5 Very High Systemic Arterial Blood Pressure
The Joint National Committee (JNC) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has defined two acute conditions of elevated systemic arterial pressure.1 A hypertensive emergency is characterized by the presence of elevated systemic blood pressure (BP) and new or progressive end-organ damage, including but not limited to the cardiac, renal, and central nervous systems. A hypertensive emergency is an infrequent clinical situation that requires immediate BP reduction (not necessarily to normal ranges). Although the absolute BP elevation is not a criterion for the diagnosis, a hypertensive emergency is typically associated with a diastolic blood pressure (DBP) above 120 mm Hg. If unrecognized or left untreated, hypertensive emergencies can lead to acute myocardial infarction (MI), pulmonary edema from left ventricular (LV) dysfunction, hypertensive encephalopathy (HE), intracranial hemorrhage, microangiopathic hemolysis, and/or acute renal failure (Box 5-1).
Box 5-1
Hypertensive Emergencies
LV, Left ventricular.
Using JNC definitions, hypertensive crises (urgency and emergency) account for more than 25% of all patient visits to a medical section of an emergency department (ED), with hypertensive emergencies accounting for one-third of the cases.2 Central nervous system (CNS) complications are the most prevalent organ system dysfunction, followed by cardiovascular dysfunction. The incidence of the disorder has remained stable at 2 to 3 cases per 100,000 population over many decades, although the prognosis associated with aggressive medical management has improved significantly.3 Most commonly, hypertensive emergencies occur in the setting of uncontrolled or unknown chronic hypertension. Hypertensive emergencies also may develop as secondary hypertension in association with such diverse etiologies as renal vascular disease, sleep apnea, hyperaldosteronism, pheochromocytoma, and pregnancy (preeclampsia).4 Postoperative hypertension occurs most often following vascular surgery procedures in patients with a background history of hypertension. Untreated postoperative hypertension can contribute to postoperative bleeding in addition to the recognized complications of hypertensive emergencies.
Additional terms used by clinicians to describe very high systemic arterial BP include accelerated hypertension, which is a severely elevated BP associated with retinal findings of ocular hemorrhages and exudates. The term malignant hypertension includes severe hypertension with the presence of ocular hemorrhages and exudates with papilledema (grade IV Kimmelstiel-Wilson retinopathy). Vascular injury to the kidney in this setting is termed malignant nephrosclerosis. The term hypertensive emergency is preferred, as end-organ dysfunction can occur in the patient with hypertension in the absence of retinal findings.5,6
 Pathophysiology
Pathophysiology
An acute elevation in systemic arterial BP most fundamentally involves an increase in systemic vascular resistance. This increase in vascular resistance is attributed to a complex interaction of circulating and local vascular mediators. Vasoconstriction is promoted by circulating catecholamines, angiotensin II (ATII), vasopressin, thromboxane (TxA2), and/or endothelin 1 (ET1). In contrast, compensatory production of local counterregulatory vasodilators, including nitric oxide (NO) and prostacyclin (PGI2), is inadequate to maintain homeostatic balance. This unregulated vasoconstriction promotes further endothelial dysfunction. A proinflammatory response, incorporating cytokine secretion, monocyte activation, and up-regulated expression of endothelial adhesion molecules, appears to occur in hypertensive emergencies, leading to promotion of endothelial hyperpermeability and activation of coagulation cascades.7 This cascade of intravascular events leads to the characteristic pathologic findings of obliterative vascular lesions. These vascular changes, evident to the clinician by examination of the retina, are mirrored by changes in the kidney, leading to a proliferative arteritis, and in advanced stages of the process, fibrinoid necrosis. Relative ischemia results in affected organs, leading to end-organ dysfunction. Early control of elevated BP is critical to prevent progression to a more advanced stage of the disease process.
Aggressive control of elevated systemic arterial BP must be undertaken with caution, however. The potential adverse effects of aggressive BP control have been most carefully considered in the cerebral circulation. Normally, cerebrovascular arteriolar tone is adjusted over a range of cerebral perfusion pressures in order to maintain a constant cerebral blood flow (CBF). Increases in cerebral perfusion pressure (CPP) promote an increase in vascular resistance, whereas decreases in CPP act to vasodilate the cerebral vasculature. In normal individuals, constant flow is therefore maintained over a range of mean arterial pressure (MAP) from approximately 60 mm Hg to 150 mm Hg.8 As MAP increases to values over 180 mm Hg, or the upper limit of autoregulation, cerebral hyperperfusion can occur, resulting in cerebral edema. Conversely, when CPP falls below the lower limit of autoregulation, CBF decreases, and tissue ischemia may occur. In patients with long-standing hypertension, a rightward shift of the CPP-CBF relationship occurs such that the lower limit of autoregulation occurs at a value higher than in normal subjects.9 Comparative studies in hypertensive and normotensive patients suggest that the lower limit of autoregulation is about 20% below the resting MAP for both, although the absolute value is higher for hypertensive patients.10 These data support the common recommendation for a maximum BP reduction in the acute setting of 20% to 25% of the MAP from the highest values, or a DBP goal typically in the 100 to 110 mm Hg range. This regulated level of BP reduction should maintain critical organ perfusion even for patients with long-standing hypertension.
 Cerebrovascular Disease
Cerebrovascular Disease
Hypertensive Encephalopathy
Acute elevations in systemic arterial BP can lead to HE, resulting from a failure of the upper level of cerebral vascular autoregulation. The most common clinical manifestations include headache, nausea and vomiting, visual disturbances, focal neurologic findings, or seizures. If left untreated, the condition can progress to coma and death. The majority of patients with HE will have a MAP significantly above the patient’s baseline BP, although not always in the range typically associated with hypertensive emergency. Retinal findings including arteriolar spasm, exudates, hemorrhages, and papilledema are often present but are not required to establish the diagnosis. Magnetic resonance imaging (MRI) studies show a characteristic edema pattern involving the subcortical white matter of the parietooccipital regions; this finding is termed posterior leukoencephalopathy.11 Best appreciated on T2-weighted images, posterior structures including the cerebellum, brainstem, and occasionally the cortex also can be affected. The findings typically are bilateral but can be asymmetric. The electroencephalogram (EEG) can show loss of the posterior dominant alpha rhythm, generalized slowing, and posterior epileptiform discharges, which resolve after appropriate therapy.12
Acute Stroke
Hypertension is present in as many as 80% of patients with acute stroke, particularly in patients with preexisting hypertension. The incidence is higher among patients with primary intracerebral hemorrhage as compared to ischemic disorders.13 The acute high systemic arterial BP most frequently declines to normal within 48 hours of presentation. The relationship between BP and mortality in patients with stroke may be “U-shaped.” According to this notion, systolic BP (SBP) values above or below 140 to 180 mm Hg are associated with increased mortality. In the International Stroke Trial, SBP above 200 mm Hg was associated with an increased risk of recurrent ischemic stroke (50% greater risk of recurrence), while low BP (particularly <120 mm Hg) was associated with an excess number of deaths from coronary heart disease.14
A number of important clinical features complicate the management of hypertension in acute stroke. First, during acute stroke, cerebral autoregulation may be compromised in ischemic tissue, and lowering of BP may further compromise CBF and extend ischemic injury. Second, medications used to treat hypertension can lead to cerebral vasodilation, augmenting CBF and leading to progression of cerebral edema.15 Ideally a “correct” level of MAP should be maintained in each patient to maintain CPP without risking worsening cerebral edema or progression of the lesion, but the clinical determination of this “ideal” value is often difficult.
A Cochrane review of 12 trials comparing an active intervention to placebo/control with 1153 total participants concluded that insufficient evidence existed to favor altering BP in acute stroke.16 Using available information, most consensus guidelines recommend that BP not be treated acutely in patients with ischemic stroke unless the hypertension is extreme (SBP >220 mm Hg or DBP >120 mm Hg) or the patient has active end-organ dysfunction in other organ systems.17 When treatment is indicated, cautious lowering of BP by approximately 15% during the first 24 hours after stroke onset is suggested. Antihypertensive medications are restarted approximately 24 hours after stroke onset in patients with preexisting hypertension who are neurologically stable, unless a specific contraindication to restarting treatment exists. Requiring special consideration are patients with extracranial or intracranial arterial stenosis and candidates for thrombolytic therapy. The former group is dependent on perfusion pressure so BP therapy may be further delayed. In contrast, before lytic therapy is started, treatment is recommended so that SBP is 185 mm Hg or less and DBP is 110 mm Hg or less. Blood pressure should be stabilized and maintained below 180/105 mm Hg for at least 24 hours after intravenous lytic therapy.17
Two recent clinical trials have suggested aggressive BP reduction limits hematoma expansion without clear benefit on mortality.18,19
 Cardiovascular Disease
Cardiovascular Disease
Acute Left Ventricular Dysfunction
The vast majority of patients presenting with acute heart failure are hypertensive on initial assessment.20 Hypertension can be the inciting event, with secondary myocardial dysfunction; or alternatively, hypertension can be a secondary component of acute pulmonary edema due to the sympathoadrenal response to hypoxemia, increased work of breathing, and anxiety. Efforts to control elevated systemic arterial pressure in this setting are essential because high systemic arterial BP in the patient with acute pulmonary edema contributes to increased myocardial workload and diastolic dysfunction. In contrast, the use of vasodilators in patients with acute pulmonary edema and normal to low BP can have deleterious effects.21,22 Similar to the patient with cerebrovascular disease, a U-shaped blood pressure/mortality relationship is expected.
In addition to the more traditional IV vasodilators, IV calcium channel antagonists have demonstrated efficacy in the treatment of acute hypertension in the setting of LV dysfunction. The dihydropyridine calcium channel antagonists nicardipine and clevidipine can reduce systemic arterial pressure while preserving coronary blood flow.23 Fenoldopam, a dopamine-1 receptor antagonist, also has been has been shown to preserve coronary blood flow during treatment to reduce systemic arterial pressure in this setting.24 Despite their demonstrated efficacy in the treatment of hypertensive emergency, limited data exist with these newer agents to suggest superiority over NTG or nitroprusside. Agent selection should first be influenced by the adverse risk profile associated with the individual agents (Table 5-1). When not contraindicated by specific risk, the agents with a more favorable cost profile (i.e., NTG, nitroprusside) should be used based upon equivalent efficacy.
 Renovascular Disease
Renovascular Disease
Scleroderma Renal Crisis
Scleroderma renal crisis is characterized by the development of acute renal failure associated with moderate to severe hypertension and a normal to minimally abnormal urine sediment. The most significant risk factor for scleroderma renal crisis is the presence of diffuse skin involvement characteristic of the disease and recent treatment with high-dose corticosteroids.25 The disorder results in marked activation of the renin-angiotensin system. Aggressive control of BP using ACE inhibitors, particularly early in the disease process, can control BP in up to 90% of patients and promote a greater rate of recovery in renal function.26
Post Kidney Transplantation
Hypertension following renal transplantation occurs in the majority of patients.27 In the immediate posttransplantation period, hypertension can be a manifestation of volume overload, graft rejection, ischemia, or toxic effects of calcineurin inhibitors used for immunosuppression. Treatment is directed primarily at the underlying mechanism. Renal artery stenosis can also complicate allograft function and should be evaluated in any patient with resistant hypertension. This complication can occur at any time within 1 month or up to 3 years after transplantation.28 In the immediate posttransplant period, BP should be regulated at the upper limits of normal to preserve graft function. In the later postoperative period, more strict control of BP is favored.29 Calcium channel blockers (CCBs) are frequently used to treat hypertension after renal transplantation, based upon their antagonism of cyclosporine-induced renal vasoconstriction. CCBs also have been studied extensively in renal transplant hypertension and are associated with preservation of allograft function in comparison to placebo.29 ACE inhibitors have the potential to exacerbate renal dysfunction and augment hyperkalemia induced by calcineurin inhibitors.
 Excess Catecholamine States
Excess Catecholamine States
Pheochromocytoma
Pheochromocytoma can result in the production of circulating mediators, leading to catecholamine excess. These mediators result in hypertension, diaphoresis, tachycardia, and paresthesias of the hands and feet. These attacks can last from minutes to days and occur as frequently as several times a day or as infrequently as once a month.30 Operative manipulation of the tumor can result in perioperative hypertension. The treatment of hypertension in this disorder must avoid the use of isolated therapy with a beta-blocker, a strategy that can lead to unopposed alpha-adrenergic stimulation, with the risk of further vasoconstriction and BP elevation. The preferred agent for treatment is phentolamine, a potent alpha-adrenergic antagonist. If needed, this medication can be combined with a beta-blocker, or a combined alpha/beta-blocker such as labetalol also can be used safely.
Pharmacologically Mediated
A broad range of medications have been associated with the development of hypertension or alternatively may limit the effectiveness of treatment for primary hypertension. A detailed medical history is required to evaluate patients with high systemic arterial BP. Attention should be paid to prescription medications as well as herbal supplements and substance abuse.31 Both administration of exogenous substances and abrupt withdrawal of substances can be associated with hypertensive crises. As an example, clonidine withdrawal can mimic the crisis of pheochromocytoma. Clonidine is a centrally acting stimulant of alpha-adrenergic receptors that reduces peripheral adrenergic system activation. Rapid withdrawal or tapering of clonidine produces a hyperadrenergic state characterized by hypertension, diaphoresis, headache, and anxiety.32 The syndrome is best treated by restarting treatment with clonidine. Extreme symptoms can be treated as outlined for the patient with pheochromocytoma. Hypertension also can occur during the withdrawal phase of alcohol abuse.
 Antihypertensive Medications
Antihypertensive Medications
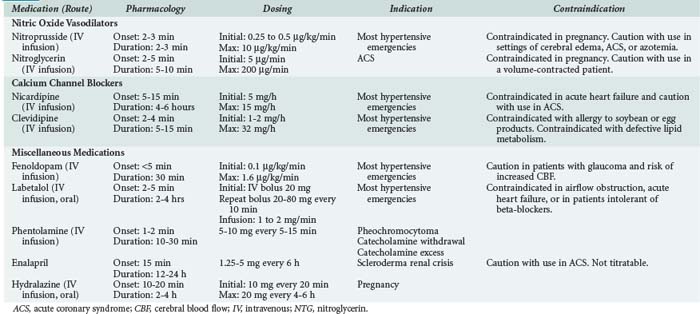
For hypertensive urgencies, oral therapy can be used to lower BP to safer levels over a 24-hour interval. These patients in general do not require monitoring in an ICU. A summary of the medications available for the treatment of hypertensive emergency is outlined in Table 5-2.
| Cerebrovascular Disease | |
| Acute ischemic stroke | Nicardipine, labetalol |
| Acute intracerebral hemorrhage | Nicardipine, labetalol |
| Cardiovascular Disease | |
| ACS | NTG |
| Acute LV dysfunction | NTG, nitroprusside |
| Acute aortic dissection | Beta-blocker followed by nitroprusside or nicardipine |
| Acute MI | Clevidipine, labetalol, nicardipine, NTG |
| Renovascular Disease | |
| Acute renal failure | Clevidipine, labetalol, nicardipine, nitroglycerin |
| Scleroderma renal crisis | ACE inhibitor |
| Endocrine Diseases | |
| Pheochromocytoma | Phentolamine, labetalol |
| Drug-Related Disorders | |
| Catecholamine toxicity | Phentolamine, labetalol |
| Perioperative hypertension | Clevidipine, nicardipine, NTG, nitroprusside |
| Preeclampsia or eclampsia | Hydralazine, labetalol |
ACE, angiotensin-converting enzyme; ACS, acute coronary syndrome; LV, left ventricular; MI, myocardial infarction; NTG, nitroglycerin.
Nitric Oxide Vasodilators
SNP’s arteriolar and venous vasodilating activity may not be uniform, however. Redistribution of oxygenated blood flow from unresponsive ischemic regions to vasodilated nonischemic coronary arteries can reduce coronary perfusion pressure, resulting in a “coronary steal” syndrome.33 A similar “cerebral steal” syndrome has been suggested with SNP as a result of preferential vasodilation in systemic vascular beds versus cerebral vessels.15 Additional concerns have been raised with the use of SNP in patients with increased ICP; dilatation of large-capacitance vessels by SNP can lead to an increase in CBF and ICP.15
Cyanide toxicity from SNP is uncommon and occurs primarily in patients receiving infusions for more than 24 to 48 hours, in the setting of underlying renal insufficiency, and/or the use of doses that exceed the capacity of the body to detoxify cyanide (>2 µg/kg/min). The treatment of cyanide intoxication involves the administration of sodium thiosulfate. Sodium thiosulfate donates its sulfane sulfur atom in a reaction catalyzed by the enzyme, rhodanese, to convert cyanide to the much less toxic thiocyanate ion, which is then excreted in the urine. For severe cases, sodium nitrite may also be administered. Sodium nitrite oxidizes hemoglobin (Hb) in the blood to methemoglobin, which binds cyanide with high affinity. Thus, methemoglobin competes with other cellular targets for cyanide, notably cytochrome a-a3 in mitochondria, and thereby decreases the toxic effects of cyanide ion. The onset of action of sodium nitrite is rapid, but the induction of methemoglobinemia decreases the oxygen-carrying capacity of blood and therefore can be harmful in patients with anemia or significant carboxyhemoglobinemia. Hydroxocobalamin (vitamin B12a), is another safe and effective antidote for cyanide intoxication. Hydroxyocobalamin administration does not affect the oxygen-carrying capacity of the blood, so this harmless agent may be preferable to sodium nitrite. Hydroxyocobalamin reacts with circulating cyanide to form cyanocobalamin, with subsequent urinary excretion. Hydroxocobalamin has been demonstrated to minimize the risk of cyanide accumulation during nitroprusside use in surgery.34
Calcium Channel Blockers
Calcium channel blockers are a heterogenous class of medications used in the treatment of hypertension emergencies. A specific class of CCB called the dihydropyridines (e.g., nicardipine, clevidipine) are selective for vascular smooth muscle over the myocardium, having little if any activity on cardiac muscle or the sinoatrial node.35 Because these drugs act to promote vascular smooth muscle relaxation without associated cardiac effects, they are attractive for the treatment of hypertensive emergencies. In contrast, CCBs from other pharmacologic classes, such as diltiazem and verapamil, affect the cardiac conduction system and myocardial calcium channels, making them less optimal choices for the treatment of hypertension.
Nicardipine hydrochloride is a dihydropyridine CCB that acts primarily as a systemic, cerebral, and coronary artery vasodilator. The greater water solubility of this drug, in comparison to other CCBs such as nifedipine, allows IV administration with a short onset and duration of action and therefore easy titration to therapeutic effect. The medication has no significant effect on cardiac inotropy and promotes afterload reduction. Nicardipine readily crosses the blood-brain barrier and relaxes vascular smooth muscle, especially in regions of ischemic tissue. The medication acts as a vasodilator of small-resistance cerebral arterioles but does not change intracranial volume or ICP; thus, cerebral oxygenation is preserved.36
Nicardipine has been studied as an alternative agent to SNP in the management of hypertension for patients with intracranial or subarachnoid hemorrhage. In comparison to SNP, nicardipine offers equal efficacy in terms of BP control. But nicardipine, avoiding problems related to the toxic metabolites of SNP, requires less frequent dose adjustments and carries less risk of increasing ICP.37 Comparative investigations of nicardipine and nitroprusside in postoperative patients with hypertension suggest therapeutic equivalency.38,39 Nicardipine is metabolized by the liver, and excretion can be impaired in patients with abnormal hepatic function.
Clevidipine has been most extensively investigated in adult patients (>18 years of age) with acute perioperative or postoperative hypertension in the setting of cardiac surgery. The antihypertensive efficacy of IV clevidipine was compared with that of SNP and NTG for perioperative hypertension, and with nicardipine for postoperative hypertension.40 All agents were administered by IV infusion. The primary endpoint was the incidence of death, stroke, MI, or renal dysfunction from study drug initiation to 30 days after surgery. BP control was a secondary endpoint evaluated; using the area under the curve (AUC) of SBP excursions above or below predetermined limits (65-135 mm Hg, intraoperatively; 75-145 mm Hg, pre- and postoperatively).
The antihypertensive efficacy of IV clevidipine in patients with acute severe hypertension has also been assessed in a large, noncomparative, open-label, multicenter, phase III study.41 Clevidipine was administered as a non–weight-based dose of 2 mg per hour for patients with acute severe hypertension with or without end-organ injury. The medication provided rapid, predictable BP control, and the majority of patients reached the target BP within 30 minutes. Prolonged administration (>18 hours) was well tolerated.
 Miscellaneous Medications
Miscellaneous Medications
The hemodynamic effects of fenoldopam and SNP were compared in a multicentric clinical trial that enrolled patients with acute hypertension. The researchers showed that fenoldopam was as effective as SNP for controlling acute systemic hypertension.42 The average decreases in BP at 6 hours of infusion were similar in the two study groups. The average maintenance infusion rate for fenoldopam was 0.41 µg/kg/min (range, 0.1 to 1.62 µg/kg/min). The time required to reach the maintenance infusion rate was similar in the two groups. In a population subset, indices of renal function, including creatinine clearance, urinary output, and sodium excretion, were better in the group randomized to fenoldopam treatment. However, the study sample was too small to draw definitive conclusions. Both drugs were equally well tolerated.
The use of fenoldopam in patients with hypertensive emergencies was evaluated in 107 patients with DBP >120 mm Hg and clinical evidence of acute vasculopathy.43 Infusion rates of 0.01, 0.03, 0.1, or 0.3 µg/kg/min for 24 hours were studied. Within this range of doses, fenoldopam was safe. Thus fenoldopam is an easily titrated drug that is effective when BP has to be reduced rapidly.
Labetalol is an oral and parenteral agent that acts as an alpha- and nonselective beta-adrenergic blocker. The BP-lowering effect is produced through a reduction in systemic vascular resistance without a compensatory increase in heart rate. Labetalol has very little effect on the cerebral circulation and is thus not associated with an increase in ICP in the normal brain.44 The drug has been used effectively in patients with end-organ dysfunction in the setting of acute neurologic injury, pheochromocytoma, cocaine intoxication, dissecting aneurysm, and eclampsia. The primary contraindication to the use of the medication relates to its nonselective beta-blocking properties. The drug should be used cautiously in patients with reactive airways disease, heart block, or decompensated LV failure.
Aronson S, et al. The ECLIPSE trials: comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107(4):1110-1121.
Lane DA, Lip GYH, Beevers DG. Improving survival of malignant hypertension patients over 40 years. Am J Hypertens. 2009;22(11):1199-1204.
Geeganage Geeganage C, Bath PM. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev 2008;(4):CD000039.
Grossman E, Messerli FH. Secondary hypertension: interfering substances. J Clin Hypertens (Greenwich). 2008;10(7):556-566.
Immink RV, et al. Cerebral hemodynamics during treatment with sodium nitroprusside versus labetalol in malignant hypertension. Hypertension. 2008;52(2):236-240.
1 Lenfant C, Chobanian A, Jones D, Roccella E. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41(6):1178-1179.
2 Zampaglione B, Pascale C, Marchisio M, Cavallo-Perin P. Hypertensive urgencies and emergencies. Prevalence and clinical presentation. Hypertension. 1996;27(1):144-147.
3 Lane DA, Lip GYH, Beevers DG. Improving survival of malignant hypertension patients over 40 years. Am J Hypertens. 2009;22(11):1199-1204.
4 Börgel J, Springer S, Ghafoor J, Arndt D, Duchna HW, Barthel A, et al. Unrecognized secondary causes of hypertension in patients with hypertensive urgency/emergency: prevalence and co-prevalence. Clin Res Cardiol. 2010;99(8):499-506.
5 Ahmed ME, Walker JM, Beevers DG, Beevers M. Lack of difference between malignant and accelerated hypertension. Br Med J (Clin Res Ed). 1986;292(6515):235-237.
6 McGregor E, Isles CG, Jay JL, Lever AF, Murray GD. Retinal changes in malignant hypertension. Br Med J (Clin Res Ed). 1986;292(6515):233-234.
7 Verhaar MC, et al. Progressive vascular damage in hypertension is associated with increased levels of circulating P-selectin. J Hypertens. 1998;16(1):45-50.
8 Lassen NA. Autoregulation of Cerebral Blood Flow. Circ Res. 1964;15(Suppl):201-204.
9 Czosnyka M, et al. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373-386.
10 Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation. 1976;53(4):720-727.
11 Hinchey J, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
12 Manfredi M, et al. Eclamptic encephalopathy: imaging and pathogenetic considerations. Acta Neurol Scand. 1997;96(5):277-282.
13 Jorgensen HS, et al. Blood pressure in acute stroke. The Copenhagen Stroke Study. Cerebrovasc Dis. 2002;13(3):204-209.
14 Leonardi-Bee J, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33(5):1315-1320.
15 Immink RV, et al. Cerebral hemodynamics during treatment with sodium nitroprusside versus labetalol in malignant hypertension. Hypertension. 2008;52(2):236-240.
16 Geeganage C, Bath PM. Interventions for deliberately altering blood pressure in acute stroke. Cochrane Database Syst Rev 2008;(4):CD000039.
17 Adams HPJr, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
18 Anderson CS, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7(5):391-399.
19 Qureshi AI, et al. Effect of systolic blood pressure reduction on hematoma expansion, perihematomal edema, and 3-month outcome among patients with intracerebral hemorrhage: results from the antihypertensive treatment of acute cerebral hemorrhage study. Arch Neurol. 2010;67(5):570-576.
20 Milo-Cotter O, et al. Acute heart failure associated with high admission blood pressure—a distinct vascular disorder? Eur J Heart Fail. 2007;9:178-183.
21 Elkayam U, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153(1):98-104.
22 Mebazaa A, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297(17):1883-1891.
23 Peacock FWt, et al. Clevidipine for severe hypertension in acute heart failure: a VELOCITY trial analysis. Congest Heart Fail. 2010;16(2):55-59.
24 Halpenny M, et al. The effects of fenoldopam on coronary conduit blood flow after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2001;15(1):72-76.
25 Denton CP, et al. Renal complications and scleroderma renal crisis. Rheumatology (Oxford). 2009;48(Suppl 3):iii32-iii35.
26 Steen V. Scleroderma renal crisis. Indian J Med Sci. 2007;61(2):71-72.
27 Paoletti E, et al. Association of arterial hypertension with renal target organ damage in kidney transplant recipients: the predictive role of ambulatory blood pressure monitoring. Transplantation. 2009;87(12):1864-1869.
28 Polak WG, et al. Incidence and outcome of transplant renal artery stenosis: single center experience. Transplant Proc. 2006;38(1):131-132.
29 Gill JS. Cardiovascular disease in transplant recipients: current and future treatment strategies. Clin J Am Soc Nephrol. 2008;3(Suppl 2):S29-S37.
30 Manger WM. The protean manifestations of pheochromocytoma. Horm Metab Res. 2009;41(9):658-663.
31 Grossman E, Messerli FH. Secondary hypertension: interfering substances. J Clin Hypertens (Greenwich). 2008;10(7):556-566.
32 Houston MC. Abrupt cessation of treatment in hypertension: consideration of clinical features, mechanisms, prevention and management of the discontinuation syndrome. Am Heart J. 1981;102(3 Pt 1):415-430.
33 Mann T, et al. Effect of nitroprusside on regional myocardial blood flow in coronary artery disease. Results in 25 patients and comparison with nitroglycerin. Circulation. 1978;57(4):732-738.
34 Cottrell JE, et al. Prevention of nitroprusside-induced cyanide toxicity with hydroxocobalamin. N Engl J Med. 1978;298(15):809-811.
35 Triggle DJ. Calcium channel antagonists: clinical uses–past, present and future. Biochem Pharmacol. 2007;74(1):1-9.
36 Narotam PK, et al. Management of hypertensive emergencies in acute brain disease: evaluation of the treatment effects of intravenous nicardipine on cerebral oxygenation. J Neurosurg. 2008;109(6):1065-1074.
37 Roitberg BZ, et al. Prospective randomized comparison of safety and efficacy of nicardipine and nitroprusside drip for control of hypertension in the neurosurgical intensive care unit. Neurosurgery. 2008;63(1):115-120. discussion 120-1
38 Dorman T, et al. Nicardipine versus nitroprusside for breakthrough hypertension following carotid endarterectomy. J Clin Anesth. 2001;13(1):16-19.
39 Hersey SL, et al. Nicardipine versus nitroprusside for controlled hypotension during spinal surgery in adolescents. Anesth Analg. 1997;84(6):1239-1244.
40 Aronson S, et al. The ECLIPSE trials: comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107(4):1110-1121.
41 Pollack CV, et al. Clevidipine, an intravenous dihydropyridine calcium channel blocker, is safe and effective for the treatment of patients with acute severe hypertension. Ann Emerg Med. 2009;53(3):329-338.
42 Panacek EA, et al. Randomized, prospective trial of fenoldopam vs sodium nitroprusside in the treatment of acute severe hypertension. Fenoldopam Study Group. Acad Emerg Med. 1995;2(11):959-965.
43 Tumlin JA, et al. Fenoldopam, a dopamine agonist, for hypertensive emergency: a multicenter randomized trial. Fenoldopam Study Group. Acad Emerg Med. 2000;7(6):653-662.
44 Olsen KS, et al. Effect of labetalol on cerebral blood flow, oxygen metabolism and autoregulation in healthy humans. Br J Anaesth. 1995;75(1):51-54.