CHAPTER 16 Ventricular system and subarachnoid space
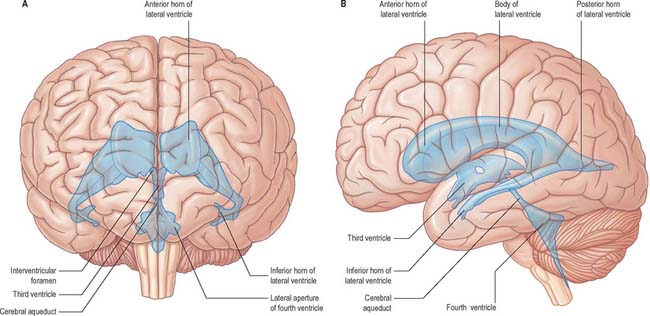
The cerebral ventricular system consists of a series of interconnecting spaces and channels within the brain (Fig. 16.1) which are derived from the central lumen of the embryonic neural tube and the cerebral vesicles to which it gives rise (Ch. 24). Each cerebral hemisphere contains a large lateral ventricle that communicates near its rostral end with the third ventricle via the interventricular foramen (foramen of Monro). The third ventricle is a midline, slit-like cavity lying between the right and left thalamus and hypothalamus. Caudally, the third ventricle is continuous with the cerebral aqueduct, a narrow tube that passes the length of the midbrain, and which is continuous in turn with the fourth ventricle, a wide tent-shaped cavity lying between the brain stem and cerebellum. The fourth ventricle communicates with the subarachnoid space of the cisterna magna though the foramen of Magendie, and of the cerebellopontine angle through the foramina of Luschka; caudally it is continuous with the vestigial central canal of the spinal cord.
TOPOGRAPHY AND RELATIONS OF THE VENTRICULAR SYSTEM
LATERAL VENTRICLE
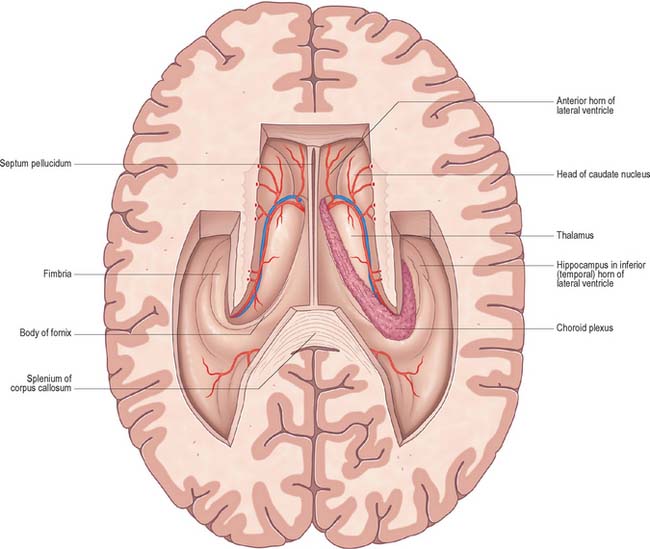
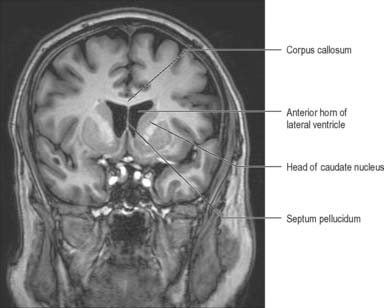
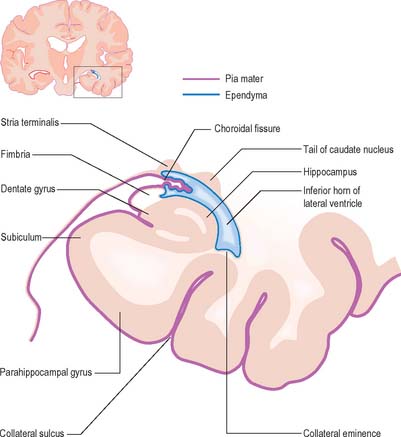
The lateral ventricle is customarily divided into a body and anterior (frontal), posterior (occipital) and inferior (temporal) horns. The anterior horn lies within the frontal lobe. It is bounded anteriorly by the posterior aspect of the genu and rostrum of the corpus callosum, and its roof is formed by the anterior part of the body of the corpus callosum. The anterior horns of the two ventricles are separated by the septum pellucidum. The coronal profile of the anterior horn is roughly that of a flattened triangle in which the rounded head of the caudate nucleus forms the lateral wall and floor (Figs 16.2, 16.3). The anterior horn extends back as far as the interventricular foramen. The body lies within the frontal and parietal lobes and extends from the interventricular foramen to the splenium of the corpus callosum. The bodies of the lateral ventricles are separated by the septum pellucidum, which contains the columns of the fornices in its lower edge. The lateral wall of the body of the ventricle is formed by the caudate nucleus superiorly and the thalamus inferiorly. The boundary between the thalamus and caudate nucleus is marked by a groove (Fig. 16.2) which is occupied by a fascicle of nerve fibres, the stria terminalis, and by the thalamostriate vein (see Fig. 22.5). The inferior limit of the body of the ventricle and its medial wall are formed by the body of the fornix (Fig. 16.4). The fornix is separated from the thalamus by the choroidal fissure. The choroid plexus occludes the choroidal fissure and covers part of the thalamus and fornix. The body of the lateral ventricle widens posteriorly to become continuous with the posterior and inferior horns at the collateral trigone or atrium. The posterior horn curves posteromedially into the occipital lobe. It is usually diamond-shaped or square in outline, and the two sides are often asymmetrical. Fibres of the tapetum of the corpus callosum separate the ventricle from the optic radiation, and form the roof and lateral wall of the posterior horn. Fibres of the splenium of the corpus callosum (forceps major) pass medially as they sweep back into the occipital lobe, and produce a rounded elevation in the upper medial wall of the posterior horn. Lower down, a second elevation, the calcar avis, corresponds to the deeply infolded cortex of the anterior part of the calcarine sulcus. The inferior horn is the largest compartment of the lateral ventricle and extends forwards into the temporal lobe. It curves round the posterior aspect of the thalamus (pulvinar), passes downwards and posterolaterally and then curves anteriorly to end within 2.5 cm of the temporal pole, near the uncus. Its position relative to the surface of the hemisphere usually corresponds to the superior temporal sulcus. The roof of the inferior horn is formed mainly by the tapetum of the corpus callosum, but also by the tail of the caudate nucleus and the stria terminalis, which extend forwards in the roof to terminate in the amygdala at the anterior end of the ventricle. The floor of the ventricle consists of the hippocampus medially and the collateral eminence, formed by the infolding of the collateral sulcus, laterally. The inferior part of the choroid fissure lies between the fimbria (a distinct bundle of efferent fibres that leaves the hippocampus) and the stria terminalis in the roof of the temporal horn (Fig. 16.5). The temporal extension of the choroid plexus fills this fissure and covers the outer surface of the hippocampus.
THIRD VENTRICLE
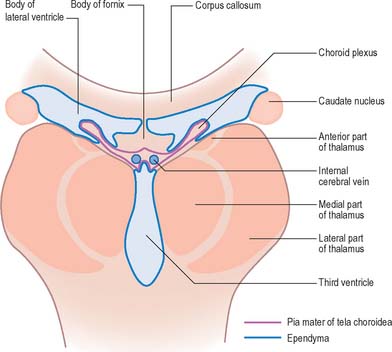
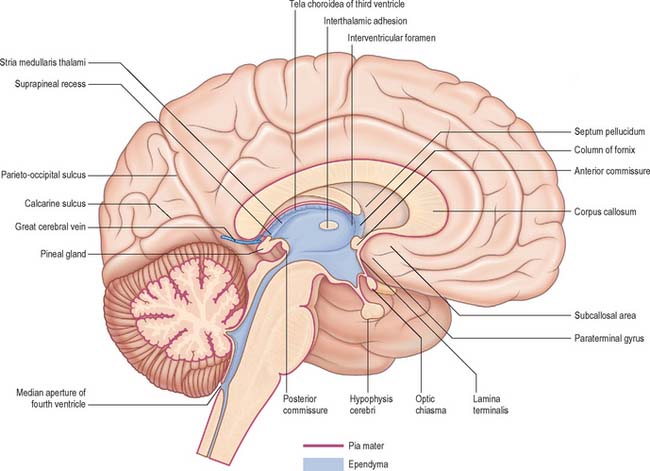
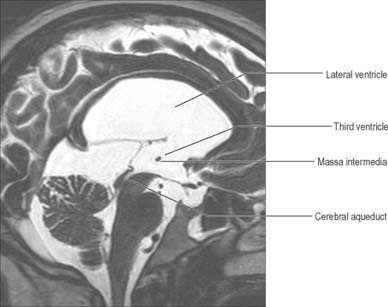
The third ventricle is a midline, slit-like cavity which is derived from the primitive forebrain vesicle (Figs 16.1, 16.4, 16.6, 16.7; see Fig. 15.8). The upper part of the lateral wall of the ventricle is formed by the medial surface of the anterior two-thirds of the thalamus, and the lower part is formed by the hypothalamus anteriorly and the subthalamus posteriorly. An indistinct hypothalamic sulcus extends horizontally on the ventricular wall between the interventricular foramen and the cerebral aqueduct, and marks the boundary between the thalamus and hypothalamus. Dorsally, the lateral wall is limited by a ridge covering the stria medullaris thalami. The lateral walls of the third ventricle are joined by an interthalamic adhesion, or massa intermedia, a band of grey matter which extends from one thalamus to the other.
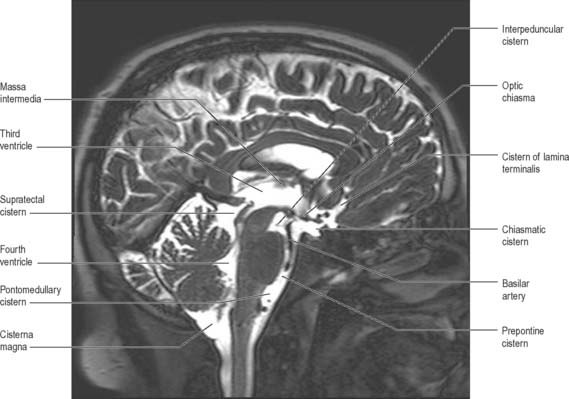
Anteriorly, the third ventricle extends to the lamina terminalis (Fig. 16.7). This thin structure stretches from the optic chiasma to the rostrum of the corpus callosum and represents the rostral boundary of the embryonic neural tube. The lamina terminalis forms the roof of the small virtual cavity lying immediately below the ventricle called the cistern of the lamina terminalis. This is important because it contains the anterior communicating artery and aneurysm formation at this site may cause intraventricular haemorrhage through the thin membrane of the lamina terminalis. Above this, the anterior wall is formed by the diverging columns of the fornices and the transversely orientated anterior commissure, which crosses the midline. The anterior and posterior commissures are important neuroradiological landmarks. Prior to the introduction of modern imaging techniques, the fact that these commissures could be identified by ventriculography led to their use as reference markers for stereotaxic surgical procedures. This convention is now universal: the positions of the anterior and posterior commissures are used as the basic reference points for surgical atlases of brain anatomy. The narrow interventricular foramen is located immediately posterior to the column of the fornix and separates the fornix from the anterior nucleus of the thalamus.
The roof of the third ventricle is a thin ependymal layer that extends from its lateral walls to the choroid plexus, which spans the choroidal fissure (Fig. 16.4). Above the roof is the body of the fornix. The posterior boundary of the ventricle is marked by a suprapineal recess above the pineal gland, a pineal (epiphysial) recess, which extends into the pineal stalk, and by the posterior commissure. Below the commissure the ventricle is continuous with the cerebral aqueduct of the midbrain.
CEREBRAL AQUEDUCT
The cerebral aqueduct is a small tube, roughly circular in transverse section and 1–2 mm in diameter. It extends throughout the dorsal quarter of the midbrain in the midline and is surrounded by the periaqueductal (central) grey matter (Fig. 16.7; see Fig. 19.19). Rostrally, it commences immediately behind and below the posterior commissure, where it is continuous with the caudal aspect of the third ventricle. Caudally, it is continuous with the lumen of the fourth ventricle at the junction of the midbrain and pons. The superior and inferior colliculi are dorsal to the aqueduct and the midbrain tegmentum is ventral.
FOURTH VENTRICLE
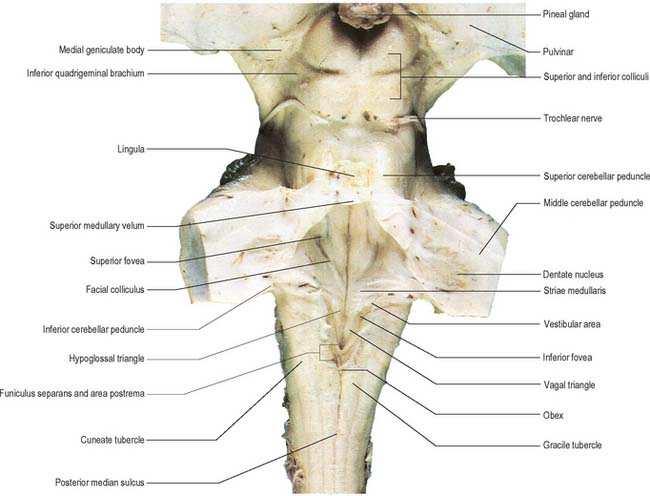
The fourth ventricle lies between the brain stem and the cerebellum (Figs 16.1B, 16.6, 16.7, 16.8; see Fig. 19.3). Rostrally it is continuous with the cerebral aqueduct, and caudally with the central canal of the spinal cord. In sagittal section, the fourth ventricle has a characteristic triangular profile, and the apex of its tented roof protrudes into the inferior aspect of the cerebellum. The ventricle is at its widest at the level of the pontomedullary junction, where a lateral recess on both sides extends to the lateral border of the brain stem. At this point the lateral aperture of the fourth ventricle (foramen of Luschka) provides access to the subarachnoid space at the cerebellopontine angle; CSF flows through it into the lateral extension of the pontine cistern. Occasionally, a lateral recess may not open.
The roof of the fourth ventricle is formed by the superior and inferior medullary veli. Superiorly, a thin sheet of tissue, the superior medullary velum, stretches across the ventricle between the converging superior cerebellar peduncles (Fig. 16.8). The superior medullary velum is continuous with the cerebellar white matter and is covered dorsally by the lingula of the superior vermis. The inferior medullary velum is more complex and is mostly composed of a thin sheet, devoid of neural tissue, formed by ventricular ependyma and the pia mater of the tela choroidea, which covers it dorsally. A large median aperture (foramen of Magendie) is present in the roof of the ventricle as a perforation in the posterior medullary velum, just inferior to the nodule of the cerebellum: CSF flows through the foramen from the ventricle into the cisterna magna.
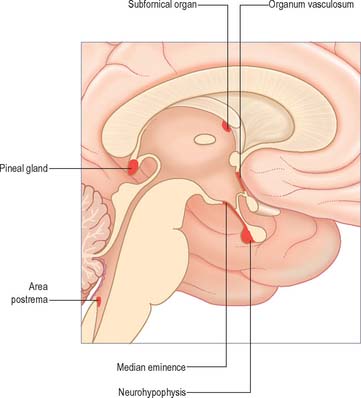
CIRCUMVENTRICULAR ORGANS
The walls of the ventricular system are lined with ependymal cells covering a subependymal layer of glia. At certain midline sites in the ventricular walls, collectively referred to as circumventricular organs, the blood–brain barrier is absent and specialized ependymal cells called tanycytes are present (Fig. 16.9). The functions of ependyma and tanycytes may include secretion into the CSF; transport of neurochemicals from subjacent neurones, glia or vessels to the CSF; transport of neurochemicals from the CSF to the same subjacent structures; chemoreception. In the adult, the ependymal and subependymal glial cell layers are the source of undifferentiated stem cells that are currently under intensive study for their potential neurorestorative properties.
The pineal gland (see Ch. 21) is a small structure approximately 8 mm in diameter situated rostro-dorsal to the superior colliculus and behind the stria medullaris. It is part of the epithalamus and consists of internal lobules of pinealocytes and sparse neurones covered by a pial capsule. It secretes melatonin which appears to be involved in the inhibition of puberty in children and in the in the regulation of the sleep–wake cycle in both children and adults.
The area postrema is a bilaterally paired structure, located at the caudal limit of the floor of the fourth ventricle. It is an important chemoreceptive area that triggers vomiting in response to the presence of emetic substances in the blood. The area postrema, along with the nucleus of the solitary tract and the dorsal motor nucleus of the vagus, makes up the so-called dorsal vagal complex, which is the major termination site of vagal afferent nerve fibres.
CHOROID PLEXUS AND CEREBROSPINAL FLUID
CHOROID PLEXUS
The vascular pia mater in the roofs of the third and fourth ventricles, and in the medial wall of the lateral ventricle along the line of the choroid fissure, is closely apposed to the ependymal lining of the ventricles, without any intervening brain tissue. It forms the tela choroidea, which gives rise to the highly vascularized choroid plexuses from which CSF is secreted into the lateral, third and fourth ventricles (Figs 16.2, 16.4, 16.5). The body or stroma of the choroid plexus consists of many capillaries, separated from the subarachnoid space by the pia mater and choroid ependymal cells.
In the lateral ventricle, the choroid plexus extends anteriorly as far as the interventricular foramen, through which it is continuous across the third ventricle with the plexus of the opposite lateral ventricle. From the interventricular foramen, the plexus passes posteriorly, in contact with the thalamus, curving round its posterior aspect to enter the inferior horn of the ventricle and reach the hippocampus. Throughout the body of the ventricle, the choroid fissure lies between the fornix superiorly and the thalamus inferiorly (Fig. 16.4).
SUBARACHNOID SPACE AND CIRCULATION OF CEREBROSPINAL FLUID
SUBARACHNOID SPACE
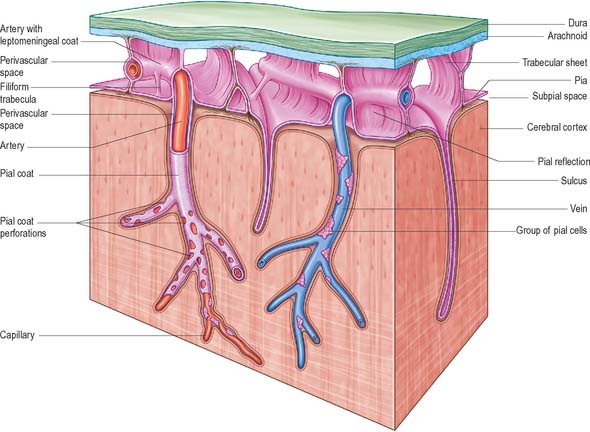
The subarachnoid space lies between the arachnoid and the pia mater. The cerebral subarachnoid space is connected with the fourth ventricle of the brain by three openings. The median aperture (foramen of Magendie) lies in the median plane in the inferior part of the roof of the fourth ventricle and provides communication with the cisterna magna. The paired lateral apertures (foramina of Luschka) are located at the ends of the lateral recesses of the fourth ventricle, and open into the subarachnoid space at the cerebellopontine angle, behind the upper roots of the glossopharyngeal nerves. The subarachnoid space therefore contains cerebrospinal fluid (CSF); it also contains the larger arteries and veins which traverse the surface of the brain (Fig. 16.10).
Trabeculae, in the form of sheets or fine filiform structures, traverse the subarachnoid space from the deep layers of the arachnoid mater to the pia mater and are also attached to large blood vessels within the subarachnoid space (Fig. 16.10). Each trabecula has a core of collagen and is coated by leptomeningeal cells. The trabeculae that cross the subarachnoid space may form compartments, particularly in the perivascular regions, which may facilitate directional flow of CSF through the subarachnoid space.
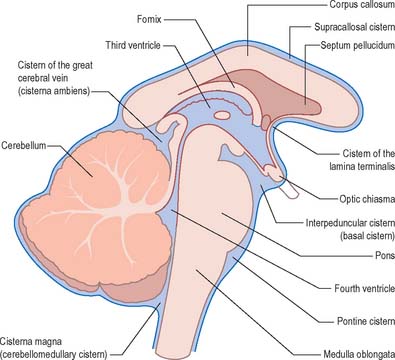
Arachnoid and pia mater are in close apposition over the convexities of the brain, such as the cortical gyri, whereas concavities are followed by the pia but spanned by the arachnoid. This arrangement produces a subarachnoid space of greatly variable depth that is location dependent. The more expansive spaces are identified as subarachnoid cisterns (Fig. 16.11), which are continuous with each other through the general subarachnoid space. Subarachnoid trabeculae that cross the subarachnoid cisterns are long and filamentous.
CIRCULATION OF CEREBROSPINAL FLUID
Arachnoid villi and granulations
Arachnoid granulations are focal outpushings of the arachnoid mater and subarachnoid space through the wall of dural venous sinuses, very often close to points where veins enter the sinus: granulations have been reported in the superior sagittal, transverse, superior petrosal and straight sinuses in decreasing frequency. They are most prominent along the margins of the great longitudinal fissure, commonly in the lateral lacunae of the superior sagittal sinus, and also protruding directly into the lumen of the sinus (see Ch. 27). They increase in number and size with increasing age and may appear as focal filling defects in the venous sinuses on angiograms and contrast enhanced CT scans and focal signal alterations in the venous sinuses on MR examination.
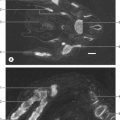
At the base of each arachnoid granulation, a thin neck of arachnoid mater projects through an aperture in the dural lining of the venous sinus and expands to form a core of collagenous trabeculae and interwoven channels (Fig. 16.12). The core is surmounted by an apical cap of arachnoid cells, some 150 μm thick. Channels extend through the cap to reach the subendothelial regions of the granulation. The cap region of each granulation is attached to the endothelium of the sinus over an area some 300 μm in diameter, whereas the rest of the core of the granulation is separated from the endothelium by a fibrous dural cupola.
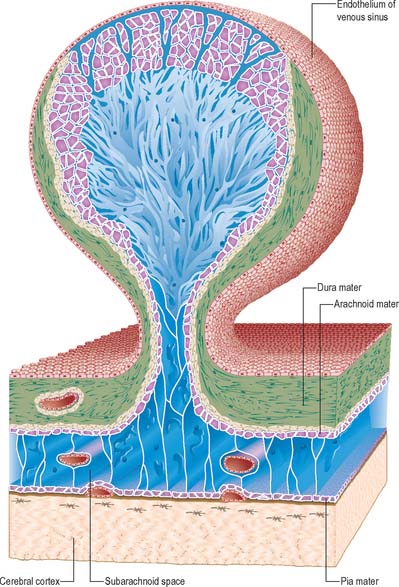
Fig. 16.12 An arachnoid granulation.
(Modified by permission from Springer-Verlag, Principles of Pediatric Neurosurgery, Vol 4: Morphology of CSF drainage pathways in man. Raimondi (ed), Kida and Weller 1994.)
The function of arachnoid granulations remains controversial. It has long been thought that arachnoid villi and arachnoid granulations constitute the major pathway for the resorption of CSF from the subarachnoid space into the blood, although the relative contribution each might make to the process is not known and the exact mechanism of resorption has not been determined. Recent studies using radionuclide cisternography and cardiac gated MRI have shown that CSF may also be resorbed throughout the ventricles and subarachnoid space, through the walls of pial and subarachnoid vessels. Moreover, there is a growing body of experimental evidence that significant volumes of CSF may be absorbed into extracranial lymphatics in animals: radiolabelled proteins injected into the CSF (rabbits, cats), brain parenchyma (rabbits) or ventricles (sheep) have been recovered from cervical lymph, possibly by traveling along extensions of the subarachnoid space that surround the olfactory and optic nerves. It has been suggested that extracranial lymphatics play a major role in CSF reabsorption at relatively low pressures, but that other routes, including arachnoid granulations, become increasingly important as CSF pressure rises. A connection between parenchymal interstitial fluid and extracranial lymphatics in humans has yet to be convincingly demonstrated.
Hydrocephalus
Obstruction of the circulation of CSF leads to accumulation of fluid within the ventricular system (hydrocephalus), which causes compression of the brain (Fig. 16.13). Within the brain, critical points at which obstruction may occur correspond to the narrow foramina and passages of the ventricular system. Thus, obstruction of the interventricular foramen causes enlargement of the lateral ventricles; obstruction of the cerebral aqueduct leads to enlargement of both the lateral ventricles and the third ventricle; and obstruction or congenital absence of the apertures of the fourth ventricle leads to enlargement of the entire ventricular system. Hydrocephalus may also occur in the presence of intact communications between the ventricular and subarachnoid spaces (communicating hydrocephalus). The conventional view is that obstructions within the subarachnoid space impair CSF circulation and lead to CSF malabsorption, causing either obstructive or communicating hydrocephalus. Analyses of the intracranial hydrodynamics related to the pulse pressure have suggested that this is an oversimplification, and that increased pulse pressure in the brain capillaries maintains the ventricular enlargement in communicating hydrocephalus and chronic obstructive hydrocephalus (see Greitz 2004 for further details of the haemodynamic theory).
PIA MATER
The pia mater is a delicate membrane which closely invests the surface of the brain, from which it is separated by a microscopic subpial space (Fig. 16.10), and it follows the contours of the brain into concavities and the depths of fissures and sulci. During development, it becomes apposed to the ependyma in the roof of the telencephalon and fourth ventricle to form the stroma of the choroid plexus. The pia mater shares a common embryological origin and structural similarity with the arachnoid mater (see Ch. 27).
Pia mater is formed from a layer of leptomeningeal cells, often only 1 to 2 cells thick, in which the cells are joined by desmosomes and gap junctions but few, if any, tight junctions. The cells are continuous with the coating of the subarachnoid trabeculae and separated from the basal lamina of the glia limitans by bundles of collagen and fibroblast-like cells, and the arteries and veins that lie in the subpial space (Fig. 16.10).
Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145-165.
Describes modern theories of CSF production and transport..
Kida S, Weller RO. Morphology of CSF drainage pathways in man. Raimondi A, editor. Principles of Pediatric Neurosurgery, vol 4. Berlin: Springer, 1994.
Klintworth GK. The ontogeny and growth of the human tentorium cerebelli. Anat Rec. 1967;158:433-442.
McKinley MJ, McAllen RM, Davern P, et al. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol. 2003;172:III-XII. 1–122.
Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451(2):170-188.
Describes the structure and ultrastructure of the basal laminae and subependymal layer..
Paulson OB. Blood-brain barrier, brain metabolism and cerebral blood flow. Eur Neuropsychopharmacol. 2002;12(6):495-501.
Strazielle N, Ghersi-Egea JF. Choroid plexus in the central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59(7):561-574.
Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vasc Pharmacol. 2002;38(6):323-337.
Zakharov A, Papaiconomou C, Koh L, Djenic J, Bozanovic-Sosic R, Johnston M. Integrating the roles of extracranial lymphatics and intracranial veins in cerebrospinal fluid absorption in sheep. Microvasc Res. 2004;67:96-104.
Zhang ET, Inman CBE, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111-123.