Vesicoureteral Reflux
Definition and Imaging Objectives
Overview: Vesicoureteral reflux (VUR) refers to the retrograde passage of urine from the urinary bladder into the ureter and often to the calyces. It is a common and potentially important childhood problem that generally is regarded as abnormal at all ages. Because of recent insights into the natural history of fetal and neonatal urinary tract development, this judgment is increasingly under discussion and review. VUR itself causes neither urinary tract infection (UTI) nor renal damage, but it may be associated with bladder dysfunction. However, VUR is a risk factor for the development of upper UTI and pyelonephritis, with consequent renal scarring and potential long-term sequelae (Box 120-1).1–15
The major objective in the evaluation of children with documented UTI traditionally has been to diagnose or exclude VUR. Today, the focus of imaging in UTI has shifted to evaluation of renal inflammatory involvement or existing renal scarring and to assessment of structural or functional abnormalities of the urinary tract that may predispose to renal damage (i.e., prenatal “hydronephrosis”), complicated UTI, and VUR, particularly early depiction of anomalies that may require prompt interventional or surgical treatment to prevent renal damage.1,16–34
Etiology
Overview: The most common cause of VUR is a developmental anomaly of the ureterovesicular junction (UVJ) in which the ureteral orifice may be lateralized or too large (“golf hole ostium”) or the submucosal ureter is too short and/or deficient in longitudinal muscle fibers. VUR often is seen in patients with other urinary tract anomalies (Box 120-2). Additionally, some sort of immaturity of the UVJ may play a role in fetal and neonatal VUR, because a large percentage of congenital VUR decreases spontaneously within the first years of life. This type of VUR, often referred to as primary or congenital VUR, is seen more frequently in girls than in boys. High-grade congenital VUR in male infants, often with severe congenital renal dysplasia (“congenital reflux nephropathy”), constitutes a different entity with a far more serious prognosis.
Secondary VUR is seen in patients with bladder outlet obstruction (e.g., posterior urethral valves) or with neurogenic bladder disease (e.g., myelomeningocele). It is caused in part by thinning and weakening of the UVJ musculature precipitated by chronically increased intravesical pressure. However, the fact that VUR in these disorders may be absent and frequently is unilateral suggests the possibility of an associated congenital weakness of the UVJ or a protective measure of a potentially thickened bladder wall that otherwise may lead to ureteral obstruction. Although lesser degrees of lower urinary tract obstruction per se do not seem to cause VUR in patients with a completely normal UVJ, they may precipitate VUR (with ascending spread of infection to the kidneys) in people with ostia of borderline competence as the result of local edema and cellular infiltration, causing further weakening of the UVJ.1,35–38
Imaging Techniques
Overview: The diagnostic imaging modalities available for the evaluation of VUR include ultrasound, voiding cystourethrogram (VCUG), and nuclear cystography (Box 120-3). A new method for VUR assessment is contrast-enhanced voiding urosonography (ce-VUS). This method uses ultrasound contrast material (e.g., shaken saline solution, air, or commercially available contrast agents) instilled into the urinary bladder via suprapubic or transurethral catheterization and bladder filling (Fig. 120-1 and e-Fig. 120-2). The reflux of contrast into the upper tracts can be appreciated readily by alternately scanning both kidneys as well as the retrovesical space during filling and before and after voiding. Ultrasound techniques such as harmonic imaging, stimulated acoustic emission, or other contrast-specific techniques have further enhanced ce-VUS potential for VUR depiction and grading, resulting in a reported sensitivity and specificity equal to VCUG (Fig. 120-3 and e-Fig. 120-4).1,39–55
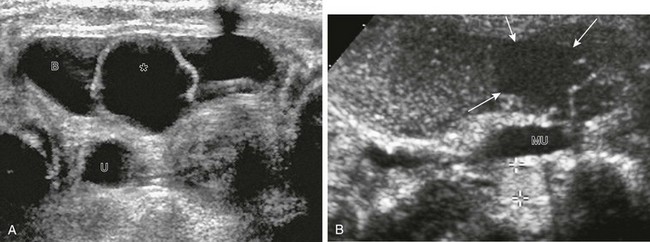
Figure 120-1 Vesicoureteral reflux demonstrated by echo-enhanced urosonography.
A, A transverse unenhanced bladder sonogram reveals a ureterocele (asterisk) within and a dilated ureter (U) behind the urinary bladder (B). B, A longitudinal sonogram after instillation of contrast material (Levovist) into the urinary bladder shows the nonrefluxing ureterocele (arrows) and corresponding megaureter (MU) and echogenic contrast material within the refluxing lower pole ureter (cursors). See also e-Figure 120-2.
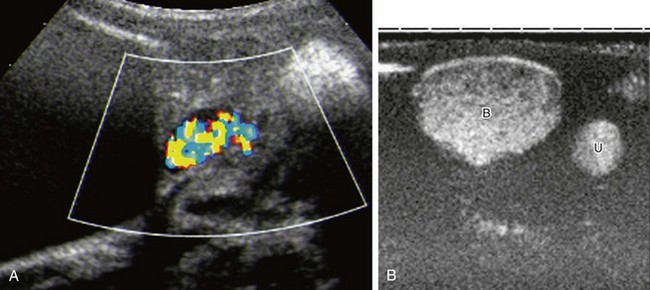
Figure 120-3 Improved detection of vesicoureteral reflux using contrast-specific techniques.
A, The same patient as in Figure 120-1 and e-Figure 120-2, with reflux into the right lower moiety. Reflux is now depicted using stimulated acoustic emission with high ultrasound power (high mechanical index) to burst the contrast bubbles, thus creating strong color signals. B, Contrast-specific imaging techniques (CDI, Siemens, Mountain View, CA) provide exquisite contrast delineation in a patient with refluxing megaureter, using double-image technique for visualization contrast image (B, bladder; U, ureter). See e-Figure 120-4 for a generic image with simultaneous contrast and harmonic imaging gray-scale information.
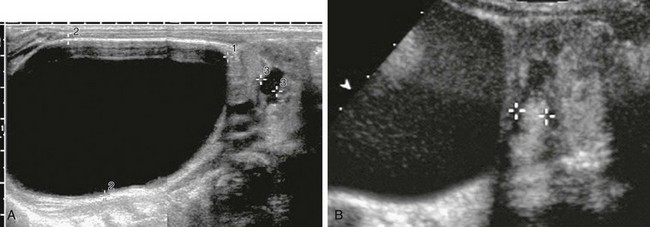
e-Figure 120-2 Vesicoureteral reflux demonstrated by echo-enhanced urosonography, in the same patient as in Figure 120-1.
A, A longitudinal sonogram of the duplex right kidney shows a markedly dilated upper moiety and only mild dilatation of the lower pole moiety. B, A longitudinal renal sonogram shows echogenic contrast in the dilated pelvocalyceal system of the lower pole moiety (cursors), thus diagnosing high-grade vesicoureteral reflux.
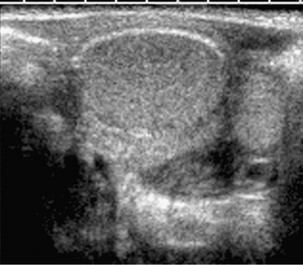
e-Figure 120-4 Improved detection of vesicoureteral reflux using contrast-specific techniques in the same patient as in Figure 120-3. A generic image shown with simultaneous contrast and harmonic imaging gray-scale information.
VCUG is and remains the basic imaging technique for VUR assessment. It uses radiopaque contrast material instilled into the catheterized and emptied urinary bladder for detection of VUR into the upper urinary tract. For a reliable assessment, the bladder must be filled to near capacity. For infants younger than 1 year of age, bladder capacity in milliliters is determined by the patient’s weight in kilograms × 7. For patients older than 1 year of age, bladder capacity in milliliters equals age in years plus 2, multiplied by 30. Fluoroscopic observation of the (early) filling phase, the distal ureters (in oblique projections), the renal collecting system, and the urethra during voiding (lateral projection in boys) enables focused imaging of critical areas and conditions such as intrarenal VUR and UVJ anatomy. Cyclic VCUG should be performed in infants to avoid missing significant VUR, because VUR may not occur during the first fill and void.1,56–69
For studying the renal parenchyma, ultrasound and dimercaptosuccinic acid (DMSA) scintigraphy commonly are used. Other methods for the evaluation of the upper tract are contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI), which may become indicated for assessment of complicated disease. The choice of imaging method for VUR assessment depends in large part on individual preference, availability, and the experience of the examiner. Other variables include the age, sex, and race of the patient; whether an initial or a follow-up examination is being done; and the cost of and time required to perform the procedure. All male infants and all patients before surgery should have a conventional VCUG performed for detailed anatomic assessment.1,20,21,23,24,28,30,31,70–85
Grading
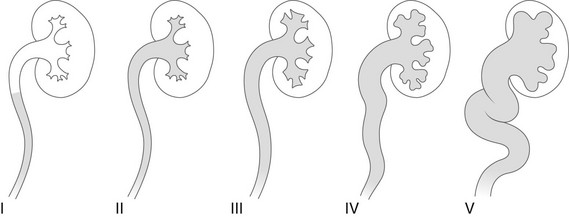
Overview: The international criteria for grading VUR is based on the VCUG (Fig. 120-5). Similar comparable grading scales exist for ce-VUS and nuclear cystography. Although simple and easy to use, this classification and other similar classifications reflect only the appearance of the upper tracts and do not take into consideration other important factors such as the age and sex of the patient, the presence of intrarenal reflux or urinary tract obstruction, renal function and scarring, high- or low-pressure VUR, early or late VUR, slow or quick clearance from the upper collecting system, cystoscopic findings, and the presence or absence of associated disorders (e.g., ureteral duplication, ureteral ectopia, ureterocele, bladder diverticula, prunebelly syndrome, urethral obstruction, megacystis-megaureter association, or neurogenic bladder). This additional information sometimes is essential for making decisions about therapy.1,86–88
VUR Imaging
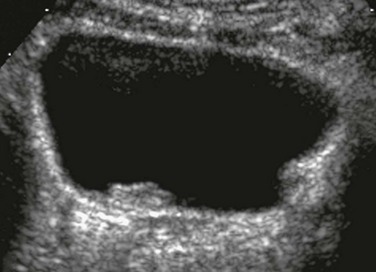
On ultrasound, the ureters and pelvocalyceal systems of patients with VUR often appear normal even if they appear dilated on VCUG (particularly when the bladder is empty or catheterized). Indirect sonographic signs for VUR are uroepithelial thickening of the ureter or renal pelvis, changing diameter of the pelvocalyceal system and ureter, quick refilling of the bladder after voiding, asymmetric ureteral inflow jets, and a lateralized position or unusual shape of the ureteral ostium (Fig. 120-6). In more severe cases, the refluxing upper tracts are grossly dilated, with clubbing of the calyces and elongated and tortuous ureters (e-Fig. 120-7). Ureteral peristalsis generally is poor, especially in the presence of urinary infection and high-grade VUR. Kinks in the proximal ureter or distal segment of the ureter are common. Direct sonographic evidence of VUR may be identified on ce-VUS as described previously.1,89–103
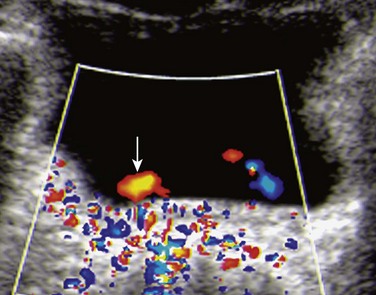
Figure 120-6 Lateralized ureteral orifice.
A transverse color Doppler image shows a laterally positioned and abnormally oriented right ureteral jet (arrow). Such indirect signs may help ultrasound diagnosis of vesicoureteral reflux.
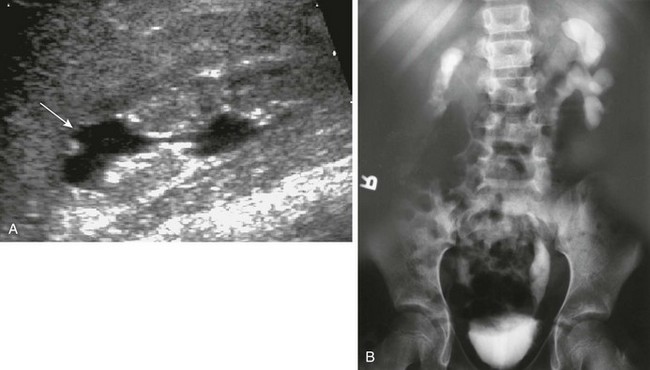
e-Figure 120-7 Reflux nephropathy.
A, A longitudinal sonogram shows features of a scarred kidney from severe vesicoureteral reflux with recurrent urinary tract infection: dilated and clubbed calyces with focal overlying parenchymal narrowing (arrow) and echogenic nondifferentiated parenchyma with irregular contours. B, An intravenous urogram image shows a small and atrophic functioning right kidney with markedly thinned parenchyma. The left kidney collecting system is distorted as well.
Voiding Cystourethrogram
The appearance of refluxing ureters and pelvocalyceal systems on VCUG is quite variable, ranging from normal-sized upper tracts to extreme upper tract dilatation and marked ureteral tortuosity (Fig. 120-8 and e-Fig. 120-9). These changes may reflect only an increased volume and decreased motility of the ureters, but in some cases a developmental defect of the ureter related either to inutero VUR or to an inadequate development of the ureteral musculature is suspected (e.g., prune-belly syndrome). In some patients, VUR is accompanied by marked ballooning of the pelvocalyceal system without evidence of UPJ obstruction (e-Fig. 120-10). The phenomenon may be transient and reflects an increased elasticity of unknown cause in the upper collecting system. Sometimes VUR may induce a kink at the UPJ, with a valvelike mechanism that may even deteriorate after antireflux procedures, producing a functional UPJ obstruction (Fig. 120-11). The obstruction may be related to local scarring from infection, anatomic kinks in the ureter, or overlying aberrant vessels or fibrous bands. Thus a primary congenital UPJ obstruction and primary VUR may coexist as associated anomalies.1,104–107
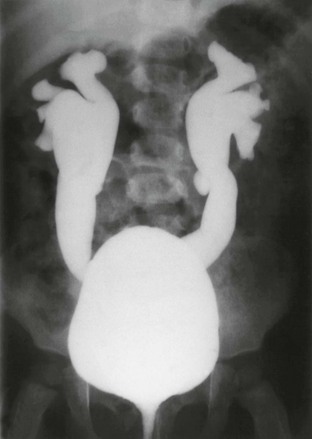
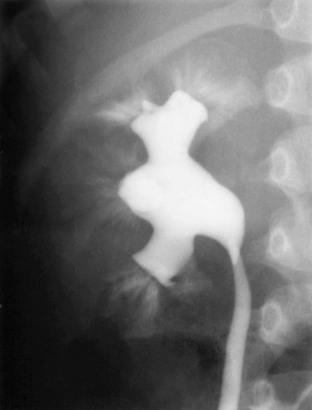
Figure 120-8 Vesicoureteral reflux.
A voiding cystourethrogram image shows bilateral grade IV vesicoureteral reflux. Also see e-Figure 120-9.
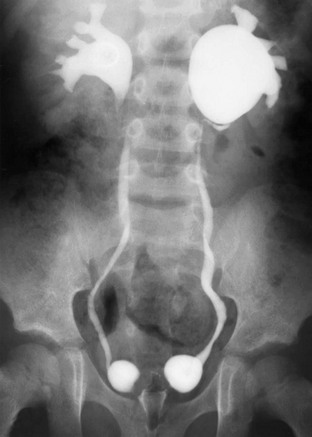
Figure 120-11 Vesicoureteral reflux and functional ureteropelvic junction obstruction.
A voiding cystourethrogram image after voiding shows bilateral vesicoureteral reflux and ballooning of the left renal pelvis. The intravenous urogram image (not shown) was entirely normal. Bilateral paraureteral (Hutch) diverticula also are present.
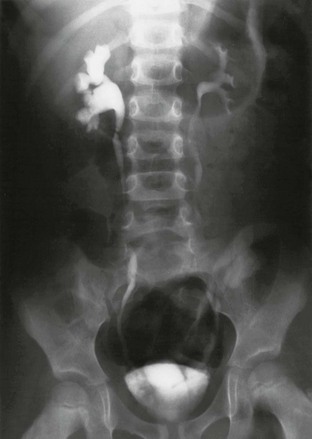
e-Figure 120-9 Vesicoureteral reflux.
A voiding cystourethrogram image shows left grade II vesicoureteral reflux with thin and delicate calyces and right grade III reflux where the calyces are dilated and blunted.
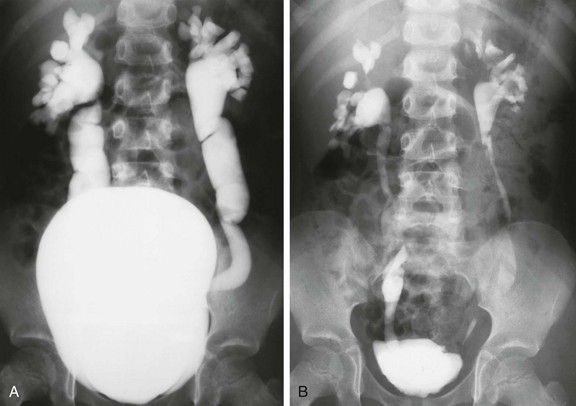
e-Figure 120-10 Vesicoureteral reflux.
A, A voiding cystourethrogram in a 5-year-old girl with recurrent urinary tract infections shows a large, smooth-walled bladder and bilateral grade V vesicoureteral reflux. B, An image after voiding shows only mild hydroureteronephrosis, along with bilateral renal parenchymal loss.
In patients with VUR to the calyces, one may observe transient pyelotubular and interstitial reflux of contrast material extending outward in a wedge-shaped pattern from one or more papillae to the renal cortical surface (Fig. 120-12). Intrarenal reflux is an important finding because of its association with renal scarring. It is believed that the morphology of the opening of the collecting ducts of Bellini on the renal papilla is partly responsible for intrarenal reflux. The opening of these ducts on compound, flat-topped papillae (more common at the poles) are round and therefore less resistant to retrograde flow than the slitlike openings of the ducts of simple or conical papillae. The fact that intrarenal reflux does not occur in all infants and that it is rarely seen after age 4 years suggests an additional local defect that improves with age.1–3,6,8,9,12,15,19,56–59,62,63,66,69,88,105,107
Natural History
Overview: VUR has a tendency to improve and to disappear spontaneously during the first decade of life, often during the preschool years. This tendency is attributed to a maturation process of the UVJ with age, with an increase in the length of the intramural ureter and strengthening of its musculature. Mild VUR (grades I and II) with normal-sized ureters and ureteral orifices has a favorable prognosis and disappears with time in more than 80% of cases, whereas more severe forms of VUR with dilated ureters have a lower incidence of spontaneous recovery (grade III, about 50%; grade IV, 30%; grade V, rarely). VUR that is associated with anatomic anomalies such as a large ureteral orifice and a short submucosal tunnel at cystoscopy is not likely to resolve. VUR occurring in an ectopically ending ureter (i.e., defective UVJ accompanying lateral ureteral ectopia of the lower pole moiety of a duplex kidney) or associated with a large paraureteral diverticulum also tends to persist, especially if the ureter ends in the diverticulum. VUR that occurs in patients with a lower urinary tract obstruction may disappear after correction of the lesion but is considered uncured if it is still present 1 year postoperatively. VUR that occurs in persons with neurogenic bladder disease or bladder dysfunction also tends to persist until the functional disturbance is treated successfully.1,108–117
Renal Scarring and VUR
Overview and Pathophysiology: Renal scarring is a common and potentially serious problem in patients with VUR and upper UTI (acute pyelonephritis). Renal scarring is characterized by one or more areas of renal cortical atrophy that is almost always associated with blunting or distortion of the underlying calyx or group of calyces, retraction of the papillae, and reduction of the medullary zone. Histologically, the affected kidneys show areas of cortical loss with tubular destruction and atrophy and interstitial fibrosis. Obliteration of glomeruli, arteriolar changes, and minor signs of interstitial inflammation also may be observed. The scarring characteristically has a focal or segmental pattern with a predisposition for the upper pole (38%) and less frequently for the lower pole. In some cases the process affects the entire kidney diffusely. The areas of scarring result in one or more clefts or depressions of various sizes in the outline of the kidney. The unaffected renal parenchyma may be hypertrophied, sometimes simulating a renal mass (pseudotumor). When the process is diffuse and severe, it results in global renal atrophy.
Imaging: The parenchymal changes may be directly depicted by ultrasound as areas of depression in the outline of the kidney with increased echogenicity of the nondifferentiated parenchyma at the dilated and distorted calyx; color Doppler imaging reveals focally reduced peripheral vascularity (Fig. 120-13). Nuclear scintigraphy, using a cortical agent such as DMSA, is especially sensitive in detecting renal cortical scars and is considered the gold standard. Magnetic resonance urography has been shown to hold great potential for diagnosis of renal involvement in persons with a UTI and renal scarring. Parenchymal changes also are visualized on contrast-enhanced CT, but this study is not indicated for the evaluation of renal scarring.
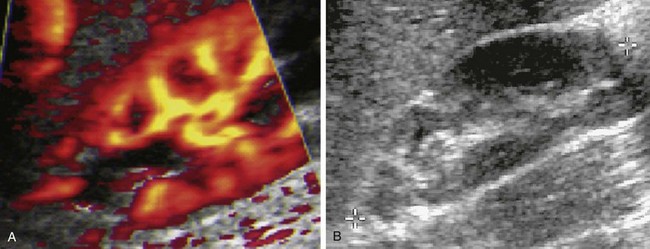
Figure 120-13 Acute pyelonephritis.
A, A longitudinal color Doppler image shows a focal perfusion defect in the upper pole associated with a dilated calyx in an infant with acute febrile upper urinary tract infection. Evaluation found grade III vesicoureteral reflux. B, A follow-up longitudinal sonogram shows the development of upper pole scarring.
It may take a few weeks to several months for a scar to become apparent on imaging. Some apparently new renal scars that are seen in follow-up examinations may represent the end stage of an old insult to the kidney that was not apparent earlier or may be a result of intercurrent infections. In addition, what appears to be a progression of a previously demonstrated scar on follow-up may only reflect continuing growth and hypertrophy of adjacent normal renal tissue contrasting with a fixed atrophic area, creating an increasing mismatch during physiologic growth.1,118–139
End-Stage Renal Disease
Overview and Pathophysiology: Severe renal scarring is associated with decreased renal function of varying severity and is a relatively common cause of end-stage renal disease (ESRD), leading to dialysis and renal transplantation. Severe renal scarring is responsible for 8% of all cases of ESRD, but establishing how many children with scarred kidneys eventually experience this complication is difficult because VUR may not be demonstrated at the time of diagnosis (as a result of previous ureteral reimplantation or spontaneous resolution of the VUR). ESRD resulting from scarring is seen most commonly in older children, adolescents, and young adults, with an increased risk in patients with hypertension. The ESRD of these patients is probably the result of a decrease in the number of nephrons, a limited growth potential of the unaffected nephrons, plus an acquired glomerulosclerosis caused by an increased workload (hyperperfusion) of these unaffected glomeruli, similar to patients with a posterior urethral valve who also have congenital renal hypodysplasia.1,140–144
Treatment
Overview: Eradication or early treatment of UTI and the prevention of recurrences are the main therapeutic objectives in children with VUR, with an emphasis on infants and small children who are at particularly high risk of experiencing renal scars. These objectives may be attained by long-term suppressive medication with antibiotics until the patient is 4 to 5 years of age or until VUR ceases. This medical treatment is thought to be often sufficient in patients with low-grade VUR. However, with the new knowledge of the natural history of VUR and the increasing number of resistant bacteria causing breakthrough infections, this strategy is increasingly under discussion, particularly in persons with low-grade VUR. An antireflux procedure may become necessary in many patients with symptomatic and persisting high-grade VUR and usually is indicated in persons with grade V VUR, the latter sometimes after temporary urinary diversion via a ureterostomy. The success rate of ureteral reimplantation is high (up to 95%) in patients with grade I and II VUR, but the success rate decreases as the size of the affected ureter increases (a 60% success rate in persons with grade V VUR has been reported). Endoscopic intravesical injection of a small amount of some material (e.g., Silicon, Teflon paste, or dextranomer/hyaluronic acid [Deflux]) into the bladder wall behind the submucosal ureteral tunnel offers an alternative to the surgical procedures (Fig. 120-14). The injected material elevates and narrows the ureteral tunnel and causes a localized protrusion of the bladder wall at the lateral angle of the trigone containing the ureteral orifice. The injected material is well visualized by ultrasound as a rounded echogenic focus (Fig. 120-15 and e-Fig. 120-16).1,145–171
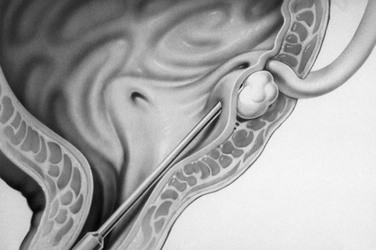
Figure 120-14 Subureteric injection.
A diagrammatic representation of injection of material under the entrance of the ureter into the bladder to eliminate vesicoureteral reflux.
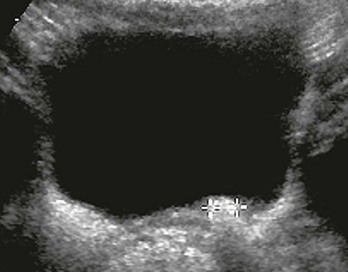
Figure 120-15 Postoperative imaging for vesicoureteral reflux.
A transverse bladder sonogram after cystoscopic injection shows echogenic material at the left ureterovesical junction (cursors). See e-Figure 120-16 for the parasagittal view.
Imaging Algorithms for Evaluation and Follow-up
Overview: Different recommendations exist for VUR imaging and imaging during or after UTI in different parts of the world. With the new knowledge and insight into the nature and pathophysiology of VUR and renal damage, many of these imaging algorithms are undergoing changes. Imaging is increasingly focused on phenomena that define prognosis and risk for long-term sequelae, such as the kidney with potential renal scars and bladder functional disturbances. We want to avoid diagnostic overimaging for economic reasons and patient concerns.
Imaging: In large parts of Europe and North America, VUR assessment is considered to be indicated in all infants and children up to 2 years of age after the first UTI and in older children if a UTI has occurred with proven renal involvement, scars, or recurrent upper UTI. Furthermore, some sort of cystogram is considered indicated in all neonates with significant hydronephrosis and in all patients with significant urinary tract malformations, particularly preoperatively. School-age patients with a UTI should undergo an initial ultrasound, potentially a urodynamic assessment, and a late DMSA scan; VUR assessment is promoted only in persons with renal scarring, severe functional disorders, and recurrent infections. In teenage girls with a UTI who are afebrile, have no previous history of urinary tract disease, and have clinical signs of cystitis, ultrasound of the upper tracts may be the only procedure necessary. Patients with a family risk can undergo ultrasound and cystography, with ce-VUS and nuclear cystography being preferred in this patient group to minimize radiation exposure.
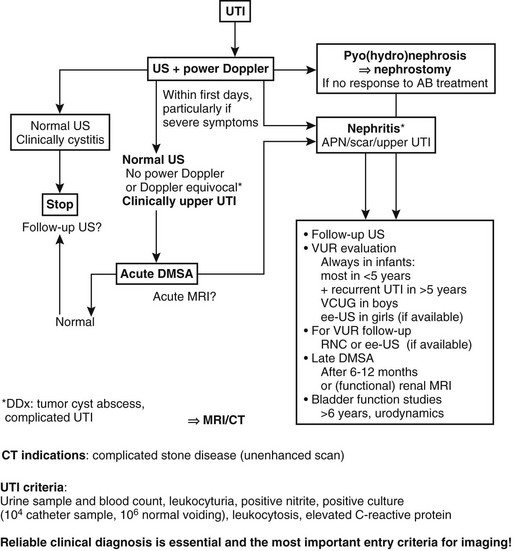
In persons with a UTI, imaging is increasingly being focused on the kidney; thus an early ultrasound or DMSA study is considered compulsory in any febrile patient with an upper UTI. To differentiate acute renal inflammatory changes from persisting scars, a DMSA study 6 to 12 months after the infection is recommended. In follow-up examinations and in studies after reimplantation, a nuclear cystogram (or ce-VUS) may replace VCUG for evaluation of VUR, and ultrasound and DMSA scans may be used to monitor the upper tracts (Box 120-4 and Fig. 120-17).1,172–185
Brandström, P, Esbjörner, E, Herthelius, M, et al. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol. 2010;184(1):286–291.
Brandström, P, Nevéus, T, Sixt, R, et al. The Swedish reflux trial in children: IV. Renal damage. J Urol. 2010;184(1):292–297.
Fernbach, SK, Feinstein, KA, Schmidt, MB. Pediatric voiding cystourethrography: a pictorial guide. Radiographics. 2000;20:155.
Fouzas, S, Krikelli, E, Vassilakos, P, et al. DMSA scan for revealing vesicoureteral reflux in young children with urinary tract infection. Pediatrics. 2010;126(3):e513–e519.
Giordano, M, Marzolla, R, Puteo, F, et al. Voiding urosonography as first step in diagnosis of vesicoureteral reflux in children: a clinical experience. Pediatr Radiol. 2007;37:674–677.
Hannula, A, Venhola, M, Renko, M, et al. Vesicoureteral reflux in children with suspected and proven urinary tract infection. Pediatr Nephrol. 2010;25(8):1463–1469.
Hernandez, RH, Goodsitt, M. Reduction of radiation dose in pediatric patients using pulsed fluoroscopy. AJR Am J Roentgenol. 1996;167:1247.
Holmdahl, G, Brandström, P, Läckgren, G, et al. The Swedish reflux trial in children: II. Vesicoureteral reflux outcome. J Urol. 2010;184(1):280–285.
Keren, R, Carpenter, MA, Hoberman, A, et al. Rationale and design issues of the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) study. Pediatrics. 2008;122(suppl 5):S240–S250.
Leslie, B, Moore, K, Salle, JL, et al. Outcome of antibiotic prophylaxis discontinuation in patients with persistent vesicoureteral reflux initially presenting with febrile urinary tract infection: time to event analysis. J Urol. 2010;184(3):1093–1098.
Montini, G, Tullus, K, Hewitt, I. Febrile urinary tract infections in children. N Engl J Med. 2011;365(3):239–250.
Peters, CA, Skoog, SJ, Arant, BS, Jr., et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J Urol. 2010;184(3):1134–1144.
Riccabona, M, Fotter, R. Reorientation and future trends in paediatric uroradiology: minutes of a symposium. Pediatr Radiol. 2004;34:295.
Riccabona, M. VUR. In Carty H, Brunelle F, Shaw D, et al, eds.: Imaging children, 2nd ed, Edinburgh: Churchill Livingstone, 2006.
Sillén, U, Brandström, P, Jodal, U, et al. The Swedish reflux trial in children: v. Bladder dysfunction. J Urol. 2010;184(1):298–304.
Skoog, SJ, Peters, CA, Arant, BS, Jr., et al. Pediatric Vesicoureteral Reflux Guidelines Panel Summary Report: Clinical Practice Guidelines for Screening Siblings of Children With Vesicoureteral Reflux and Neonates/Infants With Prenatal Hydronephrosis. J Urol. 2010;184(3):1145–1151.
Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management Roberts, KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610.
Zier, JL, Kvam, KA, Kurachek, SC, et al. Sedation with nitrous oxide compared with no sedation during catheterization for urologic imaging in children. Pediatr Radiol. 2007;37:678–684.
References
1. Riccabona, M. The ureter and vesicoureteral reflux. In Slovis T, ed.: Caffey’s pediatric diagnostic imaging, 11th ed, Philadelphia: Mosby, 2008.
2. Feld, LG, Mattoo, TK. Urinary tract infections and vesicoureteral reflux in infants and children. Pediatr Rev. 2010;31(11):451–463.
3. Bell, LE, Mattoo, TK. Update on childhood urinary tract infection and vesicoureteral reflux. Semin Nephrol. 2009;29(4):349–359.
4. Sargent, MA. What is the normal prevalence of vesicoureteral reflux? Pediatr Radiol. 2000;30:723.
5. American Academy of Pediatrics Committee on Quality Improvement, Subcommittee on UTI. Practice parameter: the diagnosis, treatment and evaluation of the initial UTI in febrile infants and young children. Pediatrics. 1999;103:843.
6. Sillen, U. Vesicoureteral reflux in infants. Pediatr Nephrol. 1999;13:355.
7. Larcombe, J. Urinary tract infection in children. BMJ. 1999;319:1173.
8. Pennington, DJ, Zerin, MJ. Imaging of the urinary tract infection. Pediatr Ann. 1999;28:678.
9. Garin, EH, Campos, A, Homsy, Y. Primary vesicoureteral reflux: review of current concepts. Pediatr Nephrol. 1998;12:249.
10. Sobel, JD. Pathogenesis of urinary tract infection. Infect Dis Clin North Am. 1997;11:531.
11. Hellerstein, S. Urinary tract infection: old and new concepts. Pediatr Clin North Am. 1995;42:1433.
12. Jodal, U. Urinary tract infections (UTI): significance, pathogenesis, clinical features and diagnosis. In Postelstwaite RJ, ed.: Butterworth and Heinneman clinical pediatric nephrology, 2nd ed, Cambridge, Butterworth and Heinnemann, 1994.
13. Anderson, PAM, Rickwood, AMK. Features of primary vesicoureteral reflux detected by prenatal ultrasound. Br J Urol. 1991;67:267.
14. Arant, BS. Vesicoureteral reflux and renal injury. Am J Kidney Dis. 1991;17:491.
15. Reid, BS, Bender, TM. Radiographic evaluation of children with urinary tract infections. Radiol Clin North Am. 1988;26:39.
16. Storm, DW, Patel, AS, Koff, SA, et al. Novel management of urinary tract infections. Curr Opin Urol. 2011;21(4):328–333.
17. Grazioli, S, Parvex, P, Merlini, L, et al. Antenatal and postnatal ultrasound in the evaluation of the risk of vesicoureteral reflux. Pediatr Nephrol. 2010;25(9):1687–1692.
18. Estrada, CR, Peters, CA, Retik, AB, et al. Vesicoureteral reflux and urinary tract infection in children with a history of prenatal hydronephrosis—should voiding cystourethrography be performed in cases of postnatally persistent grade II hydronephrosis? J Urol. 2009;181(2):801–806. [discussion 806-807].
19. Riccabona, M, Urinary tract infection. Imaging children, 2nd ed. Carty, H, Brunelle, F, Stringer, D, et al, eds. Imaging children, Philadelphia, Elsevier Science, 2005;vol I.
20. Majd, M, Nussbaum Blask, AR, Markle, BM, et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA SPECT, spiral CT, MR imaging, and power Doppler ultrasound in an experimental pig model. Radiology. 2001;218:101.
21. Biggi, A, Dardanelli, L, Pomero, G, et al. Acute renal cortical scintigraphy in children with a first urinary tract infection. Pediatr Nephrol. 2001;16:733.
22. Merguerian, PA, Jamal, MA, Agarwal, SK, et al. Utility of DMSA scanning in the evaluation of children with primary vesico-ureteral reflux. Urology. 1999;53:1024.
23. Morin, D, Veyrac, C, Kotzki, PO, et al. Comparison of ultrasound and DMSA scintigraphy changes in acute pyelonephritis. Pediatr Nephrol. 1999;13:219.
24. Lonergan, GJ, Pennington, DJ, Morrison, JC, et al. Childhood pyelonephritis: comparison of gadolinium-enhanced MR imaging and renal cortical scintigraphy for diagnosis. Radiology. 1998;207:377.
25. Yeung, CK, Godley, ML, Dhillon, HK, et al. The characteristics of primary vesico-ureteric reflux in male and female infants with pre-natal hydronephrosis. Br J Urol. 1997;80(2):319–327.
26. Rushton, HG. The evaluation of acute pyelonephritis and renal scarring with Tc DMSA scintigraphy: evolving concepts and future directions. Pediatr Nephrol. 1997;11:108.
27. Stokland, E, Hellström, M, Jacobsson, B, et al. Renal damage one year after first urinary tract infection: role of DMSA scintigraphy. J Pediatr. 1996;129:815.
28. Stokland, E, Hellstöm, M, Jacobsson, B, et al. Early 99mTc dimercaptosuccinic acid (DMSA) scintigraphy in symptomatic first-time urinary tract infection. Acta Paediatr. 1996;85:430.
29. Ditchfeld, MR, DeCampo, JF, Nolan, TM, et al. Risk factors in the development of early renal cortical defects in children with UTI. AJR Am J Roentgenol. 1994;152:1393.
30. Benador, D, Benador, N, Slosman, DO, et al. Cortical scintigraphy in the evaluation of renal parenchymal changes in children with pyelonephritis. J Pediatr. 1994;124:17.
31. Jakobsson, B, Nolstedt, L, Svensson, L, et al. 99mTc-dimercapto-succinic acid (DMSA) in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol. 1992;6:328.
32. Kenny, PJ. Imaging of chronic renal infections. AJR Am J Roentgenol. 1990;155:485.
33. Goldraich, NP, Ramos, OL, Goldraich, IH. Urography versus DMSA scan in children with vesicoureteric reflux. Pediatr Nephrol. 1989;3:1.
34. Smellie, JM, Shaw, PJ, Prescod, NP, et al. 99mTc dimercaptosuccinic acid (DMSA) scan in patients with established radiological renal scarring. Arch Dis Child. 1988;63:1315.
35. Altobelli, E, Buscarini, M, Nappo, SG, et al. Urodynamics investigation on children with vesicoureteral reflux identifies overactive bladder and poor compliance in those with voiding dysfunction. Pediatr Surg Int. 2011;27(5):517–522.
36. Carvas, F, Silva, A, Nguyen, HT. The genetics of primary, nonsyndromic vesicoureteral reflux. Curr Opin Urol. 2010;20(4):336–342.
37. Afshar, K, Malek, R, Bakhshi, M, et al. Should the presence of congenital para-ureteral diverticulum affect the management of vesicoureteral reflux? J Urol. 2005;174(4 pt 2):1590–1593.
38. Oswald, J, Brenner, E, Schwentner, C, et al. The intravesical ureter in children with vesicoureteral reflux: a morphological and immunohistochemical characterization. J Urol. 2003;170:2423.
39. Darge, K. Voiding urosonography with US contrast agent for the diagnosis of vesicoureteric reflux in children: an update. Pediatr Radiol. 2010;40(6):956–962.
40. Darge, K. Voiding urosonography with ultrasound contrast agents for the diagnosis of vesicoureteric reflux in children, I. Procedure. Pediatr Radiol. 2008;38(1):40–53.
41. Darge, K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children, II. Comparison with radiological examinations. Pediatr Radiol. 2008;38(1):54–63. [quiz 126-127].
42. Lee, SK, Chang, Y, Park, NH, et al. Magnetic resonance voiding cystography in the diagnosis of vesicoureteral reflux: comparative study with voiding cystourethrography. J Magn Reson Imaging. 2005;21(4):406–414.
43. Riccabona, M, Mache, CJ, Lindbichler, F. Echoenhanced Color Doppler cystosonography of vesico-ureteral reflux in children: improvement by stimulated acoustic emission. Acta Radiol. 2003;44:18.
44. Radmayr, C, Klauser, A, Pallwein, L, et al. Contrast enhanced reflux sonography in children: a comparison to standard radiological imaging. J Urol. 2002;167:1428.
45. Berrocal, T, Gaya, F, Arjonilla, A, et al. Vesicoureteral reflux: diagnosis and grading with echoenhanced cystosonography versus voiding cystourethrography. Radiology. 2001;221:359.
46. Darge, K, Zieger, B, Rohrschneider, W, et al. Contrast-enhanced harmonic imaging for the diagnosis of vesicoureteral reflux in pediatric patients. AJR Am J Roentgenol. 2001;177:1411.
47. Kim, B, Lim, HK, Choi, MH, et al. Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med. 2001;20:5.
48. Valenti, AL, Salvaggio, E, Manzoni, C, et al. Contrast-enhanced gray-scale and color Doppler voiding urosonography versus voiding cystourethrography in the diagnosis and grading of vesicoureteral reflux. J Clin Ultrasound. 2001;29:65.
49. Kenda, RB, Novljan, G, Kenig, A, et al. Echo-enhanced ultrasound voiding cystography in children: a new approach. Pediatr Nephrol. 2000;14:297.
50. Ascenti, G, Chimenz, R, Zimbaro, G, et al. Potential role of colour-Doppler cysto-sonography with echocontrast in the screening and follow-up of vesicoureteral reflux. Acta Pediatr. 2000;89:1336.
51. Mentzel, HJ, Vogt, S, Patzer, L, et al. Contrast enhanced sonography of vesico-ureteral reflux in children: primary results. AJR Am J Roentgenol. 1999;173:737.
52. Darge, K, Tröger, J, Duetting, T, et al. Reflux in young patients: comparison of voiding ultrasound of the bladder and the retrovesical space with echo-enhancement versus voiding cystourethrography for diagnosis. Radiology. 1999;210:201.
53. Bosio, M. Cystosonography with echocontrast: a new imaging modality to detect vesico-ureteric reflux in children. Pediatr Radiol. 1998;28:250.
54. Atala, A, Wible, JH, Share, LC, et al. Sonography with sonicated albumin in the detection of vesico-ureteral reflux. J Urol. 1993;150:756.
55. Hanbury, DC, Coulden, RA, Farman, P, et al. Ultrasound cystography in the diagnosis of vesico-ureteral reflux. Br J Urol. 1990;65:250.
56. Riccabona, M, Vesico-ureteral reflux (VUR). Imaging children, 2nd ed. Carty, H, Brunelle, F, Stringer, D, et al, eds. Imaging children, Philadelphia, Elsevier Science, 2005;vol I.
57. Riccabona, M. Cystography in infants and children—a critical appraisal of the many forms, with special regard to voiding cystourethrography. Eur Radiol. 2002;12:2910.
58. Papadopoulou, F, Efremidis, SC, Oiconomou, A, et al. Cyclic voiding cystourethrography: is vesicoureteral reflux missed with standard voiding cystourethrography? Eur Radiol. 2002;12(3):666–670.
59. Gelfand, MJ, Koch, BL, Elgazzar, AH, et al. Cyclic cystography: diagnostic yield in selected pediatric populations. Radiology. 1999;213:118.
60. Poli-Merol, M, Francois, S, Pfliger, F, et al. Interest of direct radionuclide cystography in repeated urinary tract infection exploration in children. Eur J Pediatr Surg. 1998;8:339.
61. Mandel, GA, Eggli, DF, Gilday, DL, et al. Procedure guideline for radionuclide cystography in children. J Nucl Med. 1997;38:1650.
62. Smellie, LM, Rigden, SPA, Prescod, NP, et al. Urinary tract infection: a comparison of four methods of investigation. Arch Dis Child. 1995;72:247.
63. Kleinman, PK, Diamond, DA, Karellas, A, et al. Tailored low dose fluoroscopic voiding cystourethrography for the reevaluation of vesicoureteral reflux in girls. AJR Am J Roentgenol. 1994;162:1151. [discussion 1154].
64. Mozley, D, Heyman, S, Duckett, J, et al. Direct vesicoureteral scintigraphy: quantifying early outcome predictors in children with primary reflux. J Nucl Med. 1994;35:1602.
65. Paltiel, H, Rupich, R, Kiruluta, G. Enhanced detection of VUR in infants and children with use of cycling VCUG. Radiology. 1992;184:753.
66. Lebowitz, RL. The detection and characterization of vesicoureteral reflux in the child. J Urol. 1992;148:1640.
67. MacKenzie, JR, Fowler, K, Hollman, AS, et al. The value of ultrasound in the child with an acute urinary tract infection. Br J Urol. 1994;74:240.
68. Ditchfeld, MR, DeCampo, JF, Cook, DJ, et al. Vesico-ureteric reflux: an accurate predictor of acute pyelonephritis in childhood urinary tract infection? Radiology. 1994;190:413.
69. Jequier, S, Jequier, JC. Reliability of voiding cystourethrography to detect reflux. AJR Am J Roentgenol. 1989;153:807.
70. Siomou, E, Giapros, V, Fotopoulos, A, et al. Implications of 99mTc-DMSA scintigraphy performed during urinary tract infection in neonates. Pediatrics. 2009;124(3):881–887.
71. Chan, Y, Chan, K, Roebuck, D, et al. Potential utility of MRI in the evaluation of children at risk of renal scarring. Pediatr Radiol. 1999;29:856.
72. Dacher, JN, Avni, FE, Arnaud, F, et al. Renal sinus hyperechogenicity in acute pyelonephritis; description and pathological correlation. Pediatr Radiol. 1999;29:179.
73. Pennington, DJ, Lonergan, GL, Flack, CE, et al. Experimental pyelonephritis in piglets: diagnosis with MR imaging. Radiology. 1998;207:377.
74. Poustchi-Amin, M, Leonidas, JC, Palestro, C, et al. Magnetic resonance imaging in acute pyelonephritis. Pediatr Nephrol. 1998;12:579.
75. Dacher, JN, Pfister, C, Monroc, M, et al. Power Doppler sonographic pattern of acute pyelonephritis in children: comparison with CT. AJR Am J Roentgenol. 1996;166:1451.
76. Winter, DW. Power Doppler sonographic evaluation of acute pyelonephritis in children. J Ultrasound Med. 1996;15:91.
77. Pickworth, FE, Carlin, JB, Ditchfeld, MR, et al. Ultrasound measurements of renal enlargement in children with acute pyelonephritis and time needed for resolution: implications for renal growth assessment. AJR Am J Roentgenol. 1995;165:405.
78. Stokland, E, Hellström, M, Hansson, S, et al. Reliability of ultrasound in identification of reflux nephropathy in children. BMJ. 1994;309:235.
79. Dacher, JN, Boillot, B, Eurin, D, et al. Rational use of CT in acute pyelonephritis: findings and relationships with vesico-ureteral reflux. Pediatr Radiol. 1993;23:281.
80. Eggli, KD, Eggli, D. Color Doppler US in pyelonephritis. Pediatr Radiol. 1992;22:422.
81. Kass, EJ, Fisk-Bennett, D, Cacciarelli, AA. The sensitivity of renal scintigraphy and ultrasound in detecting nonobstructive acute pyelonephritis. J Urol. 1992;148:606.
82. Björgvinsson, E, Majd, M, Eggli, KD. Diagnosis of APN in children: comparison of US and Tc-DMSA scintigraphy. AJR Am J Roentgenol. 1991;157:539.
83. Arnold, AJ, Brownless, SM, Carty, HM, et al. Detection of renal scarring by DMSA scanning: an experimental study. J Pediatr Surg. 1990;25:391.
84. Mason, WG, Jr. Urinary tract infections in children: renal ultrasound evaluation. Radiology. 1989;153:109.
85. Avni, EF, Vandemerckt, C, Braude, P, et al. US evaluation of renal inflammatory diseases in children. J Urol. 1988;6:18.
86. Darge, K, Troeger, J. Vesicoureteral reflux grading in contrast-enhanced voiding urosonography. Eur J Radiol. 2002;43(2):122–128.
87. Godley, ML, Ransley, PG, Parkhouse, HF, et al. Quantitation of vesico-ureteral reflux by radionuclide cystography and urodynamics. Pediatr Nephrol. 1990;4:485.
88. Lebowitz, RL, Olbing, H, Parkkulainen, KV, et al. International Reflux Study in children: international system of radiographic grading of vesico-ureteral reflux. Pediatr Radiol. 1985;15:105.
89. Ismaili, K, Wissing, KM, Lolin, K, et al. Characteristics of first urinary tract infection with fever in children: a prospective clinical and imaging study. Pediatr Infect Dis J. 2011;30(5):371–374.
90. Riccabona, M, Haberlik, A, Ring, E. Virtual cystoscopy in children, based on three-dimensional ultrasound (3DUS) data: preliminary results. Eur Radiol. 2006;16(supp 1):284.
91. Leroy, S, Vantalon, S, Larakeb, A, et al. Vesicoureteral reflux in children with urinary tract infection: comparison of diagnostic accuracy of renal US criteria. Radiology. 2010;255(3):890–898.
92. Novljan, G, Levart, TK, Kljucevsek, D, et al. Ultrasound detection of vesicoureteral reflux in children. J Urol. 2010;184(1):319–324.
93. Robben, GF, Boesten, M, Linmans, J, et al. Significance of thickening of the wall of the renal collecting system in children: an ultrasound study. Pediatr Radiol. 1999;29:736.
94. Haberlik, A. Detection of low-grade vesicoureteral reflux in children by color Doppler imaging mode. Pediatr Surg Int. 1998;12:38.
95. Hiraoka, M, Hashimoto, G, Hori, C, et al. Use of ultrasound in the detection of vesico-ureteral reflux in children suspected of having urinary tract infection. J Clin Ultrasound. 1997;25:195.
96. Avni, FE, Ayadi, K, Rypens, F, et al. Can careful ultrasound examination of the urinary tract exclude vesico-ureteric reflux in the neonate? Br J Radiol. 1997;70:977.
97. Matsumo, T, Fukushima Motoyama, H, Higushi, E, et al. Color flow imaging for detection of vesico-ureteral reflux. Lancet. 1996;347:757.
98. Parker, MD, Clark, RL. Urothelial striations revisited. Radiology. 1996;198:89.
99. Hiraoka, M, Kasuga, K, Hori, C, et al. Ultrasound indicators of vesico-ureteral reflux in the newborn. Lancet. 1994;343:519.
100. Salih, M, Baltaci, S, Kilic, S, et al. Color flow Doppler ultrasound in the diagnosis of vesico-ureteral reflux. Eur Urol. 1994;26:93.
101. Alton, DJ, Lequesne, GW, Gent, R, et al. Sonographically demonstrated thickening of the renal pelvis in children. Pediatr Radiol. 1992;22:426.
102. Jecquier, S, Paltiel, H, Lafortune, M. Ureterovesical jets in children and infants: duplex and color Doppler US studies. Radiology. 1990;175:349.
103. Avni, EF, VanGansbeke, D, Thoua, Y, et al. Ultrasound demonstration of pyelitis and ureteritis in children. Pediatr Radiol. 1988;18:134.
104. Koff, S, Wagner, T, Jayanthi, V. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160:1019.
105. White, RHR. Vesicoureteric reflux and renal scarring. Arch Dis Child. 1989;64:407.
106. Hollowell, JG, Altman, HG, Snyder, HM, 3rd., et al. Coexisting ureteropelvic junction obstruction and vesicoureteral reflux: diagnosis and therapeutic implications. J Urol. 1989;142:490.
107. Diard, F, Nicolau, A, Bernard, S. Intra-renal reflux: a new cause of medullary hyperechogenicity? Pediatr Radiol. 1987;17:154.
108. Sjöström, S, Sillén, U, Jodal, U, et al. Predictive factors for resolution of congenital high grade vesicoureteral reflux in infants: results of univariate and multivariate analyses. J Urol. 2010;183(3):1177–1184.
109. Estrada, CR, Jr., Passerotti, CC, Graham, DA, et al. Nomograms for predicting annual resolution rate of primary vesicoureteral reflux: results from 2,462 children. J Urol. 2009;182(4):1535–1541.
110. Avni, FE, Hall, M, Janssens, F. Urinary tract infection. In: Fotter R, ed. Pediatric uroradiology. Berlin: Springer, 2001.
111. Auringer, ST. Pediatric uroradiology update. Urol Clin North Am. 1997;24:673.
112. Goldraich, NP, Goldraich, IJ. Follow-up of conservatively treated children with high and low grade vesicoureteral reflux: a prospective study. J Urol. 1992;148:1688.
113. McLorie, GA, McKenna, PH, Jumper, BM, et al. High grade vesicoureteral reflux: analysis of observational therapy. J Urol. 1990;144:537.
114. Hellström, M, Jacobsson, B, Marild, S, et al. Voiding cystourethrography as a predictor of reflux nephropathy in children with urinary-tract infections. AJR Am J Roentgenol. 1989;152:801.
115. Ben-Ami, T, Gayer, G, Hertz, M, et al. Natural history of reflux in the lower pole of duplicated collecting systems: a controlled study. Pediatr Radiol. 1989;19:308.
116. Andriole, VT. Urinary tract infections: recent developments. J Infect Dis. 1987;156:865.
117. Lenaghan, D, Whitaker, JG, Jensen, F, et al. The natural history of reflux and long-term effects of reflux on the kidney. J Urol. 1976;115:728.
118. Zaffanello, M, Tardivo, S, Cataldi, L, et al. Genetic susceptibility to renal scar formation after urinary tract infection: a systematic review and meta-analysis of candidate gene polymorphisms. Pediatr Nephrol. 2011;26(7):1017–1029.
119. Peters, C, Rushton, HG. Vesicoureteral reflux associated renal damage: congenital reflux nephropathy and acquired renal scarring. J Urol. 2010;184(1):265–273.
120. Swerkersson, S, Jodal, U, Sixt, R, et al. Relationship among vesicoureteral reflux, urinary tract infection and renal damage in children. J Urol. 2007;178(2):647–651. [discussion 650-651].
121. Wennerström, M, Hansson, S, Jodal, U, et al. Primary and acquired renal scarring in boys and girls with urinary tract infection. J Pediatr. 2000;136:30.
122. Goldman, M, Lahat, E, Strauss, S, et al. Imaging after urinary tract infection in male neonates. Pediatrics. 2000;195:1232.
123. Assael, BM, Guez, S, Marra, G, et al. Congenital reflux nephropathy: a follow-up of 108 cases diagnosed perinatally. Br J Urol. 1998;82:252.
124. Stock, JA, Wilson, D, Hanna, MN. Congenital reflux nephropathy and severe unilateral reflux. J Urol. 1998;160:1017.
125. Dillon, MJ, Goonasekera, CDA. Reflux nephropathy. J Am Soc Nephrol. 1998;9:2377.
126. Chambers, T. An essay on the consequences of childhood urinary tract infection. Pediatr Nephrol. 1997;11:178.
127. Merrick, MC, Notghi, A, Chalmers, N, et al. Long term follow-up to determine the prognostic value of imaging after urinary tract infection: II. Scarring. Arch Dis Child. 1995;72:393.
128. Martinelli, J, Claesson, I, Lidin-Janson, G, et al. Urinary infection, reflux and renal scarring in females continuously followed for 13-18 years. Pediatr Nephrol. 1995;9:131.
129. Smellie, JM, Poulton, A, Prescod, NP. Retrospective study of children with renal scarring associated with urinary infection. BMJ. 1994;308:1193.
130. Matsuoka, H, Oshima, K, Sakamoto, K, et al. Renal pathology in patients with reflux nephropathy. Eur Urol. 1994;26:153.
131. Riccabona, M, Ring, E, Maurer, U, et al. Scintigraphy and sonography in reflux nephropathy: a comparison. Nucl Med Com. 1993;14:339.
132. Rushton, HG, Majd, M, Jantausch, B, et al. Renal scarring following vesico-ureteral reflux and non-reflux pyelonephritis in children: evaluation with Tc-DMSA. J Urol. 1992;147:1327.
133. Bernstein, J, Arant, BS. Morphological characteristics of segmental scarring in vesicoureteral reflux. J Urol. 1992;148:171.
134. Shanon, A, Feldman, W, McDonald, P, et al. Evaluation of renal scars by Tc-DMSA scan, intravenous urography and ultrasound: a comparative study. J Pediatr. 1992;120:399.
135. Farnsworth, RH, Rossleigh, MA, Leighton, DM, et al. The detection of reflux nephropathy in infants by Tc DMSA studies. J Urol. 1991;145:542.
136. Najmalin, A, Burge, DM, Atwell, JD. Reflux nephropathy secondary to intra-uterine vesico-ureteral reflux. J Pediatr Surg. 1990;25:387.
137. McLorie, GA, Alaibadi, H, Churchill, BM, et al. 99mTechnetium-dimercapto-succinic acid renal scanning and excretory urography in diagnosis of focal renal scars in children. J Urol. 1989;142:790.
138. Shimada, K, Matsui, T, Ogino, T, et al. Renal growth and progression of reflux nephropathy in children with vesicoureteral reflux. J Urol. 1988;140:1097.
139. Lerner, GR, Fleischmann, LE, Perlmutter, AD. Reflux nephropathy. Pediatr Clin North Am. 1987;34:747.
140. Novak, TE, Mathews, R, Martz, K, et al. Progression of chronic kidney disease in children with vesicoureteral reflux: the North American Pediatric Renal Trials Collaborative Studies Database. J Urol. 2009;182(suppl 4):1678–1681.
141. Brakeman, P. Vesicoureteral reflux, reflux nephropathy, and end-stage renal disease. Adv Urol. 2008:508949.
142. Vachvanichsanong, P. Urinary tract infection: one lingering effect of childhood kidney diseases—review of the literature. J Nephrol. 2007;20(1):21–28.
143. Berg, UB. Long term follow-up of renal morphology and function in children with recurrent pyelonephritis. J Urol. 1992;148:1715.
144. Goonasekera, C, Dillon, MJ. Hypertension in reflux nephropathy. Br J Urol. 1999;83:1.
145. Greenfield, SP. Antibiotic prophylaxis in pediatric urology: an update. Curr Urol Rep. 2011;12(2):126–131.
146. White, B. Diagnosis and treatment of urinary tract infections in children. Am Fam Physician. 2011;83(4):409–415.
147. Nagler, EV, Williams, G, Hodson, EM, et al. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev. (6):2011. [CD001532].
148. Park, S, Han, JY, Kim, KS. Risk factors for recurrent urinary tract infection in infants with vesicoureteral reflux during prophylactic treatment: effect of delayed contrast passage on voiding cystourethrogram. Urology. 2011;78(1):170–173.
149. Khalil, BA, Goyal, A, Dickson, AP. Surgical intervention in children with vesicoureteric reflux: are we intervening too late? Pediatr Surg Int. 2010;26(7):729–731.
150. Mattoo, TK. Evidence for and against urinary prophylaxis in vesicoureteral reflux. Pediatr Nephrol. 2010;25(12):2379–2382.
151. Szmigielska, A, Krzemien, G, Roszkowska-Blaim, M. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2010;362(6):556. [author reply 556-557].
152. Dai, B, Liu, Y, Jia, J, et al. Long-term antibiotics for the prevention of recurrent urinary tract infection in children: a systematic review and meta-analysis. Arch Dis Child. 2010;95(7):499–508.
153. Mattoo, TK. Are prophylactic antibiotics indicated after a urinary tract infection? Curr Opin Pediatr. 2009;21(2):203–206.
154. Mori, R, Fitzgerald, A, Williams, C, et al. Antibiotic prophylaxis for children at risk of developing urinary tract infection: a systematic review. Acta Paediatr. 2009;98(11):1781–1786.
155. Lee, EK, Gatti, JM, Demarco, RT, et al. Long-term followup of dextranomer/hyaluronic acid injection for vesicoureteral reflux: late failure warrants continued followup. J Urol. 2009;181(4):1869–1874. [discussion 1874-1875].
156. Sedberry-Ross, S, Rice, DC, Pohl, HG, et al. Febrile urinary tract infections in children with an early negative voiding cystourethrogram after treatment of vesicoureteral reflux with dextranomer/hyaluronic acid. J Urol. 2008;180(suppl 4):1605–1609. [discussion 1610].
157. Faust, WC, Pohl, HG. Role of prophylaxis in vesicoureteral reflux. Curr Opin Urol. 2007;17(4):252–256.
158. Hensle, TW, Grogg, AL. Part 1: Vesicoureteral reflux treatment: the past, present, and future. Curr Med Res Opin. 2007;23(suppl4):S1–S5.
159. Hensle, TW, Hyun, G, Grogg, AL, et al. Part 2: Examining pediatric vesicoureteral reflux: a real-world evaluation of treatment patterns and outcomes. Curr Med Res Opin. 2007;23(suppl 4):S7–S13.
160. Elder, JS, Shah, MB, Batiste, LR, et al. Part 3: Endoscopic injection versus antibiotic prophylaxis in the reduction of urinary tract infections in patients with vesicoureteral reflux. Curr Med Res Opin. 2007;23(suppl 4):S15–S20.
161. Heidenreich, A, Ozgur, E, Becker, T, et al. Surgical management of vesicoureteral reflux in pediatric patients. World J Urol. 2004;22(2):96–106.
162. Molitierno, JA, Scherz, HC, Kirsch, AJ. Endoscopic treatment of vesicoureteral reflux using dextranomer hyaluronic acid copolymer. J Pediatr Urol. 2008;4(3):221–228.
163. Bollgren, I. Antibacterial prophylaxis in children with UTI. Acta Pediatr Scand. 1999;431(S):48.
164. Shapiro, E, Elder, J. The office management of recurrent urinary tract infection and vesico-ureteral reflux in children. Urol Clin. 1998;4:725.
165. Stark, H. Urinary tract infection in girls: the cost-effectiveness of currently recommended investigative routines. Pediatr Nephrol. 1997;11:174.
166. Gordon, I. Vesicoureteral reflux, urinary tract infection and renal damage in children. Lancet. 1995;346:489.
167. Tamminen-Mobius, T, Brunier, E, Ebel, KD, et al. Cessation of vesicoureteral reflux for five years in infants and children allocated to medical treatment. J Urol. 1992;148:1662.
168. Arant, BS. Medical management of mild and moderate vesicoureteral reflux: follow-up studies of infants and young children—a preliminary report of the Southwest Pediatric Nephrology Group. J Urol. 1992;148:1683.
169. Farkas, A, Moriel, EZ, Lupa, S. Endoscopic correction of vesicoureteral reflux: our experience with 115 ureters. J Urol. 1990;144:534.
170. Blake, NS, O’Connell, E. Endoscopic connection of vesico-ureteric reflux by subureteric Teflon injection: follow-up ultrasound and voiding cystography. Br J Radiol. 1989;62:443.
171. Gore, MD, Fernbach, SK, Donaldson, JS, et al. Radiographic evaluation of subureteric injection of Teflon to correct VUR. AJR Am J Roentgenol. 1989;152:115.
172. Finnell, SM, Carroll, AE, Downs, SM, the Subcommittee on Urinary Tract Infection. Technical report—diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3):e749–e770.
173. Lee, JH, Kim, MK, Park, SE. Is a routine voiding cystourethrogram necessary in children after the first febrile urinary tract infection? Acta Paediatr. 2012;101(3):e105–e109.
174. Schroeder, AR, Abidari, JM, Kirpekar, R, et al. Impact of a more restrictive approach to urinary tract imaging after febrile urinary tract infection. Arch Pediatr Adolesc Med. 2011;165(11):1027–1032.
175. Koyle, MA, Elder, JS, Skoog, SJ, et al. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation. Pediatr Surg Int. 2011;27(4):337–346.
176. Mantadakis, E, Vouloumanou, EK, Georgantzi, GG, et al. Acute Tc-99m DMSA scan for identifying dilating vesicoureteral reflux in children: a meta-analysis. Pediatrics. 2011;128(1):e169–e179.
177. Soccorso, G, Moss, G, Roberts, J, et al. Infantile urinary tract infection and timing of micturating cystourethrogram. J Pediatr Urol. 2010;6(6):582–584.
178. Quirino, IG, Silva, JM, Diniz, JS, et al. Combined use of late phase dimercapto-succinic acid renal scintigraphy and ultrasound as first line screening after urinary tract infection in children. J Urol. 2011;185(1):258–263.
179. Routh, JC, Lee, RS, Chow, JS. Radiation dose and screening for vesicoureteral reflux. AJR Am J Roentgenol. 2010;194(2):W243.
180. Ajdinovi , B, Jaukovi
, B, Jaukovi , L, Krsti
, L, Krsti , Z, et al. Impact of micturating cystourethrography and DMSA renal scintigraphy on the investigation scheme in children with urinary tract infection. Ann Nucl Med. 2008;22(8):661–665.
, Z, et al. Impact of micturating cystourethrography and DMSA renal scintigraphy on the investigation scheme in children with urinary tract infection. Ann Nucl Med. 2008;22(8):661–665.
181. Keren, R. Imaging and treatment strategies for children after first urinary tract infection. Curr Opin Pediatr. 2007;19(6):705–710.
182. Mahant, S, To, T, Friedman, J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;139:568.
183. Smellie, JM, Prescod, NP, Shaw, PJ, et al. Childhood reflux and urinary infection: a follow-up of 10-41 years in 226 adults. Pediatr Nephrol. 1998;12:727.
184. Connolly, LP, Treves, ST, Zurakowski, D, et al. Natural history of vesicoureteral reflux in siblings. J Urol. 1996;156:1805.
185. Dick, PT, Feldman, W. Routine diagnostic imaging for childhood urinary tract infection: a systematic overview. J Pediatr. 1996;128:15.