CHAPTER 44 Vertebroplasty
OVERVIEW
Fracture of the intervertebral body represents structural failure of its osseous components, causing compression deformity.1 Although compression fractures can be quite painful and incapacitating, many are asymptomatic and heal undetected.2 Uncovering a vertebral body compression fracture must alert the treating clinician to properly diagnose the responsible etiology. Vertebral fracture is the most common complication of osteoporosis.3 The lifetime risk of a clinically relevant vertebral fracture due to osteoporosis is approximately 16% in white women, and this proportion varies depending on the radiographic definition utilized in the study.4 Age, history of fracture and osteoporosis, and decreased height and physical activity have all been associated with vertebral fractures in both genders.5 Less common etiologies of vertebral compression fractures include multiple myeloma, lymphoma, metastatic disease, and benign vascular lesions. Malignant compression fractures account for approximately 25% of nontraumatic vertebral compression fractures.6 Hemangiomas are even less common, occurring with an incidence of 10–12%, many of which are asymptomatic and discovered incidentally.7
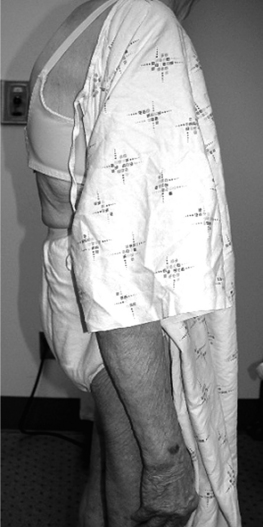
Vertebral body compression fractures typically manifest as episodes of acute and frequently explosive axial pain precipitated by routine activities of daily living. Movement is limited by pain, and flexion is restricted more than extension. This spinal pain is exacerbated by axial loading during sitting or standing, and alleviated by recumbent positioning. The process of changing position is particularly uncomfortable, leaving the afflicted patient with the desire to remain in a position of comfort; particularly resting supine in bed. Thoracic fractures can result in thoracic kyphosis (dowager’s hump) (Fig. 44.1) leading to a discrepancy between standing height and arm span, and pulmonary dysfunction. Involvement of the lumbar spine may lead to progressive loss of normal lumbar lordosis, leading to early satiety and abdominal bloating. Neurologic involvement is rare in osteoporotic compression fractures because the posterior cortical wall is rarely breached.8 When involvement of the spinal cord or cauda equina occur, it should suggest other conditions that can lead to a compression fracture such as infection, metastatic or primary bone tumors, myeloma, Paget’s disease, or lymphoma.9
Pain and disability consequent to osteoporotic vertebral fractures tax the health care, industrial, and societal systems. Approximately 750 000 new osteoporotic vertebral fractures occur in the United States each year,3 and over one-third of these become chronically painful.10 Vertebral fractures in patients aged 65 years or older account for 150 000 hospital admissions per year11 with an estimated total cost of 1.5 billion dollars.3 Patients aged 45 years or older account for 161 000 physician office visits and more than 5 million restricted-activity days per year.12 Hence, osteoporotic vertebral compression fractures are a leading cause of disability and morbidity in the elderly.13 Such outcomes are due to chronic pain, progressive vertebral collapse, and spinal kyphosis.14 Osteoporotic compression fractures have been shown to adversely affect quality of life, physical functioning, mental health, and survival.15
TRADITIONAL TREATMENT APPROACH
Traditional treatment pursuits strive to achieve pain relief, reduction of the sequelae of deconditioning, and maximal function. These objectives are largely achieved by conservative measures including relative rest, narcotic analgesics, antiosteoporotic medications, spinal orthotics, and gradual extension-biased exercises.3,16 Rarely, these fractures are associated with neurologic compromise or spinal instability necessitating surgical repair. Bed rest is prescribed in the acute phase, but must be minimized to 2 days to reduce the occurrence of contractures,17 worsening osteoporosis,18 muscle weakness and atrophy,19 and thromboembolic complications.20 Each of these entities will increase the morbidity and mortality resulting from a compression fracture. In the majority of cases, the pain will abate within 2–4 weeks to as many as 8–12 weeks. However, a significant number will remain painful,15 possibly due to incomplete healing with progressive bony collapse, altered spinal kinematics resulting from deformity, or development of a pseudoarthrosis at the involved segment.14 Analgesic medications are poorly tolerated by elderly patients due to confusion, increased fall risk, and constipation. Bracing may not be well tolerated due to decreased diaphragmatic excursion, and restriction of abdominal movement. Overall, these treatment interventions aim to ameliorate the symptoms associated with osteoporotic compression fractures, while not addressing underlying tissue injury.
Historical overview of vertebroplasty
Over the past two decades, vertebroplasty, the percutaneous injection of polymethyl methacrylate (PMMA) directly into the fractured vertebral body, has been increasingly utilized to stabilize vertebral body compression fractures. This technique was invented in 1984 by two French physicians, Galibert and Deramond, to treat symptomatic vertebral angiomas, and their findings were subsequently published in 1987.21 Nine years later, Cotton et al.22 and Weill et al.,23 in two independent studies, established the efficacy of vertebroplasty in patients suffering from metastatic spine disease or multiple myeloma. Jensen et al. published the first American trial in 1997 of applying vertebroplasty to treat osteoporotic vertebral compression fractures.24 Hence, European physicians have served the pioneering role in establishing the utility of vertebroplasty, acquiring greater experience with this new technology in pursuit of primarily treating painful spinal tumors. In contrast, North American physicians have witnessed a growth in deploying this procedure to treat painful osteoporotic patients who themselves served as the impetus for exploring this treatment option.25 Consequently, vertebroplasty has been promoted as a viable option to treat painful vertebral compression fractures in the absence of sound methodologic prospective trials supporting this contention. Although conclusive evidence is lacking, diligent studies have been and are being performed to explain vertebroplasty’s therapeutic benefit, substantiate its safety, demonstrate the durability of its efficacy, and refine its technique.
PATHOPHYSIOLOGY OF VERTEBRAL BODY COMPRESSION FRACTURE
Mechanical failure of the vertebral body represents disrupted structural integrity. The vertebral body’s ability to function properly to bear and transmit loads is a function of the health of its trabecular bone; its structural design as dictated by shape, size, and organization; and the loading conditions involving the energy it absorbs.1
Intrinsic factors
The strength of the trabecular bone is a key attribute in determining a vertebral body’s risk of fracture. Trabecular bone functions to carry the axial load transmitted to the vertebral body from the adjacent intervertebral discs. Trabecular bone will fail if its strength is less than the working stresses experienced within the vertebral body during physiologic or traumatic loads. Whole bone fractures are the consequence of such failures. Trabecular bone strength is determined by apparent density and its architecture. Reductions in apparent density occur with aging, osteoporosis, and tumor infiltration. The compressive mechanical strength of trabecular bone is proportional to the apparent density squared. Thus, a decrease in the later will cause a disproportionate reduction in trabecular bone strength.1
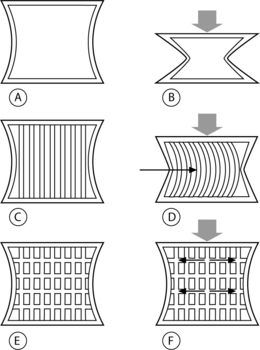
Vertebral trabeculae are arranged in vertical and horizontal columns, and the vertical columns outnumber the horizontal ones at any given density.1 Compressive loads are initially borne by the vertical trabecular struts, and when these struts begin to bow their horizontal counterparts act as cross-beams to restrain this displacement (Fig. 44.2).26 Consequently, an axial load is sustained by a combination of vertical pressure and transverse tension within the trabeculae. The ability to transfer load from vertical pressure to transverse tension confers resilience to the vertebral body.26 As the number of trabeculae decrease with aging, osteoporosis, or space-occupying lesion, the altered architecture reduces the vertebral body’s resiliency, thus lowering its threshold for fracture under load.1
A strong, positive correlation between lumbar spine bone mineral density (BMD) and failure force required to fracture a lumbar vertebra has been unequivocally established.27 Hence, BMD can be used as a convenient and specific predictor of the risk of vertebral body failure under compressive loads. This correlation appears to be a continuum without a single fracture threshold value for BMD at which a vertebral body will fail.1 A given vertebra will fatigue with repetitive loading, allowing it to fail at a lower load force than would be required during a single load application.28 The phenomenon of vertebral fatigue may be due to intraosseous crack initiation, crack growth, and final failure.29 Indeed, such bony deficits have been observed in human vertebral bone, irrespective of age, lending credence to the presence of such microdamage reducing vertebral fracture resistance.30
Extrinsic factors
Extrinsic factors such as loading conditions are as integral to vertebral body fracture as the already discussed intrinsic factors. Fractures occur at a point when external loads result in internal stresses that exceed the strength of the bone. Clinical recognition of the features of osteoporotic vertebral compression fractures can be difficult. Cooper et al. reviewed charts from 341 fracture patients over a 5-year period and found that 16% of patients were diagnosed incidentally, 33% after a fall, and 9% after routine lifting.2 Within this study cohort, approximately 50% of the vertebral fractures were reported as spontaneous or incidental,2 perhaps a result of vertebral fatigue.1 In a smaller study, Myers et al. recorded a history of a fall in 50% of osteoporotic acute fracture patients over 60 years old, a history of acute fracture in 20% of patients with routine activities of daily living, with the remainder of patients not able to recall a specific event at the time of injury.31 The apparent disparity among the causes of osteoporotic vertebral compression fractures might be explained by a combination of intrinsic and extrinsic factors. A single traumatic event leading to a load sufficient to fracture a weak vertebra might explain a fracture due to osteoporosis or a tumor. A fracture associated with routine activity or no apparent cause may represent structural fatigue due to age, osteoporosis, or tumor-related vertebral changes. A ‘factor of risk’ has been established incorporating an individual patient’s BMD to forecast the risk of vertebral fracture consequential to some routine activities of daily living.32 Factors of risk are the ratio of the estimated forces applied to the spine to the vertebral failure threshold based on lumbar BMD. A ratio equal to or greater than 1 represents a strong likelihood of fracture.1,32 Individuals with low BMD routinely operate near a ratio of 1, and women with below average BMD who lift a toddler experience a factor of risk near 1.1 For women with very low BMD, innocuous activities such as tying one’s shoes increases the risk of fracture.1
Implicit in the dynamic relationship between routine activities and fracture risk is a role for posture and neuromuscular conditioning, and degenerative disc disease. Evidence has recently been published substantiating this contention.33,34 An involutional loss of functional muscle motor units occurs with aging and predisposes an individual to poor postural control.35 A 2-year structured back extension exercise program has been shown to reduce the incidence of vertebral compression fractures and increase bone mineral density in healthy, postmenopausal women, up to 8 years after cessation of the program.33 Thus, extension-based exercise helps maintain bone mineral density and prevent vertebral compression fractures, perhaps due to improved posture and spinal stabilization.
Pollitine et al., in an elegant study, defined the relative loads experienced by the three columns of the spine in patients with degenerative discs.34 Twice as much load is assumed by the posterior column than by the anterior vertebral body in the erect standing position in patients with degenerative intervertebral discs. In contrast, the anterior vertebral body’s axial load increases almost three times during forward bending in the presence of degenerative discs. A relative ‘off-loading’ of the anterior vertebral body during upright posture will result in bone loss due to a lack of routine dynamic strain.36,37 Ultimately, the weakened anterior vertebral body is abnormally loaded above its counterpart in spines containing healthy, nondegenerate discs. The findings of Pollitine et al. suggest that in a person with severely degenerated intervertebral discs, the load placed on the anterior vertebral body increases by greater than 300% in flexion as compared to standing erect.34 Sinaki et al.’s reduction in compression fractures may be due to their patients’ increased spinal extensor conditioning as well as an independent increase in BMD. Their treatment arm utilized extension exercises without specific land-based weight-bearing exercises.33 The increased extensor conditioning may have decreased the load incurred by the anterior vertebral body during flexion activities, and may have inadvertently increased the anterior vertebral body’s resilience to fracture. However, Sinaki et al. did not remark about the presence or absence of disc degeneration.
An accurate comprehension of the pain generating mechanisms of vertebral compression fractures is indispensable if successful treatment is to be effectuated. Traditional treatment interventions assume adequate tissue healing with time, which seems to be logical given the natural history of gradual resolution of pain within 2–12 weeks. However, chronic pain may develop due to microfracture nonunion,14,38 pseudoarthrosis,3 sensitization of the basivertebral nerve,39 or spinal deformity14 leading to painful paraspinal muscle spasm. The first three of these probable pathophysiologic mechanisms can be addressed with the percutaneous injection of PMMA. Although kyphotic deformity is not maximally corrected by vertebroplasty, reduction in pain allowing extension-based spinal stabilization may reduce painful myofascial dysfunction, thus reducing kyphosis.
MECHANISM OF ACTION OF VERTEBROPLASTY
The pain relief afforded to patients having undergone vertebroplasty has not yet been successfully attributed to a primary mechanism. Thermal,40–43 chemical,44,45 and mechanical46 effects have all been reported as possible mechanisms. However, the mechanical effects of vertebroplasty have been regarded as the critical factor leading to symptom reduction.46–48 Tohmeh et al., in 1999, demonstrated increased strength and stiffness in osteoporotic cadaveric compression fractures utilizing either a unipedicular or bipedicular approach.46 However, the authors did not investigate the distribution or volume of PMMA injected relative to these mechanical parameters. Liebschner et al. subsequently established that symmetric deposition of PMMA is ideal to prevent single-sided load transfer, and that overall a small amount is needed to restore stiffness and strength.49 Belkoff et al. provided preliminary guidance for the amount of injected PMMA necessary to restore stiffness and strength;47 however, Molloy et al. later established the approximate percentage of vertebral body volume required to be filled to restore adequate strength and stiffness.48 A weak correlation has been noted between percentage of fill and restored strength and stiffness,48 which echoes previous clinical observations of minimal correlation between pain relief and the amount of PMMA injected.22 If an intravertebral cleft is present, there is a trend toward greater pain relief if these clefts are opacified during vertebroplasty.50 These findings suggest that either the singular factor of achieving a minimum threshold of stabilization is needed to abate micromotion or malunion induced pain, or that an alternate pain-reducing mechanism is yet to be demonstrated.
The polymerization process of PMMA is exothermic and its effects have been studied utilizing cadaveric vertebral bodies.42,51 Deramond et al. observed sufficiently elevated and sustained intraosseous temperatures in the central and anterior vertebral bodies during PMMA injection, and lack thereof at the anterior wall of the spinal canal, to cause thermal damage to intraosseous neural structures.42 Belkoff and Molloy subsequently observed higher temperature elevation in vertebral specimens during intraosseous injection of PMMA, perhaps due to a different temperature recording technique, but these higher temperatures did not occur at the spinal canal.51 The authors were led to believe that the risk of thermal necrosis may have been underappreciated. Yet histologic examination of four vertebral bodies, previously treated with percutaneous injection of PMMA, retrieved from human spines during spinal surgery, identified rare foci of necrosis.52 Unfortunately, histologic assessment was not performed on the intraosseous neural structures of these four samples. Further, a vast network of substance-P containing nerves has been documented within the vertebral body.39 Consequently, thermal and/or chemical denervation may contribute to the pain-relieving effect of vertebroplasty.
INDICATIONS AND CONTRAINDICATIONS
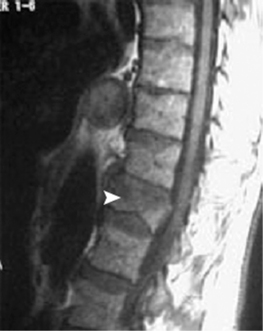
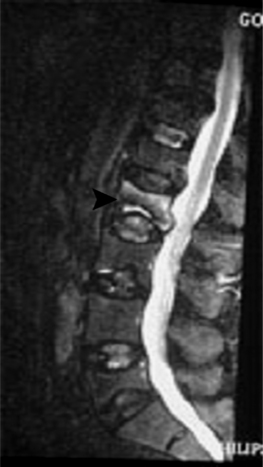
Vertebroplasty was first developed to treat symptomatic vertebral body hemangiomas.21 Since 1984, the conditions for which percutaneous injection of PMMA has been undertaken have been expanded to include primary and metastatic spinal fractures (Fig. 44.3),22,23 osteoporotic compression fractures (Fig. 44.4),24 vertebral fractures due to osteogenesis imperfecta,53 recurrent fracture or pain at a previously treated segment,54 and pseudoarthroses associated with noninfected avascular necrosis of the vertebral body.55
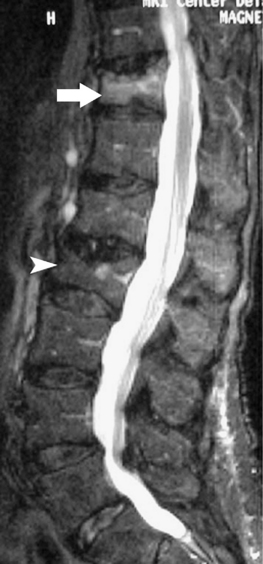
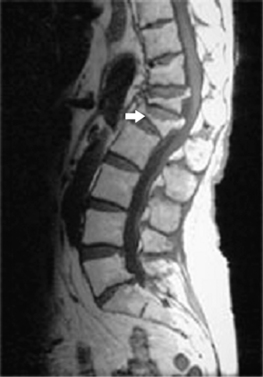
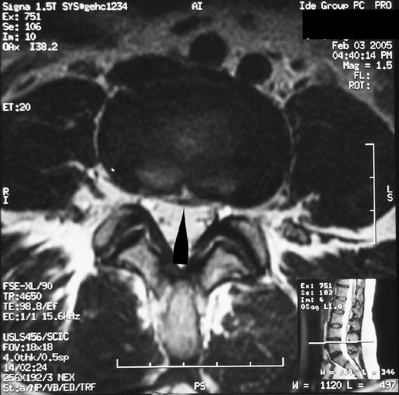
The universally accepted indications for performing percutaneous vertebroplasty (PVP) are severe, focal, intractable spinal pain, in the absence of radicular pain, recalcitrant to traditional treatment measures due to acute compression fractures.3,14,22,25,24,56–72 An acute fracture is substantiated by a typical history and evidence of new or progressive fracture by magnetic resonance imaging (MRI) (Fig. 44.5). Alternatively, persistent pain, despite appropriate conservative care, greater than 3 months due to an imaging confirmed compression fracture is a relative indication.73 In a retrospective observational investigation, Kaufmann et al.73 found that fracture age at the time of PVP was not independently associated with postprocedural pain or activity. In subsequent retrospective study, Brown et al.74 found that 80% of 41 osteoporotic fracture patients with symptom duration of greater than 12–24 months had post-PVP pain improvement; in comparison, 92% of 49 control patients, those with symptoms less than 1 year, experienced pain relief. Although the number of patients achieving partial or complete pain relief was not statistically different between groups, complete pain relief was more frequent in the control group. The ideal time frame during which to intervene with PVP to treat painful vertebral compression fractures has yet to be determined in well-controlled prospective studies. However, some contend that the likelihood of improvement probably decreases over time and appears to be low for remote, 6 months or older, fractures.3
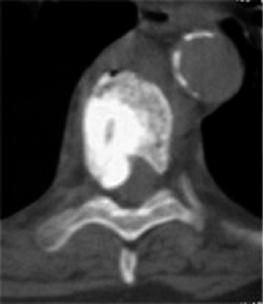
Contraindications to PVP include a fracture that breaches the posterior vertebral wall, retropulsed bone fragments, gross spinal instability, clinical evidence of myelopathy or radiculopathy, osteoblastic metastatic lesions, uncorrectable coagulopathy, systemic or spinal infection, or medical conditions that preclude safe emergent surgical decompression.3 A loss of vertebral body height of greater than 75%, has been cited as a relative contraindication to PVP (Fig. 44.6).23,56 However, Peh et al. evaluated 37 patients who underwent PVP to treat vertebra plana, defined as a collapsed vertebra to less than one-third of its original height.57 Ninety-four percent of these patients experienced pain reduction, with 51% of the 37 patients reporting complete pain relief.57 No major complications were reported, and the authors concluded that PVP of severe osteoporotic vertebral body compression fractures should not be withheld from this category of patient.57 At this point, it seems most prudent to regard severe vertebral compression fractures as relative indications in the absence of myelopathy, instability, or disruption of the posterior vertebral body wall.
EFFICACY OF PERCUTANEOUS VERTEBROPLASTY
Successful clinical outcomes have been reported since the inception of PVP in 1984.21 A plethora of studies have been published documenting the therapeutic benefit of PVP in treating painful vertebral body compression fractures.3,14,21–25,53–58–65,75 The excitement generated by a treatment intervention that achieves complete or near complete pain relief has fueled many investigations attesting to its efficacy. Many of these reports have evaluated pain reduction, and less commonly quality of life or patient satisfaction. Only one study included a control group, and no studies to date have employed a prospective, randomized trial incorporating a control group. Hence, the natural history of a certain vertebral compression fracture has not been concurrently compared to the clinical course of this condition after treatment with PVP. Rather, several investigations have seemingly established PVP’s safety and efficacy at reducing disruptive pain symptoms due to vertebral body compression fractures.
Osteoporotic compression fractures
Vertebral compression fracture is a common clinical manifestation of osteoporosis, but rarely leads to neurologic compromise.9 However, osteoporotic vertebral compression fractures are invariably associated with significant impairment in functional, physical, and psychosocial indices.76 Quality of life suffers after the first osteoporotic compression fracture and decreases further with subsequent fractures.77 Afflicted individuals experience pain, disability, loss of activity, fear of falling, and embarrassment over their appearance.78 Increased mortality has been observed after an initial osteoporotic compression fracture, and progressively increases with advancing time after the initial injury.79 As our society continues to age, refining the appropriate role of PVP in treating osteoporotic vertebral compression fractures has emerged as the multifaceted impetus for a variety of investigations.
Jensen et al. first addressed the efficacy of PVP to treat osteoporotic vertebral compression fractures in the late 1990s.24,75 Ninety percent of 29 patients treated with PVP reported significant reduction in pain within 24 hours after the procedure.24 At a mean follow-up of 281 days, 23 of these 26 patients who initially experienced significant improvement reported sustained pain reduction and increased mobility.75 In a larger study of 245 osteoporotic patients, Evans et al.58 reported significant reduction in pain, and improved ambulatory status and activities of daily living at a median follow-up interval of 7 months. Yet the 245 cases were reviewed retrospectively and represented the only cases out of 488 available for follow-up. The authors did not use a validated disability assessment tool, and it was administered at varying times after treatment intervention. Long-term observations were conducted by Grados et al.59 who documented a statistically significant reduction in visual analog scale (VAS) pain scores in 96% of osteoporotic compression fracture patients treated with PVP. The mean duration of follow-up was 48 months with a range of 12–84 months. The authors concluded that the results obtained by PVP are durable over time, as their findings did not deteriorate from 1 month to a mean of 48 months after treatment intervention. However, 15 of the original cohort of 40 were not available at follow-up, the methodology was retrospective, and clinical significance, such as quality of life or disability, of the reduced VAS ratings was not assessed. These findings have been corroborated by those of other retrospective studies incorporating smaller subgroups of osteoporotic patients with significant pain relief occurring in 75–95% of treated patients.56,60–63
Peters et al.64 prospectively observed a statistically significant decrease in VAS ratings in 84% of 42 osteoporotic compression fracture patients treated with PVP at 56 levels, and 7 patients reported no change in their pain level. All 42 patients were available at follow-up at 6 weeks, and had failed to improve despite a 6-week course of undefined medical management. The authors employed a bipedicular approach to inject PMMA into each fractured vertebrae except for six that were treated with a unipedicular technique. No relationship was disclosed between unipedicular technique and failure of pain reduction, and follow-up occurred at 6 weeks. Focusing on the efficacy of PVP in treating severe (>70% vertebral body collapse) osteoporotic compression fractures, Peh et al.57 prospectively found complete or partial pain relief in 97% of 38 patients undergoing 48 PVPs. Just 30 of the initial 38 patients were available for follow-up at a mean of 11 months (range 3–24 months). Pre-intervention symptoms ranged from 2 weeks to over 1 year. Bipedicular injection was competed in 20 levels, and 28 underwent unipedicular injection. McGraw et al.65 prospectively followed 100 patients undergoing 156 PVPs for osteoporotic (n=92), neoplastic (n=5), concomitant spinal stenosis (n=2), and osteogenesis imperfecta (n=1) related fractures. Ninety-nine patients were available for follow-up at a mean interval of 21.5 months (range 6–44 months), and 93% of patients reported improvement in their back pain with a statistically significant reduction in VAS ratings. However, the authors did not report the pre-PVP symptom interval, stratify results according to diagnostic category or how many levels were treated using a bipedicular technique. All three investigations used similar inclusion criteria including painful osteoporotic compression fractures refractory to medical management, proven by imaging and history and physical examination. The severity of collapse varied among the studies, and all three inquiries used pain reduction and mobility as the outcomes measures without incorporating a valid disability tool.
Cortet et al.66 prospectively evaluated the efficacy of PVP in treating 20 osteoporotic vertebral compression fractures in 16 patients suffering from intractable pain despite 3 months of medical management. A statistically significant improvement was noted in VAS ratings, McGill-Melzack scoring system, and five out of six dimensions of the Nottingham Health Profile at 1, 3, and 6 months after PVP. Zoarski et al.67 prospectively followed 30 patients utilizing verbal pain scores and the Musculoskeletal Outcomes Data Evaluation and Management Scale (MODEMS) spinal intervention questionnaire. All patients demonstrated a lack of improvement after a minimum of 4 weeks of conservative care prior to PVP. Twenty-nine patients reported pain relief within hours of the procedure, and at 2 weeks 80% reported feeling better and 90% reported they would undergo the procedure again. All four modules of the MODEMS data (treatment score, pain and disability, physical function, and mental function) improved with statistical significance at 2 weeks. Ninety-six percent of 23 available patients at follow-up at 15–18 months reported continued pain relief and satisfaction with their outcome. All 30 patients underwent bipedicular injection of PMMA. Findings in a long-term, prospective study by Perez-Higueras et al.68 seem to support the durability of the results afforded to patients by PVP. Thirteen out of 17 original patients treated for 39 osteoporotic vertebral compression fractures were available at a mean follow-up of 65 months (range 60–71). All patients had failed to improve after a minimum of 3 months of conservative care. Outcomes were measured by VAS ratings and the short McGill questionnaire and all 13 demonstrated significant improvement at 3 months which increased slightly at the 5-year follow-up.
These prospective studies have helped to provide greater insight regarding the efficacy of PVP in treating osteoporotic vertebral compression fractures. However, none incorporated a control group for comparison to the treatment arm. Implicit in this methodology is that a regression toward the mean has already occurred during the period of conservative care. Hence, any treatment intervention after this time frame would be attributed to the intervention itself. However, none of these studies recorded such statistical analysis. To date, one prospective study has incorporated a control group of patients treated with conventional therapy.69
In a nonrandomized fashion, Diamond et al.69 compared the clinical outcomes of 55 osteoporotic patients who underwent PVP with 24 patients who refused PVP and were conservatively managed. Inclusion criteria were acute severe vertebral compression pain unrelieved by 1–6 weeks of oral analgesics, densitometric evidence of osteoporosis, and acute fracture activity as indicated by MRI or technetium-99 m bone scan. No differences existed between the two groups regarding smoking history, alcohol intake, corticosteroid use, thyroxine therapy, vitamin D and parathyroid hormone levels, bone density, or degree of vertebral collapse. More importantly, both groups had statistically similar baseline levels of pain and disability. Fifty-three patients in the PVP group and 21 of the conservative group were available at a mean follow-up of 215 days (range 57–399), and outcomes were measured by VAS ratings and the Barthel index level of function. Statistically significant improvement in pain and physical functioning was noted at 24 hours in the PVP compared to the conservatively treated group. No differences were observed at 6 weeks or 6–12 months. A 40% reduction in the mean length of hospitalization was observed in the PVP group. These findings suggest, but do not prove, that PVP provides effective pain relief in the short term. The absence of a clinical separation between the two groups at longer follow-up may be due to the natural history of most compression fracture symptoms abating over 6–12 weeks. Hence, these cases may have been destined to clinically improve within 6 weeks after initiation of the study due to the natural history of the condition itself.
Neoplastic compression fractures
The first series limited to patients with malignant disease was published in 1989 when Kaemmerlen et al.70 briefly described their experience in treating 20 patients with 33 neoplastic vertebral body fractures. At an average follow-up of 2.8 months, 85% of patients noted substantial pain relief requiring either no analgesic medication or a reduced dose. Two patients did not respond and one patient developed spinal cord compression. In 1996, Weill et al.23 treated 37 patients for 48 metastatic lesions. At 6 months, 73% of treated patients reported marked or complete pain relief, and 21% reported moderate pain relief. The 94% reporting significant pain relief at 6 months dwindled to 65% at 1 year. Recurrent pain was attributed to the development of adjacent metastatic vertebral lesions or meningitis as identified by MRI.
Later in the same year, Cotten et al.22 prospectively evaluated 37 patients treated for 40 metastatic or multiple myelomatous lesions in a controlled trial. The enrolled patients had experienced severe spinal pain not amendable to surgical intervention. Patients were evaluated using the McGill-Melzack questionnaire at 48 hours, 3 months, and 6 months after intervention with a mean follow-up at 4.2 months. All patients received concurrent radiation therapy between 12 to 22 days after PVP. Within the first 48 hours, 13.5% were pain free, 55% reported substantial improvement, and 30% were moderately improved. These results were sustained in 89% at 3 months, and 75% at 6 months. Twelve patients were not available at long-term follow-up. In a larger study, Deramond et al.71 evaluated 101 patients treated for spinal malignancies. Over 80% of patients experienced moderate to complete pain relief, and injection of PMMA may have had an antitumoral effect as local tumor recurrence was rare during the follow-up course.71 Only one investigation has been reported in the North American literature,60 which assessed a small number of patients treated for 13 primary or metastatic lesions. Significant pain relief was observed in just 50% of eight patients at a mean follow-up of 12 months.
Due to the small number of neoplastic induced compression fractures studied by Barr et al,60 conclusive statements may be erroneous. However, it appears as if neoplastic vertebral compression fractures treated with PVP may have a less durable effect compared to their osteoporotic counterparts. Yet one must consider differences between these two patient populations. A significant proportion of patients fall out of the study cohort due to death or morbidity that precludes follow-up evaluation.72 Furthermore, one must not lose sight of the existence of a concomitant variable unique to patients with a compression fracture due to a malignancy; radiation therapy may skew the outcomes after treatment with PVP. Immediate pain relief is likely due to the percutaneous intervention itself. However, benefit months later may be partially attributable to radiation therapy.72 However, others propose that spinal segment involvement distant to the treated level may result in less durable effects.60 Percutaneous vertebroplasty appears to offer significant pain relief in a safe manner to patients suffering from neoplastic or osteoporotic spinal fractures that are not managed by conservative care or radiation therapy alone.60,72
Benign vascular lesions
Benign vascular lesions can be treated by PVP, achieving results similar those regarding osteoporotic compression fractures. Galibert et al.’s initial series of seven patients experienced immediate and sustained relief at 2-year follow-up after PVP.21 Several studies, mostly case series, have upheld the observation of significant pain relief in up to 90% of patients.56,62,71,80–83 Clinical and radiological characteristics can classify vertebral hemangiomas (VH) with treatment implications.25 Asymptomatic VH without radiologic signs of aggressiveness do not require treatment. Radiologic aggressiveness is typified by predominantly soft tissue stroma (exhibited by low signal intensity on T1-weighted MR images),84 involvement of the entire vertebral body, irregular, honeycomb vertical striations, and a poorly defined cortex.85 Vertebral hemangiomas producing severe spinal pain without radiologic signs of aggressiveness can be successfully treated with PVP.25,71,80,81 Asymptomatic patients in which aggressive VH are incidentally discovered may be monitored clinically.25 Symptomatic VH with aggressive features causing acute myelopathy or cauda equina injury can be successfully treated by preoperative PVP and subsequent surgical decompression.25,56,81–83 Progressive neurologic compromise due to a symptomatic, aggressive VH can be successfully treated with PVP.25 Spinal VHs are rarely symptomatic and occur in the spine with an incidence of less than 12%. Hence, a symptomatic lesion would be expected to respond well to a treatment that effectively embolizes the vasculature and solidifies the vertebral segment.
Specific osseous parameters
Solidifying a fracture nonunion seems to be the primary means by which PVP achieves symptom reduction in a subset of vertebral compression fractures.50,55,86 Lane et al.50 retrospectively examined pain relief in compression fracture patients with fractures demonstrated intraosseous clefts and those without clefts. Although a statistically significant difference was not found, the authors found a trend toward greater improvement in patients with intraosseous clefts versus those without. However, the calculated p-value was 0.18; thus, statistical support for a meaningful trend was not established. Regardless, this study did lend credence to the utility of PVP in treating patients with vertebral compression fractures with intraosseous clefts. Eighteen patients reported a mean VAS rating reduction of 66% at 12-months follow-up. However, 45 patients were initially enrolled, and at the time of publication only 18 were 12 months post-PVP. Perhaps this VAS reduction is an underestimation. This conjecture seems to be upheld by Peh et al.’s work86 in which 18 patients were prospectively evaluated after undergoing PVP for treatment of vertebral compression fractures with intraosseous vacuum phenomena. Post-PVP radiographic evaluation confirmed the invariable filling of the clefts with PMMA in all cases. Forty-four percent of patients enjoyed complete pain relief and 33.3% reported partial pain reduction, while 22.2% did not benefit from PVP. Again, the power of this study suffers from a small cohort, and 7 of the 18 patients were not available for follow-up beyond a mean of 4 months due to death. The 11 surviving patients were followed up at a mean of 13.6 months. Seventy-seven percent of patients reporting complete or partial pain relief at a mean follow-up of 4–13.6 months suggests that PVP can effectively reduce pain in compression fractures containing intraosseous clefts or vacuum phenomena. Larger trials will need to confirm these preliminary findings, especially in comparison to fractures without such clefts, if we are to accept the notion that such clefts represent a painful nonunion.
An interesting complication of these intraosseous clefts is dynamic instability. Jang et al.55 evaluated the efficacy of PVP in the treatment of intravertebral pseudoarthrosis caused by vertebral body avascular necrosis as demonstrated by intravertebral vacuum phenomena or fluid collection. Thirteen of 16 total cases involved the thoracolumbar junction or one segment adjacent to the T12–L1 level. It is not surprising that the preponderance of such cases occurred at the transition between the rigid thoracic spine and relatively mobile lumbar spine. As well, the flexion–extension motion is most pronounced at the thoracolumbar junction as a consequence of the changeover from relative spinal axial rotation to relatively greater spinal sagittal motion. Repetitive stress and strain at these spinal segments would likely interfere with adequate healing of vertebral insufficiency fractures occurring at these levels in contrast to other segments which are less mobile.55
Excellent outcomes may also be achieved with repeat PVP preformed at prior treated levels.54 Gaughen et al.54 retrospectively observed excellent pain relief in 83% of 6 subjects treated at the same level as prior PVP, a mean of 41 days after initial PVP. Recurrent symptoms correlated with increased radiotracer uptake at that level on bone scan in three subjects, increased radiographic compression in one, stable fracture with diffuse edema on MR images in one, and no evidence of new fracture by CT in another patient. The initial PVPs were performed via unilateral pedicular approach in five patients with four of these demonstrating bilateral hemivertebral body spread of PMMA. The authors did not report at what follow-up interval their findings were recorded. They proposed that recurrent symptoms might be due to new fractures around the PMMA cement, or poor fracture healing from insufficient filling. Although it appears that PVP may be successfully repeated at a previously treated segment for a recurrent or new pathologic condition, PVP may not have needed to be repeated if bipedicular injection was performed initially, thus preventing a unilateral toggle effect.
Other conditions in which osseous fragility manifests as fractures may result in vertebral body compression fractures. Osteogenesis imperfecta (OI) is such a condition in which defective collagen production results in weakened osseous structures. Painful spinal compression fractures due to OI may be effectively treated by PVP.53 Rami et al.53 reported a case of T10 compression fracture successfully treated with PVP. At 1 week the patient was pain free, and at a 17-month follow-up the patient still enjoyed a marked improvement in quality of life and pain relief. Ultimately, large, prospective trials are needed to confirm this preliminary finding. However, PVP should be offered as an effective treatment for persistent pain due to OI-related vertebral compression fracture.
SIDE EFFECTS AND COMPLICATIONS
Justification of an invasive treatment intervention, regardless of how minimal this intervention may be, requires an accurate risk:benefit ratio calculation. A natural consequence of the excitement for performing PVP for painful vertebral compression fractures is a blurring of the appropriate indications and neglecting to adhere to proposed exclusion criteria. Despite numerous clinical trials and investigations,14,22,24,25,58–72 most retrospective, PVP has not been adequately studied using a randomized, prospective methodology. The lack of such evidence should not serve as dissuasion to pursuing original research. Rather, such a void ought to serve as the impetus for future projects that strive to answer the questions raised by the current foundation of investigations.
Reported clinical complications related to PVP using PMMA include transitory fever, transient worsening of pain, radicular pain, rib fractures, cement pulmonary embolism, infection, spinal cord compression, and predisposition toward adjacent segmental injury.24,56,59,60,63,70,87–91 Most complications are non-neurologic, transitory, and subclinical; however, the rate of complications varies considerably with the indication. The frequency of complications is 1.3% in osteoporosis, 2.5% in spinal hemangiomas, and 10% neoplastic disease.90 Detailed analysis of most literature regarding complication rates in clinical trials reveals rates reported per patient rather than per procedure. It is more telling to cite complication rates per procedure as risk certainly inflates with increasing levels treated.
Complications in osteoporotic patients
The complication rate for osteoporotic vertebral compression fractures is relatively low, reportedly 0–4.3% per level treated.57,60,64,65,69 Among the reported complications are transient radicular pain responding to oral steroid therapy,60,63,65 subcutaneous extravasation of cement requiring surgical removal,64 fractured transverse process and psoas muscle hemorrhage in a patient on heparin therapy.69 More commonly, subclinical extravasation of cement occurred in up to 65% of treated levels.66 Follow-up CT after treatment of 20 vertebral bodies demonstrated cement deposition in the paravertebral soft tissue in 30% of cases, peridural space in 15% of cases, intradiscally in 15% of cases, and the lumbar venous plexus in 5%.66 Intradiscal leakage of PMMA has been observed in 35% of treated levels in osteoporotic fractures.57 Catastrophic neurological complications by report are much more rare. Three cases have been published of spinal cord injury due to cement encroachment into the spinal canal (Fig. 44.7).87–89 At this juncture, it appears the benefits afforded by PVP in treating osteoporotic vertebral body compression fractures outweighs the calculated risks. Extravasation of bone cement outside of the vertebral body may have few clinical ramifications in most instances. Escape of the cement into the venous system has the chance of fatal outcome if it reaches the pulmonary vasculature.90 This seems to occur more frequently in neoplastic fractures due to their vascularity.22,91
Complications in neoplastic patients
The complication rate increases in patients with malignant compression fractures consequential to the cortical destruction from the neoplastic process. Transient radiculopathy occurs in 3–6% of patients treated with PVP that responds to oral steroids or nonsteroidal antiinflammatory medications.22,71,90 Yet a minority of these patients experience persistent symptoms requiring surgical extraction of foraminal cement.22,23 Infection was reported in just one case of an immunocompromised patient.90 Spinal cord injury due to cement extravasation into the central canal has been reported71,92 but is rare. Paravertebral leakage of cement occurs more commonly with spinal metastatic disease (56%) than with myeloma (15%).22 Eighty-seven percent of epidural leaks occurred in the presence of pre-PVP posterior cortical destruction, and 87% of these leaks were asymptomatic.22 Intradiscal extension due to cortical fracture or osteolysis of the vertebral endplate occurs invariably in neoplastic fractures with no immediately overt symptoms.22
Cardiovascular side effects and complications
A fatal outcome of venous leakage of PMMA is an embolus that travels to the inferior vena cava, eventually reaching the pulmonary vasculature, resulting in pulmonary infarction.91,93 Instances of pulmonary embolism (PE) have been reported,23,91,93 one of which did not demonstrate radiographic evidence of pulmonary PMMA.23 Padovani et al.91 suspected that insufficient polymerization allowed a less viscous mixture of PMMA to be injected into a Langerhans cell histiocytosis-related vertebral fracture resulting in venous leakage. The patient in this case report responded favorably to anticoagulant therapy. More recently, Jang et al.93 reported a case series of three patients developing a PE after PVP. The authors had treated 72 vertebrae for malignant osteolytic spinal tumors. Each of the 3 patients had multiple myeloma-related vertebral compression fractures at numerous levels, and underwent PVP at four to six segments. The authors concluded that appropriate cement viscosity should be obtained prior to injection, high-resolution real-time imaging and mixing the PMMA with barium or tungsten for opacification should be undertaken, and greater caution exercised during multilevel procedures. These are prudent points of concern when performing PVP regardless of the underlying condition. However, they are particularly important when approaching fractures due to highly vascularized tumors such as renal cell carcinoma, thyroid tumor, hemangioma, hepatoma, or an extensive osteolytic tumor such as multiple myeloma.22,91
Cardiovascular instability has been observed in orthopedic procedures employing acrylic bone cement,94 and some have proposed this to be a lesser risk in PVP.62 Aebli et al.95 utilized a sheep model to study the effects of bone cement injected into healthy vertebrae. The authors observed a transient, immediate drop in heart rate and mean arterial pressure by 1 to 7 seconds after cement injection and a second hypotensive episode occurring approximately 18 seconds after injection. These hemodynamic changes returned to baseline in less than 3 minutes. The second drop in heart rate and blood pressure occurred concomitantly with the appearance of echogenic particles within the pulmonary artery. The authors concluded that the first drop in the cardiovascular parameters occurred by cardiac reflex inhibition due to intraosseous pressurization. The second drop may have been related to the systemic extravasation of bone cement. Yet the PVPs were performed in healthy bone and not monitored by real-time imaging. Thus, extravasation may very well have occurred due to a relatively high intraosseous pressure and the interventionalist not terminating injection once the cement had breached the posterior portion of the vertebra.
Aebli et al.’s follow up study evaluated the value of placing a vent hole in the contralateral pedicle when performing multilevel unipedicular PVP in healthy sheep.96 Placement of the vent hole reduced the amplitude of deterioration in heart rate and mean arterial pressure, and the amount of pulmonary intravascular fat. The fall in blood pressure appears to be due to peripheral vasodilatation resulting from the methylmethacrylate monomer, vasoactive substances released from the bony cavity or fat–platelet aggregations, or a reduction in sympathetic tone.96 Similar decrements in blood pressure and heart rate, and increased fat emboli, occur when wax is injected rather than PMMA during PVP.97 It appears that pressurization of multiple vertebral bodies leads to decreased sympathetic tone and the release of bone marrow contents into the bloodstream, resulting in transient hemodynamic abnormalities.96,97 What remains to be determined is the clinical relevance of these findings. Will injecting PMMA into a weakened vertebra lead to similar hemodynamic alterations? In a retrospective review of 142 PVPs performed in 78 patients, no such cardiovascular abnormalities were detected.98 However, one case of transient hypotension during monosegmental PVP has been reported.99 Prudence would call for stringent monitoring of cardiovascular parameters during PVP, especially when performing multiple levels.
Increased incidence of future fractures
Augmentation of weakened and fractured vertebrae alters spinal biomechanics. It has been well established that injection of PMMA into osteoporotic vertebral bodies increases their stiffness by 174%, and this effect becomes more pronounced the lower the pre-PVP bone mineral density of the treated vertebra.100 In an osteoporotic cadaveric model, Berlemann et al.101 demonstrated an increase in new vertebral body fractures in the vicinity of a PVP-treated segment compared to fracture occurring in the vicinity of an untreated level. The adjacent cranial segment was invariably injured after PVP, whereas both the cranial and caudal segments were involved adjacent to untreated levels. Lower failure thresholds correlated with increased vertebral filling with cement. The reduced ultimate failure load of a functional spinal unit containing a cemented vertebra may be due to a ‘stress-riser’ effect101 as greater load is transmitted to an adjacent weakened vertebral body via a stiffer vertebral body and an intervening degenerate intervertebral disc. Indeed, clinical studies lend support to an increased risk of a new osteoporotic vertebral compression fracture near a level treated by PVP.59,63,67,102,103 Recently, in 38 patients treated with PVP, Lin et al.102 retrospectively observed new fractures in 58% of vertebral bodies adjacent to an intervertebral disc containing extruded bone cement in contrast to 12% of vertebra adjacent to a disc without cement leakage. Grados et al.59 observed a slight but significantly increased risk of vertebral fracture in the vicinity of a cemented vertebra (odds ratio 2.27, 95% CI 1.1–4.56) compared to a new fracture in the vicinity of an uncemented vertebra (odds ratio 1.44, CI 0.82–2.55). Although these results might be impressive, the methodology was not stellar. It was a retrospective investigation of 40 patients with just 25 available at follow-up. In a larger study, Uppin et al.103 documented 36 new fractures in 177 previously treated osteoporotic patients, 67% of which involved vertebrae adjacent to a previously treated level, and 67% of the new fractures occurred within 30 days after PVP treatment of the initial fracture. Although association does not imply causality, the findings of these studies seem to be logical in that they demonstrate what may occur when increased load is transferred to the anterior vertebral body. Neither article commented whether the patients were prescribed physical therapy for extension-biased spinal stabilization and conditioning. Strong back-extensor muscles reduce vertebral fractures in estrogen-deficient women.33 It is intuitive that such a therapy program would help reduce the incidence of new compression fractures adjacent to PVP-treated fractures. However, prospective, controlled trials must confirm this intuition.
Biomechanical models have been constructed to provide confirmatory evidence of altered load bearing in augmented vertebrae.104–106 Geometrically accurate, specimen-specific, three-dimensional computer-simulated finite element models of human vertebrae have been generated from quantitative CT data. By using nonuniform bone density and nonlinear, anistropic trabecular bone material properties, Sun and Liebschner were able to create a computational vertebral body model with realistic stress and strain distributions.104 After calibrating the model for characteristics of osteoporotic vertebrae, the authors applied axial loads to the model before and after treatment with 20% volume fill of PMMA bipedicularly. Regions of high strains were identified in local areas above and below the virtually implanted PMMA, and these peaks were absent for the untreated model at the same locations. Thus, the load was centered along the longitudinal axes of the injected PMMA, confirming that the rigid PMMA cement acts as an ‘upright pillar’ ‘which amplified the stress applied in their locale.’104 Increased intradiscal pressure ensues due to this stress concentration, causing a significant inward deflection of the adjacent vertebra’s endplate.105 It appears that the shift in load distribution consequent to the PMMA injectate ultimately results in adjacent vertebral fractures.104 The key to minimizing recurrent fractures at adjacent levels may lie in choosing softer bone cement materials without sacrificing strength104 as adjusting the volume or placement of injected cement does not affect load transfer.106
CLINICAL UTILITY AND FUTURE DIRECTIONS OF PERCUTANEOUS VERTEBROPLASTY
Almost 330 clinical and basic science studies have been published on the subject of percutaneous vertebroplasty. A large proportion of these studies is retrospective, with unclear indications and nonuniform outcomes, or are editorials and review articles.107 The prospective studies are largely case series with only one including a comparison control group in a nonrandomized fashion. More discussion about this procedure seems to permeate the literature rather than rigorous and sound clinical research methodology.107 Although animal and cadaveric studies can be valuable, extrapolating findings from these studies to those of humans is a calculated leap of faith. Such investigations provide the foundation on which meaningful clinical trials ought to be constructed for determining clinical efficacy.108 The value of descriptive studies such as case series lies in provoking original and clinically relevant thought. Analytic study design, such as a case-control study, cohort study, or randomized trial with an appropriate control group, is necessary to adequately determine the utility of therapeutic interventions, and thoroughly test these hypotheses.108
The evidence underpinning the use of percutaneous vertebroplasty as a definitive treatment for a painful vertebral compression fractures is strongly suggestive, but is not definitive. Progress is underway toward producing investigations that will provide categorical evidence. The utility of computational biomedical models such as outlined by Sun and Liebschner may currently be underappreciated. Such virtual models allow the evaluation of consequences of injecting bone cement into a sophisticatedly calculated vertebral body model, allowing the study of an array of scenarios. Thus, vast knowledge can be gathered about how an intervention will probably behave, and what effects it will have on the physiology of the model’s living counterpart. Once such knowledge is honed, more educated and definitive guidance may be provided regarding technique, complications, bone cement materials such as biodegradable materials, and the role of prophylactic vertebroplasty.104
1 Myers ER, Wilson SE. Biomechanics of osteoporosis and vertebral fracture. Spine. 1997;22(24S):25S-31S.
2 Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221-227.
3 Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429-437.
4 Melton LJIII. Epidemiology of osteoporosis. Spine. 1997;22(24S):2S-11S.
5 Janott J, Hallner D, Pfeiffer A, et al. Risk factors of osteoporosis: results of EVOS in Germany. Scand J Rheumatol. 1996;25(Suppl):123.
6 Yuh WT, Zachar CK, Barloon TJ, et al. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology. 1989;172(1):215-218.
7 Fox MW, Onofrio BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg. 1993;78:36-45.
8 Heggeness MH. Spine fractures with neurologic deficit in osteoporosis. Osteoporosis Int. 1993;3:215-221.
9 Glaser DL, Kaplan FS. Osteoporosis: definition and clinical presentation. Spine. 1997;22(24S):12S-16S.
10 Riggs BL, Melton LJIII. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S-511S.
11 Jacobson SJ, Cooper C, Gottlieb MS, et al. Hospitalization with vertebral fracture among the aged: a national population-based study, 1986–1989. Epidemiology. 1992;3:515-518.
12 Holbrook TL, Grazier K, Kelsey JL, et al. The frequency of occurrence, impact and cost of selected musculoskeletal conditions in the United States. Chicago: American Academy of Orthopedic Surgeons, 1984.
13 Johnell O. Advances in osteoporosis: better identification of risk factors can reduce morbidity and mortality. J Intern Med. 1996;239:299-304.
14 Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine. 2003;28(15S):S45-S53.
15 Sliverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27-S31.
16 Sinaki M. Beneficial musculoskeletal effects of physical activity in the older woman. Geriatr Med Today. 1989;8:53.
17 Amiel D, Woo SL-Y, Harwood FL, et al. The effect of immobilization of collagen turnover in connective tissue: a biochemical–biomechanical correlation. Acta Orthop Scand. 1982;53:325-332.
18 Cann CE, Genant HK, Young DR. Comparison of vertebral and peripheral mineral losses in disuse osteoporosis in monkey. Radiology. 1980;134:525-559.
19 Haggmark T, Eriksson T, Jansson E. Muscle fiber type changes in human skeletal muscle after injuries and immobilization. J Orthop. 1986;9:181-185.
20 Slipman CW, Lipetz JS, Jackson HB, et al. Deep venous thrombosis and pulmonary embolism as a complication of bed rest for low back pain. Arch Phys Med Rehabil. 2000;81(1):127-129.
21 Galibert P, Deramond H, Rosat P, et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 1987;33:166-168.
22 Cotten A, Dewatre F, Cortet B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996;200:525-530.
23 Weill A, Chiras J, Simon JM, et al. Spinal metastases: indications for and results of percutaneous injection of acrylic cement. Radiology. 1996;199:241-247.
24 Jensen ME, Evans AJ, Mathis JM, et al. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral compression fractures: technical aspects. AJNR. 1997;18:1897-1904.
25 Murphy KJ, Deramond H. Percutaneous vertebroplasty in benign and malignant disease. Neuroimaging Clin N Am. 2000;10(3):535-545.
26 Bogduk N. The lumbar vertebrae. Clinical anatomy of the lumbar spine and sacrum, 3rd edn.. Churchill Livingstone, Edinburgh, 2002.
27 Moro M, Hecker A, Bouxsein M, et al. Failure load of thoracic vertebrate correlates with lumbar bone mineral density measured by DXA. Calcif Tissue Int. 1995;56:206-209.
28 Hansson TH, Keller TS, Spengler DM. Mechanical behavior of the human lumbar spine. II. Fatigue strength during dynamic compressive loading. J Orthop Res. 1987;5:479-487.
29 Keaveny TM, Hayes WC. Mechanical properties of cortical and trabecular bone. In: Hall BK, editor. Bone. Vol. 7. Bone growth-B. Boca Raton: CRC Press; 1992:285-344.
30 Wenzel TE, Schaffler MD, Fyhrie DP. In vivo trabecular microcracks in human vertebral bone. Bone. 1996;19:89-95.
31 Myers ER, Wilson SE, Greenspan SL. Vertebral fractures in the elderly occur with falling and bending. J Bone Miner Res. 1996;11(Suppl):S355.
32 Hayes W, Piazza S, Zysset P. Rosenthal D, editor. Biomechanics of fractures risk prediction of the hip and spine by quantitative computed tomography. WB Saunders, Philadelphia, 1991;1-18.
33 Sinaki M, Itoi E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30(6):836-841.
34 Pollitine P, Dolan P, Tobias JH, et al. Intervertebral disc degeneration can lead to ‘stress-shielding’ of the anterior vertebral body. A cause of osteoporotic vertebral fracture? Spine. 2004;29(7):774-782.
35 McCormas AJ, Fawcett PR, Campbell MJ, et al. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121.
36 Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg [Am]. 1984;66:397-402.
37 Frost HM. Bone ‘mass’ and the ‘mechanostat’: a proposal. Anat Rec. 1987;219:1-9.
38 Wilson DR, Myers ER, Mathis JM, et al. 1999 Young Investigator Research Award runner-up. Effect of augmentation on the mechanics of vertebral wedge fractures. Spine. 2000;25(2):158-165.
39 Fras C, Kravetz P, Mody DR, et al. Substance p-containing nerves within the human vertebral body: an immunohistochemical study of the basivertebral nerve. Spine J. 2003;3(1):63-67.
40 Leeson MC, Lippitt SB. Thermal aspects of the use of polymethylmethacrylate in large metaphyseal defects in bone. A clinical review and laboratory study. Clin Orthop. 1993;295:239-245.
41 Mjoberg B, Pettersson R, Rosenqvist R, et al. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand. 1984;55:597-600.
42 Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999;25(2):17S-21S.
43 Bostrom PG, Lane JM. Future directions. Augmentation of osteoporotic vertebral bodies. Spine. 1997;22(24S):38S-42S.
44 Dahl OE, Garvik LJ, Lyberg T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro [published erratum appears in Acta Orthop Scand 1995; 66:387]. Acta Orthop Scand. 1994;65:147-153.
45 Danilewicz-Stysiak Z. Experimental investigations on the cytotoxic nature of methyl methacrylate. J Prosthet Dent. 1980;44:13-16.
46 Tohmeh AG, Mathis JM, Fenton DC, et al. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine. 1999;24:1772-1776.
47 Belkoff SM, Mathis JM, Jasper LE, et al. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26(14):1537-1541.
48 Molloy S, Mathis JM, Belkoff SM. The effect of vertebral body percentage fill on mechanical behavior during percutaneous vertebroplasty. Spine. 2003;28(14):1549-1554.
49 Liebschner MAK, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. 2001;26(14):1547-1554.
50 Lane JI, Maus TP, Wald JT, et al. Intravertebral clefts opacified during vertebroplasty: pathogenesis, technical implications, and prognostic significance. AJNR. 2002;23:1642-1646.
51 Belkoff SM, Molloy S. Temperature measurement during polymerization of polymethacrylate cement used for vertebroplasty. Spine. 2003;28(14):1555-1559.
52 Togawa D, Bauer TW, Lieberman IH, et al. Histologic evaluation of human vertebral bodies after vertebral augmentation with polymethyl methacrylate. Spine. 2003;28(14):1521-1527.
53 Rami PM, McGraw JK, Heatwole EV, et al. Percutaneous vertebroplasty in the treatment of vertebral body compression fracture secondary to osteogenesis imperfecta. Skeletal Radiol. 2002;31:162-165.
54 Gaughen JR, Jensen ME, Schweickert PA, et al. The therapeutic benefit of repeat percutaneous vertebroplasty at previously treated vertebral levels. AJNR. 2002;23:1657-1661.
55 Jang JS, Kim DY, Lee SH. Efficacy of percutaneous vertebroplasty in the treatment of intravertebral pseudoarthrosis associated with noninfected avascular necrosis of the vertebral body. Spine. 2003;28(14):1588-1592.
56 Deramond H, Derpriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications, and results. Radiol Clin North Am. 1998;36:533-546.
57 Peh WCG, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121-126.
58 Evans AJ, Jensen ME, Kip KE, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty. A retrospective report of 245 cases. Radiology. 2003;226(2):366-372.
59 Grados F, Depriester C, Cayrolle G, et al. Long-term observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology. 2000;39(12):1410-1414.
60 Barr JD, Barr MS, Lemley TJ, et al. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine. 2000;25(8):923-928.
61 Martin JB, Jean B, Sugiu K, et al. Vertebroplasty: clinical experience and follow-up results. Bone. 1999;25(2):11S-15S.
62 Gangi A, Kastler BA, Dietman JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. Am J Neuroradiol. 1994;15:83-86.
63 Cyteval C, Sarrabere MP, Roux JO, et al. Acute osteoporotic vertebral collapse: open study on percutaneous injection of acrylic surgical cement in 20 patients. Am J Roetgenol. 1999;173:1685-1690.
64 Peters KR, Guiot BH, Martin PA, et al. Vertebroplasty for osteoporotic compression fractures: current practice and evolving techniques. Neurosurgery. 2002;51((5)Suppl 2):S2096-S2103.
65 McGraw JK, Lippert JA, Minkus KD, et al. Prospective evaluation of pain relief in 100 patients undergoing percutaneous vertebroplasty: results and follow-up. J Vasc Interv Radiol. 2002;13(9):883-886.
66 Cortet B, Cotton A, Boutry N, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol. 1999;26:2222-2228.
67 Zoarski GH, Snow P, Olan WJ, et al. Percutaneous vertebroplasty for osteoporotic compression fractures: quantitative prospective evaluation of long-term outcomes. J Vasc Interv Radiol. 2002;13:139-148.
68 Perez-Higueras A, Alvarez L, Rossi RE, et al. Percutaneous vertebroplasty: long-term clinical and radiological outcome. Neuroradiology. 2002;44:950-954.
69 Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: a nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Am J Med. 2003;114(4):257-265.
70 Kaemmerlen P, Thiesse P, Jonas P, et al. Percutaneous injection of orthopaedic cement in metastatic vertebral lesion [letter]. N Engl J Med. 1989;321:121.
71 Deramond H, Depriester C, Galibert P, et al. Percutaneous vertebroplasty with polymethylmethacrylate: technique, indications and results. Radiol Clin North Am. 1998;36:533-546.
72 Jensen M, Kallmes D. Percutaneous vertebroplasty in the treatment of malignant spine disease. Cancer J. 2002;8(2):194-206.
73 Kaufmann TJ, Jensen ME, Schweickert PA, et al. Age of fracture and clinical outcomes of percutaneous vertebroplasty. Am J Neuroradiol. 2001;22:1860-1863.
74 Brown DB, Gilula LA, Sehgal M, et al. Treatment of chronic symptomatic vertebral compression fractures with percutaneous vertebroplasty. Am J Roentgenol. 2004;182:319-322.
75 Jensen ME, Dion JE. Vertebroplasty relieves osteoporosis pain. Diagn Imaging. 1997;19:68. 71–72
76 Lyles KW, Gold DT, Shipp KM, et al. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med. 1993;94:595-601.
77 Kanis JA, Johnell O. The burden of osteoporosis. J Endocrinol Invest. 1999;22:583-588.
78 Ross PD, Ettinger B, Davis JW, et al. Evaluation of adverse health outcomes associated with vertebral fractures. Osteoporosis Int. 1991;1:134-140.
79 Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137:1001-1005.
80 Feydy A, Cognard C, Miaux Y, et al. Acrylic vertebroplasty in symptomatic cervical vertebral haemangiomas: report of 2 cases. Neuroradiology. 1996;38:389-391.
81 Cotten A, Deramond H, Cortet B, et al. Preoperative percutaneous injection of methyl methacrylate and n-butyl cyanoacrylate in vertebral hemangiomas. Am J Neuroradiol. 1996;17:137-142.
82 Ide C, Gangi A, Rimmelin A, et al. Vertebral haemangiomas with spinal cord compression: the place of preoperative percutaneous vertebroplasty with methyl methacrylate. Neuroradiology. 1996;38:585-589.
83 Cortet B, Cotton A, Deprez X, et al. Vertebroplasty with surgical decompression for the treatment of aggressive vertebral hemangiomas. A report of three cases. Rev Rhum Ed Fr. 1994;61(1):14-20.
84 Laredo JD, Assouline E, Gelbert F, et al. Vertebral hemangiomas: fat content as a sign of aggressiveness. Radiology. 1990;177:467-472.
85 Laredo JD, Reizine D, Bard M, et al. Vertebral hemangiomas: radiologic evaluation. Radiology. 1986;161:183-189.
86 Peh WCG, Gelbart MS, Gilula LA, et al. Percutaneous vertebroplasty: treatment of painful vertebral compression fractures with intraosseous vacuum phenomena. Am J Roentgenol. 2003;180:1411-1417.
87 Harrington KD. Major neurological complications following percutaneous vertebroplasty with polymethylmethacrylate: a case report. J Bone Joint Surg [Am]. 2001;83:1070-1073.
88 Wenger M, Markwalder TM. Surgically controlled, transpedicular methylmethacrylate vertebroplasty with fluoroscopic guidance. Acta Neurochir. 1999;141:625-631.
89 Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine. 2002;27(19):E419-E422.
90 Chiras J, Depriester C, Weill A, et al. Vertebroplasties percutanees: technique et indications. J Neuroradiol. 1997;24:45-59.
91 Padovani B, Kasriel O, Brunner P, et al. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. Am J Neuroradiol. 1999;20:375-377.
92 Ratliff J, Nguyen T, Heiss J. Root and spinal cord compression from methylmethacrylate vertebroplasty. Spine. 2001;26(13):E300-E302.
93 Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty. A report of three cases. Spine. 2002;27(19):E416-E418.
94 Philips H, Cole PV, Lettin AWF. Cardiovascular effects of implanted acrylic cement. Br Med J. 1971;3:460-461.
95 Aebli N, Krebs J, Davis G, et al. Fat embolism and acute hypotension during vertebroplasty. An experimental study in sheep. Spine. 2002;27(5):460-466.
96 Aebli N, Krebs J, Schwenke D, et al. Cardiovascular changes during multiple vertebroplasty with and without vent-hole. An experimental study in sheep. Spine. 2003;28(14):1504-1512.
97 Aebli N, Krebs J, Schwenke D, et al. Pressurization of vertebral bodies during vertebroplasty causes cardiovascular complications. An experimental study in sheep. Spine. 2003;28(14):1513-1520.
98 Kaufmann TJ, Jensen ME, Ford G, et al. Cardiovascular effects of polymethylmethacrylate use in percutaneous vertebroplasty. Am J Neuroradiol. 2002;23:601-604.
99 Vasconcelos C, Philippe G, Martin JB, et al. Transient hypotension induced by polymethylmethacrylate injection during percutaneous vertebroplasty [letter]. J Vasc Rad. 2001;12(8):1001-1002.
100 Heini PF, Berlemann U, Kaufmann M, et al. Augmentation of mechanical properties in osteoporotic vertebral bones – a biomechanical investigation of vertebroplasty efficacy with different bone cements. Eur Spine J. 2001;10(2):164-171.
101 Berlemann U, Ferguson SJ, Nolte LP, et al. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg [Br]. 2002;84:748-752.
102 Lin EP, Ekholm S, Hiwatashi A, et al. Vertebroplasty: cement leakage into the disc increases the risk of new fracture of adjacent vertebral body. Am J Neuroradiol. 2004;25:175-180.
103 Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119-124.
104 Sun K, Liebschner AK. Evolution of vertebroplasty: a biomechanical perspective. Ann Biomed Eng. 2004;32(1):77-91.
105 Baroud GJ, Nemes J, Heini P, et al. Load shift of the intervertebral disc after vertebroplasty: a finite-element study. Eur Spine J. 2003;12(4):421-426.
106 Polikeit A, Nolte LP, Ferguson SJ. The effect of cement augmentation on the load transfer in an osteoporotic functional spinal unit. Finite-element analysis. Spine. 2003;28(10):991-996.
107 Mirza SK. Point of view. Cardiovascular changes during multiple vertebroplasty with and without vent-hole. An experimental study in sheep. Spine. 2003;28(14):1521-1522.
108 Jarvik JG, Kallmes DF, Mirza SK. Vertebroplasty – learning more, but not enough. Spine. 2003;28(14):1487-1489.