whose axons either join spinal nerves or project to targets in the thoracic cavity or head; or (3) it may pass through the chain ganglion as a preganglionic fiber to form part of a splanchnic nerve (Fig. 29-4).
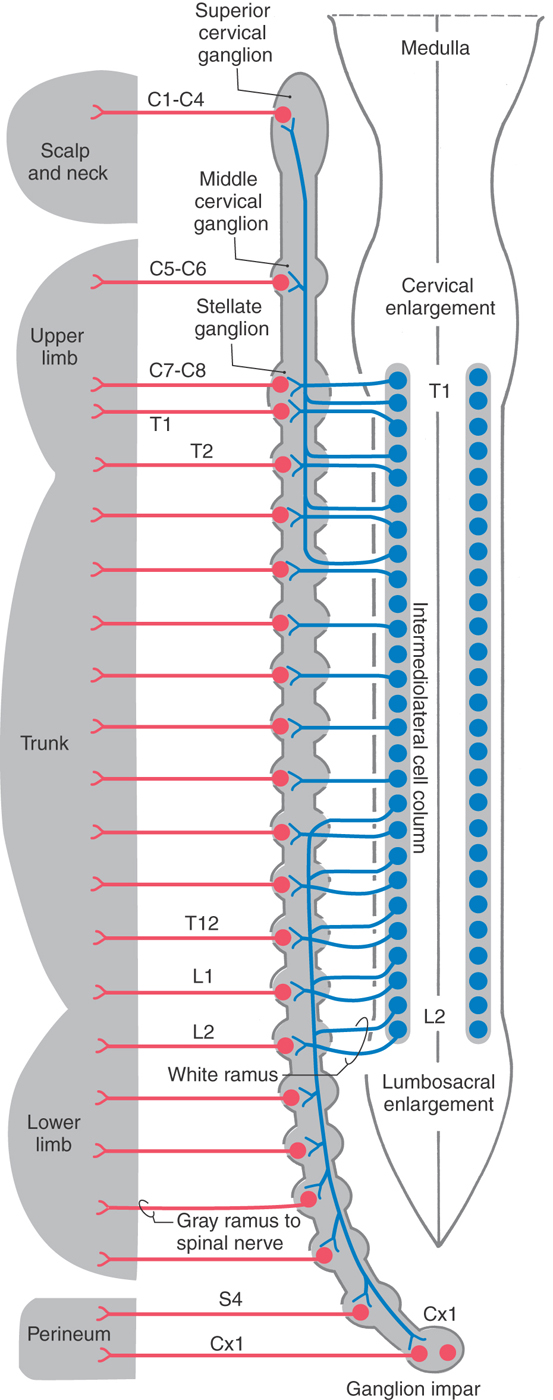
The sympathetic outflow for the entire body originates from thoracic and upper lumbar spinal cord segments. Although the segmental pattern of innervation is not straightforward, there is a general viscerotopic organization (Figs. 29-5 and 29-6). Neurons of the superior, middle, and inferior cervical ganglia receive input, via the sympathetic trunk, from preganglionic neurons of the upper thoracic spinal segments. Lower lumbar and sacral ganglia are supplied by neurons of the lower thoracic and upper lumbar spinal segments. The ganglia between these regions are supplied by their corresponding spinal levels. Thus visceral targets in the head, neck, and upper extremity as well as the viscera of the thoracic cavity are served by preganglionic sympathetic neurons in the upper thoracic segments. The main abdominal viscera and other targets in the trunk are served by the central and lower thoracic spinal cord segments, whereas the pelvic viscera, the lower trunk, and the lower extremity are served by the lower thoracic and upper lumbar spinal cord segments.
Figure 29-5. Sympathetic pathways to visceral targets in the body wall, limbs, and scalp and neck. Postganglionic sympathetic fibers that distribute to targets of the head are shown in Figure 29-6.
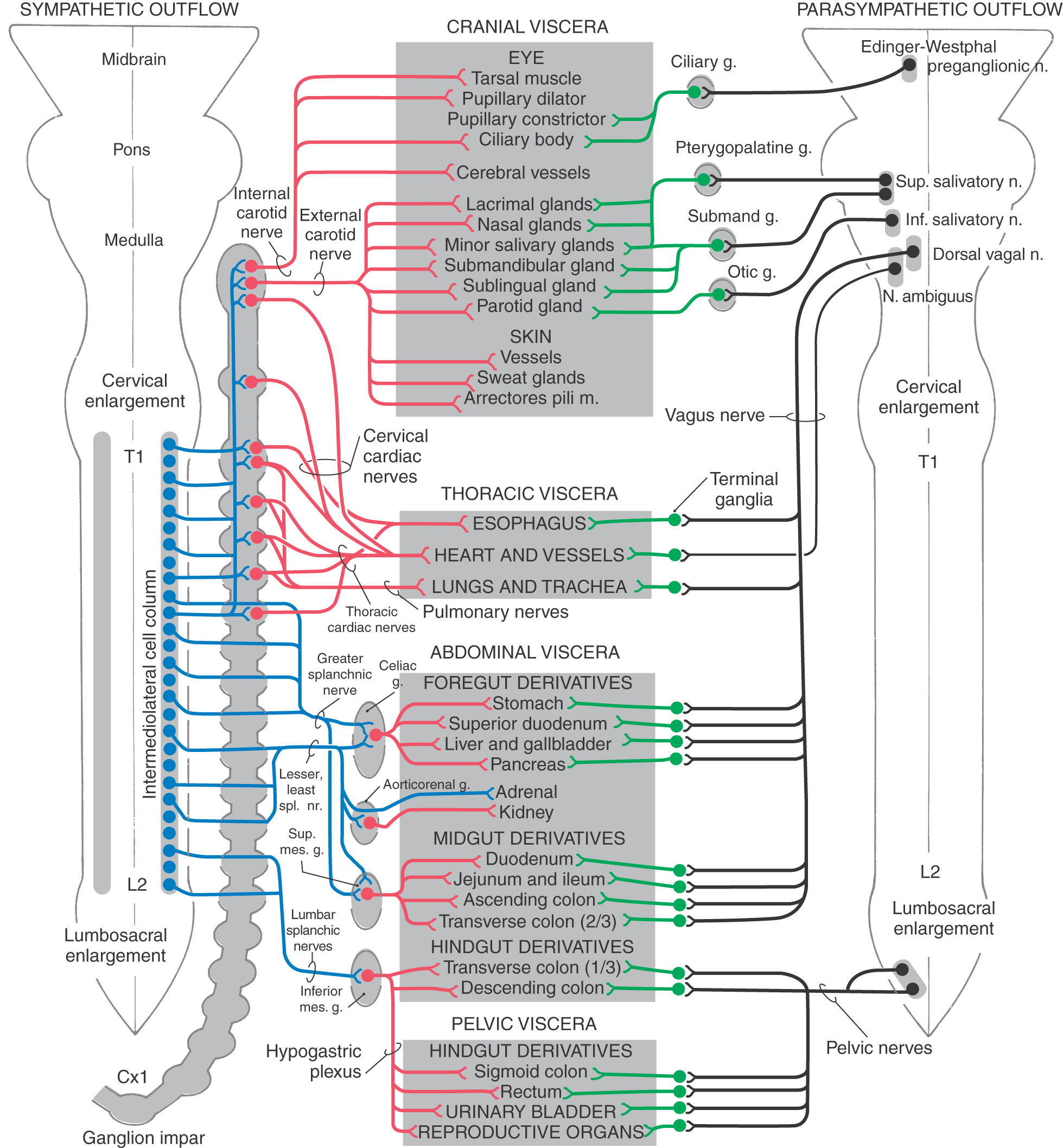
Preganglionic sympathetic neurons also target the adrenal gland (Fig. 29-6). Chromaffin cells of the adrenal medulla are related to sympathetic ganglion neurons in both derivation (neural crest) and function. These cells secrete catecholamines (mostly epinephrine) into the bloodstream in response to signals from preganglionic neurons. Thus the sympathetic system, through this endocrine pathway, regulates functions of cells that are not directly contacted by nerve terminals.
Sympathetic Ganglia
Cell bodies of sympathetic postganglionic neurons are generally grouped into discrete ganglia that are located at some distance from the target tissue. Most of them make up the sympathetic chain (paravertebral) ganglia and the prevertebral ganglia associated with the abdominal aorta or its large branches (the celiac, aorticorenal, superior mesenteric, and inferior mesenteric ganglia; see Fig. 29-6). In addition, small clusters of cell bodies are also scattered among nerve fibers in communicating rami, the sympathetic trunk between chain ganglia, and peripheral plexuses.
The sympathetic chain extends along the full length of the vertebral column, but the number of ganglia does not exactly match the number of spinal nerves. In general, there are 3 cervical, 10 to 11 thoracic, 3 to 5 lumbar, and 3 to 5 sacral sympathetic ganglia on each side and a single coccygeal ganglion (ganglion impar), where the two chains meet caudally (Figs. 29-5 and 29-6). Typically, the inferior cervical ganglion and the first thoracic ganglion fuse to form the stellate ganglion.
The sympathetic chain ganglia are connected to spinal nerves via white and gray communicating rami. The former appear white because they contain myelinated preganglionic fibers, and the latter appear gray because they are composed of unmyelinated postganglionic fibers that serve the limbs and body wall (Fig. 29-4). Consequently, only spinal nerves T1 to L2 have white rami (and contain preganglionic axons), whereas every spinal nerve is connected to the sympathetic trunk by a gray ramus that conveys postganglionic axons (Fig. 29-5).
Sympathetic postganglionic neurons in paravertebral ganglia send their axons in two general directions. First, some postganglionic fibers join the spinal nerve via a gray ramus. These fibers distribute to blood vessels, sweat glands, and arrector pili muscles in the body wall and extremities (Figs. 29-4A, B and 29-5). These structures receive little or no parasympathetic innervation, so they are exceptions to the dual arrangement of visceral innervation. Second, some of the postganglionic fibers that arise from cervical and upper thoracic sympathetic chain ganglia form cervical and thoracic cardiac nerves and pulmonary nerves that emerge directly from the ganglia (Figs. 29-4C and 29-6). These fibers innervate the vascular smooth muscle of the esophagus, heart, and lung; the glandular epithelium of respiratory structures; the smooth muscles of the esophagus; and cardiac muscle. Axons of these sympathetic neurons mingle with parasympathetic fibers of the vagus nerve to form the autonomic plexuses of the thorax.
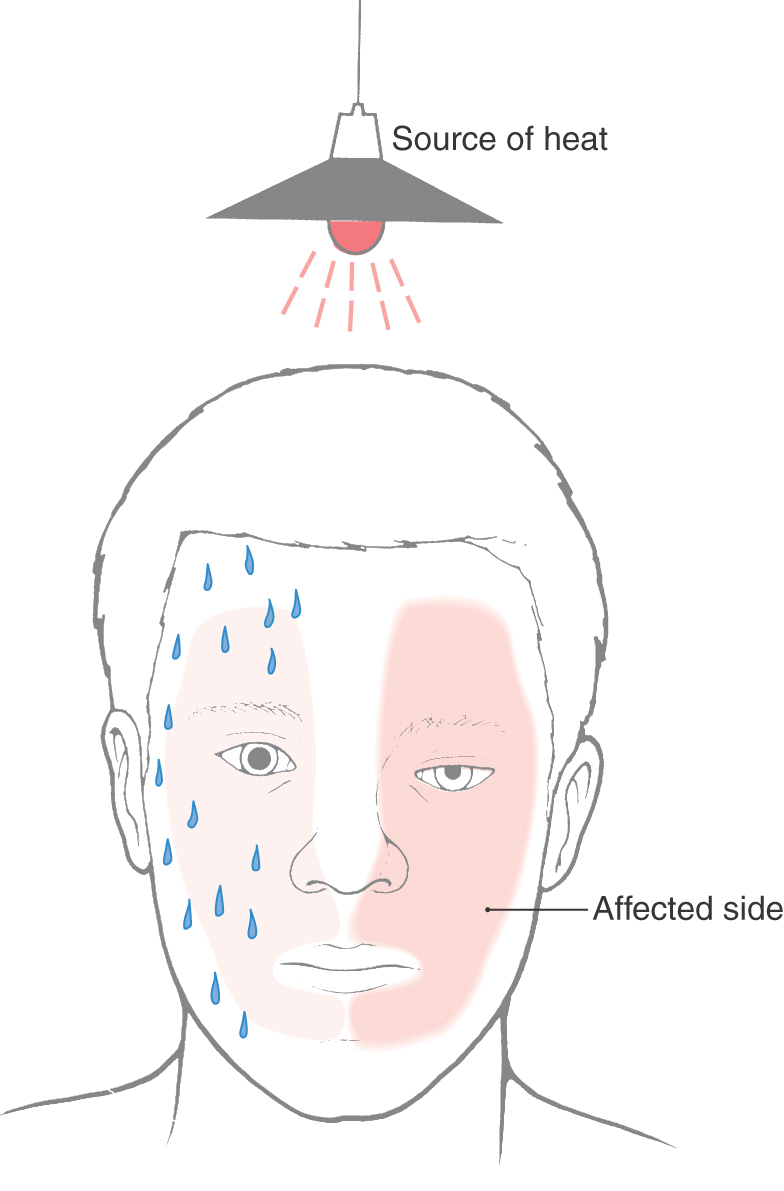
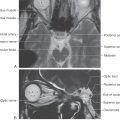
The largest of the paravertebral (sympathetic chain) ganglia is the superior cervical ganglion. Postganglionic fibers from these cells innervate blood vessels and cutaneous targets of the face, scalp, and neck of the territories supplied by the first four cervical nerves (Figs. 29-5 and 29-6). The superior cervical ganglion also innervates the salivary glands, nasal glands, lacrimal glands, and structures of the eye such as the pupillary dilator muscle and the superior and inferior tarsal muscles (Fig. 29-6). Accordingly, a constellation of signs and symptoms results from interruption (central or peripheral) of the sympathetic pathway through the superior cervical ganglion (Fig. 29-7). These include constriction of the pupil (miosis) caused by the unopposed action of the parasympathetically innervated pupillary constrictor, drooping of the upper eyelid (ptosis) resulting from paralysis of the superior tarsal muscle (of Müller), flushing of the face from loss of sympathetically mediated vascular tone, and diminished or absent sweating (anhidrosis) on the face. Collectively, these signs and symptoms are known as the Horner syndrome (Fig. 29-7).
Figure 29-7. Features of the Horner syndrome. Note the lack of sweating on the affected side in response to radiant heat.
The prevertebral sympathetic ganglia are found in visceral motor plexuses associated with the abdominal aorta and its major branches, and they receive input via the splanchnic nerves (Figs. 29-4C and 29-6). In general, the postganglionic fibers arising from each of these ganglia supply the same visceral targets as the corresponding branch of the aorta. Thus the celiac ganglion is located at the origin of the celiac artery and supplies postganglionic fibers to the spleen and to viscera derived from the embryonic foregut. The aorticorenal ganglion, associated with the renal arteries, contains cell bodies of neurons that innervate the blood vessels of the kidneys. The superior mesenteric ganglion provides postganglionic fibers that supply the territory of the superior mesenteric artery (derivatives of the midgut). Inferior mesenteric ganglion neurons project to hindgut derivatives and to the urinary bladder, urethra, and reproductive organs. All abdominal aortic plexuses contain sympathetic fibers mixed with parasympathetic preganglionic fibers of either vagal or sacral origin.
Internal Organization of Sympathetic Ganglia
Axons of preganglionic sympathetic neurons branch in the periphery and synapse on many postganglionic neurons; the output of the preganglionic neurons is thus widely divergent (Figs. 29-1 and 29-8). The number of postganglionic neurons exceeds preganglionic neurons by more than 100-fold. Each postganglionic neuron, however, receives synaptic input from a number of preganglionic neurons, so there is also considerable convergence within sympathetic ganglia.
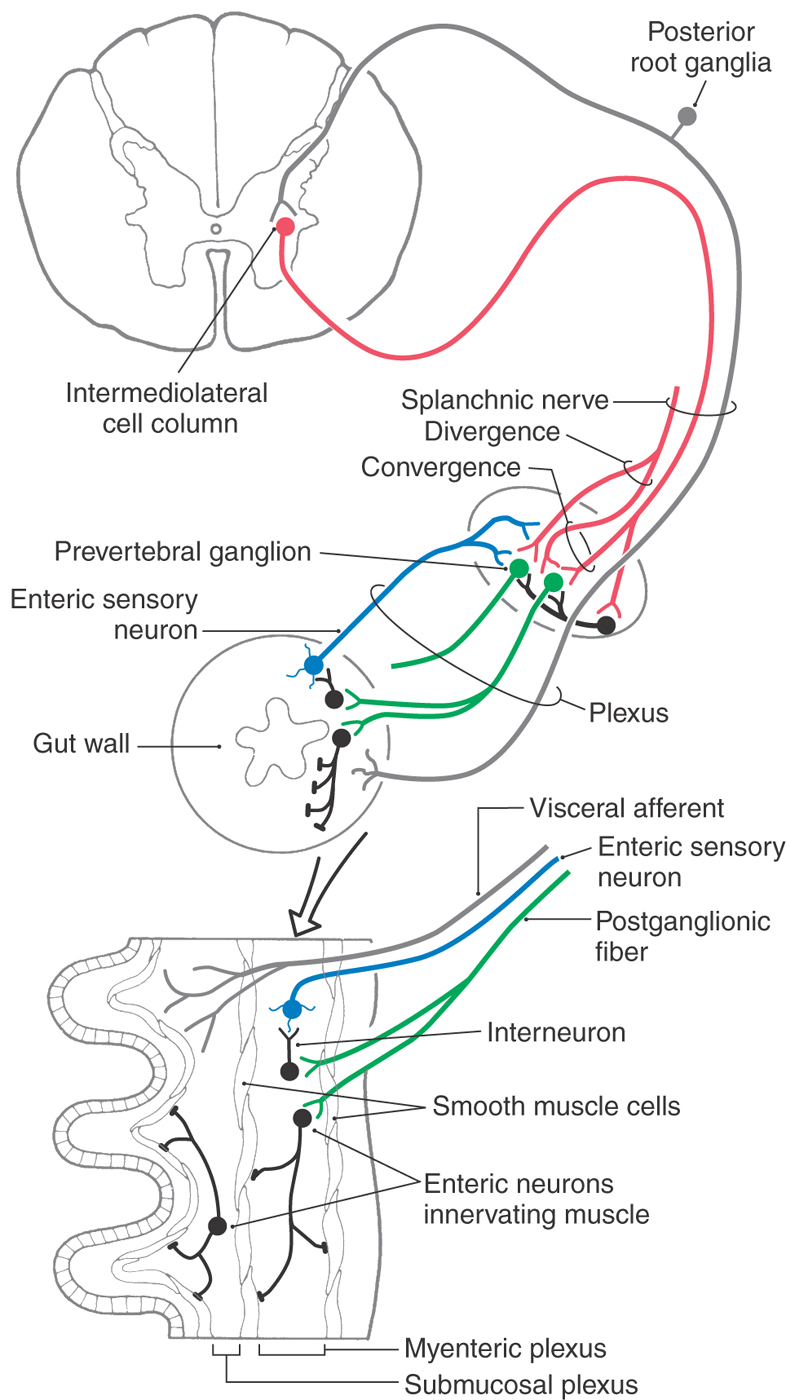
Sympathetic ganglia are commonly referred to as relay ganglia, implying that they are sites of simple signal transmission between preganglionic and postganglionic neurons. However, it is clear that more complicated signal processing takes place in prevertebral ganglia. In addition to receiving diverging and converging input from functionally coded preganglionic neurons, postganglionic neurons are influenced by a variety of other sources. These sources include synaptic inputs from collaterals of general visceral afferent fibers and from local neurons (Fig. 29-8). These connections indicate a high degree of integration in prevertebral ganglia.
Functional and Chemical Coding
Conditions of extreme excitement or exertion bring about a comprehensive (en masse) activation of sympathetic outflow, with widespread effects. These effects include increases in heart rate, blood pressure, blood flow to skeletal muscles, blood glucose level, sweating, and pupil diameter. Concurrently, there are decreases in gut motility, digestive gland secretion, and blood flow to abdominal viscera and skin (Table 29-1). This constellation of effects has led to the concept that the sympathetic system acts in a global, nonselective manner. However, in less extreme conditions, there is ongoing, selective control of function-specific and target-specific subpopulations of preganglionic and postganglionic neurons. Such selectivity can be found, for example, among cell groups that control vascular tone. For instance, sympathetic pathways to blood vessels in the skin are primarily influenced by temperature, whereas sympathetic outflow to vessels in skeletal muscle responds mainly to changes in blood pressure signaled by baroreceptors. Also, stabilization of blood flow to the head during movement from a reclining to a standing position is a function of the sympathetic division. This adaptation to posture requires rapid changes in vascular tone that must vary according to the region of the body.
Just as components of the sympathetic system can be regulated independently, there is evidence that distinct populations of preganglionic and postganglionic neurons exist. For example, even though preganglionic neurons are all cholinergic, some also express one or more neuropeptides, such as substance P and enkephalins. In addition, distinct populations of preganglionic neurons have been identified on the basis of (1) location of the cell body in the visceral motor cell groups of the spinal cord, (2) morphology of the dendritic tree, and (3) specific target cell type among ganglion cells.
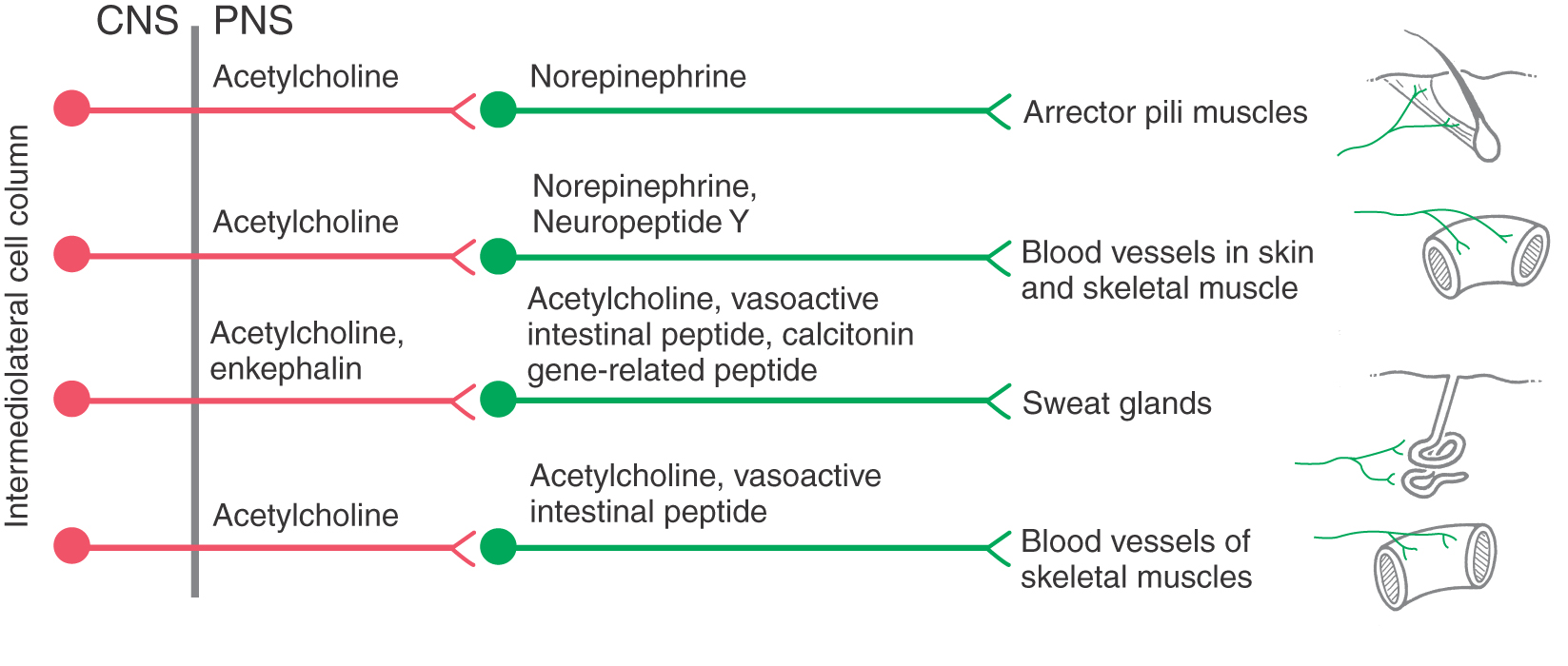
More is known about the functional significance of chemical coding in postganglionic sympathetic neurons. Although most of these cells use norepinephrine as a transmitter, some are cholinergic. The latter neurons provide secretomotor innervation to most sweat glands and possibly innervation to other targets, such as arrector pili muscles and arterioles of skeletal muscle. In addition, postganglionic cells express a variety of neuropeptides, some of which have been linked to specific functional populations of cells (Fig. 29-9; Table 29-2). Most prevalent among sympathetic peptides is neuropeptide Y, which is released along with norepinephrine by vasoconstrictor postganglionic fibers. This peptide has multiple effects at the adrenergic ending. These effects include stimulation of vascular smooth muscle contraction, potentiation of epinephrine effects, and, paradoxically, inhibition of norepinephrine release.
Receptor Types in Sympathetic Targets
The effect of a neurotransmitter on a target cell is determined by the nature of the target cell receptor and the particular signal transduction mechanism to which it is linked. Thus the effects of norepinephrine, the main neurotransmitter of most postganglionic neurons, and of epinephrine, the main hormone of the adrenal medulla, vary among different target cells according to the type or types of adrenergic receptor they express (subclasses of α- and β-adrenergic receptors). For example, α1 receptors on vascular smooth muscle cells mediate vasoconstriction, whereas activation of β2 receptors results in relaxation. Increases in heart rate and cardiac output are mediated by β1 receptors on cardiac muscle. Epinephrine is a more potent ligand than norepinephrine at most α- and β-adrenergic receptors. Consequently, epinephrine is administered to counteract symptoms of anaphylactic shock, including bronchospasm, edema, congestion of mucous membranes, and cardiovascular collapse. As mentioned, some tissues receive cholinergic sympathetic innervation (Fig. 29-9). For example, stimulation of secretion by eccrine sweat glands, but not apocrine sweat glands, is mediated by muscarinic acetylcholine receptors.
Causalgia
In special circumstances, sympathetic activation can become linked to pain. Causalgia (complex regional pain syndrome type II) is a syndrome that can result from partial injury to a peripheral nerve, typically a nerve serving an extremity. Signs and symptoms include spontaneous burning pain, hypersensitivity of the skin, pain triggered by loud noises or strong emotions, sweating and reduced temperature of the limb, mottling of the skin, and swelling of the extremity. A striking feature of causalgia is that symptoms can often be alleviated by sympathectomy or otherwise blocking sympathetic function. Thus a prevailing theory of the etiology of the pain associated with the syndrome is that sympathetic postganglionic neurons coursing in the injured nerve develop abnormal connections to nociceptive posterior root ganglion neurons. This pathologic process could occur either in the tangle of regenerated nerve fibers that form a neuroma or within the sensory ganglion. The nociceptive neurons, in turn, may develop abnormal responsiveness to adrenergic stimulation.
PARASYMPATHETIC DIVISION
Preganglionic and Postganglionic Neurons
Compared with the sympathetic division, the parasympathetic division is more restricted in its distribution. The cell bodies of parasympathetic preganglionic neurons are located either in sacral segments S2 to S4 or in the nuclei that provide the visceral efferent (VE) fibers that travel in cranial nerves III, VII, IX, and X (Table 29-3; Fig. 29-6). The parasympathetic division of the visceral motor system is accordingly called the craniosacral system, as distinct from the sympathetic division, which is the thoracolumbar.
Table 29-3 Peripheral Pathways of Parasympathetic Outflow
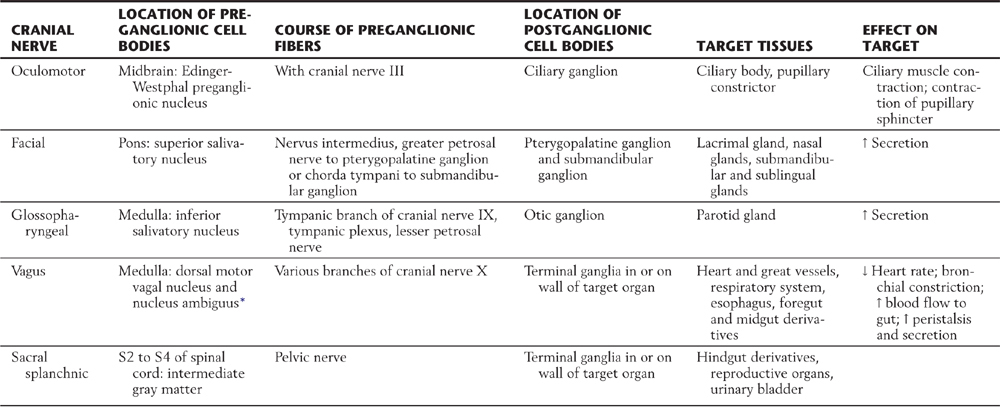
*Some visceral efferent preganglionic parasympathetic cells that innervate the heart are found in this nucleus, although its main function is to provide somatic efferent fibers that distribute on cranial nerves IX and X.
↑, increase; ↓, decrease.
The cell bodies of postganglionic parasympathetic neurons supplying cranial structures are located in discrete ganglia, and in general their axons travel distally with branches of the trigeminal nerve. Cell bodies of parasympathetic postganglionic neurons supplying viscera of the body cavities are not grouped into macroscopic ganglia. Rather, these cells are scattered within nerve plexuses of the target organ or in the wall of the gut (terminal or intramural ganglia), where they intermingle with neurons of the enteric nervous system.
Parasympathetic Outflow Pathways
The visceral motor component of the oculomotor nerve arises in the Edinger-Westphal preganglionic nucleus. These preganglionic fibers terminate in the ciliary ganglion. Axons of postganglionic cells of the ciliary ganglion innervate the sphincter muscle of the iris (for pupillary constriction) and the ciliary muscle (for near vision accommodation) (Table 29-3; Fig. 29-6).
The preganglionic parasympathetic fibers of the facial nerve originate in the superior salivatory nucleus. Some investigators distinguish a separate lacrimal nucleus that targets the lacrimal gland. Preganglionic VE axons from the superior salivatory nucleus exit the brainstem in the intermediate nerve, which is classically considered a part of the facial nerve. Some of these fibers course via the greater petrosal nerve to terminate in the pterygopalatine ganglion, which supplies the lacrimal gland and nasal and palatal mucous glands. Other preganglionic fibers travel via the chorda tympani to the submandibular ganglion, which innervates the submandibular and sublingual salivary glands (Table 29-3; Fig. 29-6).
The glossopharyngeal nerve contains preganglionic parasympathetic fibers that originate in the inferior salivatory nucleus. These fibers take a tortuous course, via the tympanic nerve and plexus, to form the lesser petrosal nerve, which ends in the otic ganglion. Postganglionic fibers from the otic ganglion join the auriculotemporal nerve to reach the parotid gland (Table 29-3; Fig. 29-6).
The visceral motor component of the vagus nerve provides parasympathetic innervation to organs of the thoracic and abdominal cavities. Preganglionic VE fibers of the vagus nerve originate in the dorsal motor vagal nucleus. In addition, a part of the nucleus ambiguus contains a few VE preganglionic cells, whose axons travel with the vagus to innervate the heart. However, the main outflow from the nucleus ambiguus consists of somatic efferent fibers to the glossopharyngeal and vagus nerves. Preganglionic fibers of the vagus terminate on postganglionic neurons located in the walls of viscera of the thorax and abdomen (Table 29-3; Fig. 29-6). Thus postganglionic neurons of the vagus nerve are not aggregated into discrete ganglia, as they are for cranial nerves III, VII, and IX.
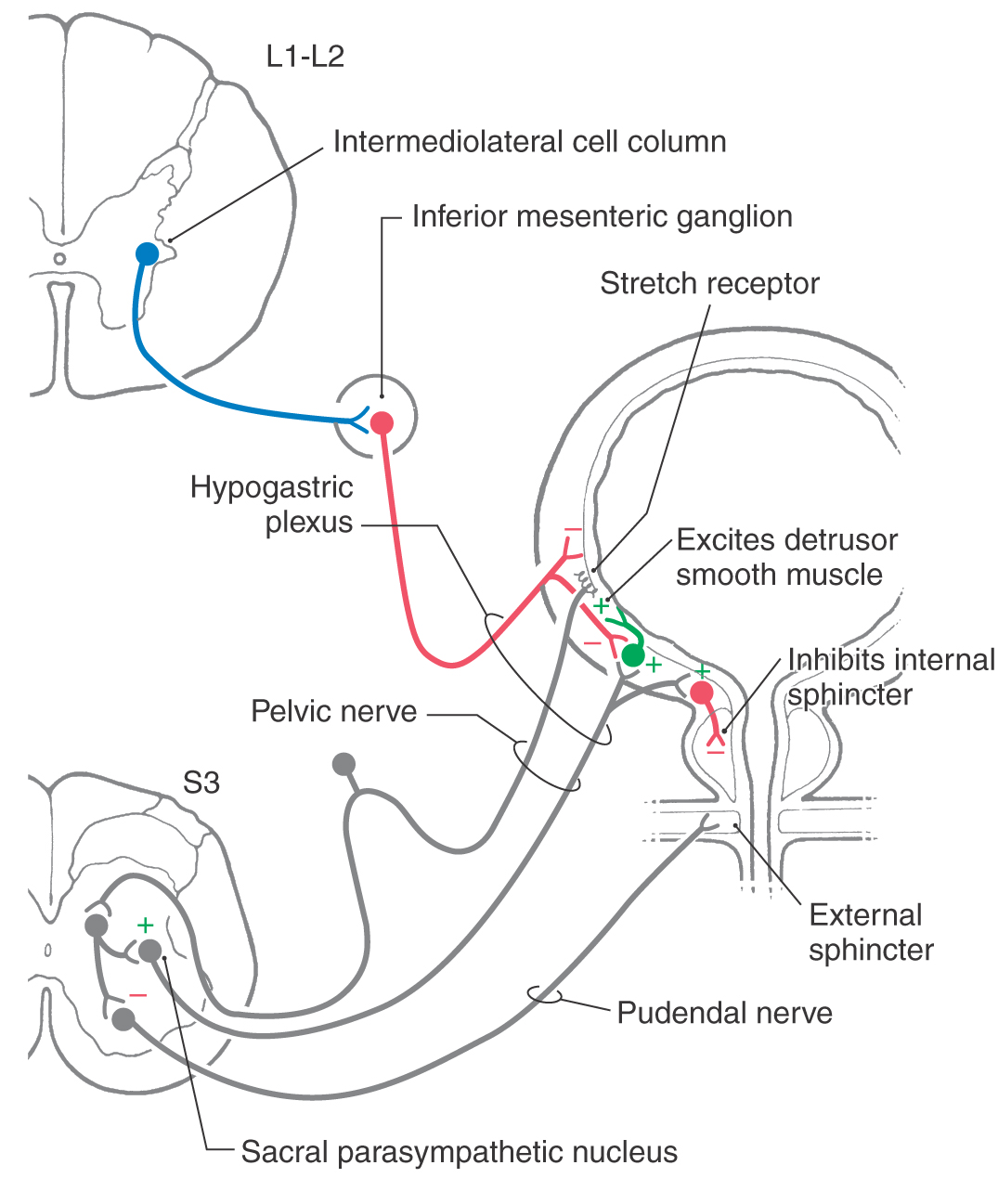
The sacral component of the parasympathetic division innervates the lower digestive tract (beginning at about the left colic flexure) and the urinary bladder, urethra, and reproductive organs. Preganglionic neurons of the sacral parasympathetic nucleus occupy a position at sacral levels S2 to S4 comparable to the intermediolateral cell column at thoracic levels (Fig. 29-10). Preganglionic fibers exit the spinal cord via ventral roots and form the pelvic nerves (nervi erigentes). These nerves mingle with sympathetic fibers of the inferior hypogastric plexuses to form the pelvic visceral plexus lateral to the rectum, bladder, and uterus (Table 29-3).
Figure 29-10. Neural pathways mediating control of the urinary bladder.
Functional and Chemical Coding
Preganglionic parasympathetic neurons, like preganglionic sympathetic neurons, use acetylcholine as their main neurotransmitter. Postganglionic parasympathetic neurons are also cholinergic. Both preganglionic and postganglionic parasympathetic neurons release molecules in addition to the principal transmitter at their terminals. These include neuropeptides, most prominently vasoactive intestinal peptides, which act as modulators of the postsynaptic response to the main transmitter.
Receptor Types in Parasympathetic Targets
Nicotinic receptors are limited to skeletal muscle and cholinergic synapses in autonomic ganglia and the CNS. Muscarinic cholinergic receptors appear to be the only class involved in the response of smooth muscle, cardiac muscle, and glandular cells to acetylcholine. The nature of the response depends on which type of muscarinic receptor (M1, M2, and so on) is expressed. For example, parasympathetic stimulation of gastric acid secretion is mediated by M1 muscarinic receptors, whereas M2 receptors mediate parasympathetic depression of heart rate and contraction of cardiac muscle.
The cholinergic receptor blocker atropine has effects that are clinically useful in certain circumstances. These effects include pupillary dilation, relaxation of bronchiolar muscle, and reduction of peristalsis and secretion in the stomach.
ENTERIC NERVOUS SYSTEM
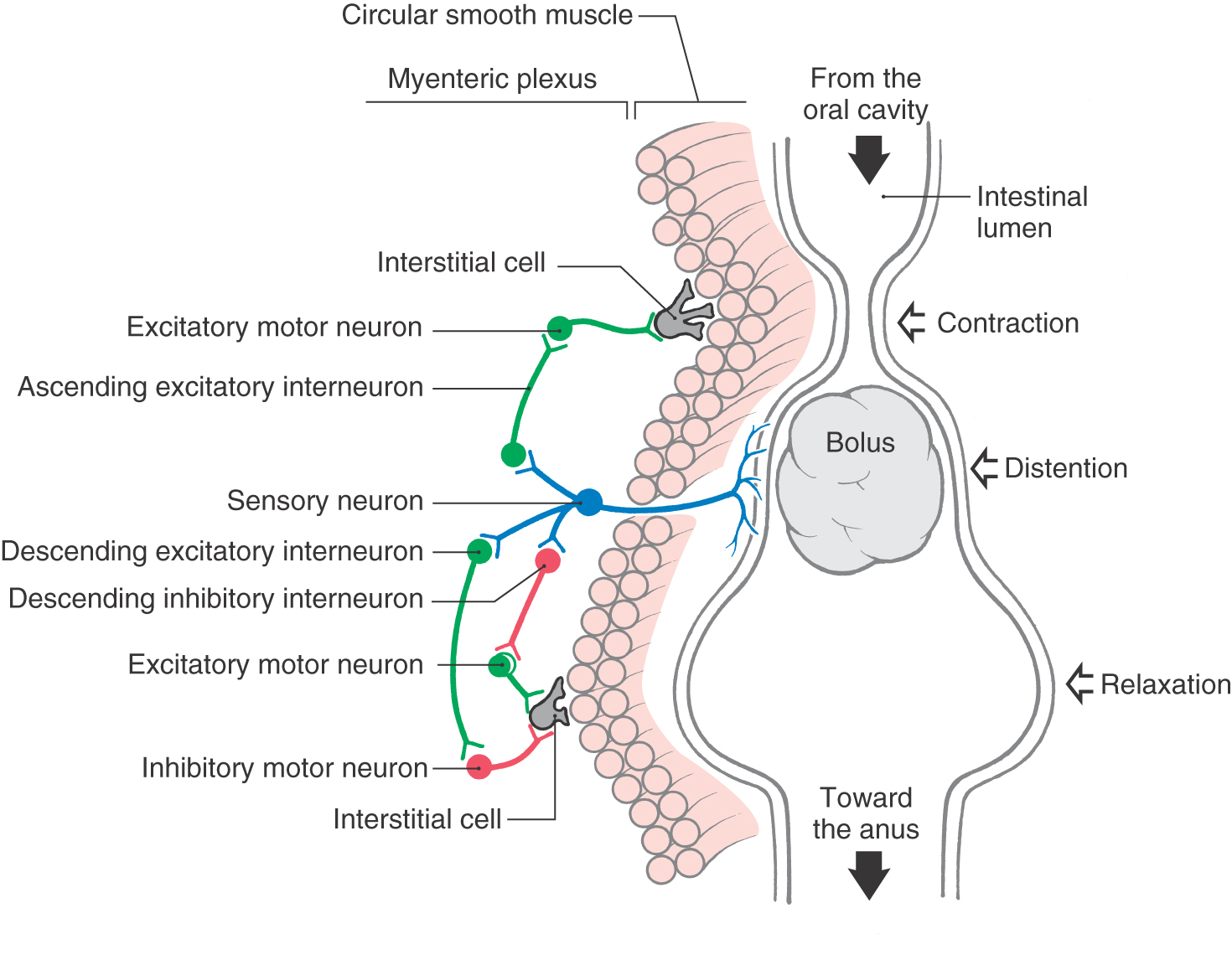
CNS influence over activities of the digestive system is conveyed by sympathetic and parasympathetic pathways. However, the digestive tract is able to perform its basic reflexive functions of secretion, absorption, and mixing and movement of luminal contents with a remarkable degree of independence from regulation by the CNS. This high degree of autonomy of digestive functions is possible because the wall of the gut (from esophagus to anus) is equipped with an elaborate intrinsic network of neurons termed the enteric nervous system or intrinsic nervous system of the gut (Fig. 29-11). The enteric nervous system is regarded by some authorities as the third division (along with the sympathetic and parasympathetic divisions) of the autonomic nervous system. It has been estimated to comprise roughly 100 million neurons, approximately the same number found in the spinal cord. The majority of enteric neurons are distributed within the myenteric and submucosal plexuses, although there are additional plexuses in the mucosa and serosa (Figs. 29-8 and 29-11). More than a dozen distinct functional types of intrinsic neurons have been identified, including several kinds of sensory neurons (mechanoreceptors, chemoreceptors, nociceptors), interneurons (excitatory, inhibitory, orally projecting, caudally projecting), and motor neurons (secretomotor, excitatory and inhibitory muscle motor).
In addition to its intrinsic nervous system, the gut wall is endowed with widely distributed smooth muscle–like cells, called interstitial cells of Cajal (ICC), that spontaneously initiate rhythmic electrical activity, much like the pacemaker cells of the heart (Fig. 29-11). These cells have extensive connections both with each other and with conventional smooth muscle cells, which are themselves electrically coupled. Thus ICC are responsible for generation and propagation of slow waves of depolarization in the smooth muscle layers of the gut wall. Although this ongoing wave pattern of electrical activity of smooth muscle is an intrinsic property of the gut wall, input from the enteric nervous system is necessary to translate the slow waves of depolarization into useful waves of contraction.
A prominent specific function of the enteric nervous system is the peristaltic reflex whereby the presence of ingested material in the intestine evokes waves of contraction and relaxation that slowly propel the material toward the anus (Fig. 29-11). The peristaltic reflex can be initiated either when the presence of a food bolus distends the intestinal wall and activates intrinsic mechanoreceptive sensory neurons or when the enteroendocrine cells in the lining epithelium respond to contents in the lumen by signaling chemoreceptive sensory neurons. In either case, the sensory neurons in turn activate populations of excitatory and inhibitory interneurons in the myenteric plexus (Fig. 29-11). Excitatory interneurons activate excitatory motor neurons upstream from the bolus and inhibitory motor neurons downstream from the bolus. Inhibitory interneurons act on excitatory motor neurons downstream. The result is propulsion of the bolus toward the anus by a combination of constriction of the segment of intestine above the bolus and relaxation (dilation) of the intestinal segment below the bolus.
In addition to intrinsic reflex pathways that generate peristalsis, additional local neural circuits bring about reflexive changes in other activities, such as absorption, local blood flow, and secretion. The last reflex is initiated in the epithelium by enteroendocrine cells that monitor the chemical composition of the luminal contents and activate sensory neurons. The sensory neurons then activate secretomotor pathways that bring about appropriate secretions by glandular cells. Although the peristaltic reflex and other basic reflexes can occur independently of external signaling, they are normally subject to extrinsic regulation by parasympathetic input (generally enhancement) and sympathetic input (generally inhibitory). Indeed, several more complex reflexes of the digestive tract, such as the defecation and enterogastric reflexes, are generated by parasympathetic and sympathetic circuits.
An extremely diverse array of neurotransmitters and neuromodulators is involved in the functions of the enteric nervous system. These include small molecules (acetylcholine, norepinephrine, adenosine triphosphate, serotonin), a gas (nitric oxide), and numerous polypeptides (substance P, calcitonin gene–related peptide, vasoactive intestinal peptide, cholecystokinin, dynorphin, enkephalins, neuropeptide Y). Some of the chemical messengers, such as serotonin (5-hydroxytryptamine), can be either excitatory or inhibitory, depending on the subtype of receptor expressed by the responding cell. The various molecular signaling systems provide important targets of present and future pharmacologic therapies for disorders of gut motility and secretion. For example, agonists and antagonists that target specific subtypes of neurotransmitter receptors are used to treat irritable bowel syndrome.
REGULATION OF VISCERAL MOTOR OUTFLOW
Sensory input occurs at every level of the visceral motor pathway, including prevertebral postganglionic neurons, preganglionic neurons, and a wide variety of CNS structures that project, either directly or indirectly, to preganglionic neurons. Many different kinds of sensory information are integrated by a series of CNS structures collectively termed the central autonomic network (CAN), which generates coordinated signals to the visceral motor, endocrine, and somatic motor outflow pathways. Although visceral motor activities are generally beyond conscious control, emotional status and mental activity clearly influence visceral structures. Accordingly, the CAN integrates input from higher CNS centers involved in cognition and complex behavioral functions.
Major Central Nervous System Components
The preganglionic neurons of the autonomic motor pathways are influenced by cells in various brainstem and forebrain areas. The hypothalamus is the highest integrator of autonomic and endocrine functions. It directly regulates the secretory activity of the anterior and posterior pituitary and has reciprocal connections with the solitary nucleus and other components of the CAN in the forebrain and brainstem. Some hypothalamic nuclei project directly to preganglionic visceral motor neurons in the dorsal vagal nucleus, nucleus ambiguus, and intermediolateral cell column. The organization and functions of the hypothalamus are considered in Chapter 30.
Because of its diverse connections, the solitary nucleus is the most important brainstem structure coordinating autonomic functions. It receives general and special visceral sensory input, and it projects to vagal motor neurons, to salivatory and reticular nuclei, and to populations of brainstem neurons, which in turn project to sympathetic preganglionic neurons. The solitary nucleus also has reciprocal connections with other components of the CAN.
Other cell groups that are important in autonomic regulation reside in the reticular formation of the brainstem. These neurons are not always restricted to specific nuclei, and they are therefore sometimes designated by their relative positions. For example, cells in the rostral ventrolateral medulla project to the intermediolateral cell column, particularly to preganglionic neurons involved in cardiovascular regulation. This area is called the vasopressor center because stimulation results in increased peripheral vascular resistance and increased cardiac output. Areas such as this have been designated centers, for example, the respiration center, micturition center, and vomiting center. Although this terminology is convenient, it should be understood that these are not well-defined anatomic entities but are components of widely distributed neural networks.
The importance of the supraspinal control of autonomic function is illustrated by some of the deficits associated with spinal cord injuries at higher levels (T6 or above). Initially, the interruption of descending reticulospinal and hypothalamospinal fibers that regulate sympathetic preganglionic neurons in the intermediolateral cell column is manifested as an overall reduction in sympathetic activity. Thus clinical signs include lowered blood pressure, orthostatic hypotension, and reduced heart rate (bradycardia). With time, hyperactivity of sympathetic reflexes (termed autonomic dysreflexia) develops, probably as a result of denervation hypersensitivity of sympathetic neurons and target tissues. Signs and symptoms include hypertension, urinary retention, piloerection, profuse sweating, and reduction of blood flow to peripheral tissues in response to any of a wide variety of noxious stimuli below the level of the spinal cord injury.
Cardiovascular System
The function of the cardiovascular system is influenced by mental activity, emotional state, posture, muscular exertion, visceral activity, body temperature, and concentrations of blood gases and electrolytes. In addition to mechanisms that regulate blood pressure, there is precise neural control of blood flow to specific organs and regions of the body.
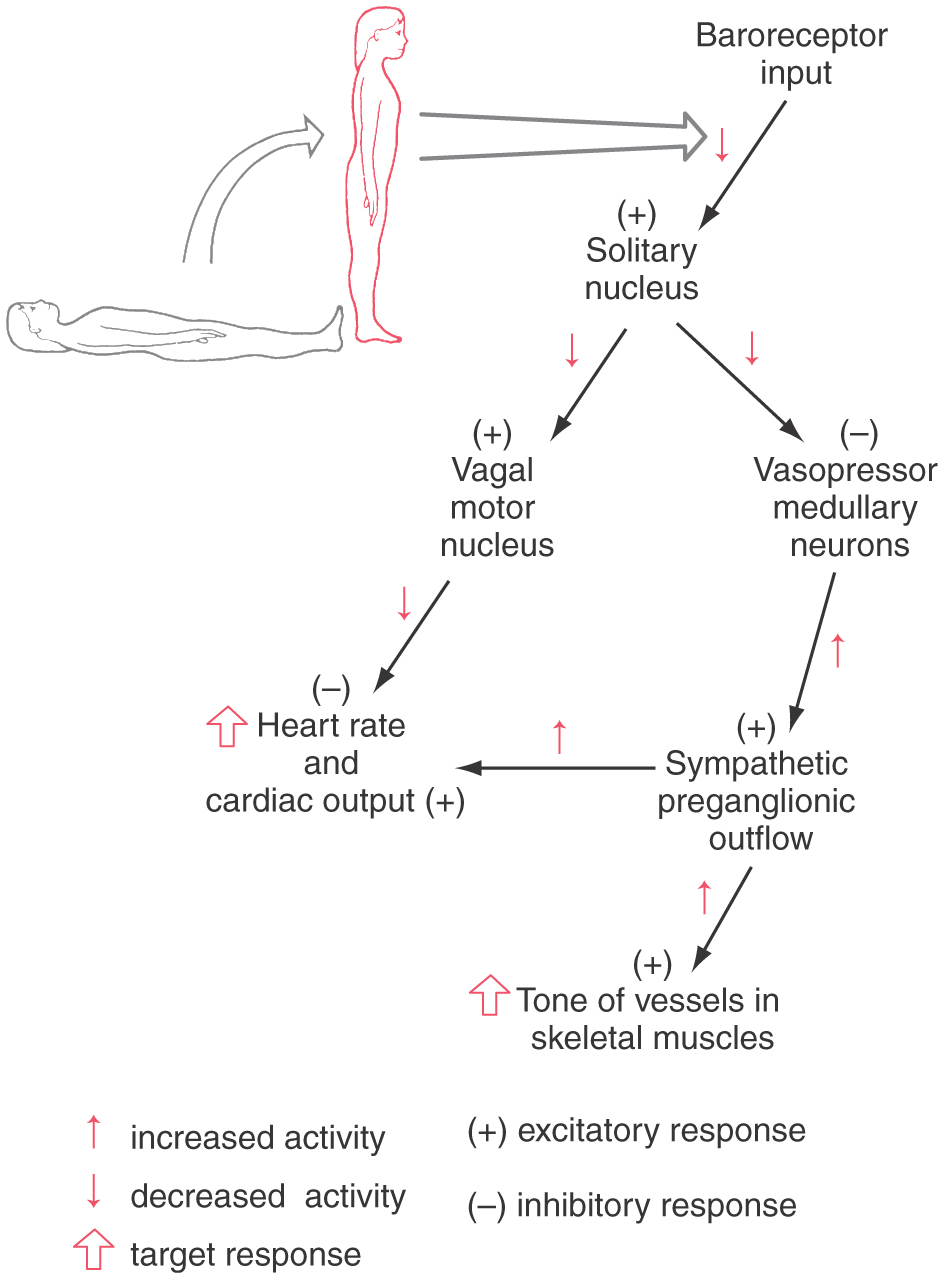
The baroreceptor reflex (Fig. 29-12) functions to buffer blood pressure against a sudden change in posture. Failure of this reflex results in orthostatic hypotension, a severe drop in blood pressure when the patient assumes an upright position. Primary visceral sensory neurons of the glossopharyngeal and vagus nerves convey signals from mechanoreceptors in the carotid and aortic sinuses centrally, where they terminate in the solitary nucleus (see Fig. 19-8). Projections of solitary neurons influence the tonic activity of parasympathetic (vagal) output to the heart and sympathetic output to the heart and peripheral vessels.
When a reclining individual stands, there is a rapid reduction in baroreceptor discharge that results in a decrease in signals from the solitary nucleus to two brainstem targets (Fig. 29-12). The first of these targets, the vagal preganglionic parasympathetic neurons that suppress heart rate and cardiac output, receive an excitatory drive from neurons of the solitary nucleus. Thus a reduction of baroreceptor discharge results in a release of the heart from this inhibitory parasympathetic drive. The second target of solitary cells consists of vasopressor neurons in the rostral ventrolateral medulla. The neurons in this region have intrinsic pacemaker features and receive inhibitory inputs from the solitary nucleus. When these rostral neurons are released from the inhibitory drive of solitary neurons, the result is increased sympathetic outflow (Fig. 29-12). This input is mediated via a major descending projection from these rostral medullary cells to the sympathetic preganglionic neurons in the intermediolateral cell column. Reduced inhibition of this excitatory projection results in increased cardiac output and increased resistance in vascular beds of skeletal muscle and abdominal visceral organs but not of the skin, heart, and brain. Some of this solitary input to vasopressor neurons may be relayed through vasodepressor cell groups in the caudal ventrolateral medulla. Thus when a person moves from a reclining to a standing posture, the resultant pooling of blood in the lower half of the body is quickly countered by increased vascular tone and increased cardiac output. Without this reflex, movement to a standing position results in dizziness or fainting because of decreased blood flow to the brain. This manifestation of orthostatic hypotension is a serious consequence of many forms of autonomic dysfunction.
The chemoreceptor reflex maintains homeostasis of blood gas composition by adjusting respiration, cardiac output, and peripheral blood flow. Decreased PO2 and increased PCO2, detected by receptors in the carotid and aortic bodies, are signaled by glossopharyngeal and vagal afferents that terminate in the solitary nucleus. Within the medulla, the reflex pathway for cardiovascular effects parallels that for the baroreceptor reflex. A decrease in blood PO2 activates this reflex and promotes increased heart rate and vascular tone. These changes result in a decreased blood flow to skeletal muscles and viscera, whereas blood flow to the brain is maintained. Thus proportionately more oxygenated blood is available to the brain than to skeletal muscle and viscera. The resulting conservation of oxygen preserves vital functioning of the CNS.
The cardiovascular component of the chemoreceptor reflex is closely coordinated with respiration, a somatic motor function coordinated by other neurons of the brainstem reticular formation. For example, if breathing is suspended (as in diving), heart rate is slowed (bradycardia) rather than accelerated (tachycardia).
Urinary Bladder and Micturition
Emptying of the urinary bladder, micturition, is brought about by contraction of smooth muscle of the bladder wall (detrusor muscle) and relaxation of smooth muscle of the internal urethral sphincter and of skeletal muscle of the external urethral sphincter (Fig. 29-10). Contraction of the detrusor and inhibition of the internal sphincter are mediated by parasympathetic outflow. Preganglionic neurons from the sacral cord innervate postganglionic neurons in the bladder wall (Fig. 29-10). The bladder wall also has a sympathetic innervation. Its influence is mainly inhibitory on both the detrusor muscle and the parasympathetic postganglionic neurons in the bladder wall (Fig. 29-10). The external urethral sphincter, which is subject to both reflex and voluntary control, is supplied by alpha motor neurons in segments S3 and S4.
During periods of urine storage, activity of bladder afferent neurons is low. The low activity of the sensory neurons results in (1) low activity of parasympathetic excitatory innervation to the detrusor and inhibitory innervation of the internal sphincter, (2) tonic activity of sympathetic neurons that inhibit both the parasympathetic ganglion cells in the bladder wall and the detrusor muscle directly, and (3) tonic activity of sacral somatic motor neurons mediating constriction of the external sphincter. As urine accumulates, pressure on the bladder wall activates tension receptors until bladder afferent activity rises to a threshold level. This increased activity of bladder afferents induces micturition by way of both spinal and brainstem reflexes that result in inhibition of sympathetic outflow, activation of parasympathetic outflow, and inhibition of somatic motor neurons supplying the external sphincter muscle. Urinary tract infections can cause irritation of the afferent innervation of the bladder lining and consequent activation of the micturition reflex. Thus the contraction of the detrusor muscle can induce a strong urge to void the bladder even when urine volume is low.
Sources and Additional Reading
Appenzeller O. The Autonomic Nervous System. ed 4 Amsterdam: Elsevier; 1990.
Brodal P. The Central Nervous System: Structure and Function. Oxford: Oxford University Press; 1998.
Gabella G. Structure of the Autonomic Nervous System. London: Chapman and Hall; 1976.
Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115.
Jänig W, Schmidt RF, eds. Reflex Sympathetic Dystrophy. New York: VHC Publishers; 1990.
Low PA, ed. Clinical Autonomic Disorders. Evaluation and Management. Boston: Little, Brown; 1992.
Shephard GM. Neurobiology. ed 3 New York: Oxford University Press; 1994.