19 Vestibular nerve
Introduction
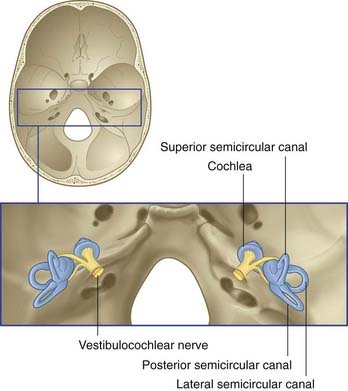
The vestibulocochlear nerve is primarily composed of the centrally directed axons of bipolar neurons housed in the petrous temporal bone (Figure 19.1). The peripheral processes are applied to neuroepithelial cells in the vestibular labyrinth and cochlea. The nerve enters the brainstem at the junctional region of pons and medulla oblongata. The functional anatomy of the vestibular division of the nerve is described in this chapter.
Vestibular System
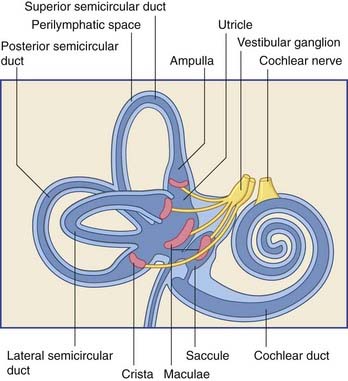
The vestibular labyrinth comprises the utricle, the saccule, and the cristae within three semicircular ducts (Figure 19.2). The utricle and saccule contain a 3 × 2 mm2 macula. Each semicircular duct contains an ampulla at one end, and the ampulla houses a crista. (It should be pointed out that clinicians commonly speak of ‘canals’ where ‘ducts’ would be strictly more appropriate.)
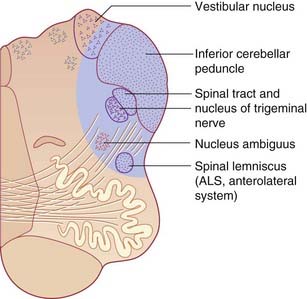
The bipolar cells of the vestibular (Scarpa’s) ganglion occupy the internal acoustic meatus. Their peripheral processes are applied to the five sensory end organs. Their central processes, which constitute the vestibular nerve, cross the subarachnoid space and synapse in the vestibular nuclei previously seen in Figures 17.14 and 17.15.
Static labyrinth: anatomy and actions
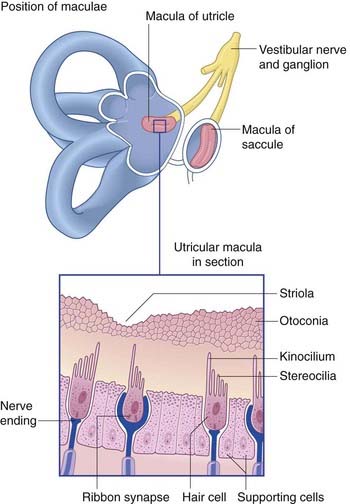
The position and structure of the maculae are shown in Figure 19.3. The utricular macula is relatively horizontal, the saccular macula is relatively vertical. The cuboidal cells lining the membranous labyrinth become columnar supporting cells in the maculae. Among the supporting cells are so-called hair cells, to which vestibular nerve endings are applied. Some hair cells are almost completely enclosed by large nerve endings, whereas others (phylogenetically older) receive only small contacts. At the cell bases are ribbon synapses, the synaptic vesicles being lined up along synaptic bars. Projecting from the free surface of each hair cell are about 100 stereocilia and, close to the cell margin, a single, long kinocilium. The hair cells discharge continuously, the resting rate being about 100 Hz.
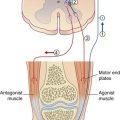
The lateral vestibulospinal tract, seen earlier in sections of medulla oblongata in Chapter 17, arises from large neurons in the lateral vestibular nucleus (of Deiters). The fibers descend in the anterior funiculus on the same side of the spinal cord and synapse upon extensor (antigravity) motor neurons. Both α and γ motor neurons are excited, and a significant part of the increased muscle tone is exerted by way of the gamma loop (Ch. 16). During standing, the tract is tonically active on both sides of the spinal cord. During walking, activity is selective for the quadriceps motor neurons of the leading leg; this commences following heel strike and continues during the stance phase (when the other leg is off the ground). Deiters’ nucleus is somatotopically organized, and the functionally appropriate neurons are selected by the flocculonodular lobe of the cerebellum. The flocculonodular lobe (Ch. 25) has two-way connections with all four vestibular nuclei.
A small, medial vestibulospinal tract arises in the medial and inferior vestibular nuclei (Figure 17.6). It descends bilaterally in the medial longitudinal fasciculus and terminates upon excitatory and inhibitory internuncials in the cervical spinal cord. It operates head-righting reflexes, which serve to keep the head – and the gaze – horizontal when the body is craned forward or to one side. Good examples of head-righting reflexes are to be seen around pool tables and in bowling alleys. An added twist can be provided, if required, by torsion of the eyeballs (up to 10°) within the orbital sockets. This eye-righting reflex is mediated by axons ascending the medial longitudinal fasciculus from the lateral vestibular nucleus to reach nuclei controlling the extraocular muscles. Evidence derived from unilateral vestibular destruction (Clinical Panel 19.1) indicates that the horizontal position of the eyes in the upright head is the result of a canceling effect of bilateral tonic activity in these Deitero-ocular pathways.
Clinical Panel 19.1 Vestibular disorders
Unilateral vestibular disease
The effects of unilateral vestibular disease are well demonstrated when the vestibular system is inactivated surgically, either during removal of an acoustic neuroma (Ch. 22) or as a last resort in treating paroxysmal attacks of vertigo. During the immediate postoperative period, the patient shows triple effects of loss of tonic input from the static labyrinth:
The medial vestibulospinal tract is also activated by the kinetic labyrinth.
The static labyrinth contributes to the sense of position. The sense of position of the body in space is normally provided by three sensory systems: the visual system, the conscious proprioceptive system, and the vestibular system. Deprived of one of the three, the individual can stand and walk by using information provided by the other two. Following loss of vision, for example, the subject can get about, although the constraints imposed by blindness are known to all. Following loss of conscious proprioception instead, the subject uses vision as a substitute for proprioceptive sense, and is disabled by closure of the eyes (sensory ataxia, Ch. 15). If the static labyrinths alone are active, closure of the eyes may lead to a heavy fall.
Kinetic labyrinth: anatomy and action
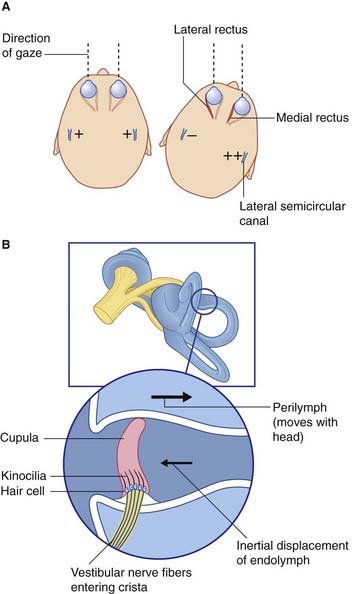
Basic features of macular epithelium are repeated in the three cristae. Again there are supporting cells, and hair cells to which vestibular nerve endings are applied. The kinocilia of the hair cells are long, penetrating into a gelatinous projection called the cupula (Figure 19.4). The cupula is bonded to the opposite wall of the ampulla.
The horizontal vestibulo-ocular reflex response to a rightward turn of the head is depicted in Figures 19.4 and 19.5, and described in their captions. Appropriate point-to-point connections also exist between the vestibular nuclei and gaze centers in the midbrain for similar reflexes in the vertical plane.
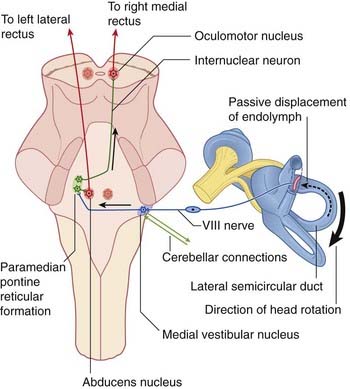
Figure 19.5 Under cerebellar guidance, the right medial vestibular nucleus responds to a rightward head turn by sending impulses to the contralateral paramedian pontine reticular formation (PPRF, Figure 17.15). The PPRF selects abducens motor neurons supplying the left lateral rectus, and sends internuclear fibers up the right medial longitudinal fasciculus to the right oculomotor nucleus, where they seek out motor neurons serving the right medial rectus.
In order to control the vestibulo-ocular reflexes, the cerebellum is informed about the initial position of the head in relation to the trunk. This information is provided by a great wealth of muscle spindles in the deep muscles surrounding the cervical vertebral column. The spindle afferents enter the rostral spinocerebellar tract and relay in the accessory cuneate nucleus on each side (Figure 17.12).
Nystagmus
Unilateral and bilateral vestibular syndromes are considered in Clinical Panel 19.1. A vascular syndrome involving the vestibular system in the medulla oblongata is described in Clinical Panel 19.2.
Clinical Panel 19.2 Lateral medullary syndrome
Lateral medullary syndrome (Figure CP 19.2.1)
Vestibulocortical connections
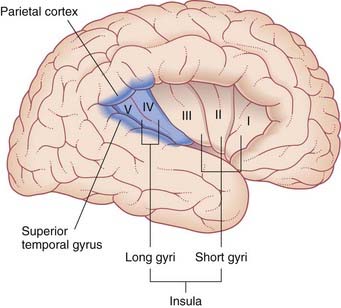
Second-order sensory neurons project from the vestibular nuclei mainly to the ipsilateral thalamus. The fibers relay via the ventral posterior nucleus to the parieto-insular vestibular cortex (PIVC) and to the adjacent region of the superior temporal gyrus, as shown in Figure 19.6. However, the PIVC cannot be named as a primary sensory area because it is in fact multisensory, receiving visual and tactile inputs as well as vestibular. (By analogy, PET studies of tactile sensation show activity throughout most of the parietal lobe, but we know from other sources that the postcentral gyrus is the primary area, being the take-off point for analysis in the posterior parietal cortex.)
The relationship of PIVC activity to hemisphere dominance is discussed in Chapter 32.
Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Ann Rev Neurosci. 2008;31:125-150.
Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511-541.
Dieterich M, Bense S, Lutz S, Drezega A, Stephan T, Bartenstein P, Brandt T. Dominance for vestibular cortical function in the non-dominant hemisphere. Cerebral Cortex. 2003;13:994-1007.
Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173-196.
Lacour M, Borel L. Vestibular control of posture and gait. Arch Ital Biol. 1993;131:81-104.
Landau ME, Barner KC. Vestibulocochlear nerve. Semin neurol. 2009;29:66-73.
Paulesu E, Frackowiak RSJ, Bottini G. Maps of somatosensory systems. In: Frackowiak RSJ, Friston KJ, Frith CD, Mazziota JC, editors. Human brain function. San Diego: Academic Press; 1997:219-231.