Viral Infections
Raegan D. Hunt, Sheila Fallon Friedlander
Introduction
Viral infections can induce a remarkable variety of cutaneous manifestations in the neonate and toddler. The clinical manifestations of any viral infection are influenced by the virulence, tissue tropism, and age at which the infection is acquired. Infections may occur in utero, perinatally (acquired between the onset of labor and the delivery), or postnatally. Skin findings may be a result of local invasion and infection, or an indirect result of viral infection of other tissues. Diagnosis of infection is based on the morphology and distribution of the skin lesions, as well as the overall clinical presentation, supported by specific laboratory studies when possible. Rapid diagnosis and appropriate antiviral therapy maximize the possibility of a positive outcome for the infant.
Herpes simplex virus
Herpes simplex virus (HSV) types 1 and 2 are pathogens for the fetus, newborn, infant and toddler, which induce a spectrum of clinical disease. Specific manifestations depend on the time and route of exposure as well as maternal immune status (primary vs recurrent maternal infection). HSV is a large, double-stranded DNA virus that can produce an acute primary infection in the susceptible host. In addition, HSV-1 and -2, like other herpes viruses, have the ability to integrate into host DNA and establish latency. Poorly understood host and environmental factors can cause reactivation of virus within latently infected sensory ganglia, leading to recurrent, active infections. Neonatal infection occurs most often as a direct result of active maternal infection, usually from primary infection acquired during pregnancy. The rate of neonatal disease has been shown to parallel the rate of genital herpes in a community.1
Epidemiology
Neonatal infection is estimated to occur at a rate of 1 in 3000 to 1 in 20 000 live births. Infection of the fetus or newborn may occur during gestation (in utero), at the time of labor and delivery (perinatally), or following the delivery (postnatally). Although the majority of neonatal infections (80–90%) are considered to be acquired perinatally, both in utero infections (4%) and postnatal infections (10%) have been well documented.2
Most affected neonates acquire infection through contact with infectious genital secretions or herpetic lesions at the time of vaginal delivery. Although primary infection in the pregnant woman usually leads to symptomatic illness, a significant proportion of women with primary infection do not have recognizable systemic or local disease.3 Primary maternal infection is usually associated with prolonged shedding (2–3 weeks) of high titers of virus from lesions, while the maternal immune response develops, in contrast to the more limited viral shedding and much shorter duration of lesions (2–5 days) with recurrent disease in women with specific humoral and cellular immunity. Neonatal infection occurs in up to 50% of infants born to mothers during primary infection, compared with an estimated 2% or less of infants born to mothers suffering from recurrent infection; pre-existing protective maternal antibodies likely explain this difference in clinical outcome. Active maternal infection at the time of delivery, based on viral culture, is thought to occur in approximately 1–7 per 1000 births.4 However, data based on polymerase chain reaction (PCR) techniques from genital specimens at delivery suggest that active maternal infection may occur up to eight times more frequently than previously appreciated.5 Prospective studies have documented that the majority of women with active infection at the time of delivery are asymptomatic, suggesting that improved rapid laboratory diagnosis and careful examination are needed to identify the at-risk mother and infant.
The optimal strategy for preventing neonatal HSV infection is controversial. Discordant HSV status of sexual partners is a risk factor if the female is susceptible, and queries regarding partner status and advice regarding abstinence near term for susceptible women with infected partners are appropriate. Routine HSV screening in pregnant women and antepartum HSV cultures in asymptomatic women with history of recurrent disease are not recommended. Based on evaluation of available limited or inconsistent scientific evidence (Level B), the American College of Obstetrics and Gynecology recommends that women with active recurrent genital herpes should be offered suppressive viral therapy at or beyond 36 weeks’ gestation and that cesarean delivery is indicated in women with active genital lesions or prodromal symptoms (e.g., vulvar pain or burning) at the time of delivery.6 Cesarean delivery is not recommended for women with a history of HSV who do not have active disease or have only non-genital disease at the time of delivery. Importantly, neonatal herpes infection has been described in infants born to mothers taking antiviral suppressive therapy and such cases may present with an atypical clinical picture and/or viral resistance patterns.2,7
Postnatal infections may be transmitted from both maternal, non-genital sites (including breast lesions) and non-maternal sources, such as other family members or nosocomial transmission from healthcare workers. In the absence of an HSV lesion on the breast, breast-feeding is not contraindicated. Active handwashing and awareness of transmission risk should be employed by all individuals with a history of or active HSV who are in contact with an infant.
Intrauterine HSV infection
Congenital (intrauterine) infection was described in 1966 by Sieber and colleagues, in an infant with culture-positive lesions, seizures, and evidence of immunity at the time of a normal delivery in which the amniotic membranes were ruptured at birth.8 In 1969, South described an infant with microcephaly, microphthalmia, seizures, and vesicular lesions on the fingers and toes following a maternal primary HSV-2 genital infection during the first month of pregnancy.9 Subsequent studies of congenital infection have documented the presence of specific cell-mediated immunity to HSV in the newborn at birth, whereas infants infected during labor and delivery do not usually develop cellular immunity until the second week of the infection.10
Infection in utero may occur either as a result of ascending infection through apparently intact membranes, or as a result of viremia occurring with a primary maternal infection. Fetal infection often leads to fetal death; however, if the fetus survives, delivery may occur at term with late sequelae in both skin and central nervous system (CNS).
Cutaneous findings
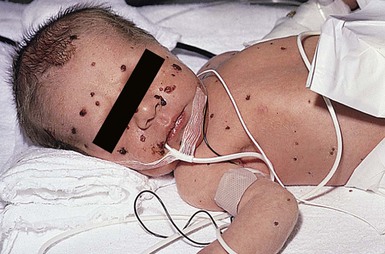
Skin manifestations at delivery are the result of residua from primary fetal infection in addition to latent virus reactivation at previous cutaneous sites of fetal infection. Skin lesions are common in the neonatal period (Fig. 13.1). In one study, 70% of infected infants had vesicular lesions, and 30% also had evidence of scar formation on the face, trunk, or extremities.11 In addition, aplasia cutis congenita-like skin findings and lesions characteristic of epidermolysis bullosa have been described.12 Atrophic limbs, previously reported with congenital varicella virus infection, have also been documented with congenital HSV infection.13
Extracutaneous findings
HSV infections acquired in utero in which the infant completes a normal gestation are almost invariably associated with CNS damage, which is easily detected by computed tomography (CT). CNS changes indicate longstanding destruction of neuronal tissue without acute inflammation. Microcephaly is present in over 50%, and chorioretinitis is present in 60% of infants with congenital HSV infection. Although skin and CNS abnormalities are present at birth, infected infants often do not show the signs of systemic toxicity and overwhelming sepsis that may occur with primary perinatal or postnatal infection.
Neonatal (perinatal) herpes simplex infection
Neonatal disease occurs in three clinically recognized syndromes, all acquired in the perinatal period: infection localized to the skin, eyes, or mouth (SEM disease); disseminated infection; and CNS infection. Exposure to maternal primary infection at the time of delivery may lead to overwhelming infection in the neonate, with a high mortality rate, or a more slowly progressive, insidious disease in which the infant has only mucocutaneous manifestations or develops slowly progressive neurologic symptoms. The incubation period varies substantially, from clinical symptoms at delivery due to presumed ascending infection through non-intact membranes, to infection presenting as late as 6 weeks of age. Infants with disseminated and SEM disease have an earlier onset, typically presenting between the first and second weeks of life. The variability of the incubation period is dependent on the integrity of the amniotic membranes, the inoculum of virus, the tissue inoculated (e.g., skin, mucous membrane), and the presence or absence of transplacental specific antibody.
Cutaneous findings
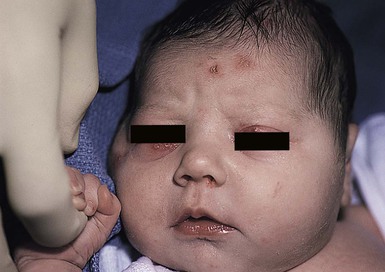
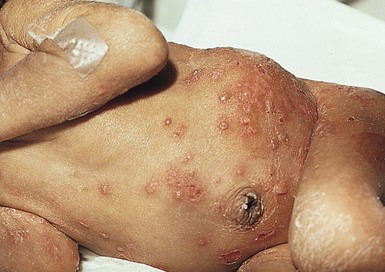
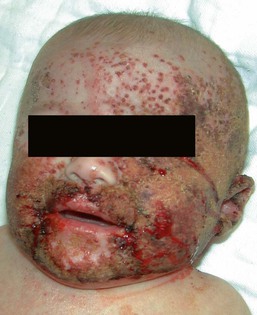
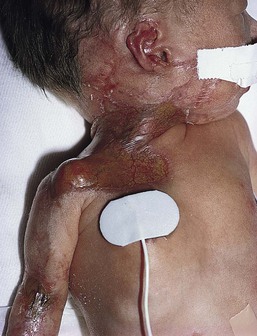
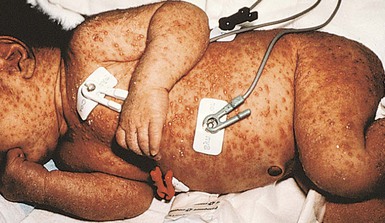
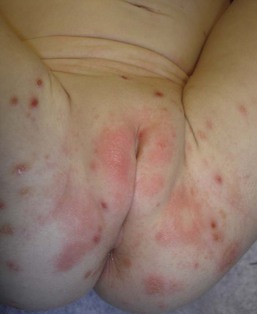
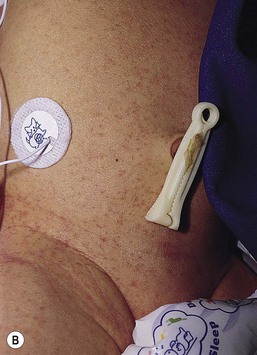
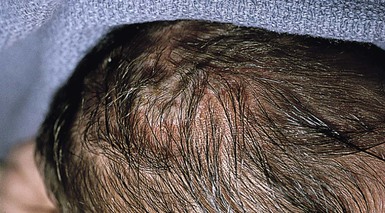
Cutaneous or mucosal lesions (mouth, nose, eye) can occur with or without signs of sepsis or encephalitis. Infants with skin, eye, or mouth disease account for 40% of all neonatal cases of HSV infection. The skin lesions appear as small, 2–4 mm vesicles, with surrounding erythema, often in herpetiform (zosteriform) clusters (Fig. 13.2). They usually occur on the part of the body which was in prolonged contact with the cervix. Often, lesions will occur at sites where the skin integrity has been breached. One of the most common sites of cutaneous infection is on the scalp vertex at the site of placement of fetal scalp monitor electrodes (Fig. 13.3). Vesicular lesions usually develop within the first 1–2 weeks of life following inoculation at this site. Lesions may progress locally, or disseminate (Fig. 13.4). In areas of mucosal involvement, a shallow ulceration with moderate inflammation is most often seen. The ulceration may be focal, with the lesion size closely resembling that of a cutaneous vesicle, or ulcerations may spread irregularly, coalescing over a much larger area. Lesions tend to follow the clinical stages of vesicle resolution seen in the older child, with pustulation 24–72 h after the appearance of the vesicle, followed by eschar formation. Skin lesions are present in most neonates with disseminated disease (77%), and in 60% of infants who present with CNS disease. In any newborn with skin or mucosal lesions of HSV, even without a history of symptomatic illness, an investigation must be undertaken to rule out disseminated or CNS disease.
Extracutaneous findings
HSV dissemination is the most devastating manifestation, presenting in the more premature infant (average gestational age at birth of 36.5 weeks) at an average chronologic age of 11 days. Multisystem involvement is analogous to overwhelming bacterial sepsis. Shock, disseminated intravascular coagulation, and multiple organ system failure are characteristic. Involvement of the lung, liver, and brain is common. The mortality rate is high. Without antiviral therapy, approximately 75% of infants will die, and even with specific antiviral therapy, mortality is still significant. Neurologic sequelae in survivors are also common, occurring in approximately 40%. Statistically, these infants have the lowest average circulating concentration of antibody to HSV.
In as many as 40% of infants ultimately diagnosed with disseminated or CNS infection, clinical disease begins with skin lesions only. Clinical and laboratory evidence of dissemination or CNS involvement not obvious at the time of presentation may develop during the first days of treatment, despite antiviral therapy.
Infants with HSV encephalitis present at a slightly older age (mean 17 days) and tend to be full term, in contrast with newborns with other clinical presentations. Antibody titers are higher in this group, leading to speculation that antibody may modify the progression of disease, with virus inoculated at delivery producing a clinically undetectable initial infection which spreads from mucosal sites to the CNS. Subtle neurologic symptoms are often present for days before the parent recognizes that the infant requires medical attention. As the child becomes more irritable and seizures become more pronounced, infants are hospitalized and evaluated. Skin lesions are only present in 60% of infants with HSV encephalitis, making the diagnosis difficult in many.
Diagnosis
The diagnosis of a herpes virus infection can be made in several ways (Table 13.1). HSV grows rapidly and easily in cell culture, and evidence of infection may be evident within the first few days after inoculation. Culture remains the most accurate means of diagnosis, but adjunctive tests may provide more rapid results. All suspected cases should have the following samples collected: (1) swab specimens from mouth, nasopharynx, conjunctiva and anus for HSV culture (the same swab can be used to test all sites, ending with the anus); (2) skin vesicle and CSF for PCR and culture analysis; (3) whole blood sample for PCR; and (4) blood sample for ALT testing. The most rapid results can be obtained from scraping of a vesicle sent for histopathologic Tzanck smear analysis, but this test is probably the most unreliable due to interpreter variability and sampling error. Direct fluorescent antibody testing (DFA) is rapid and specific, but not as sensitive as culture. CNS evaluation is necessary to rule out encephalitis. This includes traditional CSF analysis, as well as PCR. CT of the brain may be helpful in the diagnosis of encephalitis but is not considered sensitive until after 5 days of CNS symptoms. Magnetic resonance imaging (MRI) is more sensitive for CNS inflammation of the temporal lobes – the preferred sites of viral replication – and may be diagnostic within 3 days of onset of symptoms. EEG can also be helpful in CNS infections localized to the temporal lobe and may be positive earlier than any imaging study.
TABLE 13.1
Diagnosis of infection
| Virus | Culture | Histology of skin lesion | PCRa | Serology |
| HSV | Widely available and reliable; culture skin lesions, eyes, mouth, CSF, rectum; recommend setting up culture promptly after obtaining specimen | Tzanck stain of epithelial cells from the base of a vesicle is specific for alpha herpesviruses HSV and VZV; direct fluorescent antibody stains for HSV 1 or 2 are specific; DFA stains are specific and sensitive | Highly sensitive; best studied on CSF (sensitivity of skin lesions not well studied); plasma HSV PCR available in some centers | Rising antibody titer to HSV IgG is a sensitive and specific test; HSV IgM is not a sensitive test in the newborn |
| VZV | Available in many reference laboratories; culture skin lesions; recommend setting up culture promptly after obtaining specimen | Tzanck stain (see above): specific stains available for VZV | Highly sensitive, but not well studied on skin lesions; not widely available | Rising antibody titer to VSV IgG is a sensitive and specific test; VZV IgM is not a sensitive test in the newborn |
| CMV | Widely available; reliable; culture urine, saliva; urine shell vial culture technique yields results in 48–72 h | Skin lesions are due to extramedullary hematopoiesis, not due to viral replication in skin | Highly sensitive; well studied in plasma as a marker of disseminated CMV infection in immuno-compromised hosts | Rising antibody titer to CMV IgG is a sensitive and specific test; CMV IgM is not a sensitive test in the newborn; serologies are rarely used to diagnose neonatal CMV |
| Rubella | Not usually available; culture pharynx respiratory secretions, CSF, tissue | Skin lesions are due to extramedullary hematopoiesis, not due to viral replication in skin | Not well studied | Rising antibody titer to rubella IgG is a sensitive and specific test; rubella IgM is not a sensitive test in the newborn. Compare mother’s prenatal serology test results with those of the infant at the time of birth |
| Enterovirus | Widely available in viral culture laboratories; culture vesicular lesions, pharynx (during the acute phase of illness), and stool (up to 6 weeks following the illness); some strains including coxsackie virus A6 may be difficult to culture | Nonspecific | Highly sensitive; widely available | Usually not helpful |
| Parvovirus B19 | Not available in standard cell culture | Placental and fetal tissues – intranuclear inclusions in nucleated erythroid cells; EM and immunohistochemistry may be helpful | Highly sensitive; can be used on amniotic fluid, fetal plasma and tissues | IgM or IgG seroconversion helpful in pregnant female. Fetal IgM specific but not sensitive |
| HIV | Available; not highly sensitive in newborn; not recommended | Nonspecific | Sensitive means of diagnosis, can be repeated at 1–2 months and 4–6 months of age as needed; plasma PCR is the preferred test for infants <12 months of age | Maternal antibody present at birth may persist, especially under 4–6 months and up to 18 months; serologic testing may be most helpful after 12–18 months of age |
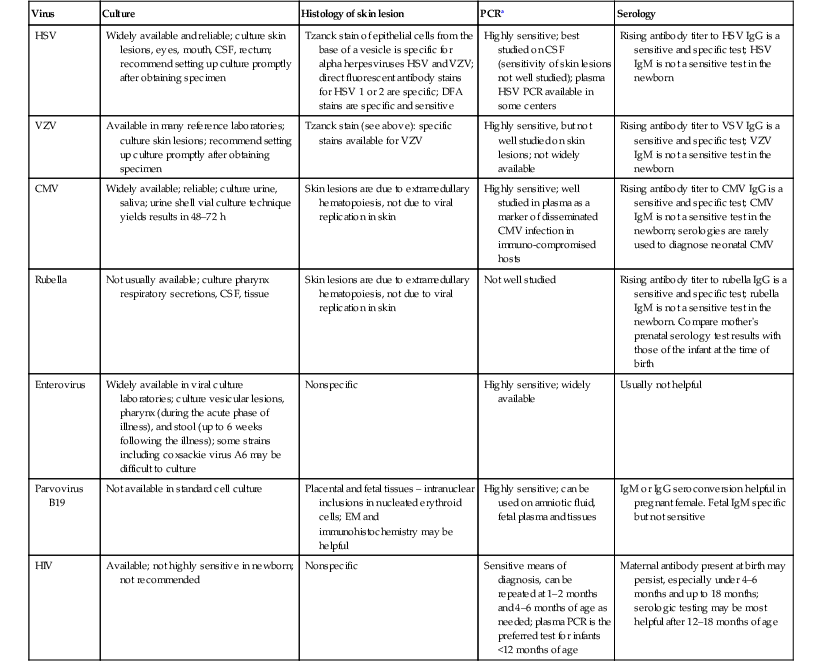
a Viral PCR panels are available in some centers and PCR diagnostic technology is rapidly evolving.
Differential diagnosis
Disseminated infection with HSV produces a clinical picture similar to that of early-onset neonatal sepsis caused by group B Streptococcus, enteric Gram-negative bacilli, and Listeria. Empiric therapy with antibiotics (standard management for the hospitalized ill neonate) will have no effect on the progression of HSV disease. For infants with progressive clinical symptoms of sepsis and sterile bacterial cultures of blood, urine, and CSF, HSV should be considered as a potential pathogen.
Other viral infections in the newborn period may also be confused with HSV. Enteroviral infection can cause a wide spectrum of clinical signs in the neonate, from fever and irritability to overwhelming sepsis with multiple organ system failure, to aseptic meningoencephalitis with minimal symptoms of systemic toxicity. Enteroviral infections may be associated with cutaneous vesiculopustular lesions. Neonatal seizures caused by enteroviral infections are the result of diffuse CNS irritation, in contrast to the focal temporal lesions of early HSV disease. Destructive changes of HSV that are appreciated on serial imaging studies of the CNS (by either CT or MRI) are not generally seen with enteroviral disease.
Perinatal varicella may produce overwhelming sepsis in the newborn. The density of cutaneous lesions in neonatal varicella usually far exceeds that seen with HSV infections, which characteristically produce a focal cluster of lesions at the site of inoculation with minimal cutaneous dissemination. However, both demonstrate the identical findings of multinucleate giant cells on Tzanck preparations. Only virus-specific staining techniques, PCR, or culture will be able to differentiate between these viruses.
Other viral pathogens occasionally cause severe acute disease in the newborn, including influenza A and B, parainfluenza 1, 2, and 3, and adenovirus. In general, the seasonal context of the infection, exposure history, and a predominance of respiratory tract symptoms help differentiate these infections. Viral cultures of the respiratory tract will assist in the identification of these pathogens.
Incontinentia pigmenti may present with localized vesicles, which may be mistaken for herpes simplex infections. These infants often have peripheral eosinophilia. Biopsy will reveal increased numbers of eosinophils, and cultures will be negative for HSV. Vesicular lesions that appear herpetic can also occur in Langerhans’ cell histiocytosis. Tzanck preparation will reveal histiocytes, and biopsy will show large numbers of histiocytes and an absence of multinucleate giant cells. Additionally, epidermolytic hyperkeratosis (EHK), epidermolysis bullosa (EB), congenital erosive and vesicular dermatosis, and ankyloblepharon-ectodermal dysplasia clefting syndrome can present with denuded eroded skin and bullae which may be confused with HSV.
Management and prognosis
The treatment of choice in neonatal herpes infections is acyclovir administered intravenously, regardless of the clinical presentation (Table 13.2). Acyclovir 60 mg/kg per day in three divided doses should be given for 14 days for SEM, and for a minimum of 21 days in those with disseminated or CNS disease.14 In infants with renal failure, the doses should be reduced accordingly. The mortality rate for disseminated disease is about 29%, even with therapy. Approximately 50% of infants will experience cutaneous recurrences. Daily suppressive doses of oral acyclovir have been recommended to prevent skin recurrences and, in infants with CNS disease surviving neonatal HSV, treatment with suppressive doses of oral acyclovir for 6 months after the initial 14–21 day parenteral acyclovir regimen led to improved neurodevelopmental outcomes at 1 year of age.15 Valacyclovir is also efficacious in the treatment of HSV, but no oral liquid formulation currently exists. Pharmacokinetic studies have shown inter-infant drug clearance variability in those less than 3 months of age, and some experts therefore do not recommend use in very young infants.16
TABLE 13.2
Herpes virus infections in neonates and infants
| Herpes virus | Age at infection | Clinical findings | Treatmenta |
| HSV | Intrauterine | Congenital HSV | |
| SKIN LESIONS | Parenteral acyclovir 60 mg/kg per day divided three times daily ×21 days (minimum) | ||
| Vesicles (~70%), scar-like lesions (~30%), reports of lesions concerning for epidermolysis bullosa or aplasia cutis congenita | |||
| ASSOCIATED ABNORMALITIES | |||
| Atrophic limbs, microcephaly (~50%), chorioretinitis (~40%) | |||
| Neonatal/perinatal | SEM disease | ||
| Infection localized to the skin, eyes, or mouth | Parenteral acyclovir 60 mg/kg per day divided three times daily ×14 days; if ocular involvement: add ophthalmic antiviral (1% trifluridine, 0.1% iododeoxyuridine, or 3% vidarabine) | ||
| SKIN LESIONS | |||
| Vesicles (2–4 mm), with surrounding erythema, often in herpetiform (zosteriform) clusters; mucosal ulcerations, small and round (2–4 mm) or large and irregular | |||
| Disseminated infection; CNS infection | |||
| SKIN LESIONS | Parenteral acyclovir 60 mg/kg per day divided three times daily ×21 days (minimum) | ||
| Vesicles and/or erosions described above may or may not be found | |||
| SYSTEMIC FINDINGS | |||
| Septic shock, disseminated intravascular coagulation, multiple organ system failure, seizures | |||
| VZV | Intrauterine | Fetal varicella syndrome/varicella embryopathy | Prevention |
| See Table 13.3, Features of fetal varicella syndrome | Vaccination of women prior to childbearing; treatment of exposed, susceptible pregnant women with VZIG as soon as possible (within 48 and up to 96 h after exposure); consideration of treatment of pregnant woman with acyclovir | ||
| Care of affected infant | |||
| Supportive | |||
| Neonatal/perinatal | Neonatal varicella | Prevention | |
| SKIN LESIONS | Neonates born to mothers who have developed varicella from 5 days before to 2 days after delivery should receive VZIG intramuscularly as soon as possible after delivery | ||
| Variable; red macules which mature into small vesicles; hemorrhagic and/or necrotic vesicles | Treatment | ||
| Parenteral acyclovir, 60 mg/kg per day divided three times daily ×10–21 days | |||
| Infant (1–23 months) | Infantile herpes zoster | ||
| SKIN LESIONS | Acyclovir 20 mg/kg per dose by mouth 4 times daily ×10 days | ||
| Grouped erythematous papules that vesiculate and crust in a dermatomal distribution | |||
| Infantile varicella | |||
| SKIN LESIONS | HEALTHY CHILDREN | ||
| Crops of vesicles on erythematous bases ‘dewdrop on a rose petal’ in different stages | Supportive care | ||
| ASSOCIATED FINDINGS | IMMUNOCOMPROMISED CHILDREN | ||
| Fever, headache, myalgias | Intravenous acyclovir should be started within 24–72 h (<1 year of age, 30 mg/kg per day divided every 8 h for 7–10 days; >1 year of age, 1500 mg/m2 per day divided every 8 h ×7–10 days). | ||
| VZV EXPOSURE OF IMMUNOCOMPROMISED PATIENTS | |||
| VZIG should be administered within 48 h of exposure (may be given up to 96 h) |
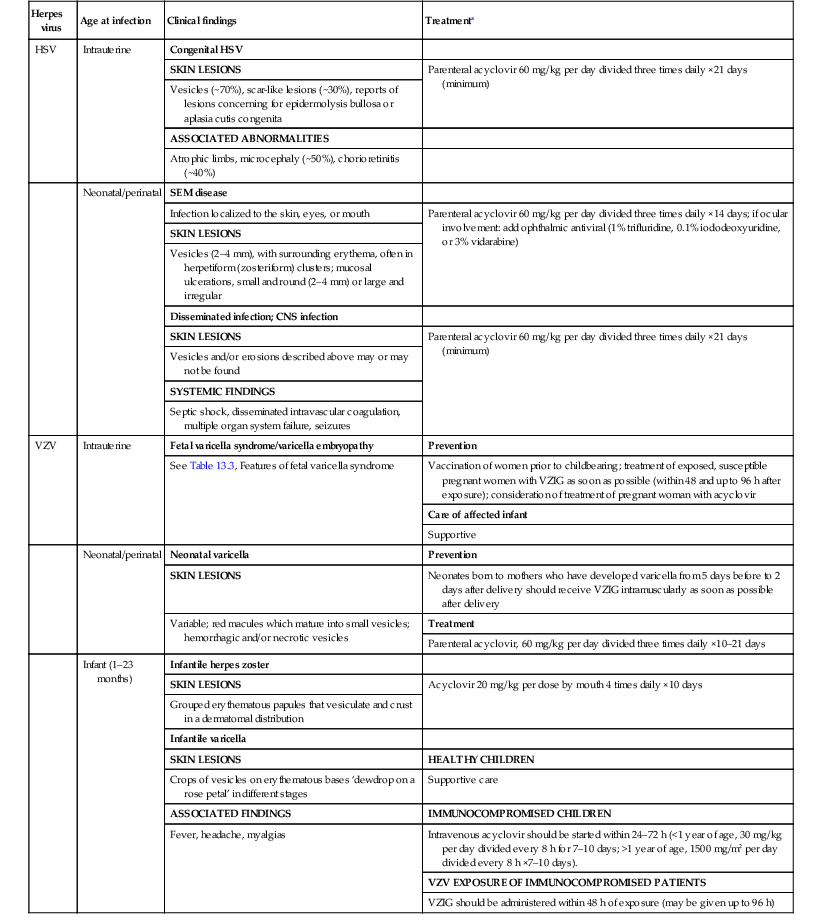
a Adapted from Red Book: 2012 Report of the Committee on Infectious Disease, American Academy of Pediatrics, 2012.
HSV in children beyond the neonatal period
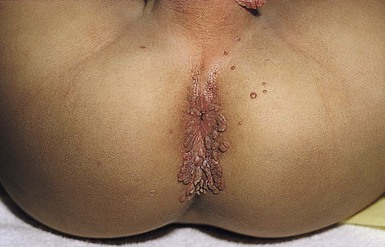
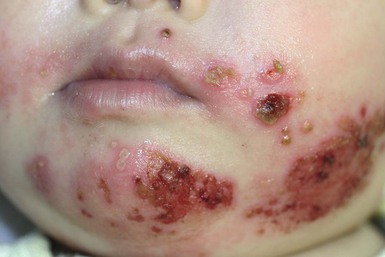
At least 25% of US children have serologic evidence of HSV-1 by age 7 years. HSV infections in young children may present with clusters of erosions and vesicles (Fig. 13.5). However, most primary, non-neonatal HSV infections are asymptomatic. Gingivostomatitis secondary to HSV-1 is the most common presentation in childhood, generally occurring from 1 to 5 years of age. Children may be quite ill, with fever, drooling, submandibular adenopathy, and diffuse edema and erythema of the gingiva and oral mucous membranes. Extension of lesions to the lips, chin and cheeks often occurs. Ulcers, erosions, and oral symptoms can be so severe that hospitalization may be required for intravenous hydration support. The differential diagnosis includes coxsackie virus infection, Stevens–Johnson disease, and aphthous ulcers.
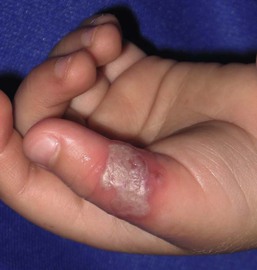
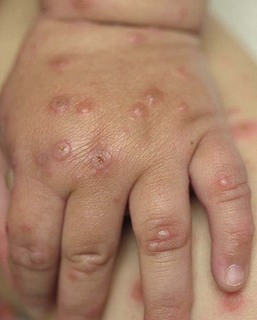
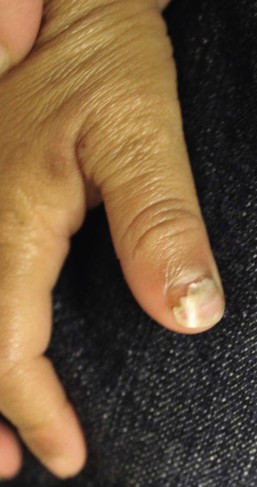
Isolated HSV labialis may also occur, but less frequently. HSV infection of the pulp of the distal phalanx of a digit, termed herpetic whitlow can occur in children as an isolated finding. Such lesions are slate-gray, vesicular, and painful (Fig. 13.6![]() ). They may result from autoinoculation or inoculation from another infected individual. Genital lesions can also occur in young children with vesicular and eroded lesions, but should always raise concern for sexual abuse.
). They may result from autoinoculation or inoculation from another infected individual. Genital lesions can also occur in young children with vesicular and eroded lesions, but should always raise concern for sexual abuse.
Eczema herpeticum
This form of severe HSV cutaneous infection develops in individuals who suffer from a chronic skin condition. Though most commonly seen in association with atopic dermatitis, it can be associated with disorders such as Darier disease or pemphigus vulgaris. Patients develop vesiculations and erosions predominantly in affected dermatitis sites where the skin barrier is presumably impaired and more vulnerable to viral invasion. The lesions often have a ‘punched-out’ monomorphous appearance, and vesicles may not be evident (Fig. 13.7). These children may have high fevers and appear ill. Secondary bacterial infection of the skin and keratoconjunctivitis may also develop. The differential diagnosis includes varicella infections, including zoster, and secondary bacterial infection of primary skin conditions. Families should be counseled regarding the risk of recurrence, and the need to protect their child from direct exposure to active herpes lesions in others.
Therapy of HSV infections in children depends on the severity of involvement and underlying immune considerations. Immunocompromised children and those with eczema herpeticum will often benefit from intravenous therapy. Bland emollients are also often utilized; in children with eczema herpeticum, topical corticosteroids are then added once the infection appears to be responding to antiviral therapy. Most other conditions can be treated with oral therapy if treatment it desired.
Varicella
Varicella (chickenpox) is usually a benign, self-limited disease when it occurs in immunocompetent individuals during childhood. The developing fetus and neonate, however, are at higher risk for an adverse outcome following infection. Fortunately, more than 90% of women are estimated to have varicella zoster virus (VZV) IgG seropositive status, thereby conferring immunity to the fetus. In addition, women planning conception are encouraged to seek VZV vaccination if they do not have evidence of prior immunity, substantially decreasing the risk of fetal infection. The widespread use of the varicella live attenuated (Oka/Merck) vaccine has led to a lower incidence of VZV disease in the general population, thereby decreasing the exposure risk to expectant mothers and infants. However, the vaccine is not 100% effective, and healthcare practitioners must be aware of the possible manifestations of varicella infection in neonates, as well as appropriate therapy.
The exact incidence of varicella during pregnancy is unknown, but is estimated to be between three and 10 cases per 10 000 pregnancies. Fetal or early neonatal exposure may result in a variety of manifestations, ranging from minimal cutaneous lesions to significant morbidity and death. Three distinct disorders may occur following intrauterine or neonatal exposure to VZV: fetal varicella syndrome, neonatal varicella, and infantile herpes zoster.
Fetal varicella syndrome
Congenital defects predominantly involving the skin, nervous system, and musculoskeletal system can occur following fetal exposure to varicella virus. Other terms for the fetal varicella syndrome include varicella embryopathy and congenital varicella syndrome.
Cutaneous findings
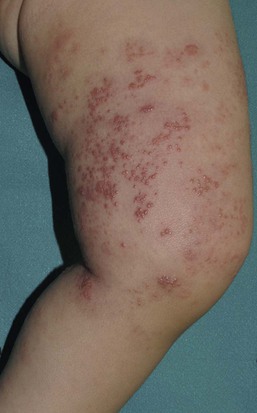
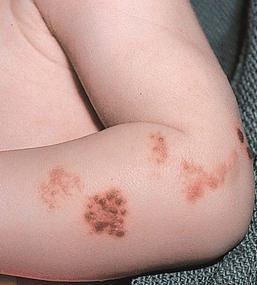
Specific anomalies include cicatricial skin lesions that correspond to a dermatomal distribution, often with hypoplasia of underlying tissues. These lesions may initially appear as denuded areas and subsequently develop stellate or angular scars (Fig. 13.8).
Extracutaneous findings
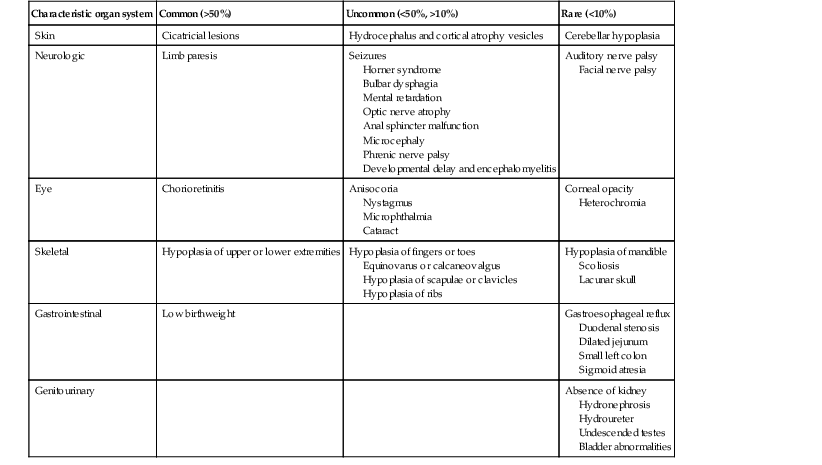
Low birthweight is a common finding in affected infants. The varied extracutaneous manifestations of this syndrome can be grouped as neurologic, musculoskeletal, ophthalmologic, gastrointestinal, and genitourinary. Limb paresis and hypoplasia of the extremities are common findings, as is chorioretinitis. Less common findings include microphthalmia, cataracts, nystagmus, hydrocephalus, and mental retardation (Table 13.3).
TABLE 13.3
Features of fetal varicella syndrome
| Characteristic organ system | Common (>50%) | Uncommon (<50%, >10%) | Rare (<10%) |
| Skin | Cicatricial lesions | Hydrocephalus and cortical atrophy vesicles | Cerebellar hypoplasia |
| Neurologic | Limb paresis | Seizures Horner syndrome Bulbar dysphagia Mental retardation Optic nerve atrophy Anal sphincter malfunction Microcephaly Phrenic nerve palsy Developmental delay and encephalomyelitis |
Auditory nerve palsy Facial nerve palsy |
| Eye | Chorioretinitis | Anisocoria Nystagmus Microphthalmia Cataract |
Corneal opacity Heterochromia |
| Skeletal | Hypoplasia of upper or lower extremities | Hypoplasia of fingers or toes Equinovarus or calcaneovalgus Hypoplasia of scapulae or clavicles Hypoplasia of ribs |
Hypoplasia of mandible Scoliosis Lacunar skull |
| Gastrointestinal | Low birthweight | Gastroesophageal reflux Duodenal stenosis Dilated jejunum Small left colon Sigmoid atresia |
|
| Genitourinary | Absence of kidney Hydronephrosis Hydroureter Undescended testes Bladder abnormalities |
Etiology and pathogenesis
Varicella zoster virus (VZV) is a herpes virus consisting of double-stranded DNA. The incubation period is usually 14 days, but ranges from 10 to 21 days after exposure. Most fetuses exposed to VZV during gestation will have no discernible sequelae. The greatest risk for fetal varicella syndrome occurs in the first 20 weeks’ gestation, with the highest risk (2%) between 13 and 20 weeks;17 a lower rate before 13 weeks (0.4%) may reflect underreporting or a higher rate of spontaneous abortion. Rare cases in the second half of pregnancy have been reported.18,19 A prospective study carried out in Germany and the UK between 1980 and 1993 identified 1373 women who had varicella and 366 who had zoster during the first 36 weeks’ gestation. Nine cases of congenital varicella syndrome were reported overall, and all occurred after maternal varicella infection in the first 20 weeks of pregnancy. The highest risk occurred between 13 and 20 weeks’ gestation, with seven cases noted in this subset. No cases of congenital varicella were noted following maternal herpes zoster during pregnancy. Herpes zoster in infancy was identified in 10 children whose mothers had varicella during pregnancy. Although 97 women who received varicella zoster immunoglobulin (VZIG) developed varicella, no cases of congenital varicella occurred in this group.17 Some believe that the failure to identify congenital varicella in late pregnancy reflects the limited statistical power of epidemiologic efforts, but nonetheless, late pregnancy is clearly a time of lower risk for the development of the classic stigmata of congenital disease.20 It has been postulated that the severe segmental anomalies that can be seen in fetal varicella syndrome are the result of reactivation of primary varicella in the developing fetus at a time when the immune system is not sufficiently developed to modify the severity of infection.
Maternal herpes zoster does not appear to pose a significant risk to the fetus. No cases of congenital varicella occurred in a prospective study of 366 women who had zoster during pregnancy, and no serological evidence of transplacental transmission was noted.17 Approximately 18 cases of congenital anomalies occurring in association with maternal herpes zoster infection have been reported; however, it is not clear that these anomalies were a result of maternal zoster infection.21 A case of cutaneous lesions and limb hypoplasia in a fetus whose mother developed disseminated herpes zoster at 12 weeks’ gestation did appear consistent with fetal varicella syndrome, but localized maternal zoster has not been clearly implicated as a cause of fetal disease.22
Diagnosis
The denuded or scarred areas seen with fetal varicella syndrome may be mistaken for aplasia cutis congenita or Bart syndrome. Other congenital viral infections should be considered in any infant presenting with microcephaly, ophthalmologic, or neurologic abnormalities.
Prenatal diagnosis of fetal varicella syndrome using viral or immunologic methods is unreliable.23 IgM may be undetectable, even in infants with classic clinical findings. Infection before 18 weeks’ gestation may lead to a suboptimal or altered immune response resulting from immaturity of the fetal immune system. Prenatal diagnosis of intrauterine exposure to varicella may be accomplished by means of cordocentesis, amniocentesis, and chorionic villus sampling.23 IgM may be detected in cord blood as early as 19–22 weeks’ gestation. Virus can be grown from amniotic fluid and fetal blood samples, and DNA probes can be utilized to evaluate placental tissue.24,25 However, transplacental transfer of virus can occur without any significant sequelae to the fetus, and the degree of fetal involvement cannot be determined by immunologic or viral evaluation. Thus, although the above-mentioned evaluations may be useful in diagnosing fetal varicella syndrome, they are neither sensitive nor specific enough to accurately determine which fetuses will suffer untoward effects.
High-quality ultrasound at 20–22 weeks’ gestation has been used as a means of surveying at-risk fetuses. Sonographic abnormalities include fetal hydrops, polyhydramnios, abnormal foci within the liver, microcephaly, and limb hypoplasia.26 Unfortunately, some findings may not be apparent until later in pregnancy. A report of a fatal case of varicella embryopathy that used ultrasonography and MRI at 26 and 32 weeks’ gestation found a high correlation between fetal imaging and subsequent pathologic findings, including limb involvement and even dermatologic features; however, MRI scanning was required to identify CNS abnormalities. The authors recommend combining prenatal ultrasonography with MRI in any woman noted to have varicella seroconversion during pregnancy.27
Varicella virus is not usually isolated from live-born infants with congenital infection, and other findings must be used to confirm the diagnosis. Criteria useful in confirming the diagnosis include clinical, virologic, or serologic evidence of maternal varicella infection during pregnancy; erosions or scars in a dermatomal distribution; and immunologic evidence of varicella infection in the infant, including IgM antibody or persistence of IgG antibody beyond 1 year of life in the absence of clinical varicella infection. The development of herpes zoster in the first year of life without a prior history of varicella infection is also good evidence that the infant was exposed to varicella zoster during gestation. In rare instances, varicella virus particles have been detected by means of electron microscopy in skin samples obtained at or near birth.24
Treatment
Prevention by eliminating natural infection during pregnancy is the best approach to this disease, and should be facilitated by the increasing use of the varicella vaccine in childhood. Ideally, pre-conception evaluation should identify at-risk women, who should then receive the varicella vaccine before conceiving. No fetal anomalies have been reported in infants born to pregnant women who have received the vaccine inadvertently. Nonetheless, the vaccine, which is a live attenuated virus, is contraindicated in pregnancy.
Therapeutic abortion is not automatically recommended to at-risk mothers as the risk of a fetal anomaly following exposure is so small.28 At-risk mothers known to have recent exposure to varicella during the first 20 weeks of pregnancy should have varicella serologic evaluation. Latex agglutination (LA), immunofluorescent assays (IFA), fluorescent antibody-to-membrane antigen assays (FAMA), and enzyme-linked immunosorbent assays (ELISA) are sensitive and specific.23 The LA test is also rapid and simple, making it quite useful in evaluating at-risk pregnant women.
Varicella zoster immunoglobulin post-exposure prophylaxis may be offered to varicella-susceptible pregnant women. It may be administered within 96 h of exposure, but is most efficacious within the first 48 h. It does not reliably prevent maternal illness, but does modify the severity of infection. It is unclear whether VZIG prevents fetal varicella syndrome or neonatal infection, but there were no cases of fetal infection in 97 pregnancies complicated by maternal exposure and treated with post-exposure VZIG prophylaxis.17,29 Because fetal varicella syndrome is so rare, larger studies would be required to confirm protection. Exposed pregnant women who are seropositive for VZV do not require VZIG.
Treatment with acyclovir should be considered in any pregnant women with varicella, particularly those in the third trimester, because of the risk of severe maternal disease, and to minimize the risk of neonatal disease in case delivery occurs during or soon after acute infection. The drug is usually well tolerated with little toxicity to the mother, but the risks and benefits to the mother and fetus have not yet been clearly delineated. The International Registry of Acyclovir Use During Pregnancy has followed at least 1246 fetal exposures to the drug, and no increased incidence of fetal abnormalities in exposed infants has been noted.30 It has not been determined whether such treatment will eliminate the risk of varicella embryopathy or infantile zoster in exposed fetuses. Ophthalmologic and neurologic evaluation of the infant born to a mother with varicella during pregnancy is indicated, as is careful examination of the musculoskeletal, genitourinary, and gastrointestinal systems for underlying anomalies.
Neonatal varicella
Neonatal varicella may result if a mother develops chickenpox before or immediately after delivery. If maternal varicella occurs from 5 days before to 2 days after delivery, the in utero-inoculated fetus is at high risk for severe disseminated disease.
Cutaneous findings
The clinical course of neonatal varicella can be quite variable. Those who are more likely to develop severe illness generally develop skin lesions within 5–10 days after delivery. Some children will develop a few cutaneous lesions, but otherwise remain well. Lesions often appear initially as small pink to red macules that relatively rapidly become papular and subsequently develop a teardrop-shaped vesicle. Other patients initially develop crops of cutaneous lesions that may evolve into hemorrhagic and/or necrotic vesicles (Fig. 13.9).
Extracutaneous findings
Disseminated infection with widespread cutaneous and visceral involvement may develop and lead to severe morbidity. The mortality rate for neonatal varicella before the use of acyclovir has been estimated at 10–30%.28,29 Death from severe pneumonitis and respiratory distress often occurs 4–6 days after onset of lesions. Hepatitis and encephalitis may also develop. A study from Thailand in 1999, evaluating 26 children with neonatal varicella, reported no mortality. Of the 26 children, 12 received intravenous acyclovir.31
Etiology and pathogenesis
Infants may develop lesions from 1 to 16 days after birth if the mother experiences active disease near the time of birth. The usual onset of rash is 9–15 days after onset of maternal rash. Administration of VZIG may prolong the incubation period to 28 days.14 In aggregate data from two studies, 23–62% of infants whose mothers developed varicella in the last 3 weeks of pregnancy developed neonatal varicella.17 The risk of severe neonatal varicella is related to the timing of maternal infection, presumably because of a critical period when transmission of virus to the infant occurs prior to the development and transplacental transfer of maternal antibodies, which modify expression of the infection in the neonate.
Diagnosis
Smears of vesicles using a Tzanck preparation will demonstrate multinucleate giant cells and margination of chromatin. VZV PCR evaluation is also helpful and is more specific for VZV; vesicular swabs, scrapings, biopsy tissue and CSF can be evaluated by PCR analysis. Direct fluorescent antibody (DFA) tests are less sensitive than PCR and occasionally false-positive in disorders such as incontinentia pigmenti and Langerhans’ cell histiocytosis; therefore positive viral culture or PCR remain the best, most reliable means of diagnosis.32 A history of maternal varicella infection during pregnancy is also helpful but not necessary to confirm the diagnosis because infants may also contract the disease from siblings, care givers, and other close contacts. The differential diagnosis of vesiculopustular lesions is discussed in Chapter 10.
Treatment
Prevention is the best intervention. Delaying delivery until sufficient time has elapsed for transplacental transfer of maternal antibody is one approach; this generally occurs 5–7 days after the onset of maternal illness. Neonates born to mothers who have developed varicella from 5 days before to 2 days after delivery should receive VZIG intramuscularly as soon as possible after delivery.14 Direct contact between the infant and maternal skin lesions should be avoided, but breastfeeding is not prohibited if such contact can be avoided. Neonates who develop lesions or signs of infection should be treated with intravenous acyclovir, 20 mg/kg every 8 h for a minimum of 10 days. More prolonged therapy of 14–21 days may be necessary for disseminated or CNS infection.14 Aggressive supportive therapy is sometimes also required. The use of prophylactic acyclovir in high-risk infants has also been suggested by some authors.
It should be borne in mind that any infant born to a woman who has had varicella within 3 weeks of delivery may be infectious at birth or shortly thereafter. If onset of maternal infection is within 1–2 weeks of delivery, many experts recommend that the child be isolated (from at-risk hospital personnel and other babies) from birth. If onset of disease occurs in the mother within 1 week of delivery, or following the birth of the infant, the infant should be isolated 7 days after the onset of maternal disease.14
If a mother develops a varicella rash 3 or more days after delivery, the infant may contract varicella but this will more likely be via the respiratory route, which theoretically leads to a smaller systemic inoculum and less severe disease. However, serious illness has been reported in the first 4 weeks of life when infants contract disease from their mother or siblings during this period. Severe infantile disease may occasionally occur even in infants born to immune mothers.33 If the mother is seronegative and the infant is exposed to an infectious sibling, many experts would recommend VZIG post-exposure prophylaxis for the infant. Post-exposure prophylaxis with acyclovir may be effective in older infants, but no data exist for its efficacy or safety in neonates. Although the mortality rate following noncongenital infantile varicella (0.008%) is higher than that in older children, it is still lower than the rate in immunocompromised individuals (7%) or following intrauterine exposure (10–30%).26
Special concerns for the neonatal nursery and intensive care unit
Varicella exposure in the neonatal intensive care unit.
Recommendations regarding prophylaxis after VZV exposure vary. The UK Advisory Group on Chickenpox recommends routine administration of VZIG to all neonates following exposure. If sensitive and rapid testing is available, exposed infants may be tested and the use of VZIG avoided for those with passive antibody to VZV. Others recommend concentrating prophylactic measures on those infants less than 30 weeks old and weighing less than 1 kg. The Report of the Committee on Infectious Diseases of the American Academy of Pediatrics recommends VZIG for all exposed, hospitalized premature infants who are less than 28 weeks’ gestation, or who weigh 1 kg or less at birth. In addition, those exposed, hospitalized premature infants of 28 weeks or more gestation, born to mothers who are seronegative or lack a history of varicella infection, should also be given VZIG.14
Infantile herpes zoster
Following primary infection, the varicella zoster virus persists in the sensory dorsal root ganglia and is kept in check by cell-mediated host immune mechanisms. Reactivation of the virus can occur and generally leads to localized involvement of skin and nerves in a dermatomal pattern corresponding to the ganglion in which reactivation took place. This disease, termed herpes zoster, has been recognized since antiquity. The term ‘zoster’ (girdle in Greek) refers to the tendency of the lesions to involve the trunk in a ‘girdle-like’ pattern. Infants may develop classic herpes zoster without prior evidence of primary varicella if exposed to varicella virus in utero. Neonatal herpes zoster has only rarely been reported. It is generally a benign disease without significant morbidity or sequelae for the infant unless the infant is immunocompromised.
Cutaneous findings
Affected infants usually have discrete lesions that involve predominantly the thoracic dermatomes. The lesions initially appear as a group of small pink to erythematous papules that subsequently vesiculate and crust (Figs 13.10, 13.11![]() ). Occasionally lesions become hemorrhagic and develop necrotic eschars. Scarring may occur in the involved area. Although the disease may disseminate, immunocompetent children usually have a benign course and an excellent outcome.
). Occasionally lesions become hemorrhagic and develop necrotic eschars. Scarring may occur in the involved area. Although the disease may disseminate, immunocompetent children usually have a benign course and an excellent outcome.
Diagnosis
The diagnosis is usually straightforward when the lesions assume a dermatomal distribution. A Tzanck preparation is the most rapid means to evaluate suspicious vesicular lesions. Zoster lesions may be initially mistaken for arthropod bites or impetigo. Herpes simplex infections may mimic herpes zoster but are usually more localized, with the potential to recur. Direct fluorescent antibody testing and PCR evaluation are also relatively rapid diagnostic techniques (see ‘Infantile varicella’, below). Varicella virus can be isolated from vesicular lesions of herpes zoster.
Etiology and pathogenesis
Pediatric herpes zoster is more common in immunocompromised children, and in immunocompetent children within the first year of life who have been exposed to varicella in utero.18 The risk of infantile herpes zoster increases if exposure to varicella zoster virus occurs in the second half of pregnancy.17 Approximately 2% of fetuses exposed to VZV during the second half of pregnancy will develop herpes zoster during infancy.17
Treatment
Many infectious disease experts treat zoster in neonates with systemic acyclovir. Immunocompetent infants with zoster generally do well and do not require antiviral therapy. Patients who develop severe hemorrhagic disease, as well as those with disseminated lesions, are likely to benefit from systemic therapy. Supportive therapy in all cases should include good hygiene at the blister sites, compresses as needed, and treatment for secondary bacterial infection if indicated.
Infantile varicella (chickenpox)
Primary varicella (primary VZV, chickenpox) was a nearly universal childhood illness, until the widespread use of the VZV (Oka strain) vaccine. The two dose vaccine series is recommended for 1- and 4-year-old children. In those infants who do not have passive immunity from the mother, and as passive humoral immunity wanes, infants aged 1–12 months may be at risk for primary VZV. However, widespread use of the VZV vaccine in the older, eligible population offers protection to this group and led to an 89.7% decrease in infantile VZV (ages 0–12 months) from 1995 to 2008.34
VZV is transmitted via respiratory droplets. Fever, headache, myalgias and arthralgias precede the earliest skin lesions, which develop 1–2 days after exposure. Lesions evolve quickly from a red papule to a small vesicle on a red base, classically described as a ‘dewdrop on a rose petal’. The vesicles occur in crops and then crust, leading to the appearance of lesions in different stages (Fig. 13.12![]() ). Painful mucosal erosions may also develop. Skin lesions may heal with scarring. The disease spontaneously resolves in healthy children, but there is a risk of secondary bacterial infection. Infants with a compromised immune system, such as those with leukemia, lymphoma, HIV, or taking immunosuppressive medications, are at increased risk of complicated disease, which may also include thrombocytopenia and respiratory compromise.
). Painful mucosal erosions may also develop. Skin lesions may heal with scarring. The disease spontaneously resolves in healthy children, but there is a risk of secondary bacterial infection. Infants with a compromised immune system, such as those with leukemia, lymphoma, HIV, or taking immunosuppressive medications, are at increased risk of complicated disease, which may also include thrombocytopenia and respiratory compromise.
In older infants (ages 12–23 months) who have received the VZV vaccine, post-vaccine vesicular eruptions may occur. PCR analysis has detected the vaccine strain VZV (Oka) in some of these lesions. Post-marketing surveillance data shows that the vaccine strain VZV (Oka) is most likely to be associated with the post-vaccine vesicular eruptions occurring between days 15 to 42 after the vaccination, whereas those occurring within 2 weeks or >42 days later are more likely secondary to wild-type VZV.35 VZV infections acquired after 42 days, termed breakthrough varicella, are milder than the native disease, with fewer lesions (usually ≤50), more morbilliform appearance, lower fever and shorter duration. Post-vaccine herpes zoster (HZ) eruptions also occur. In contrast to wild-type HZ cases, Oka-associated HZ cases showed a trend towards occurring in younger children (median age 2 years), near the site of vaccination, and with a shorter latency after vaccination.35
Primary VZV can be diagnosed with history and physical examination. Adjunctive laboratory tests including Tzank smear, PCR, direct fluorescence antibody (DFA), serologies and viral culture (described above) may be helpful if diagnosis is in doubt.
Treatment of primary VZV in healthy children is not recommended, as studies have not shown meaningful reduction in the development of new lesions or the total number of lesions with antiviral therapy (acyclovir).36 In contrast, intravenous acyclovir should be started early (within 24–72 h of skin lesions) for immunocompromised children. In high-risk individuals with known exposure to VZV, varicella zoster immunoglobulin (VZIG) should be administered within 48 h of exposure, although it may be given up to 96 h. Valacyclovir, 20 mg/kg per day, given three times daily for 5 days, has been used by some experts for treatment of varicella in high-risk and immunocompetent patients. This drug is currently licensed for children 2 years of age or older.
According to guidelines from the American Academy of Pediatrics, non-immunized infants with VZV must be excluded from childcare settings until all lesions are crusted over and those previously immunized may return when no new lesions have developed in 24 h.14 Infants with HZ may return if lesions can be adequately covered or when all lesions are crusted.14
Cytomegalovirus
Cytomegalovirus (CMV), one of the most common viral infections of the newborn, is acquired congenitally, perinatally, or postnatally. CMV, a member of the herpes virus family, is a double-stranded DNA virus. It represents the most frequently diagnosed congenital infection, occurring in 1–2% of all births.37
Cutaneous findings
The principal cutaneous manifestations of CMV infection are similar to those of congenital rubella syndrome and consist of skin lesions of extramedullary hematopoiesis (‘blueberry muffin’ spots) and petechiae secondary to thrombocytopenia, which resolve during the first weeks of life.
Extracutaneous findings
Other manifestations of congenital CMV infection syndrome include intrauterine growth retardation, microcephaly with chorioretinitis, hepatosplenomegaly, and pneumonitis.
Etiology and pathogenesis
Congenital infection occurs most often following reactivation of latent maternal infection during pregnancy, resulting in viremia and/or transplacental transmission of lymphocyte-associated virus to the fetus. The vast majority of infected infants born to immune mothers with reactivation of CMV are normal, and have no stigmata of congenital infection. However, seronegative mothers may acquire a primary CMV infection during pregnancy, leading to a far greater incidence of symptomatic disease in the neonate. The severity of infection depends on the trimester in which fetal infection has occurred, with infections early in gestation leading to more pronounced clinical findings and a poorer prognosis than those acquired during the third trimester. Infection may also occur at the time of delivery following exposure to infectious secretions during the process of vaginal birth. The infection is acquired perinatally in 40% of infants born to culture-positive mothers. Postnatal transmission has also been well-documented in breast-fed infants following the ingestion of culture-positive breast-milk.
Diagnosis
The virus can be isolated from urine, pharynx, saliva, peripheral blood leukocytes, and a host of other tissues. Culture is the most widely available method of diagnosis. Quantitative PCR techniques can be used in assessing white blood cells, CSF, and other tissues. Infants with congenital infection are, by definition, culture positive at the time of birth. For a diagnosis of congenital infection, cultures should be obtained within the first 2 weeks of life. If cultures are positive in the infant beyond 1–2 weeks of age, it is not possible to differentiate between congenital and perinatal CMV infection. This distinction is often critical, however, as the prognosis for perinatal CMV is uniformly good, whereas that of congenital CMV is not. Skin biopsies do not usually reveal evidence of active CMV infection, although specimens from liver, lung, or kidney will show clear evidence of CMV inclusions in infected parenchymal tissues. A spun sample of urine may demonstrate viral inclusions in tubular epithelial cells in up to 50% of culture-positive samples, and may demonstrate CMV by electron microscopy in up to 93% of culture-positive urine samples.38 Serologic studies can also be used to make a diagnosis of congenital/perinatal CMV infection, either by demonstrating specific CMV-IgM antibody produced by the neonate or by documenting a persistent, increasing titer of IgG antibody during the first 4–6 months of life. Although the presence of specific CMV-IgM in cord blood will verify congenital infection, the sensitivity of this test as currently performed in reference laboratories may be less than 50%. PCR testing for CMV-DNA is accepted as a very sensitive diagnostic technique for plasma and certain tissue fluids in immunocompromised hosts, and is a highly sensitive test in the newborn.37
Treatment
Vaccines have been in development for several years but have not demonstrated sufficiently adequate protection from CMV infection to justify their extensive use. Currently, treatment of CMV infections is primarily accomplished with parenteral ganciclovir or oral valganciclovir, its oral prodrug; both are nucleoside analogs with potent activity against most strains of CMV.39 The drug is associated with significant bone marrow toxicity. Clinical trials of intravenous ganciclovir for congenital CMV demonstrated a significant reduction in hearing loss at 6 months in infants with CNS (central nervous system) manifestations of congenital infection.40 Unfortunately, congenital CMV infections cannot be cured but only suppressed during the period of antiviral administration. Limited data exist on the use of oral ganciclovir and valganciclovir in the neonate.41
Rubella
The association of maternal rubella infection with congenital disease of the newborn was first recognized in 1941. Extensive investigations have resulted in delineation of the congenital rubella infection syndrome, typified by a small-for-gestational age infant with microcephaly, deafness, cataracts, heart defects, chorioretinitis, hepatosplenomegaly, and a papular rash on the face, trunk, and extremities.
Cutaneous findings
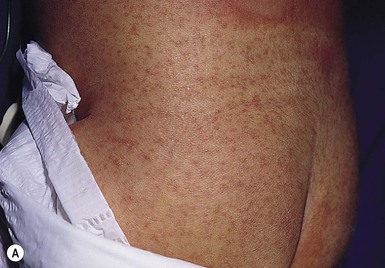
The rash can be mild or extensive, and is a manifestation of intradermal sites of extramedullary hematopoiesis (EMH). It often becomes hemorrhagic secondary to thrombocytopenia present at birth in these infants. The initial ‘cranberry muffin’ character of these 2–20 mm raised, erythematous, soft, spongy lesions changes to the more characteristic appearance of ‘blueberry muffin’ spots following intralesional hemorrhage. Petechiae may also be present in addition to the lesions of EMH. The lesions of EMH are not specific for rubella infection, and may also occur with congenital cytomegalovirus and toxoplasma infections. Petechial and purpuric lesions are evident in up to 60% of infants with congenital rubella.
Extracutaneous findings
Associated clinical findings, beyond those listed previously, include congenital heart disease (patent ductus arteriosus, pulmonic stenosis, aortic stenosis), cataracts, and pneumonia, which may actually develop and progress after birth. Psychomotor retardation and deafness occur in up to 50% of infants with documented congenital rubella syndrome.42
Etiology and pathogenesis
Maternal infection gives rise to viremia, which is transmitted to the fetus, affecting rapidly dividing fetal tissues most prominently during the first 12 weeks’ gestation. This single-stranded RNA virus usually causes a noncytolytic infection of cells, leading to cell dysfunction and defects in organogenesis. Although fetal infection may occur at any time during gestation, visible consequences of infection are most common following first-trimester infection, rare with a second-trimester infection, and virtually nonexistent following infection late in pregnancy.43
Diagnosis
Viral culture of the nasopharynx is the definitive method of confirming the diagnosis of rubella infection (Table 13.1), as virus shedding continues for several weeks to months after birth. Detection of rubella-specific IgM is helpful, but both false-positive and -negative results can occur. Assessing the maternal serologic status can be helpful, but many ‘prenatal’ serologies are actually performed at the end of the first trimester and therefore do not truly represent the mother’s immune status before pregnancy and cannot rule out infection at week 12 of gestation. If the infant is believed to have been infected, acute (cord blood) and convalescent (obtained at 4–6 months of age) blood samples should be obtained to determine antibody titers for rubella. These titers are diagnostic, as virtually all maternal transplacental antibodies will have disappeared from the infant by 6 months of age. Evaluation of the cutaneous lesions will lead only to the nonspecific diagnosis of EMH.
Prevention and treatment
No specific antiviral therapy exists for rubella virus. In the USA, universal immunization of children is specifically designed to prevent congenital rubella infection. Prenatal screening of women during early pregnancy should detect susceptible individuals, and immunization immediately following the pregnancy is indicated.
Acquired immunodeficiency syndrome
Acquired immunodeficiency syndrome (AIDS) is a multisystem disorder characterized by T-lymphocyte depletion and recurrent opportunistic infections. It results from infection with the human immunodeficiency virus (HIV), an RNA retrovirus that infects CD4 T lymphocytes, as well as other immune cells. Transmission can occur in utero, perinatally, or via breast-milk. It can also be transmitted via sexual contact, percutaneous blood exposure, mucous membrane exposure to contaminated blood, and via blood transfusion. Characteristics of the infection include a variable latency period and an extremely high mortality rate if untreated. Perinatal transmission from infected mothers is the most common cause of childhood infection.44 The number of pediatric cases decreased by 90% in 2002 compared with 1992, mainly because of a decrease in perinatal transmission due to screening for HIV during pregnancy, with antiretroviral treatment of the mother at the end of pregnancy and treatment of the newborn for the first 6 weeks of life.14,44 Most infected infants are asymptomatic in the first few months, but severe disease can occur within this time frame. Cutaneous abnormalities are among the earliest findings, and may be infectious, inflammatory, or neoplastic in nature. Failure to thrive, recurrent infections, and pulmonary disease (relating to Pneumocystis jiroveci or lymphoid interstitial pneumonia) are common findings in infants. These children are also at risk for gastrointestinal disorders, including malabsorption, hepatosplenomegaly, and CMV-mediated organ involvement such as hepatitis and marrow failure.
Cutaneous findings
Cutaneous and mucous membrane disease is very common in infants with symptomatic HIV infection. Frequently, the first indication that an infant is infected is the development of a severe or recurrent bacterial or fungal infection. In other instances, widespread and protracted seborrheic dermatitis may be the first clue to the patient’s underlying immunodeficiency. Cutaneous infections that are extensive, progressive, or difficult to treat, should raise suspicion for HIV infection. The type of cutaneous involvement that occurs with disease is generally related to the degree of immunosuppression.
Infectious skin disorders.
Mucocutaneous candidiasis is the most common dermatologic manifestation of pediatric HIV infection and occurs in the overwhelming majority of symptomatic children.45 The disease is more severe and chronic than in the immunocompetent host and frequently persists beyond the first 6 months of life. White, cheesy patches or plaques overlying an erythematous base are found on the buccal mucosa, tongue, and palate. The lesions are friable and in severe cases, may extend to involve the esophagus. The diaper area is commonly involved, with a beefy-red erythema involving the convex surfaces and creases, along with satellite papules or pustules. There may be angular cheilitis and extensive, generalized cutaneous involvement. Severe dermatophyte infections of the nails, hair, and skin may develop, and other unusual fungi, such as Cryptococcus spp. and Aspergillus spp. may cause systemic as well as cutaneous disease.46
Bacterial infections of the skin may take the form of severe and recurrent staphylococcal impetigo, folliculitis, cellulitis, or abscesses. Other more unusual pathogens may be noted, particularly when the patient is severely immunosuppressed. Although polymorphonuclear (PMN) function against bacteria is not altered, T/B-cell cooperation leading to opsonizing antibacterial function may be severely affected.
Viral infections are also atypical in course and lesion morphology. The lesions of varicella zoster infection may become chronic, hemorrhagic, ulcerating, and/or hyperkeratotic.47 Herpes zoster occurs earlier and more frequently in HIV-infected children. Herpes simplex infections may also be severe, prolonged, and/or recurrent. Molluscum contagiosum and papilloma virus infections are more frequent and may be relatively refractory to therapy.
Scabies can occur in early infancy and may present in a severe crusted form, often referred to as Norwegian scabies. Such cases are highly infectious because the affected infant usually possesses numerous generalized crusted papules that harbor large numbers of organisms.
Neoplastic skin disease.
Cancer is the presenting sign for AIDS in only 2% of children, compared with 15% of adults.48 Kaposi sarcoma (KS) is significantly less common in childhood than in adult AIDS. However, a report from Zambia noted a significant increase in the incidence of pediatric KS since 1987.49
Kaposi sarcoma has been described in a 6-day-old infant, but is rare in the neonatal period.50 Non-Hodgkin lymphomas, which are sometimes limited to the CNS, occur with increased frequency in children with AIDS, as do leiomyomas and leiomyosarcomas.51
Inflammatory skin disease.
Confluent beefy erythema with superimposed greasy thin scale first noted on the scalp and face is characteristic of seborrheic dermatitis in children with AIDS. It spreads to involve the axillae and diaper area, and occasionally may progress to a severe, generalized erythroderma. Atopic dermatitis and psoriasis can also occur in HIV-infected patients, but are not commonly diagnosed in the neonatal period. Drug eruptions, particularly from trimethoprim-sulfamethoxazole, are more frequent in children with AIDS.52 The rash usually develops within 7–10 days after the start of therapy and is a pink, papular or morbilliform eruption. Evolution to TEN (toxic epidermal necrolysis) may occur, but most eruptions resolve following discontinuation of the offending agent.
Extracutaneous manifestations
An HIV-related embryopathy described in 1986, and characterized by microcephaly and dysmorphic facial features, has not been substantiated. The papular exanthem/enanthem that develops shortly after HIV infection in adults has not been noted in perinatally acquired disease.53
Clinical conditions that should raise suspicion for HIV infection include failure to thrive, recurrent severe bacterial or opportunistic infections, hepatitis, lymphoid interstitial pneumonia, parotitis, lymphadenopathy, and hepatosplenomegaly. Pneumocystis jiroveci pneumonia is the most common serious opportunistic infection in HIV-infected children and usually develops between 3 and 6 months of age; however, infection can occur in infants as young as 4–6 weeks. Patients may also develop severe wasting, encephalopathy, developmental delay, nephropathy, cardiomyopathy, and diarrhea.
Etiology and pathogenesis
HIV is a human retrovirus containing RNA. Two forms exist: HIV-1, most prevalent in the USA, and HIV-2, a related virus more commonly seen in West Africa. The virus has a predilection for CD4 lymphocytes, glial cells, macrophages and monocytes, and infection generally leads to significant impairment in cell-mediated immunity.
HIV is transmitted by contaminated blood, semen, human milk, and cervical secretions. The virus has also been isolated from saliva, tears, urine, cerebrospinal fluid, and pleural fluid, but these have not been shown to routinely transmit infection.
Many sources have documented that perinatal transmission of HIV accounts for the overwhelming majority of pediatric cases in the USA.44 Infection may be transmitted to the infant in utero, at the time of delivery, or through breast-feeding. The risk of infection for an infant whose mother did not receive interventions to prevent transmission is approximately 13–39%.14 The risk of intrapartum transmission is higher than for intrauterine spread. In areas with high rates of breast-feeding, one-third to half of maternal transmissions occur through breast-feeding. It is thought that the majority of infections are transmitted close to or at the time of delivery. Routine prenatal screening for HIV infection and treatment of infected women and their infants with antiviral agents such as zidovudine, and other antiretrovirals, are recommended. If an infected mother is compliant with antiretroviral medications and her viral load is <1.00 copies/mL, her risk of vertical HIV transmission decreases to 1%. Cesarean section delivery decreases the risk of HIV transmission to the fetus by approximately 50%, presumably because the infant has a reduced exposure to maternal blood and cervical secretions.54 The combined use of maternal antiviral therapy and cesarean section delivery can theoretically reduce the risk of transmission to approximately 0.5%.
The incubation period for HIV infection is quite variable. Infants are generally asymptomatic during the first few months of life. The median age of onset for perinatally acquired untreated disease is 12–18 months. Conversely, severe illness can develop in the first few months of life. Approximately 10–15% of children will die before 4 years of age.14
Diagnosis
Diagnosis during infancy requires the detection of the virus or viral nucleic acid, as almost all infants born to HIV-seropositive mothers will have transplacentally acquired antibodies, which may persist for up to 18 months. It is therefore necessary to document infection using other methods in the newborn and young infant. A positive viral culture is diagnostic; however, only 25–50% of infected children will be identified at birth by means of culture. In addition, viral culture is expensive, not widely available, and requires up to a month for the results. PCR evaluation is a very sensitive and widely available method for the detection of HIV infection in the neonatal period, and is currently recommended. HIV DNA PCR is the preferred test to diagnose HIV-1 infection in infants and children younger than 18 months of age. It is highly sensitive and specific.
Approximately 30% of HIV-infected infants will have positive PCR studies within the first 48 h of life, and 93% will be positive by 2 weeks of age.55 If initial evaluation within the first 48 h is negative, repeat tests should be performed at 1–3 months, and then again at 4–6 months if necessary.55
Laboratory findings suspicious for HIV infection include a decreased ratio of helper to suppressor T cells, and hyper-gammaglobulinemia. One study suggested that elevated IgG levels and oral candidiasis in children less than 15 months of age had a high (98%) specificity for the diagnosis, but low sensitivity (37%).55 Lymphopenia is not usually seen in infants and children. Microcytic, hypochromic anemia is common, and thrombocytopenia may also be present. Because cutaneous disease in HIV-infected patients often presents in an atypical fashion, culture and biopsy of any suspicious lesion should be obtained if the diagnosis is in doubt.
Treatment
Treatment options and recommendations change with time. CDC guidelines are continuously updated, are available on the CDC website, and represent the most current consensus on treatment (see: www.aidsinfo.nih.gov for updates regarding interventions). Zidovudine therapy of the HIV-infected pregnant woman and the newborn infant can significantly decrease perinatal transmission, as can caesarean section delivery (see above). Oral administration of zidovudine to the infant for 6 weeks further reduces the risk of perinatal transmission. Most infected pregnant women are on combination antiretroviral therapy. Short-term adverse effects of the drug include anemia, neutropenia, and hepatitis. No long-term adverse side-effects have been noted.56 Breast-feeding by HIV-infected women is not recommended in areas where safe alternative options are available.
Infected infants should be referred to an HIV specialist. Chemoprophylaxis with TMP/SMX (trimethoprim-sulfamethoxazole) reduces the risk of infection with Pneumocystis jiroveci and may reduce the incidence of cutaneous bacterial infections. Cutaneous infections with fungi, bacteria, and viruses should be treated as appropriate for each disease. Candidiasis usually requires systemic therapy, and fluconazole has proved particularly useful for this purpose. Acyclovir therapy is appropriate for the treatment of herpes simplex and zoster infections, but chronic use may lead to acyclovir resistance, which is more common in the AIDS population. Foscarnet and ganciclovir have both proved to be efficacious antiviral agents in immunocompromised patients.
Human parvovirus B19 infections
Parvovirus B19 infection is classically associated with a benign viral exanthem of childhood (erythema infectiosum) consisting of a distinctive ‘slapped cheek’ appearance, followed by a reticulate, lacy truncal eruption. The virus can also less commonly manifest other findings in children and adults (Table 13.4), as well as in the developing fetus and infant.57 The range of clinical manifestations caused by parvovirus B19 is diverse. Red blood progenitor cells are particularly susceptible to the virus, but it can also affect skin, liver, and myocardial cells. Although the majority of healthy individuals who are infected have few or no symptoms, the fetus is at particular risk for significant morbidity. Infection during pregnancy has been associated with an increased risk of miscarriage, fetal hydrops, intrauterine growth retardation, and isolated pleural and pericardial effusions.58 The risk of fetal death is approximately 1–9%, with the greatest risk occurring during the first half of pregnancy.9 Recommendations for management of exposed pregnant women are summarized in Box 13.1. Symptomatic neonatal disease is rare and usually consists of persistent anemia following congenital infection.59
TABLE 13.4
Manifestations of human parvovirus B19 infection
| Host | Findings |
| Immunocompetent children | Erythema infectiosum, papular purpuric gloves and socks syndrome (adolescents) |
| Immunocompetent adults | Transient arthritis |
| Individuals with increased hemolysis, e.g., sickle cell disease | Transient aplastic crisis |
| Immunodeficient patients | Chronic anemia |
| Fetus (first 20 weeks’ gestation) | Hydrops fetalis; pleural, pericardial, or peritoneal effusions; anemia |
| Neonate | Persistent anemia, red cell aplasia; blueberry muffin lesions (rare); myocarditis (rare) |
| Infant (1–24 months) | Acral papules and purpura; myocarditis (rare) |
Fetal, congenital, and neonatal disease
Cutaneous findings
The most common findings in affected abortuses are pallor, maceration, and subcutaneous edema, all consistent with the diagnosis of hydrops. Blueberry muffin lesions showing extramedullary hematopoiesis have also been reported.
Extracutaneous findings
Increased fluid may develop in the pericardial, peritoneal and pleural cavities. The exact risk of congenital anomalies following B19 infection is controversial, but is thought to be extremely low. Bilateral cleft lip and palate, micrognathia, subcutaneous hemorrhage, and congestion of internal organs have been found in fetuses in association with characteristic nuclear inclusions within erythroid precursor cells, endothelial, and smooth muscle cells.60 Three live-born infants with severe neurologic defects whose mothers had serologically documented parvovirus infection during pregnancy have also been described.61 Significant ocular abnormalities involving the globe, retina, and cornea have been reported in one case.62 Large prospective studies have failed to note any significant risk of congenital abnormalities following maternal parvovirus infection.63 Current consensus is that the risk of congenital infection from parvovirus is less than 1% and is not yet clearly determined.64
Most cases of documented neonatal infection consist of persistent anemia following congenital infection.14 Isolated congenital red cell aplasia, which may mimic Diamond–Blackfan anemia, may be caused by parvovirus B19 infection.64 Relapsing erythroid hypoplasia in a 2-month-old infant has also been attributed to parvovirus infection. Multisystem disease in an infant who presented at birth with petechiae and thrombocytopenia and developed edema, cardiomegaly, bradycardia, and hypotension on day 2, has also been reported.65 True neonatal disease is thought to be rare, but it is possible that it may be unrecognized and underreported. Unfortunately, technical problems in identifying neonatal infection make it difficult to ascertain the true incidence (see below).
Etiology and pathogenesis
Parvovirus is one of the smallest known DNA viruses to infect humans. It is a global pathogen with increased prevalence in the late winter and early spring in temperate climates. Periodic epidemics occur. The most common mode of transmission is person-to-person contact via respiratory secretions. The incubation period is 4–14 days, but can be as long as 21 days.63 Parvovirus B19 can also be transmitted vertically from mother to fetus, and during transfusion with contaminated blood products.14 Since 2002, quantitative DNA measurement has been used to screen plasma products and thus reduce the risk of transmission through blood products.
Infection is rare in the first year of life, and the highest rate of infection occurs among children of school age. The prevalence of IgG antibodies in pregnant women is approximately 65%.57 Secondary attack rates are highest with household contact. Pregnant women with a child aged 5–7 years in the household appear to have a higher risk of becoming infected than do those with children under 2 years of age. Of particular concern is the risk to seronegative women in daycare and school settings, because infection in the first 20 weeks’ gestation can lead to increased fetal wastage and fetal hydrops. The greatest risk occurs during epidemics, and nursery school teachers appear to have a threefold increased risk of acute infection compared to other pregnant women.57 Nonetheless, routine exclusion of pregnant women from the workplace is not recommended, as risk of infection from other contacts is also likely during epidemics.
Parvovirus B19 propagates in human erythroid cells. In normal hosts, the cytotoxic effect of the virus leads to cessation of red blood cell production for approximately 4–8 days, creating a significant stress in patients with a rapidly expanding red cell mass (e.g., second-trimester fetus), or decreased red cell survival (e.g., underlying hemolytic anemia). The cellular receptor for parvovirus B19 (globoside or blood group P antigen) is located predominantly on the surface of erythroid precursor cells, thus explaining the virus’s affinity for this cell line. It is also present on myocardial cells, megakaryocytes, and endothelial cells, which may explain the thrombocytopenia, vasculitis, and myocarditis that are occasionally noted in affected fetuses and individuals. Immunocompromised hosts, presumably including fetuses, may have persistent viral infection and resultant chronic anemia.
The pathogenesis of hydrops secondary to parvovirus may be multifactorial. Severe anemia is almost always present and may lead to hypoxic injury and high output cardiac failure. Ascites, effusions, and skin edema may result. Myocarditis and diminished fetal cardiac output have also been noted in some cases, and may contribute to hydrops.
The exact risk to any pregnant woman (and her fetus) following exposure is not precisely known, although there appears to be a risk only if infection develops within the first 20 weeks’ gestation. Various studies have estimated the risk of adverse fetal outcome following maternal infection to be from 1% to 9%, with greatest risk during the first half of pregnancy.57 Prospective studies have shown an excess rate of fetal loss of 9%, confined to exposure during the first 20 weeks’ gestation, and an incidence of fetal hydrops of 2.9% with maternal infection between 11 and 18 weeks’ gestation.63,66 No significant risk for congenital anomalies has been noted. Spontaneous resolution of hydrops has been documented and complicates the issue of when and if intrauterine transfusion should be carried out (see ‘Treatment’, below).
Diagnosis
The diagnosis of acute parvovirus infection in pregnant women using IgM antibodies or IgG seroconversion is straightforward. Using radioimmunoassay or ELISA, over 90% of cases can be documented at the time of rash.14 The presence of IgM indicates that infection probably occurred within the previous 2–4 months. IgG levels are helpful to determine past exposure, and hence immunity, but are not helpful in detecting acute infection, as more than 50% of adults are seropositive. Fetuses, neonates, and immunocompromised patients may not mount an appropriate immune response following infection, and other methods may be required to document infection in these patients.
Parvovirus cannot be propagated in traditional cell culture. Virus may be identified using DNA hybridization techniques and PCR assays for B19.60 PCR is the optimal test for diagnosing chronic infection in immunocompromised patients, and may persist for up to 9 months in immunocompromised patients.
Routine histopathologic evaluation may reveal the presence of characteristic intranuclear inclusions in nucleated erythroid cells in placental or fetal tissue. Immunohistochemistry may detect viral antigen by staining techniques. Electron microscopy has been used to detect virus particles in serum, and prenatally in amniotic fluid, fetal blood, and ascitic fluid, as well as in postmortem tissue.58
Serologic evidence of infection in infants may be re-evaluated at 1 year of age, at which time maternal antibody should have disappeared and immunoglobulin detection will indicate true fetal or neonatal infection. Viral studies may fail to reveal acute infection if carried out subsequent to resolution of the infection. Viremia persists only 2–4 days in the immunocompetent host, and is generally absent by the time the classic rash develops.
Differential diagnosis
Nonimmune fetal hydrops can result from a number of diverse etiologies. It has been associated with many cardiac, infectious, hematologic, and genetic abnormalities, including anemias of diverse origins, CMV, toxoplasmosis, and syphilis.
Treatment
Management of the exposed pregnant woman.
Serologic evaluation should be offered; if the woman has high IgG titers to parvovirus B19 and lacks IgM, she is immune and not at risk. If she is seronegative, titers should be rechecked in 2 weeks for the presence of specific IgM. If evidence of acute infection exists, serial ultrasonographic evaluation is suggested.67,68 α-Fetoprotein levels have also been used as a screening tool.67,68 The risk of adverse outcome is minimal if infection occurs after 20 weeks’ gestation, and is low even if the mother becomes infected in the first two trimesters.
Management of fetal hydrops.
Fetal intrauterine digitalization and transfusion may be useful if significant fetal hydrops is observed. Although studies have shown reduced mortality with transfusion, its use is controversial because cases with spontaneous resolution of intrauterine hydrops can occur, and the procedure poses some risk to the fetus.
Congenital and neonatal infection.
Neonates with congenital infection attributed to parvovirus may respond to intravenous immunoglobulin therapy. Supportive care, including transfusion, may be required. Immunosuppressed patients may benefit from IVIG therapy.
Parvovirus infection in infants and toddlers.
Parvovirus in the older infant may present with the typical slapped cheek appearance and lacy peripheral eruption observed in school-age children, or may have less characteristic features. A juvenile variant of the papular purpuric gloves and socks syndrome (more typical of adolescents and young adults) has been described, in which infants develop erythematous and purpuric papules on the hands and feet lasting an average of 4–6 weeks. However, this presentation in infants has also been associated with other infections (EBV, CMV) and is not specific for parvovirus B19.69 Recently, in India, human parvovirus B19 IgM antibodies were detected in 15 out of 24 patients who had fever and generalized erythroderma, which resolved after 7–10 days with desquamation.70 Most of these patients were less than 1 year of age.
Parvovirus has also been associated with acute myocarditis in infants, and a prospective postmortem study of infants who succumbed to sudden infant death syndrome (SIDS) detected parvovirus B19 in cardiac tissue from 7 (11.2%) cases of SIDS.71,72
Enterovirus
The enteroviruses are a group of common, single-stranded RNA viruses that include the polioviruses, coxsackie viruses A and B, and echoviruses. Enteroviral infection in the neonate occurs most frequently during summer and early fall. Neonatal enterovirus infection can be severe, and the closely related parechovirus, a member of the same viral family (Picornaviridae), results in equally devastating disease. Neonatal enterovirus and parechovirus infections cannot be distinguished clinically.75
Cutaneous findings
Perinatal disease occurs within the first few weeks of life and results in the nonspecific clinical symptoms of sepsis (fever, irritability, poor feeding), accompanied by skin findings in approximately one- to two-thirds of infants.76,77 The rash is most often maculopapular (morbilliform), macular, or petechial (Fig. 13.13). The vesicopustular lesions that occur on intact skin (lesions analogous to those seen in hand, foot, and mouth disease) develop secondary to viremia, not from local inoculation as is seen in HSV infections. The pharynx is often erythematous, but usually without lesions. However, ulcers consistent with herpangina may appear on the soft palate. These early lesions may be indistinguishable from those of HSV infection. With progression of the infection, oral lesions remain circular (2–4 mm in diameter), confined to the soft palate, and exhibit a ‘punched-out’ appearance surrounded by a rim of erythema. Unlike those of HSV, they do not continue to enlarge or involve the hard palate, buccal mucosa, or gingival sulci.
Extracutaneous findings
Systemic manifestations of infection may be mild or severe. Disseminated infection involving the lungs, liver, and CNS (in addition to the upper respiratory tract and skin) occurs more often in the premature than in the full-term infant. The amount of transplacental antibody present is likely to affect the severity of the infection; therefore the most overwhelming infections occur in premature infants who lack significant amounts of specific maternal antibody for the infecting type of enterovirus.
In utero disease may occur rarely, with insufficient numbers of cases reported to consider a ‘congenital infection syndrome’. Findings in the neonate appear to result from residual damage to the heart, gastrointestinal tract, urogenital tract, muscle, or cutaneous tissue, rather than ongoing infection or reactivation of latent virus, with an apparent high intrauterine mortality rate.78
Etiology and pathogenesis
Estimated rates of neonatal infection are between 2 and 38 cases per 1000 births.76,79 Modes of transmission to the infant are similar to those for HSV. Acquisition from maternal sources at the time of delivery occurs in the majority of neonatal infections, with congenital infection being reported only rarely.80 Postnatal infection from sources other than the mother is also very common, leading to illness and frequent hospitalization of symptomatic infants during the first few months of life.
Diagnosis
Definitive diagnosis of enteroviral infection is most often achieved by PCR or viral culture of the pharynx or stool (Table 13.1). During acute infection, cultures of the pharynx are the most likely to yield the pathogen, whereas intestinal excretion of virus from gut-associated lymphoid tissue increases following clinical recovery. Fecal shedding of virus may continue for up to 6 weeks after acute infection, despite the presence of neutralizing antibody in serum. The PCR technique has been exceptionally useful in the diagnosis of enteroviral meningitis, and may be performed on the CSF of an infant whose rash is suspected to be enteroviral in origin.76,81 PCR of material from the pharynx, stool, or lesions has not been systematically evaluated. Histologic examination of the morbilliform skin eruptions does not yield specific cytologic information on the viral etiology of the rash. Serologies are not usually helpful, as no class-specific antibody response occurs with enteroviral infections, and at least 72 serotypes of enterovirus have been identified to date.
Treatment
Traditionally, only supportive care has been given; however, some experts recommend the administration of intravenous immunoglobulin to infants with overwhelming systemic infection.82 Specific antiviral therapy with a novel anti-picornavirus agent, pleconaril, is currently being evaluated in a double-blind, placebo-controlled trial in newborn infants with disseminated infection and has the potential to offer effective therapy for serious enteroviral infections.76
Infantile enteroviral disease
Enteroviral infections in older infants commonly manifest as hand, foot, and mouth disease (HFMD), but severe illness such as myocarditis, polio, meninigitis, and encephalitis can also develop, which may result in long-term sequelae.
Hand, foot, and mouth disease
Classic HFMD presents with fever, characteristic round to oval small vesicles on the palms and soles, and small vesicles and erosions on the oropharyngeal mucosae and tongue. In some cases, the cutaneous lesions also involve the dorsal hands, knees, buttocks, thighs and genitalia or evolve into a diffuse vesicular eruption (Fig. 13.14![]() ). The oral enanthem is often painful and places the child at risk for decreased oral intake and dehydration. Weeks after infection, nail changes such as transverse ridging (Beau’s lines) or onychomadesis may be noted (Fig. 13.15
). The oral enanthem is often painful and places the child at risk for decreased oral intake and dehydration. Weeks after infection, nail changes such as transverse ridging (Beau’s lines) or onychomadesis may be noted (Fig. 13.15![]() ).
).
Several enteroviruses cause HFMD, including coxsackie viruses A5, A7, A9, A10, A16, B1, B2, B3, B5, echovirus 4 and enterovirus 71.83 Most North American HFMD is due to coxsackie virus A16, however, more severe cutaneous disease linked to coxsackie virus A6 has been recognized in the USA since 2011 and CDC surveillance indicates a higher than expected rate of coxsackie virus A6 HFMD in children <2 years of age.84 Previously associated with international outbreaks in Finland, Taiwan, Japan, and Singapore, the coxsackie virus A6 exanthem appears to be more extensive, and may have unusual features such as erosions, an eczema herpeticum-like appearance, or a petechial or hemorrhagic appearance.85
Human papilloma virus infections
Infection with the human papilloma virus (HPV) can lead to cutaneous infections that commonly manifest as warts on the skin or mucous membranes. Lesions may be spread by direct sexual or nonsexual contact. Mother to infant transmission can be transplacental or via exposure to cervical or genital lesions. Autoinoculation or transmission via sexual abuse can also occur. There is some evidence that fomite spread is possible through the use of bidets, shared towels and undergarments.86 The incubation period for HPV has been estimated at 1–20 months, but latency periods may be in excess of 2 years.87 Although the vast majority of HPV disease results in transient lesions with a benign course, infection with certain subtypes of HPV (16, 18, 31, 33) can eventually lead to malignant metaplasia of the infected tissue.
Clinical lesions associated with HPV infections are only rarely present at birth or during the early neonatal period. Such lesions include anogenital warts (condyloma acuminata) and laryngeal papillomatosis. These lesions are more likely to become evident from 6 months to 2 years of age, and are believed to result from perinatal infection with a long latency period preceding clinical expression. An epidemiologic study of anogenital and laryngeal lesions in children under 13 years of age noted that the mean age of children with HPV was 4.5 years.88 Given that the majority of cases of anogenital HPV occur beyond 2 years of age, and that only a minority of these are sexually transmitted, it appears that many anogenital and laryngeal HPV infections in children are the result of horizontal transmission that is nonsexual.
Cutaneous findings
Condyloma acuminata favor the mucocutaneous junctions and are soft, papillomatous pink to flesh-colored lesions that may be discrete or confluent, pedunculated or flat-topped (Fig. 13.16). Areas most frequently affected in infants include the perianal skin, glans penis, vulva, and vaginal introitus (Fig. 13.17). The usual interval between exposure and the development of lesions appears to be 1–8 months, with an average of 3 months; however it is believed that much longer latency periods, perhaps up to 2 years, can occur.89,90 Lesions presenting in the neonatal period may represent in utero or perinatal exposure. Respiratory tract papillomas may affect the larynx, and less commonly the trachea, bronchial, and pulmonary epithelia. They are thought to be acquired by aspiration of infectious maternal vaginal and cervical secretions during labor. Such lesions usually present in infancy with hoarseness and respiratory distress.
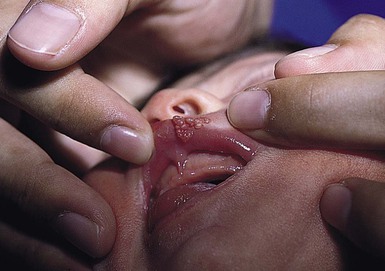
Etiology and pathogenesis
HPV is a small (55 nm), non-enveloped, circular, double-stranded DNA virus. It is expressed exclusively in fully differentiated keratinocytes and cannot be perpetuated in tissue culture. Over 130 different subtypes possessing varied oncogenic potential and tissue tropism have been identified. Although the papilloma viruses are categorized as to mucosal or cutaneous tropism, this classification is not strict, as genital types may be found on the skin, and cutaneous subtypes have been identified in anogenital lesions, particularly in children.91 The viruses have also been classified as to their malignant potential. HPV 6 and 11 are considered low-risk subtypes, whereas HPV 16, 18, 31, and 33 have been associated with anogenital cancer. HPV 30 has been associated with oral and laryngeal carcinoma, as well as anogenital carcinoma.91
HPV is one of the most common sexually transmitted diseases, and its incidence in infants and children probably reflects prevalence in the adult population.92 Most anogenital warts are subclinical and asymptomatic. The prevalence of genital lesions (condyloma) in the adult population is 0.6–13%, but molecular diagnostic studies have shown evidence of HPV infection in 11–80% of asymptomatic, sexually active young women.93–95 Transmission of infection may occur through vertical, innocent, and sexual contact. Subclinical infection of the cervix or vagina of a pregnant woman may lead to infection in her infant.96 The virus can be transmitted from mother to fetus before or during birth, and the rate of perinatal transmission from genital HPV-positive mothers to the pharyngeal mucosa of their infants is approximately 30–50%.97,98 However, studies of mother–neonate pairs have demonstrated that even when there is transmission of virus, it is most often only transient and clinical disease is unlikely to occur.97–99
Neonates appear to be at higher risk for exposure to HPV during vaginal delivery than during cesarean section.97 However, infants born by cesarean section have been found to be HPV DNA-positive for the same type as their mothers.100 Rare cases of anogenital warts present at birth following cesarean section delivery support the possibility of ascending infection.98 Transplacental exposure would explain such findings, as would small amniotic tears or leaks. The risk of a child contracting laryngeal papillomatosis is quite small.
HPV DNA has been identified in hepatic tissue from four infants with extrahepatic biliary duct atresia and three with neonatal giant cell hepatitis. Concordant HPV types were found in the infants’ mothers, supporting vertical transmission of the virus and its role in the pathologies noted in these infants.101
Epidermodysplasia verruciformis is a rare inherited disorder associated with immunodeficiency that leads to a chronic susceptibility to infection with certain subtypes of HPV. Lesions often resemble flat warts and malignant transformation can occur in later life.
Diagnosis
The clinical appearance of anogenital warts is usually diagnostic. A careful maternal history, including prior genital lesions and abnormal PAP smears, should be obtained. However, a negative history and normal maternal examination do not rule out the possibility of HPV disease. A spontaneous remission rate as high as 67% has been reported for HPV infections,92 and subclinical infection of the cervix or vagina may be present.
Histopathologic examination of anogenital HPV lesions demonstrates a slightly thickened stratum corneum, papillomatosis, and acanthosis of the epithelium, and thickening and elongation of the rete ridges. The presence of large vacuolated cells (koilocytes) in the epithelium is a characteristic sign, but is absent in approximately 50% of biopsies. HPV typing using probes against the most commonly encountered types (6, 11, 16, 18, and 33) may be useful. Such typing can now be performed using paraffin sections from routinely fixed tissue samples. PCR evaluation of suspicious areas has also been performed using specimens obtained by swabbing the site with a simple cotton swab, but this technique is not standardly available.108
Differential diagnosis
Sexual abuse should be considered in childhood HPV disease but is much less likely in the small infant. Condyloma lata should always be considered in the differential diagnosis.
Syphilitic lesions are usually moister, wider based, and frequently larger than anogenital HPV lesions. Infantile pyramidal protrusion consists of a soft tissue swelling covered by smooth erythematous skin on the perineal median raphe. These may be congenital, tend to be larger than anogenital HPV lesions, and possess a smoother surface and a broad base. Molluscum contagiosum lesions are generally smoother, with a dome-shaped configuration, and central umbilication. Pseudoverrucous papules and nodules occur following chronic irritant diaper dermatitis and can be mistaken for condyloma. Skin tags may also resemble condyloma acuminata, but are uncommon in the neonatal period, are flesh-colored, discrete, and do not spread.
Treatment
Prevention is the optimal approach, and the development of at least two human papilloma virus vaccines has increased the likelihood of success of this goal. One of these vaccines, Gardasil®, is effective against HPV types 6, 11, 16, and 18; it was FDA approved for vaccination of all females from 9–26 years in 2006 and all males from 9–26 years in 2009. This vaccine is effective only if given prior to exposure to the particular HPV subtypes represented, and therefore vaccination prior to sexual intercourse is recommended.
Many experts believe that treatment of these viral lesions may not always be necessary because warts are relatively asymptomatic, the spontaneous remission rate is quite high, and the cure rate with therapy low. However, it must be kept in mind that oncogenic forms (HPV 16, 18, 31, 33) can lead to anogenital cancers later in life. Fortunately, these subtypes are not usually found in childhood lesions. No easy, universally effective treatment exists. A number of therapeutic modalities have been used in adults and older children. These include liquid nitrogen, intralesional Candida antigen immunotherapy, podophyllin resin, trichloroacetic acid, cantharidin, podofilox, imiquimod 5% cream, sinecatechins and interferon. Physical destruction, including electrodesiccation, laser therapy, and simple excision, is an alternative therapy. The failure rate for treatment of HPV infections has been estimated at 25–50%, regardless of the method used.102 Vaccination to prevent acquisition of strains with neoplastic potential is the optimal approach.
Most experts opt for simple, less painful means of treatment in infants and young children. Agents frequently used for anogenital warts include podophyllin resin, trichloroacetic acid, and imiquimod cream. Sequential intralesional Candida injections are utilized for other sites. The family must be aware that frequent treatments are often required and that subclinical lesions in surrounding skin may become evident over time, despite the eradication of currently existing lesions.
Molluscum contagiosum
Molluscum contagiosum is a viral infection of the skin that most commonly affects young children, but can occur at any age. This disease is only rarely noted in the neonatal period, but lesions have been documented within the first week of life.103 A retrospective chart review of 302 patients found that less than 1% of affected patients were under 1 year and 28% of all affected patients were less than 36 months of age. The majority of patients who contracted the disease had fewer than 15 lesions, and children with atopic dermatitis were at higher risk for an increased number of lesions.104
Cutaneous findings
Molluscum lesions initially appear as small pink or flesh-colored pinpoint papules, which gradually enlarge and assume a pearly or white dome-shaped appearance. The papules are usually 1–5 mm in size, but giant lesions in excess of 1 cm can occur. The lesions tend to cluster, and more commonly appear on the trunk and in intertriginous areas such as the antecubital and popliteal fossae and axillae. Rarely, lesions may develop on the palms, soles, or mucous membranes. An eczematoid, red, scaling patch may surround the papules and is termed molluscum dermatitis. Autoinoculation from scratching or shaving may occur.
There are rare reports of molluscum contagiosum in the newborn (Fig. 13.18![]() ). Mandel and Lewis103 reported an infant who developed two thigh papules at 1 week of life, and another author documented multiple scalp lesions in a 6-week-old.105 However, in a series of five women with genital molluscum lesions at the time of delivery, none of their infants developed molluscum.106
). Mandel and Lewis103 reported an infant who developed two thigh papules at 1 week of life, and another author documented multiple scalp lesions in a 6-week-old.105 However, in a series of five women with genital molluscum lesions at the time of delivery, none of their infants developed molluscum.106
Etiology and pathogenesis
Molluscum contagiosum is caused by a large, approximately 300 nm, brick-shaped poxvirus, which contains double-stranded DNA. At least 43 different DNA subtypes have been identified. The entire genetic sequence of MCV type 1 has been determined, and there appears to be considerable protein homology with the smallpox virus. The virus does not grow in tissue culture, and an animal model does not exist.
Molluscum has a worldwide distribution, but is most common in tropical countries. Spread is through direct (including sexual) contact with infected persons, contaminated items, or by means of autoinoculation. The incubation period is estimated at 2 weeks to 6 months. The duration of disease is quite variable and may last just a few weeks or more than 1 year. Incidence peaks in early childhood and again in young adults, as a result of sexual transmission. An increased incidence has been noted in wrestlers and swimmers, and outbreaks have occurred in pools and waterparks.107 The disease has only rarely been noted in neonates, and it has been hypothesized that transplacental maternally derived antibody may be protective, but it can be seen in older infants. The immunocompromised, especially those with HIV infection, are subject to particularly extensive and prolonged infections that commonly involve the face. Patients with atopic dermatitis also appear to have more prolonged infections.
Diagnosis
The diagnosis is easily established when classic dome-shaped opalescent lesions with central umbilication are present. A curd-like material can be expressed from the central core and examined for the presence of molluscum bodies. These appear as monomorphous ovoid granular structures, and are best visualized with Wright’s or Giemsa stains; (KOH) potassium hydroxide staining is also an option. Histopathologic evaluation of a lesion will reveal large intracytoplasmic inclusion bodies within suprabasilar epithelial cells and lobular proliferation of the epidermis.
Differential diagnosis
Cutaneous cryptococcal lesions are occasionally mistaken for molluscum contagiosum in immunocompromised patients. Small lesions may be mistaken for common or flat warts. Giant molluscum lesions can resemble juvenile xanthogranuloma or Langerhans’ cell histiocytosis. Large inflamed lesions may resemble furuncles. The differential diagnosis for atypical giant lesions includes a number of neoplastic disorders, and biopsy is indicated in such cases.
Treatment
Molluscum is generally self-limited and frequently does not require therapy. Instances where intervention may be necessary include conjunctival lesions, which may damage the cornea, irritated, bleeding, or rapidly spreading lesions, and cosmetically disfiguring lesions, particularly in the immunosuppressed patient. Genital lesions are usually treated to prevent spread. A number of therapeutic modalities are used, including physical agents such as cryotherapy and curettage. Topical chemical treatments include cantharidin 0.7% in collodion, imiquimod 5%, podophyllin, salicylic acid, tretinoin, and silver nitrate. Cantharidin is commonly used, and is applied with a wooden applicator to each site, taking care to avoid mucosal, intertriginous, and facial areas. The site is not occluded, and the family is instructed to wash the area in 2–6 h, depending on previous sensitivity to the agent. Repeat treatments are sometimes required. Adhesive tape occlusion and systemic cimetidine therapy have been utilized, with variable results. A local anesthetic consisting of topical lidocaine (LMX-4) may be applied prior to curettage. Families should be advised that multiple visits and treatments may be required and that spread of infection may occur through shared baths, towels, and swimming pools. Genital lesions are not uncommon in young children and are thought to be the result of autoinoculation. The issue of sexual abuse may be raised in older children, but supporting evidence should be documented prior to referral, as nonsexually transmitted genital involvement is often seen in childhood infections.
Viral exanthems in infants and toddlers
Viral illnesses commonly affect infants and toddlers and can be distinguished by the morphology and distribution of skin lesions (Table 13.5), as well as associated symptoms.
TABLE 13.5
Skin findings of viral disease in children 1–24 months of age
| Infection | Skin findings |
| Measles (rubeola) | Koplik spots (transient blue-gray to white papules on buccal mucosa across from second molar), erythematous macules and papules starting on face and behind ears and spreading down trunk |
| Rubella (German measles) | Forchheimer spots (transient red papules on soft palate), pink macules and papules starting on face and spreading down trunk (measles-like), lymphadenopathy |
| Roseola | Blanchable pink macules and papules on trunk which erupt after high fever (>103°F) breaks |
| Parvovirus B19 | Rosy pink patches on cheeks (‘slapped cheeks’) and lacy interconnecting pink macules on extremities, papules and purpura on the hands and feet (young infants) |
| Enterovirus | Herpangina: erythematous round punched-out erosions on soft palate |
| Hand, foot, and mouth disease: oval superficial vesicles on hands, feet, knees, buttocks and erosions of oral mucosa, post-illness nail changes including Beau’s lines and onychomadesis | |
| Human papillomavirus | Skin-toned verrucous, flat-topped and filiform papules on skin, genital mucosa, or respiratory mucosa |
| Molluscum contagiosum | Pink to skin-toned dome shaped papules with central umbilication on skin |
Measles (rubeola)
Measles results from infection with a single-stranded RNA paramyxovirus, genus Morbillivirus. Transmission peaks in the late winter and early spring. It is highly contagious via respiratory droplets. The measles vaccination program has induced a greater than 99% decrease in the reported incidence of measles; however new measles infections in the United States continue to occur.108 Although herd immunity provides protection, it is important for practitioners to recognize this disease, which may affect infants as their passive immunity wanes before their eligibility for vaccination at 1 year of age and may also affect those who forgo immunization. Measles infection can be complicated by pneumonia, gastroenteritis, myocarditis, and encephalitis.
Clinical findings
Measles typically presents with cough, coryza, conjunctivitis and fever. Blue-white papules on erythematous buccal mucosa directly across from premolar teeth, termed Koplik’s spots, can be seen at the onset of these symptoms and are pathognomonic for the disease. Koplik’s spots typically fade as the rash develops, so their absence does not rule out the diagnosis. The skin eruption begins about 2–4 days after the prodromal symptoms with erythematous macules and papules (morbilliform) on the temples and behind the ears, which then progresses downward from the face to involve the trunk and extremities.
Differential diagnosis
Morbilliform drug eruptions, Kawasaki disease, and other viral exanthems (e.g., rubella) may enter the differential diagnosis of measles. In most cases, the clinical history of symptoms and evolution of the rash will help differentiate.
Diagnosis
Most commonly, measles can be diagnosed based on the history and clinical findings; however, laboratory confirmation is available through serologies and PCR.
Treatment
Supportive care is generally the only intervention indicated as there is no specific treatment for measles. In developing countries, vitamin A supplementation has been shown to reduce measles-related morbidity and mortality.109 While recognizing that there are limitations in the available data, the American Academy of Pediatrics recommends that vitamin A supplementation be considered in any infant (ages 6 months–2 years) hospitalized for measles and associated complications, as well as in infants more than 6 months of age diagnosed with measles who are recent immigrants, or who have immunodeficiency, malnutrition, diseases which impair intestinal absorption (e.g., cystic fibrosis, short gut syndrome), or ophthalmologic signs of vitamin A deficiency (Bitot’s spots, xerophthalmia, night blindness).110 The need for, and safety of, vitamin A supplementation in infants with measles who are <6 months old has not been established.110
Rubella (german measles)
Rubella is caused by infection with the rubella virus, an enveloped, single-stranded RNA togavirus. Similar to measles, the illness peaks in incidence in late winter and spring and is transmitted via the respiratory route. Rubella is included in the MMR vaccine, which infants should receive at 12 months with a booster to follow at 4 years. The vaccination schedule has been successful in the control of rubella. The Centers for Disease Control and Prevention determined that the USA achieved endemic rubella elimination in 2001, and more recent evidence supports that the elimination of endemic rubella is being maintained.111 However, it is important for practitioners to recognize this illness, as it remains endemic in many other parts of the world and susceptible or unvaccinated infants may potentially be exposed through contact with family members who travel globally. For example, exposure to rubella through air travel has been reported, although contact-tracing revealed no cases of a rubella-like rash.112
Clinical manifestations include low-grade fevers, mild upper respiratory symptoms, and lymphadenopathy, especially in the posterior head and neck lymphatic chains. In some cases, transient red papules termed ‘Forchheimer spots’ may be seen on the soft palate early in the infection. The skin eruption often mimics measles, with pink macules and papules starting on the face and spreading downward to the trunk and extremities. The exanthem fades in about 3 days, so the illness is sometimes referred to as ‘3-day measles’. Care is supportive and the symptoms tend to be mild; however, care must be taken to isolate children with suspected rubella from expectant mothers who are either non-immune or do not know their rubella immunity status to prevent congenital rubella syndrome (see above).
Roseola (exanthem subitum, sixth disease)
Roseola is a mild viral illness that is very common in infants aged 6–12 months. It is attributed to human herpesvirus (HHV) type 6 or 7. HHV7 infections occur less often in children <24 months of age, and most roseola cases are associated with HHV6.113 Although nearly 90% of adults demonstrate serologic immunity to these ubiquitous viruses, only about one-third of infants develop the acute illness.
The infection manifests with 3–7 days of high fever (>103°F). Febrile seizures develop in up to 10–15% of affected infants. When the fever breaks, blanchable, pink macules and papules erupt on the face, trunk and extremities. The exanthem is asymptomatic and fades over hours to days. In the febrile stage, supportive care with antipyretics is indicated, but otherwise no treatment is required for this transient viral illness.
Unilateral laterothoracic exanthem
Unilateral laterothoracic exanthem (ULE), also known as asymmetric periflexural exanthem of childhood, is a distinct skin eruption that can be seen in children ages 12–24 months. In a prospective study, the mean age of onset was 24.3 months.114 It begins with a unilateral truncal eruption that extends to the axilla or inguinal area. Less commonly, extremities may be involved. Lesion morphology varies and includes macules, papules, eczematous patches and rarely, vesicles and purpura. As the eruption progresses, it often expands to involve the contralateral side. Itching is present in about 50% of affected children. Antihistamines and topical steroids can be used to reduce symptoms, and the eruption self-resolves over 3–8 weeks.
A viral etiology for ULE is suspected, as it occurs in young children and may be associated with prodromal upper respiratory or gastrointestinal symptoms as well as low-grade fever. Studies have not been able to confirm a consistent etiologic agent, and ULE may reflect a reaction pattern to more than one microorganism.
Gianotti–Crosti syndrome
Gianotti–Crosti syndrome (GCS), also known as papular acrodermatitis of childhood, is a symmetric eruption of monomorphous, flat-topped pink or pink-brown papules on the cheeks, buttocks, and extensor extremities. Lesions range in size from 1–10 mm. Younger children tend to develop larger papules. The eruption typically lasts about 3 weeks, but clearance may be delayed as long as 2–3 months. Prodromal fever and upper respiratory symptoms occur prior to rash onset in many patients, and in some children lymphadenopathy, hepatomegaly, and splenomegaly develop. GCS occurs in infants aged 3–24 months, as well as in children and adolescents; most affected children are older than 12 months.
GCS has been reported to occur in association with Epstein–Barr virus (EBV), hepatitis B virus, cytomegalovirus, and coxsackie virus.115 In the USA, EBV is the most commonly associated viral infection and associated hepatitis B infection is rare.116 GCS has also been linked to immunizations for influenza, diphtheria, pertussis, polio, Bacillus Calmette–Guérin (BCG), and measles/mumps/rubella (MMR).117 Similar to ULE, GCS is considered an immune system mediated reaction pattern to viral infections or immunizations.
Access the full reference list at ExpertConsult.com ![]()
Figures 6, 11, 12, 14, 15 and 18 are available online at ExpertConsult.com ![]()