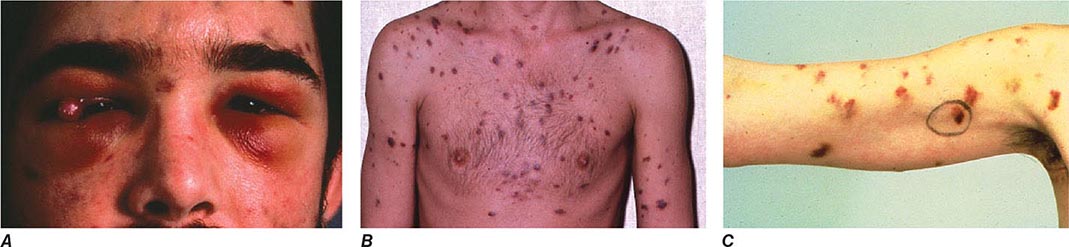
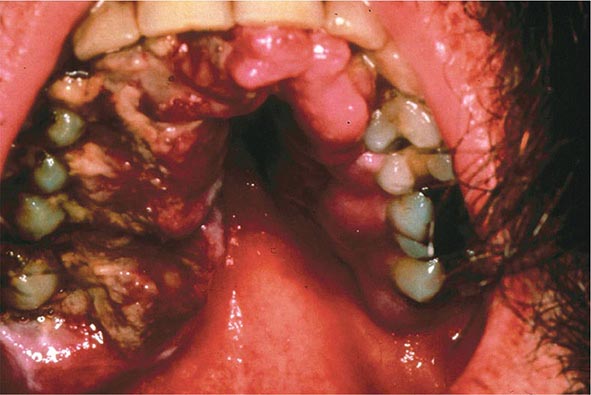
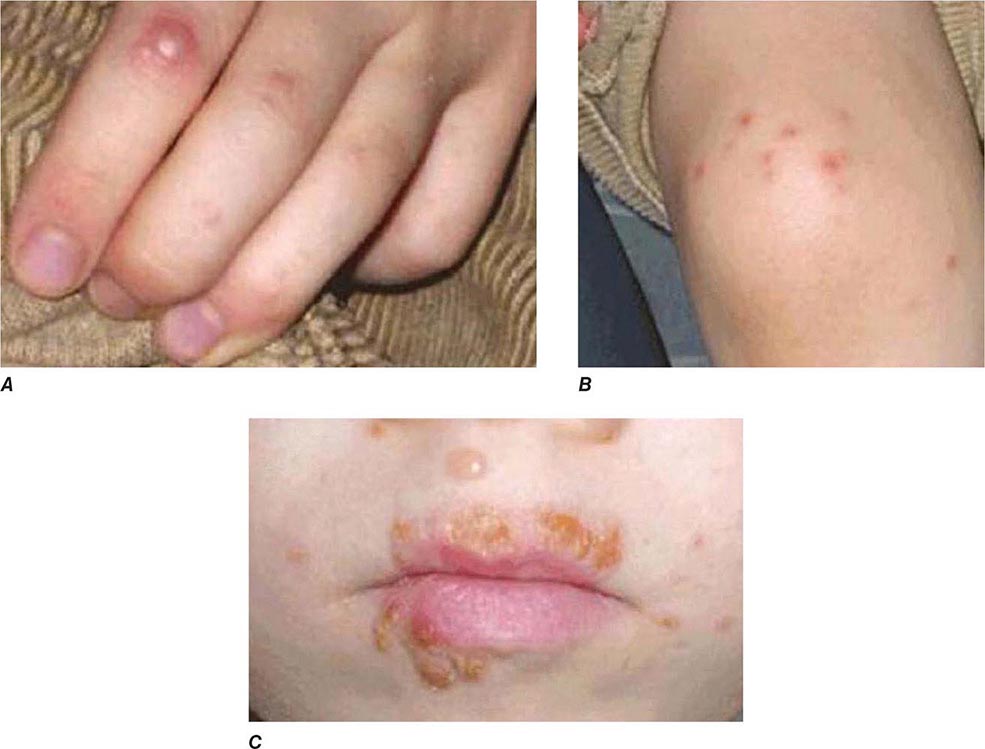
FIGURE 226-34 Various oral lesions in HIV-infected individuals. A. Thrush. B. Hairy leukoplakia. C. Aphthous ulcer. D. Kaposi’s sarcoma.
Esophagitis (Fig. 226-35) may present with odynophagia and retrosternal pain. Upper endoscopy is generally required to make an accurate diagnosis. Esophagitis may be due to Candida, CMV, or HSV. While CMV tends to be associated with a single large ulcer, HSV infection is more often associated with multiple small ulcers. The esophagus may also be the site of KS and lymphoma. Like the oral mucosa, the esophageal mucosa may have large, painful ulcers of unclear etiology that may respond to thalidomide. While achlorhydria is a common problem in patients with HIV infection, other gastric problems are generally rare. Among the neoplastic conditions involving the stomach are KS and lymphoma.
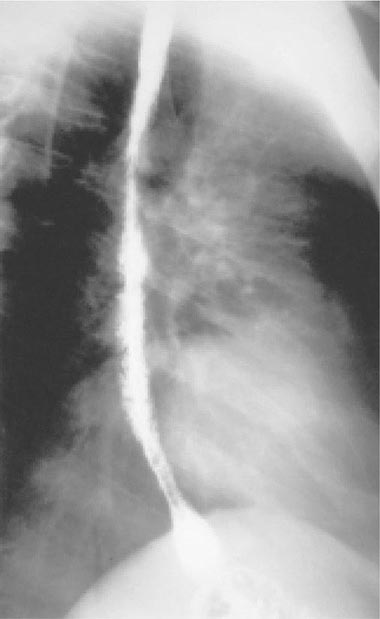
FIGURE 226-35 Barium swallow of a patient with Candida esophagitis. The flow of barium along the mucosal surface is grossly irregular.
Infections of the small and large intestine leading to diarrhea, abdominal pain, and occasionally fever are among the most significant GI problems in HIV-infected patients. They include infections with bacteria, protozoa, and viruses.
Bacteria may be responsible for secondary infections of the GI tract. Infections with enteric pathogens such as Salmonella, Shigella, and Campylobacter are more common in men who have sex with men and are often more severe and more apt to relapse in patients with HIV infection. Patients with untreated HIV have approximately a 20-fold increased risk of infection with S. typhimurium. They may present with a variety of nonspecific symptoms including fever, anorexia, fatigue, and malaise of several weeks’ duration. Diarrhea is common but may be absent. Diagnosis is made by culture of blood and stool. Long-term therapy with ciprofloxacin is the recommended treatment. HIV-infected patients also have an increased incidence of S. typhi infection in areas of the world where typhoid is a problem. Shigella spp., particularly S. flexneri, can cause severe intestinal disease in HIV-infected individuals. Up to 50% of patients will develop bacteremia. Campylobacter infections occur with an increased frequency in patients with HIV infection. While C. jejuni is the strain most frequently isolated, infections with many other strains have been reported. Patients usually present with crampy abdominal pain, fever, and bloody diarrhea. Infection may also present as proctitis. Stool examination reveals the presence of fecal leukocytes. Systemic infection can occur, with up to 10% of infected patients exhibiting bacteremia. Most strains are sensitive to erythromycin. Abdominal pain and diarrhea may be seen with MAC infection.
Fungal infections may also be a cause of diarrhea in patients with HIV infection. Histoplasmosis, coccidioidomycosis, and penicilliosis have all been identified as a cause of fever and diarrhea in patients with HIV infection. Peritonitis has been seen with C. immitis.
Cryptosporidia, microsporidia, and Isospora belli (Chap. 254) are the most common opportunistic protozoa that infect the GI tract and cause diarrhea in HIV-infected patients. Cryptosporidial infection may present in a variety of ways, ranging from a self-limited or intermittent diarrheal illness in patients in the early stages of HIV infection to a severe, life-threatening diarrhea in severely immunodeficient individuals. In patients with untreated HIV infection and CD4+ T cell counts of <300/μL, the incidence of cryptosporidiosis is ~1% per year. In 75% of cases the diarrhea is accompanied by crampy abdominal pain, and 25% of patients have nausea and/or vomiting. Cryptosporidia may also cause biliary tract disease in the HIV-infected patient, leading to cholecystitis with or without accompanying cholangitis and pancreatitis secondary to papillary stenosis. The diagnosis of cryptosporidial diarrhea is made by stool examination or biopsy of the small intestine. The diarrhea is noninflammatory, and the characteristic finding is the presence of oocysts that stain with acid-fast dyes. Therapy is predominantly supportive, and marked improvements have been reported in the setting of effective cART. Treatment with up to 2000 mg/d of nitazoxanide (NTZ) is associated with improvement in symptoms or a decrease in shedding of organisms in about half of patients. Its overall role in the management of this condition remains unclear. Patients can minimize their risk of developing cryptosporidiosis by avoiding contact with human and animal feces, by not drinking untreated water from lakes or rivers, and by not eating raw shellfish.
Microsporidia are small, unicellular, obligate intracellular parasites that reside in the cytoplasm of enteric cells (Chap. 254). The main species causing disease in humans is Enterocytozoon bieneusi. The clinical manifestations are similar to those described for cryptosporidia and include abdominal pain, malabsorption, diarrhea, and cholangitis. The small size of the organism may make it difficult to detect; however, with the use of chromotrope-based stains, organisms can be identified in stool samples by light microscopy. Definitive diagnosis generally depends on electron-microscopic examination of a stool specimen, intestinal aspirate, or intestinal biopsy specimen. In contrast to cryptosporidia, microsporidia have been noted in a variety of extraintestinal locations, including the eye, brain, sinuses, muscle, and liver, and they have been associated with conjunctivitis and hepatitis. The most effective way to deal with microsporidia in a patient with HIV infection is to restore the immune system by treating the HIV infection with cART. Albendazole, 400 mg bid, has been reported to be of benefit in some patients.
I. belli is a coccidian parasite (Chap. 254) most commonly found as a cause of diarrhea in patients from tropical and subtropical regions. Its cysts appear in the stool as large, acid-fast structures that can be differentiated from those of cryptosporidia on the basis of size, shape, and number of sporocysts. The clinical syndromes of Isospora infection are identical to those caused by cryptosporidia. The important distinction is that infection with Isospora is generally relatively easy to treat with TMP/SMX. While relapses are common, a thrice-weekly regimen of TMP/SMX appears adequate to prevent recurrence.
CMV colitis was once seen as a consequence of advanced immunodeficiency in 5–10% of patients with AIDS. It is much less common with the advent of cART. CMV colitis presents as diarrhea, abdominal pain, weight loss, and anorexia. The diarrhea is usually nonbloody, and the diagnosis is achieved through endoscopy and biopsy. Multiple mucosal ulcerations are seen at endoscopy, and biopsies reveal characteristic intranuclear and cytoplasmic inclusion bodies. Secondary bacteremias may result as a consequence of thinning of the bowel wall. Treatment is with either ganciclovir or foscarnet for 3–6 weeks. Relapses are common, and maintenance therapy is typically necessary in patients whose HIV infection is poorly controlled. Patients with CMV disease of the GI tract should be carefully monitored for evidence of CMV retinitis.
In addition to disease caused by specific secondary infections, patients with HIV infection may also experience a chronic diarrheal syndrome for which no etiologic agent other than HIV can be identified. This entity is referred to as AIDS enteropathy or HIV enteropathy. It is most likely a direct result of HIV infection in the GI tract. Histologic examination of the small bowel in these patients reveals low-grade mucosal atrophy with a decrease in mitotic figures, suggesting a hyporegenerative state. Patients often have decreased or absent small-bowel lactase and malabsorption with accompanying weight loss.
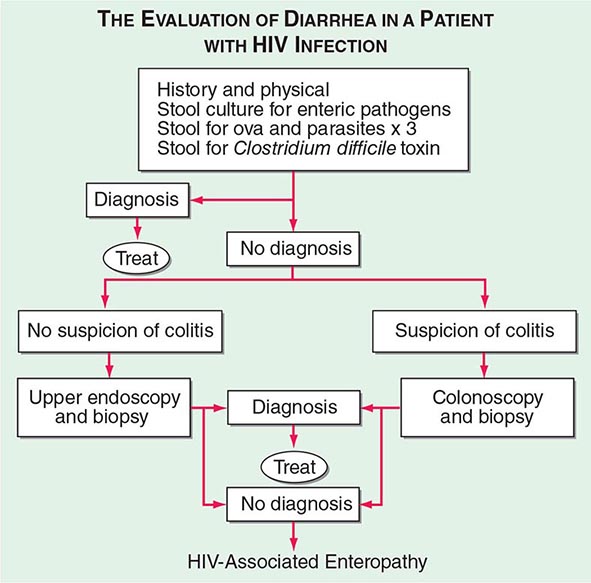
The initial evaluation of a patient with HIV infection and diarrhea should include a set of stool examinations, including culture, examination for ova and parasites, and examination for Clostridium difficile toxin. Approximately 50% of the time this workup will demonstrate infection with pathogenic bacteria, mycobacteria, or protozoa. If the initial stool examinations are negative, additional evaluation, including upper and/or lower endoscopy with biopsy, will yield a diagnosis of microsporidial or mycobacterial infection of the small intestine ~30% of the time. In patients for whom this diagnostic evaluation is nonrevealing, a presumptive diagnosis of HIV enteropathy can be made if the diarrhea has persisted for >1 month. An algorithm for the evaluation of diarrhea in patients with HIV infection is given in Fig. 226-36.
FIGURE 226-36 Algorithm for the evaluation of diarrhea in a patient with HIV infection. HIV-associated enteropathy is a diagnosis of exclusion and can be made only after other, generally treatable, forms of diarrheal illness have been ruled out.
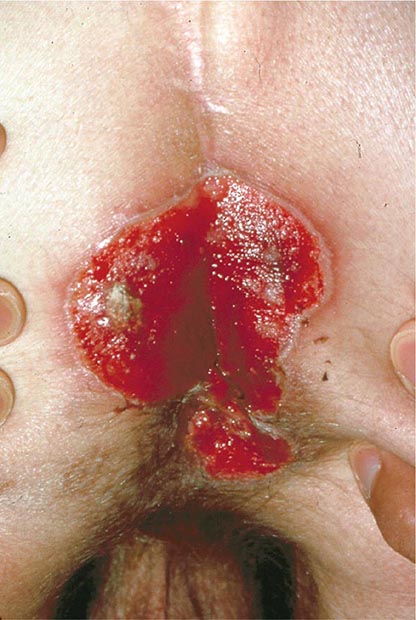
Rectal lesions are common in HIV-infected patients, particularly the perirectal ulcers and erosions due to the reactivation of HSV (Fig. 226-37). These lesions may appear quite atypical, as denuded skin without vesicles. They typically respond well to treatment with acyclovir, famciclovir, or foscarnet. Other rectal lesions encountered in patients with HIV infection include condylomata acuminata, KS, and intraepithelial neoplasia (see below).
FIGURE 226-37 Severe, erosive perirectal herpes simplex in a patient with AIDS.
Hepatobiliary Diseases Diseases of the hepatobiliary system are a major problem in patients with HIV infection. It has been estimated that approximately 15% of the deaths of patients with HIV infection are related to liver disease. While this is predominantly a reflection of the problems encountered in the setting of co-infection with hepatitis B or C, it is also a reflection of the hepatic injury, ranging from hepatic steatosis to hypersensitivity reactions to immune reconstitution, that can be seen in the context of cART.
The prevalence of co-infection with HIV and hepatitis viruses varies by geographic region. In the United States, ~90% of HIV-infected individuals have evidence of infection with HBV; 6–14% have chronic HBV infection; 5–50% of patients are co-infected with HCV; and co-infections with hepatitis D, E, and/or G viruses are common. Among IV drug users with HIV infection, rates of HCV infection range from 70% to 95%. HIV infection has a significant impact on the course of hepatitis virus infection. It is associated with approximately a threefold increase in the development of persistent hepatitis B surface antigenemia. Patients infected with both HBV and HIV have decreased evidence of inflammatory liver disease. The presumption that this is due to the immunosuppressive effects of HIV infection is supported by the observations that this situation can be reversed, and one may see the development of more severe hepatitis following the initiation of effective cART. In studies of the impact of HIV on HBV infection, four- to tenfold increases in liver-related mortality rates have been noted in patients with HIV and active HBV infection compared to rates in patients with either infection alone. There is, however, only a slight increase in overall mortality rate in HIV-infected individuals who are also hepatitis B surface antigen (HBsAg)–positive. IFN-α is less successful as treatment for HBV in patients with HIV co-infection. Lamivudine, emtricitabine, adefovir/tenofovir/entecavir, and telbivudine alone or in combination are useful in the treatment of hepatitis B in patients with HIV infection. It is important to remember that all the above-mentioned drugs also have activity against HIV and should not be used alone in patients with HIV infection, in order to avoid the emergence of quasispecies of HIV resistant to these drugs. For this reason, the need to treat hepatitis B infection in a patient with HIV infection is an indication to treat HIV infection in that same patient, regardless of CD4+ T cell count. HCV infection is more severe in the patient with HIV infection; it does not appear to affect overall mortality rates in HIV-infected individuals when other variables such as age, baseline CD4+ T cell count, and use of cART are taken into account. In the setting of HIV and HCV co-infection, levels of HCV are approximately tenfold higher than in the HIV-negative patient with HCV infection. There is a 50% higher overall mortality rate with a five-fold increased risk of death due to liver disease in patients chronically infected with both HCV and HIV. Use of directly acting agents for the treatment for HCV leads to cure rates approaching 100%, even in patients with HIV co-infection. Successful treatment of HCV in HIV-infected patients decreases mortality. Hepatitis A virus infection is not seen with an increased frequency in patients with HIV infection. It is recommended that all patients with HIV infection who have not experienced natural infection be immunized with hepatitis A and/or hepatitis B vaccines. Infection with hepatitis G virus, also known as GB virus C, is seen in ~50% of patients with HIV infection. For reasons that are currently unclear, there are data to suggest that patients with HIV infection co-infected with this virus have a decreased rate of progression to AIDS.
A variety of other infections also may involve the liver. Granulomatous hepatitis may be seen as a consequence of mycobacterial or fungal infections, particularly MAC infection. Hepatic masses may be seen in the context of TB, peliosis hepatis, or fungal infection. Among the fungal opportunistic infections, C. immitis and Histoplasma capsulatum are those most likely to involve the liver. Biliary tract disease in the form of papillary stenosis or sclerosing cholangitis has been reported in the context of cryptosporidiosis, CMV infection, and KS. When no diagnosis can be made, the term AIDS cholangiopathy is used. Hemophagocytic lymphohistiocytosis of the liver has been seen in the setting of Hodgkin’s disease.
Many of the drugs used to treat HIV infection are metabolized by the liver and can cause liver injury. Fatal hepatic reactions have been reported with a wide array of antiretrovirals including nucleoside analogues, nonnucleoside analogues, and protease inhibitors. Nucleoside analogues work by inhibiting DNA synthesis. This can result in toxicity to mitochondria, which can lead to disturbances in oxidative metabolism. This may manifest as hepatic steatosis and, in severe cases, lactic acidosis and fulminant liver failure. It is important to be aware of this condition and to watch for it in patients with HIV infection receiving nucleoside analogues. It is reversible if diagnosed early and the offending agent(s) discontinued. Nevirapine has been associated with at times fatal fulminant and cholestatic hepatitis, hepatic necrosis, and hepatic failure. Indinavir may cause mild to moderate elevations in serum bilirubin in 10–15% of patients in a syndrome similar to Gilbert’s syndrome. A similar pattern of hepatic injury may be seen with atazanavir. In the patient receiving cART with an unexplained increase in hepatic transaminases, strong consideration should be given to drug toxicity.
Pancreatic injury is most commonly a consequence of drug toxicity, notably that secondary to pentamidine or dideoxynucleosides. While up to half of patients in some series have biochemical evidence of pancreatic injury, <5% of patients show any clinical evidence of pancreatitis that is not linked to a drug toxicity.
Diseases of the Kidney and Genitourinary Tract Diseases of the kidney or genitourinary tract may be a direct consequence of HIV infection, due to an opportunistic infection or neoplasm, or related to drug toxicity. Overall, microalbuminuria is seen in ~20% of untreated HIV-infected patients; significant proteinuria is seen in closer to 2%. The presence of microalbuminuria has been associated with an increase in all-cause mortality rate. HIV-associated nephropathy (HIVAN) was first described in IDUs and was initially thought to be IDU nephropathy in patients with HIV infection; it is now recognized as a true direct complication of HIV infection. Although the majority of patients with this condition have CD4+ T cell counts <200/μL, HIV-associated nephropathy can be an early manifestation of HIV infection and is also seen in children. Over 90% of reported cases have been in African-American or Hispanic individuals; the disease is not only more prevalent in these populations but also more severe and is the third leading cause of end-stage renal failure among African Americans age 20–64 in the United States. Proteinuria is the hallmark of this disorder. Edema and hypertension are rare. Ultrasound examination reveals enlarged, hyperechogenic kidneys. A definitive diagnosis is obtained through renal biopsy. Histologically, focal segmental glomerulosclerosis is present in 80%, and mesangial proliferation in 10–15% of cases. Prior to effective antiretroviral therapy, this disease was characterized by relatively rapid progression to end-stage renal disease. Patients with HIV-associated nephropathy should be treated for their HIV infection regardless of CD4+ T cell count. Treatment with angiotensin-converting enzyme (ACE) inhibitors and/or prednisone, 60 mg/d, also has been reported to be of benefit in some cases. The incidence of this disease in patients receiving adequate cART has not been well defined; however, the impression is that it has decreased in frequency and severity. It is the leading cause of end-stage renal disease in patients with HIV infection.
Among the drugs commonly associated with renal damage in patients with HIV disease are pentamidine, amphotericin, adefovir, cidofovir, tenofovir, and foscarnet. TMP/SMX may compete for tubular secretion with creatinine and cause an increase in the serum creatinine level. Sulfadiazine may crystallize in the kidney and result in an easily reversible form of renal shutdown, while indinavir or atazanavir may form renal calculi. Adequate hydration is the mainstay of treatment and prevention for these latter two conditions.
Genitourinary tract infections are seen with a high frequency in patients with HIV infection; they present with skin lesions, dysuria, hematuria, and/or pyuria and are managed in the same fashion as in patients without HIV infection. Infections with HSV are covered below (“Dermatologic Diseases”). Infections with T. pallidum, the etiologic agent of syphilis, play an important role in the HIV epidemic. In HIV-negative individuals, genital syphilitic ulcers as well as the ulcers of chancroid are major predisposing factors for heterosexual transmission of HIV infection. While most HIV-infected individuals with syphilis have a typical presentation, a variety of formerly rare clinical problems may be encountered in the setting of dual infection. Among them are lues maligna, an ulcerating lesion of the skin due to a necrotizing vasculitis; unexplained fever; nephrotic syndrome; and neurosyphilis. The most common presentation of syphilis in the HIV-infected patient is that of condylomata lata, a form of secondary syphilis. Neurosyphilis may be asymptomatic or may present as acute meningitis, neuroretinitis, deafness, or stroke. The rate of neurosyphilis may be as high as 1% in patients with HIV infection, and one should consider a lumbar puncture to look for neurosyphilis in all patients with HIV infection and secondary syphilis. As a consequence of the immunologic abnormalities seen in the setting of HIV infection, diagnosis of syphilis through standard serologic testing may be challenging. On the one hand, a significant number of patients have false-positive Venereal Disease Research Laboratory (VDRL) tests due to polyclonal B cell activation. On the other hand, the development of a new positive VDRL may be delayed in patients with new infections, and the anti–fluorescent treponemal antibody (anti-FTA) test may be negative due to immunodeficiency. Thus, dark-field examination of appropriate specimens should be performed in any patient in whom syphilis is suspected, even if the patient has a negative VDRL. Similarly, any patient with a positive serum VDRL test, neurologic findings, and an abnormal spinal fluid examination should be considered to have neurosyphilis and treated accordingly, regardless of the CSF VDRL result. In any setting, patients treated for syphilis need to be carefully monitored to ensure adequate therapy. Approximately one-third of patients with HIV infection will experience a Jarisch-Herxheimer reaction upon initiation of therapy for syphilis.
Vulvovaginal candidiasis is a common problem in women with HIV infection. Symptoms include pruritus, discomfort, dyspareunia, and dysuria. Vulvar infection may present as a morbilliform rash that may extend to the thighs. Vaginal infection is usually associated with a white discharge, and plaques may be seen along an erythematous vaginal wall. Diagnosis is made by microscopic examination of the discharge for pseudohyphal elements in a 10% potassium hydroxide solution. Mild disease can be treated with topical therapy. More serious disease can be treated with fluconazole. Other causes of vaginitis include Trichomonas and mixed bacteria.
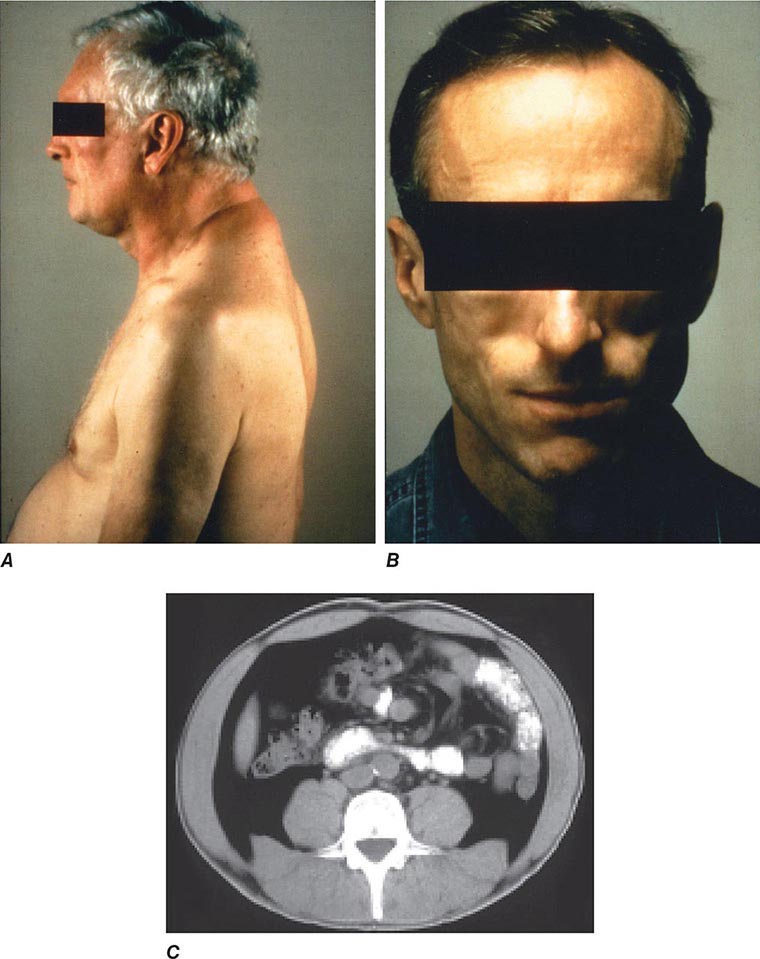
Diseases of the Endocrine System and Metabolic Disorders A variety of endocrine and metabolic disorders are seen in the context of HIV infection. These may be a direct consequence of HIV infection, secondary to opportunistic infections or neoplasms, or related to medication side effects. Between 33% and 75% of patients with HIV infection receiving thymidine analogues or protease inhibitors as a component of cART develop a syndrome often referred to as lipodystrophy, consisting of elevations in plasma triglycerides, total cholesterol, and apolipoprotein B, as well as hyperinsulinemia and hyperglycemia. Many of the patients have been noted to have a characteristic set of body habitus changes associated with fat redistribution, consisting of truncal obesity coupled with peripheral wasting (Fig. 226-38). Truncal obesity is apparent as an increase in abdominal girth related to increases in mesenteric fat, a dorsocervical fat pad (“buffalo hump”) reminiscent of patients with Cushing’s syndrome, and enlargement of the breasts. The peripheral wasting, or lipoatrophy, is particularly noticeable in the face and buttocks and by the prominence of the veins in the legs. These changes may develop at any time ranging from ~6 weeks to several years following the initiation of cART. Approximately 20% of the patients with HIV-associated lipodystrophy meet the criteria for the metabolic syndrome as defined by The International Diabetes Federation or The U.S. National Cholesterol Education Program Adult Treatment Panel III. The lipodystrophy syndrome has been reported in association with regimens containing a variety of different drugs, and while initially reported in the setting of protease inhibitor therapy, it appears that similar changes can also be induced by protease-sparing regimens. It has been suggested that the lipoatrophy changes are particularly severe in patients receiving the thymidine analogues stavudine and zidovudine. National Cholesterol Education Program (NCEP) guidelines should be followed in the management of these lipid abnormalities (Chap. 291e), and consideration should be given to changing the components of cART with avoidance of thymidine analogues (azidothymidine and stavudine) and protease inhibitors. Due to concerns regarding drug interactions, the most commonly utilized lipid-lowering agents in this setting are gemfibrozil and atorvastatin. In addition, lactic acidosis is associated with cART. This is most commonly seen with nucleoside analogue reverse transcriptase inhibitors and can be fatal (see below).
FIGURE 226-38 Characteristics of lipodystrophy. A. Truncal obesity and buffalo hump. B. Facial wasting. C. Accumulation of intraabdominal fat on CT scan.
Patients with advanced HIV disease may develop hyponatremia due to the syndrome of inappropriate antidiuretic hormone (vasopressin) secretion (SIADH) as a consequence of increased free-water intake and decreased free-water excretion. SIADH is usually seen in conjunction with pulmonary or CNS disease. Low serum sodium may also be due to adrenal insufficiency; a concomitant high serum potassium should alert one to this possibility. Hyperkalemia may be secondary to adrenal insufficiency; HIV nephropathy; or medications, particularly trimethoprim and pentamidine. Hypokalemia may be seen in the setting of tenofovir or amphotericin therapy. Adrenal gland disease may be due to mycobacterial infections, CMV disease, cryptococcal disease, histoplasmosis, or ketoconazole toxicity. Iatrogenic Cushing’s syndrome with suppression of the hypothalamic-pituitary-adrenal axis may be seen with the use of local glucocorticoids (injected or inhaled) in patients receiving ritonavir. This is due to inhibition of the hepatic enzyme CYP3A4 by ritonavir leading to prolongation of the glucocorticoid half-life.
Thyroid function may be altered in 10–15% of patients with HIV infection. Both hypo- and hyperthyroidism may be seen. The predominant abnormality is subclinical hypothyroidism. In the setting of cART, up to 10% of patients have been noted to have elevated thyroid-stimulating hormone levels, suggesting that this may be a manifestation of immune reconstitution. Immune-reconstitution Graves’ disease may occur as a late (9–48 months) complication of cART. In advanced HIV disease, infection of the thyroid gland may occur with opportunistic pathogens, including P. jiroveci, CMV, mycobacteria, Toxoplasma gondii, and Cryptococcus neoformans. These infections are generally associated with a nontender, diffuse enlargement of the thyroid gland. Thyroid function is usually normal. Diagnosis is made by fine-needle aspirate or open biopsy.
Depending on the severity of disease, HIV infection is associated with hypogonadism in 20–50% of men. While this is generally a complication of underlying illness, testicular dysfunction may also be a side effect of ganciclovir therapy. In some surveys, up to two-thirds of patients report decreased libido and one-third complain of erectile dysfunction. Androgen-replacement therapy should be considered in patients with symptomatic hypogonadism. HIV infection does not seem to have a significant effect on the menstrual cycle outside the setting of advanced disease.
Immunologic and Rheumatologic Diseases Immunologic and rheumatologic disorders are common in patients with HIV infection and range from excessive immediate-type hypersensitivity reactions (Chap. 376) to an increase in the incidence of reactive arthritis (Chap. 384) to conditions characterized by a diffuse infiltrative lymphocytosis. The occurrence of these phenomena is an apparent paradox in the setting of the profound immunodeficiency and immunosuppression that characterizes HIV infection and reflects the complex nature of the immune system and its regulatory mechanisms.
Drug allergies are the most significant allergic reactions occurring in HIV-infected patients and appear to become more common as the disease progresses. They occur in up to 65% of patients who receive therapy with TMP/SMX for PCP. In general, these drug reactions are characterized by erythematous, morbilliform eruptions that are pruritic, tend to coalesce, and are often associated with fever. Nonetheless, ~33% of patients can be maintained on the offending therapy, and thus these reactions are not an immediate indication to stop the drug. Anaphylaxis is extremely rare in patients with HIV infection, and patients who have a cutaneous reaction during a single course of therapy can still be considered candidates for future treatment or prophylaxis with the same agent. The one exception to this is the nucleoside analogue abacavir, where fatal hypersensitivity reactions have been reported with rechallenge. This hypersensitivity is strongly associated with the HLA-B5701 haplotype, and a hypersensitivity reaction to abacavir is an absolute contraindication to future therapy. For other agents, including TMP/SMX, desensitization regimens are moderately successful. While the mechanisms underlying these allergic-type reactions remain unknown, patients with HIV infection have been noted to have elevated IgE levels that increase as the CD4+ T cell count declines. The numerous examples of patients with multiple drug reactions suggest that a common pathway is involved.
HIV infection shares many similarities with a variety of autoimmune diseases, including a substantial polyclonal B cell activation that is associated with a high incidence of antiphospholipid antibodies, such as anticardiolipin antibodies, VDRL antibodies, and lupus-like anticoagulants. In addition, HIV-infected individuals have an increased incidence of antinuclear antibodies. Despite these serologic findings, there is no evidence that HIV-infected individuals have an increase in two of the more common autoimmune diseases, i.e., systemic lupus erythematosus and rheumatoid arthritis. In fact, it has been observed that these diseases may be somewhat ameliorated by the concomitant presence of HIV infection, suggesting that an intact CD4+ T cell limb of the immune response plays an integral role in the pathogenesis of these conditions. Similarly, there are anecdotal reports of patients with common variable immunodeficiency (Chap. 374), characterized by hypogammaglobulinemia, who have had a normalization of Ig levels following the development of HIV infection, suggesting a possible role for overactive CD4+ T cell immunity in certain forms of that syndrome. The one autoimmune disease that may occur with an increased frequency in patients with HIV infection is a variant of primary Sjögren’s syndrome (Chap. 383). Patients with HIV infection may develop a syndrome consisting of parotid gland enlargement, dry eyes, and dry mouth that is associated with lymphocytic infiltrates of the salivary gland and lung. One also can see peripheral neuropathy, polymyositis, renal tubular acidosis, and hepatitis. In contrast to Sjögren’s syndrome, in which the lymphocytic infiltrates are composed predominantly of CD4+ T cells, in patients with HIV infection the infiltrates are composed predominantly of CD8+ T cells. In addition, while patients with Sjögren’s syndrome are mainly women who have autoantibodies to Ro and La and who frequently have HLA-DR3 or -B8 MHC haplotypes, HIV-infected individuals with this syndrome are usually African-American men who do not have anti-Ro or anti-La and who most often are HLA-DR5. This syndrome appears to be less common with the increased use of effective cART. The term diffuse infiltrative lymphocytosis syndrome (DILS) is used to describe this entity and to distinguish it from Sjögren’s syndrome.
Approximately one-third of HIV-infected individuals experience arthralgias; furthermore, 5–10% are diagnosed as having some form of reactive arthritis, such as Reiter’s syndrome or psoriatic arthritis as well as undifferentiated spondyloarthropathy (Chap. 384). These syndromes occur with increasing frequency as the competency of the immune system declines. This association may be related to an increase in the number of infections with organisms that may trigger a reactive arthritis with progressive immunodeficiency or to a loss of important regulatory T cells. Reactive arthritides in HIV-infected individuals generally respond well to standard treatment; however, therapy with methotrexate has been associated with an increase in the incidence of opportunistic infections and should be used with caution and only in severe cases.
HIV-infected individuals also experience a variety of joint problems without obvious cause that are referred to generically as HIV– or AIDS-associated arthropathy. This syndrome is characterized by subacute oligoarticular arthritis developing over a period of 1–6 weeks and lasting 6 weeks to 6 months. It generally involves the large joints, predominantly the knees and ankles, and is nonerosive with only a mild inflammatory response. X-rays are nonrevealing. Nonsteroidal anti-inflammatory drugs are only marginally helpful; however, relief has been noted with the use of intraarticular glucocorticoids. A second form of arthritis also thought to be secondary to HIV infection is called painful articular syndrome. This condition, reported as occurring in as many as 10% of AIDS patients, presents as an acute, severe, sharp pain in the affected joint. It affects primarily the knees, elbows, and shoulders; lasts 2–24 h; and may be severe enough to require narcotic analgesics. The cause of this arthropathy is unclear; however, it is thought to result from a direct effect of HIV on the joint. This condition is reminiscent of the fact that other lentiviruses, in particular the caprine arthritis-encephalitis virus, are capable of directly causing arthritis.
A variety of other immunologic or rheumatologic diseases have been reported in HIV-infected individuals, either de novo or in association with opportunistic infections or drugs. Using the criteria of widespread musculoskeletal pain of at least 3 months’ duration and the presence of at least 11 of 18 possible tender points by digital palpation, 11% of an HIV-infected cohort containing 55% IDUs were diagnosed as having fibromyalgia (Chap. 396). While the incidence of frank arthritis was less in this population than in other studied populations that consisted predominantly of men who have sex with men, these data support the concept that there are musculoskeletal problems that occur as a direct result of HIV infection. In addition there have been reports of leukocytoclastic vasculitis in the setting of zidovudine therapy. CNS angiitis and polymyositis also have been reported in HIV-infected individuals. Septic arthritis is surprisingly rare, especially given the increased incidence of staphylococcal bacteremias seen in this population. When septic arthritis has been reported, it has usually been due to Staphylococcus aureus, systemic fungal infection with C. neoformans, Sporothrix schenckii, or H. capsulatum or to systemic mycobacterial infection with M. tuberculosis, M. haemophilum, M. avium, or M. kansasii.
Patients with HIV infection treated with cART have been found to have an increased incidence of osteonecrosis or avascular necrosis of the hip and shoulders. In a study of asymptomatic patients, 4.4% were found to have evidence of osteonecrosis on MRI. While precise cause-and-effect relationships have been difficult to establish, this complication has been associated with the use of lipid-lowering agents, systemic glucocorticoids, and testosterone; bodybuilding exercise; alcohol consumption; and the presence of anticardiolipin antibodies. Osteoporosis has been reported in 7% of women with HIV infection, with 41% of women demonstrating some degree of osteopenia. Several studies have documented decreases in bone mineral density of 2–6% in the first 2 years following the initiation of cART. This may be particularly apparent with tenofovir-containing regimens.
Immune Reconstitution Inflammatory Syndrome (IRIS)
Following the initiation of effective cART, a paradoxical worsening of preexisting, untreated, or partially treated opportunistic infections may be noted. One may also see exacerbations of pre-existing or the development of new autoimmune conditions following the initiation of antiretrovirals (Table 226-12). IRIS related to a known pre-existing infection or neoplasm is referred to as paradoxical IRIS, while IRIS associated with a previously undiagnosed condition is referred to as unmasking IRIS. The term immune reconstitution disease (IRD) is sometimes used to distinguish IRIS manifestations related to opportunistic diseases from IRIS manifestations related to autoimmune diseases. IRD is particularly common in patients with underlying untreated mycobacterial or fungal infections. IRIS is seen in 10–30% of patients, depending on the clinical setting, and is most common in patients starting therapy with CD4+ T cell counts <50 cells/μL who have a precipitous drop in HIV RNA levels following the initiation of cART. Signs and symptoms may appear anywhere from 2 weeks to 2 years after the initiation of cART and can include localized lymphadenitis, prolonged fever, pulmonary infiltrates, hepatitis, increased intracranial pressure, uveitis, sarcoidosis, and Graves’ disease. The clinical course can be protracted, and severe cases can be fatal. The underlying mechanism appears to be related to a phenomenon similar to type IV hypersensitivity reactions and reflects the immediate improvements in immune function that occur as levels of HIV RNA drop and the immunosuppressive effects of HIV infection are controlled. In severe cases, the use of immunosuppressive drugs such as glucocorticoids may be required to blunt the inflammatory component of these reactions while specific antimicrobial therapy takes effect.
|
CHARACTERISTICS OF IMMUNE RECONSTITUTION INFLAMMATORY SYNDROME (IRIS) |
Diseases of the Hematopoietic System Disorders of the hematopoietic system including lymphadenopathy, anemia, leukopenia, and/or thrombocytopenia are common throughout the course of HIV infection and may be the direct result of HIV, manifestations of secondary infections and neoplasms, or side effects of therapy (Table 226-13). Direct histologic examination and culture of lymph node or bone marrow tissue are often diagnostic. A significant percentage of bone marrow aspirates from patients with HIV infection have been reported to contain lymphoid aggregates, the precise significance of which is unknown. Initiation of cART will lead to reversal of most hematologic complications that are the direct result of HIV infection.
|
CAUSES OF BONE MARROW SUPPRESSION IN PATIENTS WITH HIV INFECTION |
Some patients, otherwise asymptomatic, may develop persistent generalized lymphadenopathy as an early clinical manifestation of HIV infection. This condition is defined as the presence of enlarged lymph nodes (>1 cm) in two or more extrainguinal sites for >3 months without an obvious cause. The lymphadenopathy is due to marked follicular hyperplasia in the node in response to HIV infection. The nodes are generally discrete and freely movable. This feature of HIV disease may be seen at any point in the spectrum of immune dysfunction and is not associated with an increased likelihood of developing AIDS. Paradoxically, a loss in lymphadenopathy or a decrease in lymph node size outside the setting of cART may be a prognostic marker of disease progression. In patients with CD4+ T cell counts >200/μL, the differential diagnosis of lymphadenopathy includes KS, TB, Castleman’s disease, and lymphoma. In patients with more advanced disease, lymphadenopathy may also be due to atypical mycobacterial infection, toxoplasmosis, systemic fungal infection, or bacillary angiomatosis. While indicated in patients with CD4+ T cell counts <200/μL, lymph node biopsy is not indicated in patients with early-stage disease unless there are signs and symptoms of systemic illness, such as fever and weight loss, or unless the nodes begin to enlarge, become fixed, or coalesce. Monoclonal gammopathy of unknown significance (MGUS) (Chap. 136), defined as the presence of a serum monoclonal IgG, IgA, or IgM in the absence of a clear cause, has been reported in 3% of patients with HIV infection. The overall clinical significance of this finding in patients with HIV infection is unclear, although it has been associated with other viral infections, non-Hodgkin’s lymphoma, and plasma cell malignancy.
Anemia is the most common hematologic abnormality in HIV-infected patients and, in the absence of a specific treatable cause, is independently associated with a poor prognosis. While generally mild, anemia can be quite severe and require chronic blood transfusions. Among the specific reversible causes of anemia in the setting of HIV infection are drug toxicity, systemic fungal and mycobacterial infections, nutritional deficiencies, and parvovirus B19 infections. Zidovudine may block erythroid maturation prior to its effects on other marrow elements. A characteristic feature of zidovudine therapy is an elevated mean corpuscular volume (MCV). Another drug used in patients with HIV infection that has a selective effect on the erythroid series is dapsone. This drug can cause a serious hemolytic anemia in patients who are deficient in glucose-6-phosphate dehydrogenase and can create a functional anemia in others through induction of methemoglobinemia. Folate levels are usually normal in HIV-infected individuals; however, vitamin B12 levels may be depressed as a consequence of achlorhydria or malabsorption. True autoimmune hemolytic anemia is rare, although ~20% of patients with HIV infection may have a positive direct antiglobulin test as a consequence of polyclonal B cell activation. Infection with parvovirus B19 may also cause anemia. It is important to recognize this possibility given the fact that it responds well to treatment with IVIg. Erythropoietin levels in patients with HIV infection and anemia are generally lower than expected given the degree of anemia. Treatment with erythropoietin may result in an increase in hemoglobin levels. An exception to this is a subset of patients with zidovudine-associated anemia in whom erythropoietin levels may be quite high.
During the course of HIV infection, neutropenia may be seen in approximately half of patients. In most instances it is mild; however, it can be severe and can put patients at risk of spontaneous bacterial infections. This is most frequently seen in patients with severely advanced HIV disease and in patients receiving any of a number of potentially myelosuppressive therapies. In the setting of neutropenia, diseases that are not commonly seen in HIV-infected patients, such as aspergillosis or mucormycosis, may occur. Both granulocyte colony-stimulating factor (G-CSF) and GM-CSF increase neutrophil counts in patients with HIV infection regardless of the cause of the neutropenia. Earlier concerns about the potential of these agents to also increase levels of HIV were not confirmed in controlled clinical trials.
Thrombocytopenia may be an early consequence of HIV infection. Approximately 3% of patients with untreated HIV infection and CD4+ T cell counts ≥400/μL have platelet counts <150,000/μL. For untreated patients with CD4+ T cell counts <400/μL, this incidence increases to 10%. In patients receiving antiretrovirals, thrombocytopenia is associated with hepatitis C, cirrhosis, and ongoing high-level HIV replication. Thrombocytopenia is rarely a serious clinical problem in patients with HIV infection and generally responds well to successful cART. Clinically, it resembles the thrombocytopenia seen in patients with idiopathic thrombocytopenic purpura (Chap. 140). Immune complexes containing anti-gp120 antibodies and anti-anti-gp120 antibodies have been noted in the circulation and on the surface of platelets in patients with HIV infection. Patients with HIV infection have also been noted to have a platelet-specific antibody directed toward a 25-kDa component of the surface of the platelet. Other data suggest that the thrombocytopenia in patients with HIV infection may be due to a direct effect of HIV on megakaryocytes. Whatever the cause, it is very clear that the most effective medical approach to this problem has been the use of cART. For patients with platelet counts <20,000/μL, a more aggressive approach combining IVIg or anti-Rh Ig for an immediate response and cART for a more lasting response is appropriate. Rituximab has been used with some success in otherwise refractory cases. Splenectomy is a rarely needed option and is reserved for patients refractory to medical management. Because of the risk of serious infection with encapsulated organisms, all patients with HIV infection about to undergo splenectomy should be immunized with pneumococcal polysaccharide. It should be noted that, in addition to causing an increase in the platelet count, removal of the spleen will result in an increase in the peripheral blood lymphocyte count, making CD4+ T cell counts unreliable markers of immunocompetence. In this setting, the clinician should rely on the CD4+ T cell percentage for making diagnostic decisions with respect to the likelihood of opportunistic infections. A CD4+ T cell percentage of 15 is approximately equivalent to a CD4+ T cell count of 200/μL. In patients with early HIV infection, thrombocytopenia has also been reported as a consequence of classic thrombotic thrombocytopenic purpura (Chap. 140). This clinical syndrome, consisting of fever, thrombocytopenia, hemolytic anemia, and neurologic and renal dysfunction, is a rare complication of early HIV infection. As in other settings, the appropriate management is the use of salicylates and plasma exchange. Other causes of thrombocytopenia include lymphoma, mycobacterial infections, and fungal infections.
The incidence of venous thromboembolic disease such as deep-vein thrombosis or pulmonary embolus is approximately 1% per year in patients with HIV infection. This is approximately 10 times higher than that seen in an age-matched population. Factors associated with an increased risk of clinical thrombosis include age over 45, history of an opportunistic infection, lower CD4 count, and estrogen use. Abnormalities of the coagulation cascade including decreased protein S activity, increases in factor VIII, anticardiolipin antibodies, or lupus-like anticoagulant have been reported in more than 50% of patients with HIV infection. The clinical significance of this increased propensity toward thromboembolic disease is likely reflected in the observation that elevations in D-dimer are strongly associated with all-cause mortality in patients with HIV infection (Table 226-9).
Dermatologic Diseases Dermatologic problems occur in >90% of patients with HIV infection. From the macular, roseola-like rash seen with the acute seroconversion syndrome to extensive end-stage KS, cutaneous manifestations of HIV disease can be seen throughout the course of HIV infection. Among the more common nonneoplastic problems are seborrheic dermatitis, folliculitis, and opportunistic infections. Extrapulmonary pneumocystosis may cause a necrotizing vasculitis. Neoplastic conditions are covered below.
Seborrheic dermatitis occurs in 3% of the general population and in up to 50% of patients with HIV infection. Seborrheic dermatitis increases in prevalence and severity as the CD4+ T cell count declines. In HIV-infected patients, seborrheic dermatitis may be aggravated by concomitant infection with Pityrosporum, a yeastlike fungus; use of topical antifungal agents has been recommended in cases refractory to standard topical treatment.
Folliculitis is among the most prevalent dermatologic disorders in patients with HIV infection and is seen in ~20% of patients. It is more common in patients with CD4+ T cell counts <200 cells/μL. Pruritic papular eruption is one of the most common pruritic rashes in patients with HIV infection. It appears as multiple papules on the face, trunk, and extensor surfaces and may improve with cART. Eosinophilic pustular folliculitis is a rare form of folliculitis that is seen with increased frequency in patients with HIV infection. It presents as multiple, urticarial perifollicular papules that may coalesce into plaquelike lesions. Skin biopsy reveals an eosinophilic infiltrate of the hair follicle, which in certain cases has been associated with the presence of a mite. Patients typically have an elevated serum IgE level and may respond to treatment with topical anthelmintics. Pruritus is a common symptom in patients with HIV infection and can lead to prurigo nodularis. Patients with HIV infection have also been reported to develop a severe form of Norwegian scabies with hyperkeratotic psoriasiform lesions.
Both psoriasis and ichthyosis, although they are not reported to be increased in frequency, may be particularly severe when they occur in patients with HIV infection. Preexisting psoriasis may become guttate in appearance and more refractory to treatment in the setting of HIV infection.
Reactivation herpes zoster (shingles) is seen in 10–20% of patients with HIV infection. This reactivation syndrome of varicella-zoster virus indicates a modest decline in immune function and may be the first indication of clinical immunodeficiency. In one series, patients who developed shingles did so an average of 5 years after HIV infection. In a cohort of patients with HIV infection and localized zoster, the subsequent rate of the development of AIDS was 1% per month. In that study, AIDS was more likely to develop if the outbreak of zoster was associated with severe pain, extensive skin involvement, or involvement of cranial or cervical dermatomes. The clinical manifestations of reactivation zoster in HIV-infected patients, although indicative of immunologic compromise, are not as severe as those seen in other immunodeficient conditions. Thus, while lesions may extend over several dermatomes, involve the spinal cord, and/or be associated with frank cutaneous dissemination, visceral involvement has not been reported. In contrast to patients without a known underlying immunodeficiency state, patients with HIV infection tend to have recurrences of zoster with a relapse rate of ~20%. Valacyclovir, acyclovir, or famciclovir is the treatment of choice. Foscarnet may be of value in patients with acyclovir-resistant virus.
Infection with herpes simplex virus in HIV-infected individuals is associated with recurrent orolabial, genital, and perianal lesions as part of recurrent reactivation syndromes (Chap. 216). As HIV disease progresses and the CD4+ T cell count declines, these infections become more frequent and severe. Lesions often appear as beefy red, are exquisitely painful, and have a tendency to occur high in the gluteal cleft (Fig. 226-37). Perirectal HSV may be associated with proctitis and anal fissures. HSV should be high in the differential diagnosis of any HIV-infected patient with a poorly healing, painful perirectal lesion. In addition to recurrent mucosal ulcers, recurrent HSV infection in the form of herpetic whitlow can be a problem in patients with HIV infection, presenting with painful vesicles or extensive cutaneous erosion. Valacyclovir, acyclovir or famciclovir is the treatment of choice in these settings. It is noteworthy that even subclinical reactivation of herpes simplex may be associated with increases in plasma HIV RNA levels.
Diffuse skin eruptions due to Molluscum contagiosum may be seen in patients with advanced HIV infection. These flesh-colored, umbilicated lesions may be treated with local therapy. They tend to regress with effective cART. Similarly, condyloma acuminatum lesions may be more severe and more widely distributed in patients with low CD4+ T cell counts. Imiquimod cream may be helpful in some cases. Atypical mycobacterial infections may present as erythematous cutaneous nodules, as may fungal infections, Bartonella, Acanthamoeba, and KS. Cutaneous infections with Aspergillus have been noted at the site of IV catheter placement.
The skin of patients with HIV infection is often a target organ for drug reactions (Chap. 74). Although most skin reactions are mild and not necessarily an indication to discontinue therapy, patients may have particularly severe cutaneous reactions, including erythroderma, Stevens-Johnson syndrome, and toxic epidermal necrolysis, as a reaction to drugs—particularly sulfa drugs, nonnucleoside reverse transcriptase inhibitors, abacavir, amprenavir, darunavir, fosamprenavir, and tipranavir. Similarly, patients with HIV infection are often quite photosensitive and burn easily following exposure to sunlight or as a side effect of radiation therapy (Chap. 75).
HIV infection and its treatment may be accompanied by cosmetic changes of the skin that are not of great clinical importance but may be troubling to patients. Yellowing of the nails and straightening of the hair, particularly in African-American patients, have been reported as a consequence of HIV infection. Zidovudine therapy has been associated with elongation of the eyelashes and the development of a bluish discoloration to the nails, again more common in African-American patients. Therapy with clofazimine may cause a yellow-orange discoloration of the skin and urine.
Neurologic Diseases Clinical disease of the nervous system accounts for a significant degree of morbidity in a high percentage of patients with HIV infection (Table 226-14). The neurologic problems that occur in HIV-infected individuals may be either primary to the pathogenic processes of HIV infection or secondary to opportunistic infections or neoplasms. Among the more frequent opportunistic diseases that involve the CNS are toxoplasmosis, cryptococcosis, progressive multifocal leukoencephalopathy, and primary CNS lymphoma. Other less common problems include mycobacterial infections; syphilis; and infection with CMV, HTLV-1, Trypanosoma cruzi, or Acanthamoeba. Overall, secondary diseases of the CNS have been reported to occur in approximately one-third of patients with AIDS. These data antedate the widespread use of cART, and this frequency is considerably lower in patients receiving effective antiretroviral drugs. Primary processes related to HIV infection of the nervous system are reminiscent of those seen with other lentiviruses, such as the Visna-Maedi virus of sheep.
|
NEUROLOGIC DISEASES IN PATIENTS WITH HIV INFECTION |
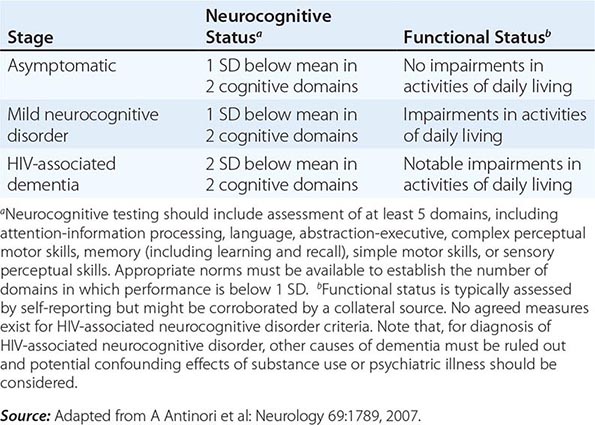
Neurologic problems directly attributable to HIV occur throughout the course of infection and may be inflammatory, demyelinating, or degenerative in nature. The term HIV-associated neurocognitive disorders (HAND) is used to describe a spectrum of disorders that range from asymptomatic neurocognitive impairment (ANI) to minor neurocognitive disorder (MND) to clinically severe dementia. The most severe form, HIV-associated dementia (HAD), also referred to as the AIDS dementia complex, or HIV encephalopathy, is considered an AIDS-defining illness. Most HIV-infected patients have some neurologic problem during the course of their disease. Even in the setting of suppressive cART, approximately 50% of HIV-infected individuals can be shown to have mild to moderate neurocognitive impairment using sensitive neuropsychiatric testing. As noted in the section on pathogenesis, damage to the CNS may be a direct result of viral infection of the CNS macrophages or glial cells or may be secondary to the release of neurotoxins and potentially toxic cytokines such as IL-1β, TNF-α, IL-6, and TGF-β. It has been reported that HIV-infected individuals with the E4 allele for apoE are at increased risk for AIDS encephalopathy and peripheral neuropathy. Virtually all patients with HIV infection have some degree of nervous system involvement with the virus. This is evidenced by the fact that CSF findings are abnormal in ~90% of patients, even during the asymptomatic phase of HIV infection. CSF abnormalities include pleocytosis (50–65% of patients), detection of viral RNA (~75%), elevated CSF protein (35%), and evidence of intrathecal synthesis of anti-HIV antibodies (90%). It is important to point out that evidence of infection of the CNS with HIV does not imply impairment of cognitive function. The neurologic function of an HIV-infected individual should be considered normal unless clinical signs and symptoms suggest otherwise.
Aseptic meningitis may be seen in any but the very late stages of HIV infection. In the setting of acute primary infection, patients may experience a syndrome of headache, photophobia, and meningismus. Rarely, an acute encephalopathy due to encephalitis may occur. Cranial nerve involvement may be seen, predominantly cranial nerve VII but occasionally V and/or VIII. CSF findings include a lymphocytic pleocytosis, elevated protein level, and normal glucose level. This syndrome, which cannot be clinically differentiated from other viral meningitides (Chap. 165), usually resolves spontaneously within 2–4 weeks; however, in some patients, signs and symptoms may become chronic. Aseptic meningitis may occur any time in the course of HIV infection; however, it is rare following the development of AIDS. This suggests that clinical aseptic meningitis in the context of HIV infection is an immune-mediated disease.
Cryptococcus is the leading infectious cause of meningitis in patients with AIDS (Chap. 239). While the vast majority of these are due to C. neoformans, up to 12% may be due to C. gattii. Cryptococcal meningitis is the initial AIDS-defining illness in ~2% of patients and generally occurs in patients with CD4+ T cell counts <100/μL. Cryptococcal meningitis is particularly common in untreated patients with AIDS in Africa, occurring in ~5% of patients. Most patients present with a picture of subacute meningoencephalitis with fever, nausea, vomiting, altered mental status, headache, and meningeal signs. The incidence of seizures and focal neurologic deficits is low. The CSF profile may be normal or may show only modest elevations in WBC or protein levels and decreases in glucose. The opening pressure in the CSF is usually elevated. In addition to meningitis, patients may develop cryptococcomas and cranial nerve involvement. Approximately one-third of patients also have pulmonary disease. Uncommon manifestations of cryptococcal infection include skin lesions that resemble molluscum contagiosum, lymphadenopathy, palatal and glossal ulcers, arthritis, gastroenteritis, myocarditis, and prostatitis. The prostate gland may serve as a reservoir for smoldering cryptococcal infection. The diagnosis of cryptococcal meningitis is made by identification of organisms in spinal fluid with india ink examination or by the detection of cryptococcal antigen. Blood cultures for fungus are often positive. A biopsy may be needed to make a diagnosis of CNS cryptococcoma. Treatment is with IV amphotericin B 0.7 mg/kg daily, or liposomal amphotericin 4–6 mg/kg daily, with flucytosine 25 mg/kg qid for at least 2 weeks if possible, continuing with amphotericin alone ideally until the CSF culture turns negative. Decreases in renal function in association with amphotericin can lead to increases in flucytosine levels and subsequent bone marrow suppression. Amphotericin is followed by fluconazole 400 mg/d PO for 8 weeks, and then fluconazole 200 mg/d until the CD4+ T cell count has increased to >200 cells/μL for 6 months in response to cART. Repeated lumbar puncture may be required to manage increased intracranial pressure. Symptoms may recur with initiation of cART as an immune reconstitution syndrome (see above). Other fungi that may cause meningitis in patients with HIV infection are C. immitis and H. capsulatum. Meningoencephalitis has also been reported due to Acanthamoeba or Naegleria.
HIV-associated dementia consists of a constellation of signs and symptoms of CNS disease. While this is generally a late complication of HIV infection that progresses slowly over months, it can be seen in patients with CD4+ T cell counts >350 cells/μL. A major feature of this entity is the development of dementia, defined as a decline in cognitive ability from a previous level. It may present as impaired ability to concentrate, increased forgetfulness, difficulty reading, or increased difficulty performing complex tasks. Initially these symptoms may be indistinguishable from findings of situational depression or fatigue. In contrast to “cortical” dementia (such as Alzheimer’s disease), aphasia, apraxia, and agnosia are uncommon, leading some investigators to classify HIV encephalopathy as a “subcortical dementia” characterized by defects in short-term memory and executive function (see below). In addition to dementia, patients with HIV encephalopathy may also have motor and behavioral abnormalities. Among the motor problems are unsteady gait, poor balance, tremor, and difficulty with rapid alternating movements. Increased tone and deep tendon reflexes may be found in patients with spinal cord involvement. Late stages may be complicated by bowel and/or bladder incontinence. Behavioral problems include apathy, irritability, and lack of initiative, with progression to a vegetative state in some instances. Some patients develop a state of agitation or mild mania. These changes usually occur without significant changes in level of alertness. This is in contrast to the finding of somnolence in patients with dementia due to toxic/metabolic encephalopathies.
HIV-associated dementia is the initial AIDS-defining illness in ~3% of patients with HIV infection and thus only rarely precedes clinical evidence of immunodeficiency. Clinically significant encephalopathy eventually develops in ~25% of untreated patients with AIDS. As immunologic function declines, the risk and severity of HIV-associated dementia increases. Autopsy series suggest that 80–90% of patients with HIV infection have histologic evidence of CNS involvement. Several classification schemes have been developed for grading HIV encephalopathy; a commonly used clinical staging system is outlined in Table 226-15.
|
CLINICAL STAGING OF HAND ACCORDING TO FRASCATI CRITERIA |
The precise cause of HIV-associated dementia remains unclear, although the condition is thought to be a result of a combination of direct effects of HIV on the CNS and associated immune activation. HIV has been found in the brains of patients with HIV encephalopathy by Southern blot, in situ hybridization, PCR, and electron microscopy. Multinucleated giant cells, macrophages, and microglial cells appear to be the main cell types harboring virus in the CNS. Histologically, the major changes are seen in the subcortical areas of the brain and include pallor and gliosis, multinucleated giant cell encephalitis, and vacuolar myelopathy. Less commonly, diffuse or focal spongiform changes occur in the white matter. Areas of the brain involved in motor function, language, and judgment are most severely affected.
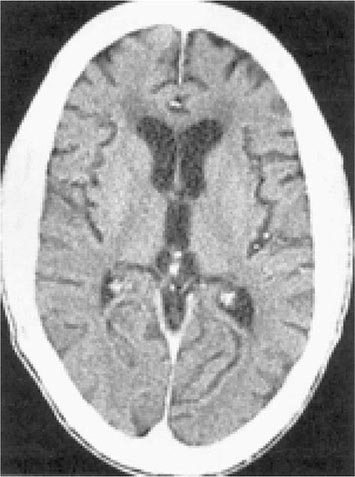
There are no specific criteria for a diagnosis of HIV-associated dementia, and this syndrome must be differentiated from a number of other diseases that affect the CNS of HIV-infected patients (Table 226-14). The diagnosis of dementia depends on demonstrating a decline in cognitive function. This can be accomplished objectively with the use of a Mini-Mental Status Examination (MMSE) in patients for whom prior scores are available. For this reason, it is advisable for all patients with a diagnosis of HIV infection to have a baseline MMSE. However, changes in MMSE scores may be absent in patients with mild HIV encephalopathy. Imaging studies of the CNS, by either MRI or CT, often demonstrate evidence of cerebral atrophy (Fig. 226-39). MRI may also reveal small areas of increased density on T2-weighted images. Lumbar puncture is an important element of the evaluation of patients with HIV infection and neurologic abnormalities. It is generally most helpful in ruling out or making a diagnosis of opportunistic infections. In HIV encephalopathy, patients may have the nonspecific findings of an increase in CSF cells and protein level. While HIV RNA can often be detected in the spinal fluid and HIV can be cultured from the CSF, this finding is not specific for HIV encephalopathy. There appears to be no correlation between the presence of HIV in the CSF and the presence of HIV encephalopathy. Elevated levels of macrophage chemoattractant protein (MCP-1), β2-microglobulin, neopterin, and quinolinic acid (a metabolite of tryptophan reported to cause CNS injury) have been noted in the CSF of patients with HIV encephalopathy. These findings suggest that these factors as well as inflammatory cytokines may be involved in the pathogenesis of this syndrome.
FIGURE 226-39 AIDS dementia complex. Postcontrast CT scan through the lateral ventricles of a 47-year-old man with AIDS, altered mental status, and dementia. The lateral and third ventricles and the cerebral sulci are abnormally prominent. Mild white matter hypodensity is seen adjacent to the frontal horns of the lateral ventricles.
Combination antiretroviral therapy is of benefit in patients with HIV-associated dementia. Improvement in neuropsychiatric test scores has been noted for both adult and pediatric patients treated with antiretrovirals. The rapid improvement in cognitive function noted with the initiation of cART suggests that at least some component of this problem is quickly reversible, again supporting at least a partial role of soluble mediators in the pathogenesis. It should also be noted that these patients have an increased sensitivity to the side effects of neuroleptic drugs. The use of these drugs for symptomatic treatment is associated with an increased risk of extrapyramidal side effects; therefore, patients with HIV encephalopathy who receive these agents must be monitored carefully. It is felt by many physicians that the decrease in the prevalence of severe cases of HAND brought about by cART has resulted in an increase in the prevalence of milder forms of this disorder.
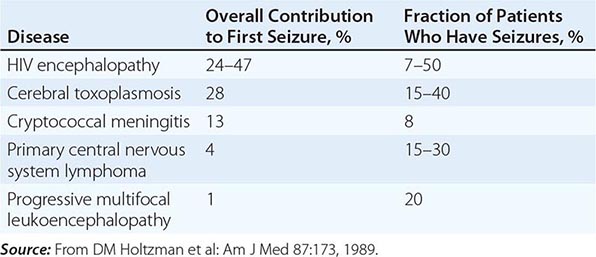
Seizures may be a consequence of opportunistic infections, neoplasms, or HIV encephalopathy (Table 226-16). The seizure threshold is often lower than normal in patients with advanced HIV infection due in part to the frequent presence of electrolyte abnormalities. Seizures are seen in 15–40% of patients with cerebral toxoplasmosis, 15–35% of patients with primary CNS lymphoma, 8% of patients with cryptococcal meningitis, and 7–50% of patients with HIV encephalopathy. Seizures may also be seen in patients with CNS tuberculosis, aseptic meningitis, and progressive multifocal leukoencephalopathy. Seizures may be the presenting clinical symptom of HIV disease. In one study of 100 patients with HIV infection presenting with a first seizure, cerebral mass lesions were the most common cause, responsible for 32 of the 100 new-onset seizures. Of these 32 cases, 28 were due to toxoplasmosis and 4 to lymphoma. HIV encephalopathy accounted for an additional 24 new-onset seizures. Cryptococcal meningitis was the third most common diagnosis, responsible for 13 of the 100 seizures. In 23 cases, no cause could be found, and it is possible that these cases represent a subcategory of HIV encephalopathy. Of these 23 cases, 16 (70%) had 2 or more seizures, suggesting that anticonvulsant therapy is indicated in all patients with HIV infection and seizures unless a rapidly correctable cause is found. While phenytoin remains the initial treatment of choice, hypersensitivity reactions to this drug have been reported in >10% of patients with AIDS, and therefore the use of phenobarbital or valproic acid should be considered as alternatives. Due to a variety of drug-drug interactions between antiseizure medications and antiretrovirals, drug levels need to be monitored carefully.
|
CAUSES OF SEIZURES IN PATIENTS WITH HIV INFECTION |
Patients with HIV infection may present with focal neurologic deficits from a variety of causes. The most common causes are toxoplasmosis, progressive multifocal leukoencephalopathy, and CNS lymphoma. Other causes include cryptococcal infections (discussed above; also Chap. 239), stroke, and reactivation of Chagas’ disease.
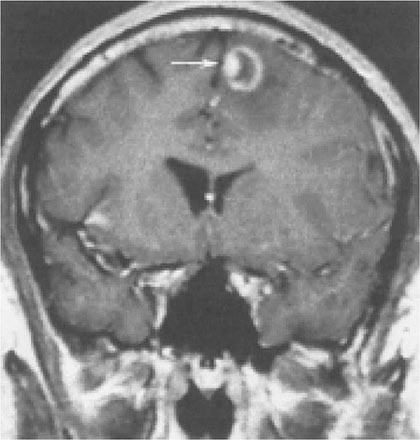
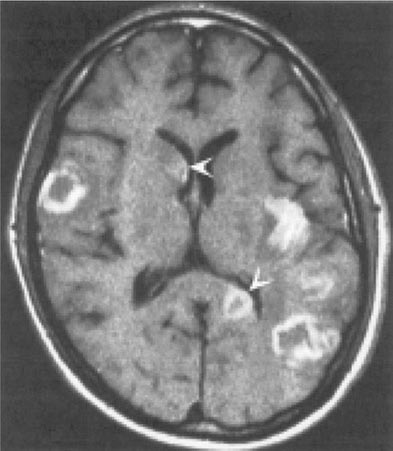
Toxoplasmosis has been one of the most common causes of secondary CNS infections in patients with AIDS, but its incidence is decreasing in the era of cART. It is most common in patients from the Caribbean and from France, where the seroprevalence of T. gondii is around 50%. This figure is closer to 15% in the United States. Toxoplasmosis is generally a late complication of HIV infection and usually occurs in patients with CD4+ T cell counts <200/μL. Cerebral toxoplasmosis is thought to represent a reactivation of latent tissue cysts. It is 10 times more common in patients with antibodies to the organism than in patients who are seronegative. Patients diagnosed with HIV infection should be screened for IgG antibodies to T. gondii during the time of their initial workup. Those who are seronegative should be counseled about ways to minimize the risk of primary infection including avoiding the consumption of undercooked meat and careful hand washing after contact with soil or changing the cat litter box. The most common clinical presentation of cerebral toxoplasmosis in patients with HIV infection is fever, headache, and focal neurologic deficits. Patients may present with seizure, hemiparesis, or aphasia as a manifestation of these focal deficits or with a picture more influenced by the accompanying cerebral edema and characterized by confusion, dementia, and lethargy, which can progress to coma. The diagnosis is usually suspected on the basis of MRI findings of multiple lesions in multiple locations, although in some cases only a single lesion is seen. Pathologically, these lesions generally exhibit inflammation and central necrosis and, as a result, demonstrate ring enhancement on contrast MRI (Fig. 226-40) or, if MRI is unavailable or contraindicated, on double-dose contrast CT. There is usually evidence of surrounding edema. In addition to toxoplasmosis, the differential diagnosis of single or multiple enhancing mass lesions in the HIV-infected patient includes primary CNS lymphoma and, less commonly, TB or fungal or bacterial abscesses. The definitive diagnostic procedure is brain biopsy. However, given the morbidity rate that can accompany this procedure, it is usually reserved for the patient who has failed 2–4 weeks of empiric therapy for toxoplasmosis. If the patient is seronegative for T. gondii, the likelihood that a mass lesion is due to toxoplasmosis is <10%. In that setting, one may choose to be more aggressive and perform a brain biopsy sooner. Standard treatment is sulfadiazine and pyrimethamine with leucovorin as needed for a minimum of 4–6 weeks. Alternative therapeutic regimens include clindamycin in combination with pyrimethamine; atovaquone plus pyrimethamine; and azithromycin plus pyrimethamine plus rifabutin. Relapses are common, and it is recommended that patients with a history of prior toxoplasmic encephalitis receive maintenance therapy with sulfadiazine, pyrimethamine, and leucovorin as long as their CD4+ T cell counts remain <200 cells/μL. Patients with CD4+ T cell counts <100/μL and IgG antibody to Toxoplasma should receive primary prophylaxis for toxoplasmosis. Fortunately, the same daily regimen of a single double-strength tablet of TMP/SMX used for P. jiroveci prophylaxis provides adequate primary protection against toxoplasmosis. Secondary prophylaxis/maintenance therapy for toxoplasmosis may be discontinued in the setting of effective cART and increases in CD4+ T cell counts to >200/μL for 6 months.
FIGURE 226-40 Central nervous system toxoplasmosis. A coronal postcontrast T1-weighted MRI scan demonstrates a peripheral enhancing lesion in the left frontal lobe, associated with an eccentric nodular area of enhancement (arrow); this so-called eccentric target sign is typical of toxoplasmosis.
JC virus, a human polyomavirus that is the etiologic agent of progressive multifocal leukoencephalopathy (PML), is an important opportunistic pathogen in patients with AIDS (Chap. 164). While ~80% of the general adult population has antibodies to JC virus, indicative of prior infection, <10% of healthy adults show any evidence of ongoing viral replication. PML is the only known clinical manifestation of JC virus infection. It is a late manifestation of AIDS and is seen in ~1–4% of patients with AIDS. The lesions of PML begin as small foci of demyelination in subcortical white matter that eventually coalesce. The cerebral hemispheres, cerebellum, and brainstem may all be involved. Patients typically have a protracted course with multifocal neurologic deficits, with or without changes in mental status. Approximately 20% of patients experience seizures. Ataxia, hemiparesis, visual field defects, aphasia, and sensory defects may occur. Headache, fever, nausea, and vomiting are rarely seen. Their presence should suggest another diagnosis. MRI typically reveals multiple, nonenhancing white matter lesions that may coalesce and have a predilection for the occipital and parietal lobes. The lesions show signal hyperintensity on T2-weighted images and diminished signal on T1-weighted images. The measurement of JC virus DNA levels in CSF has a diagnostic sensitivity of 76% and a specificity of close to 100%. Prior to the availability of cART, the majority of patients with PML died within 3–6 months of the onset of symptoms. Paradoxical worsening of PML has been seen with initiation of cART as an immune reconstitution syndrome. There is no specific treatment for PML; however, a median survival of 2 years and survival of >15 years have been reported in patients with PML treated with cART for their HIV disease. Despite having a significant impact on survival, only ~50% of patients with HIV infection and PML show neurologic improvement with cART. Studies with other antiviral agents such as cidofovir have failed to show clear benefit. Factors influencing a favorable prognosis for PML in the setting of HIV infection include a CD4+ T cell count >100/μL at baseline and the ability to maintain an HIV viral load of <500 copies/mL. Baseline HIV-1 viral load does not have independent predictive value of survival. PML is one of the few opportunistic infections that continues to occur with some frequency despite the widespread use of cART.
Reactivation American trypanosomiasis may present as acute meningoencephalitis with focal neurologic signs, fever, headache, vomiting, and seizures. Accompanying cardiac disease in the form of arrhythmias or heart failure should increase the index of suspicion. The presence of antibodies to T. cruzi supports the diagnosis. In South America, reactivation of Chagas’ disease is considered to be an AIDS-defining condition and may be the initial AIDS-defining condition. The majority of cases occur in patients with CD4+ T cell counts <200 cells/μL. Lesions appear radiographically as single or multiple hypodense areas, typically with ring enhancement and edema. They are found predominantly in the subcortical areas, a feature that differentiates them from the deeper lesions of toxoplasmosis. T. cruzi amastigotes, or trypanosomes, can be identified from biopsy specimens or CSF. Other CSF findings include elevated protein and a mild (<100 cells/μL) lymphocytic pleocytosis. Organisms can also be identified by direct examination of the blood. Treatment consists of benzimidazole (2.5 mg/kg bid) or nifurtimox (2 mg/kg qid) for at least 60 days, followed by maintenance therapy for the duration of immunodeficiency with either drug at a dose of 5 mg/kg three times a week. As is the case with cerebral toxoplasmosis, successful therapy with antiretrovirals may allow discontinuation of therapy for Chagas’ disease.
Stroke may occur in patients with HIV infection. In contrast to the other causes of focal neurologic deficits in patients with HIV infection, the symptoms of a stroke are sudden in onset. Patients with HIV infection have an increased prevalence of many classic risk factors associated with stroke, including smoking and diabetes. It has been reported that HIV infection itself can lead to an increase in carotid artery stiffness. The relative increase in risk for stroke as a consequence of HIV infection is more pronounced in women and in individuals between the ages of 18 and 29. Among the secondary infectious diseases in patients with HIV infection that may be associated with stroke are vasculitis due to cerebral varicella zoster or neurosyphilis and septic embolism in association with fungal infection. Other elements of the differential diagnosis of stroke in the patient with HIV infection include atherosclerotic cerebral vascular disease, thrombotic thrombocytopenic purpura, and cocaine or amphetamine use.
Primary CNS lymphoma is discussed below in the section on neoplastic diseases.
Spinal cord disease, or myelopathy, is present in ~20% of patients with AIDS, often as part of HIV-associated neurocognitive disorder. In fact, 90% of the patients with HIV-associated myelopathy have some evidence of dementia, suggesting that similar pathologic processes may be responsible for both conditions. Three main types of spinal cord disease are seen in patients with AIDS. The first of these is a vacuolar myelopathy, as mentioned above. This condition is pathologically similar to subacute combined degeneration of the cord, such as that occurring with pernicious anemia. Although vitamin B12 deficiency can be seen in patients with AIDS as a primary complication of HIV infection, it does not appear to be responsible for the myelopathy seen in the majority of patients. Vacuolar myelopathy is characterized by a subacute onset and often presents with gait disturbances, predominantly ataxia and spasticity; it may progress to include bladder and bowel dysfunction. Physical findings include evidence of increased deep tendon reflexes and extensor plantar responses. The second form of spinal cord disease involves the dorsal columns and presents as a pure sensory ataxia. The third form is also sensory in nature and presents with paresthesias and dysesthesias of the lower extremities. In contrast to the cognitive problems seen in patients with HIV encephalopathy, these spinal cord syndromes do not respond well to antiretroviral drugs, and therapy is mainly supportive.
One important disease of the spinal cord that also involves the peripheral nerves is a myelopathy and polyradiculopathy seen in association with CMV infection. This entity is generally seen late in the course of HIV infection and is fulminant in onset, with lower extremity and sacral paresthesias, difficulty in walking, areflexia, ascending sensory loss, and urinary retention. The clinical course is rapidly progressive over a period of weeks. CSF examination reveals a predominantly neutrophilic pleocytosis, and CMV DNA can be detected by CSF PCR. Therapy with ganciclovir or foscarnet can lead to rapid improvement, and prompt initiation of foscarnet or ganciclovir therapy is important in minimizing the degree of permanent neurologic damage. Combination therapy with both drugs should be considered in patients who have been previously treated for CMV disease. Other diseases involving the spinal cord in patients with HIV infection include HTLV-1-associated myelopathy (HAM) (Chap. 225e), neurosyphilis (Chap. 206), infection with herpes simplex (Chap. 216) or varicella-zoster (Chap. 217), TB (Chap. 202), and lymphoma (Chap. 134).
Peripheral neuropathies are common in patients with HIV infection. They occur at all stages of illness and take a variety of forms. Early in the course of HIV infection, an acute inflammatory demyelinating polyneuropathy resembling Guillain-Barré syndrome may occur (Chap. 460). In other patients, a progressive or relapsing-remitting inflammatory neuropathy resembling chronic inflammatory demyelinating polyneuropathy (CIDP) has been noted. Patients commonly present with progressive weakness, areflexia, and minimal sensory changes. CSF examination often reveals a mononuclear pleocytosis, and peripheral nerve biopsy demonstrates a perivascular infiltrate suggesting an autoimmune etiology. Plasma exchange or IVIg has been tried with variable success. Because of the immunosuppressive effects of glucocorticoids, they should be reserved for severe cases of CIDP refractory to other measures. Another autoimmune peripheral neuropathy seen in patients with AIDS is mononeuritis multiplex (Chaps. 460 and 385) due to a necrotizing arteritis of peripheral nerves. The most common peripheral neuropathy in patients with HIV infection is a distal sensory polyneuropathy (DSPN) also referred to as painful sensory neuropathy (HIV-SN), predominantly sensory neuropathy, or distal symmetric peripheral neuropathy. This condition may be a direct consequence of HIV infection or a side effect of dideoxynucleoside therapy. It is more common in taller individuals, older individuals, and those with lower CD4 counts. Two-thirds of patients with AIDS may be shown by electrophysiologic studies to have some evidence of peripheral nerve disease. Presenting symptoms are usually painful burning sensations in the feet and lower extremities. Findings on examination include a stocking-type sensory loss to pinprick, temperature, and touch sensation and a loss of ankle reflexes. Motor changes are mild and are usually limited to weakness of the intrinsic foot muscles. Response of this condition to antiretrovirals has been variable, perhaps because antiretrovirals are responsible for the problem in some instances. When due to dideoxynucleoside therapy, patients with lower extremity peripheral neuropathy may complain of a sensation that they are walking on ice. Other entities in the differential diagnosis of peripheral neuropathy include diabetes mellitus, vitamin B12 deficiency, and side effects from metronidazole or dapsone. For distal symmetric polyneuropathy that fails to resolve following the discontinuation of dideoxynucleosides, therapy is symptomatic; gabapentin, carbamazepine, tricyclics, or analgesics may be effective for dysesthesias. Treatment-naïve patients may respond to cART.
Myopathy may complicate the course of HIV infection; causes include HIV infection itself, zidovudine, and the generalized wasting syndrome. HIV-associated myopathy may range in severity from an asymptomatic elevation in creatine kinase levels to a subacute syndrome characterized by proximal muscle weakness and myalgias. Quite pronounced elevations in creatine kinase may occur in asymptomatic patients, particularly after exercise. The clinical significance of this as an isolated laboratory finding is unclear. A variety of both inflammatory and noninflammatory pathologic processes have been noted in patients with more severe myopathy, including myofiber necrosis with inflammatory cells, nemaline rod bodies, cytoplasmic bodies, and mitochondrial abnormalities. Profound muscle wasting, often with muscle pain, may be seen after prolonged zidovudine therapy. This toxic side effect of the drug is dose-dependent and is related to its ability to interfere with the function of mitochondrial polymerases. It is reversible following discontinuation of the drug. Red ragged fibers are a histologic hallmark of zidovudine-induced myopathy.
Ophthalmologic Diseases Ophthalmologic problems occur in ~50% of patients with advanced HIV infection. The most common abnormal findings on funduscopic examination are cotton-wool spots. These are hard white spots that appear on the surface of the retina and often have an irregular edge. They represent areas of retinal ischemia secondary to microvascular disease. At times they are associated with small areas of hemorrhage and thus can be difficult to distinguish from CMV retinitis. In contrast to CMV retinitis, however, these lesions are not associated with visual loss and tend to remain stable or improve over time.
One of the most devastating consequences of HIV infection is CMV retinitis. Patients at high risk of CMV retinitis (CD4+ T cell count <100/μL) should undergo an ophthalmologic examination every 3–6 months. The majority of cases of CMV retinitis occur in patients with a CD4+ T cell count <50/μL. Prior to the availability of cART, this CMV reactivation syndrome was seen in 25–30% of patients with AIDS. In the cART era this has dropped to close to 2%. CMV retinitis usually presents as a painless, progressive loss of vision. Patients may also complain of blurred vision, “floaters,” and scintillations. The disease is usually bilateral, although typically it affects one eye more than the other. The diagnosis is made on clinical grounds by an experienced ophthalmologist. The characteristic retinal appearance is that of perivascular hemorrhage and exudate. In situations where the diagnosis is in doubt due to an atypical presentation or an unexpected lack of response to therapy, vitreous or aqueous humor sampling with molecular diagnostic techniques may be of value. CMV infection of the retina results in a necrotic inflammatory process, and the visual loss that develops is irreversible. CMV retinitis may be complicated by rhegmatogenous retinal detachment as a consequence of retinal atrophy in areas of prior inflammation. Therapy for CMV retinitis consists of oral valganciclovir, IV ganciclovir, or IV foscarnet, with cidofovir as an alternative. Combination therapy with ganciclovir and foscarnet has been shown to be slightly more effective than either ganciclovir or foscarnet alone in the patient with relapsed CMV retinitis. A 3-week induction course is followed by maintenance therapy with oral valganciclovir. If CMV disease is limited to the eye, intravitreal injections of ganciclovir or foscarnet may be considered. Intravitreal injections of cidofovir are generally avoided due to the increased risk of uveitis and hypotony. Maintenance therapy is continued until the CD4+ T cell count remains >100 μL for >6 months. The majority of patients with HIV infection and CMV disease develop some degree of uveitis with the initiation of cART. The etiology of this is unknown; however, it has been suggested that this may be due to the generation of an enhanced immune response to CMV as an IRIS (see above). In some instances this has required the use of topical glucocorticoids.
Both HSV and varicella zoster virus can cause a rapidly progressing, bilateral necrotizing retinitis referred to as the acute retinal necrosis syndrome, or progressive outer retinal necrosis (PORN). This syndrome, in contrast to CMV retinitis, is associated with pain, keratitis, and iritis. It is often associated with orolabial HSV or trigeminal zoster. Ophthalmologic examination reveals widespread pale gray peripheral lesions. This condition is often complicated by retinal detachment. It is important to recognize and treat this condition with IV acyclovir as quickly as possible to minimize the loss of vision.
Several other secondary infections may cause ocular problems in HIV-infected patients. P. jiroveci can cause a lesion of the choroid that may be detected as an incidental finding on ophthalmologic examination. These lesions are typically bilateral, are from half to twice the disc diameter in size, and appear as slightly elevated yellow-white plaques. They are usually asymptomatic and may be confused with cotton-wool spots. Chorioretinitis due to toxoplasmosis can be seen alone or, more commonly, in association with CNS toxoplasmosis. KS may involve the eyelid or conjunctiva, while lymphoma may involve the retina. Syphilis may lead to a uveitis that is highly associated with the presence of neurosyphilis.
Additional Disseminated Infections and Wasting Syndrome Infections with species of the small, gram-negative, Rickettsia-like organism Bartonella (Chap. 197) are seen with increased frequency in patients with HIV infection. While it is not considered an AIDS-defining illness by the CDC, many experts view infection with Bartonella as indicative of a severe defect in cell-mediated immunity. It is usually seen in patients with CD4+ T cell counts <100/μL and is a significant cause of unexplained fever in patients with advanced HIV infection. Among the clinical manifestations of Bartonella infection are bacillary angiomatosis, cat-scratch disease, and trench fever. Bacillary angiomatosis is usually due to infection with B. henselae and is linked to exposure to flea-infested cats. It is characterized by a vascular proliferation that leads to a variety of skin lesions that have been confused with the skin lesions of KS. In contrast to the lesions of KS, the lesions of bacillary angiomatosis generally blanch, are painful, and typically occur in the setting of systemic symptoms. Infection can extend to the lymph nodes, liver (peliosis hepatis), spleen, bone, heart, CNS, respiratory tract, and GI tract. Cat-scratch disease also is due to B. henselae and generally begins with a papule at the site of inoculation. This is followed several weeks later by the development of regional adenopathy and malaise. Infection with B. quintana is transmitted by lice and has been associated with case reports of trench fever, endocarditis, adenopathy, and bacillary angiomatosis. The organism is quite difficult to culture, and diagnosis often relies on identifying the organism in biopsy specimens using the Warthin-Starry or similar stains. Treatment is with either doxycycline or erythromycin for at least 3 months.
Histoplasmosis is an opportunistic infection that is seen most frequently in patients in the Mississippi and Ohio River valleys, Puerto Rico, the Dominican Republic, and South America. These are all areas in which infection with H. capsulatum is endemic (Chap. 236). Because of this limited geographic distribution, the percentage of AIDS cases in the United States with histoplasmosis is only ~0.5. Histoplasmosis is generally a late manifestation of HIV infection; however, it may be the initial AIDS-defining condition. In one study, the median CD4+ T cell count for patients with histoplasmosis and AIDS was 33/μL. While disease due to H. capsulatum may present as a primary infection of the lung, disseminated disease, presumably due to reactivation, is the most common presentation in HIV-infected patients. Patients usually present with a 4- to 8-week history of fever and weight loss. Hepatosplenomegaly and lymphadenopathy are each seen in about 25% of patients. CNS disease, either meningitis or a mass lesion, is seen in 15% of patients. Bone marrow involvement is common, with thrombocytopenia, neutropenia, and anemia occurring in 33% of patients. Approximately 7% of patients have mucocutaneous lesions consisting of a maculopapular rash and skin or oral ulcers. Respiratory symptoms are usually mild, with chest x-ray showing a diffuse infiltrate or diffuse small nodules in ~50% of cases. The gastrointestinal tract may be involved. Diagnosis is made by silver staining of tissue, by culturing the organisms from blood, bone marrow, or tissue, or by detecting antigen in blood or urine. Treatment is typically with liposomal amphotericin B followed by maintenance therapy with oral itraconazole until the serum histoplasma antigen is <2 units, the patient has been on antiretrovirals for at least 6 months, and the CD4 count is >150 cells/μL. In the setting of mild infection, it may be appropriate to initiate therapy with itraconazole alone.
Following the spread of HIV infection to southeast Asia, disseminated infection with the fungus Penicillium marneffei was recognized as a complication of HIV infection and is considered an AIDS-defining condition in those parts of the world where it occurs. P. marneffei is the third most common AIDS-defining illness in Thailand, following TB and cryptococcosis. It is more frequently diagnosed in the rainy than the dry season. Clinical features include fever, generalized lymphadenopathy, hepatosplenomegaly, anemia, thrombocytopenia, and papular skin lesions with central umbilication. Treatment is with amphotericin B followed by itraconazole until the CD4+ T cell count is >100 cells/μL for at least 6 months.
Visceral leishmaniasis (Chap. 251) is recognized with increasing frequency in patients with HIV infection who live in or travel to areas endemic for this protozoal infection transmitted by sandflies. The clinical presentation is one of hepatosplenomegaly, fever, and hematologic abnormalities. Lymphadenopathy and other constitutional symptoms may be present. A chronic, relapsing course is seen in two-thirds of co-infected patients. Organisms can be isolated from cultures of bone marrow aspirates. Histologic stains may be negative, and antibody titers are of little help. Patients with HIV infection usually respond well initially to standard therapy with amphotericin B or pentavalent antimony compounds. Eradication of the organism is difficult, however, and relapses are common.
Patients with HIV infection are at a slightly increased risk of clinical malaria. This is particularly true for patients from nonendemic areas with presumed primary infection and in patients with lower CD4+ T cell counts. HIV-positive individuals with CD4+ T cell counts <300 cells/μL have a poorer response to malaria treatment than others. Co-infection with malaria is associated with a modest increase in HIV viral load. The risk of malaria may be decreased with TMP/SMX prophylaxis.
Generalized wasting is an AIDS-defining condition; it is defined as involuntary weight loss of >10% associated with intermittent or constant fever and chronic diarrhea or fatigue lasting >30 days in the absence of a defined cause other than HIV infection. Prior to the widespread use of cART it was the initial AIDS-defining condition in ~10% of patients with AIDS in the United States and is an indication for initiation of cART. Generalized wasting is rarely seen today with the earlier initiation of antiretrovirals. A constant feature of this syndrome is severe muscle wasting with scattered myofiber degeneration and occasional evidence of myositis. Glucocorticoids may be of some benefit; however, this approach must be carefully weighed against the risk of compounding the immunodeficiency of HIV infection. Androgenic steroids, growth hormone, and total parenteral nutrition have been used as therapeutic interventions with variable success.
Neoplastic Diseases The neoplastic diseases considered to be AIDS-defining conditions are Kaposi’s sarcoma, non-Hodgkin’s lymphoma, and invasive cervical carcinoma. In addition, there is also an increase in the incidence of a variety of non-AIDS-defining malignancies including Hodgkin’s disease; multiple myeloma; leukemia; melanoma; and cervical, brain, testicular, oral, lung, gastric, liver, renal, and anal cancers. Since the introduction of potent cART, there has been a marked reduction in the incidence of KS (Fig. 226-33) and CNS lymphoma, such that the non-AIDS-defining malignancies now account for more morbidity and mortality in patients with HIV infection than the AIDS-defining malignancies. Rates of non-Hodgkin’s lymphoma have declined as well; however, this decline has not been as dramatic as the decline in rates of KS. In contrast, cART has had little effect on human papillomavirus (HPV)-associated malignancies. As patients with HIV infection live longer, a wider array of cancers is seen in this population. While some may only reflect known risk factors (e.g., smoking, alcohol consumption, co-infection with other viruses such as hepatitis B) that are increased in patients with HIV infection, some may be a direct consequence of HIV and are clearly increased in patients with lower CD4+ T cell counts.
Kaposi’s sarcoma is a multicentric neoplasm consisting of multiple vascular nodules appearing in the skin, mucous membranes, and viscera. The course ranges from indolent, with only minor skin or lymph node involvement, to fulminant, with extensive cutaneous and visceral involvement. In the initial period of the AIDS epidemic, KS was a prominent clinical feature of the first cases of AIDS, occurring in 79% of the patients diagnosed in 1981. By 1989 it was seen in only 25% of cases, by 1992 the number had decreased to 9%, and by 1997 the number was <1%. HHV-8 (KSHV) has been strongly implicated as a viral cofactor in the pathogenesis of KS.
Clinically, KS has varied presentations and may be seen at any stage of HIV infection, even in the presence of a normal CD4+ T cell count. The initial lesion may be a small, raised reddish-purple nodule on the skin (Fig. 226-41), a discoloration on the oral mucosa (Fig. 226-34D), or a swollen lymph node. Lesions often appear in sun-exposed areas, particularly the tip of the nose, and have a propensity to occur in areas of trauma (Koebner phenomenon). Because of the vascular nature of the tumors and the presence of extravasated red blood cells in the lesions, their colors range from reddish to purple to brown and often take the appearance of a bruise, with yellowish discoloration and tattooing. Lesions range in size from a few millimeters to several centimeters in diameter and may be either discrete or confluent. KS lesions most commonly appear as raised macules; however, they can also be papular, particularly in patients with higher CD4+ T cell counts. Confluent lesions may give rise to surrounding lymphedema and may be disfiguring when they involve the face and disabling when they involve the lower extremities or the surfaces of joints. Apart from skin, the lymph nodes, GI tract, and lung are the organ systems most commonly affected by KS. Lesions have been reported in virtually every organ, including the heart and the CNS. In contrast to most malignancies, in which lymph node involvement implies metastatic spread and a poor prognosis, lymph node involvement may be seen very early in KS and is of no special clinical significance. In fact, some patients may present with disease limited to the lymph nodes. These are generally patients with relatively intact immune function and thus the patients with the best prognosis. Pulmonary involvement with KS generally presents with shortness of breath. Some 80% of patients with pulmonary KS also have cutaneous lesions. The chest x-ray characteristically shows bilateral lower lobe infiltrates that obscure the margins of the mediastinum and diaphragm (Fig. 226-42). Pleural effusions are seen in 70% of cases of pulmonary KS, a fact that is often helpful in the differential diagnosis. GI involvement is seen in 50% of patients with KS and usually takes one of two forms: (1) mucosal involvement, which may lead to bleeding that can be severe; these patients sometimes also develop symptoms of GI obstruction if lesions become large; and (2) biliary tract involvement. KS lesions may infiltrate the gallbladder and biliary tree, leading to a clinical picture of obstructive jaundice similar to that seen with sclerosing cholangitis. Several staging systems have been proposed for KS. One in common use was developed by the National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group; it distinguishes patients on the basis of tumor extent, immunologic function, and presence or absence of systemic disease (Table 226-17).
FIGURE 226-41 Kaposi’s sarcoma in three patients with AIDS demonstrating (A) periorbital edema and bruising; (B) classic truncal distribution of lesions; and (C) upper extremity lesions.
FIGURE 226-42 Chest x-ray of a patient with AIDS and pulmonary Kaposi’s sarcoma. The characteristic findings include dense bilateral lower lobe infiltrates obscuring the heart borders and pleural effusions.
|
NATIONAL INSTITUTE OF ALLERGY AND INFECTIOUS DISEASES AIDS CLINICAL TRIALS GROUP TIS STAGING SYSTEM FOR KAPOSI’S SARCOMA |
A diagnosis of KS is based on biopsy of a suspicious lesion. Histologically one sees a proliferation of spindle cells and endothelial cells, extravasation of red blood cells, hemosiderin-laden macrophages, and, in early cases, an inflammatory cell infiltrate. Included in the differential diagnosis are lymphoma (particularly for oral lesions), bacillary angiomatosis, and cutaneous mycobacterial infections.
Management of KS (Table 226-18) should be carried out in consultation with an expert since definitive treatment guidelines do not exist. In the majority of cases, effective cART will go a long way in achieving control. Antiretroviral therapy has been associated with the spontaneous regression of KS lesions. Paradoxically, it has also been associated with the initial appearance of KS as a form of IRIS. For patients in whom tumor persists or is compromising vital functions or in whom control of HIV replication is not possible, a variety of options exist. In some cases, lesions remain quite indolent, and many of these patients can be managed with no specific treatment. Fewer than 10% of AIDS patients with KS die as a consequence of their malignancy, and death from secondary infections is considerably more common. Thus, whenever possible one should avoid treatment regimens that may further suppress the immune system and increase susceptibility to opportunistic infections. Treatment is indicated under two main circumstances. The first is when a single lesion or a limited number of lesions are causing significant discomfort or cosmetic problems, such as with prominent facial lesions, lesions overlying a joint, or lesions in the oropharynx that interfere with swallowing or breathing. Under these circumstances, treatment with localized radiation, intralesional vinblastine, topical 9-cis-retinoic acid, or cryotherapy may be helpful. It should be noted that patients with HIV infection are particularly sensitive to the side effects of radiation therapy. This is especially true with respect to the development of radiation-induced mucositis; doses of radiation directed at mucosal surfaces, particularly in the head and neck region, should be adjusted accordingly. The use of systemic therapy, either IFN-α or chemotherapy, should be considered in patients with a large number of lesions or in patients with visceral involvement. The single most important determinant of response appears to be the CD4+ T cell count. This relationship between response rate and baseline CD4+ T cell count is particularly true for IFN-α. The response rate to IFN-α for patients with CD4+ T cell counts >600/μL is ~80%, while the response rate for patients with counts <150/μL is <10%. In contrast to the other systemic therapies, IFN-α provides an added advantage of having antiretroviral activity; thus, it may be the appropriate first choice for single-agent systemic therapy for early patients with disseminated disease. A variety of chemotherapeutic agents also have been shown to have activity against KS. Four of them—liposomal daunorubicin, liposomal doxorubicin, vinblastine, and paclitaxel—have been approved by the FDA for this indication. Liposomal daunorubicin is approved as first-line therapy for patients with advanced KS. It has fewer side effects than conventional chemotherapy. In contrast, liposomal doxorubicin and paclitaxel are approved only for KS patients who have failed standard chemotherapy. Response rates vary from 23 to 88%, appear to be comparable to what had been achieved earlier with combination chemotherapy regimens, and are greatly influenced by CD4+ T cell count.
|
MANAGEMENT OF AIDS-ASSOCIATED KAPOSI’S SARCOMA |
Lymphomas occur with an increased frequency in patients with congenital or acquired T cell immunodeficiencies (Chap. 374). AIDS is no exception; at least 6% of all patients with AIDS develop lymphoma at some time during the course of their illness. This is a 120-fold increase in incidence compared with the general population. In contrast to the situation with KS, primary CNS lymphoma, and most opportunistic infections, the incidence of AIDS-associated systemic lymphomas has not experienced a dramatic decrease as a consequence of the widespread use of effective cART. Lymphoma occurs in all risk groups, with the highest incidence in patients with hemophilia and the lowest incidence in patients from the Caribbean or Africa with heterosexually acquired infection. Lymphoma is a late manifestation of HIV infection, generally occurring in patients with CD4+ T cell counts <200/μL. As HIV disease progresses, the risk of lymphoma increases. The attack rate for lymphoma increases exponentially with increasing duration of HIV infection and decreasing level of immunologic function. At 3 years following a diagnosis of HIV infection, the risk of lymphoma is 0.8% per year; by 8 years after infection, it is 2.6% per year. As individuals with HIV infection live longer as a consequence of improved cART and better treatment and prophylaxis of opportunistic infections, it is anticipated that the incidence of lymphomas may increase.
Three main categories of lymphoma are seen in patients with HIV infection: grade III or IV immunoblastic lymphoma, Burkitt’s lymphoma, and primary CNS lymphoma. Approximately 90% of these lymphomas are B cell in phenotype; more than half contain EBV DNA. Some are associated with KSHV. These tumors may be either monoclonal or oligoclonal in nature and are probably in some way related to the pronounced polyclonal B cell activation seen in patients with AIDS.
Immunoblastic lymphomas account for ~60% of the cases of lymphoma in patients with AIDS. The majority of these are diffuse large B cell lymphomas (DLBCL). They are generally high grade and would have been classified as diffuse histiocytic lymphomas in earlier classification schemes. This tumor is more common in older patients, increasing in incidence from 0% in HIV-infected individuals <1 year old to >3% in those >50 years of age. Two variants of immunoblastic lymphoma that are seen primarily in HIV-infected patients are primary effusion lymphoma (PEL) and its solid variant, plasmacytic lymphoma of the oral cavity. PEL, also referred to as body cavity lymphoma, presents with lymphomatous pleural, pericardial, and/or peritoneal effusions in the absence of discrete nodal or extranodal masses. The tumor cells do not express surface markers for B cells or T cells and are felt to represent a preplasmacytic stage of differentiation. While both HHV-8 and EBV DNA sequences have been found in the genomes of the malignant cells from patients with body cavity lymphoma, KSHV is felt to be the driving force behind the oncogenesis (see above).
Small noncleaved cell lymphoma (Burkitt’s lymphoma) accounts for ~20% of the cases of lymphoma in patients with AIDS. It is most frequent in patients 10–19 years old and usually demonstrates characteristic c-myc translocations from chromosome 8 to chromosomes 14 or 22. Burkitt’s lymphoma is not commonly seen in the setting of immunodeficiency other than HIV-associated immunodeficiency, and the incidence of this particular tumor is more than 1000-fold higher in the setting of HIV infection than in the general population. In contrast to African Burkitt’s lymphoma, where 97% of the cases contain EBV genome, only 50% of HIV-associated Burkitt’s lymphomas are EBV-positive.
Primary CNS lymphoma accounts for ~20% of the cases of lymphoma in patients with HIV infection. In contrast to HIV-associated Burkitt’s lymphoma, primary CNS lymphomas are usually positive for EBV. In one study, the incidence of Epstein-Barr positivity was 100%. This malignancy does not have a predilection for any particular age group. The median CD4+ T cell count at the time of diagnosis is ~50/μL. Thus, CNS lymphoma generally presents at a later stage of HIV infection than does systemic lymphoma. This may explain, at least in part, the poorer prognosis for this subset of patients.
The clinical presentation of lymphoma in patients with HIV infection is quite varied, ranging from focal seizures to rapidly growing mass lesions in the oral mucosa (Fig. 226-43) to persistent unexplained fever. At least 80% of patients present with extranodal disease, and a similar percentage have B-type symptoms of fever, night sweats, or weight loss. Virtually any site in the body may be involved. The most common extranodal site is the CNS, which is involved in approximately one-third of all patients with lymphoma. Approximately 60% of these cases are primary CNS lymphoma. Primary CNS lymphoma generally presents with focal neurologic deficits, including cranial nerve findings, headaches, and/or seizures. MRI or CT generally reveals a limited number (one to three) of 3- to 5-cm lesions (Fig. 226-44). The lesions often show ring enhancement on contrast administration and may occur in any location. Contrast enhancement is usually less pronounced than that seen with toxoplasmosis. Locations that are most commonly involved with CNS lymphoma are deep in the white matter. The main diseases in the differential diagnosis are cerebral toxoplasmosis and cerebral Chagas’ disease. In addition to the 20% of lymphomas in HIV-infected individuals that are primary CNS lymphomas, CNS disease is also seen in HIV-infected patients with systemic lymphoma. Approximately 20% of patients with systemic lymphoma have CNS disease in the form of leptomeningeal involvement. This fact underscores the importance of lumbar puncture in the staging evaluation of patients with systemic lymphoma.
FIGURE 226-43 Immunoblastic lymphoma involving the hard palate of a patient with AIDS.
FIGURE 226-44 Central nervous system lymphoma. Postcontrast T1-weighted MRI scan in a patient with AIDS, an altered mental status, and hemiparesis. Multiple enhancing lesions, some ring-enhancing, are present. The left sylvian lesion shows gyral and subcortical enhancement, and the lesions in the caudate and splenium (arrowheads) show enhancement of adjacent ependymal surfaces.
Systemic lymphoma is seen at earlier stages of HIV infection than primary CNS lymphoma. In one series the mean CD4+ T cell count was 226/μL. In addition to lymph node involvement, systemic lymphoma may commonly involve the GI tract, bone marrow, liver, and lung. GI tract involvement is seen in ~25% of patients. Any site in the GI tract may be involved, and patients may complain of difficulty swallowing or abdominal pain. The diagnosis is usually suspected on the basis of CT or MRI of the abdomen. Bone marrow involvement is seen in ~20% of patients and may lead to pancytopenia. Liver and lung involvement are each seen in ~10% of patients. Pulmonary disease may present as a mass lesion, multiple nodules, or an interstitial infiltrate.
Both conventional and unconventional approaches have been employed in an attempt to treat HIV-related lymphomas. Systemic lymphoma is generally treated by the oncologist with combination chemotherapy. Earlier disappointing figures are being replaced with more optimistic results for the treatment of systemic lymphoma following the availability of more effective cART and the use of rituximab in CD20+ tumors. While there is some controversy regarding the use of antiretrovirals during chemotherapy, there is no question that their use overall in patients with HIV lymphoma has improved survival. Concerns regarding synergistic bone marrow toxicities with chemotherapy and cART are mitigated with the use of cART regimens that avoid bone marrow–toxic antiretrovirals. As in most situations in patients with HIV disease, those with higher CD4+ T cell counts tend to fare better. Response rates as high as 72% with a median survival of 33 months and disease-free intervals up to 9 years have been reported. Treatment of primary CNS lymphoma remains a significant challenge. Treatment is complicated by the fact that this illness usually occurs in patients with advanced HIV disease. Palliative measures such as radiation therapy provide some relief. The prognosis remains poor in this group, with a 2-year survival of 29%.
Multicentric Castleman’s disease is a KSHV-associated lymphoproliferative disorder that is seen with an increased frequency in patients with HIV infection. While not a true malignancy, it shares many features with lymphoma including generalized lymphadenopathy, hepatosplenomegaly, and systemic symptoms of fever, fatigue, and weight loss. Pulmonary symptoms may be seen in ~50% of patients. KS is present in 75–82% of cases. Lymph node biopsies reveal a predominance of interfollicular plasma cells and/or germinal centers with vascularization and an “onionskin” (hyaline vascular) appearance. Prior to the availability of cART, HIV-infected patients with multicentric Castleman’s disease had a 15-fold increased risk of developing non-Hodgkin’s lymphoma compared with HIV-infected patients in general. Treatment typically involves chemotherapy. Anecdotal reports of success with rituximab suggest that more specific treatment may be successful, although, in one series treatment with rituximab was associated with worsening of coexisting KS. The median survival of patients with treated multicentric Castleman’s disease pre-cART was initially reported as 14 months. This has increased to a 2-year survival of more than 90% in the era of cART.
Evidence of infection with human papillomavirus (HPV), associated with intraepithelial dysplasia of the cervix or anus, is approximately twice as common in HIV-infected individuals as in the general population and can lead to intraepithelial neoplasia and eventually invasive cancer. In a series of studies, HIV-infected men were examined for evidence of anal dysplasia, and Papanicolaou (Pap) smears were found to be abnormal in 20–80%. These changes tend to persist and are generally not affected by cART, raising the possibility of a subsequent transition to a more malignant condition. While the incidence of an abnormal Pap smear of the cervix is ~5% in otherwise healthy women, the incidence of abnormal cervical smears in women with HIV infection is 30–60%, and invasive cervical cancer is included as an AIDS-defining condition. While only small increases in the absolute numbers of cervical or anal cancers have been seen as a consequence of HIV infection, the relative risk of these conditions when one compares HIV-infected to -noninfected men and women is on the order of 10- to 100-fold. Given the high rates of dysplasia and relative risks for cervical and anal cancer, a comprehensive gynecologic and rectal examination, including Pap smear, is indicated at the initial evaluation and 6 months later for all patients with HIV infection. If these examinations are negative at both time points, the patient should be followed with yearly evaluations. If an initial or repeat Pap smear shows evidence of severe inflammation with reactive squamous changes, the next Pap smear should be performed at 3 months. If, at any time, a Pap smear shows evidence of squamous intraepithelial lesions, colposcopic examination with biopsies as indicated should be performed. The 2-year survival rate for HIV-infected patients with invasive cervical cancer is 64% compared with 79% in non-HIV-infected patients. In addition to rectal and cervical lesions, HPV can also lead to head and neck cancers. In one study of men who have sex with men, 25% were found to have oral HPV; high-risk HPV genotypes were three times more common in the HIV-infected men. The most common HPV genotypes in the general population and the genotypes upon which current HPV vaccines are based are 6, 11, 16, and 18. This is not the case in the HIV-infected population, where other genotypes such as 58 and 53 also are prominent. This raises concerns about the level of effectiveness of the current HPV vaccines for HIV-infected patients. Despite this, it is recommended that patients with HIV infection be vaccinated against HPV.
IDIOPATHIC CD4+ T LYMPHOCYTOPENIA
A syndrome was recognized in 1992 characterized by an absolute CD4+ T cell count of <300/μL or <20% of total T cells on a minimum of two occasions at least 6 weeks apart; no evidence of HIV-1, HIV-2, HTLV-1, or HTLV-2 on testing; and the absence of any defined immunodeficiency or therapy associated with decreased levels of CD4+ T cells. By mid-1993, ~100 patients had been described. After extensive multicenter investigations, a series of reports were published in early 1993, which together allowed a number of conclusions. Idiopathic CD4+ lymphocytopenia (ICL) is a very rare syndrome, as determined by studies of blood donors and cohorts of HIV-seronegative men who have sex with men. Cases were clearly identified as early as 1983 and were remarkably similar to the clinical features of ICL that had been identified decades earlier. The definition of ICL based on CD4+ T cell counts coincided with the ready availability of testing for CD4+ T cells in patients suspected of being immunodeficient. Although, as a result of immune deficiency, certain patients with ICL develop some of the opportunistic diseases (particularly cryptococcosis, nontuberculous mycobacterial infections, and cervical dysplasia) seen in HIV-infected patients, the syndrome is demographically, clinically, and immunologically unlike HIV infection and AIDS. Fewer than half of the reported ICL patients had risk factors for HIV infection, and there were wide geographic and age distributions. The fact that a significant proportion of patients did have risk factors probably reflects a selection bias, in that physicians who take care of HIV-infected patients are more likely to monitor CD4+ T cells. Approximately half of the patients are women, compared with approximately one-third among HIV-infected individuals in the United States. Many patients with ICL remained clinically stable, and their condition did not deteriorate progressively as is common with seriously immunodeficient HIV-infected patients. Approximately 15% of patients with ICL experience spontaneous reversal of the CD4+ T lymphocytopenia. Immunologic abnormalities in ICL are somewhat different from those of HIV infection. ICL patients often have increases in CD4+ T cell activation with decreases in CD8+ T cells and B cells. Furthermore, immunoglobulin levels are either normal or, more commonly, decreased in patients with ICL, compared with the usual hypergammaglobulinemia of HIV-infected individuals. Virologic studies of these patients have revealed no evidence of HIV-1, HIV-2, HTLV-1, or HTLV-2 or of any other mononuclear cell–tropic virus. Furthermore, there has been no epidemiologic evidence to suggest that a transmissible microbe was involved. The cases of ICL have been widely dispersed, with no clustering. Close contacts and sexual partners who were studied were clinically well and were serologically, immunologically, and virologically negative for HIV. ICL is a heterogeneous syndrome, and it is highly likely that there is no common cause; however, there may be common causes among subgroups of patients that are currently unrecognized.
Patients who present with laboratory data consistent with ICL should be worked up for underlying diseases that could be responsible for the immune deficiency. If no underlying cause is detected, no specific therapy should be initiated. However, if opportunistic diseases occur, they should be treated appropriately (see above). Depending on the level of the CD4+ T cell count, patients should receive prophylaxis for the commonly encountered opportunistic infections.
HIV AND THE HEALTH CARE WORKER
Health care workers, especially those who deal with large numbers of HIV-infected patients, have a small but definite risk of becoming infected with HIV as a result of professional activities (see “Occupational Transmission of HIV: Health Care Workers, Laboratory Workers, and the Health Care Setting,” above). The first case of HIV transmission from a patient to health care worker was reported in 1984. Occupational transmission of HIV has been reported in most countries; as noted above, the global number of HIV infections among health care workers attributable to punctures/cuts has been estimated to be 1000 cases (range, 200–5000) per year.
In the United States 57 health care workers for whom case investigations were completed as of 2010 had documented seroconversions to HIV following occupational exposures. The routes of exposure resulting in infection were as follows: 48 percutaneous (puncture/cut injury); 5 mucocutaneous (mucous membrane and/or skin); 2 both percutaneous and mucocutaneous; and 2 of unknown route. Of the 57 health care personnel, 49 were exposed to HIV-infected blood; 3 to concentrated virus in a laboratory; 1 to visibly bloody fluid; and 4 to an unspecified fluid. The individuals with documented seroconversions included 19 laboratory workers (16 of whom were clinical laboratory workers), 24 nurses, 6 physicians, 2 surgical technicians, 1 dialysis technician, 1 respiratory therapist, 1 health aide, 1 embalmer/morgue technician, and 2 housekeeper/maintenance workers. In addition, more than 140 possible cases of occupationally acquired HIV infection have been reported among health care personnel in the United States. The number of these workers who actually acquired their infection through occupational exposures is not known. Taken together, data from several large studies suggest that the risk of HIV infection following a percutaneous exposure to HIV-contaminated blood is ~0.3%, and after a mucous membrane exposure, ~0.09%. Although episodes of HIV transmission after nonintact skin exposure have been documented, the average risk for transmission by this route has not been precisely quantified but is estimated to be less than the risk for mucous membrane exposures. The risk for transmission after exposure to fluids or tissues other than HIV-infected blood also has not been quantified but is probably considerably lower than for blood exposures. A seroprevalence survey of 3420 orthopedic surgeons, 75% of whom practiced in an area with a relatively high prevalence of HIV infection and 39% of whom reported percutaneous exposure to patient blood, usually through an accident involving a suture needle, failed to reveal any cases of possible occupational infection, suggesting that the risk of infection with a suture needle may be considerably less than that with a blood-drawing (hollow-bore) needle.
Most cases of health care worker seroconversion occur as a result of needle-stick injuries. When one considers the circumstances that result in needle-stick injuries, it is immediately obvious that adhering to the standard guidelines for dealing with sharp objects would result in a significant decrease in this type of accident. In one study, 27% of needle-stick injuries resulted from improper disposal of the needle (over half of these were due to recapping the needle), 23% occurred during attempts to start an IV line, 22% occurred during blood drawing, 16% were associated with an IM or SC injection, and 12% were associated with giving an IV infusion.
Clinicians should consider potential occupational exposures to HIV as urgent medical concerns to ensure timely postexposure management and possible administration of postexposure antiretroviral prophylaxis (PEP). Recommendations regarding PEP must take into account that a variety of circumstances determine the risk of transmission of HIV following occupational exposure. In this regard, several factors have been associated with an increased risk for occupational transmission of HIV infection, including deep injury, the presence of visible blood on the instrument causing the exposure, injury with a device that had been placed in the vein or artery of the source patient, terminal illness in the source patient, and lack of postexposure cART in the exposed health care worker. Other important considerations when considering PEP in the health care worker include known or suspected pregnancy or breast-feeding, the possibility of exposure to drug-resistant virus, and toxicities of PEP regimens. Regardless of the decision to use PEP, the wound should be cleansed immediately and antiseptic applied. If a decision is made to offer PEP, U.S. Public Health Service guidelines recommend (1) a combination of two nucleoside analogue reverse transcriptase inhibitors given for 4 weeks for less severe exposures, or (2) a combination of two nucleoside analogue reverse transcriptase inhibitors plus a third drug given for 4 weeks for more severe exposures. Most clinicians administer the latter regimen in all cases in which a decision is made to treat. Detailed guidelines are available from the Updated U.S. Public Health Service Guidelines for the Management of Occupational Exposures to HIV and Recommendations for Postexposure Prophylaxis (CDC, 2005). The report emphasizes the importance of adherence to PEP when it is indicated, follow-up of exposed workers to improve PEP adherences, monitoring for adverse events (including seroconversion), and expert consultation in the management of exposures.
For consultation on the treatment of occupational exposures to HIV and other bloodborne pathogens, the clinician managing the exposed patient can call the National Clinicians’ Post-Exposure Prophylaxis Hotline (PEPline) at 888-448-4911. This service is available 24 hours a day at no charge. (Additional information on the Internet is available at www.nccc.ucsf.edu.) PEPline support may be especially useful in challenging situations, such as when drug-resistant HIV strains are suspected or the health care worker is pregnant.
Health care workers can minimize their risk of occupational HIV infection by following the CDC guidelines of July 1991, which include adherence to universal precautions, refraining from direct patient care if one has exudative lesions or weeping dermatitis, and disinfecting and sterilizing reusable devices employed in invasive procedures. The premise of universal precautions is that every specimen should be handled as if it came from someone infected with a bloodborne pathogen. All samples should be double-bagged, gloves should be worn when drawing blood, and spills should be immediately disinfected with bleach.
In attempting to put this small but definite risk to the health care worker in perspective, it is important to point out that ~200 health care workers die each year as a result of occupationally acquired hepatitis B infection. The tragedy in this instance is that these infections and deaths due to HBV could be greatly decreased by more extended use of the HBV vaccine. The risk of HBV infection following a needle-stick injury from a hepatitis antigen–positive patient is much higher than the risk of HIV infection (see “Transmission,” above). There are multiple examples of needle-stick injuries where the patient was positive for both HBV and HIV and the health care worker became infected only with HBV. For these reasons, it is advisable, given the high prevalence of HBV infection in HIV-infected individuals, that all health care workers dealing with HIV-infected patients be immunized with the HBV vaccine.
TB is another infection common to HIV-infected patients that can be transmitted to the health care worker. For this reason, all health care workers should know their PPD status, have it checked yearly, and receive 6 months of isoniazid treatment if their skin test converts to positive. In addition, all patients in whom a diagnosis of TB is being entertained should be placed immediately in respiratory isolation, pending results of the diagnostic evaluation. The emergence of drug-resistant organisms, including the extensively drug-resistant TB strains that have been identified in Africa, has made TB an increasing problem for health care workers. This is particularly true for the health care worker with preexisting HIV infection.
One of the most charged issues ever to come between health care workers and patients is that of transmission of infection from HIV-infected health care workers to their patients. This is discussed in “Occupational Transmission of HIV: Health Care Workers, Laboratory Workers, and the Health Care Setting,” above. Theoretically, the same universal precautions that are used to protect the health care worker from the HIV-infected patient will also protect the patient from the HIV-infected health care worker.
A PREVENTIVE VACCINE AGAINST HIV INFECTION
Given that human behavior, especially human sexual behavior, is extremely difficult to change, a critical modality for preventing the spread of HIV infection is the development of a safe and effective vaccine. Historically, vaccines have provided a safe, cost-effective, and efficient means of preventing illness, disability, and death from infectious diseases. Successful vaccines for the most part are predicated on the assumptions that the body can mount an adequate immune response to the microbe or virus in question during natural infection and that the vaccine will mimic the natural response to infection. Even with serious diseases, such as smallpox, poliomyelitis, measles, and influenza among others, the body in the vast majority of cases clears the infectious agent and provides protection, which is usually life-long against future exposure. Unfortunately, this is not the case with HIV infection since the natural immune response to HIV infection is unable to clear the virus from the body and cases of superinfection are not uncommon. Some of the factors that contribute to the problematic nature of development of a preventive HIV vaccine are the high mutability of the virus, the fact that the infection can be transmitted by cell-free or cell-associated virus, the fact that the HIV provirus integrates itself into the genome of the target cell and may remain in a latent form unexposed to the immune system, the likely need for the development of effective mucosal immunity, and the fact that it has been difficult to establish the precise correlates of protective immunity to HIV infection. A fraction of a percent of HIV-infected individuals are “elite controllers” in that they maintain extremely low and even undetectable levels of viremia in the absence of cART, and a number of individuals have been exposed to HIV multiple times but remain uninfected; these facts suggest that there are elements of host defense or an HIV-specific immune response that have the potential to be protective. Early attempts to develop a vaccine with the envelope protein gp120 aimed at inducing neutralizing antibodies in humans were unsuccessful in that the elicited antisera failed to neutralize primary isolates of HIV cultured and tested in fresh peripheral blood mononuclear cells. In this regard, two phase 3 trials were undertaken in the United States and Thailand using soluble gp120, and the vaccines failed to protect human volunteers from HIV infection. In addition, two separate vaccine trials aimed at eliciting CD8+ T cell responses to prevent infection and, if unsuccessful in preventing infection to control postinfection viremia, also failed at both goals. Recently, a vaccine using a poxvirus vector prime expressing various viral proteins followed by an envelope protein boost was tested in a 16,000-person clinical trial (RV144) conducted in Thailand among predominantly low-prevalence heterosexuals. The vaccine provided the first positive, albeit very modest, signal ever reported in an HIV vaccine trial, showing 31% protection against acquisition of infection. Such a result is certainly not sufficient justification for clinical use of the vaccine, but it served as an important first step in the direction of the development of a safe and effective vaccine against HIV infection. Follow-up studies of RV144 indicate that nonneutralizing or weakly neutralizing antibody responses against certain constant epitopes in the otherwise highly variable V1-V2 region of the HIV envelope may be associated with the modest degree of protection observed in that clinical trial. Additional studies are planned in attempts to improve on the results of RV144 by a variety of approaches, including increasing the number of vaccine boosts with envelope protein.
An area of HIV vaccine research that is currently being actively pursued is the attempt to induce broadly neutralizing antibodies by developing as immunogens for vaccination certain epitopes on the HIV envelope that are the targets of naturally occurring broadly neutralizing antibodies during HIV infection. It is curious that only about 20% of HIV-infected individuals develop broadly neutralizing antibodies in response to natural infection and they do so only after 2 to 3 years of ongoing infection. By the time these antibodies appear, they can neutralize a broad range of primary HIV isolates, but they appear to be ineffective against the autologous virus in the infected subject. Upon close examination, these broadly neutralizing antibodies manifest a high degree of somatic mutations that were accumulated over time and are responsible for their affinity maturation and broadly neutralizing capacity. The goal of current efforts is to develop the conformationally correct HIV envelope epitopes that, when used as immunogens, would direct the immune response of an uninfected individual to the production of broadly neutralizing antibodies over a reasonable time frame by sequential immunizations. It remains to be seen whether this approach will be feasible.
PREVENTION
Education, counseling, and behavior modification are the cornerstones of any HIV prevention strategy. A major problem in the United States and elsewhere is that many infections are passed on by those who do not know that they are infected. Of the ~1.1 million persons in the United States who are HIV-infected, it is estimated that ~16–18% do not know their HIV status and approximately 49% of all new infections are transmitted by those people who are not aware that they are infected. In this regard, the CDC has recommended that HIV testing become part of routine medical care and that all individuals between the ages of 13 and 64 years be tested at least one time. These individuals should be informed of the testing and be tested without the need for written informed consent. Each individual could “opt out” of testing, but testing would otherwise be routinely administered. Individuals who are practicing high-risk behavior should be tested more often. In addition to identifying individuals who might benefit from cART, information gathered from such an approach should serve as the basis for behavior-modification programs, both for infected individuals who may be unaware of their HIV status and who could infect others and for uninfected individuals practicing high-risk behavior. The practice of “safer sex” is the most effective way for sexually active uninfected individuals to avoid contracting HIV infection and for infected individuals to avoid spreading infection. Abstinence from sexual relations is the only absolute way to prevent sexual transmission of HIV infection. However, for many individuals this may not be feasible, and there are a number of relatively safe practices that can markedly decrease the chances of transmission of HIV infection. Partners engaged in monogamous sexual relationships who wish to be assured of safety should both be tested for HIV antibody. If both are negative, it must be understood that any divergence from monogamy puts both partners at risk; open discussion of the importance of honesty in such relationships should be encouraged. When the HIV status of either partner is not known, or when one partner is positive, there are a number of options. Use of condoms can markedly decrease the chance of HIV transmission. It should be remembered that condoms are not 100% effective in preventing transmission of HIV infection, and there is a ~10% failure rate of condoms used for contraceptive purposes. Most condom failures result from breakage or improper usage, such as not wearing the condom for the entire period of intercourse. Latex condoms are preferable, since virus has been shown to leak through natural skin condoms. Petroleum-based gels should never be used for lubrication of the condom, since they increase the likelihood of condom rupture. Some men who have sex with men practice fellatio as a “minimal risk” activity compared with anal intercourse. It should be emphasized that receptive fellatio is definitely not safe sex, and although the incidence of transmission via fellatio is considerably less than that of rectal or vaginal intercourse, there has been documentation of transmission of HIV where receptive fellatio was the only sexual act performed (see “Transmission,” above). Topical microbicides composed of gels containing antiretroviral drugs have been shown to be efficacious in preventing acquisition of HIV infection in women engaging in vaginal intercourse. However, there has been a considerable degree of variability in efficacy related to the variable adherence of participants to the use of the intervention. In general, it is felt that microbicides can be quite efficacious; however, adherence is a major stumbling block to their broad effectiveness. Pre-exposure prophylaxis (PreP) using oral antiretroviral drugs on a daily basis in uninfected men who have sex with men and transgender women has been shown to be efficacious in preventing acquisition of HIV infection. The degree of efficacy can be very high (>90%) if subjects adhere strictly to the regimen. However, adherence has proven to be a problem in maximizing the overall effectiveness of this approach.
Adult male circumcision has been shown to result in a 50% to 65% reduction in HIV acquisition in the circumcised subject. Clearly, this approach has considerable potential as a preventive strategy for HIV infection and is currently being pursued, particularly in developing nations, as a component of HIV prevention. The most effective way to prevent transmission of HIV infection among IDUs is to stop the use of injectable drugs. Unfortunately, that is extremely difficult to accomplish unless the individual enters a treatment program. For those who will not or cannot participate in a drug treatment program and who will continue to inject drugs, the avoidance of sharing of needles and other paraphernalia (“works”) is the next best way to avoid transmission of infection. However, the cultural and social factors that contribute to the sharing of paraphernalia are complex and difficult to overcome. In addition, needles and syringes may be in short supply. Under these circumstances, paraphernalia should be cleaned after each usage with a virucidal solution, such as undiluted sodium hypochlorite (household bleach). Programs that provide sterile needles to addicts in exchange for used needles have resulted in a marked decrease in HIV transmission without increasing the use of injection drugs. It is important for IDUs to be tested for HIV infection and counseled to avoid transmission to their sexual partners. Oral PreP also is effective in preventing acquisition of HIV infections among IDUs. Prevention of transmission through blood or blood products and prevention of mother-to-child transmission are discussed in “Transmission,” above.
SECTION 15 |
INFECTIONS DUE TO RNA VIRUSES |
227 |
Viral Gastroenteritis |
Acute infectious gastroenteritis is a common illness that affects persons of all ages worldwide. It is a leading cause of mortality among children in developing countries, accounting for an estimated 0.7 million deaths each year, and is responsible for up to 10–12% of all hospitalizations among children in industrialized countries, including the United States. Elderly persons, especially those with debilitating health conditions, also are at risk of severe complications and death from acute gastroenteritis. Among healthy young adults, acute gastroenteritis is rarely fatal but incurs substantial medical and social costs, including those of time lost from work.
Several enteric viruses have been recognized as important etiologic agents of acute infectious gastroenteritis (Table 227-1, Fig. 227-1). Although most viral gastroenteritis is caused by RNA viruses, the DNA viruses that are occasionally involved (e.g., adenovirus types 40 and 41) are included in this chapter. Illness caused by these viruses is characterized by the acute onset of vomiting and/or diarrhea, which may be accompanied by fever, nausea, abdominal cramps, anorexia, and malaise. As shown in Table 227-2, several features can help distinguish gastroenteritis caused by viruses from that caused by bacterial agents. However, the distinction based on clinical and epidemiologic parameters alone is often difficult, and laboratory tests are required to confirm the diagnosis.
|
VIRAL CAUSES OF GASTROENTERITIS AMONG HUMANS |
FIGURE 227-1 Viral agents of gastroenteritis. NV, norovirus; SV, sapovirus.
|
CHARACTERISTICS OF GASTROENTERITIS CAUSED BY VIRAL AND BACTERIAL AGENTS |
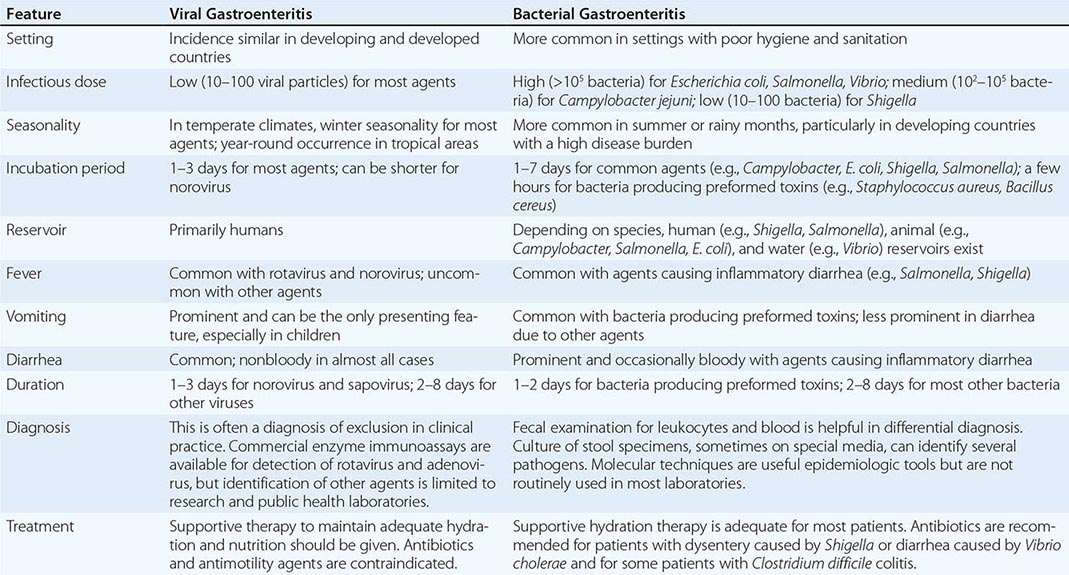
HUMAN CALICIVIRUSES
Etiologic Agent The Norwalk virus is the prototype strain of a group of small (27–40 nm), nonenveloped, round, icosahedral viruses with relatively amorphous surface features on visualization by electron microscopy. These viruses have been difficult to classify because they have not been adapted to growth in cell culture and no animal models are available. Molecular cloning and characterization have demonstrated that the viruses have a single, positive-strand RNA genome ~7.5 kb in length and possess a single virion-associated protein—similar to that of typical caliciviruses—with a molecular mass of 60 kDa. On the basis of these molecular characteristics, these viruses are presently classified in two genera belonging to the family Caliciviridae: the noroviruses and the sapoviruses (previously called Norwalk-like viruses and Sapporo-like viruses, respectively).
![]() Epidemiology Infections with the Norwalk and related human caliciviruses are common worldwide, and most adults have antibodies to these viruses. Antibody is acquired at an earlier age in developing countries—a pattern consistent with the presumed fecal-oral mode of transmission. Infections occur year-round, although, in temperate climates, a distinct increase has been noted in cold-weather months. Noroviruses may be the most common infectious agents of mild gastroenteritis in the community and affect all age groups, whereas sapoviruses primarily cause gastroenteritis in children. Noroviruses also cause traveler’s diarrhea, and outbreaks have occurred among military personnel deployed to various parts of the world. The limited data available indicate that norovirus may be the second most common viral agent (after rotavirus) among young children and the most common agent among older children and adults. In the United States, with the decline in severe rotavirus disease following implementation of rotavirus vaccines, norovirus has become the leading cause of medically attended gastroenteritis in young children. Noroviruses are also recognized as the major cause of epidemics of gastroenteritis worldwide. In the United States, >90% of outbreaks of nonbacterial gastroenteritis are caused by noroviruses.
Epidemiology Infections with the Norwalk and related human caliciviruses are common worldwide, and most adults have antibodies to these viruses. Antibody is acquired at an earlier age in developing countries—a pattern consistent with the presumed fecal-oral mode of transmission. Infections occur year-round, although, in temperate climates, a distinct increase has been noted in cold-weather months. Noroviruses may be the most common infectious agents of mild gastroenteritis in the community and affect all age groups, whereas sapoviruses primarily cause gastroenteritis in children. Noroviruses also cause traveler’s diarrhea, and outbreaks have occurred among military personnel deployed to various parts of the world. The limited data available indicate that norovirus may be the second most common viral agent (after rotavirus) among young children and the most common agent among older children and adults. In the United States, with the decline in severe rotavirus disease following implementation of rotavirus vaccines, norovirus has become the leading cause of medically attended gastroenteritis in young children. Noroviruses are also recognized as the major cause of epidemics of gastroenteritis worldwide. In the United States, >90% of outbreaks of nonbacterial gastroenteritis are caused by noroviruses.
Virus is transmitted predominantly by the fecal-oral route but is also present in vomitus. Because an inoculum with very few viruses can be infectious, transmission can occur by aerosolization, by contact with contaminated fomites, and by person-to-person contact. Viral shedding and infectivity are greatest during the acute illness, but challenge studies with Norwalk virus in volunteers indicate that viral antigen may be shed by asymptomatically infected persons and also by symptomatic persons before the onset of symptoms and for several weeks after the resolution of illness. Viral shedding can be prolonged in immunocompromised individuals.
Pathogenesis The exact sites and cellular receptors for attachment of viral particles have not been determined. Data suggest that carbohydrates that are similar to human histo-blood group antigens and are present on the gastroduodenal epithelium of individuals with the secretor phenotype may serve as ligands for the attachment of Norwalk virus. Additional studies must more fully elucidate norovirus-carbohydrate interactions, including potential strain-specific variations. After the infection of volunteers, reversible lesions are noted in the upper jejunum, with broadening and blunting of the villi, shortening of the microvilli, vacuolization of the lining epithelium, crypt hyperplasia, and infiltration of the lamina propria by polymorphonuclear neutrophils and lymphocytes. The lesions persist for at least 4 days after the resolution of symptoms and are associated with malabsorption of carbohydrates and fats and a decreased level of brush-border enzymes. Adenylate cyclase activity is not altered. No histopathologic changes are seen in the stomach or colon, but gastric motor function is delayed, and this alteration is believed to contribute to the nausea and vomiting that are typical of this illness.
Clinical Manifestations Gastroenteritis caused by Norwalk and related human caliciviruses has a sudden onset following an average incubation period of 24 h (range, 12–72 h). The illness generally lasts 12–60 h and is characterized by one or more of the following symptoms: nausea, vomiting, abdominal cramps, and diarrhea. Vomiting is more prevalent among children, whereas a greater proportion of adults develop diarrhea. Constitutional symptoms are common, including headache, fever, chills, and myalgias. The stools are characteristically loose and watery, without blood, mucus, or leukocytes. White cell counts are generally normal; rarely, leukocytosis with relative lymphopenia may be observed. Death is a rare outcome and usually results from severe dehydration in vulnerable persons (e.g., elderly patients with debilitating health conditions).
Immunity Approximately 50% of persons challenged with Norwalk virus become ill and acquire short-term immunity against the infecting strain. Immunity to Norwalk virus appears to correlate inversely with level of antibody; i.e., persons with higher levels of preexisting antibody to Norwalk virus are more susceptible to illness. This observation suggests that some individuals have a genetic predisposition to illness. Specific ABO, Lewis, and secretor blood group phenotypes may influence susceptibility to norovirus infection.
Diagnosis Cloning and sequencing of the genomes of Norwalk and several other human caliciviruses have allowed the development of assays based on polymerase chain reaction (PCR) for detection of virus in stool and vomitus. Virus-like particles produced by expression of capsid proteins in a recombinant baculovirus vector have been used to develop enzyme immunoassays (EIAs) for detection of virus in stool or a serologic response to a specific viral antigen. These newer diagnostic techniques are considerably more sensitive than previous detection methods, such as electron microscopy, immune electron microscopy, and EIAs based on reagents derived from humans. However, no currently available single assay can detect all human caliciviruses because of their great genetic and antigenic diversity. In addition, the assays are still cumbersome and are available primarily in research laboratories, although they are increasingly being adopted by public health laboratories for routine screening of fecal specimens from patients affected by outbreaks of gastroenteritis. Commercial EIA kits have limited sensitivity and usefulness in clinical practice and are of greatest utility in outbreaks, in which many specimens are tested and only a few need be positive to identify norovirus as the cause.
Prevention Epidemic prevention relies on situation-specific measures, such as control of contamination of food and water, exclusion of ill food handlers, and reduction of person-to-person spread through good personal hygiene and disinfection of contaminated fomites. The role of immunoprophylaxis is not clear, given the lack of long-term immunity from natural disease, but efforts to develop norovirus vaccines are ongoing. In a clinical study, a candidate virus-like particle norovirus vaccine was shown to protect against homologous viral challenge.
ROTAVIRUS
Etiologic Agent Rotaviruses are members of the family Reoviridae. The viral genome consists of 11 segments of double-strand RNA that are enclosed in a triple-layered, nonenveloped, icosahedral capsid 75 nm in diameter. Viral protein 6 (VP6), the major structural protein, is the target of commercial immunoassays and determines the group specificity of rotaviruses. There are seven major groups of rotavirus (A through G); human illness is caused primarily by group A and, to a much lesser extent, by groups B and C. Two outer-capsid proteins, VP7 (G-protein) and VP4 (P-protein), determine serotype specificity, induce neutralizing antibodies, and form the basis for binary classification of rotaviruses (G and P types). The segmented genome of rotavirus allows genetic reassortment (i.e., exchange of genome segments between viruses) during co-infection—a property that may play a role in viral evolution and that has been utilized in the development of reassortant animal-human rotavirus–based vaccines.
![]() Epidemiology Worldwide, nearly all children are infected with rotavirus by 3–5 years of age. Neonatal infections are common but are often asymptomatic or mild, presumably because of protection by maternal antibody or breast milk. Compared with rotavirus disease in industrialized countries, disease in developing countries occurs at a younger age, is less seasonal, and is more frequently caused by uncommon rotavirus strains. Moreover, because of suboptimal access to hydration therapy, rotavirus is a leading cause of diarrheal death among children in the developing world, with the highest mortality rates among children in sub-Saharan Africa and South Asia (Fig. 227-2).
Epidemiology Worldwide, nearly all children are infected with rotavirus by 3–5 years of age. Neonatal infections are common but are often asymptomatic or mild, presumably because of protection by maternal antibody or breast milk. Compared with rotavirus disease in industrialized countries, disease in developing countries occurs at a younger age, is less seasonal, and is more frequently caused by uncommon rotavirus strains. Moreover, because of suboptimal access to hydration therapy, rotavirus is a leading cause of diarrheal death among children in the developing world, with the highest mortality rates among children in sub-Saharan Africa and South Asia (Fig. 227-2).
FIGURE 227-2 Rotavirus mortality rates by country, per 100,000 children <5 years of age. (Reproduced with permission from UD Parashar et al: J Infect Dis 200:S9, 2009.)
First infections after 3 months of age are likely to be symptomatic, and the incidence of disease peaks among children 4–23 months of age. Reinfections are common, but the severity of disease decreases with each repeat infection. Therefore, severe rotavirus infections are less common among older children and adults than among younger individuals. Nevertheless, rotavirus can cause illness in parents and caretakers of children with rotavirus diarrhea, immunocompromised persons, travelers, and elderly individuals and should be considered in the differential diagnosis of gastroenteritis among adults.
In tropical settings, rotavirus disease occurs year-round, with less pronounced seasonal peaks than in temperate settings, where rotavirus disease occurs predominantly during the cooler fall and winter months. Before the introduction of rotavirus vaccine in the United States, the rotavirus season each year began in the Southwest during the autumn and early winter (October through December) and migrated across the continent, peaking in the Northeast during late winter and spring (March through May). The reasons for this characteristic pattern are not clear but may be correlated with state-specific differences in birth rates, which could influence the rate of accumulation of susceptible infants after each rotavirus season. After the implementation of routine vaccination of U.S. infants against rotavirus in 2006, the characteristic prevaccine geotemporal pattern of U.S. rotavirus was dramatically altered, and these changes were accompanied by substantial declines in rotavirus detections by a national network of sentinel laboratories (Fig. 227-3). During the latest two seasons with available data (spanning 2010–2012), the number of rotavirus detections declined by 74–90% from the prevaccine baseline, and the annual proportion of rotavirus tests that were positive was below 10% in both seasons (compared with a prevaccine baseline median of 26%). A pattern of biennial increases in rotavirus activity has emerged during the five postvaccine seasons (2007–2012), but activity has remained substantially below prevaccine levels in each season.
FIGURE 227-3 Percentage of rotavirus tests with positive results, by week of year, July–June, 2000–2012. The maximal or minimal percentage of rotavirus-positive tests for 2000–2006 may have occurred during any of the six baseline seasons. Data are from the National Respiratory and Enteric Virus Surveillance System. (Adapted from Centers for Disease Control and Prevention, 2012.)
During episodes of rotavirus-associated diarrhea, virus is shed in large quantities in stool (107–1012/g). Viral shedding detectable by EIA usually subsides within 1 week but may persist for >30 days in immunocompromised individuals; it may be detected for longer periods by sensitive molecular assays, such as PCR. The virus is transmitted predominantly through the fecal-oral route. Spread through respiratory secretions, person-to-person contact, or contaminated environmental surfaces has been postulated to explain the rapid acquisition of antibody in the first 3 years of life, regardless of sanitary conditions.
At least 10 different G serotypes of group A rotavirus have been identified in humans, but only 5 types (G1 through G4 and G9) are common. While human rotavirus strains that possess a high degree of genetic homology with animal strains have been identified, animal-to-human transmission appears to be uncommon.
Group B rotaviruses have been associated with several large epidemics of severe gastroenteritis among adults in China since 1982 and have also been identified in India. Group C rotaviruses have been associated with a small proportion of pediatric gastroenteritis cases in several countries worldwide.
Pathogenesis Rotaviruses infect and ultimately destroy mature enterocytes in the villous epithelium of the proximal small intestine. The loss of absorptive villous epithelium, coupled with the proliferation of secretory crypt cells, results in secretory diarrhea. Brush-border enzymes characteristic of differentiated cells are reduced, and this change leads to the accumulation of unmetabolized disaccharides and consequent osmotic diarrhea. Studies in mice indicate that a nonstructural rotavirus protein, NSP4, functions as an enterotoxin and contributes to secretory diarrhea by altering epithelial cell function and permeability. In addition, rotavirus may evoke fluid secretion through activation of the enteric nervous system in the intestinal wall. Data indicate that rotavirus antigenemia and viremia are common among children with acute rotavirus infection, although the antigen and RNA levels in serum are substantially lower than those in stool.
Clinical Manifestations The clinical spectrum of rotavirus infection ranges from subclinical infection to severe gastroenteritis leading to life-threatening dehydration. After an incubation period of 1–3 days, the illness has an abrupt onset, with vomiting frequently preceding the onset of diarrhea. Up to one-third of patients may have a temperature of >39°C. The stools are characteristically loose and watery and only infrequently contain red or white cells. Gastrointestinal symptoms generally resolve in 3–7 days.
Respiratory and neurologic features in children with rotavirus infection have been reported, but causal associations have not been proven. Moreover, rotavirus infection has been associated with a variety of other clinical conditions (e.g., sudden infant death syndrome, necrotizing enterocolitis, intussusception, Kawasaki’s disease, and type 1 diabetes), but no causal relationship has been confirmed with any of these syndromes.
Rotavirus does not appear to be a major opportunistic pathogen in children with HIV infection. In severely immunodeficient children, rotavirus can cause protracted diarrhea with prolonged viral excretion and, in rare instances, can disseminate systemically. Persons who are immunosuppressed for bone marrow transplantation also are at risk for severe or even fatal rotavirus disease.
Immunity Protection against rotavirus disease is correlated with the presence of virus-specific secretory IgA antibodies in the intestine and, to some extent, the serum. Because virus-specific IgA production at the intestinal surface is short lived, complete protection against disease is only temporary. However, each infection and subsequent reinfection confers progressively greater immunity; thus severe disease is most common among young children with first or second infections. Immunologic memory is believed to be important in the attenuation of disease severity upon reinfection.
Diagnosis Illness caused by rotavirus is difficult to distinguish clinically from that caused by other enteric viruses. Because large quantities of virus are shed in feces, the diagnosis can usually be confirmed by a wide variety of commercially available EIAs or by techniques for detecting viral RNA, such as gel electrophoresis, probe hybridization, or PCR.
![]() Prevention Efforts to develop rotavirus vaccines were pursued because it was apparent—given the similar rates in less developed and industrialized nations—that improvements in hygiene and sanitation were unlikely to reduce disease incidence. The first rotavirus vaccine licensed in the United States in 1998 was withdrawn from the market within 1 year because it was linked with a low incidence of intussusception, a severe bowel obstruction.
Prevention Efforts to develop rotavirus vaccines were pursued because it was apparent—given the similar rates in less developed and industrialized nations—that improvements in hygiene and sanitation were unlikely to reduce disease incidence. The first rotavirus vaccine licensed in the United States in 1998 was withdrawn from the market within 1 year because it was linked with a low incidence of intussusception, a severe bowel obstruction.
In 2006, promising safety and efficacy results for two new rotavirus vaccines were reported from large clinical trials conducted in North America, Europe, and Latin America. Both vaccines are now recommended for routine immunization of all U.S. infants, and their use has rapidly led to a >70–80% decline in rotavirus hospitalizations and emergency department visits at hospitals across the United States. Indirect benefits from vaccination (i.e., herd immunity) have also been documented in many settings. In April 2009, the World Health Organization recommended the use of rotavirus vaccines in all countries worldwide. As of May 2013, a total of 42 countries, including 5 low-income countries in Africa and Asia, have incorporated rotavirus vaccine into their national childhood immunization programs. In Mexico and in Brazil, a decline in deaths from childhood diarrhea following introduction of rotavirus vaccines has been documented. Postmarketing surveillance has identified a low risk of intussusception in some countries; however, the benefits of vaccination exceed the risks, and no changes in vaccine administration policy have been implemented.
![]() The different epidemiology of rotavirus disease and the greater prevalence of co-infection with other enteric pathogens, of comorbidities, and of malnutrition in developing countries may adversely affect the performance of oral rotavirus vaccines, as is the case with oral vaccines against poliomyelitis, cholera, and typhoid in these regions. Therefore, evaluation of the efficacy of rotavirus vaccines in resource-poor settings of Africa and Asia was specifically recommended, and these trials have now been completed. As anticipated, the efficacy of rotavirus vaccines was moderate (50–65%) in these settings when compared with that in industrialized countries. Nevertheless, even a moderately efficacious rotavirus vaccine would be likely to have substantial public health benefits in these areas with a high disease burden.
The different epidemiology of rotavirus disease and the greater prevalence of co-infection with other enteric pathogens, of comorbidities, and of malnutrition in developing countries may adversely affect the performance of oral rotavirus vaccines, as is the case with oral vaccines against poliomyelitis, cholera, and typhoid in these regions. Therefore, evaluation of the efficacy of rotavirus vaccines in resource-poor settings of Africa and Asia was specifically recommended, and these trials have now been completed. As anticipated, the efficacy of rotavirus vaccines was moderate (50–65%) in these settings when compared with that in industrialized countries. Nevertheless, even a moderately efficacious rotavirus vaccine would be likely to have substantial public health benefits in these areas with a high disease burden.
OTHER VIRAL AGENTS OF GASTROENTERITIS
Enteric adenoviruses of serotypes 40 and 41 belonging to subgroup F are 70- to 80-nm viruses with double-strand DNA that cause ~2–12% of all diarrhea episodes in young children. Unlike adenoviruses that cause respiratory illness, enteric adenoviruses are difficult to cultivate in cell lines, but they can be detected with commercially available EIAs. Adenovirus types 31 and 42–49 have been linked to diarrhea in HIV-infected and other immunocompromised persons.
Astroviruses are 28- to 30-nm viruses with a characteristic icosahedral structure and a positive-sense, single-strand RNA. At least seven serotypes have been identified, of which serotype 1 is most common. Astroviruses are primarily pediatric pathogens, causing ~2–10% of cases of mild to moderate gastroenteritis in children. The availability of simple immunoassays to detect virus in fecal specimens and of molecular methods to confirm and characterize strains will permit more comprehensive assessment of the etiologic role of these agents.
Toroviruses are 100- to 140-nm, enveloped, positive-strand RNA viruses that are recognized as causes of gastroenteritis in horses (Berne virus) and cattle (Breda virus). Their role as a cause of diarrhea in humans is still unclear, but studies from Canada have demonstrated associations between torovirus excretion and both nosocomial gastroenteritis and necrotizing enterocolitis in neonates. These associations require further evaluation.
Picobirnaviruses are small, bisegmented, double-strand RNA viruses that cause gastroenteritis in a variety of animals. Their role as primary causes of gastroenteritis in humans remains unclear, but several studies have found an association between picobirnaviruses and gastroenteritis in HIV-infected adults.
Several other viruses (e.g., enteroviruses, reoviruses, pestiviruses, and parvovirus B) have been identified in the feces of patients with diarrhea, but their etiologic role in gastroenteritis has not been proven. Diarrhea has also been noted as a manifestation of infection with recently recognized viruses that primarily cause severe respiratory illness: the severe acute respiratory syndrome–associated coronavirus (SARS-CoV), influenza A/H5N1 virus, and the current pandemic strain of influenza A/H1N1 virus.
228 |
Enterovirus, Parechovirus, and Reovirus Infections |
ENTEROVIRUSES
CLASSIFICATION AND CHARACTERIZATION
Enteroviruses, members of the family Picornaviridae, are so designated because of their ability to multiply in the gastrointestinal tract. Despite their name, these viruses are not a prominent cause of gastroenteritis. Enteroviruses encompass more than 100 human serotypes: 3 serotypes of poliovirus, 21 serotypes of coxsackievirus A, 6 serotypes of coxsackievirus B, 28 serotypes of echovirus, enteroviruses 68–71, and multiple new enteroviruses (beginning with enterovirus 73) that have been identified by molecular techniques. Human enteroviruses have been reclassified into four species designated A–D. Echoviruses 22 and 23 have been reclassified as parechoviruses 1 and 2 on the basis of low nucleotide homology and differences in viral proteins. Enterovirus surveillance conducted in the United States by the Centers for Disease Control and Prevention (CDC) in 2007–2008 showed that the most common enterovirus serotype, coxsackievirus B1, was followed in frequency by echoviruses 18, 9, and 6; together, these four viruses accounted for 52% of all isolates.
Human enteroviruses contain a single-stranded RNA genome surrounded by an icosahedral capsid comprising four viral proteins. These viruses have no lipid envelope and are stable in acidic environments, including the stomach. They are susceptible to chlorine-containing cleansers but resistant to inactivation by standard disinfectants (e.g., alcohol, detergents) and can persist for days at room temperature.
PATHOGENESIS AND IMMUNITY
Much of what is known about the pathogenesis of enteroviruses has been derived from studies of poliovirus infection. After ingestion, poliovirus is thought to infect epithelial cells in the mucosa of the gastrointestinal tract and then to spread to and replicate in the submucosal lymphoid tissue of the tonsils and Peyer’s patches. The virus next spreads to the regional lymph nodes, a viremic phase ensues, and the virus replicates in organs of the reticuloendothelial system. In some cases, a second episode of viremia occurs and the virus replicates further in various tissues, sometimes causing symptomatic disease.
It is uncertain whether poliovirus reaches the central nervous system (CNS) during viremia or whether it also spreads via peripheral nerves. Since viremia precedes the onset of neurologic disease in humans, it has been assumed that the virus enters the CNS via the bloodstream. The poliovirus receptor is a member of the immunoglobulin superfamily. Poliovirus infection is limited to primates, largely because their cells express the viral receptor. Studies demonstrating the poliovirus receptor in the end-plate region of muscle at the neuromuscular junction suggest that, if the virus enters the muscle during viremia, it could travel across the neuromuscular junction up the axon to the anterior horn cells. Studies of monkeys and of transgenic mice expressing the poliovirus receptor show that, after IM injection, poliovirus does not reach the spinal cord if the sciatic nerve is cut. Taken together, these findings suggest that poliovirus can spread directly from muscle to the CNS by neural pathways.
Poliovirus can usually be cultured from the blood 3–5 days after infection, before the development of neutralizing antibodies. While viral replication at secondary sites begins to slow 1 week after infection, it continues in the gastrointestinal tract. Poliovirus is shed from the oropharynx for up to 3 weeks after infection and from the gastrointestinal tract for as long as 12 weeks; hypogammaglobulinemic patients can shed poliovirus for >20 years. During replication in the gastrointestinal tract, attenuated oral poliovirus can mutate, reverting to a more neurovirulent phenotype within a few days; however, additional mutations are probably required for full neurovirulence. One patient with hypogammaglobulinemia who had been infected 12 years earlier and was receiving IV immune globulin suddenly developed quadriplegia and respiratory muscle paralysis and died; analysis showed that the virus had reverted to a more wild-type sequence.
Humoral and secretory immunity in the gastrointestinal tract is important for the control of enterovirus infections. Enteroviruses induce specific IgM, which usually persists for <6 months, and specific IgG, which persists for life. Capsid protein VP1 is the predominant target of neutralizing antibody, which generally confers lifelong protection against subsequent disease caused by the same serotype but does not prevent infection or virus shedding. Enteroviruses also induce cellular immunity whose significance is uncertain. Patients with impaired cellular immunity are not known to develop unusually severe disease when infected with enteroviruses. In contrast, the severe infections in patients with agammaglobulinemia emphasize the importance of humoral immunity in controlling enterovirus infections. Disseminated enterovirus infections have occurred in hematopoietic cell transplant recipients. IgA antibodies are instrumental in reducing poliovirus replication in and shedding from the gastrointestinal tract. Breast milk contains IgA specific for enteroviruses and can protect humans from infection.
EPIDEMIOLOGY
![]() Enteroviruses have a worldwide distribution. More than 50% of nonpoliovirus enterovirus infections and more than 90% of poliovirus infections are subclinical. When symptoms do develop, they are usually nonspecific and occur in conjunction with fever; only a minority of infections are associated with specific clinical syndromes. The incubation period for most enterovirus infections ranges from 2 to 14 days but usually is <1 week.
Enteroviruses have a worldwide distribution. More than 50% of nonpoliovirus enterovirus infections and more than 90% of poliovirus infections are subclinical. When symptoms do develop, they are usually nonspecific and occur in conjunction with fever; only a minority of infections are associated with specific clinical syndromes. The incubation period for most enterovirus infections ranges from 2 to 14 days but usually is <1 week.
Enterovirus infection is more common in socioeconomically disadvantaged areas, especially in those where conditions are crowded and in tropical areas where hygiene is poor. Infection is most common among infants and young children; serious illness develops most often during the first few days of life and in older children and adults. In developing countries, where children are infected at an early age, poliovirus infection has less often been associated with paralysis; in countries with better hygiene, older children and adults are more likely to be seronegative, become infected, and develop paralysis. Passively acquired maternal antibody reduces the risk of symptomatic infection in neonates. Young children are the most frequent shedders of enteroviruses and are usually the index cases in family outbreaks. In temperate climates, enterovirus infections occur most often in the summer and fall; no seasonal pattern is apparent in the tropics.
Most enteroviruses are transmitted primarily by the fecal-oral or oral-oral route. Patients are most infectious shortly before and after the onset of symptomatic disease, when virus is present in the stool and throat. The ingestion of virus-contaminated food or water also can cause disease. Certain enteroviruses (such as enterovirus 70, which causes acute hemorrhagic conjunctivitis) can be transmitted by direct inoculation from the fingers to the eye. Airborne transmission is important for some viruses that cause respiratory tract disease, such as coxsackievirus A21. Enteroviruses can be transmitted across the placenta from mother to fetus, causing severe disease in the newborn. The transmission of enteroviruses through blood transfusions or insect bites has not been documented. Nosocomial spread of coxsackievirus and echovirus has taken place in hospital nurseries.
CLINICAL FEATURES
Poliovirus Infection Most infections with poliovirus are asymptomatic. After an incubation period of 3–6 days, ~5% of patients present with a minor illness (abortive poliomyelitis) manifested by fever, malaise, sore throat, anorexia, myalgias, and headache. This condition usually resolves in 3 days. About 1% of patients present with aseptic meningitis (nonparalytic poliomyelitis). Examination of cerebrospinal fluid (CSF) reveals lymphocytic pleocytosis, a normal glucose level, and a normal or slightly elevated protein level; CSF polymorphonuclear leukocytes may be present early. In some patients, especially children, malaise and fever precede the onset of aseptic meningitis.
PARALYTIC POLIOMYELITIS The least common presentation is that of paralytic disease. After one or several days, signs of aseptic meningitis are followed by severe back, neck, and muscle pain and by the rapid or gradual development of motor weakness. In some cases the disease appears to be biphasic, with aseptic meningitis followed first by apparent recovery but then (1–2 days later) by the return of fever and the development of paralysis; this form is more common among children than among adults. Weakness is generally asymmetric, is proximal more than distal, and may involve the legs (most commonly); the arms; or the abdominal, thoracic, or bulbar muscles. Paralysis develops during the febrile phase of the illness and usually does not progress after defervescence. Urinary retention may also occur. Examination reveals weakness, fasciculations, decreased muscle tone, and reduced or absent reflexes in affected areas. Transient hyperreflexia sometimes precedes the loss of reflexes. Patients frequently report sensory symptoms, but objective sensory testing usually yields normal results. Bulbar paralysis may lead to dysphagia, difficulty in handling secretions, or dysphonia. Respiratory insufficiency due to aspiration, involvement of the respiratory center in the medulla, or paralysis of the phrenic or intercostal nerves may develop, and severe medullary involvement may lead to circulatory collapse. Most patients with paralysis recover some function weeks to months after infection. About two-thirds of patients have residual neurologic sequelae.
Paraytic disease is more common among older individuals, pregnant women, and persons exercising strenuously or undergoing trauma at the time of CNS symptoms. Tonsillectomy predisposes to bulbar poliomyelitis, and IM injections increase the risk of paralysis in the involved limb(s).
VACCINE-ASSOCIATED POLIOMYELITIS The risk of developing poliomyelitis after oral vaccination is estimated at 1 case per 2.5 million doses. The risk is ~2000 times higher among immunodeficient persons, especially in persons with hypo- or agammaglobulinemia. Before 1997, an average of eight cases of vaccine-associated poliomyelitis occurred—in both vaccinees and their contacts—in the United States each year. With the change in recommendations first to a sequential regimen of inactivated poliovirus vaccine (IPV) and oral poliovirus vaccine (OPV) in 1997 and then to an all-IPV regimen in 2000, the number of cases of vaccine-associated polio declined. From 1997 to 1999, six such cases were reported in the United States; no cases have been reported since 1999.
POSTPOLIO SYNDROME The postpolio syndrome presents as a new onset of weakness, fatigue, fasciculations, and pain with additional atrophy of the muscle group involved during the initial paralytic disease 20–40 years earlier. The syndrome is more common among women and with increasing time after acute disease. The onset is usually insidious, and weakness occasionally extends to muscles that were not involved during the initial illness. The prognosis is generally good; progression to further weakness is usually slow, with plateau periods of 1–10 years. The postpolio syndrome is thought to be due to progressive dysfunction and loss of motor neurons that compensated for the neurons lost during the original infection and not to persistent or reactivated poliovirus infection.
Other Enteroviruses An estimated 5–10 million cases of symptomatic disease due to enteroviruses other than poliovirus occur in the United States each year. Among neonates, enteroviruses are the most common cause of aseptic meningitis and nonspecific febrile illnesses. Certain clinical syndromes are more likely to be caused by certain serotypes (Table 228-1).
|
MANIFESTATIONS COMMONLY ASSOCIATED WITH ENTEROVIRUS SEROTYPES |
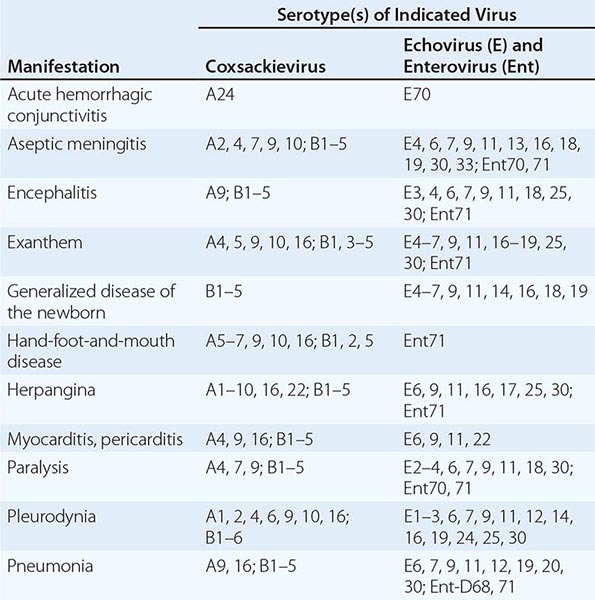
NONSPECIFIC FEBRILE ILLNESS (SUMMER GRIPPE) The most common clinical manifestation of enterovirus infection is a nonspecific febrile illness. After an incubation period of 3–6 days, patients present with an acute onset of fever, malaise, and headache. Occasional cases are associated with upper respiratory symptoms, and some cases include nausea and vomiting. Symptoms often last for 3–4 days, and most cases resolve in a week. While infections with other respiratory viruses occur more often from late fall to early spring, febrile illness due to enteroviruses frequently occurs in the summer and early fall.
GENERALIZED DISEASE OF THE NEWBORN Most serious enterovirus infections in infants develop during the first week of life, although severe disease can occur up to 3 months of age. Neonates often present with an illness resembling bacterial sepsis, with fever, irritability, and lethargy. Laboratory abnormalities include leukocytosis with a left shift, thrombocytopenia, elevated values in liver function tests, and CSF pleocytosis. The illness can be complicated by myocarditis and hypotension, fulminant hepatitis and disseminated intravascular coagulation, meningitis or meningoencephalitis, or pneumonia. It may be difficult to distinguish neonatal enterovirus infection from bacterial sepsis, although a history of a recent virus-like illness in the mother provides a clue.
ASEPTIC MENINGITIS AND ENCEPHALITIS In children and young adults, enteroviruses are the cause of up to 90% of cases of aseptic meningitis in which an etiologic agent can be identified. Patients with aseptic meningitis typically present with an acute onset of fever, chills, headache, photophobia, and pain on eye movement. Nausea and vomiting also are common. Examination reveals meningismus without localizing neurologic signs; drowsiness or irritability may also be apparent. In some cases, a febrile illness may be reported that remits but returns several days later in conjunction with signs of meningitis. Other systemic manifestations may provide clues to an enteroviral cause, including diarrhea, myalgias, rash, pleurodynia, myocarditis, and herpangina. Examination of the CSF invariably reveals pleocytosis; the CSF cell count shows a shift from neutrophil to lymphocyte predominance within 1 day of presentation, and the total cell count does not exceed 1000/μL. The CSF glucose level is usually normal (in contrast to the low CSF glucose level in mumps), with a normal or slightly elevated protein concentration. Partially treated bacterial meningitis may be particularly difficult to exclude in some instances. Enteroviral meningitis is more common in summer and fall in temperate climates, while viral meningitis of other etiologies is more common in winter and spring. Symptoms ordinarily resolve within a week, although CSF abnormalities can persist for several weeks. Enteroviral meningitis is often more severe in adults than in children. Neurologic sequelae are rare, and most patients have an excellent prognosis.
Enteroviral encephalitis is much less common than enteroviral aseptic meningitis. Occasional highly inflammatory cases of enteroviral meningitis may be complicated by a mild form of encephalitis that is recognized on the basis of progressive lethargy, disorientation, and sometimes seizures. Less commonly, severe primary encephalitis may develop. An estimated 10–35% of cases of viral encephalitis are due to enteroviruses. Immunocompetent patients generally have a good prognosis.
Patients with hypogammaglobulinemia, agammaglobulinemia, or severe combined immunodeficiency may develop chronic meningitis or encephalitis; about half of these patients have a dermatomyositis-like syndrome, with peripheral edema, rash, and myositis. They may also have chronic hepatitis. Patients may develop neurologic disease while receiving immunoglobulin replacement therapy. Echoviruses (especially echovirus 11) are the most common pathogens in this situation.
Paralytic disease due to enteroviruses other than poliovirus occurs sporadically and is usually less severe than poliomyelitis. Most cases are due to enterovirus 70 or 71 or to coxsackievirus A7 or A9. Guillain-Barré syndrome is also associated with enterovirus infection. While earlier studies suggested a link between enteroviruses and chronic fatigue syndrome, most recent studies have not demonstrated such an association.
PLEURODYNIA (BORNHOLM DISEASE) Patients with pleurodynia present with an acute onset of fever and spasms of pleuritic chest or upper abdominal pain. Chest pain is more common in adults, and abdominal pain is more common in children. Paroxysms of severe, knifelike pain usually last 15–30 min and are associated with diaphoresis and tachypnea. Fever peaks within an hour after the onset of paroxysms and subsides when pain resolves. The involved muscles are tender to palpation, and a pleural rub may be detected. The white blood cell count and chest x-ray results are usually normal. Most cases are due to coxsackievirus B and occur during epidemics. Symptoms resolve in a few days, and recurrences are rare. Treatment includes the administration of nonsteroidal anti-inflammatory agents or the application of heat to the affected muscles.
MYOCARDITIS AND PERICARDITIS Enteroviruses are estimated to cause up to one-third of cases of acute myocarditis. Coxsackievirus B and its RNA have been detected in pericardial fluid and myocardial tissue in some cases of acute myocarditis and pericarditis. Most cases of enteroviral myocarditis or pericarditis occur in newborns, adolescents, or young adults. More than two-thirds of patients are male. Patients often present with an upper respiratory tract infection that is followed by fever, chest pain, dyspnea, arrhythmias, and occasionally heart failure. A pericardial friction rub is documented in half of cases, and the electrocardiogram shows ST-segment elevations or ST- and T-wave abnormalities. Serum levels of myocardial enzymes are often elevated. Neonates commonly have severe disease, while most older children and adults recover completely. Up to 10% of cases progress to chronic dilated cardiomyopathy. Chronic constrictive pericarditis may also be a sequela.
EXANTHEMS Enterovirus infection is the leading cause of exanthems in children in the summer and fall. While exanthems are associated with many enteroviruses, certain types have been linked to specific syndromes. Echoviruses 9 and 16 have frequently been associated with exanthem and fever. Rashes may be discrete or confluent, beginning on the face and spreading to the trunk and extremities. Echovirus 9 is the most common cause of a rubelliform (discrete) rash. Unlike the rash of rubella, the enteroviral rash occurs in the summer and is not associated with lymphadenopathy. Roseola-like rashes develop after defervescence, with macules and papules on the face and trunk. The Boston exanthem, caused by echovirus 16, is a roseola-like rash. A variety of other rashes have been associated with enteroviruses, including erythema multiforme (see Fig. 25e-25) and vesicular, urticarial, petechial, or purpuric lesions. Enanthems also occur, including lesions that resemble the Koplik’s spots seen with measles (see Fig. 25e-2).
HAND-FOOT-AND-MOUTH DISEASE (Fig. 228-1) After an incubation period of 4–6 days, patients with hand-foot-and-mouth disease present with fever, anorexia, and malaise; these manifestations are followed by the development of sore throat and vesicles (see Fig. 25e-23) on the buccal mucosa and often on the tongue and then by the appearance of tender vesicular lesions on the dorsum of the hands, sometimes with involvement of the palms. The vesicles may form bullae and quickly ulcerate. About one-third of patients also have lesions on the palate, uvula, or tonsillar pillars, and one-third have a rash on the feet (including the soles) or on the buttocks. The disease is highly infectious, with attack rates of close to 100% among young children. The lesions usually resolve in 1 week. Most cases are due to coxsackievirus A16 or enterovirus 71.
FIGURE 228-1 Vesicular eruptions of the hand (A), foot (B), and mouth (C) of a 6-year-old boy with coxsackievirus A6 infection. (Images reprinted courtesy of Centers for Disease Control and Prevention/Emerging Infectious Diseases.)
![]() An epidemic of enterovirus 71 infection in Taiwan in 1998 resulted in thousands of cases of hand-foot-and-mouth disease or herpangina (see below). Severe complications included CNS disease, myocarditis, and pulmonary hemorrhage. About 90% of those who died were children ≤5 years old, and death was associated with pulmonary edema or pulmonary hemorrhage. CNS disease included aseptic meningitis, flaccid paralysis (similar to that seen in poliomyelitis), and rhombencephalitis with myoclonus and tremor or ataxia. The mean age of patients with CNS complications was 2.5 years, and MRI in cases with encephalitis usually showed brain-stem lesions. Follow-up of children at 6 months showed persistent dysphagia, cranial nerve palsies, hypoventilation, limb weakness, and atrophy; at 3 years, persistent neurologic sequelae were documented, with delayed development and impaired cognitive function.
An epidemic of enterovirus 71 infection in Taiwan in 1998 resulted in thousands of cases of hand-foot-and-mouth disease or herpangina (see below). Severe complications included CNS disease, myocarditis, and pulmonary hemorrhage. About 90% of those who died were children ≤5 years old, and death was associated with pulmonary edema or pulmonary hemorrhage. CNS disease included aseptic meningitis, flaccid paralysis (similar to that seen in poliomyelitis), and rhombencephalitis with myoclonus and tremor or ataxia. The mean age of patients with CNS complications was 2.5 years, and MRI in cases with encephalitis usually showed brain-stem lesions. Follow-up of children at 6 months showed persistent dysphagia, cranial nerve palsies, hypoventilation, limb weakness, and atrophy; at 3 years, persistent neurologic sequelae were documented, with delayed development and impaired cognitive function.
Another epidemic of enterovirus 71 infection occurred in China in 2008–2010, with nearly 500,000 infections and 126 deaths. Infections were associated with fever, rash, brain-stem encephalitis with myoclonic jerks, and limb trembling; some cases progressed to seizures and coma. Lung findings included pulmonary edema and hemorrhage; while the level of creatine kinase MB was sometimes elevated, myocardial necrosis was generally not found.
Cyclic epidemics occur every 2–3 years in other Asian countries. However, the virus circulates at lower rates in the United States, Europe, and Africa. In the United States, hand-foot-and-mouth disease is most commonly associated with coxsackievirus A16. Between November 2011 and February 2012, outbreaks of hand-foot-and-mouth disease due to coxsackievirus A6 occurred in several U.S. states, and 19% of the affected persons were hospitalized.
HERPANGINA Herpangina is usually caused by coxsackievirus A and presents as acute-onset fever, sore throat, odynophagia, and grayish-white papulovesicular lesions on an erythematous base that ulcerate. The lesions can persist for weeks; are present on the soft palate, anterior pillars of the tonsils, and uvula; and are concentrated in the posterior portion of the mouth. In contrast to herpes stomatitis, enteroviral herpangina is not associated with gingivitis. Acute lymphonodular pharyngitis associated with coxsackievirus A10 presents as white or yellow nodules surrounded by erythema in the posterior oropharynx. The lesions do not ulcerate.
ACUTE HEMORRHAGIC CONJUNCTIVITIS Patients with acute hemorrhagic conjunctivitis present with an acute onset of severe eye pain, blurred vision, photophobia, and watery discharge from the eye. Examination reveals edema, chemosis, and subconjunctival hemorrhage and often shows punctate keratitis and conjunctival follicles as well (Fig. 228-2). Preauricular adenopathy is often found. Epidemics and nosocomial spread have been associated with enterovirus 70 and coxsackievirus A24. Systemic symptoms, including headache and fever, develop in 20% of cases, and recovery is usually complete in 10 days. The sudden onset and short duration of the illness help to distinguish acute hemorrhagic conjunctivitis from other ocular infections, such as those due to adenovirus and Chlamydia trachomatis. Paralysis has been associated with some cases of acute hemorrhagic conjunctivitis due to enterovirus 70 during epidemics.
FIGURE 228-2 Acute hemorrhagic conjunctivitis due to enterovirus 70. (Image reprinted with permission from Red Book 2012: Committee on Infectious Diseases, 29th ed. Used with permission of the American Academy of Pediatrics.)
OTHER MANIFESTATIONS Enteroviruses are an infrequent cause of childhood pneumonia and the common cold. In the fall of 2014, enterovirus D68 infection was confirmed in more than 500 persons with mild to severe respiratory illnesses in 43 U.S. states. Nearly all reported cases were in children, many of whom had asthma. Enterovirus D68 was detected in upper respiratory tract specimens from some patients with unexplained acute neurologic disease during outbreaks of infection with this virus; however, the virus was not detected in the CSF, and at present the link between the virus and neurologic disease is uncertain. Coxsackievirus B has been isolated at autopsy from the pancreas of a few children presenting with type 1 diabetes mellitus; however, most attempts to isolate the virus have been unsuccessful. Other diseases that have been associated with enterovirus infection include parotitis, bronchitis, bronchiolitis, croup, infectious lymphocytosis, polymyositis, acute arthritis, and acute nephritis.
DIAGNOSIS
Isolation of enterovirus in cell culture is the traditional diagnostic procedure. While cultures of stool, nasopharyngeal, or throat samples from patients with enterovirus diseases are often positive, isolation of the virus from these sites does not prove that it is directly associated with disease because these sites are frequently colonized for weeks in patients with subclinical infections. Isolation of virus from the throat is more likely to be associated with disease than is isolation from the stool since virus is shed for shorter periods from the throat. Cultures of CSF, serum, fluid from body cavities, or tissues are positive less frequently, but a positive result is indicative of disease caused by enterovirus. In some cases, the virus is isolated only from the blood or only from the CSF; therefore, it is important to culture multiple sites. Cultures are more likely to be positive earlier than later in the course of infection. Most human enteroviruses can be detected within a week after inoculation of cell cultures. Cultures may be negative because of the presence of neutralizing antibody, lack of susceptibility of the cells used, or inappropriate handling of the specimen. Coxsackievirus A may require inoculation into special cell-culture lines or into suckling mice.
Identification of the enterovirus serotype is useful primarily for epidemiologic studies and, with a few exceptions, has little clinical utility. It is important to identify serious infections with enterovirus during epidemics and to distinguish the vaccine strain of poliovirus from the other enteroviruses in the throat or in the feces. Stool and throat samples for culture as well as acute- and convalescent-phase serum specimens should be obtained from all patients with suspected poliomyelitis. In the absence of a positive CSF culture, a positive culture of stool obtained within the first 2 weeks after the onset of symptoms is most often used to confirm the diagnosis of poliomyelitis. If polio-virus infection is suspected, two or more fecal and throat swab samples should be obtained at least 1 day apart and cultured for enterovirus as soon as possible. If poliovirus is isolated, it should be sent to the CDC for identification as either wild-type or vaccine virus.
Reverse-transcriptase polymerase chain reaction (PCR) has been used to amplify viral nucleic acid from CSF, serum, urine, stool, conjunctiva, throat swabs, and tissues. A pan-enterovirus PCR assay can detect all human enteroviruses. With the proper controls, PCR of the CSF is highly sensitive (70–100%) and specific (>80%) and is more rapid than culture. PCR of the CSF is less likely to be positive when patients present ≥3 days after the onset of meningitis or with enterovirus 71 infection; in these cases, PCR of throat or rectal swabs—although less specific than PCR of CSF—should be considered.