CHAPTER 82 Vertebral Artery Injuries Associated with Cervical Spine Trauma
Epidemiology
As improved, higher-resolution imaging protocols have been developed and instituted, the incidence of detectable VAIs has increased. Although VAI has been reported as a result of low-energy movements such as chiropractic manipulation,1,2 yoga, and sudden head turning,3–5 discussion of incidence is most appropriate for consideration in high-energy cervical spine trauma such as motor vehicle collisions and falls.
Investigation into incidence of VAI began as reports on small cohort groups and then later matured into larger, prospective studies. Louw and colleagues6 were the first to investigate VAI in bilateral facet dislocation in a prospective fashion using digital subtraction angiography (DSA) and detected arterial occlusion in 9 of 12 (75%) patients. Willis and colleagues7 defined “high-risk” groups as those with criteria of either cervical spine fracture involving the transverse foramen or bilateral facet dislocations and, using DSA, detected VAI in 12 of 26 (46%) patients. Miller and colleagues8 described a broader definition of “high-risk” criteria including (1) cervical spine fracture/dislocation, (2) neurologic findings not explained by brain imaging, (3) Horner syndrome, (4) Le Fort II or III fracture, (5) skull base fracture, or (6) expanding neck soft tissue injury. Of 216 patients who met the criteria, they found 49 (22.7%) VAIs. Biffl and colleagues’9 selection criteria was even more inclusive and found 92 VAI among 605 patients (15.2%). Using magnetic resonance angiography (MRA), Vaccaro and colleagues10 noted a 37% rate of VAI with unilateral and bilateral cervical facet dislocations. In a follow-up study Vaccaro found a 20% (12/61) incidence of VA occlusion in patients with subaxial cervical spine fractures.11
Later epidemiologic studies have used large, level I trauma registries to more accurately define overall incidence of VAI in blunt trauma admissions. The incidence of VAI among total blunt trauma admissions ranged from 0.075% to 0.77%.8,12–13 The studies had inherent variations due to location and referral pattern of the center and different imaging modalities used including digital subtraction angiography, computed tomography angiogram, and magnetic resonance arteriography. Despite the relatively low overall incidence of blunt VAI in the overall trauma population, this small population of patients has a substantial risk of stroke and mortality,14 thereby demanding screening criteria and structured protocols for detection of clinically silent lesions.
Mechanism and Types of Arterial Injury
Although VAI has been described at all levels, the V2 segment is the most commonly cited level of injury due to its confinement and tethering within the foramen transversarium, making it susceptible to shear forces during forceful cervical rotation or direct injury from fracture fragments.15,16 Although less common, the tortuous V3 segment is vulnerable to injury due to its tethering between the foramen transversarium of the atlas and the atlantal-occipital membrane.17
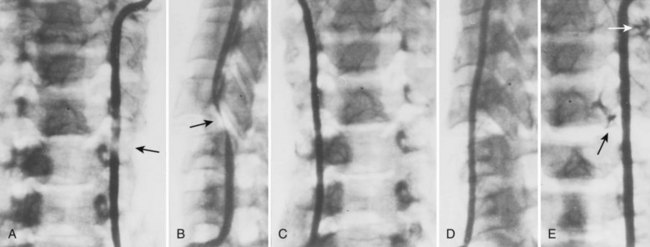
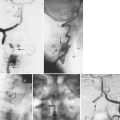
Cervical flexion-distraction fracture patterns most commonly result in VAIs, followed by flexion-compression injuries (Figs. 82-1 and 82-2).11 A review of the literature suggests that a rotational component to the cervical spine may also be a risk factor for VA occlusion.
In an attempt to better refine patient screening criteria for VAI, Cothren and colleagues13 reviewed 17,007 blunt trauma patients of whom 125 had cerebral vascular injuries associated with cervical spine injuries. The injuries were characterized as subluxations in 56 (48%) patients, C1 to C3 cervical spine fractures in 42 (36%), and extension of the fracture through the foramen transversarium in 19 (16%). Cervical spine fractures were the sole indication for screening in 90% of the study population. Screening yield of all patients admitted with one of these three fracture patterns was 37%. Therefore they recommended focused screening for patients with cervical subluxations, fractures through the transverse foramen, or fractures of C1 through C3.
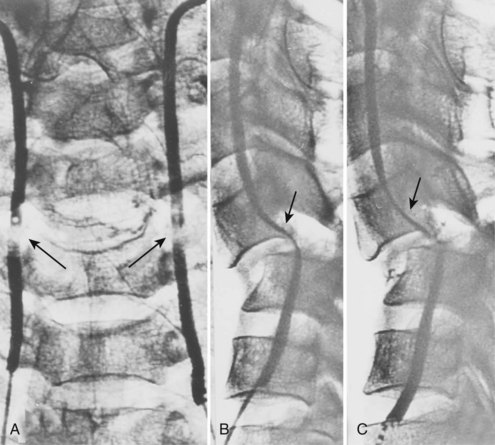
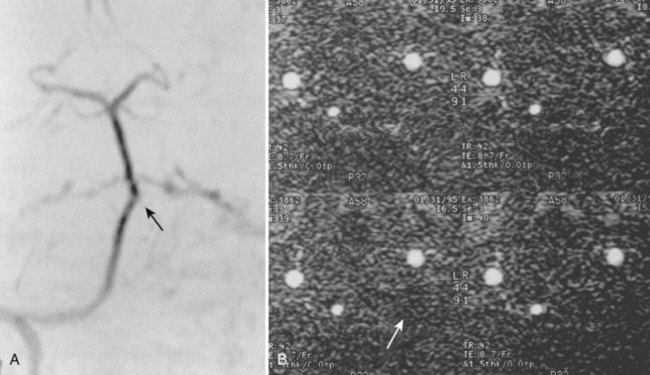
Injury to the VA in the setting of nonpenetrating cervical trauma is most likely due to a stretching mechanism.18 Sim and colleagues19 demonstrated in a cadaveric cervical spine model that the static deformity of a flexion-distraction stage II to IV subaxial cervical injury resulted in significant compression/stretch of the vertebral arterial vasculature (Figs. 82-3 to 82-5). Initially the vessel, or a portion of its intimal lining, is injured through excessive distraction in a flexion-distraction injury owing to the soft tissue attachments of the vessel in the foramina transversaria. Secondary events such as thrombus formation may then lead to occlusion of the vessel lumen. Recanalization over time after blunt injury to the VA was not a feature observed in a long-term follow-up study reported by Vaccaro and colleagues10 of image-confirmed vertebral vessel disruption. The lack of recanalization suggests that significant vessel intimal disruption occurs and that thrombus formation is not the only factor involved in vessel occlusion.
The intima of the VA is highly sensitive to shear forces experienced during high-energy blunt trauma. Because the vessel adventitia is more resilient, isolated intimal disruption can lead to a spectrum of clinical entities including an intimal flap with resultant thrombus formation or a dissection as the intima and adventitia separates cranially. Thrombus and dissection can lead to occlusion. If the energy transmitted is high enough, the adventitia can fail, leading to a pseudoaneurysm formation20 or transection if complete.
DSA provides the most anatomic detail of arterial injuries. A dissection of the VA has been shown to be the most common pattern of VAI, followed by occlusion. Multiple studies have demonstrated that dissections tend to resolve, whereas occlusions, as a rule, do not recanalize regardless of therapeutic attempts.10,21,22
Clinical Diagnoses
Abundant collateral circulation feeding the vertebrobasilar system and posterior circulation of the brain often allows unilateral disruption of the VA to remain asymptomatic. When this collateral circulation is diminished in situations of atherosclerosis or anatomic variations, patients may initially present with symptoms of vertebrobasilar insufficiency. Symptoms of VA insufficiency may manifest as dizziness, dysarthria, dysphagia, diplopia, blurred vision, and tinnitus.17
Initially asymptomatic patients can have progression of symptoms either acutely or delayed due to embolism, thrombus extension, or dissection. Heros and colleagues23 described a patient who sustained a VAI with a resultant cerebellar infarction despite having a normal contralateral artery. To explain the infarction, the authors postulated that the thrombosis had extended distally to the intracranial portion of the VA. Interestingly, Six and colleagues24 reported a case of an asymptomatic post-traumatic bilateral VA occlusion. Angiography demonstrated occlusion of both vertebral arteries but with the presence of blood flow through the intramuscular collateral vessels of the thyrocervical trunk and by collaterals from the superficial occipital artery. In fact, Blam and colleagues25 conducted a retrospective review of 1283 patients with cervical spine trauma and determined that a normal neurologic examination is not predictive of VA patency after significant cervical spine trauma. These authors also noted that VAI was observed in similar frequency in both neurologically intact and motor incomplete patients (ASIA C and D).25
The interval between spinal injury and the development of vertebrobasilar symptoms varies greatly from immediately after trauma to up to 3 months later.17,26 The late onset of symptoms suggests a process of vertebral vessel thrombus formation at the injury site with clot propagation or embolism and subsequent infarction. Therefore clinicians should be aware that late symptoms and signs of vertebrobasilar insufficiency after cervical spine trauma may be a manifestation of VAI that occurred at the time of trauma.11 Vaccaro and colleagues10 demonstrated that 83% of patients with a known traumatic vertebral vessel injury demonstrated no flow reconstitution after an average follow-up of 25.8 months. The one patient who did show reconstitution of flow was thought to have a resolution of vertebral arterial spasm that initially compromised flow.
Imaging Modalities
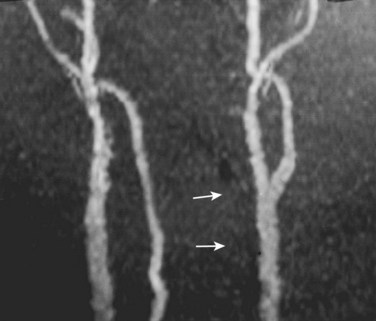
CTA is a technique that combines precisely timed high-speed, multidetector computed tomography (CT) imaging shortly after a bolus of contrast dye through a peripheral intravenous line. Using bolus tracking, a specific anatomic site, in this example the vertebral arteries, can be imaged without the need for cannulating a catheter directly to the site, thereby eliminating many possible complications associated with DSA. In addition, the amount of dye used in CTA is substantially less then that used in DSA. The majority of high-energy trauma patients require head and cervical spine scans; therefore this imaging modality can be done in the same clinical setting as other required trauma-specific imaging studies. The previous generation, four-detector CT scanner was widely found to be inadequate for VAI injury. Newer high-speed, 16-detector CT scanner imaging is far superior in resolution and in its ability to bolus track. Therefore this technique has experienced near universal acceptance within the radiology community as a replacement for DSA. Eastman and colleagues27 found that CTA was nearly identical to DSA for detection of VAI in 162 patients determined to be at risk of injury.
Although these less-invasive imaging techniques are attractive for the reasons stated earlier, and despite wide acceptance in the radiology literature, some trauma surgeons remain skeptical of CTA accuracy. Biffl and colleagues21 have questioned their ability to detect all vascular lesions when compared with the “gold-standard” DSA. The investigators developed a grading scale to evaluate the performance of MRA and CTA: grade I, dissection with less than 25% luminal narrowing; grade II, dissection with more than 25% luminal narrowing; grade III, pseudoaneurysm; grade IV, occlusion; and grade V, transection. Forty-six consecutive patients underwent CTA or MRA in conjunction with DSA. Although adequate for dramatic lesions, the more subtle grade I, II, and III lesions were routinely missed by CTA or MRA.
Malhotra and colleagues28 also noted that increasingly CTA has been replacing DSA in suspected VAI injuries and selected 119 patients who met screening criteria (facial/cervical-spinal fractures; unexplained neurologic deficit; anisocoria; lateral neck soft tissue injury; clinical suspicion) out of 7000 level I blunt trauma admissions and performed DSA and CTA. In agreement with Biffl’s study they found an alarming rate of missed VAIs using CTA that were visualized using DSA. The authors went as far as to say using CTA was dangerous due to the risk of missed injuries. Miller and colleagues’ study also found CTA and MRA to be inadequate screening tools. Although many investigators have shown failures of CTA to detect subtle lesions, Berne and colleagues29 have asserted CTA is sufficient for detection of clinically relevant injuries (i.e., high-grade injuries). Although screening criteria may be employed to direct a dedicated CTA of the cerebrovascular system, another consideration is to integrate a CTA of the VA into the standard CT trauma series protocol. Langner and colleagues30 developed such a protocol and found that an optimized craniocervical CTA can be easily integrated into a whole-body CT protocol for polytrauma patients. They assert that no additional screening technique is necessary to identify clinically relevant vascular injuries. This type of protocol could lead to a more liberalized screening of all trauma patients as recommended by some investigators.31
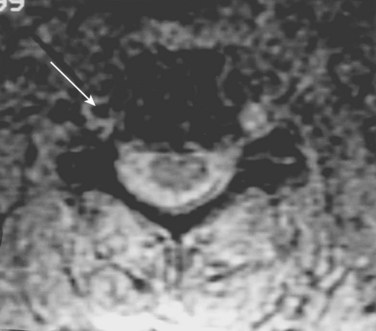
Veras and colleagues32 performed a retrospective review of patients with VAI to determine the ideal MRA imaging sequence. They noted that two-dimensional time-of-flight sequencing was a more effective imaging sequence than the three-dimensional time-of-flow because of a reduced false-positive signal dropout. Axial T1-weighted MRI was useful because of its ability to delineate the absence of flow from an intimal occlusion including an intraluminal clot. In a dog model Ren and colleagues33 found that MRA was comparable with DSA for evaluation of VAI.
Treatment
Several different methods of therapy have been used to treat symptomatically occluded VAs. The American Association of Neurological Surgeons/Congress of Neurological Surgeons Guidelines consensus concluded that patients with posterior circulation stroke and VAI have a better outcome when treated with intravenous heparin than patients who do not receive this treatment.34 Fibrinolysis with streptokinase has also been described to successfully restore flow with varied results.35
Although the exact mechanism is ill defined, be it ischemia or embolic, the outcome of patients who develop symptoms of posterior circulation ischemia without stroke and are treated with intravenous heparin1,36–38 is similar to that of patients receiving no treatment.6,39 In the symptomatic patient, Schellinger and colleagues37 advocated the initial use of heparin with a target activated partial thromboplastin time of at least twice the baseline value followed by oral anticoagulation (warfarin) for at least 3 to 6 months as a secondary prophylaxis.
Complications associated with anticoagulation in the trauma population range from 25% to 54%.40 Bleeding complications include intracranial hemorrhage, gastrointestinal bleeding, retroperitoneal hemorrhage, and excessive surgical site bleeding. In fact, in a study by Eachempati and colleagues,41 only 14% of trauma patients were deemed candidates for full anticoagulation. These facts have led physicians to focus more on antiplatelet therapy as opposed to heparinization.
In appropriately selected candidates with a symptomatic dissecting VA, surgical intervention may include vertebral vessel resection followed by grafting. Another method of arresting vessel flow is through the use of catheter embolization with Guglielmi detachable coils.42–44 Today the gold standard surgical procedure for a symptomatic vertebral dissection remains ligation of the injured VA proximal and distal to the site of the lesion rather than primary repair.43 As mentioned previously, ligation is well tolerated if there is adequate collateral flow.
The treatment of asymptomatic VAIs is controversial. Today, in the majority of level I trauma centers, formal treatment is not recommended for a patient with an asymptomatic VAI after trauma.24,44 Biffl and colleagues2 found, in a retrospective review, that systemic heparin improved the long-term neurologic outcome of both initially symptomatic and asymptomatic patients sustaining blunt VAI. The authors postulated that this treatment approach may prevent the arteriographic progression of lesions, protect against postinjury stroke, and prevent neurologic deterioration from treatment to discharge. However, the authors also noted that systemic anticoagulation introduces risks of hemorrhagic complications, especially in multisystem trauma victims with a reported 10% complication rate. Recently, Spaniolas and colleagues45 reviewed the National Trauma Data Bank entries for 2001 to 2005 and identified 574 VAIs among the 761,385 blunt trauma admissions (0.075% overall incidence). The investigation found no improvement in the modified functional independence measure (FIM) score during this time despite progressively increasing rates of detection over that period (annual incidence .053% in 2001 to 0.1% in 2005 (P < 0.001)). There are definite dangers associated with anticoagulation administration such as hemorrhage, worsening neurologic function, and increasing infarction size.19 Routine anticoagulation administration also precludes surgical intervention that may be necessary to restore spinal stability and to relieve neural compression. A potential treatment choice may be the use of oral antiplatelet therapy (i.e., aspirin following surgery or nonoperative care for 3 months in the asymptomatic patient), although this has not been supported with any clinical trials to date.
Transection of the VA is a life-threatening emergency due to hemorraghic shock and/or airway compromise. Treatment is airway management, resuscitation, and emergent interventional neuroradiology embolization.46 An additional reason to routinely evaluate for the presence of a vertebral vessel disruption in an asymptomatic patient is to ensure safety to the intact vessel if surgical intervention with implants that may put the vertebral vasculature at risk are to be used.
Summary
Pearls
Pitfalls
Key Points
1 Giacobetti F, Vaccaro A, Bos-Giacobetti M, et al. Vertebral artery occlusion associated with cervical spine trauma: A prospective analysis. Spine. 1997;22:188-192.
2 Vaccaro AR, Klein GR, Flanders AE, et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine. 1998;23:789-796.
3 Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55:811-813.
4 Biffl WL, Ray CEJr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53:850-856.
5 American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Section of Disorders of the Spine and Peripheral Nerves. Management of vertebral artery injuries after nonpenetrating cervical trauma. Neurosurgery. 2002 Mar;50(3 Suppl):S173-S178.
1 Lee KP, Carlini WG, McCormick GF, Albers GW. Neurologic complications following chiropractic manipulation: a survey of California neurologists. Neurology. 1995;45:1213-1215.
2 Oehler J, Gandjour J, Fiebach J, Schwab S. Bilateral vertebral artery dissection after chiropractic treatment. Orthopade. 2003;32:911-915. [in German]
3 Traflet RF, Babaria AR, Bell RD, et al. Vertebral artery dissection after rapid head turning. AJNR Am J Neuroradiol. 1989;10:650-651.
4 DeBehnke DJ, Brady W. Vertebral artery dissection due to minor neck trauma. J Emerg Med. 1994;12:27-31.
5 McCrory P. Vertebral artery dissection causing stroke in sport. J Clin Neurosci. 2000;7:298-300.
6 Louw JA, Mafoyane NA, Small B, Neser CP. Occlusion of the vertebral artery in cervical spine dislocations. J Bone Joint Surg Br. 1990;72:679-681.
7 Willis BK, Greiner F, Orrison WW, Benzel EC. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34:435-442.
8 Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386-395.
9 Biffl WL, Moore EE, Elliott JP, et al. The devastating potential blunt vertebral arterial injuries. Ann Surg. 2000;231:672-681.
10 Vaccaro AR, Klein GR, Flanders AE, et al. Long-term evaluation of vertebral artery injuries following cervical spine trauma using magnetic resonance angiography. Spine. 1998;23:789-794.
11 Giacobetti FB, Vaccaro AR, Bos-Giacobetti MA, et al. Vertebral artery occlusion associated with cervical spine trauma: A prospective analysis. Spine. 1997;22:188-192.
12 Berne JD, Norwood SH, McAuley CE, Villareal DH. Helical computed tomographic angiography: an excellent screening test for blunt cerebrovascular injury. J Trauma. 2004;57:11-19.
13 Cothren CC, Moore EE, Biffl WL, et al. Cervical spine fracture patterns predictive of blunt vertebral artery injury. J Trauma. 2003;55:811-813.
14 Berne JD, Norwood SH, McAuley CE, et al. The high morbidity of blunt cerebrovascular injury in an unscreened population: more evidence of the need for mandatory screening protocols. J Am Coll Surg. 2001;192:314-321.
15 Parent AD, Harkey HL, Touchstone DA, et al. Lateral cervical spine dislocation and vertebral artery injury. Neurosurgery. 1992;31:501-509.
16 Biffl WL, Ray CEJr, Moore EE, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002;235:699-707.
17 Deen HGJr, McGirr SJ. Vertebral artery injury associated with cervical spine fracture. Spine. 1992;17:230-234.
18 Bula WI, Loes DJ. Trauma to the cerebrovascular system. Neuroimaging Clin North Am. 1994;4:753-772.
19 Sim E, Vaccaro AR, Berzlanovich A, Pienaar S. The effects of staged static cervical flexion-distraction deformities on the patency of the vertebral arterial vasculature. Spine. 2000;25:2180-2186.
20 Saito T, Kamisawa O, Kaminishi Y, et al. Isolated traumatic vertebral pseudoaneurysm: report of a case. Surg Today. 2003;33:145-147.
21 Biffl WL, Ray CEJr, Moore EE, et al. Noninvasive diagnosis of blunt cerebrovascular injuries: a preliminary report. J Trauma. 2002;53:850-856.
22 Miller PR, Fabian TC, Bee TK, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001;51:279-286.
23 Heros RC. Cerebellar infarction resulting from traumatic occlusion of a vertebral artery. J Neurosurg. 1979;51:111-113.
24 Six EG, Stringer WL, Cowley AR, Davis CH. Posttraumatic bilateral vertebral artery occlusion: Case report. J Neurosurg. 1981;54:814-817.
25 Blam O, Torina P, Vaccaro AR, et al. Incidence of vertebral artery thrombosis in cervical spine trauma. Unpublished data. Submitted to Cervical Spine Research Society Miami Beach, FL, December 5-7. 2002.
26 Quint DJ, Spickler EM. Magnetic resonance demonstration of vertebral artery dissection: Report of two cases. J Neurosurg. 1990;72:964-967.
27 Eastman AL, Chason DP, Perez CL, et al. Computed tomographic angiography for the diagnosis of blunt cervical vascular injury: is it ready for primetime? J Trauma. 2006 May;60:925-929.
28 Malhotra AK, Camacho M, Ivatury RR, et al. Computed tomographic angiography for the diagnosis of blunt carotid/vertebral artery injury: a note of caution. Ann Surg. 2007 Oct;246:632-642.
29 Berne JD, Reuland KS, Villarreal DH, et al. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006 Jun;60:1204-1209.
30 Langner S, Fleck S, Kirsch M, et al. Whole-body CT trauma imaging with adapted and optimized CT angiography of the craniocervical vessels: Do we need an extra screening examination? AJNR Am J Neuroradiol. 2008;29:1902-1907.
31 Kerwin AJ, Bynoe RP, Murray J, et al. Liberalized screening for blunt carotid and vertebral artery injuries is justified. Neurosurgery. 2002 Mar;50(3 Suppl):S173-S178.
32 Veras L, Gutierrez S, Castellanos J, et al. Vertebral artery occlusion after acute cervical spine trauma. Spine. 2000;25:1171-1177.
33 Ren X, Wang W, Zhang X, et al. Clinical study and comparison of magnetic resonance angiography (MRA) and angiography diagnosis of blunt vertebral artery injury. J Trauma. 2007 Dec;63:1249-1253.
34 American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Section of Disorders of the Spine and Peripheral Nerves. Management of vertebral artery injuries after nonpenetrating cervical trauma. Neurosurgery. 2002 Mar;50(3 Suppl):S173-S178.
35 Schwarz N, Buchinger W, Gaudernak T, et al. Injuries to the cervical spine causing vertebral artery trauma: Case reports. J Trauma. 1991;31:127-133.
36 Prabhu V, Kizer J, Patil A, et al. Vertebrobasilar thrombosis associated with nonpenetrating cervical spine trauma. J Trauma. 1996;40:130-137.
37 Schellinger PD, Schwab S, Krieger D, et al. Masking of vertebral artery dissection by severe trauma to the cervical spine. Spine. 2001;26:314-319.
38 Thibodeaux LC, Hearn AT, Peschiera JL, et al. Extracranial vertebral artery dissection after trauma: A five year review. Br J Surg. 1997;84:94.
39 Schwarz N, Buchinger W, Gaudernak T, et al. Injuries to the cervical spine causing vertebral artery trauma: Case reports. J Trauma. 1991;31:127-133.
40 Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries: early diagnosis improves neurologic outcome. Ann Surg. 1998 Oct;228:462-470.
41 Eachempati SR, Vaslef SN, Sebastian MW, Reed RL2nd. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma. 1998 Dec;45:997-1004.
42 Alexander JJ, Glagov S, Zarins CK. Repair of a vertebral artery dissection. J Neurosurg. 1986;64:662-666.
43 Blickenstaff KL, Weaver FA, Yellin AE, et al. Trends in the management of traumatic vertebral arteries injuries. Am J Surg. 1989;158:101-106.
44 Bose B, Northrup BE, Osterholm JL. Delayed vertebrobasilar insufficiency following cervical spine injury. Spine. 1985;18:108-110.
45 Spaniolas K, Velmahos GC, Alam HB, et al. Does improved detection of blunt vertebral artery injuries lead to improved outcomes? Analysis of the National Trauma Data Bank. World J Surg. 2008;32:2190-2194.
46 Willis BK, Greiner F, Orrison WW, Benzel EC. The incidence of vertebral artery injury after midcervical spine fracture or subluxation. Neurosurgery. 1994;34:435-442.