Chapter 5 Ventricular System and Cerebrospinal Fluid
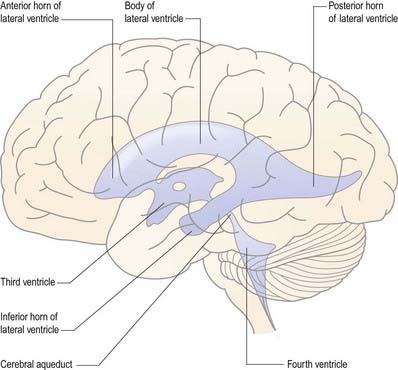
The cerebral ventricular system consists of a series of interconnecting spaces and channels within the brain that are derived from the central lumen of the embryonic neural tube and the cerebral vesicles to which it gives rise (Ch. 3). Within each cerebral hemisphere lies a large C-shaped lateral ventricle (Figs. 5.1, 5.2). Near its rostral end the lateral ventricle communicates through the interventricular foramen (foramen of Monro) with the third ventricle, which is a midline, slit-like cavity lying between the right and left halves of the thalamus and hypothalamus. Caudally, the third ventricle is continuous with the cerebral aqueduct, a narrow tube that passes the length of the midbrain; this, in turn, is continuous with the fourth ventricle, a wide, tent-shaped cavity lying between the brain stem and cerebellum. Caudally, the fourth ventricle is continuous with the vestigial central canal of the spinal cord.
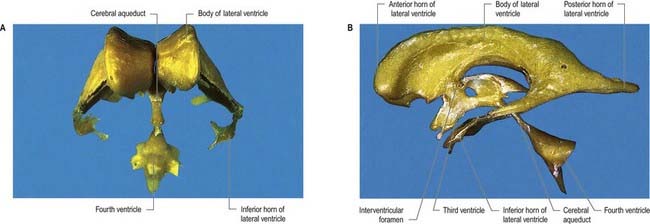
Fig. 5.1 Resin casts of the ventricular system of the human brain. A, Anterior view. B, Left lateral view.
(Prepared by D. H. Tompsett, Royal College of Surgeons of England.)
Topography and Relations of the Ventricular System
Lateral Ventricle
Viewed from its lateral aspect, the lateral ventricle has a roughly C-shaped profile, with an occipital tail (see Fig. 5.1). The shape is a consequence of the developmental expansion of the frontal, parietal and occipital regions of the hemisphere (Ch. 3), which displaces the temporal lobe inferiorly and anteriorly. Both the caudate nucleus and the fornix, which lie in the wall of the ventricle, have adopted a similar morphology, so the tail of the caudate nucleus encircles the thalamus in a C shape, and the fornix traces the outline of the ventricle forward to the interventricular foramen.
The lateral ventricle is customarily divided into a body and anterior, posterior and inferior horns (Figs. 5.1, 5.3). The anterior (frontal) horn lies within the frontal lobe. It is bounded anteriorly by the posterior aspect of the genu and rostrum of the corpus callosum, and its roof is formed by the anterior part of the body of the corpus callosum. The anterior horns of the two ventricles are separated by the septum pellucidum. The coronal profile of the anterior horn is roughly that of a flattened triangle in which the rounded head of the caudate nucleus forms the lateral wall and floor (Fig. 5.4). The anterior horn extends back as far as the interventricular foramen.
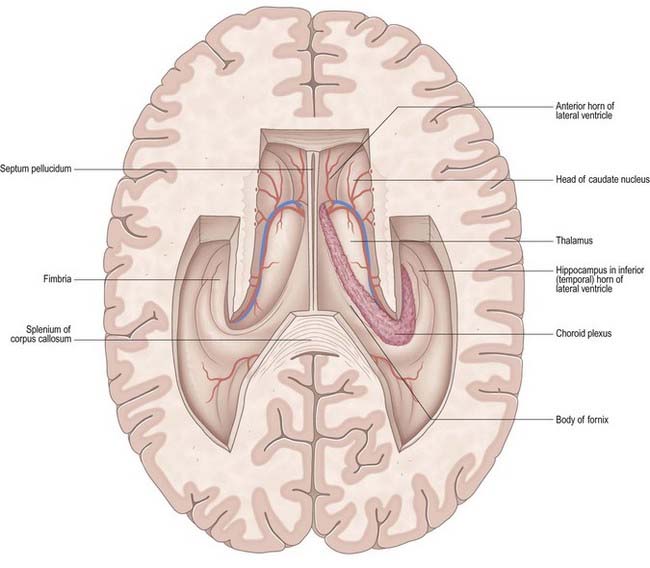
Fig. 5.3 Horizontal section of the cerebrum dissected to remove the roofs of the lateral ventricles.
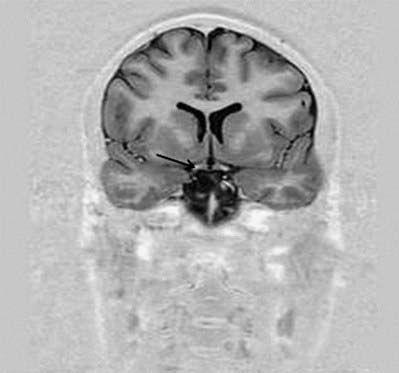
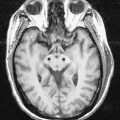
Fig. 5.4 Transverse MRI scan, at the level of the anterior horn of the lateral ventricle.
(Courtesy of Professor Alan Jackson, Department of Neuroradiology, University of Manchester, United Kingdom.)
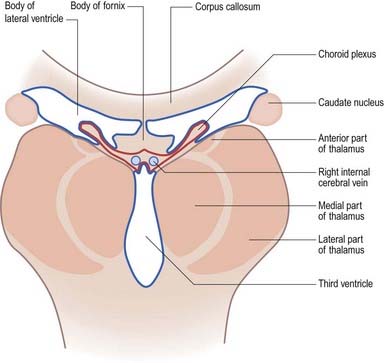
The body lies within the frontal and parietal lobes and extends from the interventricular foramen to the splenium of the corpus callosum. The bodies of the lateral ventricles are separated by the septum pellucidum, which contains the columns of the fornices in its lower edge. The coronal profile of the body of the ventricle is a flattened triangle with an inward-bulging lateral wall, formed by the thalamus inferiorly and the tail of the caudate nucleus superiorly. The boundary between the thalamus and caudate nucleus is marked by a groove (see Fig. 5.3), which is occupied by a fascicle of nerve fibres, the stria terminalis, and the thalamostriate vein. The inferior limit of the body of the ventricle and its medial wall are formed by the body of the fornix. The fornix is separated from the thalamus by the choroid fissure. The choroid plexus occludes the choroid fissure and covers part of the thalamus and fornix. The body of the lateral ventricle widens posteriorly to become continuous with the posterior and inferior horns at the collateral trigone or atrium.
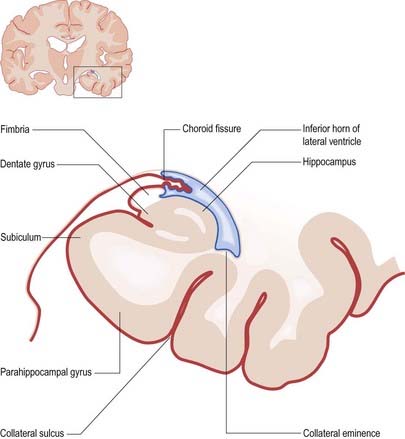
The floor of the ventricle consists of the hippocampus medially and the collateral eminence, formed by the infolding of the collateral sulcus, laterally. The inferior part of the choroid fissure lies between the fimbria (a distinct bundle of efferent fibres that leaves the hippocampus) and the stria terminalis in the roof of the temporal horn (Fig. 5.5). The temporal extension of the choroid plexus fills this fissure and covers the outer surface of the hippocampus.
Third Ventricle
The third ventricle is a midline, slit-like cavity derived from the primitive forebrain vesicle (Figs. 5.1, 5.2, 5.6–5.8). The upper part of the lateral wall of the ventricle is formed by the medial surface of the anterior two-thirds of the thalamus, and the lower part is formed by the hypothalamus anteriorly and the subthalamus posteriorly. An indistinct hypothalamic sulcus extends horizontally on the ventricular wall between the interventricular foramen and the cerebral aqueduct, marking the boundary between the thalamus and hypothalamus. Dorsally, the lateral wall is limited by a ridge covering the stria medullaris thalami. The lateral walls of the third ventricle are joined by an interthalamic adhesion, or massa intermedia, a band of grey matter that extends from one thalamus to the other.
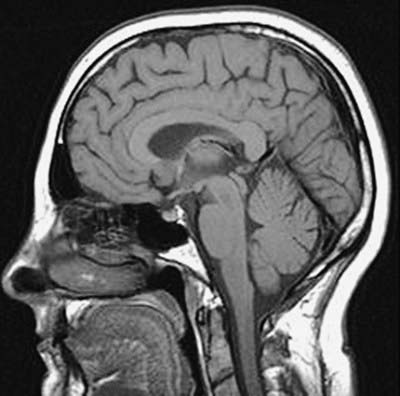
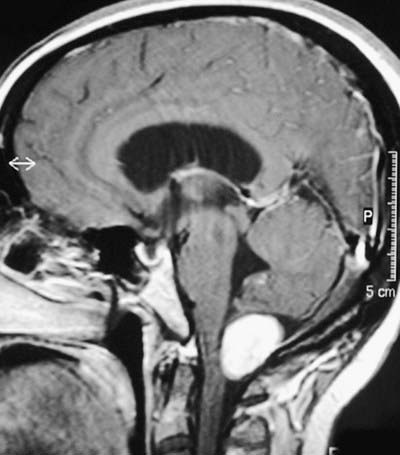
Fig. 5.7 MRI scan of the head in the sagittal plane.
(Courtesy of Professor Alan Jackson, Department of Neuroradiology, University of Manchester, United Kingdom.)
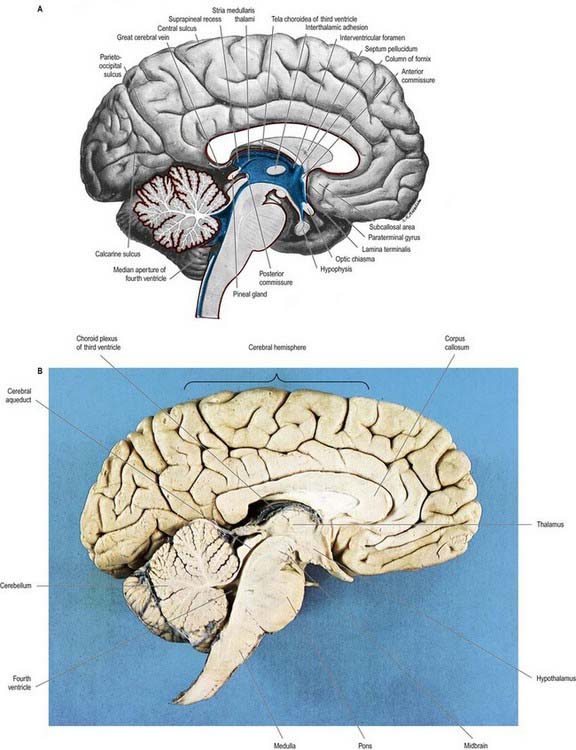
Fig. 5.8 A, Sagittal hemisection of the brain showing the third and fourth ventricles. Pia mater, red; ependyma, blue. B, Sagittal hemisection through the brain.
(Hemisection by E. L. Rees; photograph by Kevin Fitzpatrick on behalf of GKT School of Medicine, London.)
Anteriorly, the third ventricle extends to the lamina terminalis (see Fig. 5.8). This thin structure stretches from the optic chiasma to the rostrum of the corpus callosum and represents the rostral boundary of the embryonic neural tube. The lamina terminalis forms the roof of the small virtual cavity lying immediately below the ventricle, called the cistern of the lamina terminalis. This is important because it contains the anterior communicating artery, and aneurysm formation at this site may cause intraventricular haemorrhage through the thin membrane of the lamina terminalis. Above this, the anterior wall is formed by the diverging columns of the fornices and the transversely oriented anterior commissure, which crosses the midline. The anterior and posterior commissures are important neuroradiological landmarks. Before the introduction of modern imaging techniques, the anterior and posterior commissures could be identified by ventriculography. This led to their use as markers of the baseline for stereotactic surgical procedures. This convention is now universal, and the positions of the anterior and posterior commissures are the basic reference points for most surgical atlases of brain anatomy. The narrow interventricular foramen is located immediately posterior to the column of the fornix and separates the fornix from the anterior nucleus of the thalamus.
The roof of the third ventricle is a thin ependymal layer that extends from its lateral walls to the choroid plexus, which spans the choroid fissure (see Fig. 5.6). Above this is the body of the fornix. The posterior boundary of the ventricle is marked by a suprapineal recess above the pineal gland, by a pineal (epiphyseal) recess that extends into the pineal stalk and by the posterior commissure. Below the commissure, the ventricle is continuous with the cerebral aqueduct of the midbrain.
Cerebral Aqueduct
The cerebral aqueduct is a small tube, roughly circular in transverse section and approximately 2 mm in diameter. It extends throughout the dorsal quarter of the midbrain in the midline and is surrounded by the central, periaqueductal grey matter (see Fig. 5.8). Rostrally, it commences immediately behind and below the posterior commissure, where it is continuous with the caudal aspect of the third ventricle. Caudally, it is continuous with the lumen of the fourth ventricle at the junction of the midbrain and pons. The superior and inferior colliculi are dorsal to the aqueduct, and the midbrain tegmentum is ventral.
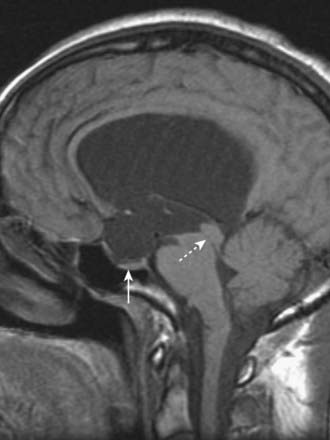
CASE 1 AQUEDUCTAL STENOSIS
Imaging demonstrates symmetric enlargement of the lateral and third ventricles (Fig. 5.9). The aqueduct is not visualized, and the fourth ventricle and cisterna magna are normal. A diagnosis of aqueductal stenosis is made, and ventricular shunting results in remarkable clinical improvement.
Fourth Ventricle
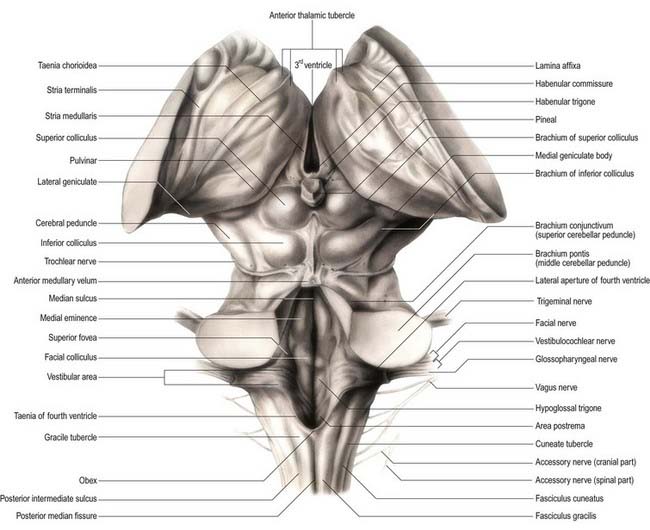
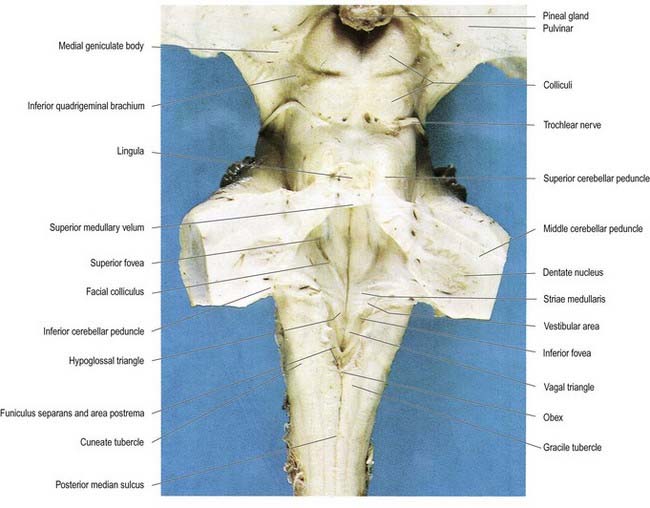
The fourth ventricle lies between the brain stem and the cerebellum (Figs. 5.10, 5.11). It is continuous rostrally with the cerebral aqueduct and caudally with the central canal of the spinal cord. In sagittal section, the fourth ventricle has a characteristic triangular profile, and the apex of its tented roof protrudes into the inferior aspect of the cerebellum. The ventricle is at its widest at the level of the pontomedullary junction, where a lateral recess on both sides extends to the lateral border of the brain stem. At this point, the lateral aperture of the fourth ventricle (foramen of Luschka) provides access to the subarachnoid space at the cerebellopontine angle, and CSF flows through it into the lateral extension of the pontine cistern. Occasionally, a lateral recess may not open.
The roof of the fourth ventricle is formed by the superior and inferior medullary veli. Superiorly, a thin sheet of tissue, the superior medullary velum, stretches across the ventricle between the converging superior cerebellar peduncles (see Fig. 5.10). The superior medullary velum is continuous with the cerebellar white matter and is covered dorsally by the lingula of the superior vermis. The inferior medullary velum is more complex and is composed mostly of a thin sheet, devoid of neural tissue, formed by ventricular ependyma and the pia mater of the tela choroidea, which covers it dorsally. A large median aperture (foramen of Magendie) is present in the roof of the ventricle as a perforation in the posterior medullary velum, just inferior to the nodule of the cerebellum. CSF flows from the ventricle through the foramen into the cerebellomedullary cistern.
Circumventricular Organs
The walls of the ventricular system are lined with ependymal cells, beneath which lies a subependymal layer of glia. At certain sites, collectively referred to as circumventricular organs (Fig. 5.12), specialized ependymal cells called tanycytes are also present. Ependyma and tanycytes may be involved in secretion into the CSF; transport of neurochemicals from subjacent neurones, glia or vessels to the CSF; transport of neurochemicals from CSF to the same subjacent structures; and chemoreception. In addition, in the adult, the ependymal and subependymal glial cell layers are the source of undifferentiated stem cells (Mercier, Kitasako, and Hatton 2002), currently under intensive study for their potential neurorestorative properties.
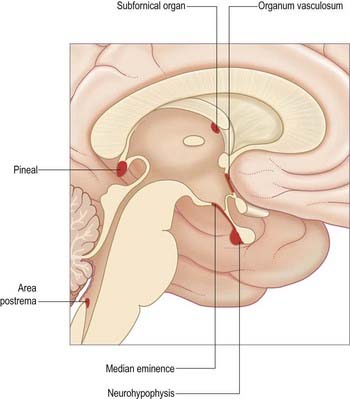
Fig. 5.12 Median sagittal section of the brain indicating the locations of the circumventricular organs.
The circumventricular organs are midline sites in the ventricular walls (McKinley et al 2003), where the blood–brain barrier is absent. They include the vascular organ (organum vasculosum), subfornical organ, neurohypophysis, median eminence, subcommissural organ, pineal gland and area postrema.
Choroid Plexus and Cerebrospinal Fluid
Choroid Plexus
Choroid plexuses are located in the lateral ventricles, the third ventricle and the fourth ventricle (see Figs. 5.3, 5.5, 5.6).
In the lateral ventricle, the choroid plexus extends anteriorly as far as the interventricular foramen, through which it is continuous across the third ventricle with the plexus of the opposite lateral ventricle. From the interventricular foramen, the plexus passes posteriorly, in contact with the thalamus, curving around its posterior aspect to enter the inferior horn of the ventricle and reach the hippocampus. Throughout the body of the ventricle, the choroid fissure lies between the fornix superiorly and the thalamus inferiorly (see Fig. 5.6).
From above, the tela choroidea is triangular, with a rounded apex between the interventricular foramina, often indented by the anterior columns of the fornices (see Fig. 5.3). Its lateral edges are irregular and contain choroid vascular fringes. At the posterior basal angles of the tela, these fringes continue and curve into the inferior horn of the ventricle; centrally, the pial layers depart from each other as described earlier. When the tela is removed, a transverse slit (the transverse fissure) is left between the splenium and the junction of the ventricular roof and the tectum. It marks the posterior limit of the extracerebral space enclosed by the posterior extensions of the corpus callosum above the third ventricle. The latter contains the roots of the choroid plexus of the third ventricle and of the lateral ventricles, enclosed between the two layers of pia mater (see Fig. 5.6). The choroid plexus of the third ventricle is attached to the tela choroidea, which is, in effect, the thin roof of the third ventricle as it develops during fetal life. In coronal sections of the cerebral hemispheres, the choroid plexus of the third ventricle can be seen in continuity with the choroid plexus of the lateral ventricles (see Fig. 5.6).
The choroid plexus of the fourth ventricle is similar in structure to that of the lateral and third ventricles. Thus, the roof of the inferior part of the fourth ventricle develops as a thin sheet in which the pia mater is in direct contact with the ependymal lining of the ventricle. This thin sheet forms the tela choroidea of the fourth ventricle, lying between the cerebellum and the inferior part of the roof of the ventricle. The choroid plexus of the fourth ventricle is T-shaped, having vertical and horizontal limbs, but this form varies widely. The vertical (longitudinal) limb is double, flanks the midline and is adherent to the roof of the ventricle. The limbs fuse at the superior margin of the median aperture (foramen of Magendie) and are often prolonged on the ventral aspect of the cerebellar vermis. The horizontal limbs of the plexus project into the lateral recesses of the ventricle. Small tufts of plexus pass through the lateral apertures (foramina of Luschka) and emerge, still covered by ependyma, in the subarachnoid space of the cerebellopontine angle.
Cerebrospinal Fluid
Hydrocephalus
Obstruction of the circulation of CSF leads to the accumulation of fluid (hydrocephalus), which causes compression of the brain (Fig. 5.13). Within the brain, critical points at which obstruction may occur correspond to the narrow foramina and passages of the ventricular system. Thus, obstruction of the interventricular foramen, as with an intraventricular tumour, causes enlargement of one or both lateral ventricles. Obstruction of the cerebral aqueduct, which may be congenital, due to atresia of the aqueduct, or acquired, as in ependymitis accompanying chronic infection (e.g. tuberculous meningitis), leads to enlargement of both lateral ventricles and the third ventricle. Obstruction or congenital absence of the apertures of the fourth ventricle leads to enlargement of the entire ventricular system. Obstruction or restriction of CSF circulation can also occur within the subarachnoid space as a result of meningeal adhesions caused by meningitis. When this occurs at the level of the tentorial notch, passage of CSF from the posterior fossa to its sites of reabsorption is restricted.
McKinley M.J., McAllen R.M., Davern P., Giles M.E., Penschow J., Sunn N., Uschakov A., Oldfield B.J. The sensory circumventricular organs of the mammalian brain. Adv. Anat. Embryol. Cell Biol. 2003;172:III-XII. 1–122
Mercier, Kitasako, Hatton, 2002Mercier F. Kitasako J.T. Hatton G.I. Anatomy of the brain neurogenic zones revisited: fractones and the ?broblast/macrophage network. J. Comp. Neurol. 451:2002;170-188.
Describes the structure and ultrastructure of the basal laminae and subependymal layer.
Paulson O.B. Blood–brain barrier, brain metabolism and cerebral blood flow. Eur. Neuropsychopharmacol. 2002;12:495-501.
Russell D.S. Observations on the pathology of hydrocephalus. Medical Research Council; 1949.
Strazielle, Ghersi-Egea, 2000Strazielle N. Ghersi-Egea J.F. Choroid plexus in the central nervous system: biology and physiopathology. J. Neuropathol. Exp. Neurol. 59:2000;561-574.
Wolburg, Lippoldt, 2002Wolburg H. Lippoldt A. Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul. Pharmacol. 38:2002;323-337.
Reviews the molecular properties of the tight junctions between endothelial cells that constitute the blood–brain barrier.