PART 14: Disorders of the Gastrointestinal System
SECTION 1 |
DISORDERS OF THE ALIMENTARY TRACT |
344 |
Approach to the Patient with Gastrointestinal Disease |
ANATOMIC CONSIDERATIONS
The gastrointestinal (GI) tract extends from the mouth to the anus and is composed of several organs with distinct functions. Specialized independently controlled thickened sphincters that assist in gut compartmentalization separate the organs. The gut wall is organized into well-defined layers that contribute to functional activities in each region. The mucosa is a barrier to luminal contents or a site for transfer of fluids or nutrients. Gut smooth muscle in association with the enteric nervous system mediates propulsion from one region to the next. Many GI organs possess a serosal layer that provides a supportive foundation but that also permits external input.
Interactions with other organ systems serve the needs both of the gut and the body. Pancreaticobiliary conduits deliver bile and enzymes into the duodenum. A rich vascular supply is modulated by GI tract activity. Lymphatic channels assist in gut immune activities. Intrinsic gut wall nerves provide the basic controls for propulsion and fluid regulation. Extrinsic neural input provides volitional or involuntary control to degrees that are specific for each gut region.
FUNCTIONS OF THE GASTROINTESTINAL TRACT
The GI tract serves two main functions—assimilating nutrients and eliminating waste. The gut anatomy is organized to serve these functions. In the mouth, food is processed, mixed with salivary amylase, and delivered to the gut lumen. The esophagus propels the bolus into the stomach; the lower esophageal sphincter prevents oral reflux of gastric contents. The esophageal mucosa has a protective squamous histology, which does not permit significant diffusion or absorption. Propulsive esophageal activities are exclusively aboral and coordinate with relaxation of the upper and lower esophageal sphincters on swallowing.
The stomach furthers food preparation by triturating and mixing the bolus with pepsin and acid. Gastric acid also sterilizes the upper gut. The proximal stomach serves a storage function by relaxing to accommodate the meal. The distal stomach exhibits phasic contractions that propel solid food residue against the pylorus, where it is repeatedly propelled proximally for further mixing before it is emptied into the duodenum. Finally, the stomach secretes intrinsic factor for vitamin B12 absorption.
The small intestine serves most of the nutrient absorptive function of the gut. The intestinal mucosa exhibits villus architecture to provide maximal surface area for absorption and is endowed with specialized enzymes and transporters. Triturated food from the stomach mixes with pancreatic juice and bile in the duodenum to facilitate digestion. Pancreatic juice contains the main enzymes for carbohydrate, protein, and fat digestion as well as bicarbonate to optimize the pH for activation of these enzymes. Bile secreted by the liver and stored in the gallbladder is essential for intestinal lipid digestion. The proximal intestine is optimized for rapid absorption of nutrient breakdown products and most minerals, whereas the ileum is better suited for absorption of vitamin B12 and bile acids. The small intestine also aids in waste elimination. Bile contains by-products of erythrocyte degradation, toxins, metabolized and unmetabolized medications, and cholesterol. Motor function of the small intestine delivers indigestible food residue and sloughed enterocytes into the colon for further processing. The small intestine terminates in the ileocecal junction, a sphincteric structure that prevents coloileal reflux and maintains small-intestinal sterility.
The colon prepares the waste material for controlled evacuation. The colonic mucosa dehydrates the stool, decreasing daily fecal volumes from 1000–1500 mL delivered from the ileum to 100–200 mL expelled from the rectum. The colonic lumen possesses a dense bacterial colonization that ferments undigested carbohydrates and short-chain fatty acids. Whereas transit times in the esophagus are on the order of seconds and times in the stomach and small intestine range from minutes to a few hours, propagation through the colon takes more than 1 day in most individuals. Colonic motor patterns exhibit a to-and-fro character that facilitates slow fecal desiccation. The proximal colon serves to mix and absorb fluid, while the distal colon exhibits peristaltic contractions and mass actions that function to expel the stool. The colon terminates in the anus, a structure with volitional and involuntary controls to permit retention of the fecal bolus until it can be released in a socially convenient setting.
EXTRINSIC MODULATION OF GUT FUNCTION
GI function is modified by influences outside of the gut. Unlike other organ systems, the gut is in continuity with the outside environment. Thus, protective mechanisms are vigilant against deleterious effects of foods, medications, toxins, and infectious organisms. Mucosal immune mechanisms include chronic lymphocyte and plasma cell populations in the epithelial layer and lamina propria backed up by lymph node chains to prevent noxious agents from entering the circulation. Antimicrobial peptides secreted by Paneth cells in the intestine further contribute to the defense mechanisms against pathogens in the lumen. All substances absorbed into the bloodstream are filtered through the liver via the portal venous circulation. In the liver, many drugs and toxins are detoxified by a variety of mechanisms. Although intrinsic nerves control most basic gut activities, extrinsic neural input modulates many functions. Two activities under voluntary control are swallowing and defecation. Many normal GI reflexes involve extrinsic vagus or splanchnic nerve pathways. The brain-gut axis further alters function in regions not under volitional regulation. As an example, stress has potent effects on gut motor, secretory, and sensory functions.
OVERVIEW OF GASTROINTESTINAL DISEASES
GI diseases develop as a result of abnormalities within or outside of the gut and range in severity from those that produce mild symptoms and no long-term morbidity to those with intractable symptoms or adverse outcomes. Diseases may be localized to one organ or exhibit diffuse involvement at many sites.
CLASSIFICATION OF GI DISEASES
GI diseases are manifestations of alterations in nutrient assimilation or waste evacuation or in the activities supporting these main functions.
Impaired Digestion and Absorption Diseases of the stomach, intestine, biliary tree, and pancreas can disrupt digestion and absorption. The most common intestinal maldigestion syndrome, lactase deficiency, produces gas and diarrhea after ingestion of dairy products and has no adverse outcomes. Other intestinal enzyme deficiencies produce similar symptoms after ingestion of other simple sugars. Conversely, celiac disease, bacterial overgrowth, infectious enteritis, Crohn’s ileitis, and radiation damage, which affect digestion and/or absorption more diffusely, produce anemia, dehydration, electrolyte disorders, or malnutrition. Gastric hypersecretory conditions such as Zollinger-Ellison syndrome damage the intestinal mucosa, impair pancreatic enzyme activation, and accelerate transit due to excess gastric acid. Biliary obstruction from stricture or neoplasm impairs fat digestion. Impaired pancreatic enzyme release in chronic pancreatitis or pancreatic cancer decreases intraluminal digestion and can lead to malnutrition.
Altered Secretion Selected GI diseases result from dysregulation of gut secretion. Gastric acid hypersecretion occurs in Zollinger-Ellison syndrome, G cell hyperplasia, retained antrum syndrome, and some individuals with duodenal ulcers. Conversely, patients with atrophic gastritis or pernicious anemia release little or no gastric acid. Inflammatory and infectious small-intestinal and colonic diseases produce fluid loss through impaired absorption or enhanced secretion. Common intestinal and colonic hypersecretory conditions cause diarrhea and include acute bacterial or viral infection, chronic Giardia or cryptosporidia infections, small-intestinal bacterial overgrowth, bile salt diarrhea, microscopic colitis, diabetic diarrhea, and abuse of certain laxatives. Less common causes include large colonic villus adenomas and endocrine neoplasias with tumor overproduction of secretagogue transmitters like vasoactive intestinal polypeptide.
Altered Gut Transit Impaired gut transit may be secondary to mechanical obstruction. Esophageal occlusion often results from acid-induced stricture or neoplasm. Gastric outlet obstruction develops from peptic ulcer disease or gastric cancer. Small-intestinal obstruction most commonly results from adhesions but may also occur with Crohn’s disease, radiation- or drug-induced strictures, and less likely malignancy. The most common cause of colonic obstruction is colon cancer, although inflammatory strictures develop in patients with inflammatory bowel disease, after certain infections such as diverticulitis, or with some drugs.
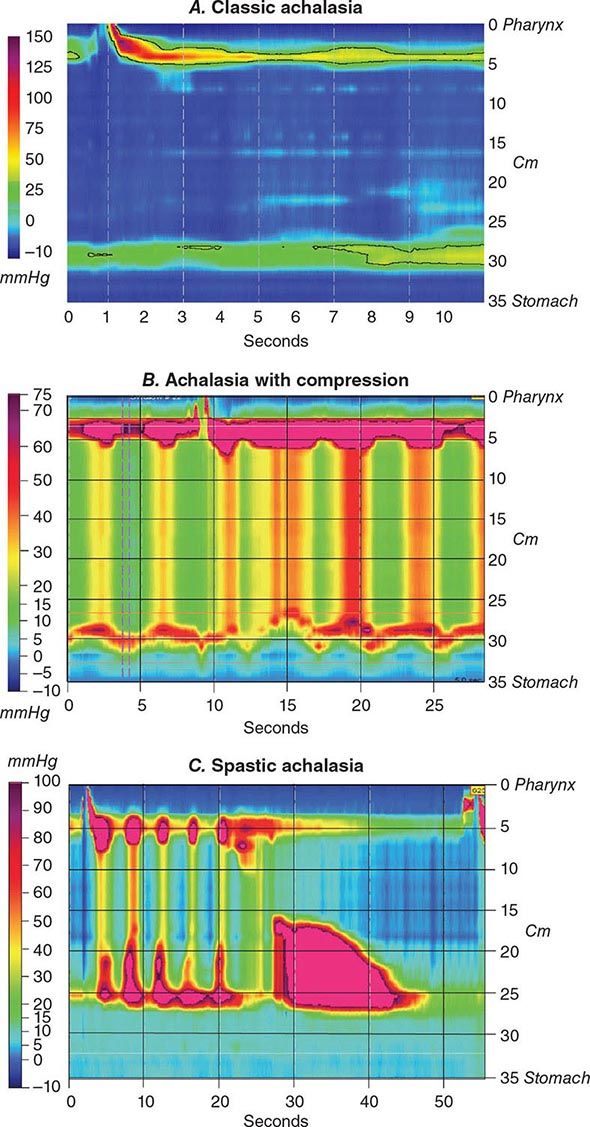
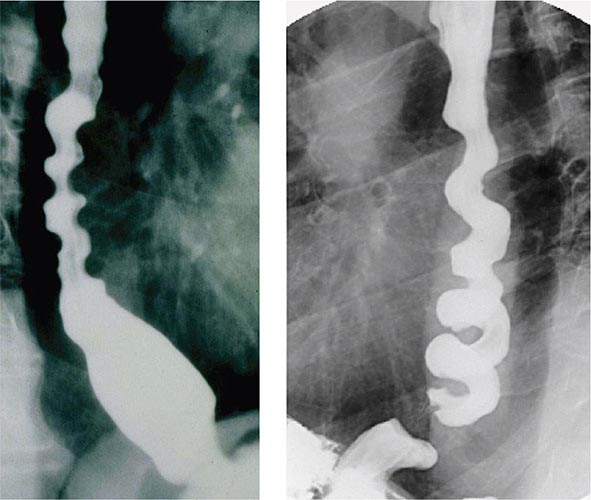
Retardation of propulsion also develops from disordered motor function. Achalasia is characterized by impaired esophageal body peristalsis and incomplete lower esophageal sphincter relaxation. Gastroparesis is the symptomatic delay in gastric emptying of meals due to impaired gastric motility. Intestinal pseudoobstruction causes marked delays in small-bowel transit due to enteric nerve or intestinal smooth-muscle injury. Slow-transit constipation is produced by diffusely impaired colonic propulsion. Constipation also is produced by outlet abnormalities such as rectal prolapse, intussusception, or dyssynergia—a failure of anal or puborectalis relaxation upon attempted defecation.
Disorders of rapid propulsion are less common than those with delayed transit. Rapid gastric emptying occurs in postvagotomy dumping syndrome, with gastric hypersecretion, and in some cases of functional dyspepsia and cyclic vomiting syndrome. Exaggerated intestinal or colonic motor patterns may be responsible for diarrhea in irritable bowel syndrome. Accelerated transit with hyperdefecation is noted in hyperthyroidism.
Immune Dysregulation Many inflammatory GI conditions are consequences of altered gut immune function. The mucosal inflammation of celiac disease results from dietary ingestion of gluten-containing grains. Some patients with food allergy also exhibit altered immune populations. Eosinophilic esophagitis and eosinophilic gastroenteritis are inflammatory disorders with prominent mucosal eosinophils. Ulcerative colitis and Crohn’s disease are disorders of uncertain etiology that produce mucosal injury primarily in the lower gut. The microscopic colitides, lymphocytic and collagenous colitis, exhibit colonic subepithelial infiltrates without visible mucosal damage. Bacterial, viral, and protozoal organisms may produce ileitis or colitis in selected patient populations.
Impaired Gut Blood Flow Different GI regions are at variable risk for ischemic damage from impaired blood flow. Rare cases of gastroparesis result from blockage of the celiac and superior mesenteric arteries. More commonly encountered are intestinal and colonic ischemia that are consequences of arterial embolus, arterial thrombosis, venous thrombosis, or hypoperfusion from dehydration, sepsis, hemorrhage, or reduced cardiac output. These may produce mucosal injury, hemorrhage, or even perforation. Chronic ischemia may result in intestinal stricture. Some cases of radiation enterocolitis exhibit reduced mucosal blood flow.
Neoplastic Degeneration All GI regions are susceptible to malignant degeneration to varying degrees. In the United States, colorectal cancer is most common and usually presents after age 50 years. Worldwide, gastric cancer is prevalent especially in certain Asian regions. Esophageal cancer develops with chronic acid reflux or after an extensive alcohol or tobacco use history. Small-intestinal neoplasms are rare and occur with underlying inflammatory disease. Anal cancers arise after prior anal infection or inflammation. Pancreatic and biliary cancers elicit severe pain, weight loss, and jaundice and have poor prognoses. Hepatocellular carcinoma usually arises in the setting of chronic viral hepatitis or cirrhosis secondary to other causes. Most GI cancers exhibit carcinomatous histology; however, lymphomas and other cell types also are observed.
Disorders Without Obvious Organic Abnormalities The most common GI disorders show no abnormalities on biochemical or structural testing and include irritable bowel syndrome, functional dyspepsia, functional chest pain, and functional heartburn. These disorders exhibit altered gut motor function; however, the pathogenic relevance of these abnormalities is uncertain. Exaggerated visceral sensory responses to noxious stimulation may cause discomfort in these disorders. Symptoms in other patients result from altered processing of visceral pain sensations in the central nervous system. Functional bowel patients with severe symptoms may exhibit significant emotional disturbances on psychometric testing. Subtle immunologic defects may contribute to functional symptoms as well.
Genetic Influences Although many GI diseases result from environmental factors, others exhibit hereditary components. Family members of inflammatory bowel disease patients show a genetic predisposition to disease development themselves. Colonic and esophageal malignancies arise in certain inherited disorders. Rare genetic dysmotility syndromes are described. Familial clustering is even observed in the functional bowel disorders, although this may be secondary learned familial illness behavior rather than a true hereditary factor.
SYMPTOMS OF GASTROINTESTINAL DISEASE
The most common GI symptoms are abdominal pain, heartburn, nausea and vomiting, altered bowel habits, GI bleeding, and jaundice (Table 344-1). Others are dysphagia, anorexia, weight loss, fatigue, and extraintestinal symptoms.
|
COMMON CAUSES OF COMMON GASTROINTESTINAL (GI) SYMPTOMS |
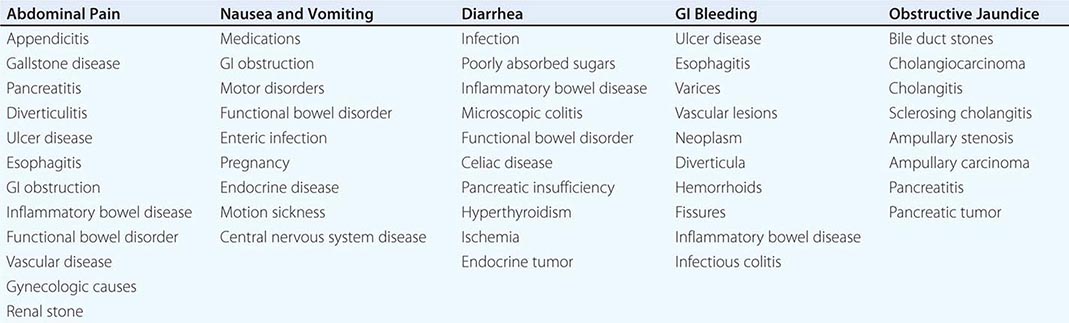
Abdominal Pain Abdominal pain results from GI disease and extra-intestinal conditions involving the genitourinary tract, abdominal wall, thorax, or spine. Visceral pain generally is midline in location and vague in character, whereas parietal pain is localized and precisely described. Common inflammatory diseases with pain include peptic ulcer, appendicitis, diverticulitis, inflammatory bowel disease, and infectious enterocolitis. Other intraabdominal causes of pain include gallstone disease and pancreatitis. Noninflammatory visceral sources include mesenteric ischemia and neoplasia. The most common causes of abdominal pain are irritable bowel syndrome and functional dyspepsia.
Heartburn Heartburn, a burning substernal sensation, is reported intermittently by at least 40% of the population. Classically, heartburn is felt to result from excess gastroesophageal reflux of acid. However, some cases exhibit normal esophageal acid exposure and may result from reflux of nonacidic material or heightened sensitivity of esophageal mucosal nerves.
Nausea and Vomiting Nausea and vomiting are caused by GI diseases, medications, toxins, acute and chronic infection, endocrine disorders, labyrinthine conditions, and central nervous system disease. The best-characterized GI etiologies relate to mechanical obstruction of the upper gut; however, disorders of propulsion including gastroparesis and intestinal pseudoobstruction also elicit prominent symptoms. Nausea and vomiting also are commonly reported by patients with irritable bowel syndrome and functional disorders of the upper gut (including chronic idiopathic nausea and functional vomiting).
Altered Bowel Habits Altered bowel habits are common complaints of patients with GI disease. Constipation is reported as infrequent defecation, straining with defecation, passage of hard stools, or a sense of incomplete fecal evacuation. Causes of constipation include obstruction, motor disorders of the colon, medications, and endocrine diseases such as hypothyroidism and hyperparathyroidism. Diarrhea is reported as frequent defecation, passage of loose or watery stools, fecal urgency, or a similar sense of incomplete evacuation. The differential diagnosis of diarrhea is broad and includes infections, inflammatory causes, malabsorption, and medications. Irritable bowel syndrome produces constipation, diarrhea, or an alternating bowel pattern. Fecal mucus is common in irritable bowel syndrome, whereas pus characterizes inflammatory disease. Steatorrhea develops with malabsorption.
GI Bleeding Hemorrhage may develop from any gut organ. Most commonly, upper GI bleeding presents with melena or hematemesis, whereas lower GI bleeding produces passage of bright red or maroon stools. However, briskly bleeding upper sites can elicit voluminous red rectal bleeding, whereas slowly bleeding ascending colon sites may produce melena. Chronic slow GI bleeding may present with iron deficiency anemia. The most common upper GI causes of bleeding are ulcer disease, gastroduodenitis, and esophagitis. Other etiologies include portal hypertensive causes, malignancy, tears across the gastroesophageal junction, and vascular lesions. The most prevalent lower GI sources of hemorrhage include hemorrhoids, anal fissures, diverticula, ischemic colitis, and arteriovenous malformations. Other causes include neoplasm, inflammatory bowel disease, infectious colitis, drug-induced colitis, and other vascular lesions.
Jaundice Jaundice results from prehepatic, intrahepatic, or posthepatic disease. Posthepatic causes of jaundice include biliary diseases, such as choledocholithiasis, acute cholangitis, primary sclerosing cholangitis, other strictures, and neoplasm, and pancreatic disorders, such as acute and chronic pancreatitis, stricture, and malignancy.
Other Symptoms Other symptoms are manifestations of GI disease. Dysphagia, odynophagia, and unexplained chest pain suggest esophageal disease. A globus sensation is reported with esophagopharyngeal conditions, but also occurs with functional GI disorders. Weight loss, anorexia, and fatigue are nonspecific symptoms of neoplastic, inflammatory, gut motility, pancreatic, small-bowel mucosal, and psychiatric conditions. Fever is reported with inflammatory illness, but malignancies also evoke febrile responses. GI disorders also produce extraintestinal symptoms. Inflammatory bowel disease is associated with hepatobiliary dysfunction, skin and eye lesions, and arthritis. Celiac disease may present with dermatitis herpetiformis. Jaundice can produce pruritus. Conversely, systemic diseases can have GI consequences. Systemic lupus may cause gut ischemia, presenting with pain or bleeding. Overwhelming stress or severe burns may lead to gastric ulcer formation.
EVALUATION OF THE PATIENT WITH GASTROINTESTINAL DISEASE
Evaluation of the patient with GI disease begins with a careful history and examination. Subsequent investigation with a variety of tools designed to test gut structure or function are indicated in selected cases. Some patients exhibit normal findings on diagnostic testing. In these individuals, validated symptom profiles are used to confidently diagnose a functional bowel disorder.
HISTORY
The history of the patient with suspected GI disease has several components. Symptom timing suggests specific etiologies. Symptoms of short duration commonly result from acute infection, toxin exposure, or abrupt inflammation or ischemia. Long-standing symptoms point to underlying chronic inflammatory or neoplastic conditions or functional bowel disorders. Symptoms from mechanical obstruction, ischemia, inflammatory bowel disease, and functional bowel disorders are worsened by meals. Conversely, ulcer symptoms may be relieved by eating or antacids. Symptom patterns and duration may suggest underlying etiologies. Ulcer pain occurs at intermittent intervals lasting weeks to months, whereas biliary colic has a sudden onset and lasts up to several hours. Pain from acute inflammation as with acute pancreatitis is severe and persists for days to weeks. Meals elicit diarrhea in some cases of inflammatory bowel disease and irritable bowel syndrome. Defecation relieves discomfort in inflammatory bowel disease and irritable bowel syndrome. Functional bowel disorders are exacerbated by stress. Sudden awakening from sound sleep suggests organic rather than functional disease. Diarrhea from malabsorption usually improves with fasting, whereas secretory diarrhea persists without oral intake.
Symptom relation to other factors narrows the list of diagnostic possibilities. Obstructive symptoms with prior abdominal surgery raise concern for adhesions, whereas loose stools after gastrectomy or gallbladder excision suggest dumping syndrome or postcholecystectomy diarrhea. Symptom onset after travel prompts a search for enteric infection. Medications may produce pain, altered bowel habits, or GI bleeding. Lower GI bleeding likely results from neoplasms, diverticula, or vascular lesions in an older person and from anorectal abnormalities or inflammatory bowel disease in a younger individual. Celiac disease is prevalent in people of northern European descent, whereas inflammatory bowel disease is more common in certain Jewish populations. A sexual history may raise concern for sexually transmitted diseases or immunodeficiency.
For more than two decades, working groups have been convened to devise symptom criteria to improve the confident diagnosis of functional bowel disorders and to minimize the numbers of unnecessary diagnostic tests performed. The most widely accepted symptom-based criteria are the Rome criteria. When tested against findings of structural investigations, the Rome criteria exhibit diagnostic specificities exceeding 90% for many of the functional bowel disorders.
PHYSICAL EXAMINATION
The physical exam complements information from the history. Abnormal vital signs provide diagnostic clues and determine the need for acute intervention. Fever suggests inflammation or neoplasm. Orthostasis is found with significant blood loss, dehydration, sepsis, or autonomic neuropathy. Skin, eye, or joint findings may point to specific diagnoses. Neck exam with swallowing assessment evaluates dysphagia. Cardiopulmonary disease may present with abdominal pain or nausea; thus lung and cardiac exams are important. Pelvic examination tests for a gynecologic source of abdominal pain. Rectal exam may detect blood, indicating gut mucosal injury or neoplasm or a palpable inflammatory mass in appendicitis. Metabolic conditions and gut motor disorders have associated peripheral neuropathy.
Inspection of the abdomen may reveal distention from obstruction, tumor, or ascites or vascular abnormalities with liver disease. Ecchymoses develop with severe pancreatitis. Auscultation can detect bruits or friction rubs from vascular disease or hepatic tumors. Loss of bowel sounds signifies ileus, whereas high-pitched, hyperactive sounds characterize intestinal obstruction. Percussion assesses liver size and can detect shifting dullness from ascites. Palpation assesses for hepatosplenomegaly as well as neoplastic or inflammatory masses. Abdominal exam is helpful in evaluating unexplained pain. Intestinal ischemia elicits severe pain but little tenderness. Patients with visceral pain may exhibit generalized discomfort, whereas those with parietal pain or peritonitis have directed pain, often with involuntary guarding, rigidity, or rebound. Patients with musculoskeletal abdominal wall pain may note tenderness exacerbated by Valsalva or straight-leg lift maneuvers.
TOOLS FOR PATIENT EVALUATION
Laboratory, radiographic, and functional tests can assist in diagnosis of suspected GI disease. The GI tract also is amenable to internal evaluation with upper and lower endoscopy and to examination of luminal contents. Histopathologic exams of GI tissues complement these tests.
Laboratory Selected laboratory tests facilitate the diagnosis of GI disease. Iron-deficiency anemia suggests mucosal blood loss, whereas vitamin B12 deficiency results from small-intestinal, gastric, or pancreatic disease. Either also can result from inadequate oral intake. Leukocytosis and increased sedimentation rates and C-reactive protein levels are found in inflammatory conditions, whereas leukopenia is seen in viremic illness. Severe vomiting or diarrhea elicits electrolyte disturbances, acid-base abnormalities, and elevated blood urea nitrogen. Pancreaticobiliary or liver disease is suggested by elevated pancreatic or liver chemistries. Thyroid chemistries, cortisol, and calcium levels are obtained to exclude endocrinologic causes of GI symptoms. Pregnancy testing is considered for women with unexplained nausea. Serologic tests can screen for celiac disease, inflammatory bowel disease, rheumatologic diseases like lupus or scleroderma, and paraneoplastic dysmotility syndromes. Hormone levels are obtained for suspected endocrine neoplasia. Intraabdominal malignancies produce other tumor markers including the carcinoembryonic antigen CA 19-9 and α-fetoprotein. Blood testing also monitors medication therapy in some diseases, as with thiopurine metabolite levels in inflammatory bowel disease. Other body fluids are sampled under certain circumstances. Ascitic fluid is analyzed for infection, malignancy, or findings of portal hypertension. Cerebrospinal fluid is obtained for suspected central nervous system causes of vomiting. Urine samples screen for carcinoid, porphyria, and heavy metal intoxication.
Luminal Contents Luminal contents can be examined for diagnostic clues. Stool samples are cultured for bacterial pathogens, examined for leukocytes and parasites, or tested for Giardia antigen. Duodenal aspirates can be examined for parasites or cultured for bacterial overgrowth. Fecal fat is quantified in possible malabsorption. Stool electrolytes can be measured in diarrheal conditions. Laxative screens are done when laxative abuse is suspected. Gastric acid is quantified to rule out Zollinger-Ellison syndrome. Esophageal pH testing is done for refractory symptoms of acid reflux, whereas impedance techniques assess for nonacidic reflux. Pancreatic juice is analyzed for enzyme or bicarbonate content to exclude pancreatic exocrine insufficiency.
Endoscopy The gut is accessible with endoscopy, which can provide the diagnosis of the causes of bleeding, pain, nausea and vomiting, weight loss, altered bowel function, and fever. Table 344-2 lists the most common indications for the major endoscopic procedures. Upper endoscopy evaluates the esophagus, stomach, and duodenum, whereas colonoscopy assesses the colon and distal ileum. Upper endoscopy is advocated as the initial structural test performed in patients with suspected ulcer disease, esophagitis, neoplasm, malabsorption, and Barrett’s metaplasia because of its ability to directly visualize as well as biopsy the abnormality. Colonoscopy is the procedure of choice for colon cancer screening and surveillance as well as diagnosis of colitis secondary to infection, ischemia, radiation, and inflammatory bowel disease. Sigmoidoscopy examines the colon up to the splenic flexure and is currently used to exclude distal colonic inflammation or obstruction in young patients not at significant risk for colon cancer. For elusive GI bleeding secondary to arteriovenous malformations or superficial ulcers, small-intestinal examination is performed with push enteroscopy, capsule endoscopy, or double-balloon enteroscopy. Capsule endoscopy also can visualize small-intestinal Crohn’s disease in individuals with negative barium radiography. Endoscopic retrograde cholangiopancreaticography (ERCP) provides diagnoses of pancreatic and biliary disease. Endoscopic ultrasound is useful for evaluating extent of disease in GI malignancy as well as exclusion of choledocholithiasis, evaluation of pancreatitis, drainage of pancreatic pseudocysts, and assessment of anal continuity.
|
COMMON INDICATIONS FOR ENDOSCOPY |
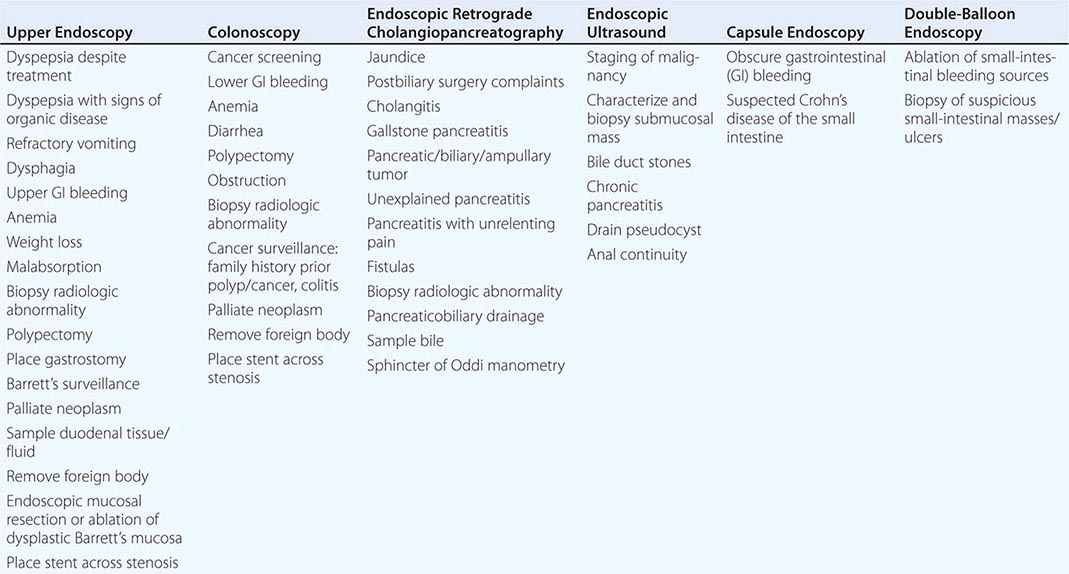
Radiography/Nuclear Medicine Radiographic tests evaluate diseases of the gut and extraluminal structures. Oral or rectal contrast agents like barium provide mucosal definition from the esophagus to the rectum. Contrast radiography also assesses gut transit and pelvic floor dysfunction. Barium swallow is the initial procedure for evaluation of dysphagia to exclude subtle rings or strictures and assess for achalasia, whereas small-bowel contrast radiology reliably diagnoses intestinal tumors and Crohn’s ileitis. Contrast enemas are performed when colonoscopy is unsuccessful or contraindicated. Ultrasound and computed tomography (CT) evaluate regions not accessible by endoscopy or contrast studies, including the liver, pancreas, gallbladder, kidneys, and retroperitoneum. These tests are useful for diagnosis of mass lesions, fluid collections, organ enlargement, and, in the case of ultrasound, gallstones. CT and magnetic resonance (MR) colonography are being evaluated as alternatives to colonoscopy for colon cancer screening. MR imaging assesses the pancreaticobiliary ducts to exclude neoplasm, stones, and sclerosing cholangitis, and the liver to characterize benign and malignant tumors. Specialized CT or MR enterography can assess intensity of inflammatory bowel disease. Angiography excludes mesenteric ischemia and determines spread of malignancy. Angiographic techniques also access the biliary tree in obstructive jaundice. CT and MR techniques can be used to screen for mesenteric occlusion, thereby limiting exposure to angiographic dyes. Positron emission tomography can facilitate distinguishing malignant from benign disease in several organ systems.
Scintigraphy both evaluates structural abnormalities and quantifies luminal transit. Radionuclide bleeding scans localize bleeding sites in patients with brisk hemorrhage so that therapy with endoscopy, angiography, or surgery may be directed. Radiolabeled leukocyte scans can search for intraabdominal abscesses not visualized on CT. Biliary scintigraphy is complementary to ultrasound in the assessment of cholecystitis. Scintigraphy to quantify esophageal and gastric emptying is well established, whereas techniques to measure small-intestinal or colonic transit are less widely used.
Histopathology Gut mucosal biopsies obtained at endoscopy evaluate for inflammatory, infectious, and neoplastic disease. Deep rectal biopsies assist with diagnosis of Hirschsprung’s disease or amyloid. Liver biopsy is indicated in cases with abnormal liver chemistries, in unexplained jaundice, following liver transplant to exclude rejection, and to characterize the degree of inflammation in patients with chronic viral hepatitis prior to initiating antiviral therapy. Biopsies obtained during CT or ultrasound can evaluate for other intraabdominal conditions not accessible by endoscopy.
Functional Testing Tests of gut function provide important data when structural testing is nondiagnostic. In addition to gastric acid and pancreatic function testing, functional testing of motor activity is provided by manometric techniques. Esophageal manometry is useful for suspected achalasia, whereas small-intestinal manometry tests for pseudoobstruction. A wireless motility capsule is now available to measure transit and contractile activity in the stomach, small intestine, and colon in a single test. Anorectal manometry with balloon expulsion testing is used for unexplained incontinence or constipation from outlet dysfunction. Anorectal manometry and electromyography also assess anal function in fecal incontinence. Biliary manometry tests for sphincter of Oddi dysfunction with unexplained biliary pain. Measurement of breath hydrogen while fasting and after oral mono- or oligosaccharide challenge can screen for carbohydrate intolerance and small-intestinal bacterial overgrowth.
345 |
Gastrointestinal Endoscopy |
Gastrointestinal endoscopy has been attempted for over 200 years, but the introduction of semirigid gastroscopes in the middle of the twentieth century marked the dawn of the modern endoscopic era. Since then, rapid advances in endoscopic technology have led to dramatic changes in the diagnosis and treatment of many digestive diseases. Innovative endoscopic devices and new endoscopic treatment modalities continue to expand the use of endoscopy in patient care.
Current flexible endoscopes provide an electronic video image generated by a charge-coupled device in the tip of the endoscope. Operator controls permit deflection of the endoscope tip; fiberoptic bundles or light-emitting diodes bring light to the tip of the endoscope; and working channels allow washing, suctioning, and the passage of instruments. Progressive changes in the diameter and stiffness of endoscopes have improved the ease and patient tolerance of endoscopy.
ENDOSCOPIC PROCEDURES
UPPER ENDOSCOPY
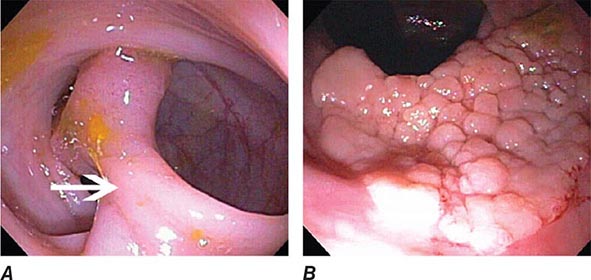
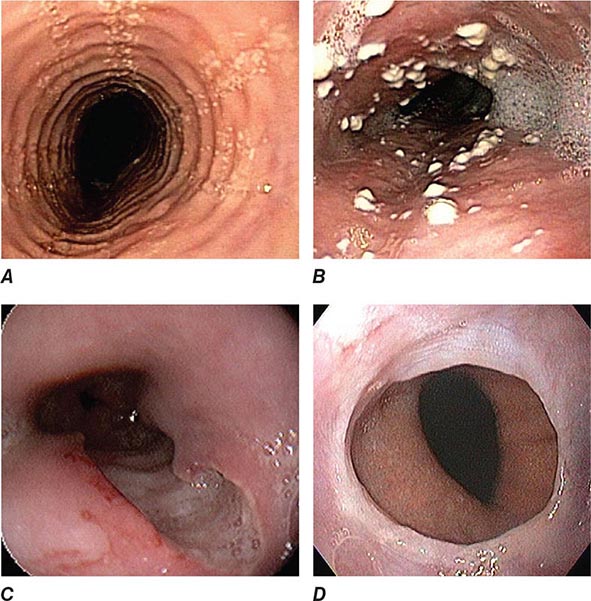
Upper endoscopy, also referred to as esophagogastroduodenoscopy (EGD), is performed by passing a flexible endoscope through the mouth into the esophagus, stomach, and duodenum. The procedure is the best method for examining the upper gastrointestinal mucosa. While the upper gastrointestinal radiographic series has similar accuracy for diagnosis of duodenal ulcer (Fig. 345-1), EGD is superior for detection of gastric ulcers (Fig. 345-2) and flat mucosal lesions such as Barrett’s esophagus (Fig. 345-3), and it permits directed biopsy and endoscopic therapy. Intravenous conscious sedation is given to most patients in the United States to ease the anxiety and discomfort of the procedure, although in many countries EGD is routinely performed with topical pharyngeal anesthesia only. Patient tolerance of unsedated EGD is improved by the use of an ultrathin, 5-mm diameter endoscope that can be passed transorally or transnasally.
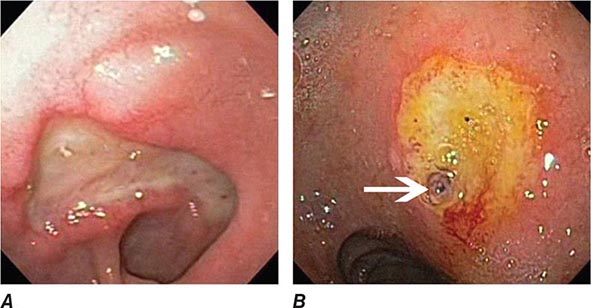
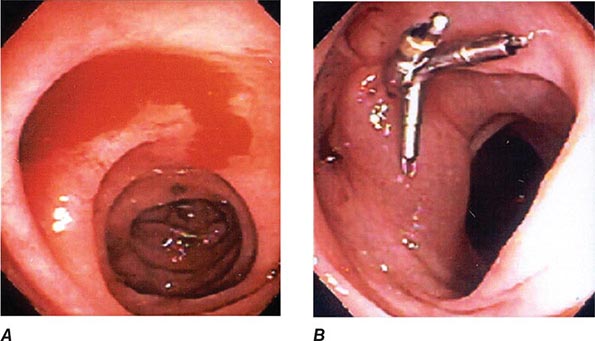
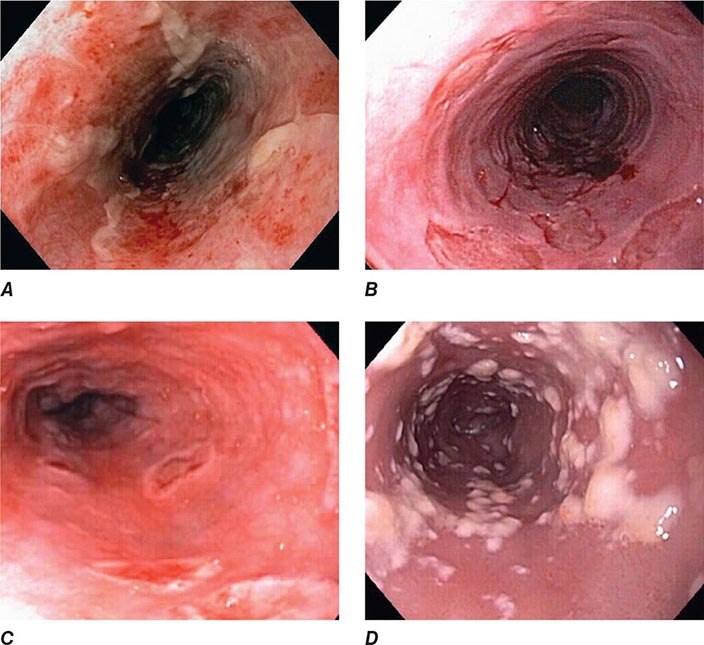
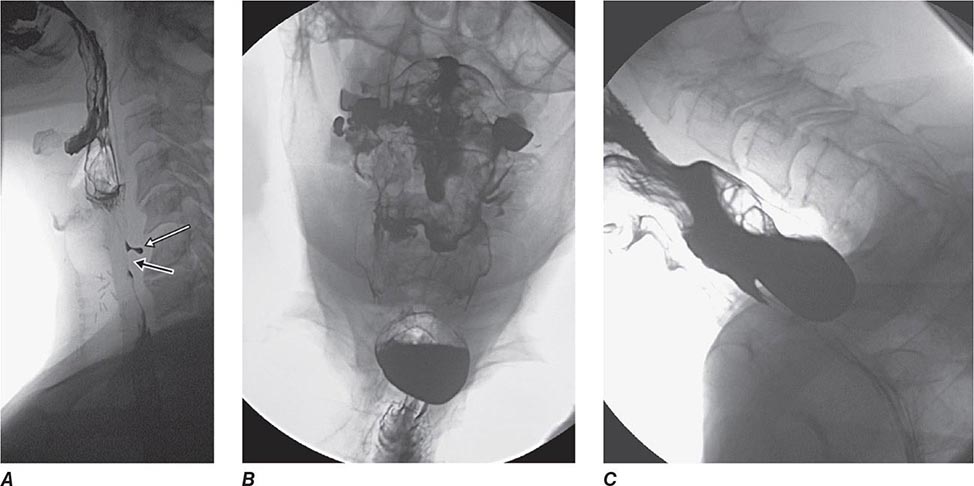
FIGURE 345-1 Duodenal ulcers. A. Ulcer with a clean base. B. Ulcer with a visible vessel (arrow) in a patient with recent hemorrhage.
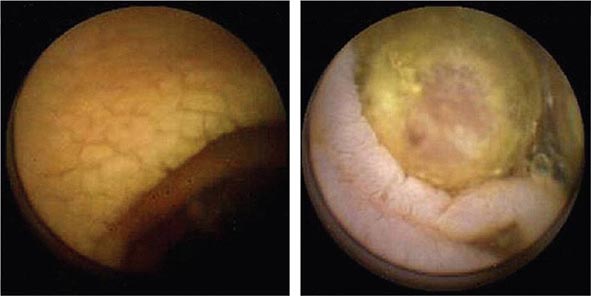
FIGURE 345-2 Gastric ulcers. A. Benign gastric ulcer. B. Malignant gastric ulcer involving greater curvature of stomach.
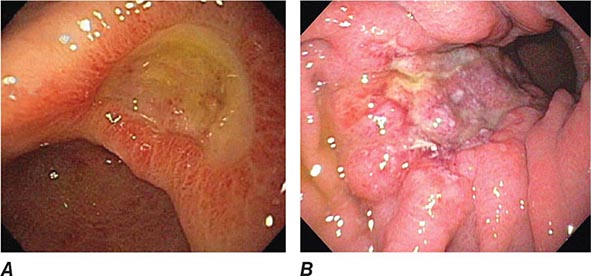
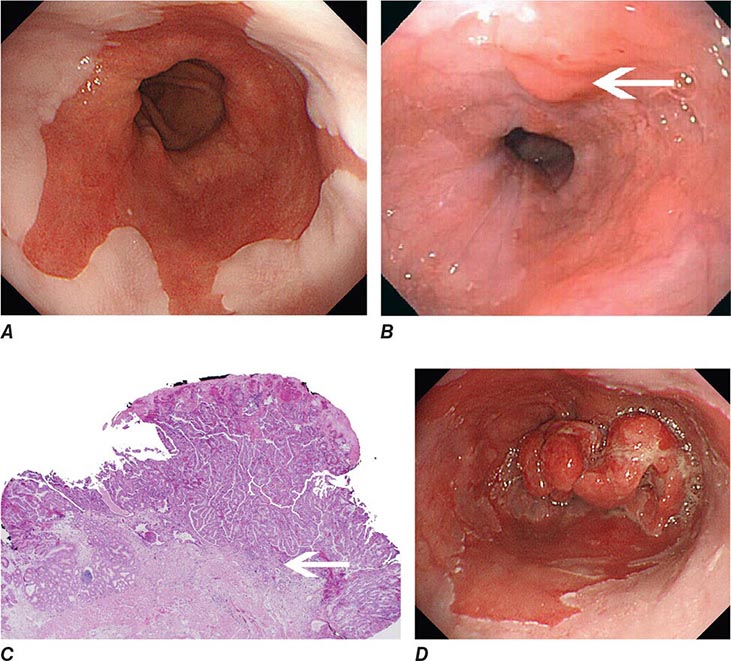
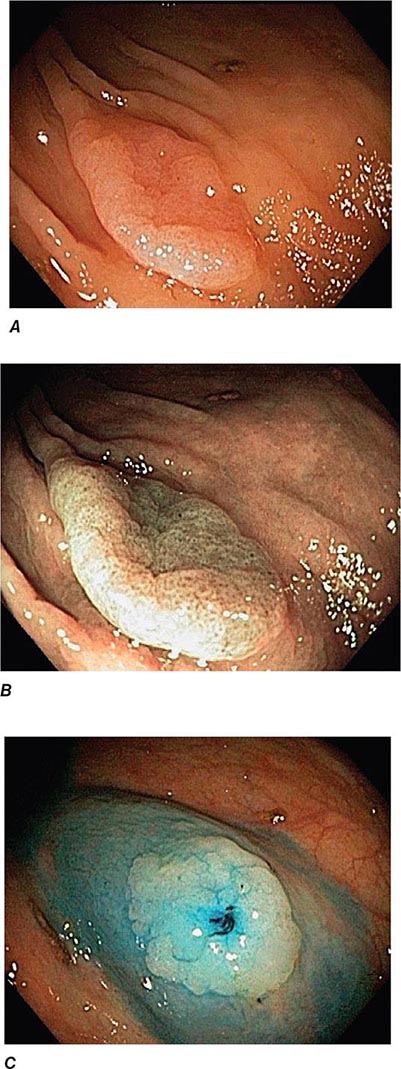
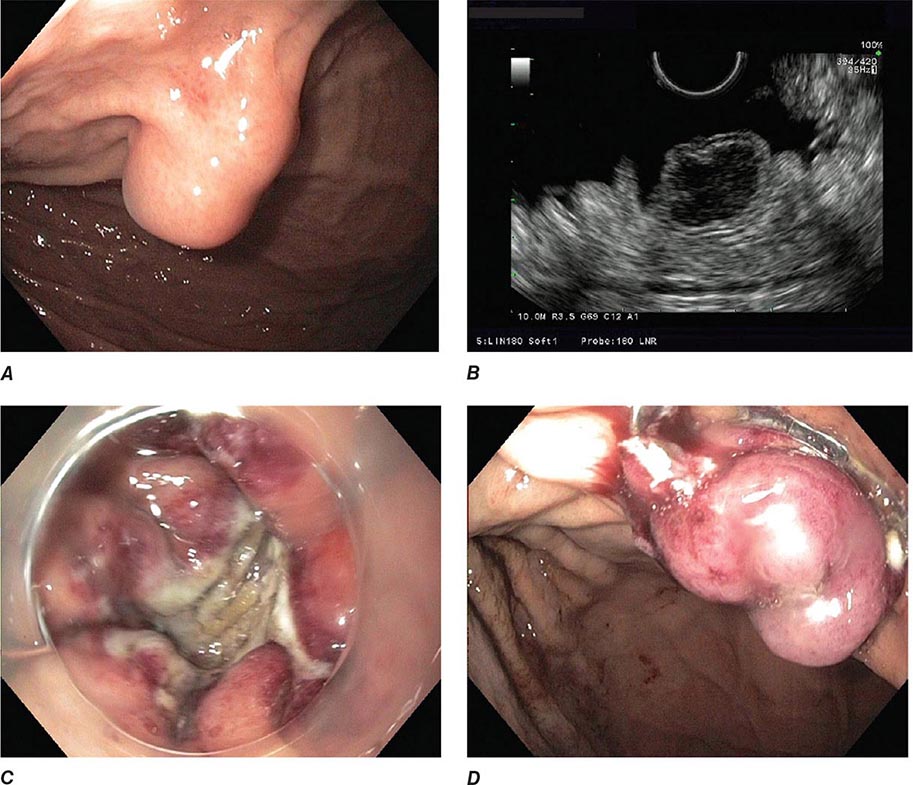
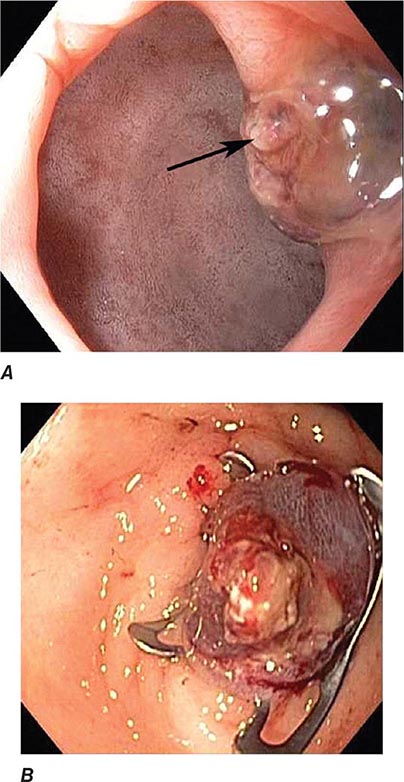
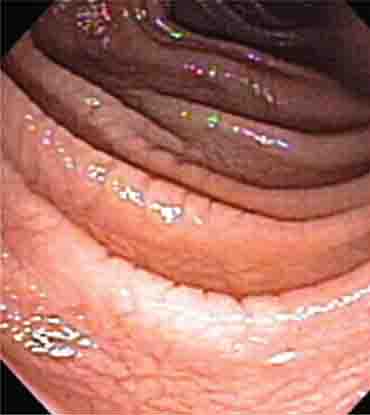
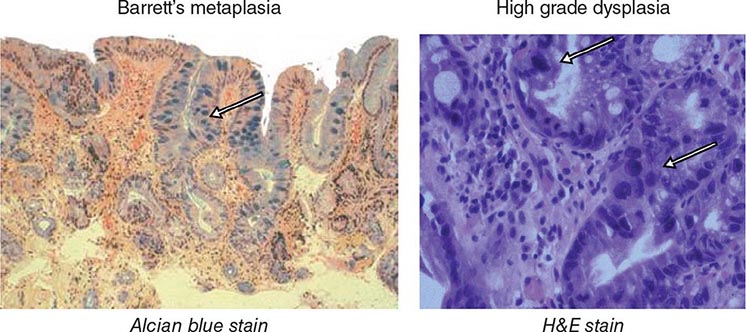
FIGURE 345-3 Barrett’s esophagus. A. Pink tongues of Barrett’s mucosa extending proximally from the gastroesophageal junction. B. Barrett’s esophagus with a suspicious nodule (arrow) identified during endoscopic surveillance. C. Histologic finding of intramucosal adenocarcinoma in the endoscopically resected nodule. Tumor extends into the esophageal submucosa (arrow). D. Barrett’s esophagus with locally advanced adenocarcinoma.
COLONOSCOPY
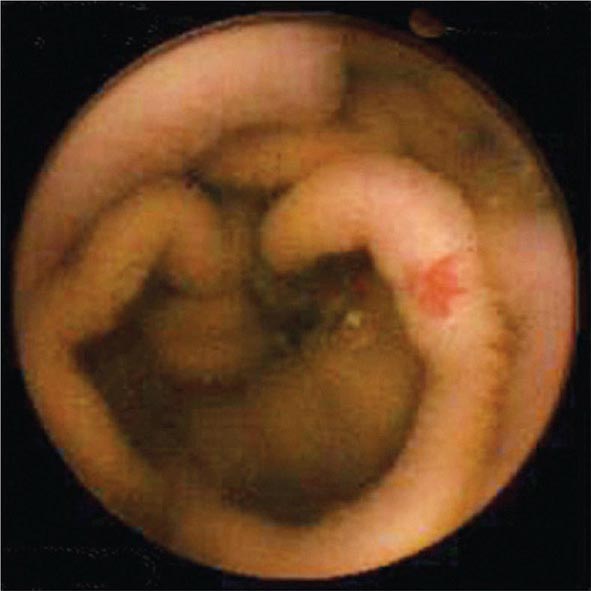
Colonoscopy is performed by passing a flexible colonoscope through the anal canal into the rectum and colon. The cecum is reached in >95% of cases, and the terminal ileum can often be examined. Colonoscopy is the gold standard for imaging the colonic mucosa. Colonoscopy has greater sensitivity than barium enema for colitis (Fig. 345-4), polyps (Fig. 345-51), and cancer (Fig. 345-6). Computed tomography (CT) colonography is an emerging technology that rivals the accuracy of colonoscopy for detection of some polyps and cancer, although it may not be sensitive for the detection of flat lesions, such as serrated polyps (Fig. 345-7). Conscious sedation is usually given before colonoscopy in the United States, although a willing patient and a skilled examiner can complete the procedure without sedation in many cases.
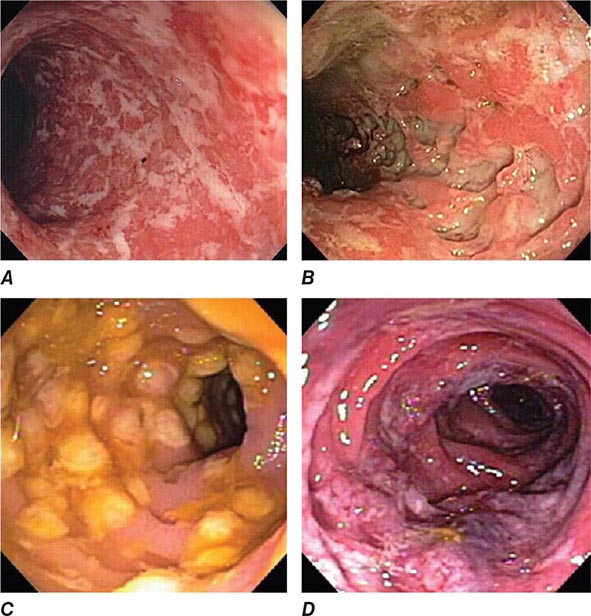
FIGURE 345-4 Causes of colitis. A. Chronic ulcerative colitis with diffuse ulcerations and exudates. B. Severe Crohn’s colitis with deep ulcers. C. Pseudomembranous colitis with yellow, adherent pseudomembranes. D. Ischemic colitis with patchy mucosal edema, subepithelial hemorrhage, and cyanosis.
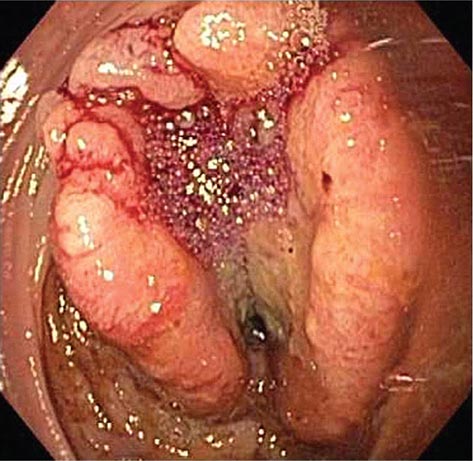
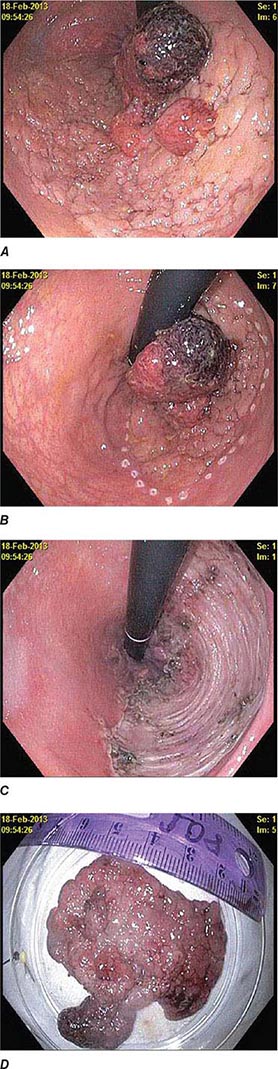
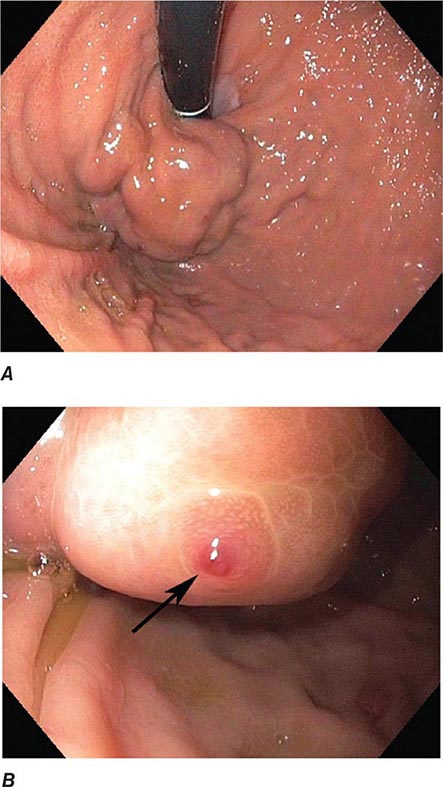
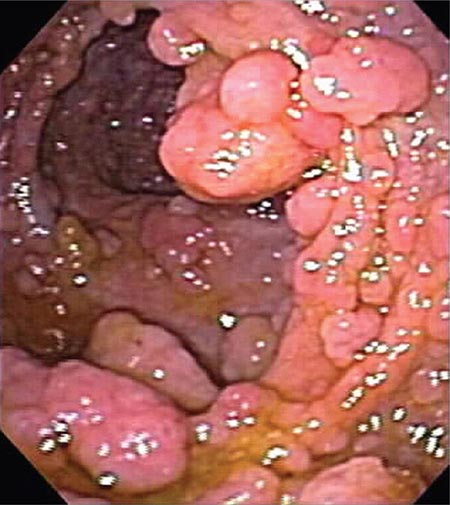
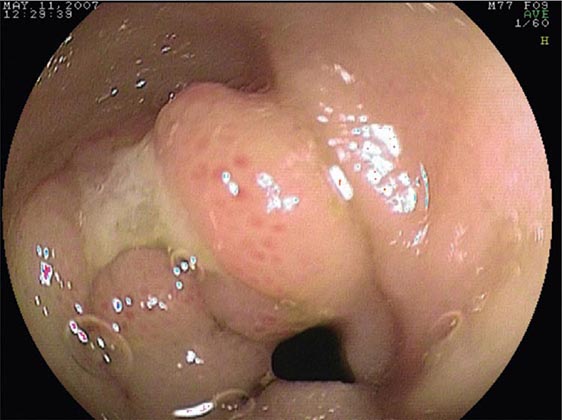
FIGURE 345-5 Colonic polyps. A. Pedunculated colon polyp on a thick stalk covered with normal mucosa (arrow). B. Sessile rectal polyp.
FIGURE 345-6 Colon adenocarcinoma growing into the lumen.
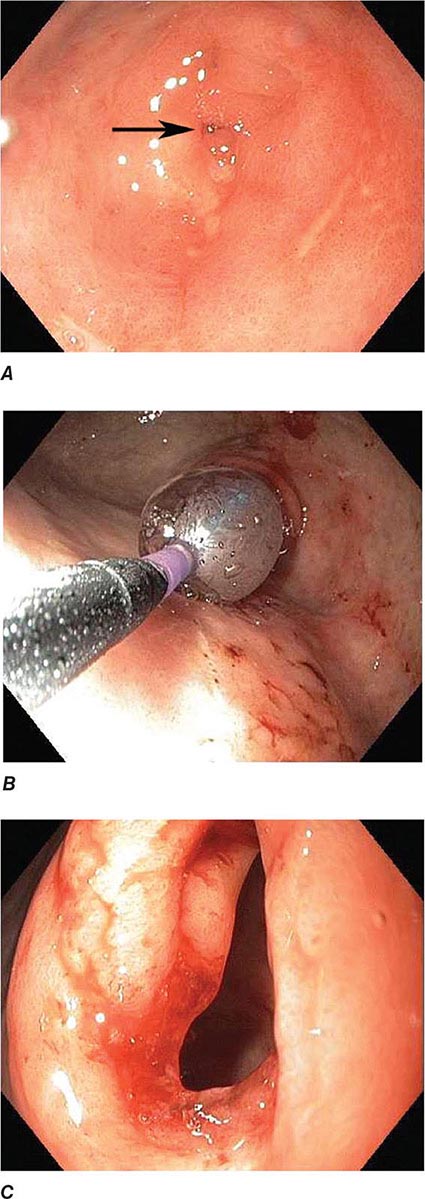
FIGURE 345-7 Flat serrated polyp in the cecum. A. Appearance of the lesion under conventional white-light imaging. B. Mucosal patterns and boundary of the lesion enhanced with narrow band imaging. C. Submucosal lifting of the lesion with dye (methylene blue) injection prior to resection.
FLEXIBLE SIGMOIDOSCOPY
Flexible sigmoidoscopy is similar to colonoscopy, but visualizes only the rectum and a variable portion of the left colon, typically to 60 cm from the anal verge. This procedure causes abdominal cramping, but it is brief and is usually performed without sedation. Flexible sigmoidoscopy is primarily used for evaluation of diarrhea and rectal outlet bleeding.
SMALL-BOWEL ENDOSCOPY
Three endoscopic techniques are currently used to evaluate the small intestine, most often in patients presenting with presumed small-bowel bleeding. For capsule endoscopy, the patient swallows a disposable capsule that contains a complementary metal oxide silicon (CMOS) chip camera. Color still images (Fig. 345-8) are transmitted wirelessly to an external receiver at several frames per second until the capsule’s battery is exhausted or it is passed into the toilet. Capsule endoscopy enables visualization of the small-bowel mucosa beyond the reach of a conventional endoscope and, at present, is solely a diagnostic procedure.
FIGURE 345-8 Capsule endoscopy image of jejunal vascular ectasia.
Push enteroscopy is performed with a long endoscope similar in design to an upper endoscope. The enteroscope is pushed down the small bowel, sometimes with the help of a stiffening overtube that extends from the mouth to the small intestine. The proximal to mid-jejunum is usually reached, and the instrument channel of the endoscope allows for biopsy or endoscopic therapy.
Deeper insertion into the small bowel can be accomplished by single– or double-balloon enteroscopy or spiral enteroscopy (Fig. 345-9). These instruments enable pleating of the small intestine onto an overtube (see Video 346e-1). With balloon-assisted enteroscopy, the entire intestinal tract can be visualized in some patients when both the oral and anal routes of insertion are used. Biopsies and endoscopic therapy can be performed throughout the visualized small bowel (Fig. 345-10).
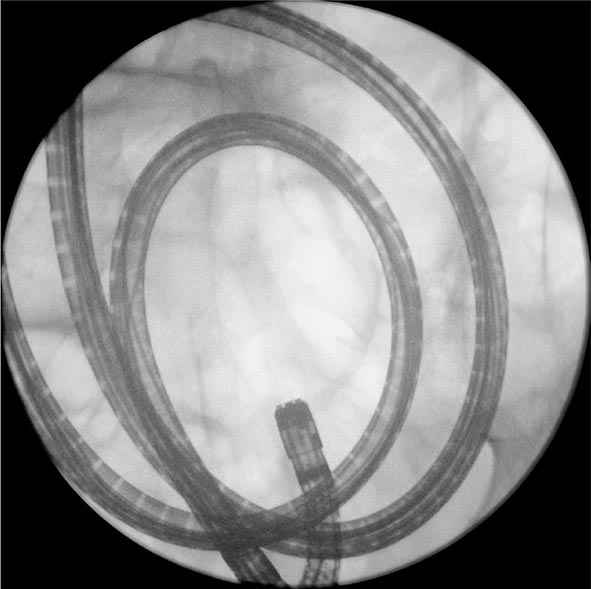
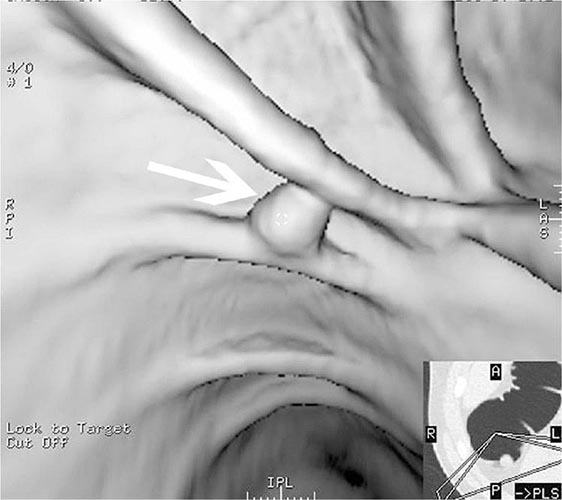
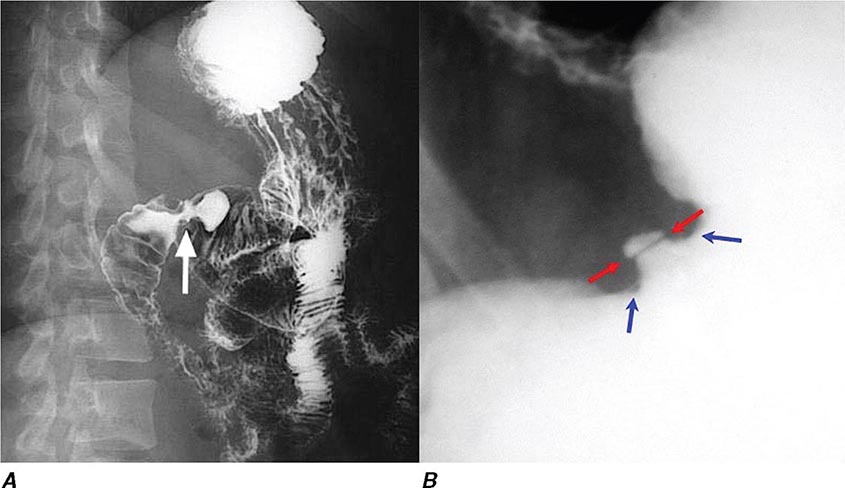
FIGURE 345-9 Radiograph of a double-balloon enteroscope in the small intestine.
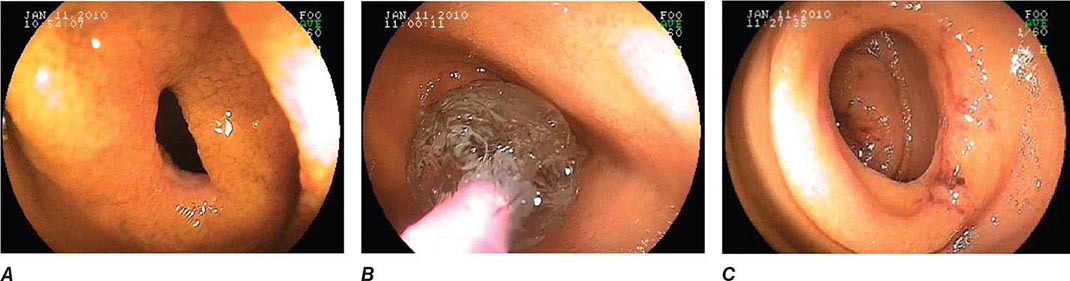
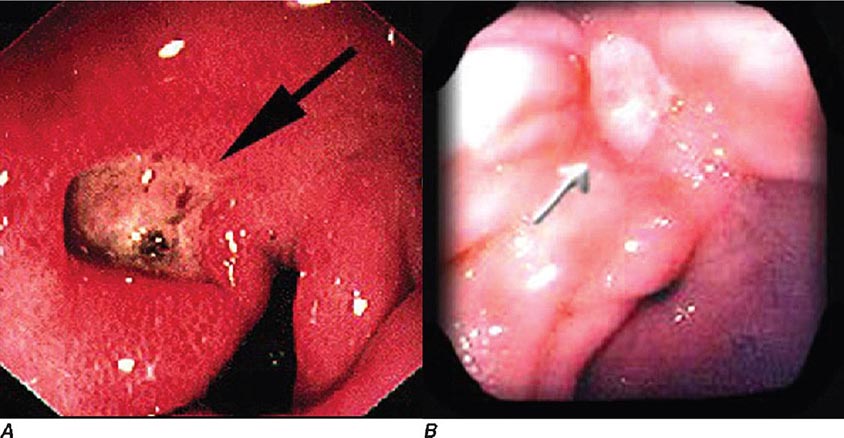
FIGURE 345-10 Nonsteroidal anti-inflammatory drug (NSAID)–induced proximal ileal stricture diagnosed by double-balloon endoscopy. A. Ileal stricture causing obstructive symptoms. B. Balloon dilatation of the ileal stricture. C. Appearance of stricture after dilatation.
ENDOSCOPIC RETROGRADE CHOLANGIOPANCREATOGRAPHY (ERCP)
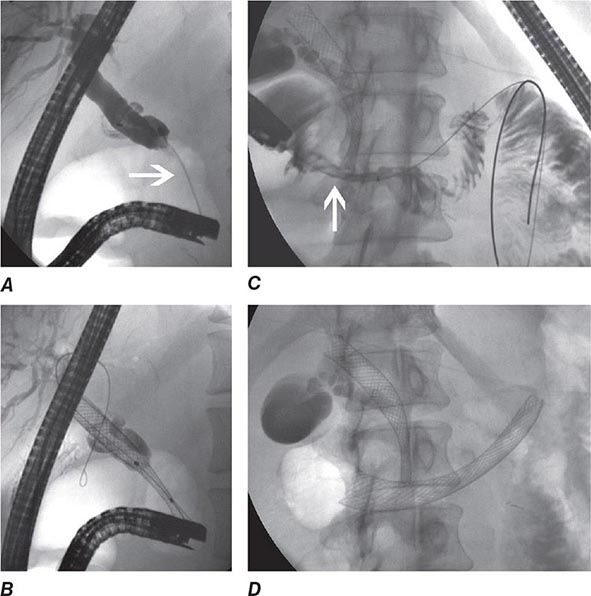
During ERCP a side-viewing endoscope is passed through the mouth to the duodenum, the ampulla of Vater is identified and cannulated with a thin plastic catheter, and radiographic contrast material is injected into the bile duct and pancreatic duct under fluoroscopic guidance (Fig. 345-11). When indicated, the sphincter of Oddi can be opened using the technique of endoscopic sphincterotomy (Fig. 345-12). Stones can be retrieved from the ducts (see Video 346e-15), biopsies can be performed, strictures can be dilated and/or stented (Fig. 345-13), and ductal leaks can be stented (Fig. 345-14). ERCP is often performed for therapy but remains important in diagnosis, especially for sphincter of Oddi dysfunction and for tissue sampling of ductal strictures.
FIGURE 345-11 Endoscopic retrograde cholangiopancreatography (ERCP) for bile duct stones with cholangitis. A. Faceted bile duct stones are demonstrated in the common bile duct. B. After endoscopic sphincterotomy, the stones are extracted with a Dormia basket. A small abscess communicates with the left hepatic duct.
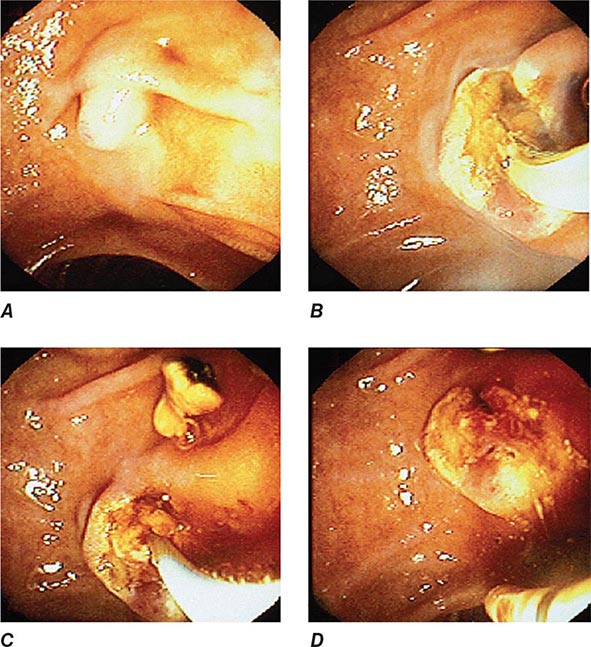
FIGURE 345-12 Endoscopic sphincterotomy. A. A normal-appearing ampulla of Vater. B. Sphincterotomy is performed with electrocautery. C. Bile duct stones are extracted with a balloon catheter. D. Final appearance of the sphincterotomy.
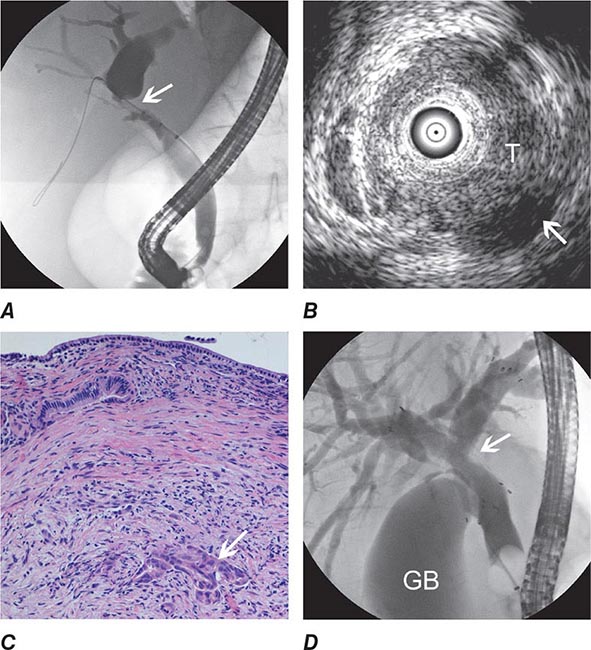
FIGURE 345-13 Endoscopic diagnosis, staging, and palliation of hilar cholangiocarcinoma. A. Endoscopic retrograde cholangiopancreatography (ERCP) in a patient with obstructive jaundice demonstrates a malignant-appearing stricture of the biliary confluence extending into the left and right intrahepatic ducts. B. Intraductal ultrasound of the biliary stricture demonstrates marked bile duct wall thickening due to tumor (T) with partial encasement of the hepatic artery (arrow). C. Intraductal biopsy obtained during ERCP demonstrates malignant cells infiltrating the submucosa of the bile duct wall (arrow). D. Endoscopic placement of bilateral self-expanding metal stents (arrow) relieves the biliary obstruction. GB, gallbladder. (Image C courtesy of Dr. Thomas Smyrk; with permission.)
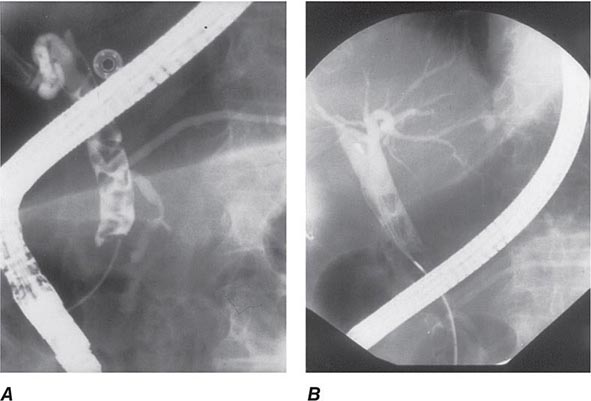
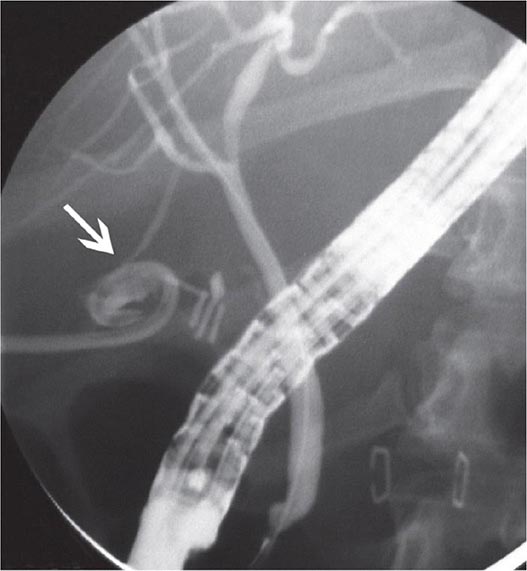
FIGURE 345-14 Bile leak (arrow) from a duct of Luschka after laparoscopic cholecystectomy. Contrast leaks from a small right intrahepatic duct into the gallbladder fossa and then flows into the pigtail of a percutaneous drainage catheter.
ENDOSCOPIC ULTRASOUND (EUS)
EUS utilizes high-frequency ultrasound transducers incorporated into the tip of a flexible endoscope. Ultrasound images are obtained of the gut wall and adjacent organs, vessels, and lymph nodes. By sacrificing depth of ultrasound penetration and bringing the ultrasound transducer close to the area of interest via endoscopy, high-resolution images are obtained. EUS provides the most accurate preoperative local staging of esophageal, pancreatic, and rectal malignancies (Fig. 345-15), although it does not detect most distant metastases. EUS is also useful for diagnosis of bile duct stones, gallbladder disease, submucosal gastrointestinal lesions, and chronic pancreatitis. Fine-needle aspirates and core biopsies of masses and lymph nodes in the posterior mediastinum, abdomen, pancreas, retroperitoneum, and pelvis can be obtained under EUS guidance (Fig. 345-16). EUS-guided therapeutic procedures are increasingly performed, including drainage of abscesses, pseudocysts, and pancreatic necrosis into the gut lumen (see Video 346e-2), celiac plexus neurolysis for treatment of pancreatic pain, ethanol ablation of pancreatic neuroendocrine tumors, treatment of gastrointestinal hemorrhage, and drainage of obstructed biliary and pancreatic ducts.
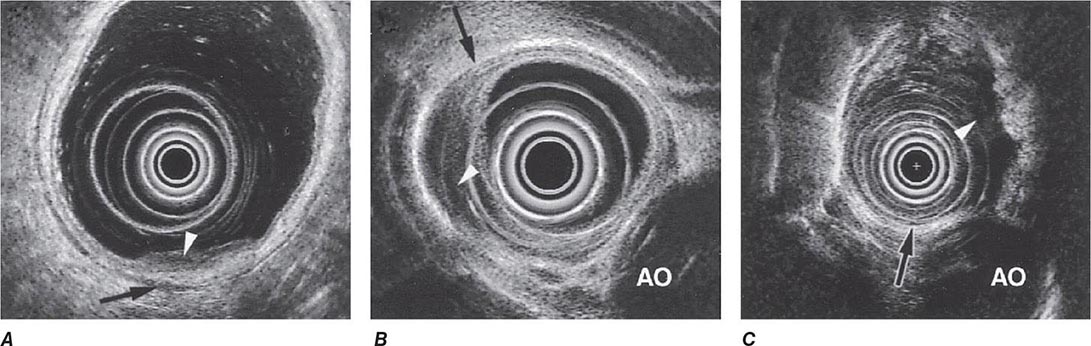
FIGURE 345-15 Local staging of gastrointestinal cancers with endoscopic ultrasound. In each example, the white arrowhead marks the primary tumor and the black arrow indicates the muscularis propria of the intestinal wall. A. T1 gastric cancer. The tumor does not invade the mp. B. T2 esophageal cancer. The tumor invades the muscularis propria. C. T3 esophageal cancer. The tumor extends through the muscularis propria into the surrounding tissue and focally abuts the aorta. AO, aorta.
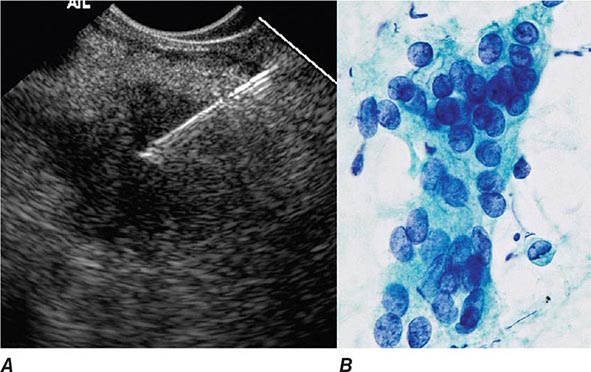
FIGURE 345-16 Endoscopic ultrasound (EUS)–guided fine-needle aspiration (FNA). A. Ultrasound image of a 22-gauge needle passed through the duodenal wall and positioned in a hypoechoic pancreatic head mass. B. Micrograph of aspirated malignant cells. (Image B courtesy of Dr. Michael R. Henry; with permission.)
NATURAL ORIFICE TRANSLUMINAL ENDOSCOPIC SURGERY (NOTES)
NOTES is an evolving collection of endoscopic methods that entail passage of an endoscope or its accessories into or through the wall of the gastrointestinal tract to perform diagnostic or therapeutic interventions. Some NOTES procedures, such as percutaneous endoscopic gastrostomy (PEG) or endoscopic necrosectomy of pancreatic necrosis, are well-established clinical procedures (see Video 346e-2); others, such as per-oral endoscopic myotomy (POEM) and endoscopic full-thickness resection of gastrointestinal mural lesions (Fig. 345-17, see Video 346e-3), are emerging as viable clinical therapeutic options; and still others, such as endoscopic appendectomy, cholecystectomy, and tubal ligation, are in development, and their ultimate clinical application is presently unclear. NOTES is currently an area of intense innovation and endoscopic research.
FIGURE 345-17 Endoscopic full-thickness resection of a gastrointestinal stromal tumor. A. Subepithelial lesion in the proximal stomach. B. Hypoechoic lesion arising from the fourth layer (muscularis propria) at endoscopic ultrasound. C. Full-thickness resection defect. D. Closure of defect using an over-the-scope clip.
ENDOSCOPIC RESECTION AND CLOSURE TECHNIQUES
Endoscopic mucosal resection (EMR) (see Video 346e-4) and endoscopic submucosal dissection (ESD) (Fig. 345-18, see Video 346e-5) are two commonly used techniques for the resection of benign and early-stage malignant gastrointestinal neoplasms. In addition to providing larger specimens for more accurate histopathologic assessment and diagnosis, these techniques can be potentially curative for certain dysplastic lesions and focal intramucosal carcinomas involving the esophagus, stomach, and colon. Several devices are also available for closure of EMR and ESD defects, as well as gastrointestinal fistulas and perforations. Endoscopic clips deployed through the working channel of an endoscope have been used for many years to treat bleeding lesions, but the development of more robust over-the-scope clips has facilitated endoscopic closure of gastrointestinal fistulas and perforations not previously amenable to endoscopic therapy (see Video 346e-6). Endoscopic suturing is also feasible, and the technique can be used to close perforations and large defects (Fig. 345-19, see Video 346e-7), anastomotic leaks, and fistulas. Other potential indications for endoscopic suturing include stent fixation to prevent its migration (Fig. 345-20), and endoscopic bariatric procedures. These technologies are likely to have an expanding role in patient care.
FIGURE 345-18 Endoscopic submucosal dissection. A. Large flat distal rectal adenoma with central lobulation. B. Marking the periphery of the lesion with coagulation dots. C. Rectal defect following endoscopic submucosal dissection. D. Specimen resected en bloc.
FIGURE 345-19 Closure of large defect using an endoscopic suturing device. A. Ulcerated inflammatory fibroid polyp in the antrum. B. Large defect following endoscopic submucosal dissection of the lesion. C. Closure of the defect using endoscopic sutures (arrows). D. Resected specimen.
FIGURE 345-20 Prevention of stent migration using endoscopic sutures. A. Esophagogastric anastomotic stricture refractory to balloon dilation. B. Temporary placement of covered esophageal stent. C. Endoscopic suturing device to anchor stent to esophageal wall. D. Stent fixation with endoscopic sutures (arrows).
RISKS OF ENDOSCOPY
Medications used during conscious sedation may cause respiratory depression or allergic reactions. All endoscopic procedures carry some risk of bleeding and gastrointestinal perforation. The risk is small with diagnostic upper endoscopy and colonoscopy (<1:1000 procedures), but ranges from 0.5 to 5% when therapeutic procedures, such as EMR and ESD, control of hemorrhage, or stricture dilatation, are performed. Bleeding and perforation are rare adverse events with flexible sigmoidoscopy. The risk of adverse events for diagnostic EUS (without needle aspiration) is similar to that for diagnostic upper endoscopy.
Infectious complications are uncommon with most endoscopic procedures. Some procedures carry a higher incidence of postprocedure bacteremia, and prophylactic antibiotics may be indicated (Table 345-1). Management of antithrombotic agents prior to endoscopic procedures should take into account the procedural risk of hemorrhage, the agent, and the patient condition, as summarized in Table 345-2.
|
ANTIBIOTIC PROPHYLAXIS FOR ENDOSCOPIC PROCEDURES |
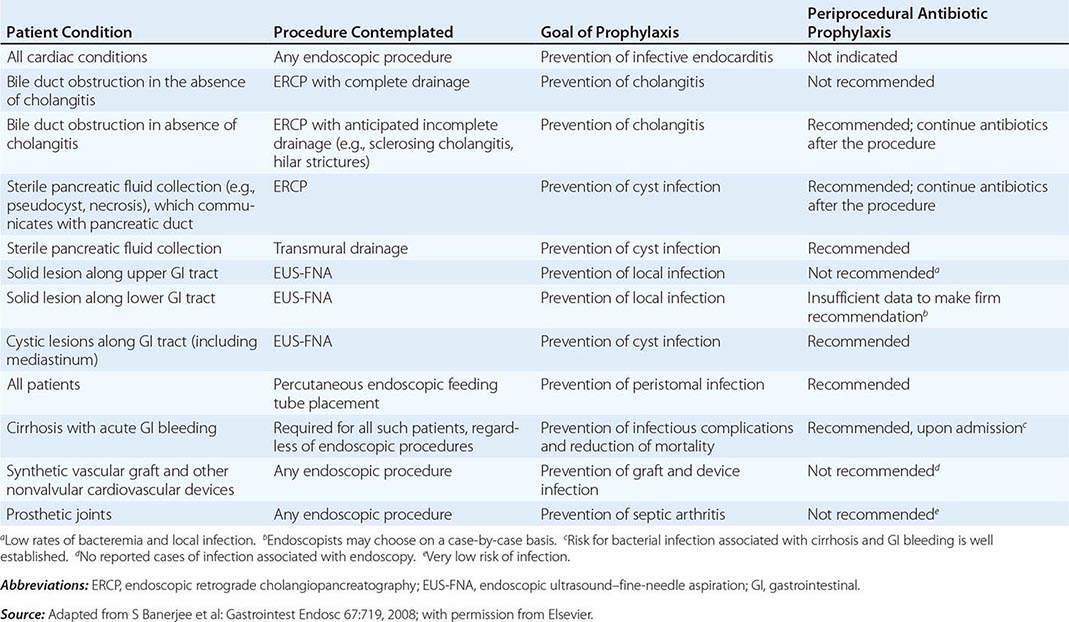
|
MANAGEMENT OF ANTITHROMBOTIC DRUGS BEFORE ENDOSCOPIC PROCEDURES |
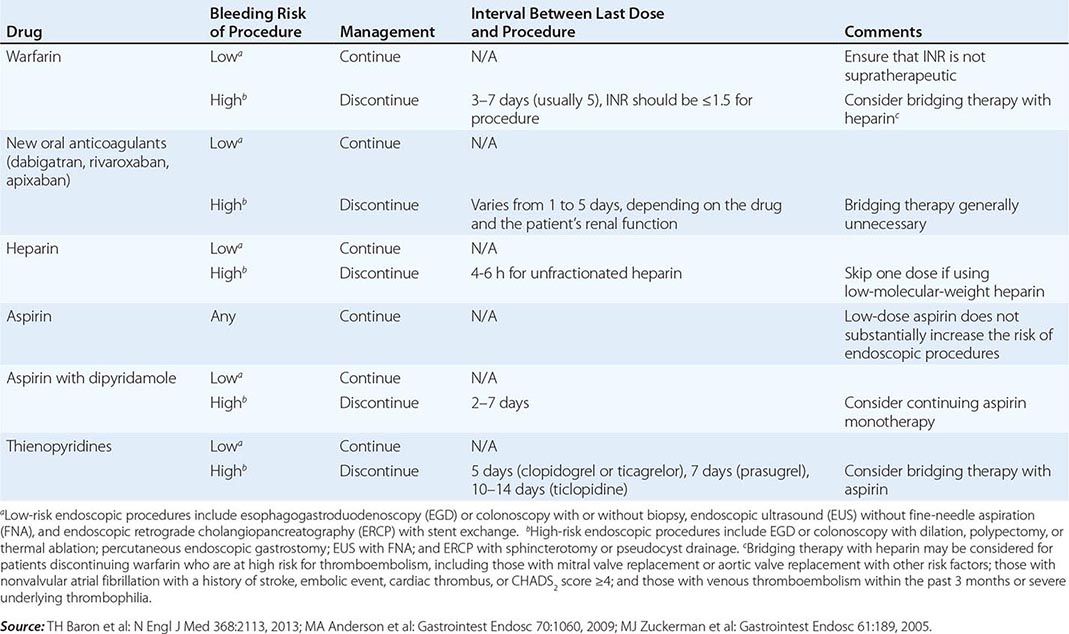
ERCP carries additional risks. Pancreatitis occurs in about 5% of patients undergoing the procedure and in up to 30% of patients with sphincter of Oddi dysfunction. Young anicteric patients with normal ducts are at increased risk. Post-ERCP pancreatitis is usually mild and self-limited, but may result in prolonged hospitalization, surgery, diabetes, or death when severe. Bleeding occurs in 1% of endoscopic sphincterotomies. Ascending cholangitis, pseudocyst infection, retroperitoneal perforation, and abscess formation may occur as a result of ERCP.
Percutaneous gastrostomy tube placement during EGD is associated with a 10–15% incidence of adverse events, most often wound infections. Fasciitis, pneumonia, bleeding, buried bumper syndrome, and colonic injury may result from gastrostomy tube placement.
URGENT ENDOSCOPY
ACUTE GASTROINTESTINAL HEMORRHAGE
Endoscopy is an important diagnostic and therapeutic technique for patients with acute gastrointestinal hemorrhage. Although gastrointestinal bleeding stops spontaneously in most cases, some patients will have persistent or recurrent hemorrhage that may be life-threatening. Clinical predictors of rebleeding help identify patients most likely to benefit from urgent endoscopy and endoscopic, angiographic, or surgical hemostasis.
Initial Evaluation The initial evaluation of the bleeding patient focuses on the severity of hemorrhage as reflected by the postural vital signs, the frequency of hematemesis or melena, and (in some cases) findings on nasogastric lavage. Decreases in hematocrit and hemoglobin lag behind the clinical course and are not reliable gauges of the magnitude of acute bleeding. This initial evaluation, completed well before the bleeding source is confidently identified, guides immediate supportive care of the patient, triage to the ward or intensive care unit, and timing of endoscopy. The severity of the initial hemorrhage is the most important indication for urgent endoscopy, since a large initial bleed increases the likelihood of ongoing or recurrent bleeding. Patients with resting hypotension or orthostatic change in vital signs, repeated hematemesis, or bloody nasogastric aspirate that does not clear with large-volume lavage, or those requiring blood transfusions, should be considered for urgent endoscopy. In addition, patients with cirrhosis, coagulopathy, or respiratory or renal failure and those over 70 years of age are more likely to have significant rebleeding.
Bedside evaluation also suggests an upper or lower gastrointestinal source of bleeding in most patients. Over 90% of patients with melena are bleeding proximal to the ligament of Treitz, and about 85% of patients with hematochezia are bleeding from the colon. Melena can result from bleeding in the small bowel or right colon, especially in older patients with slow colonic transit. Conversely, some patients with massive hematochezia may be bleeding from an upper gastrointestinal source, such as a gastric Dieulafoy lesion or duodenal ulcer, with rapid intestinal transit. Early upper endoscopy should be considered in such patients.
Endoscopy should be performed after the patient has been resuscitated with intravenous fluids and transfusions, as necessary. Marked coagulopathy or thrombocytopenia is usually treated before endoscopy, since correction of these abnormalities may lead to resolution of bleeding, and techniques for endoscopic hemostasis are limited in such patients. Metabolic derangements should also be addressed. Tracheal intubation for airway protection should be considered before upper endoscopy in patients with repeated recent hematemesis, encephalopathy, and suspected variceal hemorrhage.
Most patients with significant hematochezia can undergo colonoscopy after a rapid colonic purge with a polyethylene glycol solution; the preparation fluid may be administered via a nasogastric tube. Colonoscopy has a higher diagnostic yield than radionuclide bleeding scans or angiography in lower gastrointestinal bleeding, and endoscopic therapy can be applied in some cases. In a minority of cases, endoscopic assessment is hindered by poor visualization due to persistent vigorous bleeding with recurrent hemodynamic instability, and other techniques (such as angiography or emergent subtotal colectomy) must be employed. In such patients, massive bleeding originating from an upper gastrointestinal source should also be considered and excluded by upper endoscopy. The anal and rectal mucosa should be visualized endoscopically early in the course of massive rectal bleeding, because bleeding lesions in or close to the anal canal may be identified that are amenable to endoscopic or surgical transanal hemostatic techniques.
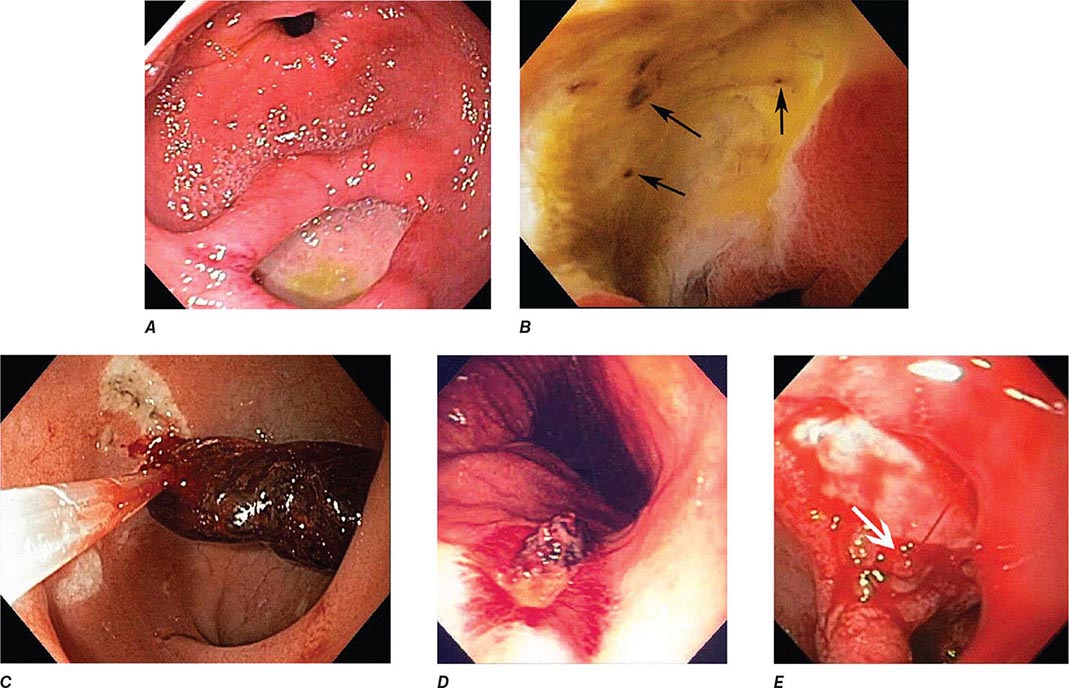
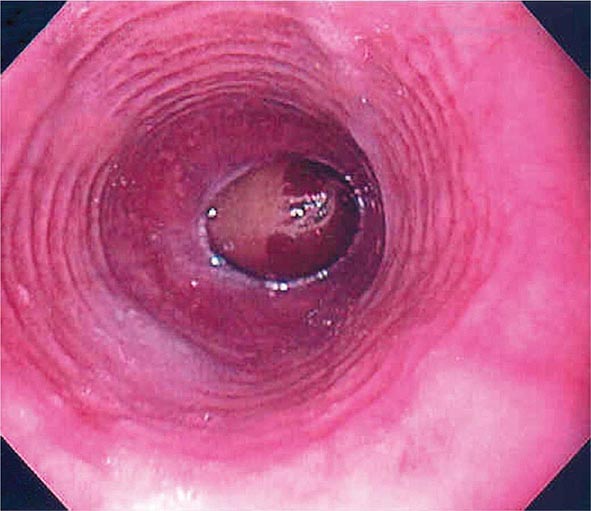
Peptic Ulcer The endoscopic appearance of peptic ulcers provides useful prognostic information and guides the need for endoscopic therapy in patients with acute hemorrhage (Fig. 345-21). A clean-based ulcer is associated with a low risk (3–5%) of rebleeding; patients with melena and a clean-based ulcer are often discharged home from the emergency room or endoscopy suite if they are young, reliable, and otherwise healthy. Flat pigmented spots and adherent clots covering the ulcer base have a 10% and 20% risk of rebleeding, respectively. Endoscopic therapy is often considered for an ulcer with an adherent clot. When a fibrin plug is seen protruding from a vessel wall in the base of an ulcer (so-called sentinel clot or visible vessel), the risk of rebleeding from the ulcer is 40%. This finding generally leads to endoscopic therapy to decrease the rebleeding rate. Occasionally, active spurting from an ulcer is seen, with >90% risk of ongoing bleeding without therapy.
FIGURE 345-21 Stigmata of hemorrhage in peptic ulcers. A. Gastric antral ulcer with a clean base. B. Duodenal ulcer with flat pigmented spots (arrows). C. Duodenal ulcer with a dense adherent clot. D. Gastric ulcer with a pigmented protuberance/visible vessel. E. Duodenal ulcer with active spurting (arrow).
Endoscopic therapy of ulcers with high-risk stigmata typically lowers the rebleeding rate to 5–10%. Several hemostatic techniques are available, including injection of epinephrine or a sclerosant into and around the vessel, “coaptive coagulation” of the vessel in the base of the ulcer using a thermal probe that is pressed against the site of bleeding, placement of hemoclips (Fig. 345-22), or a combination of these modalities (see Video 346e-8). In conjunction with endoscopic therapy, the administration of a proton pump inhibitor decreases the risk of rebleeding and improves patient outcome.
FIGURE 345-22 Endoscopic hemostasis of ulcer bleeding. A. Pyloric channel ulcer with visible vessel (arrow). B. Ulcer hemostasis with placement of an over-the-scope clip.
Varices Two complementary strategies guide therapy of bleeding varices: local treatment of the bleeding varices and treatment of the underlying portal hypertension. Local therapies, including endoscopic variceal band ligation, endoscopic variceal sclerotherapy, and balloon tamponade with a Sengstaken-Blakemore tube, effectively control acute hemorrhage in most patients, although therapies that decrease portal pressure (pharmacologic treatment, surgical shunts, or radiologically placed intrahepatic portosystemic shunts) also play an important role.
Endoscopic variceal ligation (EVL) is indicated for the prevention of a first bleed (primary prophylaxis) from large esophageal varices (Figs. 345-23 and 24), particularly in patients in whom beta blockers are contraindicated or not tolerated. EVL is also the preferred endoscopic therapy for control of active esophageal variceal bleeding and for subsequent eradication of esophageal varices (secondary prophylaxis). During EVL, a varix is suctioned into a cap fitted on the end of the endoscope, and a rubber band is released from the cap, ligating the varix (Fig. 345-24, see Video 346e-9). EVL controls acute hemorrhage in up to 90% of patients. Complications of EVL, such as postbanding ulcer bleeding and esophageal stenosis, are uncommon. Endoscopic variceal sclerotherapy (EVS) involves the injection of a sclerosing, thrombogenic solution into or next to esophageal varices. EVS also controls acute hemorrhage in most patients, but it is generally used as salvage therapy when band ligation fails because of its higher complication rate compared to EVL. These techniques are used when varices are actively bleeding during endoscopy or (more commonly) when varices are the only identifiable cause of acute hemorrhage. Bleeding from large gastric fundic varices (Fig. 345-25) is best treated with endoscopic cyanoacrylate (“glue”) injection (see Video 346e-10), because EVL or EVS of these varices is associated with a high rebleeding rate. Complications of cyanoacrylate injection include infection and glue embolization to other organs, such as the lungs, brain, and spleen.
FIGURE 345-23 Esophageal varices.
FIGURE 345-24 Endoscopic band ligation of esophageal varices. A. Large esophageal varices with stigmata of recent bleeding. B. Band ligation of varices.
FIGURE 345-25 Gastric varices. A. Large gastric fundal varices. B. Stigmata of recent bleeding from the same gastric varices (arrow).
After treatment of the acute hemorrhage, an elective course of endoscopic therapy can be undertaken with the goal of eradicating esophageal varices and preventing rebleeding months to years later. However, this chronic therapy is less successful, preventing long-term rebleeding in ~50% of patients. Pharmacologic therapies that decrease portal pressure have similar efficacy, and the two modalities may be combined.
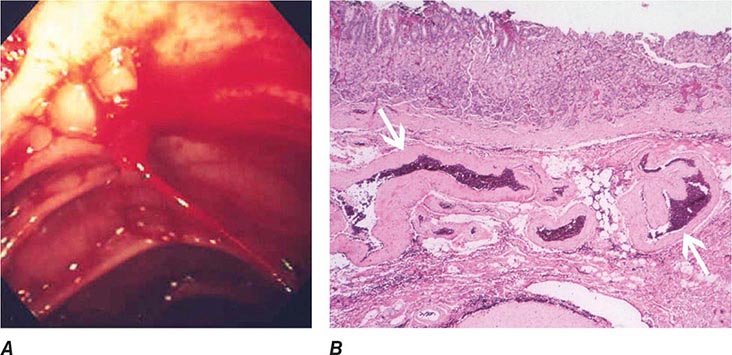
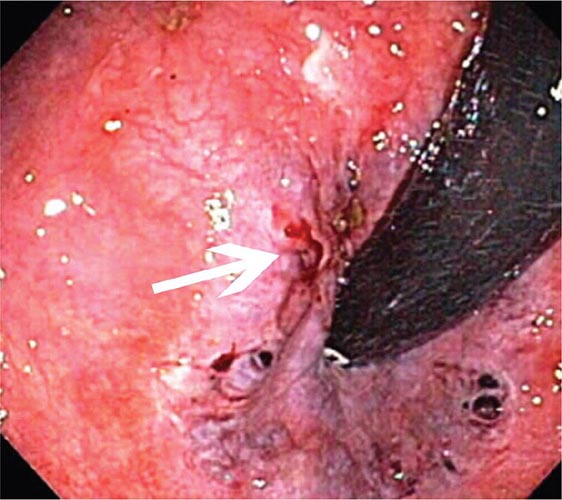
Dieulafoy’s Lesion This lesion, also called persistent caliber artery, is a large-caliber arteriole that runs immediately beneath the gastrointestinal mucosa and bleeds through a pinpoint mucosal erosion (Fig. 345-26). Dieulafoy’s lesion is seen most commonly on the lesser curvature of the proximal stomach, causes impressive arterial hemorrhage, and may be difficult to diagnose; it is often recognized only after repeated endoscopy for recurrent bleeding. Endoscopic therapy, such as thermal coagulation or band ligation, is typically effective for control of bleeding and ablation of the underlying vessel once the lesion has been identified (see Video 346e-11). Rescue therapies, such as angiographic embolization or surgical oversewing, are considered in situations where endoscopic therapy has failed.
FIGURE 345-26 Dieulafoy’s lesion. A. Actively spurting jejunal Dieulafoy’s lesion. There is no underlying mucosal lesion. B. Histology of a gastric Dieulafoy’s lesion. A persistent caliber artery (arrows) is present in the gastric submucosa, immediately beneath the mucosa.
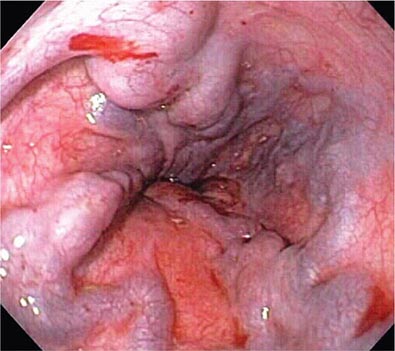
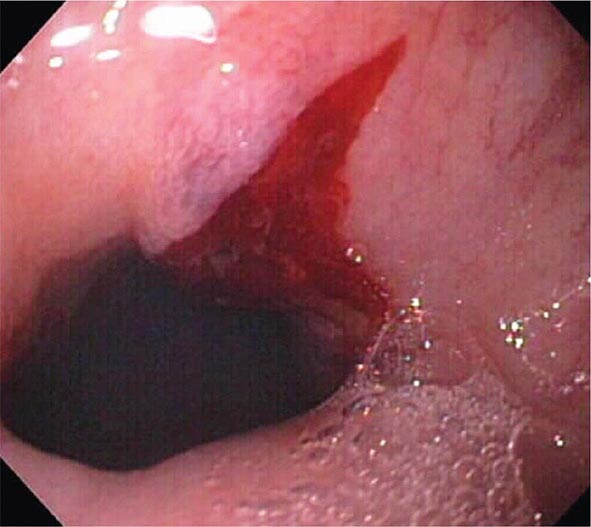
Mallory-Weiss Tear A Mallory-Weiss tear is a linear mucosal rent near or across the gastroesophageal junction that is often associated with retching or vomiting (Fig. 345-27). When the tear disrupts a submucosal arteriole, brisk hemorrhage may result. Endoscopy is the best method of diagnosis, and an actively bleeding tear can be treated endoscopically with epinephrine injection, coaptive coagulation, band ligation, or hemoclips (see Video 346e-12). Unlike peptic ulcer, a Mallory-Weiss tear with a nonbleeding sentinel clot in its base rarely rebleeds and thus does not necessitate endoscopic therapy.
FIGURE 345-27 Mallory-Weiss tear at the gastroesophageal junction.
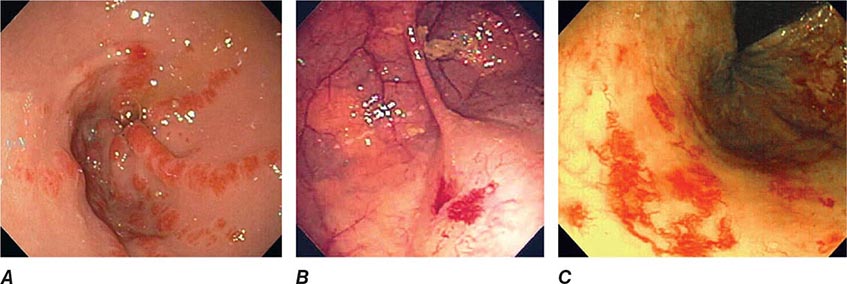
Vascular Ectasias Vascular ectasias are flat mucosal vascular anomalies that are best diagnosed by endoscopy. They usually cause slow intestinal blood loss and occur either in a sporadic fashion or in a well-defined pattern of distribution (e.g., gastric antral vascular ectasia [GAVE] or “watermelon stomach”) (Fig. 345-28). Cecal vascular ectasias, GAVE, and radiation-induced rectal ectasias are often responsive to local endoscopic ablative therapy, such as argon plasma coagulation (see Video 346e-13). Patients with diffuse small-bowel vascular ectasias (associated with chronic renal failure and with hereditary hemorrhagic telangiectasia) may continue to bleed despite endoscopic treatment of easily accessible lesions by conventional endoscopy. These patients may benefit from deep enteroscopy with endoscopic therapy, pharmacologic treatment with octreotide or estrogen/progesterone therapy, or intraoperative enteroscopy.
FIGURE 345-28 Gastrointestinal vascular ectasias. A. Gastric antral vascular ectasia (“watermelon stomach”) characterized by stripes of prominent flat or raised vascular ectasias. B. Cecal vascular ectasias. C. Radiation-induced vascular ectasias of the rectum in a patient previously treated for prostate cancer.
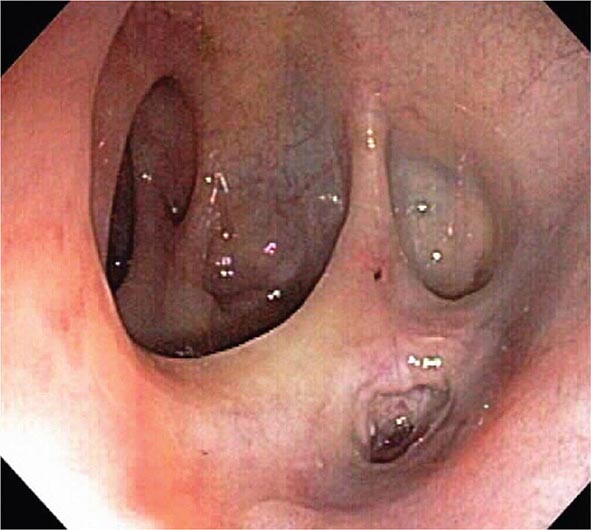
Colonic Diverticula Diverticula form where nutrient arteries penetrate the muscular wall of the colon en route to the colonic mucosa (Fig. 345-29). The artery found in the base of a diverticulum may bleed, causing painless and impressive hematochezia. Colonoscopy is indicated in patients with hematochezia and suspected diverticular hemorrhage, because other causes of bleeding (such as vascular ectasias, colitis, and colon cancer) must be excluded. In addition, an actively bleeding diverticulum may be seen and treated during colonoscopy (Fig. 345-30, see Video 346e-14).
FIGURE 345-29 Colonic diverticula.
FIGURE 345-30 Diverticular hemorrhage. A. Actively bleeding sigmoid diverticulum. B. Hemostasis achieved using endoscopic clips.
GASTROINTESTINAL OBSTRUCTION AND PSEUDOOBSTRUCTION
Endoscopy is useful for evaluation and treatment of some forms of gastrointestinal obstruction. An important exception is small-bowel obstruction due to surgical adhesions, which is generally not diagnosed or treated endoscopically. Esophageal, gastroduodenal, and colonic obstruction or pseudoobstruction can all be diagnosed and often managed endoscopically.
Acute Esophageal Obstruction
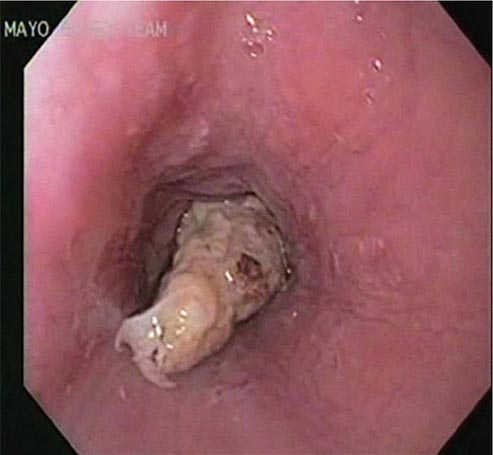
Esophageal obstruction by impacted food (Fig. 345-31) or an ingested foreign body is a potentially life-threatening event and represents an endoscopic emergency. Left untreated, the patient may develop esophageal ulceration, ischemia, and perforation. Patients with persistent esophageal obstruction often have hypersalivation and are usually unable to swallow water; endoscopy is generally the best initial test in such patients, because endoscopic removal of the obstructing material is usually possible, and the presence of an underlying esophageal pathology can often be determined. Radiographs of the chest and neck should be considered before endoscopy in patients with fever, obstruction for ≥24 h, or ingestion of a sharp object, such as a fishbone. Radiographic contrast studies interfere with subsequent endoscopy and are not advisable in most patients with a clinical picture of esophageal obstruction. Sips of a carbonated beverage, sublingual nifedipine or nitrates, or intravenous glucagon may resolve an esophageal food impaction, but in most patients, an underlying web, ring, or stricture is present and endoscopic removal of the obstructing food bolus is necessary.
FIGURE 345-31 Esophageal food (meat) impaction.
Gastric Outlet Obstruction Obstruction of the gastric outlet is commonly caused by gastric, duodenal, or pancreatic malignancy or chronic peptic ulceration with stenosis of the pylorus (Fig. 345-32). Patients vomit partially digested food many hours after eating. Gastric decompression with a nasogastric tube and subsequent lavage for removal of retained material is the first step in treatment. The diagnosis can then be confirmed with a saline load test, if desired. Endoscopy is useful for diagnosis and treatment. Patients with benign pyloric stenosis may be treated with endoscopic balloon dilatation of the pylorus, and a course of endoscopic dilatation results in long-term relief of symptoms in about 50% of patients. Malignant gastric outlet obstruction can be relieved with endoscopically placed expandable stents in patients with inoperable malignancy (Fig. 345-33).
FIGURE 345-32 Gastric outlet obstruction due to pyloric stenosis. A. Sequela of nonsteroidal anti-inflammatory drug (NSAID)–induced ulcer disease with severe stenosis of the pylorus (arrow). B. Balloon dilation of the stenosis. C. Appearance of pyloric ring after dilation.
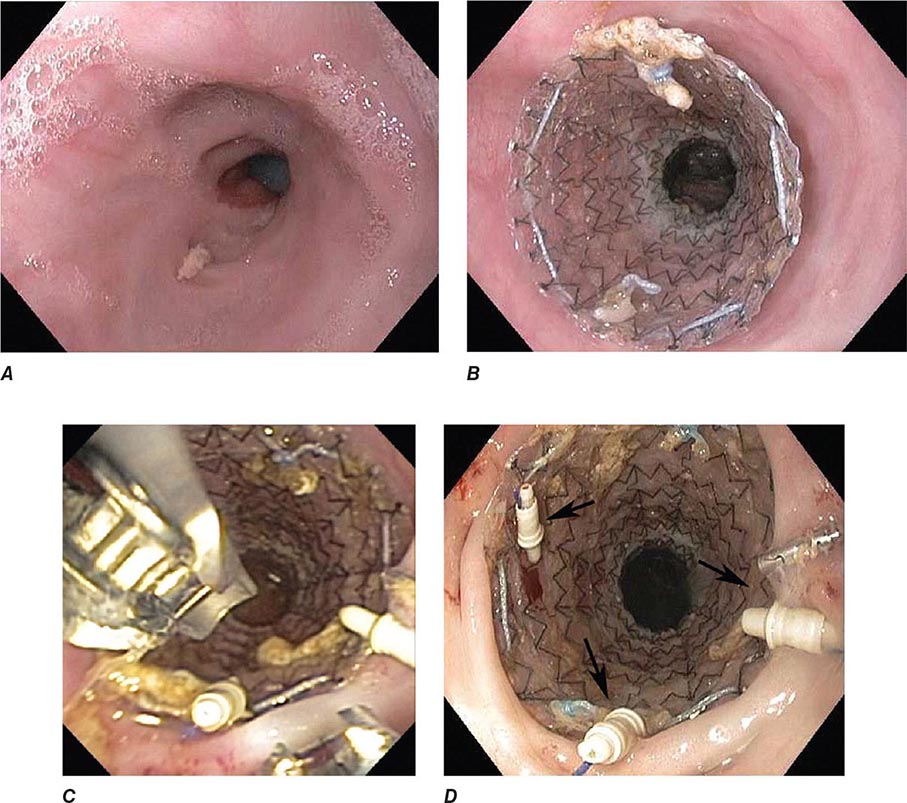
FIGURE 345-33 Biliary and duodenal self-expanding metal stents (SEMS) for obstruction caused by pancreatic cancer. A. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrates a distal bile duct stricture (arrow). B. A biliary SEMS is placed. C. Contrast injection demonstrates a duodenal stricture (arrow). D. Biliary and duodenal SEMS in place.
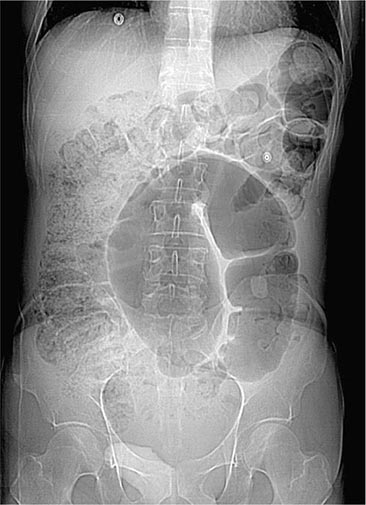
Colonic Obstruction and Pseudoobstruction These both present with abdominal distention and discomfort; tympany; and a dilated, air-filled colon on plain abdominal radiography. The radiographic appearance can be characteristic of a particular condition, such as sigmoid volvulus (Fig. 345-34). Both structural obstruction and pseudoobstruction may lead to colonic perforation if left untreated. Acute colonic pseudoobstruction is a form of colonic ileus that is usually attributable to electrolyte disorders, narcotic and anticholinergic medications, immobility (as after surgery), and retroperitoneal hemorrhage or mass. Multiple causative factors are often present. Colonoscopy, water-soluble contrast enema, or CT may be used to assess for an obstructing lesion and differentiate obstruction from pseudoobstruction. One of these diagnostic studies should be strongly considered if the patient does not have clear risk factors for pseudoobstruction, if radiographs do not show air in the rectum, or if the patient fails to improve when underlying causes of pseudoobstruction have been addressed. The risk of cecal perforation in pseudoobstruction rises when the cecal diameter exceeds 12 cm, and decompression of the colon may be achieved using intravenous neostigmine or via colonoscopic decompression (Fig. 345-35). Most patients should receive a trial of conservative therapy (with correction of electrolyte disorders, removal of offending medications, and increased mobilization) before undergoing an invasive decompressive procedure for colonic pseudoobstruction.
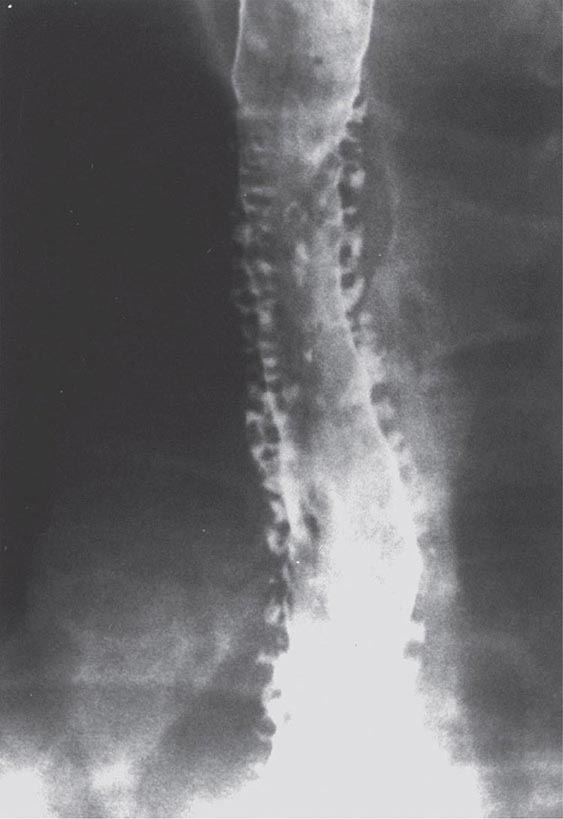
FIGURE 345-34 Sigmoid volvulus with the characteristic radiologic appearance of a “bent inner tube.”
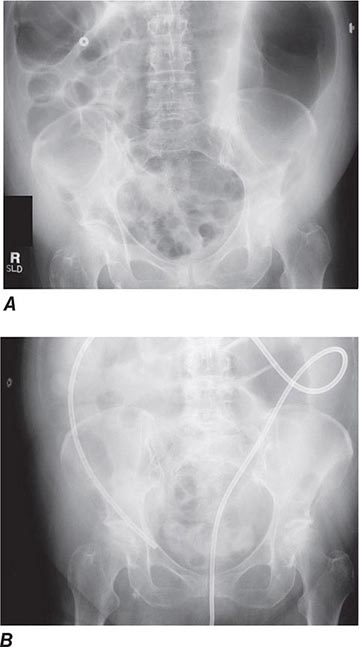
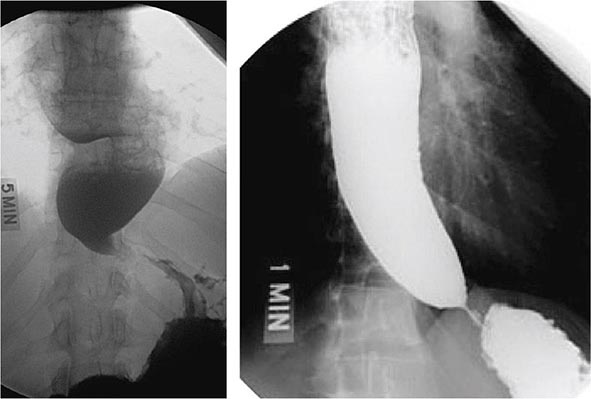
FIGURE 345-35 Acute colonic pseudoobstruction. A. Acute colonic dilatation occurring in a patient soon after knee surgery. B. Colonoscopic placement of decompression tube with marked improvement in colonic dilatation.
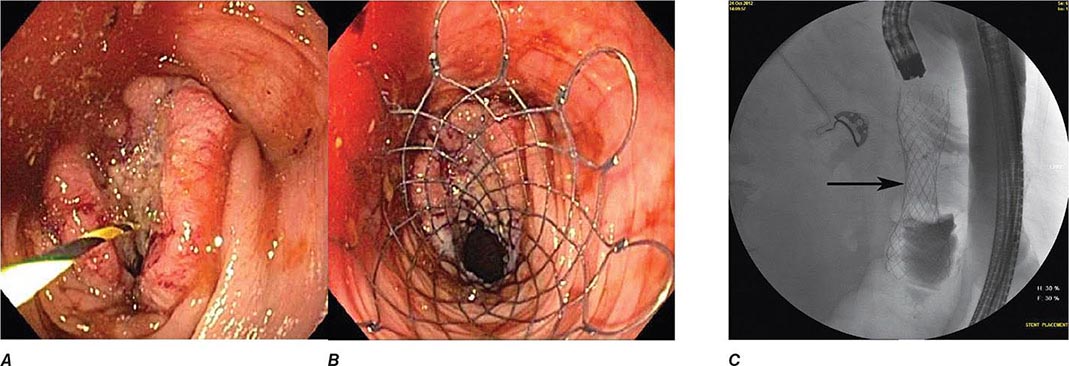
Colonic obstruction is an indication for urgent intervention. In the past, emergent diverting colostomy was usually performed with a subsequent second operation after bowel preparation to treat the underlying cause of obstruction. Colonoscopic placement of an expandable stent is now a widely used alternative that can relieve malignant colonic obstruction without emergency surgery and permit bowel preparation for an elective one-stage operation (Fig. 345-36, see Video 346e-15).
FIGURE 345-36 Obstructing colonic carcinoma. A. Colonic adenocarcinoma causing marked luminal narrowing of the distal transverse colon. B. Endoscopic placement of a self-expandable metal stent. C. Radiograph of expanded stent across the obstructing tumor with a residual waist (arrow).
ACUTE BILIARY OBSTRUCTION
The steady, severe pain that occurs when a gallstone acutely obstructs the common bile duct often brings patients to a hospital. The diagnosis of a ductal stone is suspected when the patient is jaundiced or when serum liver tests or pancreatic enzyme levels are elevated; it is confirmed by EUS, magnetic resonance cholangiography (MRCP), or direct cholangiography (performed endoscopically, percutaneously, or during surgery). ERCP is currently the primary means of diagnosing and treating common bile duct stones in most hospitals in the United States (Figs. 345-11 and 345-12).
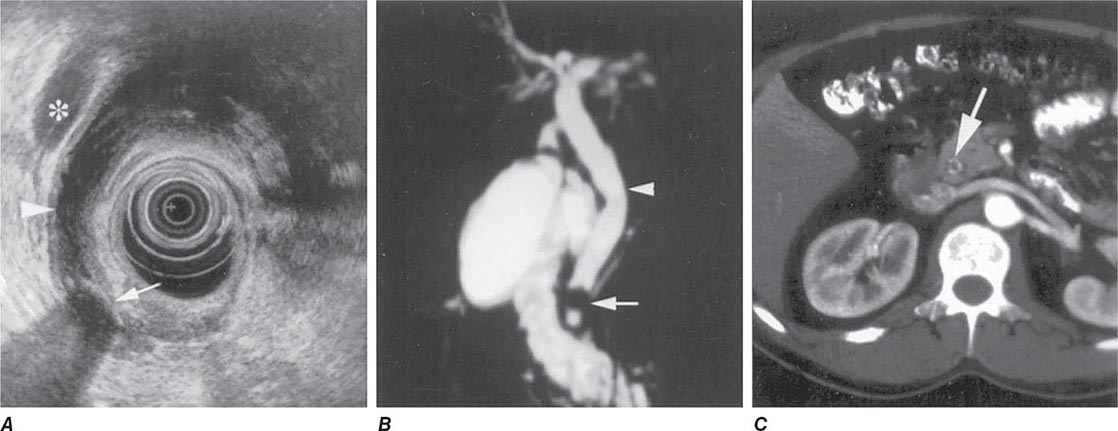
Bile Duct Imaging Whereas transabdominal ultrasound diagnoses only a minority of bile duct stones, MRCP and EUS are >90% accurate and have an important role in diagnosis. Examples of these modalities are shown in Fig. 345-37.
FIGURE 345-37 Methods of bile duct imaging. Arrows mark bile duct stones. Arrowheads indicate the common bile duct, and the asterisk marks the portal vein. A. Endoscopic ultrasound (EUS). B. Magnetic resonance cholangiopancreatography (MRCP). C. Helical computed tomography (CT).
If the suspicion for a bile duct stone is high and urgent treatment is required (as in a patient with obstructive jaundice and biliary sepsis), ERCP is the procedure of choice, because it remains the gold standard for diagnosis and allows for immediate treatment (see Video 346e-16). If a persistent bile duct stone is relatively unlikely (as in a patient with gallstone pancreatitis), ERCP may be supplanted by less invasive imaging techniques, such as EUS, MRCP, or intraoperative cholangiography performed during cholecystectomy, sparing patients the risk and discomfort of ERCP.
Ascending Cholangitis Charcot’s triad of jaundice, abdominal pain, and fever is present in about 70% of patients with ascending cholangitis and biliary sepsis. These patients are managed initially with fluid resuscitation and intravenous antibiotics. Abdominal ultrasound is often performed to assess for gallbladder stones and bile duct dilation. However, the bile duct may not be dilated early in the course of acute biliary obstruction. Medical management usually improves the patient’s clinical status, providing a window of approximately 24 h during which biliary drainage should be established, typically by ERCP. Undue delay can result in recrudescence of overt sepsis and increased morbidity and mortality rates. In addition to Charcot’s triad, the additional presence of shock and confusion (Reynolds’s pentad) is associated with high mortality rate and should prompt urgent intervention to restore biliary drainage.
Gallstone Pancreatitis Gallstones may cause acute pancreatitis as they pass through the ampulla of Vater. The occurrence of gallstone pancreatitis usually implies passage of a stone into the duodenum, and only about 20% of patients harbor a persistent stone in the ampulla or the common bile duct. Retained stones are more common in patients with jaundice, rising serum liver tests following hospitalization, severe pancreatitis, or superimposed ascending cholangitis.
Urgent ERCP decreases the morbidity rate of gallstone pancreatitis in a subset of patients with retained bile duct stones. It is unclear whether the benefit of ERCP is mainly attributable to treatment and prevention of ascending cholangitis or to relief of pancreatic ductal obstruction. ERCP is warranted early in the course of gallstone pancreatitis if ascending cholangitis is suspected, especially in a jaundiced patient. Urgent ERCP may also benefit patients predicted to have severe pancreatitis using a clinical index of severity, such as the Glasgow or Ranson score. Because the benefit of ERCP is limited to patients with a retained bile duct stone, a strategy of initial MRCP or EUS for diagnosis decreases the utilization of ERCP in gallstone pancreatitis and improves clinical outcomes by limiting the occurrence of ERCP-related adverse events.
ELECTIVE ENDOSCOPY
DYSPEPSIA
Dyspepsia is a chronic or recurrent burning discomfort or pain in the upper abdomen that may be caused by diverse processes such as gastroesophageal reflux, peptic ulcer disease, and “nonulcer dyspepsia,” a heterogeneous category that includes disorders of motility, sensation, and somatization. Gastric and esophageal malignancies are less common causes of dyspepsia. Careful history-taking allows accurate differential diagnosis of dyspepsia in only about half of patients. In the remainder, endoscopy can be a useful diagnostic tool, especially in patients whose symptoms are not resolved by an empirical trial of symptomatic treatment. Endoscopy should be performed at the outset in patients with dyspepsia and alarm features, such as weight loss or iron-deficiency anemia.
GASTROESOPHAGEAL REFLUX DISEASE (GERD)
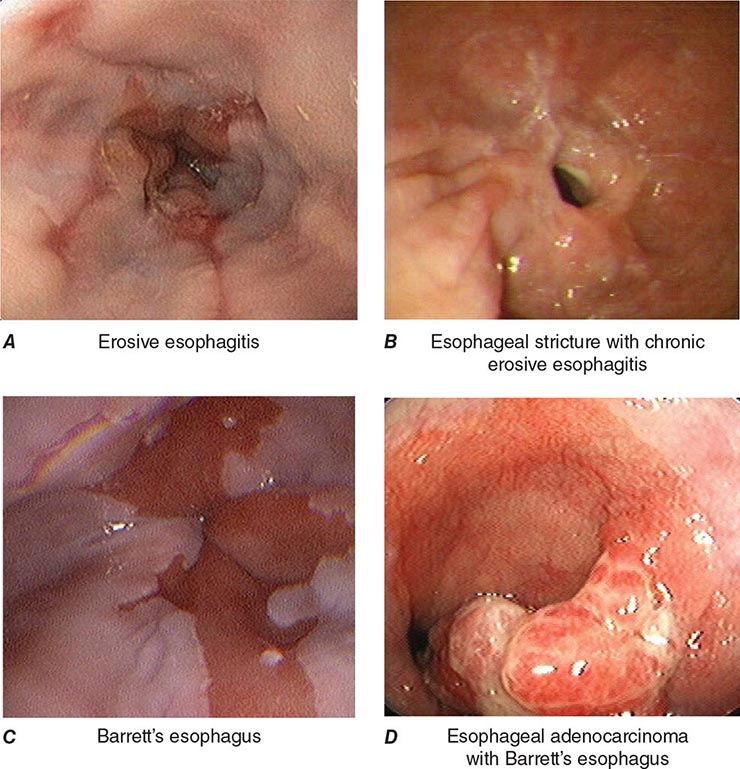
When classic symptoms of gastroesophageal reflux are present, such as water brash and substernal heartburn, presumptive diagnosis and empirical treatment are often sufficient. Endoscopy is a sensitive test for diagnosis of esophagitis (Fig. 345-38), but will miss nonerosive reflux disease (NERD) because some patients have symptomatic reflux without esophagitis. The most sensitive test for diagnosis of GERD is 24-h ambulatory pH monitoring. Endoscopy is indicated in patients with reflux symptoms refractory to antisecretory therapy; in those with alarm symptoms, such as dysphagia, weight loss, or gastrointestinal bleeding; and in those with recurrent dyspepsia after treatment that is not clearly due to reflux on clinical grounds alone. Endoscopy should be considered in patients with long-standing (≥10 years) GERD, because they have a sixfold increased risk of harboring Barrett’s esophagus compared to a patient with <1 year of reflux symptoms. Patients with Barrett’s esophagus (Fig. 345-3) generally undergo a surveillance program of periodic endoscopy with biopsies to detect dysplasia or early carcinoma.
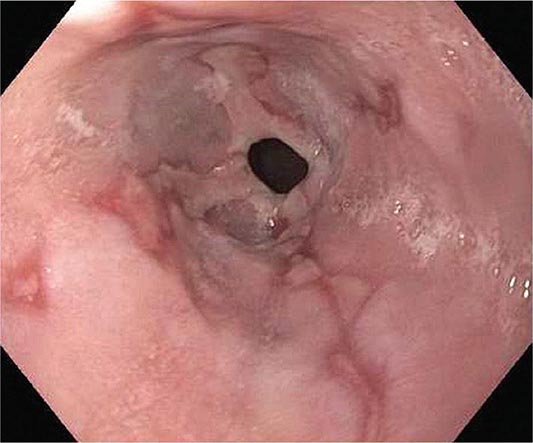
FIGURE 345-38 Causes of esophagitis. A. Severe reflux esophagitis with mucosal ulceration and friability. B. Cytomegalovirus esophagitis. C. Herpes simplex virus esophagitis with target-type shallow ulcerations. D. Candida esophagitis with white plaques adherent to the esophageal mucosa.
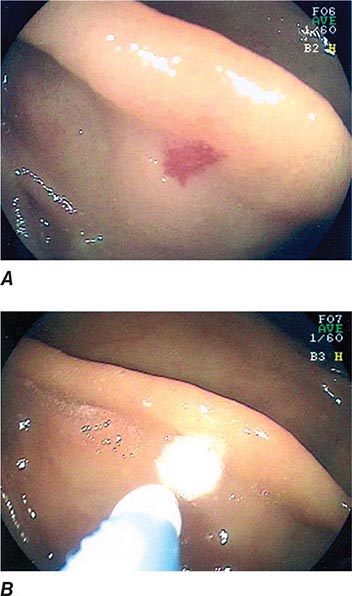
Barrett’s Esophagus Barrett’s esophagus is specialized columnar metaplasia that replaces the normal squamous mucosa of the distal esophagus in some persons with GERD. Barrett’s epithelium is a major risk factor for adenocarcinoma of the esophagus and is readily detected endoscopically, due to proximal displacement of the squamocolumnar junction (Fig. 345-3). A screening EGD for Barrett’s esophagus should be considered in patients with a chronic (≥10 year) history of GERD symptoms. Endoscopic biopsy is the gold standard for confirmation of Barrett’s esophagus and for dysplasia or cancer arising in Barrett’s mucosa.
PEPTIC ULCER
Peptic ulcer classically causes epigastric gnawing or burning, often occurring nocturnally and promptly relieved by food or antacids. Although endoscopy is the most sensitive diagnostic test for peptic ulcer, it is not a cost-effective strategy in young patients with ulcer-like dyspeptic symptoms unless endoscopy is available at low cost. Patients with suspected peptic ulcer should be evaluated for Helicobacter pylori infection. Serology (past or present infection), urea breath testing (current infection), and stool tests are noninvasive and less costly than endoscopy with biopsy. Patients with alarm symptoms and those with persistent symptoms despite treatment should undergo endoscopy to exclude gastric malignancy and other etiologies.
NONULCER DYSPEPSIA
Nonulcer dyspepsia may be associated with bloating and, unlike peptic ulcer, tends not to remit and recur. Most patients describe marginal relief on acid-reducing, prokinetic, or anti-Helicobacter therapy, and are referred for endoscopy to exclude a refractory ulcer and assess for other causes. Although endoscopy is useful for excluding other diagnoses, its impact on the treatment of patients with nonulcer dyspepsia is limited.
DYSPHAGIA
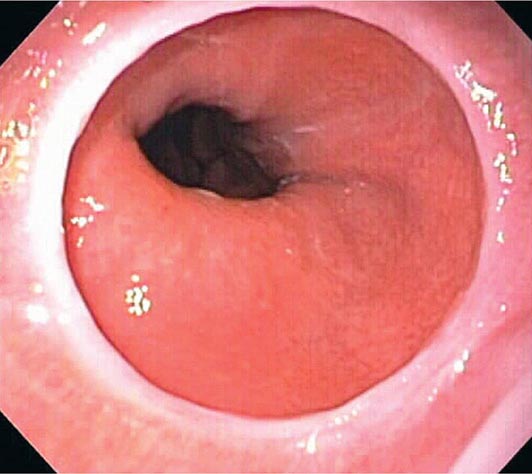
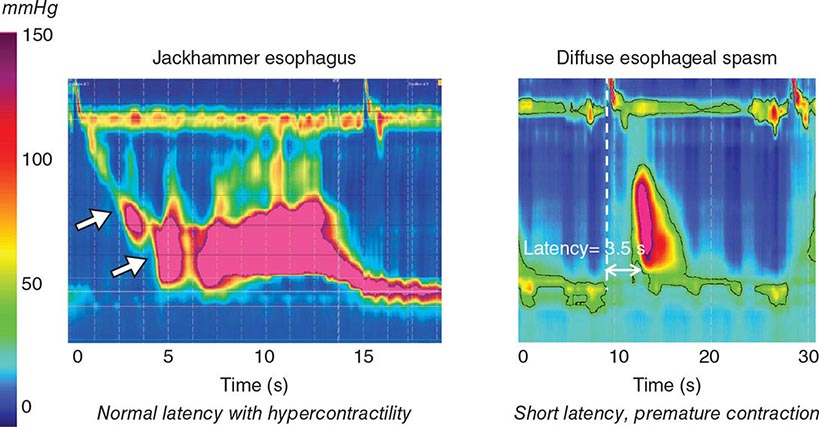
About 50% of patients presenting with difficulty swallowing have a mechanical obstruction; the remainder has a motility disorder, such as achalasia or diffuse esophageal spasm. Careful history-taking often points to a presumptive diagnosis and leads to the appropriate use of diagnostic tests. Esophageal strictures (Fig. 345-39) typically cause progressive dysphagia, first for solids, then for liquids; motility disorders often cause intermittent dysphagia for both solids and liquids. Some underlying disorders have characteristic historic features: Schatzki’s ring (Fig. 345-40) causes episodic dysphagia for solids, typically at the beginning of a meal; oropharyngeal motor disorders typically present with difficulty initiating deglutition (transfer dysphagia) and nasal reflux or coughing with swallowing; and achalasia may cause nocturnal regurgitation of undigested food.
FIGURE 345-39 Peptic esophageal stricture associated with esophagitis.
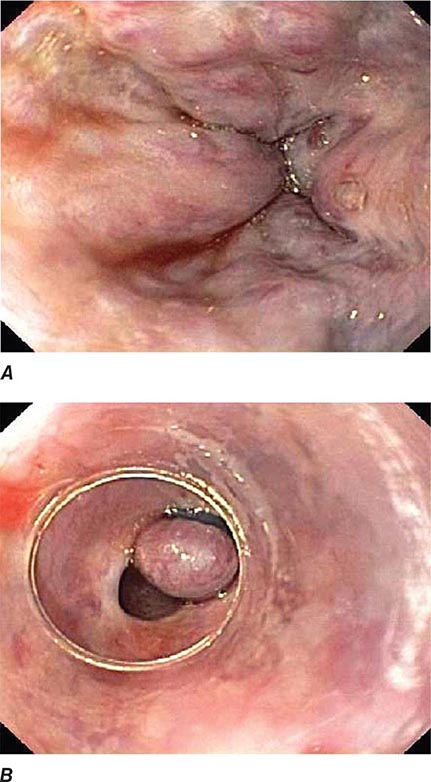
FIGURE 345-40 Schatzki’s ring at the gastroesophageal junction.
When mechanical obstruction is suspected, endoscopy is a useful initial diagnostic test, because it permits immediate biopsy and/or dilatation of strictures, masses, or rings. The presence of linear furrows and multiple corrugated rings throughout a narrowed esophagus (feline esophagus) should raise suspicion for eosinophilic esophagitis, an increasingly recognized cause for recurrent dysphagia and food impaction (Fig. 345-41). Blind or forceful passage of an endoscope may lead to perforation in a patient with stenosis of the cervical esophagus or a Zenker’s diverticulum, but gentle passage of an endoscope under direct visual guidance is reasonably safe. Endoscopy can miss a subtle stricture or ring in some patients.
FIGURE 345-41 Eosinophilic esophagitis with multiple circular rings of the esophagus creating a corrugated appearance, and an impacted grape at the narrowed esophagogastric junction. The diagnosis requires biopsy with histologic finding of > 15–20 eosinophils per high-power field.
When transfer dysphagia is evident or an esophageal motility disorder is suspected, esophageal radiography and/or a video-swallow study are the best initial diagnostic tests. The oropharyngeal swallowing mechanism, esophageal peristalsis, and the lower esophageal sphincter can all be assessed. In some disorders, subsequent esophageal manometry may also be important for diagnosis.
TREATMENT OF MALIGNANCIES
Endoscopy plays an important role in the treatment of gastrointestinal malignancies. Early-stage malignancies limited to the superficial layers of the gastrointestinal mucosa may be resected using the techniques of endoscopic mucosal resection (EMR) (see Video 346e-4) or endoscopic submucosal dissection (ESD) (see Video 346e-5). Photodynamic therapy (PDT) and radiofrequency ablation (RFA) are effective modalities for ablative treatment of high-grade dysplasia and intramucosal cancer in Barrett’s esophagus. Gastrointestinal stromal tumors can be removed en bloc by endoscopic full-thickness resection (see Video 346e-3). In general, endoscopic techniques offer the advantage of a minimally invasive approach to treatment, but rely on other imaging techniques (such as CT, magnetic resonance imaging [MRI], positron emission tomography [PET], and EUS) to exclude distant metastases or locally advanced disease better treated by surgery or other modalities. The decision to treat an early-stage gastrointestinal malignancy endoscopically is often made in collaboration with a surgeon and/or oncologist.
Endoscopic palliation of gastrointestinal malignancies relieves symptoms and in many cases prolongs survival. Malignant obstruction can be relieved by endoscopic stent placement (Figs. 345-13, 345-33, and 345-36; see Video 346e-15), and malignant gastrointestinal bleeding can often be palliated endoscopically as well. EUS-guided celiac plexus neurolysis may relieve pancreatic cancer pain.
ANEMIA AND OCCULT BLOOD IN THE STOOL
Iron-deficiency anemia may be attributed to poor iron absorption (as in celiac sprue) or, more commonly, chronic blood loss. Intestinal bleeding should be strongly suspected in men and postmenopausal women with iron-deficiency anemia, and colonoscopy is indicated in such patients, even in the absence of detectable occult blood in the stool. Approximately 30% will have large colonic polyps, 10% will have colorectal cancer, and a few additional patients will have colonic vascular lesions. When a convincing source of blood loss is not found in the colon, upper gastrointestinal endoscopy should be considered; if no lesion is found, duodenal biopsies should be obtained to exclude sprue (Fig. 345-42). Small-bowel evaluation with capsule endoscopy (Fig. 345-43), CT or magnetic resonance (MR) enterography, or balloon-assisted enteroscopy may be appropriate if both EGD and colonoscopy are unrevealing.
FIGURE 345-42 Scalloped duodenal folds in a patient with celiac sprue.
FIGURE 345-43 Capsule endoscopy images of a mildly scalloped jejunal fold (left) and an ileal tumor (right) in a patient with celiac sprue. (Images courtesy of Dr. Elizabeth Rajan; with permission.)
Tests for occult blood in the stool detect hemoglobin or the heme moiety and are most sensitive for colonic blood loss, although they will also detect larger amounts of upper gastrointestinal bleeding. Patients over age 50 with occult blood in normal-appearing stool should undergo colonoscopy to diagnose or exclude colorectal neoplasia. The diagnostic yield is lower than in iron-deficiency anemia. Whether upper endoscopy is also indicated depends on the patient’s symptoms.
The small intestine may be the source of chronic intestinal bleeding, especially if colonoscopy and upper endoscopy are not diagnostic. The utility of small-bowel evaluation varies with the clinical setting and is most important in patients in whom bleeding causes chronic or recurrent anemia. In contrast to the low diagnostic yield of small-bowel radiography, positive findings on capsule endoscopy are seen in 50–70% of patients with suspected small intestinal bleeding. The most common finding is mucosal vascular ectasias. CT or MR enterography accurately detects small-bowel masses and inflammation and is also useful for initial small-bowel evaluation. Deep enteroscopy may follow capsule endoscopy for biopsy of lesions or to provide specific therapy, such as argon plasma coagulation of vascular ectasias (Fig. 345-44).
FIGURE 345-44 A. Mid-jejunal vascular ectasia identified by double-balloon endoscopy. B. Ablation of vascular ectasia with argon plasma coagulation.
COLORECTAL CANCER SCREENING
The majority of colon cancers develop from preexisting colonic adenomas, and colorectal cancer can be largely prevented by the detection and removal of adenomatous polyps (see Video 346e-17). The choice of screening strategy for an asymptomatic person depends on personal and family history. Individuals with inflammatory bowel disease, a history of colorectal polyps or cancer, family members with adenomatous polyps or cancer, or certain familial cancer syndromes (Fig. 345-45) are at increased risk for colorectal cancer. An individual without these factors is generally considered at average risk.
FIGURE 345-45 Innumerable colon polyps of various sizes in a patient with familial adenomatous polyposis syndrome.
Screening strategies are summarized in Table 345-3. Although stool tests for occult blood have been shown to decrease mortality rate from colorectal cancer, they do not detect some cancers and many polyps, and direct visualization of the colon is a more effective screening strategy. Either sigmoidoscopy or colonoscopy may be used for cancer screening in asymptomatic average-risk individuals. The use of sigmoidoscopy was based on the historical finding that the majority of colorectal cancers occurred in the rectum and left colon and that patients with right-sided colon cancers had left-sided polyps. Over the past several decades, however, the distribution of colon cancers has changed in the United States, with proportionally fewer rectal and left-sided cancers than in the past. Large American studies of colonoscopy for screening of average-risk individuals show that cancers are roughly equally distributed between left and right colon and half of patients with right-sided lesions have no polyps in the left colon. Visualization of the entire colon thus appears to be the optimal strategy for colorectal cancer screening and prevention.
|
COLORECTAL CANCER SCREENING STRATEGIES |
Virtual colonoscopy (VC) is a radiologic technique that images the colon with CT following rectal insufflation of the colonic lumen. Computer rendering of CT images generates an electronic display of a virtual “flight” along the colonic lumen, simulating colonoscopy (Fig. 345-46). Comparative studies of virtual and routine colonoscopy have shown conflicting results, but technical refinements have improved the performance characteristics of VC. The use of VC for colorectal cancer screening may become more widespread in the future, particularly at institutions with demonstrated skill with this technique. Findings detected during virtual colonoscopy often require subsequent conventional colonoscopy for confirmation and treatment.
FIGURE 345-46 Virtual colonoscopy image of a colon polyp (arrow). (Image courtesy of Dr. Jeff Fidler; with permission.)
DIARRHEA
Most cases of diarrhea are acute, self-limited, and due to infections or medication. Chronic diarrhea (lasting >6 weeks) is more often due to a primary inflammatory, malabsorptive, or motility disorder; is less likely to resolve spontaneously; and generally requires diagnostic evaluation. Patients with chronic diarrhea or severe, unexplained acute diarrhea often undergo endoscopy if stool tests for pathogens are unrevealing. The choice of endoscopic testing depends on the clinical setting.
Patients with colonic symptoms and findings such as bloody diarrhea, tenesmus, fever, or leukocytes in stool generally undergo sigmoidoscopy or colonoscopy to assess for colitis (Fig. 345-4). Sigmoidoscopy is an appropriate initial test in most patients. Conversely, patients with symptoms and findings suggesting small-bowel disease, such as large-volume watery stools, substantial weight loss, and malabsorption of iron, calcium, or fat, may undergo upper endoscopy with duodenal aspirates for assessment of bacterial overgrowth and biopsies for assessment of mucosal diseases, such as celiac sprue.
Many patients with chronic diarrhea do not fit either of these patterns. In the setting of a long-standing history of alternating constipation and diarrhea dating to early adulthood, without findings such as blood in the stool or anemia, a diagnosis of irritable bowel syndrome may be made without direct visualization of the bowel. Steatorrhea and upper abdominal pain may prompt evaluation of the pancreas rather than the gut. Patients whose chronic diarrhea is not easily categorized often undergo initial colonoscopy to examine the entire colon and terminal ileum for inflammatory or neoplastic disease (Fig. 345-47).
FIGURE 345-47 Ulcerated ileal carcinoid tumor.
MINOR HEMATOCHEZIA
Bright red blood passed with or on formed brown stool usually has a rectal, anal, or distal sigmoid source (Fig. 345-48). Patients with even trivial amounts of hematochezia should be investigated with flexible sigmoidoscopy and anoscopy to exclude polyps or cancers in the distal colon. Patients reporting red blood on the toilet tissue only, without blood in the toilet or on the stool, are generally bleeding from a lesion in the anal canal. Careful external inspection, digital examination, and proctoscopy with anoscopy are sufficient for diagnosis in most cases.
FIGURE 345-48 Internal hemorrhoids with bleeding (arrow) as seen on a retroflexed view of the rectum.
PANCREATITIS
About 20% of patients with pancreatitis have no identified cause after routine clinical investigation (including a review of medication and alcohol use, measurement of serum triglyceride and calcium levels, abdominal ultrasonography, and CT). Endoscopic assessment leads to a specific diagnosis in the majority of such patients, often altering clinical management. Endoscopic investigation is particularly appropriate if the patient has had more than one episode of pancreatitis.
Microlithiasis, or the presence of microscopic crystals in bile, is a leading cause of previously unexplained acute pancreatitis and is sometimes seen during abdominal ultrasonography as layering sludge or flecks of floating, echogenic material in the gallbladder. Gallbladder bile can be obtained for microscopic analysis by administering a cholecystokinin analogue during endoscopy, causing contraction of the gallbladder. Bile is suctioned from the duodenum as it drains from the papilla, and the darkest fraction is examined for cholesterol crystals or bilirubinate granules. The combination of EUS of the gallbladder and bile microscopy is probably the most sensitive means of diagnosing microlithiasis.
Previously undetected chronic pancreatitis, pancreatic malignancy, or pancreas divisum may be diagnosed by either ERCP or EUS. Sphincter of Oddi dysfunction or stenosis is a potential cause for pancreatitis and can be diagnosed by manometric studies performed during ERCP. Autoimmune pancreatitis may require EUS-guided pancreatic biopsy for histologic diagnosis.
Severe pancreatitis often results in pancreatic fluid collections. Both pseudocysts and areas of walled-off pancreatic necrosis can be drained into the stomach or duodenum endoscopically, using transpapillary and transmural endoscopic techniques. Pancreatic necrosis can be treated by direct endoscopic necrosectomy (see Video 346e-2).
CANCER STAGING
Local staging of esophageal, gastric, pancreatic, bile duct, and rectal cancers can be obtained with EUS (Fig. 345-15). EUS with fine-needle aspiration (Fig. 345-16) currently provides the most accurate preoperative assessment of local tumor and nodal staging, but it does not detect most distant metastases. Details of the local tumor stage can guide treatment decisions including resectability and need for neoadjuvant therapy. EUS with transesophageal needle biopsy may also be used to assess the presence of non-small-cell lung cancer in mediastinal nodes.
OPEN-ACCESS ENDOSCOPY
Direct scheduling of endoscopic procedures by primary care physicians without preceding gastroenterology consultation, or open-access endoscopy, is common. When the indications for endoscopy are clear-cut and appropriate, the procedural risks are low, and the patient understands what to expect, open-access endoscopy streamlines patient care and decreases costs.
Patients referred for open-access endoscopy should have a recent history, physical examination, and medication review. A copy of such an evaluation should be available when the patient comes to the endoscopy suite. Patients with unstable cardiovascular or respiratory conditions should not be referred directly for open-access endoscopy. Patients with particular conditions and undergoing certain procedures should be prescribed prophylactic antibiotics prior to endoscopy (Table 345-1). In addition, patients taking anticoagulants and/or antiplatelet drugs may require adjustment of these agents before endoscopy based on the procedure risk for bleeding and condition risk for a thromboembolic event (Table 345-2).
Common indications for open-access EGD include dyspepsia resistant to a trial of appropriate therapy; dysphagia; gastrointestinal bleeding; and persistent anorexia or early satiety. Open-access colonoscopy is often requested in men or postmenopausal women with iron-deficiency anemia, in patients over age 50 with occult blood in the stool, in patients with a previous history of colorectal adenomatous polyps or cancer, and for colorectal cancer screening. Flexible sigmoidoscopy is commonly performed as an open-access procedure.
When patients are referred for open-access colonoscopy, the primary care provider may need to choose a colonic preparation. Commonly used oral preparations include polyethylene glycol lavage solution, with or without citric acid. A “split-dose” regimen improves the quality of colonic preparation. Sodium phosphate purgatives may cause fluid and electrolyte abnormalities and renal toxicity, especially in patients with renal failure or congestive heart failure and those over 70 years of age.
346e |
Video Atlas of Gastrointestinal Endoscopy |
Gastrointestinal endoscopy is an increasingly important method for diagnosis and treatment of disease. This atlas demonstrates endoscopic findings in a variety of gastrointestinal infectious, inflammatory, vascular, and neoplastic conditions. Cancer screening and prevention are common indications for gastrointestinal endoscopy, and the premalignant conditions of Barrett’s esophagus and colonic polyps are illustrated. Endoscopic treatment modalities for gastrointestinal bleeding, polyps, and biliary stones are demonstrated in video clips. The images shown in this atlas are also found in Chap. 345 of the book.
VIDEO 346e-1 Methods of deep enteroscopy. (Animations courtesy of Dr. Mark Stark and Dr. Jonathan Leighton; with permission.)
VIDEO 346e-2 Pancreatic necrosis treated by transduodenal endoscopic drainage and necrosectomy.
VIDEO 346e-3 Endoscopic full-thickness resection of a gastric subepithelial lesion.
VIDEO 346e-4 Endoscopic submucosal dissection of a large rectal adenoma.
VIDEO 346e-5 Over-the-scope clip closure of a spontaneous esophageal perforation.
VIDEO 346e-6 Endoscopic suturing for stent fixation.
VIDEO 346e-7 Actively bleeding duodenal ulcer treated with dilute epinephrine injection, thermal probe application, and hemoclips. (Video courtesy of Dr. Navtej Buttar; with permission.)
VIDEO 346e-8 Actively bleeding esophageal varices treated with endoscopic band ligation.
VIDEO 346e-9 Large, bleeding gastric varix treated with endoscopic cyanoacrylate injection.
VIDEO 346e-10 Dieulafoy’s lesion treated endoscopically.
VIDEO 346e-11 Bleeding Mallory-Weiss tear treated with hemoclip placement.
VIDEO 346e-12 Radiation proctopathy treated with argon plasma coagulation.
VIDEO 346e-13 Actively bleeding colonic diverticulum treated with dilute epinephrine injection and band ligation.
VIDEO 346e-14 Stent placement for palliation of malignant colonic obstruction.
VIDEO 346e-15 Bile duct stones removed after endoscopic sphincterotomy.
VIDEO 346e-16 Barrett’s esophagus with high-grade dysplasia treated with endoscopic mucosal resection.
VIDEO 346e-17 Pedunculated and sessile colonic polyps removed with snare cautery during colonoscopy.
347 |
Diseases of the Esophagus |
ESOPHAGEAL STRUCTURE AND FUNCTION
The esophagus is a hollow, muscular tube coursing through the posterior mediastinum joining the hypopharynx to the stomach with a sphincter at each end. It functions to transport food and fluid between these ends, otherwise remaining empty. The physiology of swallowing, esophageal motility, and oral and pharyngeal dysphagia are described in Chap. 53. Esophageal diseases can be manifested by impaired function or pain. Key functional impairments are swallowing disorders and excessive gastroesophageal reflux. Pain, sometimes indistinguishable from cardiac chest pain, can result from inflammation, infection, dysmotility, or neoplasm.
SYMPTOMS OF ESOPHAGEAL DISEASE
The clinical history remains central to the evaluation of esophageal symptoms. A thoughtfully obtained history will often expedite management. Important details include weight gain or loss, gastrointestinal bleeding, dietary habits including the timing of meals, smoking, and alcohol consumption. The major esophageal symptoms are heartburn, regurgitation, chest pain, dysphagia, odynophagia, and globus sensation.
Heartburn (pyrosis), the most common esophageal symptom, is characterized by a discomfort or burning sensation behind the sternum that arises from the epigastrium and may radiate toward the neck. Heartburn is an intermittent symptom, most commonly experienced after eating, during exercise, and while lying recumbent. The discomfort is relieved with drinking water or antacid but can occur frequently interfering with normal activities including sleep. The association between heartburn and gastroesophageal reflux disease (GERD) is so strong that empirical therapy for GERD has become accepted management. However, the term “heartburn” is often misused and/or referred to with other terms such as “indigestion” or “repeating,” making it important to clarify the intended meaning.
Regurgitation is the effortless return of food or fluid into the pharynx without nausea or retching. Patients report a sour or burning fluid in the throat or mouth that may also contain undigested food particles. Bending, belching, or maneuvers that increase intraabdominal pressure can provoke regurgitation. A clinician needs to discriminate among regurgitation, vomiting, and rumination. Vomiting is preceded by nausea and accompanied by retching. Rumination is a behavior in which recently swallowed food is regurgitated and then reswallowed repetitively for up to an hour. Although there is some linkage between rumination and mental deficiency, the behavior is also exhibited by unimpaired individuals who sometimes even find it pleasurable.
Chest pain is a common esophageal symptom with characteristics similar to cardiac pain, sometimes making this distinction difficult. Esophageal pain is usually experienced as a pressure type sensation in the mid chest, radiating to the mid back, arms, or jaws. The similarity to cardiac pain is likely because the two organs share a nerve plexus and the nerve endings in the esophageal wall have poor discriminative ability among stimuli. Esophageal distention or even chemostimulation (e.g., with acid) will often be perceived as chest pain. Gastroesophageal reflux is the most common cause of esophageal chest pain.
Esophageal dysphagia (Chap. 53) is often described as a feeling of food “sticking” or even lodging in the chest. Important distinctions are between uniquely solid food dysphagia as opposed to liquid and solid, episodic versus constant dysphagia, and progressive versus static dysphagia. If the dysphagia is for liquids as well as solid food, it suggests a motility disorder such as achalasia. Conversely, uniquely solid food dysphagia is suggestive of a stricture, ring, or tumor. Of note, a patient’s localization of food hang-up in the esophagus is notoriously imprecise. Approximately 30% of distal esophageal obstructions are perceived as cervical dysphagia. In such instances, the absence of concomitant symptoms generally associated with oropharyngeal dysphagia such as aspiration, nasopharyngeal regurgitation, cough, drooling, or obvious neuromuscular compromise should suggest an esophageal etiology.
Odynophagia is pain either caused by or exacerbated by swallowing. Although typically considered distinct from dysphagia, odynophagia may manifest concurrently with dysphagia. Odynophagia is more common with pill or infectious esophagitis than with reflux esophagitis and should prompt a search for these entities. When odynophagia does occur in GERD, it is likely related to an esophageal ulcer or deep erosion.
Globus sensation, alternatively labeled “globus hystericus,” is the perception of a lump or fullness in the throat that is felt irrespective of swallowing. Although such patients are frequently referred for an evaluation of dysphagia, globus sensation is often relieved by the act of swallowing. As implied by its alternative name (globus hystericus), globus sensation often occurs in the setting of anxiety or obsessive-compulsive disorders. Clinical experience teaches that it is often attributable to GERD.
Water brash is excessive salivation resulting from a vagal reflex triggered by acidification of the esophageal mucosa. This is not a common symptom. Afflicted individuals will describe the unpleasant sensation of the mouth rapidly filling with salty thin fluid, often in the setting of concomitant heartburn.
DIAGNOSTIC STUDIES
ENDOSCOPY
Endoscopy, also known as esophagogastroduodenoscopy (EGD), is the most useful test for the evaluation of the proximal gastrointestinal tract. Modern instruments produce high-quality, color images of the esophageal, gastric, and duodenal lumen. Endoscopes also have an instrumentation channel through which biopsy forceps, injection catheters for local delivery of therapeutic agents, balloon dilators, or hemostatic devices can be used. The key advantages of endoscopy over barium radiography are: (1) increased sensitivity for the detection of mucosal lesions, (2) vastly increased sensitivity for the detection of abnormalities mainly identifiable by color such as Barrett’s metaplasia or vascular lesions, (3) the ability to obtain biopsy specimens for histologic examination of suspected abnormalities, and (4) the ability to dilate strictures during the examination. The main disadvantages of endoscopy are cost and the utilization of sedatives or anesthetics.
RADIOGRAPHY
Contrast radiography of the esophagus, stomach, and duodenum can demonstrate reflux of the contrast media, hiatal hernia, mucosal granularity, erosions, ulcerations, and strictures. The sensitivity of radiography compared with endoscopy for detecting reflux esophagitis reportedly ranges from 22–95%, with higher grades of esophagitis (i.e., ulceration or stricture) exhibiting greater detection rates. Conversely, the sensitivity of barium radiography for detecting esophageal strictures is greater than that of endoscopy, especially when the study is done in conjunction with barium-soaked bread or a 13-mm barium tablet. Barium studies also provide an assessment of esophageal function and morphology that may be undetected on endoscopy. Tracheoesophageal fistula, altered postsurgical anatomy, and extrinsic esophageal compression are conditions where radiographic imaging complements endoscopic assessment. Hypopharyngeal pathology and disorders of the cricopharyngeus muscle are better appreciated on radiographic examination than with endoscopy, particularly with rapid sequence or video fluoroscopic recording. The major shortcoming of barium radiography is that it rarely obviates the need for endoscopy. Either a positive or a negative study is usually followed by an endoscopic evaluation either to obtain biopsies, provide therapy, or clarify findings in the case of a positive examination or to add a level of certainty in the case of a negative one.
ENDOSCOPIC ULTRASOUND
Endoscopic ultrasound (EUS) instruments combine an endoscope with an ultrasound transducer to create a transmural image of the tissue surrounding the endoscope tip. The key advantage of EUS over alternative radiologic imaging techniques is much greater resolution attributable to the proximity of the ultrasound transducer to the area being examined. Available devices can provide either radial imaging (360-degree, cross-sectional) or a curved linear image that can guide fine-needle aspiration of imaged structures such as lymph nodes or tumors. Major esophageal applications of EUS are to stage esophageal cancer, to evaluate dysplasia in Barrett’s esophagus, and to assess submucosal lesions.
ESOPHAGEAL MANOMETRY
Esophageal manometry, or motility testing, entails positioning a pressure-sensing catheter within the esophagus and then observing the contractility following test swallows. The upper and lower esophageal sphincters appear as zones of high pressure that relax on swallowing, while the intersphincteric esophagus exhibits peristaltic contractions. Manometry is used to diagnose motility disorders (achalasia, diffuse esophageal spasm) and to assess peristaltic integrity prior to the surgery for reflux disease. Technologic advances have enhanced esophageal manometry as high-resolution esophageal pressure topography (Fig. 347-1). Manometry can also be combined with intraluminal impedance monitoring. Impedance recordings use a catheter with a series of paired electrodes. Esophageal luminal contents in contact with the electrodes decrease (liquid) or increase (air) the impedance signal, allowing detection of anterograde or retrograde esophageal bolus transit.
FIGURE 347-1 High-resolution esophageal pressure topography (right) and conventional manometry (left) of a normal swallow. E, esophageal body; LES, lower esophageal sphincter; UES, upper esophageal sphincter.
REFLUX TESTING
GERD is often diagnosed in the absence of endoscopic esophagitis, which would otherwise define the disease. This occurs in the settings of partially treated disease, an abnormally sensitive esophageal mucosa, or without obvious explanation. In such instances, reflux testing can demonstrate excessive esophageal exposure to refluxed gastric juice, the physiologic abnormality of GERD. This can be done by ambulatory 24- to 48-h esophageal pH recording using either a wireless pH-sensitive transmitter that is anchored to the esophageal mucosa or a transnasally positioned wire electrode with the tip stationed in the distal esophagus. Either way, the outcome is expressed as the percentage of the day that the pH was less than 4 (indicative of recent acid reflux), with values exceeding 5% indicative of GERD. Reflux testing is useful with atypical symptoms or an inexplicably poor response to therapy. Intraluminal impedance monitoring can be added to pH monitoring to detect reflux events irrespective of whether or not they are acidic, potentially increasing the sensitivity of the study.
STRUCTURAL DISORDERS
HIATAL HERNIA
Hiatus hernia is a herniation of viscera, most commonly the stomach, into the mediastinum through the esophageal hiatus of the diaphragm. Four types of hiatus hernia are distinguished with type I, or sliding hiatal hernia, comprising at least 95% of the overall total. A sliding hiatal hernia is one in which the gastroesophageal junction and gastric cardia translocate cephalad as a result of weakening of the phrenoesophageal ligament attaching the gastroesophageal junction to the diaphragm at the hiatus and dilatation of the diaphragmatic hiatus. The incidence of sliding hernia increases with age. True to its name, sliding hernias enlarge with increased intraabdominal pressure, swallowing, and respiration. Conceptually, sliding hernias are the result of wear and tear: increased intraabdominal pressure from abdominal obesity, pregnancy, etc., along with hereditary factors predisposing to the condition. The main significance of sliding hernias is the propensity of affected individuals to have GERD.
Types II, III, and IV hiatal hernias are all subtypes of paraesophageal hernia in which the herniation into the mediastinum includes a visceral structure other than the gastric cardia. With type II and III paraesophageal hernias, the gastric fundus also herniates with the distinction being that in type II, the gastroesophageal junction remains fixed at the hiatus, whereas type III is a combined sliding and paraesophageal hernia. With type IV hiatal hernias, viscera other than the stomach herniate into the mediastinum, most commonly the colon. With type II and III paraesophageal hernias, the stomach inverts as it herniates and large paraesophageal hernias can lead to an upside down stomach, gastric volvulus, and even strangulation of the stomach. Because of this risk, surgical repair is often advocated for large paraesophageal hernias.
RINGS AND WEBS
A lower esophageal mucosal ring, also called a B ring, is a thin membranous narrowing at the squamocolumnar mucosal junction (Fig. 347-2). Its origin is unknown, but B rings are demonstrable in about 10–15% of the general population and are usually asymptomatic. When the lumen diameter is less than 13 mm, distal rings are usually associated with episodic solid food dysphagia and are called Schatzki rings. Patients typically present older than 40 years, consistent with an acquired rather than congenital origin. Schatzki ring is one of the most common causes of intermittent food impaction, also known as “steakhouse syndrome” because meat is a typical instigator. Symptomatic rings are easily treated by dilation.
FIGURE 347-2 Radiographic anatomy of the gastroesophageal junction.
Web-like constrictions higher in the esophagus can be of congenital or inflammatory origin. Asymptomatic cervical esophageal webs are demonstrated in about 10% of people and typically originate along the anterior aspect of the esophagus. When circumferential, they can cause intermittent dysphagia to solids similar to Schatzki rings and are similarly treated with dilatation. The combination of symptomatic proximal esophageal webs and iron-deficiency anemia in middle-aged women constitutes Plummer-Vinson syndrome.
DIVERTICULA
Esophageal diverticula are categorized by location with the most common being epiphrenic, hypopharyngeal (Zenker’s), and midesophageal. Epiphrenic and Zenker’s diverticula are false diverticula involving herniation of the mucosa and submucosa through the muscular layer of the esophagus. These lesions result from increased intraluminal pressure associated with distal obstruction. In the case of Zenker’s, the obstruction is a stenotic cricopharyngeus muscle (upper esophageal sphincter), and the hypopharyngeal herniation most commonly occurs in an area of natural weakness proximal to the cricopharyngeus known as Killian’s triangle (Fig. 347-3). Small Zenker’s diverticula are usually asymptomatic, but when they enlarge sufficiently to retain food and saliva they can be associated with dysphagia, halitosis, and aspiration. Treatment is by surgical diverticulectomy and cricopharyngeal myotomy or a marsupialization procedure in which an endoscopic stapling device is used to divide the cricopharyngeus.
FIGURE 347-3 Examples of small (A) and large (B, C) Zenker’s diverticula arising from Killian’s triangle in the distal hypopharynx. Smaller diverticula are evident only during the swallow, whereas larger ones retain food and fluid.
Epiphrenic diverticula are usually associated with achalasia or a distal esophageal stricture. Midesophageal diverticula may be caused by traction from adjacent inflammation (classically tuberculosis) in which case they are true diverticula involving all layers of the esophageal wall, or by pulsion associated with esophageal motor disorders. Midesophageal and epiphrenic diverticula are usually asymptomatic until they enlarge sufficiently to retain food and cause dysphagia and regurgitation. Symptoms attributable to the diverticula tend to correlate more with the underlying esophageal disorder than the size of the diverticula. Large diverticula can be removed surgically, usually in conjunction with a myotomy if the underlying cause is achalasia. Diffuse intramural esophageal diverticulosis is a rare entity that results from dilatation of the excretory ducts of submucosal esophageal glands (Fig. 347-4). Esophageal candidiasis and proximal esophageal strictures are commonly found in association with this disorder.
FIGURE 347-4 Intramural esophageal pseudodiverticulosis associated with chronic obstruction. Invaginations of contrast into the esophageal wall outline deep esophageal glands.
TUMORS
Esophageal cancer occurs in about 4.5:100,000 people in the United States with the associated mortality being only slightly less at 4.4:100,000. It is about 10 times less common than colorectal cancer but kills about one-quarter as many patients. These statistics emphasize both the rarity and lethality of esophageal cancer. One notable trend is the shift of dominant esophageal cancer type from squamous cell to adenocarcinoma, strongly linked to reflux disease and Barrett’s metaplasia. Other distinctions between cell types are the predilection for adenocarcinoma to affect the distal esophagus in white males and squamous cell to affect the more proximal esophagus in black males with the added risk factors of smoking, alcohol consumption, caustic injury, and human papilloma virus infection (Chap. 109).
The typical presentation of esophageal cancer is of progressive solid food dysphagia and weight loss. Associated symptoms may include odynophagia, iron deficiency, and, with midesophageal tumors, hoarseness from left recurrent laryngeal nerve injury. Generally, these are indications of locally invasive or even metastatic disease manifest by tracheoesophageal fistulas and vocal cord paralysis. Even when detected as a small lesion, esophageal cancer has poor survival because of the abundant esophageal lymphatics leading to regional lymph node metastases.
Benign esophageal tumors are uncommon and usually discovered incidentally. In decreasing frequency of occurrence, cell types include leiomyoma, fibrovascular polyps, squamous papilloma, granular cell tumors, lipomas, neurofibromas, and inflammatory fibroid polyps. These generally become symptomatic only when they are associated with dysphagia and merit removal only under the same circumstances.
CONGENITAL ANOMALIES
The most common congenital esophageal anomaly is esophageal atresia, occurring in about 1 in 5000 live births. Atresia can occur in several permutations, the common denominator being developmental failure of fusion between the proximal and distal esophagus associated with a tracheoesophageal fistula, most commonly with the distal segment excluded. Alternatively, there can be an H-type configuration in which esophageal fusion has occurred, but with a tracheoesophageal fistula. Esophageal atresia is usually recognized and corrected surgically within the first few days of life. Later life complications include dysphagia from anastomotic strictures or absent peristalsis and reflux, which can be severe. Less common developmental anomalies include congenital esophageal stenosis, webs, and duplications.
Dysphagia can also result from congenital abnormalities that cause extrinsic compression of the esophagus. In dysphagia lusoria, the esophagus is compressed by an aberrant right subclavian artery arising from the descending aorta and passing behind the esophagus. Alternatively vascular rings may surround and constrict the esophagus.
Heterotopic gastric mucosa, also known as an esophageal inlet patch, is a focus of gastric type epithelium in the proximal cervical esophagus; the estimated prevalence is 4.5%. The inlet patch is thought to result from incomplete replacement of embryonic columnar epithelium with squamous epithelium. The majority of inlet patches are asymptomatic, but acid production can occur as most contain fundic type gastric epithelium with parietal cells.
ESOPHAGEAL MOTILITY DISORDERS
Esophageal motility disorders are diseases attributable to esophageal neuromuscular dysfunction commonly associated with dysphagia, chest pain, or heartburn. The major entities are achalasia, diffuse esophageal spasm (DES), and GERD. Motility disorders can also be secondary to broader disease processes as is the case with pseudoachalasia, Chagas’ disease, and scleroderma. Not included in this discussion are diseases affecting the pharynx and proximal esophagus, the impairment of which is almost always part of a more global neuromuscular disease process.
ACHALASIA
Achalasia is a rare disease caused by loss of ganglion cells within the esophageal myenteric plexus with a population incidence of about 1:100,000 and usually presenting between age 25 and 60. With longstanding disease, aganglionosis is noted. The disease involves both excitatory (cholinergic) and inhibitory (nitric oxide) ganglionic neurons. Functionally, inhibitory neurons mediate deglutitive lower esophageal sphincter (LES) relaxation and the sequential propagation of peristalsis. Their absence leads to impaired deglutitive LES relaxation and absent peristalsis. Increasing evidence suggests that the ultimate cause of ganglion cell degeneration in achalasia is an autoimmune process attributable to a latent infection with human herpes simplex virus 1 combined with genetic susceptibility.
Long-standing achalasia is characterized by progressive dilatation and sigmoid deformity of the esophagus with hypertrophy of the LES. Clinical manifestations may include dysphagia, regurgitation, chest pain, and weight loss. Most patients report solid and liquid food dysphagia. Regurgitation occurs when food, fluid, and secretions are retained in the dilated esophagus. Patients with advanced achalasia are at risk for bronchitis, pneumonia, or lung abscess from chronic regurgitation and aspiration. Chest pain is frequent early in the course of achalasia, thought to result from esophageal spasm. Patients describe a squeezing, pressure-like retrosternal pain, sometimes radiating to the neck, arms, jaw, and back. Paradoxically, some patients complain of heartburn that may be a chest pain equivalent. Treatment of achalasia is less effective in relieving chest pain than it is in relieving dysphagia or regurgitation.
The differential diagnosis of achalasia includes DES, Chagas’ disease, and pseudoachalasia. Chagas’ disease is endemic in areas of central Brazil, Venezuela, and northern Argentina and spread by the bite of the reduviid (kissing) bug that transmits the protozoan, Trypanosoma cruzi. The chronic phase of the disease develops years after infection and results from destruction of autonomic ganglion cells throughout the body, including the heart, gut, urinary tract, and respiratory tract. Tumor infiltration, most commonly seen with carcinoma in the gastric fundus or distal esophagus, can mimic idiopathic achalasia. The resultant “pseudoachalasia” accounts for up to 5% of suspected cases and is more likely with advanced age, abrupt onset of symptoms (<1 year), and weight loss. Hence, endoscopy is a necessary part of the evaluation of achalasia. When the clinical suspicion for pseudoachalasia is high and endoscopy nondiagnostic, computed tomography (CT) scanning or EUS may be of value. Rarely, pseudoachalasia can result from a paraneoplastic syndrome with circulating antineuronal antibodies.
Achalasia is diagnosed by barium swallow x-ray and/or esophageal manometry; endoscopy has a relatively minor role other than to exclude pseudoachalasia. The barium swallow x-ray appearance is of a dilated esophagus with poor emptying, an air-fluid level, and tapering at the LES giving it a beak-like appearance (Fig. 347-5). Occasionally, an epiphrenic diverticulum is observed. In long-standing achalasia, the esophagus may assume a sigmoid configuration. The diagnostic criteria for achalasia with esophageal manometry are impaired LES relaxation and absent peristalsis. High-resolution manometry has somewhat advanced this diagnosis; three subtypes of achalasia are differentiated based on the pattern of pressurization in the nonperistaltic esophagus (Fig. 347-6). Because manometry identifies early disease before esophageal dilatation and food retention, it is the most sensitive diagnostic test.
FIGURE 347-5 Achalasia with esophageal dilatation, tapering at the gastroesophageal junction, and an air-fluid level within the esophagus. The example on the left shows sigmoid deformity with very advanced disease.
FIGURE 347-6 Three subtypes of achalasia: classic (A), with esophageal compression (B), and spastic achalasia (C) imaged with pressure topography. All are characterized by impaired lower esophageal sphincter (LES) relaxation and absent peristalsis. However, classic achalasia has minimal pressurization of the esophageal body, whereas substantial fluid pressurization is observed in achalasia with esophageal compression, and spastic esophageal contractions are observed with spastic achalasia.
There is no known way of preventing or reversing achalasia. Therapy is directed at reducing LES pressure so that gravity and esophageal pressurization promote esophageal emptying. Peristalsis rarely, if ever, recovers. However, in many instances, remnants of peristalsis masked by esophageal pressurization and dilatation prior to therapy are demonstrable following effective treatment. LES pressure can be reduced by pharmacologic therapy, pneumatic balloon dilatation, or surgical myotomy. No large, controlled trials of the therapeutic alternatives exist, and the optimal approach is debated. Pharmacologic therapies are relatively ineffective but are often used as temporizing therapies. Nitrates or calcium channel blockers are administered before eating, advising caution because of their effects on blood pressure. Botulinum toxin, injected into the LES under endoscopic guidance, inhibits acetylcholine release from nerve endings and improves dysphagia in about 66% of cases for at least 6 months. Sildenafil and alternative phosphodiesterase inhibitors effectively decrease LES pressure, but practicalities limit their clinical use in achalasia.
The only durable therapies for achalasia are pneumatic dilatation and Heller myotomy. Pneumatic dilatation, with a reported efficacy ranging from 32–98%, is an endoscopic technique using a noncompliant, cylindrical balloon dilator positioned across the LES and inflated to a diameter of 3–4 cm. The major complication is perforation with a reported incidence of 0.5–5%. The most common surgical procedure for achalasia is laparoscopic Heller myotomy, usually performed in conjunction with an antireflux procedure (partial fundoplication); good to excellent results are reported in 62–100% of cases. A European randomized controlled trial demonstrated an equivalent response rate of approximately 90% for both pneumatic dilation and laparoscopic Heller myotomy at 2-year follow-up. Occasionally, patients with advanced disease fail to respond to pneumatic dilatation or Heller myotomy. In such refractory cases, esophageal resection with gastric pull-up or interposition of a segment of transverse colon may be the only option other than gastrostomy feeding.
An endoscopic approach to LES myotomy has been introduced, referred to as per oral esophageal myotomy. This technique involves the creation of a tunnel within the esophageal wall through which the circular muscle of the LES and distal esophagus are transected with electrocautery. Short-term studies of efficacy have been favorable. Potential advantages over the conventional laparoscopic approach include avoidance of surgical disruption of the diaphragmatic hiatus and more rapid recovery.
In untreated or inadequately treated achalasia, esophageal dilatation predisposes to stasis esophagitis. Prolonged stasis esophagitis is the likely explanation for the association between achalasia and esophageal squamous cell cancer. Tumors develop after years of achalasia, usually in the setting of a greatly dilated esophagus with the overall squamous cell cancer risk increased 17-fold compared to controls.
DIFFUSE ESOPHAGEAL SPASM (DES)
DES is manifested by episodes of dysphagia and chest pain attributable to abnormal esophageal contractions with normal deglutitive LES relaxation. Beyond that, there is little consensus. The pathophysiology and natural history of DES are ill defined. Radiographically, DES has been characterized by tertiary contractions or a “corkscrew esophagus” (Fig. 347-7), but in many instances, these abnormalities are actually indicative of achalasia. Manometrically, a variety of defining features have been proposed including uncoordinated (“spastic”) activity in the distal esophagus, spontaneous and repetitive contractions, or high-amplitude and prolonged contractions. The current consensus, derived from high-resolution manometry studies, is to define spasm by the occurrence of contractions in the distal esophagus with short latency relative to the time of the pharyngeal contraction, a dysfunction indicative of impairment of inhibitory myenteric plexus neurons. When defined in this restrictive fashion (Fig. 347-8), DES is actually much less common than achalasia.
FIGURE 347-7 Diffuse esophageal spasm. The characteristic “corkscrew” esophagus results from spastic contraction of the circular muscle in the esophageal wall; more precisely, this is actually a helical array of muscle. These findings are also seen with spastic achalasia.
FIGURE 347-8 Esophageal pressure topography of the two major variants of esophageal spasm: jackhammer esophagus (left) and diffuse esophageal spasm (right). Jackhammer esophagus is defined by the extraordinarily vigorous and repetitive contractions with normal peristaltic onset and normal latency of the contraction. Diffuse esophageal spasm is similar but primarily defined by a short latency (premature) contraction.
Esophageal chest pain closely mimics angina pectoris. Features suggesting esophageal pain include pain that is nonexertional, prolonged, interrupts sleep, meal-related, relieved with antacids, and accompanied by heartburn, dysphagia, or regurgitation. However, all of these features exhibit overlap with cardiac pain, which still must be the primary consideration. Furthermore, even within the spectrum of esophageal diseases, both chest pain and dysphagia are also characteristic of peptic or infectious esophagitis. Only after these more common entities have been excluded by evaluation and/or treatment should a diagnosis of DES be pursued.
Although the defining criteria are in flux, DES is diagnosed by manometry. Endoscopy is useful to identify alternative structural and inflammatory lesions that may cause chest pain. Radiographically, a “corkscrew esophagus,” “rosary bead esophagus,” pseudodiverticula, or curling can be indicative of DES, but these are also found with spastic achalasia. Given these vagaries of defining DES, and the resultant heterogeneity of patients identified for inclusion in therapeutic trials, it is not surprising that trial results have been disappointing. Only small, uncontrolled trials exist, reporting response to nitrates, calcium channel blockers, hydralazine, botulinum toxin, and anxiolytics. The only controlled trial showing efficacy was with an anxiolytic. Surgical therapy (long myotomy or even esophagectomy) should be considered only with severe weight loss or unbearable pain. These indications are extremely rare.
NONSPECIFIC MANOMETRIC FINDINGS
Manometric studies done to evaluate chest pain and/or dysphagia often report minor abnormalities (e.g., hypertensive or hypotensive peristalsis, hypertensive LES) that are insufficient to diagnose either achalasia or DES. These findings are of unclear significance. Reflux and psychiatric diagnoses, particularly anxiety and depression, are common among such individuals. A lower visceral pain threshold and symptoms of irritable bowel syndrome are noted in more than half of such patients. Consequently, therapy for these individuals should either target the most common esophageal disorder, GERD, or more global conditions such as depression or somatization neurosis that are found to be coexistent.
GASTROESOPHAGEAL REFLUX DISEASE (GERD)
The current conception of GERD is to encompass a family of conditions with the commonality that they are caused by gastroesophageal reflux resulting in either troublesome symptoms or an array of potential esophageal and extraesophageal manifestations. It is estimated that 15% of adults in the United States are affected by GERD, although such estimates are based only on population studies of self-reported chronic heartburn. With respect to the esophagus, the spectrum of injury includes esophagitis, stricture, Barrett’s esophagus, and adenocarcinoma (Fig. 347-9). Of particular concern is the rising incidence of esophageal adenocarcinoma, an epidemiologic trend that parallels the increasing incidence of GERD. There were about 8000 incident cases of esophageal adenocarcinoma in the United States in 2013 (half of all esophageal cancers); it is estimated that this disease burden has increased two- to sixfold in the last 20 years.
FIGURE 347-9 Endoscopic appearance of (A) peptic esophagitis, (B) a peptic stricture, (C) Barrett’s metaplasia, and (D) adenocarcinoma developing within an area of Barrett’s esophagus.
PATHOPHYSIOLOGY
The best-defined subset of GERD patients, albeit a minority overall, have esophagitis. Esophagitis occurs when refluxed gastric acid and pepsin cause necrosis of the esophageal mucosa causing erosions and ulcers. Note that some degree of gastroesophageal reflux is normal, physiologically intertwined with the mechanism of belching (transient LES relaxation), but esophagitis results from excessive reflux, often accompanied by impaired clearance of the refluxed gastric juice. Restricting reflux to that which is physiologically intended depends on the anatomic and physiologic integrity of the esophagogastric junction, a complex sphincter comprised of both the LES and the surrounding crural diaphragm. Three dominant mechanisms of esophagogastric junction incompetence are recognized: (1) transient LES relaxations (a vagovagal reflex in which LES relaxation is elicited by gastric distention), (2) LES hypotension, or (3) anatomic distortion of the esophagogastric junction inclusive of hiatus hernia. Of note, the third factor, esophagogastric junction anatomic disruption, is both significant unto itself and also because it interacts with the first two mechanisms. Transient LES relaxations account for about 90% of reflux in normal subjects or GERD patients without hiatus hernia, but patients with hiatus hernia have a more heterogeneous mechanistic profile. Factors tending to exacerbate reflux regardless of mechanism are abdominal obesity, pregnancy, gastric hypersecretory states, delayed gastric emptying, disruption of esophageal peristalsis, and gluttony.
After acid reflux, peristalsis returns the refluxed fluid to the stomach and acid clearance is completed by titration of the residual acid by bicarbonate contained in swallowed saliva. Consequently, two causes of prolonged acid clearance are impaired peristalsis and reduced salivation. Impaired peristaltic emptying can be attributable to disrupted peristalsis or superimposed reflux associated with a hiatal hernia. With superimposed reflux, fluid retained within a sliding hiatal hernia refluxes back into the esophagus during swallow-related LES relaxation, a phenomenon that does not normally occur.
Inherent in the pathophysiologic model of GERD is that gastric juice is harmful to the esophageal epithelium. However, gastric acid hypersecretion is usually not a dominant factor in the development of esophagitis. An obvious exception is with Zollinger-Ellison syndrome, which is associated with severe esophagitis in about 50% of patients. Another caveat is with chronic Helicobacter pylori gastritis, which may have a protective effect by inducing atrophic gastritis with concomitant hypoacidity. Pepsin, bile, and pancreatic enzymes within gastric secretions can also injure the esophageal epithelium, but their noxious properties are either lessened without an acidic environment or dependent on acidity for activation. Bile warrants attention because it persists in refluxate despite acid-suppressing medications. Bile can transverse the cell membrane, imparting severe cellular injury in a weakly acidic environment, and has also been invoked as a cofactor in the pathogenesis of Barrett’s metaplasia and adenocarcinoma. Hence, the causticity of gastric refluxate extends beyond hydrochloric acid.
SYMPTOMS
Heartburn and regurgitation are the typical symptoms of GERD. Somewhat less common are dysphagia and chest pain. In each case, multiple potential mechanisms for symptom genesis operate that extend beyond the basic concepts of mucosal erosion and activation of afferent sensory nerves. Specifically, hypersensitivity and functional pain are increasingly recognized as cofactors. Nonetheless, the dominant clinical strategy is empirical treatment with acid inhibitors, reserving further evaluation for those who fail to respond. Important exceptions to this are patients with chest pain or persistent dysphagia, each of which may be indicative of more morbid conditions. With chest pain, cardiac disease must be carefully considered. In the case of persistent dysphagia, chronic reflux can lead to the development of a peptic stricture or adenocarcinoma, each of which benefits from early detection and/or specific therapy.
Extraesophageal syndromes with an established association to GERD include chronic cough, laryngitis, asthma, and dental erosions. A multitude of other conditions including pharyngitis, chronic bronchitis, pulmonary fibrosis, chronic sinusitis, cardiac arrhythmias, sleep apnea, and recurrent aspiration pneumonia have proposed associations with GERD. However, in both cases, it is important to emphasize the word association as opposed to causation. In many instances, the disorders likely coexist because of shared pathogenetic mechanisms rather than strict causality. Potential mechanisms for extraesophageal GERD manifestations are either regurgitation with direct contact between the refluxate and supraesophageal structures or via a vagovagal reflex wherein reflux activation of esophageal afferent nerves triggers efferent vagal reflexes such as bronchospasm, cough, or arrhythmias.
DIFFERENTIAL DIAGNOSIS
Although generally quite characteristic, symptoms from GERD need to be distinguished from symptoms related to infectious, pill, or eosinophilic esophagitis, peptic ulcer disease, dyspepsia, biliary colic, coronary artery disease, and esophageal motility disorders. It is especially important that coronary artery disease be given early consideration because of its potentially lethal implications. The remaining elements of the differential diagnosis can be addressed by endoscopy, upper gastrointestinal series, or biliary tract ultrasonography as appropriate. The distinction among etiologies of esophagitis is usually easily made by endoscopy with mucosal biopsies, which are necessary to evaluate for infection or eosinophilic inflammation. In terms of endoscopic appearance, infectious esophagitis is diffuse and tends to involve the proximal esophagus far more frequently than does reflux esophagitis. The ulcerations seen in peptic esophagitis are usually solitary and distal, whereas infectious ulcerations are punctate and diffuse. Eosinophilic esophagitis characteristically exhibits multiple esophageal rings, linear furrows, or white punctate exudate. Esophageal ulcerations from pill esophagitis are usually singular and deep at points of luminal narrowing, especially near the carina, with sparing of the distal esophagus.
COMPLICATIONS
The complications of GERD are related to chronic esophagitis (bleeding and stricture) and the relationship between GERD and esophageal adenocarcinoma. However, both esophagitis and peptic strictures have become increasingly rare in the era of potent antisecretory medications. Conversely, the most severe histologic consequence of GERD is Barrett’s metaplasia with the associated risk of esophageal adenocarcinoma, and the incidence of these lesions has increased, not decreased, in the era of potent acid suppression. Barrett’s metaplasia, endoscopically recognized by tongues of reddish mucosa extending proximally from the gastroesophageal junction (Fig. 347-9) or histopathologically by the finding of specialized columnar metaplasia, is associated with a substantially increased risk for development of esophageal adenocarcinoma.
Barrett’s metaplasia can progress to adenocarcinoma through the intermediate stages of low- and high-grade dysplasia (Fig. 347-10). Owing to this risk, areas of Barrett’s and especially any included areas of mucosal irregularity should be extensively biopsied. The rate of cancer development is estimated at 0.1–0.3% per year, but vagaries in definitional criteria and of the extent of Barrett’s metaplasia requisite to establish the diagnosis have contributed to variability and inconsistency in this risk assessment. The group at greatest risk is obese white males in their sixth decade of life. However, despite common practice, the utility of endoscopic screening and surveillance programs intended to control the adenocarcinoma risk has not been established. Also of note, no high-level evidence confirms that aggressive antisecretory therapy or antireflux surgery causes regression of Barrett’s esophagus or prevents adenocarcinoma.
FIGURE 347-10 Histopathology of Barrett’s metaplasia and Barrett’s with high-grade dysplasia. H&E, hematoxylin and eosin.
Although the management of Barrett’s esophagus remains controversial, the finding of dysplasia in Barrett’s, particularly high-grade dysplasia, mandates further intervention. In addition to the high rate of progression to adenocarcinoma, there is also a high prevalence of unrecognized coexisting cancer with high-grade dysplasia. Nonetheless, treatment remains controversial. Esophagectomy, intensive endoscopic surveillance, and mucosal ablation have all been advocated. Currently, esophagectomy is the gold standard treatment for high-grade dysplasia in an otherwise healthy patient with minimal surgical risk. However, esophagectomy has a mortality ranging from 3–10%, along with substantial morbidity. That, along with increasing evidence of the effectiveness of endoscopic therapy with purpose-built radiofrequency ablation devices, has led many to favor this therapy as a preferable management strategy.
EOSINOPHILIC ESOPHAGITIS
Eosinophilic esophagitis (EoE) is increasingly recognized in adults and children around the world. Current prevalence estimates identified 4–6 cases per 10,000 with a predilection for white males. The increasing prevalence of EoE is attributable to a combination of an increasing incidence and a growing recognition of the condition. There is also an incompletely understood, but important, overlap between EoE and GERD that confuses diagnosis of the disease.
EoE is diagnosed based on the combination of typical esophageal symptoms and esophageal mucosal biopsies demonstrating squamous epithelial eosinophil-predominant inflammation. Alternative etiologies of esophageal eosinophilia include GERD, drug hypersensitivity, connective tissue disorders, hypereosinophilic syndrome, and infection. Current evidence indicates that EoE is an immunologic disorder induced by antigen sensitization in susceptible individuals. Dietary factors play an important role in both the pathogenesis and treatment of EoE. Aeroallergens may also contribute, but the evidence is weaker. The natural history of EoE is unclear, but an increased risk of esophageal stricture development paralleling the duration of untreated disease has been noted.
EoE should be strongly considered in children and adults with dysphagia and esophageal food impactions. In preadolescent children, symptom presentations of EoE include chest or abdominal pain, nausea, vomiting, and food aversion. Other symptoms in adults may include atypical chest pain and heartburn, particularly heartburn that is refractory to PPI therapy. An atopic history of food allergy, asthma, eczema, or allergic rhinitis is present in the majority of patients. Peripheral blood eosinophilia is demonstrable in up to 50% of patients, but the specificity of this finding is problematic in the setting of concomitant atopy. The characteristic endoscopic esophageal findings are loss of vascular markings (edema), multiple esophageal rings, longitudinally oriented furrows, and punctate exudate (Fig. 347-11). Histologic confirmation is made with the demonstration of esophageal mucosal eosinophilia (greatest density ±15 eosinophils per high-power field) (Fig. 347-12). Complications of EoE include esophageal stricture, narrow-caliber esophagus, food impaction, and esophageal perforation.
FIGURE 347-11 Endoscopic features of (A) eosinophilic esophagitis (EoE), (B) Candida esophagitis, (C) giant ulcer associated with HIV, (D) and a Schatzki ring.
FIGURE 347-12 Histopathology of eosinophilic esophagitis (EoE) showing infiltration of the esophageal squamous epithelium with eosinophils. Additional features of basal cell hyperplasia and lamina propria fibrosis are present. Eosinophilic inflammation can also be seen with gastroesophageal reflux disease.
The goals of EoE management are symptom control and the prevention of complications. Once esophageal eosinophilia is demonstrated, patients typically undergo a trial of PPI therapy as a practical means of excluding a contribution of GERD to the esophageal mucosal inflammation. PPI-responsive esophageal eosinophilia, characterized by elimination of mucosal eosinophilia, occurs in 30–50% of cases of suspected EoE. Patients with persistent symptoms and eosinophilic inflammation following PPI therapy are subsequently considered for EoE treatments such as elimination diets or swallowed topical glucocorticoids. Elemental formula diets are a highly effective therapy that have primarily been studied in children but are limited by palatability. Notably, allergy testing by means of either serum IgE or skin prick testing has demonstrated poor sensitivity and specificity in the identification of foods that incite the esophageal inflammatory response. Allergy testing combining skin prick and atopy patch testing has been effective in children with EoE, but additional validation is needed. Empiric elimination of common food allergies (milk, wheat, egg, soy, nuts, and seafood) followed by systematic reintroduction has been an effective diet therapy in both children and adults with EoE. The intent of the elimination diet approach is the identification of a single food trigger or a small number of food triggers. Swallowed, topical glucocorticoids (fluticasone propionate or budesonide) are highly effective, but recurrence of disease is common following the cessation of therapy. Systemic glucocorticoids are reserved for severely afflicted patients refractory to less morbid treatments. Esophageal dilation is very effective at relieving dysphagia in patients with fibrostenosis. Dilation should be approached conservatively because of the risk of deep, esophageal mural laceration or perforation in the stiff-walled esophagus that is characteristic of the disease.
INFECTIOUS ESOPHAGITIS
With the increased use of immunosuppression for organ transplantation as well as chronic inflammatory diseases and chemotherapy along with the AIDS epidemic, infections with Candida species, herpesvirus, and cytomegalovirus (CMV) have become relatively common. Although rare, infectious esophagitis also occurs among the nonimmunocompromised, with herpes simplex and Candida albicans being the most common pathogens. Among AIDS patients, infectious esophagitis becomes more common as the CD4 count declines; cases are rare with a CD4 count >200 and common when <100. HIV itself may also be associated with a self-limited syndrome of acute esophageal ulceration with oral ulcers and a maculopapular skin rash at the time of seroconversion. Additionally, some patients with advanced disease have deep, persistent esophageal ulcers treated with oral glucocorticoids or thalidomide. However, with the widespread use of protease inhibitors, a reduction in these HIV complications has been noted.
Regardless of the infectious agent, odynophagia is a characteristic symptom of infectious esophagitis; dysphagia, chest pain, and hemorrhage are also common. Odynophagia is uncommon with reflux esophagitis, so its presence should always raise suspicion of an alternative etiology.
CANDIDA ESOPHAGITIS
Candida is normally found in the throat, but can become pathogenic and produce esophagitis in a compromised host; C. albicans is most common. Candida esophagitis also occurs with esophageal stasis secondary to esophageal motor disorders and diverticula. Patients complain of odynophagia and dysphagia. If oral thrush is present, empirical therapy is appropriate, but co-infection is common, and persistent symptoms should lead to prompt endoscopy with biopsy, which is the most useful diagnostic evaluation. Candida esophagitis has a characteristic appearance of white plaques with friability. Rarely, Candida esophagitis is complicated by bleeding, perforation, stricture, or systemic invasion. Oral fluconazole (200–400 mg on the first day, followed by 100–200 mg daily) for 14–21 days is the preferred treatment. Patients refractory to fluconazole may respond to itraconazole, voriconazole, or posaconazole. Alternatively, poorly responsive patients or those who cannot swallow medications can be treated with an intravenous echinocandin (caspofungin 50 mg daily for 7–21 days).
HERPETIC ESOPHAGITIS
Herpes simplex virus type 1 or 2 may cause esophagitis. Vesicles on the nose and lips may coexist and are suggestive of a herpetic etiology. Varicella-zoster virus can also cause esophagitis in children with chickenpox or adults with zoster. The characteristic endoscopic findings are vesicles and small, punched-out ulcerations. Because herpes simplex infections are limited to squamous epithelium, biopsies from the ulcer margins are most likely to reveal the characteristic ground-glass nuclei, eosinophilic Cowdry’s type A inclusion bodies, and giant cells. Culture or polymerase chain reaction (PCR) assays are helpful to identify acyclovir-resistant strains. Acyclovir (200 mg orally five times a day for 7–10 days) can be used for immunocompetent hosts, although the disease is typically self-limited after a 1- to 2-week period in such patients. Immunocompromised patients are treated with acyclovir (400 mg orally five times a day for 14–21 days), famciclovir (500 mg orally three times a day), or valacyclovir (1 g orally three times a day). In patients with severe odynophagia, intravenous acyclovir, 5 mg/kg every 8 h for 7–14 days, reduces this morbidity.
CYTOMEGALOVIRUS
CMV esophagitis occurs primarily in immunocompromised patients, particularly organ transplant recipients. CMV is usually activated from a latent stage. Endoscopically, CMV lesions appear as serpiginous ulcers in an otherwise normal mucosa, particularly in the distal esophagus. Biopsies from the ulcer bases have the greatest diagnostic yield for finding the pathognomonic large nuclear or cytoplasmic inclusion bodies. Immunohistology with monoclonal antibodies to CMV and in situ hybridization tests are useful for early diagnosis. Data on therapy for CMV esophagitis are limited. Treatment studies of CMV gastrointestinal disease have demonstrated effectiveness of both ganciclovir (5 mg/kg every 12 h intravenously) and foscarnet (90 mg/kg every 12 h intravenously). Valganciclovir (900 mg two times a day), an oral formulation of ganciclovir, can also be used. Therapy is continued until healing, which may take 3–6 weeks. Maintenance therapy may be needed for patients with relapsing disease.
MECHANICAL TRAUMA AND IATROGENIC INJURY
ESOPHAGEAL PERFORATION
Most cases of esophageal perforation are from instrumentation of the esophagus or trauma. Alternatively, forceful vomiting or retching can lead to spontaneous rupture at the gastroesophageal junction (Boerhaave’s syndrome). More rarely, corrosive esophagitis or neoplasms lead to perforation. Instrument perforation from endoscopy or nasogastric tube placement typically occurs in the hypopharynx or at the gastroesophageal junction. Perforation may also occur at the site of a stricture in the setting of endoscopic food disimpaction or esophageal dilation. Esophageal perforation causes pleuritic retrosternal pain that can be associated with pneumomediastinum and subcutaneous emphysema. Mediastinitis is a major complication of esophageal perforation, and prompt recognition is key to optimizing outcome. CT of the chest is most sensitive in detecting mediastinal air. Esophageal perforation is confirmed by a contrast swallow, usually Gastrografin followed by thin barium. Treatment includes nasogastric suction and parenteral broad-spectrum antibiotics with prompt surgical drainage and repair in noncontained leaks. Conservative therapy with NPO status and antibiotics without surgery may be appropriate in cases of contained perforation that are detected early. Endoscopic clipping or stent placement may be indicated in nonoperated iatrogenic perforations or nonoperable cases such as perforated tumors.
MALLORY-WEISS TEAR
Vomiting, retching, or vigorous coughing can cause a nontransmural tear at the gastroesophageal junction that is a common cause of upper gastrointestinal bleeding. Most patients present with hematemesis. Antecedent vomiting is anticipated but not always evident. Bleeding usually abates spontaneously, but protracted bleeding may respond to local epinephrine or cauterization therapy, endoscopic clipping, or angiographic embolization. Surgery is rarely needed.
RADIATION ESOPHAGITIS
Radiation esophagitis can complicate treatment for thoracic cancers, especially breast and lung, with the risk proportional to radiation dosage. Radiosensitizing drugs such as doxorubicin, bleomycin, cyclophosphamide, and cisplatin also increase the risk. Dysphagia and odynophagia may last weeks to months after therapy. The esophageal mucosa becomes erythematous, edematous, and friable. Submucosal fibrosis and degenerative tissue changes and stricturing may occur years after the radiation exposure. Radiation exposure in excess of 5000 cGy has been associated with increased risk of esophageal stricture. Treatment for acute radiation esophagitis is supportive. Chronic strictures are managed with esophageal dilation.
CORROSIVE ESOPHAGITIS
Caustic esophageal injury from ingestion of alkali or, less commonly, acid can be accidental or from attempted suicide. Absence of oral injury does not exclude possible esophageal involvement. Thus, early endoscopic evaluation is recommended to assess and grade the injury to the esophageal mucosa. Severe corrosive injury may lead to esophageal perforation, bleeding, stricture, and death. Glucocorticoids have not been shown to improve the clinical outcome of acute corrosive esophagitis and are not recommended. Healing of more severe grades of caustic injury is commonly associated with severe stricture formation and often requires repeated dilatation.
PILL ESOPHAGITIS
Pill-induced esophagitis occurs when a swallowed pill fails to traverses the entire esophagus and lodges within the lumen. Generally, this is attributed to poor “pill taking habits”: inadequate liquid with the pill or lying down immediately after taking a pill. The most common location for the pill to lodge is in the mid-esophagus near the crossing of the aorta or carina. Extrinsic compression from these structures halts the movement of the pill or capsule. Since initially reported in 1970, more than 1000 cases of pill esophagitis have been reported, suggesting that this is not an unusual occurrence. A wide variety of medications are implicated with the most common being doxycycline, tetracycline, quinidine, phenytoin, potassium chloride, ferrous sulfate, nonsteroidal anti-inflammatory drugs (NSAIDs), and bisphosphonates. However, virtually any pill can result in pill esophagitis if taken carelessly.
Typical symptoms of pill esophagitis are the sudden onset of chest pain and odynophagia. Characteristically, the pain will develop over a period of hours or will awaken the individual from sleep. A classic history in the setting of ingestion of recognized pill offenders obviates the need for diagnostic testing in most patients. When endoscopy is performed, localized ulceration or inflammation is evident. Histologically, acute inflammation is typical. Chest CT imaging will sometimes reveal esophageal thickening consistent with transmural inflammation. Although the condition usually resolves within days to weeks, symptoms may persist for months and stricture can develop in severe cases. No specific therapy is known to hasten the healing process, but antisecretory medications are frequently prescribed to remove concomitant reflux as an aggravating factor. When healing results in stricture formation, dilation is indicated.
FOREIGN BODIES AND FOOD IMPACTION
Food or foreign bodies may lodge in the esophagus causing complete obstruction, which in turn can cause an inability to handle secretions (foaming at the mouth) and severe chest pain. Food impaction may occur due to stricture, carcinoma, Schatzki ring, eosinophilic esophagitis, or simply inattentive eating. If it does not spontaneously resolve, impacted food can be dislodged endoscopically. Use of meat tenderizer enzymes to facilitate passage of a meat bolus is discouraged because of potential esophageal injury. Glucagon (1 mg IV) is sometimes tried before endoscopic dislodgement. After emergent treatment, patients should be evaluated for potential causes of the impaction with treatment rendered as indicated.
ESOPHAGEAL MANIFESTATIONS OF SYSTEMIC DISEASE
SCLERODERMA AND COLLAGEN VASCULAR DISEASES
Scleroderma esophagus (hypotensive LES and absent esophageal peristalsis) was initially described as a manifestation of scleroderma or other collagen vascular diseases and thought to be specific for these disorders. However, this nomenclature subsequently proved unfortunate and has been discarded because an estimated half of qualifying patients do not have an identifiable systemic disease, and reflux disease is often the only identifiable association. When scleroderma esophagus occurs as a manifestation of a collagen vascular disease, the histopathologic findings are of infiltration and destruction of the esophageal muscularis propria with collagen deposition and fibrosis. The pathogenesis of absent peristalsis and LES hypotension in the absence of a collagen vascular disease is unknown. Regardless of the underlying cause, the manometric abnormalities predispose patients to severe GERD due to inadequate LES barrier function combined with poor esophageal clearance of refluxed acid. Dysphagia may also be manifest but is generally mild and alleviated by eating in an upright position and using liquids to facilitate solid emptying.
DERMATOLOGIC DISEASES
A host of dermatologic disorders (pemphigus vulgaris, bullous pemphigoid, cicatricial pemphigoid, Behçet’s syndrome, and epidermolysis bullosa) can affect the oropharynx and esophagus, particularly the proximal esophagus with blisters, bullae, webs, and strictures. Glucocorticoid treatment is usually effective. Erosive lichen planus, Stevens-Johnson syndrome, and graft-versus-host disease can also involve the esophagus. Esophageal dilatation may be necessary to treat strictures.
348 |
Peptic Ulcer Disease and Related Disorders |
PEPTIC ULCER DISEASE
Burning epigastric pain exacerbated by fasting and improved with meals is a symptom complex associated with peptic ulcer disease (PUD). An ulcer is defined as disruption of the mucosal integrity of the stomach and/or duodenum leading to a local defect or excavation due to active inflammation. Ulcers occur within the stomach and/or duodenum and are often chronic in nature. Acid peptic disorders are very common in the United States, with 4 million individuals (new cases and recurrences) affected per year. Lifetime prevalence of PUD in the United States is ~12% in men and 10% in women. PUD significantly affects quality of life by impairing overall patient well-being and contributing substantially to work absenteeism. Moreover, an estimated 15,000 deaths per year occur as a consequence of complicated PUD. The financial impact of these common disorders has been substantial, with an estimated burden on direct and indirect health care costs of ~$6 billion per year in the United States, with $3 billion spent on hospitalizations, $2 billion on physician office visits, and $1 billion in decreased productivity and days lost from work.
GASTRIC PHYSIOLOGY
Despite the constant attack on the gastroduodenal mucosa by a host of noxious agents (acid, pepsin, bile acids, pancreatic enzymes, drugs, and bacteria), integrity is maintained by an intricate system that provides mucosal defense and repair.
Gastric Anatomy The gastric epithelial lining consists of rugae that contain microscopic gastric pits, each branching into four or five gastric glands made up of highly specialized epithelial cells. The makeup of gastric glands varies with their anatomic location. Glands within the gastric cardia comprise <5% of the gastric gland area and contain mucous and endocrine cells. The 75% of gastric glands are found within the oxyntic mucosa and contain mucous neck, parietal, chief, endocrine, enterochromaffin, and enterochromaffin-like (ECL) cells (Fig. 348-1). Pyloric glands contain mucous and endocrine cells (including gastrin cells) and are found in the antrum.
FIGURE 348-1 Diagrammatic representation of the oxyntic gastric gland. (Adapted from S Ito, RJ Winchester: J Cell Biol 16:541, 1963.doi:10.1083/jcb.16.3.541. © 1963 Ito and Winchester.)
The parietal cell, also known as the oxyntic cell, is usually found in the neck, or isthmus, or in the oxyntic gland. The resting, or unstimulated, parietal cell has prominent cytoplasmic tubulovesicles and intracellular canaliculi containing short microvilli along its apical surface (Fig. 348-2). H+K+-adenosine triphosphatase (ATPase) is expressed in the tubulovesicle membrane; upon cell stimulation, this membrane, along with apical membranes, transforms into a dense network of apical intracellular canaliculi containing long microvilli. Acid secretion, a process requiring high energy, occurs at the apical canalicular surface. Numerous mitochondria (30–40% of total cell volume) generate the energy required for secretion.
FIGURE 348-2 Gastric parietal cell undergoing transformation after secretagogue-mediated stimulation. cAMP, cyclic adenosine monophosphate. (Adapted from SJ Hersey, G Sachs: Physiol Rev 75:155, 1995.)
Gastroduodenal Mucosal Defense The gastric epithelium is under constant assault by a series of endogenous noxious factors, including hydrochloric acid (HCl), pepsinogen/pepsin, and bile salts. In addition, a steady flow of exogenous substances such as medications, alcohol, and bacteria encounter the gastric mucosa. A highly intricate biologic system is in place to provide defense from mucosal injury and to repair any injury that may occur.
The mucosal defense system can be envisioned as a three-level barrier, composed of preepithelial, epithelial, and subepithelial elements (Fig. 348-3). The first line of defense is a mucus-bicarbonate-phospholipid layer, which serves as a physicochemical barrier to multiple molecules, including hydrogen ions. Mucus is secreted in a regulated fashion by gastroduodenal surface epithelial cells. It consists primarily of water (95%) and a mixture of phospholipids and glycoproteins (mucin). The mucous gel functions as a nonstirred water layer impeding diffusion of ions and molecules such as pepsin. Bicarbonate, secreted in a regulated manner by surface epithelial cells of the gastroduodenal mucosa into the mucous gel, forms a pH gradient ranging from 1 to 2 at the gastric luminal surface and reaching 6 to 7 along the epithelial cell surface.
FIGURE 348-3 Components involved in providing gastroduodenal mucosal defense and repair. CCK, cholecystokinin; CRF, corticotropin-releasing factor; EGF, epidermal growth factor; HCl, hydrochloride; IGF, insulin-like growth factor; TGFα, transforming growth factor α; TRF, thyrotropin releasing factor. (Modified and updated from Tarnawski A. Cellular and molecular mechanisms of mucosal defense and repair. In: Yoshikawa T, Arakawa T. Bioregulation and Its Disorders in the Gastrointestinal Tract. Tokyo, Japan: Blackwell Science, 1998:3–17.)
Surface epithelial cells provide the next line of defense through several factors, including mucus production, epithelial cell ionic transporters that maintain intracellular pH and bicarbonate production, and intracellular tight junctions. Surface epithelial cells generate heat shock proteins that prevent protein denaturation and protect cells from certain factors such as increased temperature, cytotoxic agents, or oxidative stress. Epithelial cells also generate trefoil factor family peptides and cathelicidins, which also play a role in surface cell protection and regeneration. If the preepithelial barrier were breached, gastric epithelial cells bordering a site of injury can migrate to restore a damaged region (restitution). This process occurs independent of cell division and requires uninterrupted blood flow and an alkaline pH in the surrounding environment. Several growth factors, including epidermal growth factor (EGF), transforming growth factor (TGF) α, and basic fibroblast growth factor (FGF), modulate the process of restitution. Larger defects that are not effectively repaired by restitution require cell proliferation. Epithelial cell regeneration is regulated by prostaglandins and growth factors such as EGF and TGF-α. In tandem with epithelial cell renewal, formation of new vessels (angiogenesis) within the injured microvascular bed occurs. Both FGF and vascular endothelial growth factor (VEGF) are important in regulating angiogenesis in the gastric mucosa.
An elaborate microvascular system within the gastric submucosal layer is the key component of the subepithelial defense/repair system, providing HCO3–, which neutralizes the acid generated by the parietal cell. Moreover, this microcirculatory bed provides an adequate supply of micronutrients and oxygen while removing toxic metabolic by-products.
Prostaglandins play a central role in gastric epithelial defense/repair (Fig. 348-4). The gastric mucosa contains abundant levels of prostaglandins that regulate the release of mucosal bicarbonate and mucus, inhibit parietal cell secretion, and are important in maintaining mucosal blood flow and epithelial cell restitution. Prostaglandins are derived from esterified arachidonic acid, which is formed from phospholipids (cell membrane) by the action of phospholipase A2. A key enzyme that controls the rate-limiting step in prostaglandin synthesis is cyclooxygenase (COX), which is present in two isoforms (COX-1, COX-2), each having distinct characteristics regarding structure, tissue distribution, and expression. COX-1 is expressed in a host of tissues, including the stomach, platelets, kidneys, and endothelial cells. This isoform is expressed in a constitutive manner and plays an important role in maintaining the integrity of renal function, platelet aggregation, and gastrointestinal (GI) mucosal integrity. In contrast, the expression of COX-2 is inducible by inflammatory stimuli, and it is expressed in macrophages, leukocytes, fibroblasts, and synovial cells. The beneficial effects of nonsteroidal anti-inflammatory drugs (NSAIDs) on tissue inflammation are due to inhibition of COX-2; the toxicity of these drugs (e.g., GI mucosal ulceration and renal dysfunction) is related to inhibition of the COX-1 isoform. The highly COX-2–selective NSAIDs have the potential to provide the beneficial effect of decreasing tissue inflammation while minimizing toxicity in the GI tract. Selective COX-2 inhibitors have had adverse effects on the cardiovascular system, leading to increased risk of myocardial infarction. Therefore, the U.S. Food and Drug Administration (FDA) has removed two of these agents (valdecoxib and rofecoxib) from the market (see below).
FIGURE 348-4 Schematic representation of the steps involved in synthesis of prostaglandin E2 (PGE2) and prostacyclin (PGI2). Characteristics and distribution of the cyclooxygenase (COX) enzymes 1 and 2 are also shown. TXA2, thromboxane A2.
Nitric oxide (NO) is important in the maintenance of gastric mucosal integrity. The key enzyme NO synthase is constitutively expressed in the mucosa and contributes to cytoprotection by stimulating gastric mucus, increasing mucosal blood flow, and maintaining epithelial cell barrier function. The central nervous system (CNS) and hormonal factors also play a role in regulating mucosal defense through multiple pathways (Fig. 348-3).
Physiology of Gastric Secretion Hydrochloric acid and pepsinogen are the two principal gastric secretory products capable of inducing mucosal injury. Gastric acid and pepsinogen play a physiologic role in protein digestion; absorption of iron, calcium, magnesium, and vitamin B12; and killing ingested bacteria. Acid secretion should be viewed as occurring under basal and stimulated conditions. Basal acid production occurs in a circadian pattern, with highest levels occurring during the night and lowest levels during the morning hours. Cholinergic input via the vagus nerve and histaminergic input from local gastric sources are the principal contributors to basal acid secretion. Stimulated gastric acid secretion occurs primarily in three phases based on the site where the signal originates (cephalic, gastric, and intestinal). Sight, smell, and taste of food are the components of the cephalic phase, which stimulates gastric secretion via the vagus nerve. The gastric phase is activated once food enters the stomach. This component of secretion is driven by nutrients (amino acids and amines) that directly stimulate the G cell to release gastrin, which in turn activates the parietal cell via direct and indirect mechanisms. Distention of the stomach wall also leads to gastrin release and acid production. The last phase of gastric acid secretion is initiated as food enters the intestine and is mediated by luminal distention and nutrient assimilation. A series of pathways that inhibit gastric acid production are also set into motion during these phases. The GI hormone somatostatin is released from endocrine cells found in the gastric mucosa (D cells) in response to HCl. Somatostatin can inhibit acid production by both direct (parietal cell) and indirect mechanisms (decreased histamine release from ECL cells and gastrin release from G cells). Additional neural (central and peripheral) and humoral (amylin, atrial natriuretic peptide [ANP], cholecystokinin, ghrelin, interleukin 11 [IL-11], obestatin, secretin, and serotonin) factors play a role in counterbalancing acid secretion. Under physiologic circumstances, these phases occur simultaneously. Ghrelin, the appetite-regulating hormone expressed in Gr cells in the stomach, may increase gastric acid secretion through stimulation of histamine release from ECL cells, but this remains to be confirmed.
The acid-secreting parietal cell is located in the oxyntic gland, adjacent to other cellular elements (ECL cell, D cell) important in the gastric secretory process (Fig. 348-5). This unique cell also secretes intrinsic factor (IF) and IL-11. The parietal cell expresses receptors for several stimulants of acid secretion, including histamine (H2), gastrin (cholecystokinin B/gastrin receptor), and acetylcholine (muscarinic, M3). Binding of histamine to the H2 receptor leads to activation of adenylate cyclase and an increase in cyclic adenosine monophosphate (AMP). Activation of the gastrin and muscarinic receptors results in activation of the protein kinase C/phosphoinositide signaling pathway. Each of these signaling pathways in turn regulates a series of downstream kinase cascades that control the acid-secreting pump, H+,K+-ATPase. The discovery that different ligands and their corresponding receptors lead to activation of different signaling pathways explains the potentiation of acid secretion that occurs when histamine and gastrin or acetylcholine are combined. More importantly, this observation explains why blocking one receptor type (H2) decreases acid secretion stimulated by agents that activate a different pathway (gastrin, acetylcholine). Parietal cells also express receptors for ligands that inhibit acid production (prostaglandins, somatostatin, and EGF). Histamine also stimulates gastric acid secretion indirectly by activating the histamine H3 receptor on D-cells, which inhibits somatostatin release.
FIGURE 348-5 Regulation of gastric acid secretion at the cellular level. ACh, acetylcholine; ANP, atrial natriuretic peptide; CGRP, calcitonin gene-related peptide; EC, enterochromaffin; ECL, enterochromaffin-like; GRP, gastrin-releasing peptide; PACAP, pituitary adenylate-cyclase activating peptide; SST, somatostatin; VIP, vasoactive intestinal peptide.
The enzyme H+,K+-ATPase is responsible for generating the large concentration of H+. It is a membrane-bound protein that consists of two subunits, α and β. The active catalytic site is found within the α subunit; the function of the β subunit is unclear. This enzyme uses the chemical energy of adenosine triphosphate (ATP) to transfer H+ ions from parietal cell cytoplasm to the secretory canaliculi in exchange for K+. The H+,K+-ATPase is located within the secretory canaliculus and in nonsecretory cytoplasmic tubulovesicles. The tubulovesicles are impermeable to K+, which leads to an inactive pump in this location. The distribution of pumps between the nonsecretory vesicles and the secretory canaliculus varies according to parietal cell activity (Fig. 348-2). Proton pumps are recycled back to the inactive state in cytoplasmic vesicles once parietal cell activation ceases. Small G proteins of the Rab family and secretory carrier membrane proteins (SCAMPS) are postulated to participate in parietal cell membrane translocation. In addition, acid secretion requires a number of apical and basolateral parietal cell membrane chloride and potassium channels.
The chief cell, found primarily in the gastric fundus, synthesizes and secretes pepsinogen, the inactive precursor of the proteolytic enzyme pepsin. The acid environment within the stomach leads to cleavage of the inactive precursor to pepsin and provides the low pH (<2) required for pepsin activity. Pepsin activity is significantly diminished at a pH of 4 and irreversibly inactivated and denatured at a pH of ≥7. Many of the secretagogues that stimulate acid secretion also stimulate pepsinogen release. The precise role of pepsin in the pathogenesis of PUD remains to be established.
PATHOPHYSIOLOGIC BASIS OF PEPTIC ULCER DISEASE
PUD encompasses both gastric and duodenal ulcers. Ulcers are defined as breaks in the mucosal surface >5 mm in size, with depth to the submucosa. Duodenal ulcers (DUs) and gastric ulcers (GUs) share many common features in terms of pathogenesis, diagnosis, and treatment, but several factors distinguish them from one another. Helicobacter pylori and NSAIDs are the most common risk factors for PUD, with estimated odds ratios in the United States of 3.7 and 3.3, respectively. Additional risk factors (odds ratio) include chronic obstructive lung disease (2.34), chronic renal insufficiency (2.29), current tobacco use (1.99), former tobacco use (1.55), older age (1.67), three or more doctor visits in a year (1.49), coronary heart disease (1.46), former alcohol use (1.29), African-American race (1.20), obesity (1.18), and diabetes (1.13). The mechanisms by which some of these risk factors lead to ulcer disease are highlighted below.
Epidemiology • DUODENAL ULCERS DUs are estimated to occur in 6–15% of the Western population. The incidence of DUs declined steadily from 1960 to 1980 and has remained stable since then. The death rates, need for surgery, and physician visits have decreased by >50% over the past 30 years. The reason for the reduction in the frequency of DUs is likely related to the decreasing frequency of H. pylori. Before the discovery of H. pylori, the natural history of DUs was typified by frequent recurrences after initial therapy. Eradication of H. pylori has greatly reduced these recurrence rates.
GASTRIC ULCERS GUs tend to occur later in life than duodenal lesions, with a peak incidence reported in the sixth decade. More than one-half of GUs occur in males and are less common than DUs, perhaps due to the higher likelihood of GUs being silent and presenting only after a complication develops. Autopsy studies suggest a similar incidence of DUs and GUs.
Pathology • DUODENAL ULCERS DUs occur most often in the first portion of the duodenum (>95%), with ~90% located within 3 cm of the pylorus. They are usually ≤1 cm in diameter but can occasionally reach 3–6 cm (giant ulcer). Ulcers are sharply demarcated, with depth at times reaching the muscularis propria. The base of the ulcer often consists of a zone of eosinophilic necrosis with surrounding fibrosis. Malignant DUs are extremely rare.
GASTRIC ULCERS In contrast to DUs, GUs can represent a malignancy and should be biopsied upon discovery. Benign GUs are most often found distal to the junction between the antrum and the acid secretory mucosa. Benign GUs are quite rare in the gastric fundus and are histologically similar to DUs. Benign GUs associated with H. pylori are also associated with antral gastritis. In contrast, NSAID-related GUs are not accompanied by chronic active gastritis but may instead have evidence of a chemical gastropathy, typified by foveolar hyperplasia, edema of the lamina propria, and epithelial regeneration in the absence of H. pylori. Extension of smooth-muscle fibers into the upper portions of the mucosa, where they are not typically found, may also occur.
Pathophysiology • DUODENAL ULCERS H. pylori and NSAID-induced injury account for the majority of DUs. Many acid secretory abnormalities have been described in DU patients. Of these, average basal and nocturnal gastric acid secretion appears to be increased in DU patients as compared to controls; however, the level of overlap between DU patients and control subjects is substantial. The reason for this altered secretory process is unclear, but H. pylori infection may contribute. Bicarbonate secretion is significantly decreased in the duodenal bulb of patients with an active DU as compared to control subjects. H. pylori infection may also play a role in this process (see below).
GASTRIC ULCERS As in DUs, the majority of GUs can be attributed to either H. pylori or NSAID-induced mucosal damage. GUs that occur in the prepyloric area or those in the body associated with a DU or a duodenal scar are similar in pathogenesis to DUs. Gastric acid output (basal and stimulated) tends to be normal or decreased in GU patients. When GUs develop in the presence of minimal acid levels, impairment of mucosal defense factors may be present. GUs have been classified based on their location: Type I occur in the gastric body and tend to be associated with low gastric acid production; type II occur in the antrum and gastric acid can vary from low to normal; type III occur within 3 cm of the pylorus and are commonly accompanied by DUs and normal or high gastric acid production; and type IV are found in the cardia and are associated with low gastric acid production.
H. PYLORI AND ACID PEPTIC DISORDERS Gastric infection with the bacterium H. pylori accounts for the majority of PUD (Chap. 188). This organism also plays a role in the development of gastric mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma. Although the entire genome of H. pylori has been sequenced, it is still not clear how this organism, which resides in the stomach, causes ulceration in the duodenum, or whether its eradication will lead to a decrease in gastric cancer.
The bacterium The bacterium, initially named Campylobacter pyloridis, is a gram-negative microaerophilic rod found most commonly in the deeper portions of the mucous gel coating the gastric mucosa or between the mucous layer and the gastric epithelium. It may attach to gastric epithelium but under normal circumstances does not appear to invade cells. It is strategically designed to live within the aggressive environment of the stomach. It is S-shaped (~0.5–3 μm in size) and contains multiple sheathed flagella. Initially, H. pylori resides in the antrum but, over time, migrates toward the more proximal segments of the stomach. The organism is capable of transforming into a coccoid form, which represents a dormant state that may facilitate survival in adverse conditions. The genome of H. pylori (1.65 million base pairs) encodes ~1500 proteins. Among this multitude of proteins there are factors that are essential determinants of H. pylori–mediated pathogenesis and colonization such as the outer membrane protein (Hop proteins), urease, and the vacuolating cytotoxin (Vac A). Moreover, the majority of H. pylori strains contain a genomic fragment that encodes the cag pathogenicity island (cag-PAI). Several of the genes that make up cag-PAI encode components of a type IV secretion island that translocates Cag A into host cells. Once in the cell, Cag A activates a series of cellular events important in cell growth and cytokine production. H. pylori also has extensive genetic diversity that in turn enhances its ability to promote disease. The first step in infection by H. pylori is dependent on the bacteria’s motility and its ability to produce urease. Urease produces ammonia from urea, an essential step in alkalinizing the surrounding pH. Additional bacterial factors include catalase, lipase, adhesins, platelet-activating factor, and pic B (induces cytokines). Multiple strains of H. pylori exist and are characterized by their ability to express several of these factors (Cag A, Vac A, etc.). It is possible that the different diseases related to H. pylori infection can be attributed to different strains of the organism with distinct pathogenic features.
Epidemiology The prevalence of H. pylori varies throughout the world and depends largely on the overall standard of living in the region. In developing parts of the world, 80% of the population may be infected by the age of 20, whereas the prevalence is 20–50% in industrialized countries. In contrast, in the United States this organism is rare in childhood. The overall prevalence of H. pylori in the United States is ~30%, with individuals born before 1950 having a higher rate of infection than those born later. About 10% of Americans <30 years of age are colonized with the bacteria. The rate of infection with H. pylori in industrialized countries has decreased substantially in recent decades. The steady increase in the prevalence of H. pylori noted with increasing age is due primarily to a cohort effect, reflecting higher transmission during a period in which the earlier cohorts were children. It has been calculated through mathematical models that improved sanitation during the latter half of the nineteenth century dramatically decreased transmission of H. pylori. Moreover, with the present rate of intervention, the organism will be ultimately eliminated from the United States. Two factors that predispose to higher colonization rates include poor socioeconomic status and less education. These factors, not race, are responsible for the rate of H. pylori infection in blacks and Hispanic Americans being double the rate seen in whites of comparable age. Other risk factors for H. pylori infection are (1) birth or residence in a developing country, (2) domestic crowding, (3) unsanitary living conditions, (4) unclean food or water, and (5) exposure to gastric contents of an infected individual.
Transmission of H. pylori occurs from person to person, following an oral-oral or fecal-oral route. The risk of H. pylori infection is declining in developing countries. The rate of infection in the United States has fallen by >50% when compared to 30 years ago.
Pathophysiology H. pylori infection is virtually always associated with a chronic active gastritis, but only 10–15% of infected individuals develop frank peptic ulceration. The basis for this difference is unknown, but is likely due to a combination of host and bacterial factors some of which are outlined below. Initial studies suggested that >90% of all DUs were associated with H. pylori, but H. pylori is present in only 30–60% of individuals with GUs and 50–70% of patients with DUs. The pathophysiology of ulcers not associated with H. pylori or NSAID ingestion (or the rare Zollinger-Ellison syndrome [ZES]) is becoming more relevant as the incidence of H. pylori is dropping, particularly in the Western world (see below).
The particular end result of H. pylori infection (gastritis, PUD, gastric MALT lymphoma, gastric cancer) is determined by a complex interplay between bacterial and host factors (Fig. 348-6).
FIGURE 348-6 Outline of the bacterial and host factors important in determining H. pylori–induced gastrointestinal disease. MALT, mucosal-associated lymphoid tissue.
1. Bacterial factors: H. pylori is able to facilitate gastric residence, induce mucosal injury, and avoid host defense. Different strains of H. pylori produce different virulence factors. A specific region of the bacterial genome, the pathogenicity island (cag-PAI), encodes the virulence factors Cag A and pic B. Vac A also contributes to pathogenicity, although it is not encoded within the pathogenicity island. These virulence factors, in conjunction with additional bacterial constituents, can cause mucosal damage, in part through their ability to target the host immune cells. For example, Vac A targets human CD4 T cells, inhibiting their proliferation and in addition can disrupt normal function of B cells, CD8 T cells, macrophages, and mast cells. Multiple studies have demonstrated that H. pylori strains that are cag-PAI positive are associated with a higher risk of PUD, premalignant gastric lesions, and gastric cancer than are strains that lack the cag-PAI. In addition, H. pylori may directly inhibit parietal cell H+,K+-ATPase activity through a Cag A–dependent mechanism, leading in part to the low acid production observed after acute infection with the organism. Urease, which allows the bacteria to reside in the acidic stomach, generates NH3, which can damage epithelial cells. The bacteria produce surface factors that are chemotactic for neutrophils and monocytes, which in turn contribute to epithelial cell injury (see below). H. pylori makes proteases and phospholipases that break down the glycoprotein lipid complex of the mucous gel, thus reducing the efficacy of this first line of mucosal defense. H. pylori expresses adhesins (OMPs like BabA), which facilitate attachment of the bacteria to gastric epithelial cells. Although lipopolysaccharide (LPS) of gram-negative bacteria often plays an important role in the infection, H. pylori LPS has low immunologic activity compared to that of other organisms. It may promote a smoldering chronic inflammation.
2. Host factors: Studies in twins suggest that there may be genetic predisposition to acquire H. pylori. The inflammatory response to H. pylori includes recruitment of neutrophils, lymphocytes (T and B), macrophages, and plasma cells. The pathogen leads to local injury by binding to class II major histocompatibility complex (MHC) molecules expressed on gastric epithelial cells, leading to cell death (apoptosis). Moreover, bacterial strains that encode cag-PAI can introduce Cag A into the host cells, leading to further cell injury and activation of cellular pathways involved in cytokine production and repression of tumor-suppressor genes. Elevated concentrations of multiple cytokines are found in the gastric epithelium of H. pylori–infected individuals, including interleukin (IL) 1α/β, IL-2, IL-6, IL-8, tumor necrosis factor (TNF) α, and interferon (IFN) γ. H. pylori infection also leads to both a mucosal and a systemic humoral response, which does not lead to eradication of the bacteria but further compounds epithelial cell injury. Additional mechanisms by which H. pylori may cause epithelial cell injury include (1) activated neutrophil-mediated production of reactive oxygen or nitrogen species and enhanced epithelial cell turnover and (2) apoptosis related to interaction with T cells (T helper 1, or TH1, cells) and IFN-γ. Finally, the human stomach can be colonized by a host of commensal organisms that may affect the likelihood of H. pylori–mediated mucosal injury.
The reason for H. pylori–mediated duodenal ulceration remains unclear. Studies suggest that H. pylori associated with duodenal ulceration may be more virulent. In addition, certain specific bacterial factors such as the DU-promoting gene A (dupA), may be associated with the development of DUs. Another potential contributing factor is that gastric metaplasia in the duodenum of DU patients, which may be due to high acid exposure (see below), permits H. pylori to bind to it and produce local injury secondary to the host response. Another hypothesis is that H. pylori antral infection could lead to increased acid production, increased duodenal acid, and mucosal injury. Basal and stimulated (meal, gastrin-releasing peptide [GRP]) gastrin release are increased in H. pylori–infected individuals, and somatostatin-secreting D cells may be decreased. H. pylori infection might induce increased acid secretion through both direct and indirect actions of H. pylori and proinflammatory cytokines (IL-8, TNF, and IL-1) on G, D, and parietal cells (Fig. 348-7). GUs, in contrast, are associated with H. pylori–induced pangastritis and normal or low gastric acid secretion. H. pylori infection has also been associated with decreased duodenal mucosal bicarbonate production. Data supporting and contradicting each of these interesting theories have been demonstrated. Thus, the mechanism by which H. pylori infection of the stomach leads to duodenal ulceration remains to be established.
FIGURE 348-7 Summary of potential mechanisms by which H. pylori may lead to gastric secretory abnormalities. D, somatostatin cell; ECL, enterochromaffin-like cell; G, G cell. (Adapted from J Calam et al: Gastroenterology 113:543, 1997.)
In summary, the final effect of H. pylori on the GI tract is variable and determined by microbial and host factors. The type and distribution of gastritis correlate with the ultimate gastric and duodenal pathology observed. Specifically, the presence of antral-predominant gastritis is associated with DU formation; gastritis involving primarily the corpus predisposes to the development of GUs, gastric atrophy, and ultimately gastric carcinoma (Fig. 348-8).
FIGURE 348-8 Natural history of H. pylori infection. MALT, mucosal-associated lymphoid tissue. (Used with permission from S Suerbaum, P Michetti: N Engl J Med 347:1175, 2002.)
NSAID-INDUCED DISEASE
Epidemiology NSAIDs represent a group of the most commonly used medications in the United States. More than 30 billion over-the-counter tablets and over 100 million prescriptions are sold yearly in the United States alone. In fact, after the introduction of COX-2 inhibitors in the year 2000, the number of prescriptions written for NSAIDs was >111 million at a cost of $4.8 billion. Side effects and complications due to NSAIDs are considered the most common drug-related toxicities in the United States. The spectrum of NSAID-induced morbidity ranges from nausea and dyspepsia (prevalence reported as high as 50–60%) to a serious GI complication such as endoscopy-documented peptic ulceration (15–30% of individuals taking NSAIDs regularly) complicated by bleeding or perforation in as many as 1.5% of users per year. It is estimated that NSAID-induced GI bleeding accounts for 60,000–120,000 hospital admissions per year, and deaths related to NSAID-induced toxicity may be as high as 16,000 per year in the United States. Approximately 4–5% of patients develop symptomatic ulcers within 1 year. Unfortunately, dyspeptic symptoms do not correlate with NSAID-induced pathology. Over 80% of patients with serious NSAID-related complications did not have preceding dyspepsia. In view of the lack of warning signs, it is important to identify patients who are at increased risk for morbidity and mortality related to NSAID usage. Even 75 mg/d of aspirin may lead to serious GI ulceration; thus, no dose of NSAID is completely safe. In fact, the incidence of mucosal injury (ulcers and erosions) in patients taking low-dose aspirin (75–325 mg) has been estimated to range from as low as 8% to as high as 60%. It appears that H. pylori infection increases the risk of PUD-associated GI bleeding in chronic users of low-dose aspirin. Established risk factors include advanced age, history of ulcer, concomitant use of glucocorticoids, high-dose NSAIDs, multiple NSAIDs, concomitant use of anticoagulants, clopidogrel, and serious or multisystem disease. Possible risk factors include concomitant infection with H. pylori, cigarette smoking, and alcohol consumption.
Pathophysiology Prostaglandins play a critical role in maintaining gastroduodenal mucosal integrity and repair. It therefore follows that interruption of prostaglandin synthesis can impair mucosal defense and repair, thus facilitating mucosal injury via a systemic mechanism. Animal studies have demonstrated that neutrophil adherence to the gastric microcirculation plays an essential role in the initiation of NSAID-induced mucosal injury. A summary of the pathogenetic pathways by which systemically administered NSAIDs may lead to mucosal injury is shown in Fig. 348-9. Single nucleotide polymorphisms (SNPs) have been found in several genes, including those encoding certain subtypes of cytochrome P450 (see below), interleukin-1β (IL-1β), angiotensinogen (AGT), and an organic ion transporting polypeptide (SLCO1B1), but these findings need confirmation in larger scale studies.
FIGURE 348-9 Mechanisms by which nonsteroidal anti-inflammatory drugs may induce mucosal injury. (Adapted from J Scheiman et al: J Clin Outcomes Management 3:23, 1996. Copyright 2003 Turner White Communications, Inc., www.turner-white.com. Used with permission.)
Injury to the mucosa also occurs as a result of the topical encounter with NSAIDs. Aspirin and many NSAIDs are weak acids that remain in a nonionized lipophilic form when found within the acid environment of the stomach. Under these conditions, NSAIDs migrate across lipid membranes of epithelial cells, leading to cell injury once trapped intracellularly in an ionized form. Topical NSAIDs can also alter the surface mucous layer, permitting back diffusion of H+ and pepsin, leading to further epithelial cell damage. Moreover, enteric-coated or buffered preparations are also associated with risk of peptic ulceration.
The interplay between H. pylori and NSAIDs in the pathogenesis of PUD is complex. Meta-analysis supports the conclusion that each of these aggressive factors is independent and synergistic risk factors for PUD and its complications such as GI bleeding. For example, eradication of H. pylori reduces the likelihood of GI complications in high-risk individuals to levels observed in individuals with average risk of NSAID-induced complications.
PATHOGENETIC FACTORS UNRELATED TO H. PYLORI AND NSAIDs IN ACID PEPTIC DISEASE Cigarette smoking has been implicated in the pathogenesis of PUD. Not only have smokers been found to have ulcers more frequently than do nonsmokers, but smoking appears to decrease healing rates, impair response to therapy, and increase ulcer-related complications such as perforation. The mechanism responsible for increased ulcer diathesis in smokers is unknown. Theories have included altered gastric emptying, decreased proximal duodenal bicarbonate production, increased risk for H. pylori infection, and cigarette-induced generation of noxious mucosal free radicals. Genetic predisposition may play a role in ulcer development. First-degree relatives of DU patients are three times as likely to develop an ulcer; however, the potential role of H. pylori infection in contacts is a major consideration. Increased frequencies of blood group O and of the nonsecretor status have also been implicated as genetic risk factors for peptic diathesis. However, H. pylori preferentially binds to group O antigens. Additional genetic factors have been postulated to predispose certain individuals to developing PUD and/or upper GI bleeding. Specifically, genes encoding the NSAID-metabolizing enzymes cytochrome P450 2C9 and 2C8 (CYP2C9 and CYP2C8) are potential susceptibility genes for NSAID-induced PUD, but unfortunately, the studies have not been consistent in demonstrating this association. In a United Kingdom study, the CYP2C19*17 gain-of-function polymorphism was associated with PUD in a Caucasian cohort, irrespective of ulcer etiology. These findings need to be confirmed in broader studies. Psychological stress has been thought to contribute to PUD, but studies examining the role of psychological factors in its pathogenesis have generated conflicting results. Although PUD is associated with certain personality traits (neuroticism), these same traits are also present in individuals with nonulcer dyspepsia (NUD) and other functional and organic disorders.
Diet has also been thought to play a role in peptic diseases. Certain foods and beverages can cause dyspepsia, but no convincing studies indicate an association between ulcer formation and a specific diet. Specific chronic disorders have been shown to have a strong association with PUD: (1) advanced age, (2) chronic pulmonary disease, (3) chronic renal failure, (4) cirrhosis, (5) nephrolithiasis, (6) α1-antitrypsin deficiency, and (7) systemic mastocytosis. Disorders with a possible association are (1) hyperparathyroidism, (2) coronary artery disease, (3) polycythemia vera, (4) chronic pancreatitis, (5) former alcohol use, (6) obesity, (7) African-American race, and (8) three or more doctor visits in a year.
Multiple factors play a role in the pathogenesis of PUD. The two predominant causes are H. pylori infection and NSAID ingestion. PUD not related to H. pylori or NSAIDs is increasing. Other less common causes of PUD are shown in Table 348-1. These etiologic agents should be considered as the incidence of H. pylori is decreasing. Independent of the inciting or injurious agent, peptic ulcers develop as a result of an imbalance between mucosal protection/repair and aggressive factors. Gastric acid plays an important role in mucosal injury.
|
CAUSES OF ULCERS NOT CAUSED BY HELICOBACTER PYLORI AND NSAIDs |
Abbreviations: Hp, H. pylori; NSAIDs, nonsteroidal anti-inflammatory drugs.
CLINICAL FEATURES
History Abdominal pain is common to many GI disorders, including DU and GU, but has a poor predictive value for the presence of either DU or GU. Up to 10% of patients with NSAID-induced mucosal disease can present with a complication (bleeding, perforation, and obstruction) without antecedent symptoms. Despite this poor correlation, a careful history and physical examination are essential components of the approach to a patient suspected of having peptic ulcers.
Epigastric pain described as a burning or gnawing discomfort can be present in both DU and GU. The discomfort is also described as an ill-defined, aching sensation or as hunger pain. The typical pain pattern in DU occurs 90 minutes to 3 hours after a meal and is frequently relieved by antacids or food. Pain that awakes the patient from sleep (between midnight and 3 A.M.) is the most discriminating symptom, with two-thirds of DU patients describing this complaint. Unfortunately, this symptom is also present in one-third of patients with NUD (see below). Elderly patients are less likely to have abdominal pain as a manifestation of PUD and may instead present with a complication such as ulcer bleeding or perforation. The pain pattern in GU patients may be different from that in DU patients, where discomfort may actually be precipitated by food. Nausea and weight loss occur more commonly in GU patients. Endoscopy detects ulcers in <30% of patients who have dyspepsia.
The mechanism for development of abdominal pain in ulcer patients is unknown. Several possible explanations include acid-induced activation of chemical receptors in the duodenum, enhanced duodenal sensitivity to bile acids and pepsin, or altered gastroduodenal motility.
Variation in the intensity or distribution of the abdominal pain, as well as the onset of associated symptoms such as nausea and/or vomiting, may be indicative of an ulcer complication. Dyspepsia that becomes constant, is no longer relieved by food or antacids, or radiates to the back may indicate a penetrating ulcer (pancreas). Sudden onset of severe, generalized abdominal pain may indicate perforation. Pain worsening with meals, nausea, and vomiting of undigested food suggest gastric outlet obstruction. Tarry stools or coffee-ground emesis indicate bleeding.
Physical examination Epigastric tenderness is the most frequent finding in patients with GU or DU. Pain may be found to the right of the midline in 20% of patients. Unfortunately, the predictive value of this finding is rather low. Physical examination is critically important for discovering evidence of ulcer complication. Tachycardia and orthostasis suggest dehydration secondary to vomiting or active GI blood loss. A severely tender, board-like abdomen suggests a perforation. Presence of a succussion splash indicates retained fluid in the stomach, suggesting gastric outlet obstruction.
PUD-Related Complications • GASTROINTESTINAL BLEEDING GI bleeding is the most common complication observed in PUD. Bleeding is estimated to occur in 19.4–57 per 100,000 individuals in a general population or in approximately 15% of patients. Bleeding and complications of ulcer disease occur more often in individuals >60 years of age. The 30-day mortality rate is as high as 5–10%. The higher incidence in the elderly is likely due to the increased use of NSAIDs in this group. In addition, up to 80% of the mortality in PUD-related bleeding is due to nonbleeding causes such as multiorgan failure (24%), pulmonary complications (24%), and malignancy (34%).
Up to 20% of patients with ulcer-related hemorrhage bleed without any preceding warning signs or symptoms.
PERFORATION The second most common ulcer-related complication is perforation, being reported in as many as 6–7% of PUD patients with an estimated 30-day mortality of over 20%. As in the case of bleeding, the incidence of perforation in the elderly appears to be increasing secondary to increased use of NSAIDs. Penetration is a form of perforation in which the ulcer bed tunnels into an adjacent organ. DUs tend to penetrate posteriorly into the pancreas, leading to pancreatitis, whereas GUs tend to penetrate into the left hepatic lobe. Gastrocolic fistulas associated with GUs have also been described.
GASTRIC OUTLET OBSTRUCTION Gastric outlet obstruction is the least common ulcer-related complication, occurring in 1–2% of patients. A patient may have relative obstruction secondary to ulcer-related inflammation and edema in the peripyloric region. This process often resolves with ulcer healing. A fixed, mechanical obstruction secondary to scar formation in the peripyloric areas is also possible. The latter requires endoscopic (balloon dilation) or surgical intervention. Signs and symptoms relative to mechanical obstruction may develop insidiously. New onset of early satiety, nausea, vomiting, increase of postprandial abdominal pain, and weight loss should make gastric outlet obstruction a possible diagnosis.
Differential Diagnosis The list of GI and non-GI disorders that can mimic ulceration of the stomach or duodenum is quite extensive. The most commonly encountered diagnosis among patients seen for upper abdominal discomfort is NUD. NUD, also known as functional dyspepsia or essential dyspepsia, refers to a group of heterogeneous disorders typified by upper abdominal pain without the presence of an ulcer. Dyspepsia has been reported to occur in up to 30% of the U.S. population. Up to 60% of patients seeking medical care for dyspepsia have a negative diagnostic evaluation. The etiology of NUD is not established, and the potential role of H. pylori in NUD remains controversial.
Several additional disease processes that may present with “ulcer-like” symptoms include proximal GI tumors, gastroesophageal reflux, vascular disease, pancreaticobiliary disease (biliary colic, chronic pancreatitis), and gastroduodenal Crohn’s disease.
Diagnostic Evaluation In view of the poor predictive value of abdominal pain for the presence of a gastroduodenal ulcer and the multiple disease processes that can mimic this disease, the clinician is often confronted with having to establish the presence of an ulcer. Documentation of an ulcer requires either a radiographic (barium study) or an endoscopic procedure. However, a large percentage of patients with symptoms suggestive of an ulcer have NUD; testing for H. pylori and antibiotic therapy (see below) is appropriate for individuals who are otherwise healthy and <45 years of age, before embarking on a diagnostic evaluation (Chap. 54).
Barium studies of the proximal GI tract are still occasionally used as a first test for documenting an ulcer. The sensitivity of older single-contrast barium meals for detecting a DU is as high as 80%, with a double-contrast study providing detection rates as high as 90%. Sensitivity for detection is decreased in small ulcers (<0.5 cm), with presence of previous scarring, or in postoperative patients. A DU appears as a well-demarcated crater, most often seen in the bulb (Fig. 348-10A). A GU may represent benign or malignant disease. Typically, a benign GU also appears as a discrete crater with radiating mucosal folds originating from the ulcer margin (Fig. 348-10B). Ulcers >3 cm in size or those associated with a mass are more often malignant. Unfortunately, up to 8% of GUs that appear to be benign by radiographic appearance are malignant by endoscopy or surgery. Radiographic studies that show a GU must be followed by endoscopy and biopsy.
FIGURE 348-10 Barium study demonstrating (A) a benign duodenal ulcer and (B) a benign gastric ulcer.
Endoscopy provides the most sensitive and specific approach for examining the upper GI tract (Fig. 348-11). In addition to permitting direct visualization of the mucosa, endoscopy facilitates photographic documentation of a mucosal defect and tissue biopsy to rule out malignancy (GU) or H. pylori. Endoscopic examination is particularly helpful in identifying lesions too small to detect by radiographic examination, for evaluation of atypical radiographic abnormalities, or to determine if an ulcer is a source of blood loss.
FIGURE 348-11 Endoscopy demonstrating (A) a benign duodenal ulcer and (B) a benign gastric ulcer.
Although the methods for diagnosing H. pylori are outlined in Chap. 181, a brief summary will be included here (Table 348-2). Several biopsy urease tests have been developed (PyloriTek, CLOtest, Hpfast, Pronto Dry) that have a sensitivity and specificity of >90–95%. Several noninvasive methods for detecting this organism have been developed. Three types of studies routinely used include serologic testing, the 13C- or 14C-urea breath test, and the fecal H. pylori (Hp) antigen test. A urinary Hp antigen test, as well as a refined monoclonal antibody stool antigen test, appears promising.
|
TESTS FOR DETECTION OF H. PYLORI |
Occasionally, specialized testing such as serum gastrin and gastric acid analysis or sham feeding may be needed in individuals with complicated or refractory PUD (see “Zollinger-Ellison Syndrome [ZES],” below). Screening for aspirin or NSAIDs (blood or urine) may also be necessary in refractory H. pylori–negative PUD patients.